Abstract
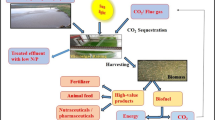
The world is facing the threat of global warming. This is associated with the modernization and increasing dependency of human beings on the fossil fuel. Fossil fuel are necessary for providing the increasing demand on energy. However, their combustion produces greenhouse gases such as carbon dioxide, methane, ozone, NOx, water vapour etc. (Kumar K et al., Bioresour Technol 102(8):4945–4953, 2011). CO2 is also continuously being added into the earth’s atmosphere through several natural sources such as volcanic eruptions, combustion of organic matters, autotrophic and heterotrophic respiration (Kumar K, Das D, Carbon dioxide sequestration by biological processes. In: Bhanage BM, Arai M (eds) Transformation and utilization of carbon dioxide. Springer, Heidelberg, pp 303–334, 2014; Sharma A et al., Enzyme Microb Technol 48:416–426, 2011). However, robust natural mechanisms of CO2 capture could maintain the balance of CO2 in the earth’s atmosphere. Global carbon cycle is disturbed mainly due to anthropogenic emissions of CO2 because of human activity (Kumar K, Das D, Carbon dioxide sequestration by biological processes. In: Bhanage BM, Arai M (eds) Transformation and utilization of carbon dioxide. Springer, Heidelberg, pp 303–334, 2014). Coal is the major contributor of CO2, which is in the range of 14–17 % depending upon its quality. Therefore, coal-based industries such as cement, steel and thermal power plants pollute the earth’s environment to a greater extent.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
The world is facing the threat of global warming. This is associated with the modernization and increasing dependency of human beings on the fossil fuel. Fossil fuel are necessary for providing the increasing demand on energy. However, their combustion produces greenhouse gases such as carbon dioxide, methane, ozone, NOx, water vapour etc. (Kumar et al., 2011). CO2 is also continuously being added into the earth’s atmosphere through several natural sources such as volcanic eruptions, combustion of organic matters, autotrophic and heterotrophic respiration (Kumar and Das, 2014; Sharma et al., 2011). However, robust natural mechanisms of CO2 capture could maintain the balance of CO2 in the earth’s atmosphere. Global carbon cycle is disturbed mainly due to anthropogenic emissions of CO2 because of human activity (Kumar and Das, 2014). Coal is the major contributor of CO2, which is in the range of 14–17 % depending upon its quality. Therefore, coal-based industries such as cement, steel and thermal power plants pollute the earth’s environment to a greater extent.
The greenhouse gases trap the solar heat and prevent it from dissipating into the space. This increases the earth’s temperature. The ecosystem of earth is affected by even a little increase in the global mean temperature. Melting glaciers, sea level rise, unpredicted rainfall and drought are some examples of global warming. Besides these, global warming increases the soil microbe’s respiration, which further adds CO2 into the earth’s atmosphere (Bardgett et al., 2008). CO2 is the major greenhouse gas of the flue gas (Kumar and Das, 2014). Keeling curve indicates a sharp rise in the global CO2 concentration after 1950 (Kumar et al., 2011). In July, 2014, CO2 concentration in the earth’s atmosphere was 399.00 ppm as measured at Mauna Loa Observatory, Hawaii, USA (http://www.esrl.noaa.gov/gmd/ccgg/trends/). This is significantly higher than the range of the CO2 concentration (180–300 ppm) measured over the history of earth. Similarly, CO2 concentration was found to increase at the rate of 2.05 ppm per year in 2013 compared to 0.94 ppm per year in 1959 (http://www.esrl.noaa.gov/gmd/ccgg/trends/).
Thus, CO2 sequestration is imperative to combat the challenges of global warming. Several natural processes continuously remove CO2 from the atmosphere. For example, large mass of oceanic water dissolves atmospheric CO2 and forms weak acids, which eventually forms bicarbonate due to reaction between carbonate anions and water (Kumar and Das, 2014; Falkowski et al., 2000). Similarly, large quantity of CO2 is continuously sent to the bottom of the ocean due to differential solubility of CO2 in cold and hot saline water. Besides these natural processes of CO2 capture, CO2 can be sequestered artificially, either by physical or biological methods. Physical methods involve natural mineral carbonation, scrubbing and geological (depleted oil and gas reservoirs) and oceanic injection (deep aquifers) etc. (Mirjafari et al., 2007; Allen et al., 2005). These methods are expensive as it also involves the collection, transportation and storage of CO2 gas. In addition, it poses potential threat to the environment due to possibility of the accidental leakage of CO2 (Bachu, 2000). Biological methods are another alternative, which mainly involves the photosynthesis process by oceanic phytoplanktons, terrestrial plants, algae and use of carbonic anhydrase (CA) enzyme. In addition, some non-photosynthetic bacteria also utilize CO2 for their metabolic activity (Kumar and Das, 2014).
As an aggressive move, CO2 sequestration through algal biomass production is a promising option. CO2 sequestered algal biomass can be further utilized for the biofuel productions (biodiesel, bioethanol, biohydrogen) and several other value added products of industrial importance (Kumar et al., 2011; Loubiere et al., 2009). Microalgae can be cultivated in non-agriculture land or even in brown fields. Contrary to energy crops such as soybeans, algae can use various water sources ranging from wastewater to saline water. Furthermore, waste containing different volatile fatty acids can also be used for the supplementation of nutrient required for the algal growth. The photosynthetic efficiency of microalgae is nearly 10 to 50 times higher than that of terrestrial plants (Kumar and Das, 2014; Li et al., 2008). This results into higher specific growth rate and biomass productivity. Moreover, their cultivation is relatively easier and biomass can be easily managed during its processing for the extraction of value-added products. In addition, genetic modification targeting desired products is easy. Terrestrial plants are effective in sequestering CO2 at low CO2 concentration. Contrary to this, microalgae are efficient in growing at a relatively higher CO2 concentration such as flue gas. Algal biomass can be directly used as food or food supplements as these are rich in protein, carbohydrates, essential fatty acids, antioxidants and several other biomolecules beneficial for the body. In fact, Nostoc sp. was used as food nearly 2000 years back during Chinese famine (Kumar and Das, 2014; Spolaore et al., 2006).
Thus, the present study aimed to summarize the different bottlenecks and the present state of art in CO2 sequestration process through algal biomass production. It also highlights the mechanisms of CO2 sequestration process. The microalgal cultivation systems such as open ponds and photobioreactors are discussed in details.
2.2 Microbiology
Microalgae and cyanobacteria are distributed throughout the biosphere with an immense range of genetic diversity. Up until now, nearly 35,000 species have been described in the literature (Sydney et al., 2014). However, their actual number may be much higher. They can be found as unicells, colonies and extended filaments, growing even under extreme climatic conditions from aquatic to terrestrial places (Kumar et al., 2011). Their uniqueness is due to the presence of chlorophyll and their CO2 fixing ability through photosynthesis in a single cell.
It is always desired to isolate a robust strain of algae, which can withstand the fluctuating physico-chemical conditions such as temperature, pH, light and CO2 concentration and can grow efficiently in the open cultivation system. The low chances of contamination, the high CO2 fixation rate and the product formation rate are the other desired characteristics of the algae. In the closed photobioreactor such as airlift photobioreactor, shear stress varies in different sections. Therefore, algal species having tolerance to high shear stress are other desired characteristics of the robust strain. Some of well known microalgal species used for the large scale production are described below.
Chlorella spp. are simple, non-motile and spherical unicellular eukaryotic green microalgae that have a thick cell wall (100–200 nm). Chlorella sp. measure between 2 and 10 μm. Chlorella sp. have a high concentration of chlorophyll and photosynthetic ability compared to higher plants. The optimal CO2 concentration for the growth of Chlorella sp. was found in the range of 5–6 % (Kumar and Das, 2012; de Morais and Costa, 2007). They are known to have a high specific growth rate (μ): 0.08 h−1 for Chlorella vulgaris and 0.11 h−1 for Chlorella sorokiniana (Cordero et al., 2011). Chlorella sp. are known as an attractive source of food due to high concentration of protein (nearly 50 %) and essential nutrients. Scenedesmus spp. are freshwater, non-motile microalgae. Various biotic and abiotic factors determine the extent of aggregation of these microalgae. For example, they are unicellular in axenic condition and known to form colonies in xenic system of the natural system (Geng et al., 2014). Botryococcus spp. are found in colony held together within a lipid biofilm matrix. They are characterized by their slow growth rate and accumulation of the high amount of hydrocarbons (up to 50 %, w/w), which is released outside the cells.
Chlorococcum spp. are spherical unicellular marine green microalgae with cells of diameter about 10 μm. They have a high tolerance to CO2 concentrations and can be grown to a very large cell density (Iwasaki et al., 1998). Dunaliella spp. are biflagellated, motile, unicellular marine green microalgae. They are mostly round in shape of diameter 9 to 11 μm. They have a high tolerance for salts, temperature and light (Segovia et al., 2003). Dunaliella lacks the cell walls, which make them an extremely fragile microorganism. They reproduce by binary fission with no evidence of cell lysis, encystment or spore formation (Segovia et al., 2003). Dunaliella is well known for the production of wide varieties of commercial products such as β-carotene (up to 14 % w/w). β-carotene can be easily extracted due to the easy rupture of their cell wall. Nannochloropsis spp. are small, non-motile spherical shaped and mostly marine in nature. They have only chlorophyll-a and completely lack chlorophyll-b and c, which make them distinct from other related microalgae. N. salina, N. Gaditana and N. oceanic are some of the well known species of Nannochloropsis. Along with CO2 sequestration, they have wide industrial applications. They are oleaginous strains, rich in polyunsaturated fatty acids and other value-added products such as astaxanthin, zeaxanthin and canthaxanthin (Lubian et al., 2000). In addition, they are choice of researchers because of easier genetic manipulation aimed for the genetic improvement. Haematococcus sp. are mobile, single-celled green algae (Chlorophyta), capable of synthesizing high amount of astaxanthin in response to environmental conditions, ranging from 1 to 5 % of dry cell weight (Wan et al., 2014). They have two stages of growth: growth stage (green cell) followed by astaxanthin accumulation stage (red cell). Therefore, Haematococcus cell changes its resistance to shear stress along the growth cycle. They have a slow growth rate (nearly 1.20 div d−1), which make them prone to contamination (González‐López et al., 2012).
Cyanobacteria are Gram-negative bacteria having a thin peptidoglycan layer. Cyanobacteria can be single cellular or multicellular. Filamentous colonies of cyanobacteria can have different type of cells such as vegetative cells, akinetes and heterocysts, which have a uniqueness to carry out complete oxygenic photosynthesis, storing food reserves and nitrogen fixing ability, respectively (Kumar et al., 2011). These are the only prokaryotic microorganism having the ability to reduce both atmospheric CO2 and nitrogen into carbohydrates and ammonia, respectively. Similar to microalgae and plants, cyanobacteria carry out oxygenic photosynthesis, involving both PSII and PSI. In anaerobic conditions, they use only PSI, causing anoxygenic photosynthesis. Nitrogen is fixed by the enzymes nitrogenase in the heterocysts of cyanobacteria. Carbohydrates and ammonia are utilized for the growth of cyanobacteria. Synechocystis is unicellular cyanobacteria. Anabaena and Aphanothece are linear multi-cellular, nitrogen fixing cyanobacteria. Spirulina sp. are freshwater, long, thin spiral thread like cyanobacteria, largely cultivated for the mass scale production. Spirulina has filaments (or trichomes) of helical shape in liquid medium and spiral shape in solid medium, which is the characteristics of the genus. Their large size (up to 1 mm in length and 12 μm in diameter) makes them easier in harvesting. Chlorophyll and phycocyanin are their main photosynthetic pigments. Spirulina sp. usually grow efficiently at alkaline pH with optimal pH in the range of 8.5 to 11. They are known for the food or food supplements as Spirulina cells are rich in protein, polyunsaturated fatty acids (PUFAs), vitamins, antioxidants and several important minerals.
2.3 Mechanism of CO2 Fixation
2.3.1 Photosynthetic Pathway
Photosynthesis is the process in which CO2 is fixed into carbohydrates using the light energy and water. Algae use this process to prepare their food for growth and survival. Chloroplast is the site of photosynthesis in the microalgae. Whereas, in cyanobacteria, photosynthetic apparatus is found in the cytoplasm. It has two stages: light dependent and light independent. Chlorophyll and light harvesting complex harness the light energy and conserve it in the form of energy currencies such as ATP and NADPH formed in the light dependent stage. In the light independent stage, ATP and NADPH are consumed during the synthesis of carbohydrates. Photosynthetic apparatus consisting of protein complexes, electron carriers and lipid molecules are located in or around the thylakoid membranes. Thylakoid membrane has two reaction centers called PSII and PSI, connected with several electron carriers such as plastoquinone, cytochrome b6f complex and plastocyanin (PC) in the order of increasing redox potential. This arrangement of electron carriers allows the flow of electron from negative redox potential to positive redox potential. PSII and PSI are specialized to absorb the light of wavelength 680 nm and 700 nm, respectively. Electron carriers are arranged in the order of increasing redox potential. The water split at PSII into protons, electrons and oxygen molecule with the help of light of 680 nm (Eq. 2.1). Protons generated by splitting of water are accumulated into the lumen of the thylakoid membrane and oxygen escapes from the cell. A proton gradient is generated across the thylakoid membranes due to accumulation of the protons in the lumen. These protons eventually escape from the lumen into the stroma generating ATP with the help of ATP synthase. On the other hand, electron gets excited at the PSII and travels to PSI by the linear electron transport chain. At PSI, it is further excited with the help of light of 700 nm. Antenna chlorophyll molecules help in harnessing the light at PSI. Excited electron reduces Ferredoxin (Fd), which is loosely bounded to the thylakoid membrane from outside (Kumar and Das, 2014). Reduced Fd transfers its electron to the ferredoxin NADP+ reductase (FNR), which catalyses the formation of NADPH using NADP+ and H+ coming from the lumen.
It should be noted that electron carriers transfer only one electron at a time with the help of one photon at each of the reaction center. Eventually the light energy is stored in the form of NAD(P)H and utilized in the light independent phase during CO2 fixation using Calvin cycle. The production of two moles of NAD(P)H requires eight photons and two moles of water as shown in Eq. 2.2.
2.3.2 Calvin Cycle
CO2 is reduced as carbohydrates into the algal cells during the light independent phase through the process called Calvin cycle. Calvin cycle takes place in the stroma of chloroplast and all the participating proteins are found residing outside the thylakoid membrane in the aqueous phase (Kumar and Das, 2014; Whitmarsh and Govindjee, 1999). Calvin cycle has broadly three steps: carboxylation, reduction and regeneration. Several complex reactions are involved in the Calvin cycle. However, only major reactions are shown in the diagram (Fig. 2.1).
Schematic diagram of the Calvin cycle. Only major intermediates have been shown. (Modified from Kumar and Das, 2014)
Ribulose-1,5-bisphosphate (RuBP), a 5-carbon compound, is the starting compound of the Calvin cycle (Calvin and Benson, 1948). Ribulose 1,5-bisphosphate carboxylase(RuBisCO) catalyzed the carboxylation reaction between RuBP and CO2 forming two molecules of glycerate 3-phosphate. In the reduction reaction, glycerate 3-phosphate is reduced to glyceraldehyde 3-phosphate (Eq. 2.3). It is followed by regeneration reaction, where five molecules of glyceraldehyde 3-phosphate is needed to regenerate one molecule of RuBP to start the Calvin cycle again and remaining one molecule is channeled for the synthesis of cellular biosynthetic materials for immediate energy source. Carbohydrates such as sucrose is transported to the cytosol, where these are stored in the form of glycogen and starch in cyanobacteria and green algae, respectively (Kumar and Das, 2014).
Calvin cycle is the most energy intensive processes among all the known pathways of CO2 capture (Fast and Papoutsakis, 2012). It requires seven molecules of ATP and four molecules of NAD(P)H to transform two molecules of CO2 into acetyl–CoA through the Calvin cycle as shown in Eq. 2.4 (Kumar and Das, 2014; Fast and Papoutsakis, 2012).
One reduced CO2 molecule stores energy equivalent to 1400 kJ, which is produced at the expense of two moles of NAD(P)H. A synthesis of two moles of NAD(P)H in the light dependent phase requires eight moles of photons containing total energy of 3840 kJ mol−1. In this way, maximum theoretical efficiency of the process can be calculated as 36.5 % (Kumar and Das, 2014).
2.3.3 CO2 Concentrating Mechanisms
Cells of microalgae and cyanobacteria are simple in structure, due to which they cannot stop the diffusion of CO2 to outside (Kumar and Das, 2014). They have low affinity for CO2. In addition, CO2 diffuses very slowly (nearly 10,000 times) in the aqueous solution compared to that in air (Kumar and Das, 2014). These factors limit the CO2 availability to the RuBisCO to discharge carboxylase activity of fixing CO2 into cellular components. In fact, under normal atmospheric condition, RuBisCO is only half saturated with the CO2. Most of the algal cells have developed carbon concentrating mechanisms (CCMs) to cope with the low partial pressure of CO2 outside the cell. The purpose of CCMs is to enhance the local partial pressure of CO2 (up to 1000 times higher than the outside culture) near the RuBisCO (carboxysome in cyanobacteria and pyrenoid in microalgae). The affinity constant (Km) of cyanobacteria and microalgae for CO2 are generally ≥200 μM and ~20 μM, respectively. This may be the reason of need to store large amount of bicarbonate within the cells of cyanobacteria (100 fold) compared to microalgae (20 fold).
CO2 is available inside the liquid culture in the form of gaseous dissolved CO2 and different other chemical forms such as carbonic acids (H2CO3), bicarbonate (HCO3 −), carbonate (CO3 2−) depending upon the pH of the culture. Most of algal species were found to assimilate both CO2 or HCO3 −. However, only HCO3 − is delivered into the cytosol regardless of the chemical species assimilated by the whole cells. This may have several reasons. For example, intracellular pH of cytoplasm is near 8, hence HCO3 − predominant inside the cytoplasm. HCO3 −, being a charged species, is unable to diffuse out the lipid bilayer of the cell membrane. CCMs have been extensively studied in the cyanobacteria. DIC is transported across the membranes by several mechanisms: transport of HCO3 − into the cytosol by the ABC type transporter utilizing ATP, transport of HCO3 − into the cytosol with the help of HCO3 −/Na+symporter or Na+/H+antiporter, transport of CO2 by NADH dehydrogenase having constitutively low or inducible high affinity for CO2. Besides these, HCO3 − can have direct access to the cytoplasm; however its transfer rate across the membrane does not contribute significantly in the DIC accumulation compared to its active transport (Kumar and Das, 2014). The HCO3 − transported to cytosol is further delivered into the carboxysome/pyrenoids. The pyrenoid is a densely packed compartment in the chloroplast containing RuBisCO. Both the carboxysome and pyrenoids have limited permeability of CO2 and act as a store house for CO2. Cyanobacteria have several carboxysomes (5 to 20 in number) into each cell depending upon species and growth conditions. RuBisCO enzymes have affinity only for gaseous CO2. Therefore, inorganic carbon must be converted back into gaseous CO2. Carbonic anhydrase (CA) does this job and catalyses the interconversion of inorganic carbon back to gaseous CO2 as shown in Eq. 2.5. Under low partial pressure of CO2 outside the algal cells, CA over-expresses to maintain high local CO2 concentration. High concentration of local CO2 activates the carboxylase activity of RuBisCO and fix the CO2 more efficiently. CCMs have been less studied in the microalgae compared to cyanobacteria. Contrary to HCO3 − in cyanobacteria, CO2 is the predominant species entering into the microalgae such as Chlamydomonas reinhardtii (Spalding, 2008). The uptake of CO2 into the whole cell and through the chloroplast of microalgae is due to diffusion and mediated transfer, respectively (Kumar and Das, 2014).
2.3.4 CO2 Fixation Through Algal Biomass Production
The CO2 is fixed during algal growth through several processes such as biomass formation, mineralization (transformation of gaseous CO2 into chemical species such as bicarbonates, carbonates) and production of extracellular products such as polysaccharides, volatile organic compounds, organohalogens, hormones etc. (Sydney et al., 2014). Among them, biomass formation is the major factor, which was found to account for 70–88 % of total CO2 fixation during algal cultivation (Sydney et al., 2014). CO2 fixed during other processes are wasted or easily lost to the environment. Therefore, most of the researchers quantify the CO2 fixation taking only biomass formation into account. The CO2 fixation ability of microalgae and cyanobacteria varies (Table 2.1).
The elemental composition of biomass of algal species such as Chlorella sp. changes depending upon experimental conditions such as the concentration of CO2 used for growing algae. C, H, N and S were reported in the range of 46.1 %–50.1 %, 6.1 %–7.74 %, 6.7 %–8.52 % and <0.5 % (w/w), respectively (Kumar et al., 2014; Rizzo et al., 2013; Friis et al., 1998). The molecular formula of the algal biomass of microalgae can be determined from the relative percent of C, H and N present in the algal biomass and can be represented in the form of CHxNyOz. The corresponding molecular weight of algal biomass of C. sorokiniana was in the range of 24–26 g (Kumar et al., 2014). For example, 24.04 g per mole of biomass of C. sorokiniana was obtained, when the air was the sole carbon source. Material balance can be written in the form of Eq. 2.6 (Kumar et al., 2014).
Assuming 50 % carbon content in the algal cells, 1.83 kg of CO2 from the air is fixed for the production of one kg of algal biomass along with the release of nearly 1.9 kg of oxygen (Kumar et al., 2014; Kumar and Das, 2012).
2.4 Bottlenecks in the Algal Biomass Production
2.4.1 Temperature
Temperature is one of the most important factors influencing all the biological systems including algae. Each of the microalgal species has an optimal temperature at which they grow most suitably. The temperature affects the cellular enzymatic reactions, including the cytosolic pH. The optimal temperature of most of the algal species varies in the range of 25–35 °C. Algal growth is more sensitive to high temperature compared to low temperature. In fact, a little increase over the optimum temperature may severely affect the algal growth. The low temperature reduces the cellular activities such as limiting the electron transport chain (ETC), activity of CA etc. (Kumar et al., 2014). The high temperature affects the CO2 fixation due to inhibitory effects on the cellular physiology and denaturing of essential proteins/enzymes. For example, photosystem II is very thermolabile and prone to denature at higher temperature. Besides these, an increase in photorespiration, a decrease in RuBisCO affinity for CO2 and a decrease in CO2 to O2 solubility ratio are the other vital phenomena negatively affecting the CO2 fixation at higher temperature (Kumar et al., 2014; Kumar et al., 2011).
The target of most of the CO2 capture using microalgae is from the flue gas, generally having higher temperature. In addition, outdoor cultivation of algae is influenced by the local climatic temperature, which is higher for the countries closer to the equator and lower for the countries located far away from the equator. Further, the temperature exposed to algal cells depends upon the time of light exposure and the season variations. The use of thermophilic algae is an alternative for the fixation of CO2 coming from the flue gas having high temperature.
2.4.2 pH
Most of the algae has neutral or slightly alkaline cytosolic pH as cellular enzymes are pH sensitive and become inactive at acidic conditions (Kumar et al., 2014). Therefore, the optimal pH of most of the algal species has been found in the range of 7 to 9. The extreme pH negatively affects the growth and CO2 fixation by disrupting cellular processes. The introduction of CO2 and other acidic gases such as Sox and NOx (generally present in the flue gas) in the culture medium decreases the pH. CO2 dissolves in water by forming carbonic acids. The availability of carbonaceous species (aqueous CO2, carbonic acids, bicarbonate and carbonate) to the algal cells depends upon the pH of the culture as the equilibrium shifts among them on change in the culture pH.
Along with the growth of the algae, pH generally increases. This may be due to consumption of CO2 and other volatile fatty acids (if present in the medium). The rise in pH along the growth is higher at the inlet gas stream with low CO2 concentration. This may be because of the release of hydroxide ions outside the cell at the expense of capture of H+ ions inside the thylakoid membranes during the activation of CCMs (Kumar and Das, 2012; Jacob-Lopes et al., 2008). The presence of nitrogen source in the medium also influences the pH of the culture broth. For example, the consumption of nitrate from the medium helps in the increase of alkalinity via OH− production (Kumar et al., 2014; Hulatt and Thomas, 2011). Contrary to this, uptake of NH4 + by the microalgae leads to H+ production (Goldman and Brewer, 1980).
2.4.3 Light
Algae fix the CO2 in the presence of light during photosynthesis. Therefore, light is crucial in CO2 capture through algal biomass production. Algal cells in the culture can experience broadly three different phases of light such as light limitation, light saturation and light inhibition (Kumar et al., 2013). The light utilization efficiency and biomass productivity proportionally increases with the light intensity till the cells become light saturated. The light saturation value of algae is in the range of 80–200 μ molphotos m−2 s−1 (Kumar et al., 2014).
At low light intensity, photosynthesis becomes limiting. Lee and Pirt (1981) reported an increase in the cellular maintenance requirements during long time exposure of dark, which causes reduction in the biomass productivity. At high light intensity, photosynthesis is disrupted due to photoinhibition. The excess light energy is dissipated as fluorescence or heat through non-photochemical quenching (NPQ) (Kumar et al., 2014; Yamakawa and Itoh, 2013). The photoinhibition disrupts the synthesis and degradation cycle (D1/D2 proteins) of light harvesting complex at PSII, eventually leading to inhibition of photosynthesis (Kumar et al., 2011). Along with the quantity of light, quality of light (light of a specific wavelength or light regime) also affects the algal biomass production. For example, in a study by Kim et al. (2014), the red light regime was found favoring the CO2 fixation in N. gaditana followed by blue and white light regime. The pigmentation of algal cells exponentially reduces the light intensity inside the culture broth. The reduction of the size of the antenna molecules of algal cells (antenna size mutant) by molecular tools was found an effective way to decrease the pigment content and thus increasing the extent of light penetration inside the algal culture. This will eventually lead to better light energy utilization causing higher CO2 fixation and biomass productivity. Proper mixing and efficient photobioreactor design are the other strategies for the effective distribution of light energy inside the algal culture and to improve the light utilization efficiency (Kumar et al., 2011).
2.4.4 Mixing
The importance of CO2 for algal cultivation can be understood by the fact that the cost of the carbon accounts for 8–27 % of the total production cost of the algal biomass (Li et al., 2013). The bottlenecks of CO2 transfer to the algal cells is due to higher resistance to the mass transfer of CO2. RuBisCO is the crucial enzyme for the Calvin cycle used in CO2 fixation. However, RuBisCO has low affinity for CO2 compared with O2 (Kumar and Das, 2013; Kumar et al., 2011). Moreover, CO2 gas faces resistance to transfer from the gas phase to the liquid phase and from the liquid phase to inside the algal cells. Therefore, adequate supply of CO2 in the culture is necessary. It has been estimated that CO2 concentration greater than 65 μmol L−1 at a pH of 8.5 is necessary for the optimal productivity of majority of microalgae (Weissman et al., 1988). However, the dissolved CO2 concentration in water in equilibrium with air is nearly 10 μmol L−1 at 25 °C.
2.4.5 Substrate Inhibition
An optimal CO2 concentration in the inlet gas stream increases the specific growth rate and enhances the biomass productivity. The optimal CO2 concentration was found in the range of 2 to 10 % in most of the microalgae. However, ability to tolerate CO2 can be as high as 100 %. High CO2 concentration causes substrate inhibition and environmental stress causing reduction in the algal growth. In addition to the environmental stress, high CO2 concentration decreases the pH of the culture. Algae has higher CO2 tolerance capability compared to terrestrial plants and therefore more effective in CO2 sequestration. CO2 tolerance ability of algal cells can be enhanced by the method of adaptation in gradually higher CO2 concentration. According to Miyairi (1995) drop in pH is less at higher temperature due to decrease in the solubility of CO2 in the culture.
2.4.6 Product Inhibition
2.4.6.1 Biomass Concentration
Cell growth causes exponential decrease in the light penetration inside the photobioreactor due to self-shading effect by the microalgal cells. This is similar to the product inhibition. At fixed light intensity, algal cells may be in a light inhibition zone at low cell density and light limiting phase at higher cell density. The biomass concentration in the range of 1–2 g L−1 was found optimum taking both biomass productivity and self-shading effect into consideration (Hu et al., 1996; Zhang et al., 2001). The continuous or semi-continuous method of algal cultivation may be an effective way to maintain optimum cell density.
2.4.6.2 Oxygen Accumulation
Accumulation of high concentration of oxygen leads to photooxidative damage to the algal cells. It has been reported that maximum tolerable dissolved oxygen concentration should not increase more than 400 % (>35 mg L−1) of air saturation value (Kumar et al., 2011; Carvalho et al., 2006; Lee and Lee, 2003). RuBisCO enzyme has affinity for oxygen as well as carbon dioxide. In fact, affinity constant (Km) for oxygenase activity is significantly lower than that of carboxylase activity. Therefore, RuBisCO participate in the CO2 fixation only when the CO2 concentration in the compartment is significantly higher. In case of oxygenase reaction, algal cells negatively affect the CO2 fixation. For example, glycolate 2-phosphate is the end product when oxygenase dependent reaction of RuBisCO is active (Eq. 2.7). A significant amount of cellular energy is wasted during the formation of glycolate 2-phosphate, which is not required by algal cells. Further, glycolate 2-phosphate breaks down into glycine, which forms serine by combining with another glycine molecule resulting in a loss of CO2− (Kumar and Das, 2014; Zeng et al., 2011). The loss of CO2 may be up to 50 % of the algal biomass (Giordano et al., 2005). Furthermore, RuBP synthesis is affected because of loss of fixed carbon.
Shapes and sizes of the photobioreactor greatly influence the rise in dissolved oxygen (DO). For example, an increase in DO is a severe problem for the horizontal tubular photobioreactor but for the photobioreactor such as airlift, it is not a critical issue due to having an open gas disengagement zone (Kumar and Das, 2014; Kumar et al., 2011; Lee and Lee, 2003).
2.5 Algal Biomass Cultivation System
2.5.1 Open Ponds
Open ponds have shallow configuration, which allow maximum light penetration inside the culture. Open ponds can be categorized into four types: shallow big ponds, tanks, circular ponds and raceway ponds (Rao et al., 2012). Among them, raceway pond is considered as an attractive cultivation system. This is because of ease of mixing and better biomass productivity. In fact, nearly 95 % of the total world algal biomass facility is based on the raceway ponds (Mendoza et al., 2013). The large scale production of microalgae was started by Oswald and Golueke (1960) in the 1950s in a raceway pond using Chlorella sp.
Algal cultivation in open ponds has several advantages such as ease in operational handling, practical way of scaling up microalgal production facility, low initial investment cost, free solar radiation, low energy requirement for mixing etc. (Jorquera et al., 2010; Mendoza et al., 2013). The product extracted from the algal biomass obtained from the open ponds are comparatively cheaper than the photobioreactor. For example, the cost of biodiesel obtained from raceway ponds was calculated as 12.73 USD per gallon, which was nearly 2.5 times lower to that of photobioreactor (Richardson et al., 2012). Open ponds also have several drawbacks: areal biomass productivity is in the range of 0.12–0.48 g L−1 d−1, which is nearly 2 to 8 times lower than the photobioreactor (Ketheesan and Nirmalakhandan, 2012; Brennan and Owende, 2010). Algal culture is susceptible to contamination, which decreases the biomass productivity and makes algal biomass unfit for the extraction of high value product. Only few selected algal species such as Chlorella, Haematococcus, Dunaliella, Nannochloropsis, Spirulina, etc. were found successful in growing in the open system. Further, the shallow depth of the open ponds allows low residence time to the introduced CO2 gas, which in turn reduces CO2 transformation efficiency into cellular constituents. In fact, nearly 80–90 % of the sparged gas is lost to the atmosphere (Richmond, 2004; Weissman et al., 1988). Besides these, open system has disadvantages in controlling the physico-chemical parameters such as availability of light, agitation, pH, temperature and nutrient concentrations (Kumar et al., 2011). The fluctuation in temperature and light availability during diurnal cycles and seasonal variations are other challenges with the open systems (Brennan and Owende, 2010).
Open ponds are generally light and carbon limited. The pond depth of the culture influence the light utilization efficiency, temperature variation in the culture, mixing (especially vertical mixing) and associated power consumption by the paddle-wheel. An increase in the pond depth enhances the total areal productivity of the algal biomass. For example, nearly 134–200 % increase in the areal productivity was obtained, when pond depth was 40 cm compared to 20 cm deep raceway ponds (Sutherland et al., 2014). Similarly, mixing is very important for algal biomass productivity as it determines the nutrient and light availability to the algal cells. It has been found that optimum mixing enhances the biomass productivity by nearly 10 fold (Hreiz et al., 2014). The vertical mixing in the raceway ponds is more important as it limits the culture depth and hence the total areal biomass productivity. The vertical mixing causes cells to move from bottom (dark zone) to surface (light zone) of the open ponds. Therefore, it ensures the frequency by which each of the algal cells experience solar light exposure. Further, vertical mixing also prevents cells from either photolimitation or photoinhibition (Chiaramonti et al., 2013).
2.5.2 General Raceway Ponds in Operation
2.5.2.1 The Paddle-Wheel Driven Raceway Ponds
Paddle-wheels are mechanically simple to construct, require little maintenance and generate gentle mixing. These are traditionally used in the raceway ponds. In the raceway ponds, clearance zone between paddle wheel and the bottom floor is kept small (Chiaramonti et al., 2013). In this way, paddle-wheel acts as a positive displacement pump due to no backflow of the liquid. A wide recirculation flow of liquid can be visualized long after the bend of the raceway ponds showing the effectiveness of the raceway ponds (Chiaramonti et al., 2013). However, paddle-wheel driven raceway ponds have disadvantage of high power consumption and low vertical mixing (Xu et al., 2014). Paddle-wheel driven raceway pond in operation is shown in Fig. 2.2.
2.5.2.2 Sump-Assisted Raceway Ponds
Most of the design of the raceway ponds is focused on the enhancement of CO2-liquid contact time. For example, a sump was incorporated in the raceway ponds and gas bubbles were introduced at the bottom of the sump (Mendoza et al., 2013). Sump is a long tubular dig, at the bottom of which gas bubbles are introduced. In this way, CO2 bubbles have to diffuse out into the atmosphere by passing through a long water column. This increases the CO2-liquid contact time and thus better CO2 utilization efficiency and minimum CO2 loss into the atmosphere (Craggs et al., 2012). Areal biomass productivity in the sump-assisted raceway pond was found 17 g m−2 d−1 with CO2 capture of nearly 66 % from the inlet gas stream (deGodos et al. 2014).
2.5.2.3 Airlift/Split Sump-Assisted Raceway Ponds
The sump-assisted raceway pond was further modified by introducing baffles in the sump. Baffle partitioned the sump into two halves. In this way, this acts as an airlift reactor with one side as riser and the other side as downcomer. The CO2-air gas mixture is introduced from the riser. The density difference between the two portions of the split sump was the driving force for the liquid movement in the raceway ponds. For further enhancing the CO2-liquid contact time, normal air was used to circulate and generate culture velocity to the liquid and CO2 was introduced in the counter-current direction at half the height of the downcomer (deGodos et al., 2014). The airlift driven raceway pond was compared with the traditional paddle-wheel driven raceway ponds. Ketheesan and Nirmalakhandan (2012) claimed the reduction of nearly 80 % energy consumption in airlift-driven raceway ponds at same culture velocity.
2.5.3 Photobioreactors
Microalgae are cultivated in photobioreactor to overcome the bottlenecks of the open ponds. Better control of process parameters such as pH, temperature, mixing, light penetration etc., maintaining axenic culture and necessity to obtain higher biomass productivity are some of the advantages of growing algae in the photobioreactor. Photobioreactors are the closed system, similar to bioreactors with additional provision of supply of light into the photobioreacotr. This requires a high surface area to volume ratio (S/V ratio) and transparent surface of the photobioreactor. Necessity to consider these two criteria make photobioreactors difficult to scale-up. Photobioreactors are generally made up of transparent materials such as glass, plexiglass or polycarbonate (Kumar et al., 2011). Closed surface of the photobioreactor helps in introducing the gaseous CO2 in a controlled way and with increased CO2 gas-liquid contact time. Higher residence time of the CO2 into the photobioreactor enhances its mass transfer and correspondingly CO2 sequestration efficiency and algal biomass productivity. Photobioreactors designed for CO2 sequestration take advantage of using gaseous CO2 for the supply of carbon source as well as ensuring sufficient mixing. However, in some photobioreactors such as stirred tank, agitation is provided by mechanical devices as well as sparging with CO2-rich gas. It is always desirable to introduce the CO2-rich gas mixture in order to reduce energy consumption. Mixing with CO2 rich gas stream also helps in the removal of oxygen generated during the algal growth.
2.5.3.1 Vertical Tubular Photobioreactor
Vertical tubular photobioreactors are most commonly used for the CO2 sequestration. Height to diameter ratio is generally kept greater than two (Kumar et al., 2011). The CO2-rich gas stream is passed at the bottom of the cylindrical vessel through sparger. Long liquid column in the cylindrical vessel increases the CO2-liquid contact time. Very tiny gas bubbles can be produced by using sparger with suitable pore size, which further enhances the volumetric mass transfer of gaseous CO2. Advantages of vertical tubular photobioreactors are in low infrastructure cost, ease in the design and handle, absence of moving parts, relatively homogeneous culture environment, higher S/V ratio and efficient removal of unused gas mixture and photosynthetically produced O2 (Kumar et al., 2011). Working volume of this type of reactor is generally 80 % of the total volume. Based on the placement of the light source, vertical tubular photobioreactors can be called as externally or internally illuminated photobioreactor.
Airlift and bubble column photobioreactors are the well known examples of vertical tubular photobioreactor. Airlift and bubble column are different from each other due to difference in mixing (Kumar and Das, 2014). Airlift photobioreactors have additionally draft tube placed at the center of the bubble column. In this way, airlift reactor has two different zones: one light zone (annular area) and another dark zone (central area). The CO2-rich gas stream can be passed either through a central tube or through the annular space. The zone of airlift, where sparger is placed, is named as riser whereas the other one is called downcomer (Kumar and Das, 2013; Kumar and Das, 2014). Riser is also called gassed zone, where liquid has less density compared to downcomer. The density difference between the two sides drives the liquid culture in axial circular and oriented motion along the length of the photobioreactor. The direction of movement of the culture broth is from riser to downcomer. It is to be noted that maintaining the liquid level above the draft tube is necessary in order to experience the axial movement of liquid. Algal cells periodically move through the light and dark zone due to elliptical movement of the culture along the length of the reactor (Kumar and Das, 2013; Kumar et al., 2011). This gives flashing light effect to algal cells (Barbosa et al., 2003). The liquid moves in zig-zag and haphazardly on removing the draft tube (bubble column), leaving many dead zones in the photobioreactor. Vertical tubular photobioreactors have the disadvantages of poor control of temperature and low surface area for receiving the sunlight.
2.5.3.2 Horizontal Tubular Photobioreactor
Horizontal tubular reactors are long tubular tubes placed horizontally generally made up of transparent polypropylene acrylic or polyvinylchloride pipes with small internal diameter. The CO2-rich gas stream is introduced from one side of the tube through the dedicated gas exchange unit. The longer length of pipes gives higher residence time to the CO2 gas bubbles. This type of reactor is mostly used for algal cultivation in the outdoor. Parallel tubes are oriented in such a way to receive a maximum of solar light. Disadvantages of this type of photobioreactor are poor gas disengagement, wall growth and cleaning problem. This becomes more severe as the length of the tubes increases. Oxygen produced during the photosynthesis get trapped inside the photobioreactor. Lee and Lee (2003) observed an increase in photosynthetically produced DO concentration up to 400 % in horizontal tubular photobioreactor. Temperature is controlled by sparging the cold water over the surface of the tubes or overlapping tubes of cold water and photobioreactor or putting the light harvesting unit in the pool of cold water (Kumar and Das, 2014).
2.5.3.3 Helical Tubular Photobioreactor
Helical tubular photobioreactor is a flexible, transparent and a coiled shape structure placed vertically. It has separate gas engagement unit attached at the top of the coil. The CO2-rich gas stream is passed from the bottom opening of the helical reactor. The long helical unit gives a high S/V ratio. In addition, CO2 gas has high residence time inside the reactor, which increases the CO2 sequestration efficiency. The light source can be placed co-axially at the center of the photobioreactor to minimize the loss of light energy and better biomass productivity. Helical photobioreactor can be scaled-up by increasing the number of coils in it. However, accumulation of oxygen increases proportionally with the increase in the coil of helical tubular photobioreactor (Kumar and Das, 2014). It has the advantage of requiring a small land footprint. Helical tubular photobioreactor suffers from many disadvantages such as fouling inside the tube, shear stress during the introducing of the gas, trapping of photosynthetically produced oxygen, most importantly design and handling of the photobioreactor. Helical reactors were further modified and transformed into a cone shape for better solar light utilization efficiency (Kumar and Das, 2014; Kumar et al., 2011; Watanabe and Hall, 1996).
2.5.3.4 Flat Panel Photobioreactor
The flat panel reactor has a cuboidal shape with flat surface at two sides for receiving the light. Most of the studies are focused on the flat panel photobioreactor. It has a high S/V ratio and better illuminated light path length. The agitation is carried out by the CO2-rich gas stream, which is introduced from the bottom through tubes with fine holes. Some researchers placed the tubes upto half of the length of the photobioreactor to induce spiral mixing pattern. Flat panel photobioreactors have been further modified by putting draft tubes inside it. Similar to airlift photobioreacter, flat panel photobioreactor can have the riser and downcomer. The simple arrangement of flat plate reactor makes it easy to construct with any desired light path length. However, wall growth, difficulty in cleaning and low biomass productivity per unit space are some of the problems with this type of reactor. An increase in the hydrostatic pressure with the increase in the volume of the reactor during scale-up is another problem associated with this type of arrangement.
2.6 Conclusions
Large scale microalgal cultivation has potential to sequester significant amounts of CO2 through biomass production and thus contributing in reducing the global warming effect. Several physico-chemical parameters influence the algal biomass productivity. Therefore, it is important to optimize these parameters and grow the microalgae at optimal environmental conditions. All the cultivation systems have their own merits and demerits. The raceway ponds are more suited for the cheap and large scale algal cultivation. Closed photobioreactor is used for growing the algae in controlled physico-chemical conditions, which will result into better biomass productivity. Various shapes and design of the closed photobioreactor have an influence on the algal biomass productivity. In addition, longer residence time of CO2 in the closed photobioreactor can improve the CO2 sequestration efficiency of microalgae. However, the cost effective biomass production in large scale is yet to achieve in the closed photobioreactor.
References
Allen, D.E., Strazisar, B.R., Soong, Y. and Hedges, S.W. (2005). Modeling carbon dioxide sequestration in saline aquifers: Significance of elevated pressures and salinities. Fuel Process Technol., 86(14), 1569–1580.
Bachu, S. (2000). Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Mgmt., 41, 953–970.
Barbosa, M.J., Janssen, M., Ham, N., Tramper, J. and Wijffels, R.H. (2003). Microalgae cultivation in airlift reactors: Modeling biomass yield and growth rate as a function of mixing frequency. Biotechnol. Bioeng., 82(2), 170–179.
Bardgett, R.D., Freeman, C. and Ostle, N.J. (2008). Microbial contributions to climate change through carbon cycle feedbacks. The ISME Journal, 2, 805–814.
Basu, S., Roy, A.S., Mohanty, K. and Ghoshal, A.K. (2013). Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresour. Technol., 143, 369–377.
Brennan, L. and Owende, P. (2010). Biofuels from microalgae – A review of technologies for production, processing and extractions of biofuels and co-products. Renew. Sustain. Energy Rev., 14, 217–232.
Calvin, M. and Benson, A.A. (1948). The path of carbon in photosynthesis. Science, 107, 476–480.
Carvalho, A.P., Luis, A., Meireles, A. and Malcata, F.X. (2006). Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog., 22, 1490–1506.
Chiaramonti, D., Prussi, M., Casini, D., Tredici, M.R., Rodolfi, L., Bassi, N., Zittelli, G.C. and Bondioli, P. (2013). Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Appl. Energy, 102, 101–111.
Chiu, S.Y., Kao, C.Y., Tsai, M.T., Ong, S.C., Chen, C.H. and Lin, C.S. (2009). Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol., 100(2), 833–838.
Cordero, B.F., Obraztsova, I., Couso, I., Leon, R., Vargas, M.A. and Rodrigue, H. (2011). Enhancement of lutein production in Chlorella sorokiniana (Chlorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs., 9, 1607–1624.
Craggs, R., Sutherland, D. and Campbell, H. (2012). Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol., 24, 329–337.
Dayananda, C., Sarada, R., Kumar, V. and Ravishankar, G.A. (2007). Isolation and characterization of hydrocarbon producing green alga Bortyococcus braunii from Indian freshwater bodies. Electron. J. Biotechnol., 10, 78–91.
deGodos, I., Mendoza, J.L., Acién, F.G., Molina, E., Banks, C.J., Heaven, S. and Rogalla, F. (2014). Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour. Technol., 153, 307–314.
de Morais, M.G. and Costa, J.A.V. (2007). Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett., 29, 1349–1352.
Falkowski, P., Scholes, R.J., Boyle, E., Canadell, J., Canfield, D., Elser, J., Gruber, N., Hibbard, K., Hogberg, P., Linder, S., Mackenzie, F.T., Moore III, B., Pedersen,T., Rosenthal, Y., Seitzinger, S., Smetacek, V. and Steffen, W. (2000). The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System. Science, 290, 291–296.
Fast, A.G. and Papoutsakis, E.T. (2012). Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr. Opin. Chem. Eng., 1, 1–16.
Friis, J.C., Holm, C. and Sorensen, B.H. (1998). Evaluation of elemental composition of algal biomass as toxical endpoint. Chemosphere., 37(13), 2665–2676.
Geng, L., Zhang, J., Qin, B. and Yang, Z. (2014). No difference in colony formation of Scenedesmus obliquus exposed to lakes with different nutrient levels. Biochem. Sys. Ecol., 57, 178–182.
Giordano, M., Beardall, J. and Raven, J.A. (2005). Mechanisms in algae: Mechanisms, environmental modulation and evolution. Annu. Rev. Plant Biol., 56, 99–131.
Goldman, J. and Brewer, P. (1980). Effect of nitrogen source and growth rate on phytoplankton-mediated changes in alkalinity. Limnol. Oceanogr., 25, 352–357.
González‐López, C. V., Acién Fernández, F. G., Fernández‐Sevilla, J. M., Sánchez Fernández, J. F., & Molina Grima, E. (2012). Development of a process for efficient use of CO2 from flue gases in the production of photosynthetic microorganisms. Biotechnology and bioengineering, 109(7), 1637–1650.
Hreiz, R., Sialve, B., Morchain, J., Escudié, R., Steyer, J.-P. and Guiraud, P. (2014). Experimental and numerical investigation of hydrodynamics in raceway reactors used for algaculture. Chem. Eng. J., 250, 230–239.
Hu, Q., Guterman, H. and Richmond, A. (1996). A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnol. Bioeng., 51, 51–60.
Hulatt, C.J. and Thomas, D.N. (2011). Productivity, carbon dioxide uptake and net energy return of microalgal bubble column photobioreactors. Bioresour. Technol., 102, 5775–5787.
Iwasaki, I., Hu, Q., Kurano, N. and Miyachi, S. (1998). Effect of extremely high-CO2 stress on energy distribution between photosystem I and photosystem II in a ‘high-CO2’ tolerant green alga, Chlorococcum littorale and the intolerant green alga Stichococcus bacillaris. J Photochem. Photobiol. B-Biol., 44(3), 184–190.
Jacob-Lopes, E., Revah, S., Hernandez, S., Shirai, K. and Franco, T.T. (2009). Development of operational strategies to remove carbon dioxide in photobioreactors. Chem. Eng. J., 153(1–3), 120–126.
Jacob-Lopes, E., Scoparo, C.H.G. and Franco, T.T. (2008). Rates of CO2 removal by Aphanothece microscopic Nageli in tubular photobioreactors. Chem. Eng. Prog., 47, 1365–1373.
Jorquera, O., Kiperstok, A., Sales, E.A., Embiruçu, M. and Ghirardi, M.L. (2010). Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol., 101, 1406–1413.
Ketheesan, B. and Nirmalakhandan, N. (2012). Feasibility of microalgal cultivation in a pilot-scale airlift-driven raceway reactor. Bioresour. Technol., 108, 196–202.
Kim, C.W., Sung, M-G., Nam, K., Moon, M., Kwon, J-H. and Yang, J-W. (2014). Effect of monochromatic illumination on lipid accumulation of Nannochloropsis gaditana under continuous cultivation. Bioresour. Technol., 159, 30–35.
Kumar, K. and Das, D. (2012). Growth characteristics of Chlorella sorokiniana in airlift and bubble column photobioreactors. Bioresour. Technol., 116, 307–313.
Kumar, K. and Das, D. (2013). CO2 sequestration and hydrogen production using cyanobacteria and green algae. In: Razeghifard, R. (ed.), Natural and Artificial Photosynthesis Solar power as an Energy Source. John Wiley and Sons, Inc., pp. 173–215.
Kumar, K. and Das, D. (2014). Carbon Dioxide Sequestration by Biological Processes. In: Bhanage, B.M. and Arai, M. (eds), Transformation and Utilization of Carbon Dioxide. Springer, pp. 303–334.
Kumar, K., Dasgupta, C.N. and Das, D. (2014). Cell growth kinetics of Chlorella sorokiniana and nutritional values of its biomass. Bioresour. Technol., 167, 358–366.
Kumar, K., Dasgupta, C.N., Nayak, BK., Lindblad, P. and Das, D. (2011). Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour. Technol., 102(8), 4945–4953.
Kumar, K., Sirasale, A. and Das, D. (2013). Use of image analysis tool for the development of light distribution pattern inside the photobioreactor for the algal cultivation. Bioresour. Technol., 143, 88–95.
Kurano, N., Ikemoto, H., Miyashita, H., Hasegawa, T., Hata, H. and Miyachi, S. (1995). Fixation and Utilization of Carbon-Dioxide by Microalgal Photosynthesis. Energy Convers. Mgmt., 36(6–9), 689–692.
Lee, J.S. and Lee, J.P. (2003). Review of advances in biological CO2 mitigation technology. Biotechnol. Bioprocess Eng, 8, 354–359.
Lee, Y.K. and Pirt, S.J. (1981). Energetics of photosynthetic algal growth: Influence of intermittent illumination in short (40s) cycles. J. Gen. Microbiol., 24, 43–52.
Li, S., Luo, S. and Guo, R. (2013). Efficiency of CO2 fixation by microalgae in a closed raceway pond. Bioresour. Technol., 136, 267–272.
Li, Y., Horsman, M., Wu, N., Lan, C.Q. and Dubois-Calero, N. (2008). Biofuels from microalgae. Biotechnol. Prog., 24, 815–820.
Lopez, C.V.G., Fernandez, F.G.A., Sevilla, J.M.F., Fernandez, J.F.S., Garcia, M.C.C. and Grima, E.M. (2009). Utilization of the cyanobacteria Anabaena sp ATCC 33047 in CO2 removal processes. Bioresour. Technol., 100(23), 5904–5910.
Loubiere, K., Olivo, E., Bougaran, G., Pruvost, J., Robert, R. and Legrand, J. (2009). A new photobioreactor for continuous microalgal production in hatcheries based on external-loop airlift and swirling flow. Biotech. Bioeng., 102(1), 132–147.
Lubian, L.M., Montero, O., Moreno-Garrido, I., Huertas, I.E., Sobrino, C., Gonzalez-del Valle, M. and Pares, G. (2000). Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol., 12(3–5), 249–255.
Mendoza, J.L., Granados, M.R., de Godos, I., Acién, F.G., Molina, E., Banks, C. and Heaven, S. (2013). Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass Bioenerg., 54, 267–275.
Mirjafari, P., Asghari, K. and Mahinpey, N. (2007). Investigating the Application of Enzyme Carbonic Anhydrase for CO2 Sequestration Purposes. Ind. Eng. Chem. Res., 46, 921–926.
Miyairi, S. (1995). CO2 assimilation in a thermophilic cyanobacterium. Energy Convers. Mgmt., 36(6–9), 763–766.
Oswald, W.J. and Golueke, C.G. (1960). Biological transformation of solar energy. Adv. Appl. Microbiol., 11, 223–242.
Rao, A.R., Ravishankar, G.A. and Sarada, R.(2012). Cultivation of green alga Botryococcus braunii in raceway, circular ponds under outdoor conditions and its growth, hydrocarbon production. Bioresour. Technol., 123, 528–533.
Richardson, J.W., Johnson, M.D. and Outlaw, J.L. (2012). Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Research., 1, 93–100.
Richmond, A. (2004). Principles for attaining maximal microalgal productivity in photobioreactors: An overview. Hydrobiologia., 512, 33–37.
Rizzo, A.M., Prussi, M., Bettucci, L., Libelli, I.M. and Chiaramonti, D. (2013). Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior. Appl. Energy, 102, 24–31.
Scragg, A.H., Illman, A.M., Carden, A. and Shales, S.W. (2002). Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenerg., 23(1), 67–73.
Segovia, M., Haramaty, L., Berges, J.A. and Falkowski, P.G. (2003). Cell death in the unicellular chlorophyte Dunaliella tertiolecta. A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiol., 132(1), 99–105.
Sharma, A., Bhattacharya, A. and Shrivastava, A. (2011). Biomimetic CO2 sequestration using purified carbonic anhydrase from indigenous bacterial strains immobilized on biopolymeric materials. Enzyme Microb. Technol., 48, 416–426.
Spalding, M.H. (2008). Microalgal carbon dioxide concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J. Exp. Biol., 59(7), 1463–1473.
Spolaore, P., Joannis-Cassan, C., Duran, E. and Isambert, A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng., 101(2), 87–96.
Sutherland, D.L., Turnbull, M.H. and Craggs, R.J. (2014). Increased pond depth improves algal productivity and nutrient removal in wastewater treatment high rate algal ponds. Water Research, 53, 271–281.
Sydney, E.B., Sturm, W., de Carvalho, J.C., Soccol, V.T., Larroche, C., Pandey, A. and Soccol, C.R. (2010). Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol., 88(10), 3291–3294.
Sydney, E.B., Novak, A.C., de Carcalho, J.C. and Soccol, C.R. (2014). Respirometric balance and carbon fixation of industrially important algae. In: A. Paney, D.-J. Lee, Y. Chisti and C.R. Soccol (eds.), Biofuels from algae. Elsevier, MA, USA, pp. 67–84.
Wan, M., Zhang, J., Hou, D., Fan, J., Li, Y., Huang, J. and Jun Wang, J. (2014). The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol., 167, 276–283.
Wang, B., Li, Y.Q., Wu, N. and Lan, C.Q. (2008). CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol., 79(5), 707–718.
Watanabe, Y. and Hall, D.O. (1996). Photosynthetic production of the filamentous Cyanobacterium Spirulina platensis in a cone-shaped helical tubular photo-bioreactor. Appl. Microbiol. Biotechnol., 44, 693–698.
Weissman, J.C., Goebel, R.P. and Benemann, J.R. (1988). Photobioreactor design: mixing, carbon utilization and oxygen accumulation. Biotechnol. Bioeng., 31, 336–344.
Whitmarsh, J. and Govindjee (1999). The photosynthetic process. In: Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee (eds), Concepts in Photobiology: Photosynthesis and Photomorphogenesis. Narosa Publishers, New Delhi and Kluwer Academic, Dordrecht, pp 11–51.
Xu, B., Li, P. and Waller, P. (2014). Study of the flow mixing in a novel ARID raceway for algae production. Renew. Energy., 62, 249–257.
Yamakawa, H. and Itoh, S. (2013). Dissipation of excess excitation energy by drought-induced nonphotochemical quenching in two species of drought-tolerant moss: Desiccation-induced acceleration of photosystem II fluorescence decay. Biochemistry., 52, 4451–4459.
Yang, X., Xiang, W., Zhang, F., Wu, H., He, H. and Fan, J. (2013). Adaptability of oleaginous microalgae Chlorococcum alkaliphilus MC-1 cultivated with flue gas. Sheng Wu Gong Cheng Xue Bao., 29, 370–381.
Yoo, C., Jun, S.Y., Lee, J.Y., Ahn, C.Y. and Oh, H.M. (2010). Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol., 101 Suppl 1, S71–74.
Yun, Y.S., Lee, S.B., Park, J.M., Lee, C.I. and Yang, J.W. (1997). Carbon dioxide fixation by algal cultivation using wastewater nutrients. J. Chem. Technol. Biotechnol., 69(4), 451–455.
Zeng, X., Danquah, M.K., Chen, X.D. and Lu, Y. (2011). Microalgae bioengineering: From CO2 fixation to biofuel production. Renew. Sust. Energ Rev., 15, 3252–3260.
Zhang, K., Miyachi, S. and Kurano, N. (2001). Evaluation of a vertical flat-plate photobioreactor for outdoor biomass production and carbon dioxide bio-fixation: Effects of reactor dimensions, irradiation and cell concentration on the biomass productivity and irradiation utilization efficiency. Appl. Microbiol. Biotechnol., 55(4), 428–433.
Acknowledgements
This work was supported by the Advanced Biomass R&D Center (ABC) of Korea Grant funded by the Ministry of Science, ICT and Future Planning (ABC-2010-0029728) and the New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) Grant funded by the Korea government Ministry of Knowledge Economy (2012T100201665).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Capital Publishing Company
About this chapter
Cite this chapter
Kumar, K., Mishra, S.K., Choi, GG., Yang, JW. (2015). CO2 Sequestration Through Algal Biomass Production. In: Das, D. (eds) Algal Biorefinery: An Integrated Approach. Springer, Cham. https://doi.org/10.1007/978-3-319-22813-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-22813-6_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22812-9
Online ISBN: 978-3-319-22813-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)