Abstract
During hemodiafiltration, a large amount of substitution fluid, which has the same electrolyte and buffer concentration as the dialysate, is infused into the patients. Since the composition of the dialysis fluid is considerable different from the blood, bidirectional transmembrane exchanges occur which affect the final balance of vital anions and cations. These dynamics are greatly influenced by both the site of the infusion, i.e. before, halfway or after the dialyzer, and the magnitude of the convection volume. The current chapter describes the effect of hemodiafiltration on a variety of small molecular weight substances, such as sodium, potassium, calcium, magnesium, bicarbonate, chloride, phosphate and acetate. In addition, the influence of hemodiafiltration on some of the key parameters involved in CKD-MBD, including vitamin D, parathyroid hormone and fibroblast growth factor 23, is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electrolytes
- Sodium
- Potassium
- Magnesium
- Bicarbonate
- Chloride
- Phosphate
- Acetate
- Vitamin D
- Parathyroid hormone
- FGF23
- CKD-MBD
Sodium
Sodium is a small positively charged cation. Besides covalent binding, sodium will also bind electrostatically to negatively charged proteins and lipids and as such there is a difference between the absolute sodium concentration in serum and that reported by standard laboratory potentiometry methods which determine sodium activity. Thus, as blood passes through a dialyzer, only that sodium which is freely available to diffuse will cross the dialyzer membrane into dialysate if there is a positive gradient between serum sodium activity and that in the dialysate. As such sodium chemically or electrically bonded to other molecules is not freely available for diffusion. In addition, diffusion of molecules must maintain electrical neutrality (Gibbs-Donnan effect). On the other hand, during convection sodium can move across the dialyzer membrane with convective plasma water movement, which includes “free” sodium but also sodium bound to small molecular weight complexes. During extracorporeal therapies, the dialyzer membrane surface becomes coated with proteins. As such, sodium movement is then reduced by binding to proteins in this dialyzer membrane boundary layer. Thus, the amount of sodium in the ultrafiltrate will be slightly lower than that in plasma water [1]. The sieving coefficient (the ratio of ultrafiltrate to serum concentration) varies with predilution compared to postdilution haemofiltration, due to the diluting effect of infusing fluid and differences in membrane protein deposition. Although the difference in sieving coefficient appears small (Fig. 11.1), this will potentially make a difference in sodium balance when large volumes of fluid are exchanged (Fig. 11.2). The site of the replacement fluid has a much greater potential effect on the sodium balance compared to the replacement/substitution fluid sodium concentration [2].
HDF combines both convective and diffusive clearance [3]. The convective element reduces net diffusion, particularly with the predilution mode by diluting solute concentrations. Depending upon the gradient between dialysate and serum sodium concentrations, sodium may be additionally lost or gained by diffusion [4]. Pedrini proposed a formula to estimate sodium changes with different forms of dialysis to account for different predialysis plasma sodium concentrations and different plasma to dialysate sodium gradients [5]. Acetate free hemodiafiltration or biofiltration (AFB), in which a bicarbonate free dialysate is used in combination with postdilution infusion of sodium bicarbonate, leads to a greater predicted positive sodium balance, followed by postdilution HDF with lower convective exchange volumes (Fig. 11.3). In theory, high volume postdilution HDF would be predicted to lead to an increased positive sodium balance compared to standard bicarbonate haemodialysis (HD), as larger convective volumes will increase protein deposition on the dialyzer membrane surface so increasing the charged protein polarisation boundary layer and so restricting sodium movement from the plasma water into the ultrafiltrate by reducing the sieving coefficient.
Theoretical predictive differences in sodium balance based on the Pedrini equation [5] between haemodialysis and haemodiafiltration treatments, for a range of different dialysate (Dsodium) to plasma (Psodium) sodium gradients
Although convective modes appear to result in a positive sodium balance, it must be recognised this relates to isovolaemic treatments [6], whereas in standard clinical practice most patients gain weight between treatment sessions and so will require ultrafiltration, which will result in a net overall sodium loss. However, HDF treatments, particularly when operated in the postdilution mode, are more likely to lead to a positive sodium balance than equivalent predilution HDF treatments (Fig. 11.4) [5], and this needs to be considered when choosing a sodium concentration for a given on-line HDF modality. In clinical practice, when patients are switched from standard haemodialysis to haemodiafiltration, a lower dialysate sodium concentration should be selected.
Theoretical predictive differences in sodium balance based on the Pedrini equation [5] between pre and postdilution modes of haemodiafiltration treatments, for a range of different dialysate (Dsodium) to plasma (Psodium) sodium gradients
Potassium
Potassium is a small positively charged cation that is rapidly cleared during HD, as clearance is predominantly by diffusion. As with sodium, the sieving coefficient of potassium for convective clearance is less than one [6], and similarly the sieving coefficient is slightly higher for predilution HDF compared to the postdilution mode [2]. Compared to hemodialysis, less potassium will be removed by pure convective treatments. Although the sieving coefficient of potassium is higher with predilution, as the replacement/substitution fluids contain a much lower potassium concentration than the serum, the diluting effect of predialyzer fluid administration results in a lower potassium clearance compared to postdilution mode. Although HDF adds a diffusive element to potassium clearance, the convective clearance reduces the diffusive potassium clearance during passage through the dialyzer, and as such reduces potassium loss. As such, HDF, particularly in the predilution mode, is not as effective in total potassium removal compared to HD [7]. Meanwhile, the changes in electrocardiography QTc intervals, QRS dispersion and supraventricular premature beats depend more on the gradient between plasma and dialysate concentrations, rather than dialysis mode [8, 9], and, as with HD, modelling of potassium in the dialysate to minimise the potassium gradient reduces the risk of arrhythmias during HDF [10].
Although potassium removal during HDF is not affected by dialysate sodium concentration, the post-treatment rebound in plasma potassium is faster and greater when a positive sodium gradient (dialysate to plasma sodium) has been used compared to a negative sodium gradient [11].
Calcium
Extracorporeal calcium clearance is more complex than that of sodium and potassium due to greater protein binding of calcium. Approximately 40 % of serum calcium is bound, predominantly to albumin, but also to other negatively charged proteins and solutes. The equilibrium dynamic between free and bond calcium is not only affected by albumin concentration but also pH. Thus, when considering calcium clearance during extracorporeal therapies, changes in serum bicarbonate and pH have to be considered. Due to protein binding, the sieving coefficient for calcium is lower than that for sodium and potassium (Fig. 11.5). The difference in calcium mass transfer between predilution and postdilution modes is greater for calcium compared to sodium and potassium, as the calcium concentration of the replacement/ substitution fluids is typically higher than serum ionised calcium [2]. As such, with isovolaemic higher convection treatments (no net ultrafiltration) then net calcium balance becomes more positive with postdilution mode [2, 12] (Fig. 11.6). Although calcium balance will also depend upon the calcium composition of the replacement/substitution fluids, as with sodium, the infusion site of replacement/substitution fluids has a potentially greater effect on calcium balance [13]. Although some calcium will be removed by net ultrafiltration, most patients will be in a positive calcium balance when treated with convective techniques, and this accounts for the reports of lower PTH and greater response to calcifediol in patients treated by HDF [14].
Although adding a diffusive clearance with HDF will alter calcium balance depending upon the gradient between the respective dialysate and serum ionised concentrations, convective clearance of calcium has a greater effect on net calcium balance [15]. Predilution mode will have the least effect on diffusive clearance. Depending upon the calcium concentration chosen, administration of fluid in predilution mode will either increase or reduce the ionised plasma calcium concentration entering the dialyzer, but as the same calcium concentration is present in both the dialysate and replacement/substitution fluids, this will minimise the differential calcium gradient, between calcium entering the hemofilter and calcium in the dialysate. The gradient for diffusion will potentially be greater in postdilution mode [13]. As such, calcium mass transfer in HDF is also affected by the infusion mode. For a given concentration gradient between blood and dialysate, calcium balance in low volume post-dilution HDF may be similar to conventional HD [15], or positive, depending upon the amount of ultrafiltration [16]. Thus some clinicians have suggested to lower the dialysate calcium concentration during postdilution HDF to reduce the risk of a positive calcium balance [12].
As net calcium balance could be negative in the pre-dilution mode, especially with higher ultrafiltration rates and targeted weight loss, it has been suggested that the dialysate calcium concentration should be increased by approximately 0.25 mmol/l to maintain a comparable balance, when switching treatment from HD to pre-dilution HDF [15].
When choosing a dialysate calcium concentration for HDF, ideally this should be prescribed taking into consideration both the predicted HDF dialysis calcium mass balance, and the other concomitant therapies (calcium containing medications, vitamin D analogues) and the underlying type of mineral bone disease.
Magnesium
Magnesium, similar to calcium, has significant plasma protein binding, with some 40–50 % protein bound. As magnesium is widely present in the diet, healthy dialysis patients are more likely to develop hypermagnesaemia, and as such most dialysate and replacement/ substitution fluids contain equivalent normal or low ionised levels of magnesium [12]. Magnesium has a similar sieving coefficient to calcium, and as the replacement/substitution fluids have an equivalent magnesium concentration to the plasma ionised magnesium, predilutional modes may result in a negative magnesium balance, whereas postdilutional convective modes will potentially result in a positive magnesium balance [6].
The difference between calcium and magnesium balance is that most centres use a standard dialysate magnesium concentration of 0.5 mmol/l, which is around the lower limit of the normal plasma ionised magnesium concentration of 0.55–0.75 mmol/l, whereas on the other hand most dialysate calcium concentrations have a relatively high ionised dialysate calcium concentration of ≥1.0–1.25 mmol/l compared to the plasma ionised calcium concentration of 1.1–1.4 mmol/l [17]. As such, there is usually diffusive loss of magnesium during conventional HD. Adding a diffusive clearance with HDF may reduce diffusive losses in predilutional mode by diluting down the concentration gradient, and on the other hand increase diffusional losses in the postdilutional mode. However as with calcium, convection plays a greater role in determining magnesium balance. Predilution leads to greater convective losses, which increase with higher convection volumes [2].
Bicarbonate
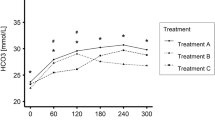
Bicarbonate is a small negatively charged anion which requires transport across cell membranes, yet due to the protein boundary layer deposited on the dialyzer surface readily passes across the dialyzer, with a slight positive sieving coefficient. As such the sieving coefficient is greater in predilutional mode compared to postdilution [18]. Most replacement/substitution fluids contain supraphysiological levels of bicarbonate, and those for HDF will also contain a small amount of acetate, to prevent calcium carbonate deposition [19]. Predilution convection will result in less overall base accumulation [20], as there will be increased convective losses of both bicarbonate and acetate compared to the postdilution mode. Infusion of bicarbonate into the plasma leads to an increase in plasma pH, which then results in both an increased influx of calcium into cells and also increased plasma protein binding, so increasing the overall net calcium balance.
Bicarbonate moves rapidly from dialysate into plasma by diffusion down a concentration gradient. As such, adding a diffusional element with HDF improves correction of acidosis compared to pure convection techniques. Although in theory predilution by reducing the concentration gradient could potentially reduce the net bicarbonate influx compared to postdilution mode, in clinical practice there is no discernible difference between pre- and postdilution modes due to the predominance of diffusive bicarbonate movement compared to that of convective transport [21].
Chloride
As with bicarbonate, the sieving coefficient for chloride convection is just above one with predilution HDF and falls slightly with post-dilution [2]. Although most chloride concentrations for HD dialysates are around 110 mmol/l, commercially available replacement/substitution fluids for continuous forms of hemofiltration and hemodiafiltration have a wide range of concentrations from 105 to 115 mmol/l. Depending upon the relative difference between serum and HDF replacement/substitution fluids, chloride balance may be negative or positive [6, 20]. The use of replacement fluids with lower concentrations of chloride increase the incidence of hypochloraemia, but improve the correction of metabolic acidosis, whereas higher chloride replacement solutions may lead to hyperchloraemia and metabolic acidosis. For the same chloride concentration predilution will tend to reduce chloride losses compared to postdilution [22].
As with bicarbonate, chloride quickly diffuses across the dialyzer membrane during HDF. Although in theory predilution HDF mode will reduce any chloride gains and losses in hypochloraemic and hyperchloraemic patients respectively, compared to postdilution mode, in clinical practice chloride balance is predominantly determined by diffusion. Most commercially available dialysate fluids contain a chloride of 110 mmol/l, and this will determine net chloride gains and losses during a treatment session [21].
Phosphate
Although phosphate is a relatively small molecule, due to its charges it has a larger water shell and so moves somewhat slower, such that whereas urea concentration in a red blood cell will fall during the time it takes to pass through the dialyzer, phosphate will not. As phosphate is predominantly intracellular, phosphate clearance by extracorporeal therapies is limited by the rate of movement from intracellular stores into plasma water, rather than by clearance from plasma water [23]. As replacement/substitution fluids for hemofiltration or hemodiafiltration traditionally contain no phosphate, more phosphate is cleared with higher convection volumes [24] and postdilutional convective therapies clear more phosphate than predilution modes. Similarly, as dialysates do not contain phosphate, adding a diffusional clearance with HDF, then more phosphate is cleared by diffusion than convection [25]. This diffusional element is lower in the predilutional mode as compared to postdilution HDF, and also reduced with increasing haematocrit [25]. Several observational studies have reported that serum phosphate concentrations are lower when switching patients from HD to HDF, or comparing cohorts of HDF to HD patients with similar small solute clearance [26, 27]. In clinical trials, predialysis phosphate levels either did not differ between patients treated with online postdilution hemodiafiltration or (mainly) high-flux hemodialysis [28, 29], or was slightly but significantly lower in patients treated with postdilution HDF as compared to low-flux hemodialysis [30]. However it has to be remembered that serum phosphate is a composite of dietary phosphate intake, gastrointestinal binding with phosphate binders, residual renal clearance and dialyzer clearance.
Acetate
Dialysates and on-line infusion fluids are made by mixing treated potable water with acid and bicarbonate concentrates. The mixture then contains calcium and carbonate which can precipitate out within the dialysate circuitry in the haemodialysis machine. To prevent or minimise such precipitation, a small amount of acetate (3–4 mmol/l) is typically added to the dialysate. Historically in the 1960s–1980s standard dialysates for low flux haemodialysis contained acetate rather than bicarbonate as the anionic base. Although meta-analysis of randomised trials did not show an overall benefit for bicarbonate dialysate, several studies linked acetate based dialysates with a greater risk for intra-dialytic hypotension. During on-line HDF, re-infusion of an acetate containing fluid will lead to increased acetate delivery to the patient. Predilution fluid replacement will reduce acetate delivery compared to postdilution at similar infusion rates. There will also be an additional acetate influx due to the presence of acetate in the dialysate. During on-line HDF the serum acetate will typically be limited to around a maximum of 0.5 mmol/l [31], but this hides the fact that there is a net acetate flux, with acetate passing into cells. Alternatives to on-line HDF include HDF using pre-prepared sterile bags of substitution fluid, or acetate free biofiltration, in which a bicarbonate free dialysate is used in combination with reinfusion of sodium bicarbonate in postdilution mode [32]. Small studies have reported that acetate free treatments cause less leukocyte and monocyte activation and lower inflammatory cytokine releases [33]. However there have been no clinical studies showing any differences in intra-treatment cardiovascular stability or longer term nutritional or survival differences between acetate containing fluids and acetate free fluids for HDF [5, 34].
Teaching Points I
-
High volume HDF may result in a net zero sodium balance, depending on the sodium concentration of the substitution fluid used
-
The infusion site of both calcium and sodium has a greater effect on ionic balance than its concentrations in the substitution fluid
-
High volume HDF may result in a positive calcium balance, depending on dialysate calcium concentration and prescribed medication
-
Predilution HDF may lead to undesirable magnesium losses
-
In HDF, both bicarbonate and chloride transport are mainly determined by diffusion
-
As far as serum levels of phosphate are concerned, HDF offers no advantage over high-flux HD.
Vitamin D
Since the lipophylic vitamin D metabolites like cholecalciferol, calcitriol, and its catabolic products 24,25 dihydroxycholecalciferol and 1,24,25 (OH)3 cholecalciferol are all nearly completely bound to Vitamin D Binding Protein (VDBP), their clearance by either diffusion or convection is negligible. Although in peritoneal dialysis some vitamin D may be lost in the dialysate, along with VDBP, this dialysis technique too does not lead to a clinically relevant decline in its plasma concentrations [35, 36]. Nevertheless, due to changes in calcium and phosphate homeostasis specifically induced by HDF techniques as described above, altered vitamin D levels could be induced by biological feedback systems. In addition, improved clearance by HDF of (yet unidentified) middle-molecules involved in vitamin D metabolism could change its levels.
Observational data do show indeed that online HDF is associated with higher 25(OH)D3 levels as compared to conventional HD [37]. Although no correction for potential differences in calcium balance was carried out, the presumed impact of calcium balance on this storage form of vitamin D is probably limited, and therefore this finding suggests either improved gastrointestinal uptake or cutaneous production of 25(OH)D3, or delayed catabolism during HDF. The clearance of fibroblast growth factor 23 (FGF23), a vitamin D catabolic hormone, is higher for HDF, see next paragraph [38]. However, this does not explain the higher levels of 25(OH)D3, as the assays used to detect this vitamin D compound do not differentiate 25(OH)D3 from its catabolic product 24,25(OH)2D3. FGF23 could however increase the ratio 24,25(OH)2D3/25(OH)D3, which may remain unnoticed due to the lack of specificity of the assay. Nevertheless, the observation that supplementation of non-active vitamin D to patients treated by on-line HDF induces a higher peak level than those treated by conventional HD does suggest a “vitamin D sparing effect” of the former technique, see Fig. 11.7 [14]. This assumption is also supported by the evolution of 25(OH)D3 levels in non-supplemented patients in the two different dialysis modalities, showing a steeper decline for conventional HD [14].
Response to treatment with calcifediol. Left panel: Levels of 25(OH)vit D3 before and after 4 months of treatment with iv calcifediol (266 μg once a week after treatment) in 23 patients treated with high-flux hemodialysis and 13 patients treated with postdilution online hemodiafiltration. Right panel: Levels of 25(OH)vit D3 without supplementation during the same time frame in 15 patients treated with high-flux hemodialysis and 8 patients treated with postdilution online hemodiafiltration (Reprinted from Perez-Garcia et al. [14]. With permission from Revista Nefrología)
Since the skin is the most important source of vitamin D by far, due to local production under the influence of UV-B light, a potentially important advantage of HDF may be the improvement of skin hyperpigmentation as compared to conventional HD [39]. This improvement is likely related to improved clearance of middle-molecules involved in regulation of melanin since this feature is also present in peritoneal dialysis [40]. The attenuated hyperpigmentation probably facilitates the penetration of UV-B light [41], although the hyperpigmentation in CKD may not be due solely to a melanin dependent mechanism.
Parathyroid Hormone (PTH)
PTH is a polypeptide hormone with a molecular weight of 9.4 kDa, and as such it classifies as a middle molecule. These properties predict improved dialysability of PTH in HDF and indeed this hormone can be detected in the dialysate, although the amounts of intact PTH are low [42]. The most important method of clearance appears to be adsorption onto the dialyser membrane and is dependent on the material of the membrane used [43]. At least when compared to high-flux HD, online HDF does not lead to increased clearance of PTH in either observational studies [44] or prospective randomized trials [29]. Based on its molecular weight, improved clearance during HDF over low-flux HD is likely, as high-flux HD, even after correcting for ionized calcium, leads to lower PTH concentrations than low-flux HD [45].
Besides direct increased clearance during HDF, this technique can influence PTH levels by modifying PTH secretion from the parathyroid glands. Several humoral factors are involved in PTH regulation, like calcium and phosphate concentrations, and active vitamin D and FGF23 levels. The latter two hormones are both inhibitors of PTH production and secretion, but their kinetics diverge during HDF. As vitamin D (at least 25(OH)D3) tends to increase and FGF23 tends to decline (see next paragraph), the net effect on PTH secretion may be balanced. As outlined above, HDF can impact calcium homeostasis in a complex fashion, depending not only on calcium concentrations in the dialysate and replacement fluids, but also on to the mode of HDF, i.e. predilution or postdilution. Since calcium is the single most important immediate regulator of PTH secretion, and its effects are swift [46], the consequences of HDF on PTH are to a large extent mediated by changes in either calcium concentration or balance. Furthermore, some clearance of PTH by absorption onto the dialyser membrane or filtration across highly permeable dialysers also occurs [43]. Indeed, targeting calcium balance directly by modifying calcium concentration in dialysate or replacement fluid directly affects PTH concentrations [47], and therefore, an overall decline in PTH can be anticipated during HDF. This also applies to children where a low calcium concentration of 1.25 mmol/l can be advised to prevent fracture risk due to PTH oversuppression when using calcium concentration of 1.5 mmol/l [48]. On the contrary, when a replacement fluid not containing calcium is used, calcium balance will be negative and PTH will increase accordingly [49]. An optimal calcium concentration during HDF, defined by a neutral effect on PTH, is between 1.25 and 1.5 mmol/l [50].
Theoretically, in addition to calcium, differential effects of HDF as compared to conventional HD on phosphate concentrations or phosphate balance could impact PTH, as phosphate stimulates PTH, at least so in healthy subjects. However, as outlined in the previous section of this chapter, in contrast to observational studies or non-randomized trials [26, 27], well-designed prospective trials show no or only very limited enhanced phosphate clearance by HDF as compared to HD [29, 51, 52]. Therefore, altered clearance of phosphate during HDF probably does not induce meaningful changes in PTH.
In conclusion, during HDF a slight decline in PTH can be expected, as a consequence of increased clearance, absorption onto the membrane and a tendency for a slight positive calcium balance when using the same calcium concentration of the replacement solution as the dialysate in conventional HD. Given the increasing prevalence of adynamic bone disease in HD populations, and the association of that specific bone disease with dismal cardiovascular outcome, a slightly lower calcium concentration for the ultrapure dialysate and infusate during HDF could be considered.
Fibroblast Growth Factor 23 (FGF23)
Like PTH, FGF23 qualifies as a middle-molecule. As the molecular weight of the biological active compound is 32 kD and this polypeptide is hydrophilic, theoretically increased clearance during HDF can be expected. Although the metabolic fate of FGF23 during end- stage renal disease is unclear, limited data point to diminished catabolism [53]. Therefore, the relative importance of renal replacement therapy in clearing this phosphate-regulating hormone may be of high importance, given the strong predictive power of high levels of FGF23 to all cause and cardiovascular mortality [54]. Despite the above-mentioned properties, FGF23 levels decline with more intensive low-flux HD schedules as well [55]. This observation can likely be explained by improved control of circulating factors of importance for FGF23 production, like phosphate itself. As predicted from its properties, HDF leads to a decline of FGF23 of 56 % percent from its value directly prior to the treatment session, as compared to a 36 % reduction during high-flux HD, pointing to the relative importance of convective clearance of this compound [38]. Likewise, in paediatric HDF, a substantial reduction of FGF23 was observed [48]. When compared to low-flux HD, clearance of FGF23 by HDF is even more impressive, with negligible decline during the former modality [56]. In a small subset of the prospective CONTRAST trial, comparing low-flux HD with HDF, the former technique led to a 10 % reduction of FGF23, while patients randomized to HDF had a decline of almost 50 % (Den Hoedt, ASN 2010 PO1436). Despite the striking consistency that exists among large observational studies showing strong independent associations between FGF23 and a range of clinically important outcomes measures, no evidence yet has shown improved outcome when directly targeting FGF23 by either modifying dietary phosphate intake or the use of phosphate binder therapy. If FGF23 decline induced by high-volume HDF improves clinical outcome is currently unknown.
Teaching Points II
-
In CKD, the level of vitamin D, which is highly protein bound, is decreased
-
Due to its protein binding, vitamin D concentrations are hardly influenced by convective transport
-
Since vitamin D levels increase after HDF, other mechanisms, such as changes vitamin D regulating factors, and attenuation of CKD-related hyperpigmentation, might play a role
-
PTH is a MMW substance, which, on theoretical grounds, may be removed by convection. Yet, in large HDF studies, reductions in PTH were modest or absent compared to high-flux HD.
-
FGF23, which has an even larger MW than PTH, is reduced by HDF up to 50 % of its initial value
-
Currently, data showing an association between reductions in both FGF23 and mortality are lacking
References
Shaldon S. Role of small molecule removal in the control of treatment morbidity with haemodialysis and haemofiltration. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1981;18:249–55.
Uchino S, Cole L, Morimatsu H, Goldsmith D, Ronco C, Bellomo R. Solute mass balance during isovolaemic high volume haemofiltration. Intensive Care Med. 2003;29:1541–6.
Gotch FA, Lam MA, Prowitt M, Keen M. Preliminary clinical results with sodium-volume modeling of hemodialysis therapy. Proc Clin Dial Transplant Forum. 1980;10:12–7.
Shaldon S, Baldamus CA, Beau MC, Koch KM, Mion CM, Lysaght MJ. Acute and chronic studies of the relationship between sodium flux in hemodialysis and hemofiltration. Trans Am Soc Artif Intern Organs. 1983;29:641–4.
Pedrini LA, De Cristofaro V, Pagliari B, Ruggiero P. Dialysate/infusate composition and infusion mode in on-line hemodiafiltration. Contrib Nephrol. 2002;137:344–9.
Tan HK, Uchino S, Bellomo R. Electrolyte mass balance during CVVH: lactate vs. bicarbonate-buffered replacement fluids. Ren Fail. 2004;26:149–53.
Severi S, Vecchietti S, Cavalcanti S, Mancini E, Santoro A. Electrocardiographic changes during hemodiafiltration with different potassium removal rates. Blood Purif. 2003;21:381–8.
Munoz RI, Montenegro J, Salcedo A, et al. Effect of acetate-free biofiltration with a potassium-profiled dialysate on the control of cardiac arrhythmias in patients at risk: a pilot study. Hemodial Int. 2008;12:108–13.
Coppolino G, Bolignano D, Parisi S, et al. Experimental therapies in renal replacement: the effect of two different potassium acetate-free biofiltration protocols on striated muscle fibers. Ther Apher Dial. 2007;11:375–81.
Buemi M, Aloisi E, Coppolino G, et al. The effect of two different protocols of potassium haemodiafiltration on QT dispersion. Nephrol Dial Transplant. 2005;20:1148–54.
De Nicola L, Bellizzi V, Minutolo R, et al. Effect of dialysate sodium concentration on interdialytic increase of potassium. J Am Soc Nephrol. 2000;11:2337–43.
Severi S, Bolasco P, Badiali F, et al. Calcium profiling in hemodiafiltration: a new way to reduce the calcium overload risk without compromising cardiovascular stability. Int J Artif Organs. 2014;37:206–14.
Karamperis N, Jensen D, Sloth E, Jensen JD. Comparison of predilution hemodiafiltration and low-flux hemodialysis at temperature-controlled conditions using high calcium-ion concentration in the replacement and dialysis fluid. Clin Nephrol. 2007;67:230–9.
Perez-Garcia R, Albalate M, de Sequera P, et al. On-line haemodiafiltration improves response to calcifediol treatment. Nefrologia. 2012;32:459–66.
Malberti F, Ravani P. The choice of the dialysate calcium concentration in the management of patients on haemodialysis and haemodiafiltration. Nephrol Dial Transplant. 2003;18 Suppl 7:vii37–40; discussion vii57.
Floccari F, Aloisi E, Nostro L, et al. QTc interval and QTc dispersion during haemodiafiltration. Nephrology. 2004;9:335–40.
Vitale C, Marangella M, Ramello A. Dialysate/infusate calcium and magnesium. Contrib Nephrol. 2002;137:350–6.
Sternby JP, Nilsson A, Garred LJ. Diffusive-convective mass transfer rates for solutes present on both sides of a dialyzer membrane. ASAIO J. 2005;51:246–51.
Hernandez-Jaras J, Garcia H, Ferrero JA. Changes in the anion gap in patients undergoing hemodiafiltration. Nefrologia. 2000;20:66–71.
Cole L, Bellomo R, Baldwin I, Hayhoe M, Ronco C. The impact of lactate-buffered high-volume hemofiltration on acid-base balance. Intensive Care Med. 2003;29:1113–20.
Ahrenholz P, Winkler RE, Ramlow W, Tiess M, Thews O. On-line hemodiafiltration with pre- and postdilution: impact on the acid-base status. Int J Artif Organs. 1998;21:321–7.
Davenport A, Worth DP, Will EJ. Hypochloraemic alkalosis after high-flux continuous haemofiltration and continuous arteriovenous haemofiltration with dialysis. Lancet. 1988;1:658.
Spalding EM, Chamney PW, Farrington K. Phosphate kinetics during hemodialysis: evidence for biphasic regulation. Kidney Int. 2002;61:655–67.
Troyanov S, Cardinal J, Geadah D, et al. Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003;18:961–6.
Spalding EM, Pandya P, Farrington K. Effect of high haematocrit on the efficiency of high-flux dialysis therapies. Nephron Clin Pract. 2008;110:c86–92.
Movilli E, Camerini C, Gaggia P, et al. Effect of post-dilutional on-line haemodiafiltration on serum calcium, phosphate and parathyroid hormone concentrations in uraemic patients. Nephrol Dial Transplant. 2011;26:4032–7.
Davenport A, Gardner C, Delaney M, Pan Thames Renal Audit G. The effect of dialysis modality on phosphate control: haemodialysis compared to haemodiafiltration. The Pan Thames Renal Audit. Nephrol Dial Transplant. 2010;25:897–901.
Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Ok E, Asci G, Toz H, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202.
Grooteman MP, van den Dorpel MA, Bots ML, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96.
Pizzarelli F, Cerrai T, Dattolo P, Ferro G. On-line haemodiafiltration with and without acetate. Nephrol Dial Transplant. 2006;21:1648–51.
Santoro A, Ferrari G, Spongano M, Badiali F, Zucchelli P. Acetate-free biofiltration: a viable alternative to bicarbonate dialysis. Artif Organs. 1989;13:476–9.
Todeschini M, Macconi D, Fernandez NG, et al. Effect of acetate-free biofiltration and bicarbonate hemodialysis on neutrophil activation. Am J Kidney Dis. 2002;40:783–93.
Pizzarelli F, Cerrai T, Dattolo P, Tetta C, Maggiore Q. Convective treatments with on-line production of replacement fluid: a clinical experience lasting 6 years. Nephrol Dial Transplant. 1998;13:363–9.
Joffe P, Heaf JG. Vitamin D and vitamin-D-binding protein kinetics in patients treated with continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int. 1989;9:281–4.
Prytula A, Wells D, McLean T, et al. Urinary and dialysate losses of vitamin D-binding protein in children on chronic peritoneal dialysis. Pediatr Nephrol. 2012;27:643–9.
Gracia-Iguacel C, Gallar P, Qureshi AR, et al. Vitamin D deficiency in dialysis patients: effect of dialysis modality and implications on outcome. J Ren Nutr. 2010;20:359–67.
Patrier L, Dupuy AM, Granger Vallee A, et al. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J Nephrol. 2013;26:342–9.
Shibata M, Nagai K, Usami K, Tawada H, Taniguchi S. The quantitative evaluation of online haemodiafiltration effect on skin hyperpigmentation. Nephrol Dial Transplant. 2011;26:988–92.
Lai CF, Kao TW, Tsai TF, et al. Quantitative comparison of skin colors in patients with ESRD undergoing different dialysis modalities. Am J Kidney Dis. 2006;48:292–300.
Shoenfeld N, Amital H, Shoenfeld Y. The effect of melanism and vitamin D synthesis on the incidence of autoimmune disease. Nat Clin Pract Rheumatol. 2009;5:99–105.
De Francisco AL, Amado JA, Prieto M, et al. Dialysis membranes and PTH changes during hemodialysis in patients with secondary hyperparathyroidism. Nephron. 1994;66:442–6.
Balducci A, Coen G, Manni M, et al. In vivo assessment of intact parathyroid hormone adsorption by different dialysis membranes during hemodialysis. Artif Organs. 2004;28:1067–75.
Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol. 2009;4:1944–53.
Makar SH, Sawires HK, Farid TM, Ali WM, Schaalan M. Effect of high-flux versus low-flux dialysis membranes on parathyroid hormone. Iran J Kidney Dis. 2010;4:327–32.
Messa P, Vallone C, Mioni G, et al. Direct in vivo assessment of parathyroid hormone-calcium relationship curve in renal patients. Kidney Int. 1994;46:1713–20.
Jean G, Mayor B, Hurot JM, et al. Biological impact of targeted dialysate calcium changes in haemodialysis patients: the key role of parathyroid hormone. Nephrol Dial Transplant. 2013;28:176–82.
Perouse de Montclos T, Ranchin B, Leclerc AL, et al. Online hemodiafiltration in children and hypoparathyroidism: a single-centre series of cases. Nephrol Ther. 2014;10:35–8.
Rius A, Hernandez-Jaras J, Pons R, et al. Kinetic of calcium, phosphate, magnesium and PTH variations during hemodiafiltration. Nefrologia. 2007;27:593–8.
Argiles A, Mourad G, Lorho R, et al. Medical treatment of severe hyperparathyroidism and its influence on anaemia in end-stage renal failure. Nephrol Dial Transplant. 1994;9:1809–12.
Locatelli F, Altieri P, Andrulli S, et al. Phosphate levels in patients treated with low-flux haemodialysis, pre-dilution haemofiltration and haemodiafiltration: post hoc analysis of a multicentre, randomized and controlled trial. Nephrol Dial Transplant. 2014;29:1239–46.
Penne EL, van der Weerd NC, van den Dorpel MA, et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis. 2010;55:77–87.
Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–85.
Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92.
Zaritsky J, Rastogi A, Fischmann G, et al. Short daily hemodialysis is associated with lower plasma FGF23 levels when compared with conventional hemodialysis. Nephrol Dial Transplant. 2014;29:437–41.
Miao LY, Zhu B, He XZ, et al. Effects of three blood purification methods on serum fibroblast growth factor-23 clearance in patients with hyperphosphatemia undergoing maintenance hemodialysis. Exp Ther Med. 2014;7:947–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Davenport, A., Vervloet, M. (2016). Effects of Convective Dialysis Techniques on Electrolytes and Mineral Metabolism. In: Nubé, M., Grooteman, M., Blankestijn, P. (eds) Hemodiafiltration. Springer, Cham. https://doi.org/10.1007/978-3-319-23332-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-23332-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23331-4
Online ISBN: 978-3-319-23332-1
eBook Packages: MedicineMedicine (R0)