Abstract
As the use and acceptance of glass-ionomer cement (GIC) increase, the scientific community will endeavour to improve current limitations due to their relatively low physical properties compared to other materials. This chapter discusses a range of future improvements in glass-ionomer cements which will increase their longevity and allow them to be used in place of other materials such as the widely used amalgam.
To improve their material properties, many paths can be investigated. New glass filler systems, including a variety of additions, modifications and pre-reacted GIC filler particles, and their effect on physical properties are detailed in this chapter. Other categories of filler particles, including spherical particles, glass fibre reinforcement and nanoparticle developments, as well as their effect on improving GIC properties such as fracture toughness, wear and other physical and aesthetic properties are documented.
Technologies utilising GIC materials as controlled-release vehicles for different materials are discussed. The importance of new mechanisms, such as self-healing technologies and self-cleaning glass technology, is documented in efforts to improve the longevity of GICs and their physical properties. Novel polymer networks, developed for improvements in strength and other properties, and technologies related to porosity reduction, methods to improve fracture toughness and improvements in adhesion durability are also be provided. Future delivery systems provide the user with an insight of what could be the new delivery systems of GICs. Important avenues for the improvement of GIC wear properties, and improvements in aesthetic properties are discussed.
Other topics focus on the future use of GIC participating in pharmacological approaches to caries reduction and restorative dentistry and include biomineralisation and biopromoting improvements, biofilm alterations, the antimicrobial/bioprotection properties of GICs and the possibility of antibiotic additions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glass ionomer cement
- Future
- Biomineralisation
- Wear
- Delivery systems
- Nanoparticle
- Controlled-release vehicle
- Self-healing
- Fracture toughness
7.1 Introduction
As permanent restorative materials, glass-ionomer cements (GICs) (both resin-modified and conventional self-cure versions) still have limitations when placed in stress-bearing restorations due to their relatively low fracture strength, toughness and wear properties (Lohbauer 2009). GICs are generally inferior in aesthetics and physical properties compared to composite resin materials.
Although the GIC bond is considered to be one of the most resilient with a lower annual failure rate compared to adhesively bonded composite resins, the bulk material mechanical properties still hinder its wide usage (Peumans et al. 2005). Variants of GICs are now available to restore different classes of cavities; however, amalgam and adhesively bonded composite resins remain the popular choice for restorative materials. A recent study has highlighted the importance of a dental material’s ability to preserve existing tooth structure and prevent secondary caries, which places GICs in a good position against other materials (Seemann et al. 2014). It is unlikely that in the short term, GICs will achieve wear, strength or longevity properties seen in amalgam and composite resins, but the focus of future developments may deliver GICs with great improvements in these properties.
7.2 GIC as an Alternative to Amalgam
GICs are specifically used for their dynamic interaction with the tooth structure, compared to the relatively “inert” and static relationship that composite resin and amalgam have with the adjacent tooth structure interface. It is this “dynamic” environment and distinct interface created by GIC materials that has enabled their use to grow.
As more advanced analytical techniques are employed to investigate and critique restorative material interfaces, GICs continue to find an unique position in terms of their technical offering to the clinician. Glass-ionomer cements have gained relevance due to their ability to self-adhere to tooth structure, biomimetic/re-mineralising properties, anti-cariogenic potential and their relative ease of use (Peumans et al. 2005; Benelli et al. 1993). Currently, bulk-fill composite resin restorations are replacing amalgam as an accepted choice for a permanent restoration, and it is this area where new GIC advances are likely to play a role.
Furthermore, as a response to the Minamata Convention on Mercury (Mackey et al. 2014), the use of amalgams is decreasing, and clinicians are now choosing alternative materials. Currently, GIC has been recommended for small, non-load-bearing permanent restorations. Although lacking the strength or wear properties achieved by amalgam, the use of modern GIC materials is similar in many ways to amalgam. For example, both amalgam and GIC placement does not require the use of an adhesive. As GICs contain water in their formulations, they can be placed on moist dentine.
Furthermore, new-generation GICs such as ChemfilTM Rock (Dentsply DeTrey GmbH, Konstanz, Germany), KetacTM Universal AplicapTM (3MESPE Dental Products, St. Paul, MN, USA) and Equi Forte (GC Corporation, Itabashi-ku, Tokyo, Japan) do not necessarily require cavity conditioning and can be placed in cavity preparations similar to amalgam. Modern “condensable” high viscosity GIC materials are immediately condensable, and as their setting reaction proceeds, they become even more condensable, which facilitates their “packing” into the cavity in a similar way to amalgam. In addition, resin-modified GICs (RMGICs) have also been shown to place minimum polymerisation stress on cavity walls, which could further explain their durable bonding mechanism (Cheetham et al. 2014a).
In contrast, variables such as adhesive placement, isolation procedures, visible light-curing and polymerisation shrinkage stress make adhesively bonded composites more difficult to use (Gerdolle et al. 2008). Prior to 2012, the majority of GIC manufacturers recommended conditioning the prepared cavity (both enamel and dentine) with some form of acid solution (Zhang et al. 2013; Altunsoy et al. 2014; Powis et al. 1982).
Recently, a high viscosity, nonsticky, packable GIC was released that did not require cavity conditioning, i.e. placement is directly into the cavity as in the case of amalgam (Giray et al. 2014; Huo et al. 2011). This represented the first attempt to provide a viable encapsulated high viscosity GIC alternative to amalgam for certain cavity classes.
7.3 Improvements in Glass Filler Systems
The current filler present in glass-ionomer materials typically consists of ground glass particles. Their composition is based on silica and alumina as well as calcium, strontium and zinc oxide as well as other components. Fluorine, phosphate and sodium are also incorporated into multicomponent glass structures (Lohbauer 2009; Moshaverinia et al. 2011). Additions and modifications to powder systems have been described previously, but few have been incorporated into commercial GICs (Moshaverinia et al. 2011). The fillers vary in size depending on the manufacturer and are typically produced by some form of attrition (grinding) process.
Glass filler systems are then subject to processing (such as thermal or chemical treatment) and drying processes that produce a filler particle suitable for use. For encapsulated GIC products, the filler system and powder to liquid ratio creates rheological properties that influence the handling of the glass-ionomers. For example, a high viscosity product can obtain higher instrument penetration forces compared to a lower viscosity product. As these materials are multicomponent and their formulations vary significantly, several components of the formulation may influence the handling of the material.
Pre-reacted GIC glass systems have been reported, although these particles are typically transported into resin or adhesive systems to provide “GIC”-like properties (i.e. fluoride release) (Kamijo et al. 2009; Han and Okiji 2011; Shimazu et al. 2012). Glass-ionomer formulations themselves could benefit from this technology, as it allows a certain part of the final matrix to achieve ultimate strength in a controlled and reproducible environment. For example, the setting reaction of these “secondary” particles can be completed prior to the mixing stage, which can potentially give improvements in immediate wear, strength, handling and other properties to the GIC. However, microfiller (0.01–0.1 μm) agglomerated systems, and nanofillers (5–100 nm) have not been widely used as the primary glass filler size range for GIC systems.
7.4 Nanoparticle Technology
Although nano and nano-hybrid filler systems in composites have been available commercially for some time, only one “nano” particle GIC (KetacTM Nano, 3 M ESPE, St Paul, MN, USA) has become available, although its filler component claims to contain a combination of fluoroaluminosilicate glass, nanoparticles and nanoclusters. The use of smaller nanoparticles is based on the premise that some properties such as gloss retention or wear resistance may be improved. Researchers have found that this type of material has reduced surface roughness values after polishing, although the hardness of the material has not been found to be significantly different from another GIC material (Bala et al. 2012).
However, when transferring nanoparticles or nanocluster technology into encapsulated GIC formulations, difficulties arise in maintaining a usable paste consistency whilst maintaining adequate filler volume content. The evolution of the filler system technologies seen in composite resin material development has potential for translation into future filler types for glass-ionomer systems (Ferracane 2011). As there is a perceived need for GIC materials to possess more aesthetic properties similar to composite resins, it is envisaged that smaller particle types will be introduced.
Various technologies can be employed to achieve nanoparticle systems for use in GICs; these include agglomeration, pre-polymerisation, resin infiltration and the joining of glass systems to nanoparticles. Although other material factors influence optical properties such as gloss, smaller (i.e. nanosize) glass particle composites typically achieve better initial gloss and gloss retention. For GICs, the “nano” phase of research and development is imminent and could provide some exceptional properties particularly in the aesthetic and wear values of GICs. However, it is yet to be shown how a nanoparticle with appropriate chemical properties can adequately replace the current “multicomponent” fluoride-containing glasses commonly employed in commercial GIC formulations.
Although many nanoparticle additions to GIC formulations have been attempted, no nanosized (i.e. <100 nm) universal “multicomponent” reactive glass system has been employed so far. Furthermore, using a combination of two different types of filler or fillers of different compositions could be employed to overcome the problems associated with using nanoparticles as the only filler type. Nano-clays have also been reported to reinforce GIC systems although not yet employed in commercial GICs (Fareed and Stamboulis 2014a, b). Nano-sheets, nano-rods, nano-tubes, nano-films and a diverse range of reported micro-nano structures could be incorporated into GICs to investigate the improvement of various properties.
7.5 Spherical Particles
At present, most GIC materials contain irregularly shaped glass particles. By incorporating spherical particles, it is possible to alter the filler to volume ratio. Some research (Gu et al. 2004) has delved into these spherical glass systems, although they have not been commercially introduced to GICs. Spherical GIC glasses can be produced in different ways, and methods such as sol–gel technology and plasma treatment have been employed. Spheroidisation of glass powders has also been performed by using flame spraying and inductively coupled radio frequency plasma spraying techniques (Gu et al. 2004). Spherical silica additions to RMGICs have also been investigated and have demonstrated improved marginal gap reduction, as well as improvements in compressive, diametral tensile and flexural strength (Irie et al. 2011; Hatanaka et al. 2006).
A potential way of reducing biofilm on glass-ionomers could be by providing a smoother material surface, possibly facilitated by smaller nanosized filler systems, or by different surface chemistries, although this is yet to be introduced (Busscher et al. 2010; Hengtrakool et al. 2006).
7.6 Glass Fibre Reinforcement
Glass fibre composite technology has enabled engineers and chemists to develop advanced composites used in many high technology and demanding situations (Bakis et al. 2002). Reactive glass fibres have been employed in experimental GICs to create fibre-reinforced GICs (FRGIC). They have been found to increase the flexural strength and increases in the work of fracture primarily due to the additional energy required to “pull” the fibres out of the fracture surface (Lohbauer et al. 2003).
Fibres of SiO2–Al2O3–CaF2–Na3 AlF6 (430 μm long) have been shown to significantly increase the fracture toughness, as well as providing large increases in the total energy release rate (Lohbauer et al. 2004). However, glass fibres are usually large compared to glass particles, which can be disadvantageous. Short fibres (1000 μm long × 10 μm wide) have also been shown to increase diametral tensile strength, flexural strength, flexural modulus and fracture toughness of GIC (Hammouda 2009). Short fibres from CaO–P2O5–Si–O2–Al2O3 glass systems maintain higher flexural strengths after thermo-cycling than unreinforced formulations, as expected from fibre-reinforced materials, although these strengths are inferior to those typically achieved by composites (Kawano et al. 2001).
However, the aesthetic properties of FRGIC materials are compromised, and this together with exposure of fibres due to wear makes the current fibre-reinforced formulations currently non-commercially viable.
Nanofibres with diameters less than 100 nm are now available, although their incorporation into GICs has not become commercially available. Electro-spun inorganic nanofibres based on TiO2, SiO2, Zr02 and Al2O3 have been developed (Qizheng et al. 2006; Wessel et al. 2010). This electro-spinning technology developed for the textile industry (Zhou and Gong 2008) could be utilised to produce suitable nanofibres to be incorporated both physically and chemically with GIC systems. Furthermore, as research into nano-tubes and nano-wires continues, the availability of these nano-products will become available for determining their effect on GICs (Zhang et al. 1999). Finally, hybrid organic–inorganic nanofibres are also being investigated in other material science areas and could possibly introduce novel properties to different classes of GICs (Fischer 2003; Wu et al. 2010).
7.7 Incorporation of Polymeric Filler Carriers
Polymer carriers are particles loaded with active molecules or compounds, which are designed to be released or influence the surrounding environment of the restoration/tooth surface interface. These nanosized, monodispersed, spherical particles with functionalities for the immobilisation of chemicals or biomolecules have been used for tissue engineering and controlled-release drug delivery and have been classified as “molecularly imprinted polymer systems”. The possibilities for these carrier fillers are endless when applied to GIC systems, and they provide novel systems for releasing future compounds not actively involved in the GIC setting reaction. These particles, loaded with zinc chloride and incorporated into adhesives, have been proposed to inhibit the degradation of collagen by matrix metalloproteinases (MMPs) (Osorio et al. 2014). Their incorporation into GICs could also prove a viable research path for further improving the GIC bond to dentine as it is well known that MMP action can compromise the dentine-adhesive interfaces (Tezvergil-Mutluay et al. 2013).
7.8 Controlled-Release Vehicle Additions to GIC
The controlled release of active ingredients from the GIC matrix could also be facilitated by other technologies. For example, microencapsulated particles have been proposed in order to facilitate tooth mineralisation. Typically these are designed for concentrated release of calcium (Ca(NO3)2), fluoride (NaF) or phosphate (K2HPO4) salts directly to the tooth. Using a semipermeable shell wall, the salt ion permeates through the shell wall at a controlled rate, and these types of particles can provide a diverse delivery platform for different materials (Latta et al. 2014).
New types of interpenetrating polymer networks could also be incorporated in GICs, as they have proven their use in the delivery of a large range of compounds and drugs through different novel carrier systems. These materials are typically based on hydrogels, microspheres, microbeads, microparticles, nanoparticles and other platforms in order to effectively deliver active ingredients (Lohani et al. 2014).
Controlled-release polymer technologies are commonly employed in drug and therapeutic formulations to deliver active ingredients over different time periods. Many of these technologies could be incorporated into GICs to improve the targeted release of future compounds. Typically, nanotechnology delivery systems can be used for improved delivery of poorly soluble drugs, targeted delivery of large molecule actives and the co-delivery of two active compounds (Farokhzad and Langer 2009).
Hydrogel technology, i.e. three-dimensional cross-link networks, can be formulated into micro- or nanoparticles and contain highly porous structures. Their characteristic porosity permits the loading of compounds which can be released at rate-dependent diffusion coefficients through the hydrogel network (Hoare and Kohane 2008). Gelatin hydrogels have also been used for the delivery of bioactive molecules allowing their use in tissue engineering, gene therapy and controlled drug release and could be adapted for use in GICs (Young et al. 2005).
Gold nanoparticles have also been used as controlled-release vehicles, and they are assumed to provide a non-toxic delivery system for active materials coated on their surface (Ghosh et al. 2008). Furthermore, block copolymer micelles have also been utilised as delivery systems for drugs and can provide different mechanisms for delivering micelle-forming polymeric drugs and other compounds (Kazunori et al. 1993). All of these controlled-release technologies could be employed for future use in GIC materials in order to provide the release of specific chemicals beneficial to protecting and repairing the remaining tooth structure.
7.9 Self-Healing Technology
The GIC setting reaction in both the conventional and resin-modified GICs is facilitated by ion-binding and gelation between poly(alkenoic acid)s and metal ions, and the kinetics and reactions have been described in detail previously (Wilson and Nicholson 2005; Wilson 1996; Smith 1998; Nicholson 1998; Walls 1986). A new research topic in polymer science, and one that could be applied to future GIC polymer chemistry and development, involves the study of “self-healing” polymer structures. Self-healing, or autonomously healing polymers and coatings, is a new technology that allows the automatic repair of undetected defects such as cracks, fractures and other damaged areas within a polymer or adhesive interface, which have resulted from chemical, thermal or mechanical fatigue processes. This type of technology could easily be incorporated into GIC materials to provide “smart” multifunctional materials that when subjected to “excess” stress could repair any resultant damage themselves. Glass-ionomers are good candidates for this technology as they already have a significant interaction (i.e. ionic and water transport) with the surrounding oral environment. There are different methods of inducing self-healing technologies, none of which as yet have been applied to GIC chemistry. Several reviews have described the different methods to facilitate self-healing technologies into polymers (Samadzadeh et al. 2010; Yuan et al. 2008; Wu et al. 2008; Wilson et al. 2010).
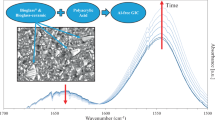
The incorporation of micro- or nano-capsules releases “repair agents” (Figs. 7.1 and 7.2) specific to the polymer architecture after crack propagation in coatings. In practice, these capsules are presented in the polymer network in sizes ranging from 3 to 800 μm, and they can contain a solid, a liquid or gas components as a “core” material, surrounded by a capsule or shell material (Wu et al. 2008; Samadzadeh et al. 2010).
Proposed self-healing concept containing nano-encapsulated healing agent. (a) Crack formed due to damage, (b) nano-capsule is ruptured releasing healing agent that migrates to crack, and (c) healing agent is then polymerised after contacting a catalyst (Reprinted from Samadzadeh et al. (2010). With permission from Elsevier)
SEM micrographs of micro-crack healing. (a) Coating containing scratch defect (arrowed) and (b) self-healed scratch defect after healing process (Reprinted from Samadzadeh et al. (2010). With permission from Elsevier)
Interpenetrating polymer networks incorporating linear polymers (e.g. poly(methacrylated phenyl glycidyl ether)) has shown diffusion of the linear polymer phase into the crack interface as a form of self-healing process. SEM images (Fig. 7.2) of damaged areas clearly demonstrate that healing of micro-cracks in the order of 20 μm can be achieved, from mobile chain interdiffusion and entanglement (Peterson et al. 2012).
Polymers incorporating embedded microcapsules of liquid monomers are also used to induce self-healing properties. When the material is damaged, the microcapsules release monomers into the damaged areas and subsequently repair the damage and prevent further fracture. Other approaches include the “mechanochemical” activation of a catalyst in a polymer chain, i.e. when mechanically induced chain scission occurs, a catalytic site is activated, thereby facilitating polymerisation of acrylic monomers. Other approaches are supra-molecular (i.e. non-covalent interactions) and also methods to exploit covalent bond forming repair processes (Colquhoun and Klumperman 2013).
Both intrinsic and extrinsic mechanisms can be used to develop self-healing properties. Polymer architecture such as chain stiffness and cross-link functionality, cross-linking density and content or reversible groups and multiphase polymer structure all have an influence on the self-healing ability of various polymer systems (Garcia 2014). Other approaches incorporating shape memory materials, swollen materials or passivation can also be utilised to impart self-healing properties, and a possible mechanism for GIC repair is shown in Fig. 7.3. The investigation of poly(ethylene-co-methacrylic acid)-based ionomers has also indicated its ability to self-heal (Wu et al. 2008). However, it is yet to be seen how manufacturers will incorporate these new technologies into GICs in an effort to improve their mechanical and adhesive performance.
Theoretical mechanism of self-healing in glass-ionomer materials (Reprinted from Wu et al. (2008). With permission from Elsevier)
7.10 Other Novel Polymer Networks for Improvements in Strength and Other Properties
In order to increase the physical properties of GICs, improvements can be achieved by modifying polymer chemistry contained in the liquid component or in the solid form present in the powder component (Guggenberger et al. 1998). Multi-arm poly(acrylic acid-co-itaconic) acids have been prepared and have demonstrated improvements in compressive strength for conventional cured GICs (Xie et al. 2010), and star-shaped poly(carboxylic acid) polymers have been proposed for resin-modified GICs (Weng et al. 2014). Vinyl-containing poly(acrylic acid-co-itaconic acid) copolymers have also shown higher flexural strength leading to a tougher fracture surface and plastic deformation properties (Wu et al. 2003). The polymer chemistry here is complex (Yelamanchili and Darvell 2008), and future improvements in results rely on the interactions between the multicomponent nature of different GIC systems which contain many components, including complex initiating systems.
7.11 Porosity Reduction
As glass-ionomers are mixed together in order for the acid–base reaction to proceed, typically, small voids or air pockets are incorporated into the mix. Although the effects of these voids are not exactly understood, it is assumed that these have a negative effect on the GIC strengths. One method of reducing these voids is to provide both parts of a glass-ionomer in a paste version, which can be hand-mixed by a spatula or through some form of a static mixer (Boehm et al. 2011). However, compared to encapsulated delivery systems, this form of delivery is typically uneconomical and is not widely used. A major advancement for encapsulated versions would be the reduction of voids during the extrusion of the mixed paste, although no such capsule version has yet to be developed.
7.12 Improvements in Fracture Toughness
Fracture toughness is known to be an important material property for successful posterior load-bearing restorations (Lloyd and Adamson 1987). To date, it has been questioned whether the survival of GICs will reach levels achieved by composite resin materials in load-bearing situations (Burke 2013). However, in some studies modern GICs used to restore load-bearing cavities have been demonstrated to perform satisfactorily up to 10 years. This demonstrates that wear properties simulated in a laboratory do not necessarily translate to reduction of anatomical form or surface roughness in real-life situations (Burke 2014).
For primary molar restorations, recent studies have indicated that the survival rate for amalgam restorations is no different from that of atraumatic restorative treatment with a high viscosity (HV) GIC. For both amalgam and HVGIC, the main reason for failure is mechanical, and it has been suggested that for single surfaces of primary teeth, HVGIC is a viable alternative to amalgam (Yamazaki et al. 2006).
Modifications to the polymer component of the GIC have shown to improve the fracture toughness of GICs. Acrylic acid-itaconic acid-N-vinylcaprolactam (NVC) ter-polymers have been synthesised and have shown significantly higher plane-strain fracture toughness (KIc) at 1-day and 1-week storage time periods compared to the control (Fuji IX, GC Corp, Tokyo, Japan) (Moshaverinia et al. 2010). However, issues associated with viscosity increases need to be overcome for the incorporation of any of these new polymers.
Improvements of fracture toughness depend on many variables. It has been shown that modification of the polymer components, such as amino acid-containing GICs, methacryloyl amino acid reactive diluents and a series of other new chemistries are viable paths to improving properties such as fracture toughness of the final cement (Culbertson 2001).
Other novel polymer systems such as 6-arm star-shaped poly(acrylic acid) polymers have also shown significant increases in fracture toughness compared to commercial RMGICs, similar in value to composite material (Zhao et al. 2009). Adjustment of the molecular weight (MW) of polymers has also been investigated for improving properties, although it is unknown how these changes will affect clinical outcomes.
Viscoelastic (i.e. viscous and elastic response of materials to deformation) properties of GICs have also been reported in an effort to understand the mechanical behaviour of these materials. Fracture may involve wear and adhesive de-bonding modes of failure, as well as the typical bulk fracture of the various GIC materials; both GIC and RMGIC exhibited viscoelastic properties (Yamazaki et al. 2006). Modifications in the powder, for example, particle size reductions and powder to liquid ratios, have also been shown to increase fracture toughness in GIC formulations (Mitsuhashi et al. 2003).
Interfacial fracture toughness has also been studied in order to provide paths for GIC improvements related to failure by fracture. Formulations are investigated at adhesive interfaces, and typically the fracture then continues into the bulk material (Setien et al. 2005; Cheetham et al. 2014b). These studies are extremely relevant for development of improved GIC materials. To improve the longevity of GICs, properties such as fracture toughness of the bulk material and the adhesive properties (interfacial fracture toughness or interfacial work of fracture) need to be improved.
7.13 Improvements in Adhesion to Dentine and Enamel
The critical issue with adhesive dentistry is to place the adhesive material using a method that gives the best chance for long-term stability and the creation of an “effective seal” to the adhesive–tooth interface. In the case of resin-based adhesive systems, there are several strategies that have been proposed in order to optimise the probability of success, and these include application of MMP inhibitors and collagen cross-linkers, additional enamel etching, agitation of the adhesive for deeper penetration, etc. (Manuja et al. 2012). These approaches are also very relevant when trying to improve the bonding reliability of GIC materials. Many original and review articles are available to explain the different concepts involved in adhesion to dentine and enamel (Tyas 2003; Manuja et al. 2012; Atmeh et al. 2012).
Adhesion to dentine and enamel is a complex science and similar to adhesively bonded composite dentistry; successful outcomes depend on many variables (Perdigão 2010; De Munck et al. 2012). Glass-ionomers have also been shown to give a more durable bond compared to other adhesive systems (Van Meerbeek et al. 2010). However, much is unknown about this bond and most importantly the degradation mechanisms of the bond.
The bonding mechanism of new-generation GICs has now demonstrated resin tag formation. Studies investigating the differences between dentine conditioned with phosphoric or polyacrylic acid solutions have shown that both protocols create a surface that allows RMGIC resin tags (Fig. 7.4) to form (Hamama et al. 2014; Korkmaz et al. 2010; El-Askary and Nassif 2011). Other studies demonstrate the intimate chemical interaction with dentine and enamel surfaces (Fig. 7.5) with exposure of collagen due to dentine pretreatment, with the remaining hydroxyapatite being used as “receptors” for chemical bonding of the GIC. Micropores have also been shown to increase the ability for micro-mechanical bonding (Van Meerbeek et al. 2003).
SEM micrographs showing the interface between RMGIC and dentine. The top image (a) had dentine treated with 37 % phosphoric acid, the bottom image (b) was treated with a solution of 25–30 % polyacrylic acid conditioner (D denotes dentine). The hand pointer indicates the presence of resin tags (Reprinted from Hamama et al. (2014). With permission from John Wiley & Sons)
(a) SEM image showing the effect of polyacrylic acid conditioner on dentine and (b) TEM photomicrograph showing the hybrid layer and gel phase created after RMGIC placement (Reprinted from van Meerbeek et al. (2003). With permission from Operative Dentisitry, Inc.)
The chemical interaction and formation of ionic bonds between carboxylic acid groups with hydroxyapatite have been described previously, and future GICs will use this information to provide materials with stronger, more resilient bonding interfaces (Yoshida et al. 2000).
Further improvements of the bonding to hydroxyapatite, enamel and dentine components will continue to be focused on the incorporation of new polymer chemistries into the GIC formulations. Pioneering work investigating the binding energies of functional groups to these structures as well as the molecular structure of the polyalkenoic acid component has shown that these factors significantly affect the chemical bonding to hydroxyapatite structures (Fukuda et al. 2003; Sennou et al. 1999). This process to gather information on surface interaction will continue to be used to modify resin formulations and optimise chemical adhesion processes for GICs.
Apart from chemically related improvements, other “macro” physical observations of GICs may be utilised to achieve improvements in adhesion. Spherical bodies (Figs. 7.6 and 7.7) have also been shown to be involved in GIC adhesive surfaces which could provide fracture initiation sites for bond failure, although their participation in the adhesive process is not yet fully understood (Yiu et al. 2004a, b). New materials will try to eliminate these porous, randomly incorporated features which disrupt a relatively homogenous and compact solid material. Further understanding of the interfacial properties of GICs with enamel and dentine will inevitably lead to improving the longevity of the GIC bond.
SEM micrograph showing a RMGIC surface fractured adjacent to the GIC–dentine interface, showing the presence of spherical bodies (hand pointer). Dehydration cracks are identified with an arrow (Reprinted from Yiu et al. (2004a). With permission from Elsevier)
SEM image of a spherical body showing brittle fracture of the spherical structure. The handpointer identifies a fractured eggshell-like structure within the GIC at the GIC–dentine interface [Reprinted from Yiu et al. (2004b). With permission from Sage Publications]
Research on GIC adhesion provides information for understanding the fundamental mechanics and kinetics of the bond formation and subsequent path of bond degradation in order for new adhesive strategies to be devised. Short-term, static bond strengths give limited information when comparing the potential longevity of a GIC bond and understate its adhesive abilities. A fracture mechanics approach, including interfacial fracture toughness and work of fracture testing (Fig. 7.8) showing the “total energy” required to initiate fracture, provides more relevant information on the possible improvements of the bond durability (Cheetham et al. 2014b).
Comparison of the interfacial work of fracture (γ wofint) results (J/m2) together with standard deviations for glass-ionomer bonded to dentine (Reprinted from Cheetham et al. (2014b). With permission from Elsevier)
7.14 Future Delivery Systems
The delivery format of glass-ionomers varies from powder–liquid bottle versions and single-use encapsulated systems to dual-barrel syringes (and unit-dose) for paste–paste GICs. Paste–paste systems generally reduce the porosity of the final GIC paste, and single-use formats (Fig. 7.9) have been recently commercialised (Boehm et al. 2011). However, these formats have not been as widely accepted as single-use encapsulated delivery formats for powder–liquid versions of GICs.
Example of a single-use dual-barrel paste–paste delivery system (Reprinted from Boehm et al. (2011))
Encapsulated systems eliminate the need for measurements to be made by the user, resulting in a dispensed material with more reproducible features such as setting reaction (gel and set time), strength and paste consistency. The other benefits of current GIC capsule systems are the ease of direct delivery and their single use. New-generation GIC capsules (Fig. 7.10) will be able to mix “ultra” high viscosity GICs, which could also allow for a new GIC with very high powder to liquid ratios and strengths not seen in GICs before (Cheetham 2014).
Example of a single-use powder–liquid delivery system (Reprinted from Cheetham (2014))
However, a disadvantage of “capsules” is that during the mixing process, they typically introduce some form of subclinical micro-porosity in the paste. Various methods, such as centrifuging the capsule after the mixing process, or vacuum mixers, have been employed to reduce this with limited effect (Suzuki et al. 2004). Theoretically, the reduction of this porosity will increase the physical properties of GICs. The clinician is yet to see the direction of future designs of GIC delivery systems, but it is likely that ease of the use in terms of delivery and reduction of porosity are areas where improvements can be made.
7.15 Wear Improvements
Currently, it is popular to place a coating over the setting GIC in order to protect it during the initial setting stage, which improves the immediate aesthetics of the restoration and acts as a method to fill in any porosity created on the surface (Zoergiebel and Ilie 2013). However, the effect of this coating on GICs placed on occlusal surfaces has not always been found to be significant when comparing the wear of uncoated surfaces (Diem et al. 2014). Enamel–GIC margins may also be protected with resin coatings, although this adds an extra step in the placement of GICs (Hokii et al. 2014).
Recent laboratory tests (Fig. 7.11) have demonstrated that the current commonly used commercial GIC materials continue to have inferior wear resistance to amalgam. However, in two-body wear studies, the wear resistance of some GICs is now similar to compomers, with recommendations that these GICs may be used adequately in occlusal restorations of primary teeth (Lazaridou et al. 2015). Furthermore, it was found that GIC materials covered with a filled polymer did not perform better in terms of GIC vertical loss (μm) and volume loss (mm3). The mean vertical loss of the best performing GIC was still nearly three times worse than amalgam, and when comparing volume loss, the amalgam had six times less volume loss (Lazaridou et al. 2015). Hence, commercial GIC materials remain inferior in wear situations when compared to amalgam. What is interesting from these studies is that when compared to other posterior materials, some GICs can now be viewed as an alternative option.
Mean volume loss (mm3) and standard deviations of dental materials after an oral simulated wear test. Note superscript a refers to conventional GIC, b refers to RMGIC, and c refers to polyacid-modified resin composite material (compomer) and d resin composite material (Reprinted from Lazaridou et al. (2015). With permission from Springer Science)
For some time, manufacturers and researchers have been attempting to improve the wear characteristics of GIC materials. Significant advances have been made since initial tests demonstrated that GICs were brittle and showed catastrophic failure during wear events, concluding that they were not acceptable for posterior occlusal applications (McKinney et al. 1987). Future efforts to improve the difference between amalgam and GIC wear properties will continue to be focused on by researchers. Wear can be improved by increasing the hardness of the GIC surface prior to any wear event. This can be achieved in a number of ways, and increasing the powder to liquid ratio, concentration of polyacids or the molecular weight of polyacids have been reported as possible ways (Guggenberger et al. 1998; Smith 1998). As commercial materials contain undisclosed proprietary compounds and are produced in novel processes, much of these advances are not documented in the public domain.
Silver particles have been utilised to provide a means of lubrication during wear events, and commercial products containing silver alloys have been shown to provide lower wear resistance (McKinney et al. 1988; Xie et al. 2000). However, from an aesthetic perspective, the addition of metal particles compromises the clinical outcome, and these materials are not currently widely accepted.
Studies by Xie et al. in 2000 have also shown that the more integrated the GIC microstructure is, the higher the mechanical properties. Furthermore, large glass particle systems have been described as contributing to lower wear properties. The integrity of the interface between the glass filler system and the polymer, the particle sizes of each of the filler systems and the number of voids created during mixing can all influence the wear properties of current GICs (Xie et al. 2000). As GICs age, their wear rate decreases, and differences in wear rates have been described in terms of polyalkenoic acid content, the “overall” chemical composition of the GIC and differences in the filler system and size of fillers used (van Duinen et al. 2005).
Avenues for future improvements in the wear properties of GICs will focus on innovations in these areas described above, in order to provide a harder external surface of GIC restorations, although it is unlikely that the wear properties in the short term will reach those obtained by modern amalgam alternatives.
7.16 Aesthetic Improvements
Compared to modern composite resin materials, the aesthetics of GICs are typically inferior, and much work is being performed in order for GICs to produce aesthetic restorations that blend in with tooth structure. For the future of GICs, the aesthetic properties are an important feature as patients in the “post amalgam age” are demanding that their teeth are restored with more tooth-like materials.
Aesthetics with GICs is likely to continue to evolve. For example, the colour change over time may be reduced with the addition of new polymer and surface chemistries that allow GICs to resist colour change even when subjected to staining media such as beverages or certain foods.
The initial translucency and optical properties (Fig. 7.12) of RMGICs are now similar to leading composite resin materials (Ogledzki et al. 2012). The optical properties, such as translucency and opacity values, together with closer colour matching of shades to Vita™ shades may be improved to follow composite resins. Conventional-cured GICs still lack the translucency properties (Fig. 7.13) of modern-day composites, although improvements are being introduced.
Opacity comparison of commercial GIC demonstrating that they have higher opacities compared to RMGICs shown in Fig. 7.12. All materials are conventional cured type (Image courtesy of SDI Limited R&D)
7.17 Fluoride Release
It is well documented that GIC materials release fluoride sourced from their glass system or from additional additives such as sodium fluoride in the set cements (Williams et al. 2002; Jones et al. 2003; Guida et al. 2002). Recent 1-year fluoride release data (Fig. 7.14) of commercial GICs has shown significant differences in fluoride release between current commercially available GICs (Shiozawa et al. 2013). Furthermore, the release of Sr, Na, Al and Si ions has been shown to affect the properties of the glass-ionomers and demonstrate how the GIC can interact with its aqueous environment. This ionic transfer is a unique feature of glass-ionomers not seen in composite resin materials. Although most manufacturers highlight the fluoride release of their material, future glass-ionomers could be designed to release high levels of calcium, phosphate or other ionic species considered beneficial to certain cavity conditions (Forsten 1998).
Cumulative fluoride release (μg/cm2) of various commercial GIC materials up to 1 year. Standard deviations are in parentheses, and the same superscript letter denotes homogenous subsets (p > 0.05) (FEX: Fuji IX GP EXTRA, GC; FIX: Fuji IX GP, GC; GFX: GlasIonomer FX-II, Shofu; KME: Ketac Molar Easymix, 3MESPE; RSC: Riva Self Cure, SDI) (Reprinted from Shiozawa et al. (2013). With permission from Springer Science)
The fluoride release from GICs has been shown to decrease with time, which in turn decreases their antimicrobial effectiveness (Dionysopoulos et al. 2013). To counteract this, it has been demonstrated that GICs can “recharge” their fluoride release by topical fluoride application (e.g. fluoride-containing toothpaste) (Dionysopoulos et al. 2013; Arbabzadeh-Zavareh et al. 2012). Studies have shown that covering aged GIC with 0.1 % fluoride toothpaste or 1.25 % fluoride gel significantly increases the fluoride release (Seppa et al. 1993).
Glass-ionomer cements based on strontium glass systems have also been shown to release strontium ions originating from the glass structure to the adjacent tooth structure. Although these ions do not have any antibacterial effect, it is envisaged that they are rapidly exchanged for calcium ions, and a synergistic relationship with fluoride could facilitate antimicrobial properties (Dabsie et al. 2009). New-generation GICs will aim to maintain a constant relevant supply of fluoride ions over their life, without degradation of the cement, and any improvement in the release of fluoride should be incorporated into future GICs.
Addition of potassium and fluoride has also been previously made to the liquid components of GICs, and this has demonstrated ion release up to 500 days (Williams et al. 1999). Glass-ionomers immersed into potassium fluoride exhibited ion release twenty times greater than specimens that had additions to their liquid component, and this process could be adapted for pre-reacted GIC filler systems that act as reservoirs for specific ions (Williams et al. 1999).
7.18 Bioremineralisation/Biopromoting Improvements
Although most manufacturers highlight the fluoride release of their GIC material/s, future GICs could be designed to release high levels of calcium, phosphate or other ionic species considered beneficial to certain cavity conditions. This release could originate from (a) glass systems involved in the main setting reaction, (b) glass systems not involved in the setting reaction or (c) other additives (e.g. amorphous calcium phosphate).
Calcium phosphate has also been added to two low viscosity GICs in the form of nano-amorphous calcium phosphate and ACP-CPP. Although the benefits of these additives have not yet been clinically proven, it shows attempts at using the GIC matrix as a vehicle for delivering specific agents to the tooth. Spray-dried amorphous calcium phosphate (ACP) nanoparticles incorporated in composite materials have been shown to release calcium ions when subjected to acidic conditions and decreasing the pH resulted in greater ion release (Xu et al. 2011).
Although ACP has been used in GICs for some time, a newly developed GIC contains casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) incorporated in the powder component. This has been shown to release fluoride, calcium and phosphate ions under acidic conditions, potentially minimising the effects of demineralisation resulting from caries attacks (Zalizniak et al. 2013).
Micro- or nano-hydroxyapatite nanoparticles have also been proposed as additives to further promote bioremineralisation in GICs and have been found to increase GIC properties (Moshaverinia et al. 2008). Biomimetic fluorhydroxyapatite/polyacrylic acid nanostructures have also been proposed as additive structures, which have structures very similar to human enamel (Roche and Stanton 2015). However, these promising compounds have not yet been incorporated into commercial GICs.
7.19 Biofilm Alteration
Biofilms are formed over tooth and GIC surfaces due to the diverse range of microbial species and communities present in the oral cavity. There are various stages of film formation, including the formation of a pellicle, colonisation, propagation and finally steady-state existence on the surface (Steinberg 2000; Teughels et al. 2006). GICs have been shown to have rougher surfaces than composite resin materials (Carlén et al. 2001). Their surface chemistry is complex, and the surface free energy components of these surfaces (i.e. total surface free energy, nonpolar, acid–base components as well as acid and basic contribution) changes when comparing polished and unpolished materials. Unpolished GIC surfaces have also been shown to contain more positively charged components, which collect more proteins and increase bacterial adhesion compared to composite resin (Carlén et al. 2001). This colonisation causes a deterioration of the GIC surface and eventually the development of caries around the interface of the material and the tooth (Busscher et al. 2010). In vitro studies have shown that the release of fluoride from GIC is not adequate to prevent biofilm formation, possibly due to the low levels of fluoride release (Al-Naimi et al. 2008). It would be advantageous for GICs to improve their protective mechanisms from biofilm attack, and a number of strategies could be employed including increased fluoride release, antimicrobial properties and the incorporation of other additives.
7.20 Self-Cleaning Glass Technology
A technology known as “self-cleaning” glass has been developed, but has not been previously adapted to biomaterials in order to reduce biofilm accumulation or to at least favourably alter the biofilm. Generally, these glass surfaces are coated with a thin layer of material that facilitates photooxidative self-cleaning or some other cleaning process. For example, titanium dioxide (TiO2) has been used to coat commercial glasses in layers less than 10 nm, and with photo-activation, it has been shown to be capable of killing gram-positive and gram-negative bacteria, although it is unknown how much energy is required to produce this effect (Foster et al. 2011; Guan 2005). Other self-cleaning surfaces have been described, but the hydrophobicity resulting from their properties may not allow them to be incorporated into GICs. However, it could be assumed that investigations into incorporating some form of “self-cleaning” mechanisms or technologies into GICs could be introduced and may potentially reduce biofilm accumulation in critical areas such as the enamel-restoration margin whilst maintaining biocompatible properties (Blossey 2003).
7.21 Antimicrobial Properties/Bio-protection of GICs
Topical antimicrobial therapy has been used as a technique to reduce and manage caries. For early childhood caries, these materials are used to reduce caries progression, as well as actively reduce the pathogenic oral microorganism levels. Antimicrobials employed and reported are fluoride, silver diamine fluoride, chlorhexidene, povidone iodine and xylitol (Jayabal and Mahesh 2014; Mei et al. 2013). Some in situ anti-cariogenic potential properties of glass-ionomers have been documented (Benelli et al. 1993), and the cariostatic effect of GICs has been demonstrated clinically, with reduced rates of secondary caries and marginal staining compared to composite. However, it is questionable whether this “inhibiting” effect can completely arrest the carious process (Van Amerongen 1996).
Incorporating additional antimicrobial compounds into the GIC formulation could be beneficial in maximising the protection of an already vulnerable tooth interface damaged by a cariogenic attack (Tyas 1991). Incorporating antimicrobial compounds into GICs and using GICs as a “carrier” has been suggested, although these highly antimicrobial materials face a difficult task of passing biocompatibility tests and material registration issues required for dental materials (Dimkov et al. 2009; Yli-Urpo et al. 2003). It has also been shown that placing antimicrobials such as benzalkonium chloride into GICs releases chloride ions as well as fluoride ions, further increasing the antimicrobial efficacy of GICs (Dimkov et al. 2009).
Other strategies have employed bioactive glasses, known to exhibit antimicrobial properties in GIC formulations. GICs incorporating these glasses at levels up to 30 % have demonstrated antimicrobial effects on Candida albicans and Streptococcus mutans and generally reducing bacterial growth levels (Yli-Urpo et al. 2003).
Macromolecules containing quaternary ammonium salts have also been investigated by incorporating poly(acrylic acid-co-itaconic acid) with pendant ammonium salts. Studies have shown that the antimicrobial effect of these polymers can be longer lasting than compounds that leach out of GICs over time (Weng 2011).
Other synthesised poly(quaternary ammonium salt)-containing polyacids have also demonstrated antimicrobial properties of GICs, although other considerations such as strength need to be taken into account when incorporating such polymers (Xie et al. 2011).
Loading GICs with nanoparticle antimicrobial release agents has also been proposed. Nanoparticles of chlorhexidine hexametaphosphate have been added to filler systems in 1–20 wt% range to provide a “broad spectrum antimicrobial agent”, which would theoretically release chlorhexidine in a controlled manner (Hook et al. 2014). Chlorhexidine is reported to provide antimicrobial action against oral bacteria. However, due to the fact that GIC restorations still fail from secondary caries, it could be concluded that fluoride release alone is not adequate to inhibit bacterial ingress into cavities.
Current data suggests that the antimicrobial effect of GICs diminishes after 1 month (El-Baky and Hussien 2013). With the addition of 1 % chlorhexidine diacetate powder (Fig. 7.15), the antibacterial effect of the GICs is significantly increased up to 84 days (El-Baky and Hussien 2013). Although these tests are laboratory based, they demonstrate the effectiveness of GIC matrices as carriers for antimicrobial additives and offer many avenues for future material improvements, particularly in high caries prevalence situations.
Means of free Streptococcus mutans areas (mm) in GIC materials containing 1 % chlorhexidine treatment; circular inhibition zones (y axis) produced around the GIC samples are demonstrated up to 84 days. The three materials are convention cured GICs (Reprinted from El-Baky and Hussien (2013). With permission from Synergy Publishers)
7.22 Antibiotic Additions
Glass-ionomers generally possess some form of antimicrobial properties compared to other inert dental materials. Considering a carious lesion is formed primarily from the acidic by-product attack of microbial species, antimicrobial properties can be viewed as a positive feature of glass-ionomers. As GIC materials are required to pass biocompatibility tests defined in standards such as ISO 7405 (ISO 2008) and ISO 10993 (ISO 2009), there will be limits to the level of antimicrobial action a GIC can possess. Antibiotics (ciprofloxacin and metronidazole) have been previously added to GICs and have been shown to increase the inhibitory effect of Streptococcus mutans and Lactobacillus casei. Furthermore, the addition of antibiotics increased the fluoride release of the GIC, possibly by the creation of voids that allow water ingress into the material (Prabhakar et al. 2013). Initial clinical trials have also shown the benefits of using GIC loaded with metronidazole, ciprofloxacin and cefaclor antibiotics in the sealing of infected dentine in atraumatic restoration in primary molars. However, to date, no commercial GIC is available that contains an antibiotic, and other additives considered safer could be the preferred path for future developments (Ferreira et al. 2013).
Silver nanoparticles (Ag NPs) have been known to possess antimicrobial properties by their ability to attach to the surface of bacterial cell membranes, thereby disturbing the functions of the cell. These have been incorporated into many different materials and demonstrate inhibitory effects against a range of bacteria. Polymer-silver nanoparticles, poly(vinyl alcohol)-silver nanoparticles as well as silver-doped hydroxyapatite have been reported (Sharma et al. 2009). Furthermore, through a micro-emulsion approach, AgNPs have been encapsulated in silica nano containers which allow for additional properties to be provided by the shell wall (Priebe and Fromm 2014). Non-nanoparticle strategies to deliver silver have also been used for coating nanofibres with silver ion releasing polymer coatings, which could be adapted for the use in GIC through the incorporation of similar nanofibrous scaffolds (Mohiti-Asli et al. 2014).
7.23 Future Pit and Fissure Sealing
Pit and fissure sealing of occlusal surfaces has principally been performed by resin sealants. Future GICs may possess “ideal” rheological and wetting properties to replace resin sealants, as well as all of the other properties that GICs possess, including the option of visible light-curing (VLC). The progressive stages of occlusal lesion formation have been described since well over a century ago (Bate 1864). Sealing fossae with an anti-cariogenic material is a logical protocol. Although low viscosity GICs lack the wear resistance of resin sealants, their dynamic interaction with the tooth together with excellent adhesive properties and bond longevity could make VLC and self-cured low viscosity GIC sealants for pit and fissure sealing a standard protocol in the future.
7.24 Participation in Future Pharmacological Approaches to Caries Reduction
The treatment of carious lesions can follow a number of protocols that generally involve the removal of the infected and affected carious regions. Although the “pharmacological” treatment of caries is not common, several treatment methods have been proposed. Silver fluoride has been used for some time to safely manage caries. The use of silver diamine fluoride, coated with potassium iodide to prevent staining processes, has been proposed (Knight 2007, 2008). These treatments have been shown to prevent biofilm formation (Knight et al. 2009) and have demonstrated that GICs can adequately bond to treated surfaces. Other pharmacological treatments may become available, and GICs are excellent choices for subsequent covering and sealing of the cavity, adding extra protection against further caries progression.
7.25 Conclusions
Future developments in GICs are likely to deliver improvements in strength, wear resistance and aesthetic properties. These improvements will allow the material to increase its longevity in load-bearing situations, thus allowing the material to become a viable alternative to amalgam restorations. Other new features likely to be focused on include optimising biomimetic, biomineralising or antimicrobial properties to provide new-generation GICs. Glass-ionomer cements will continue to be considered as “smart” materials due to their dynamic interaction with tooth structure and the surrounding environment.
References
Al-Naimi O, Itota T, Hobson R, Mccabe J. Fluoride release for restorative materials and its effect on biofilm formation in natural saliva. J Mater Sci Mater Med. 2008;19:1243–8.
Altunsoy M, Botsali MS, Korkut E, Kucukyilmaz E, Sener Y. Effect of different surface treatments on the shear and microtensile bond strength of resin-modified glass ionomer cement to dentin. Acta Odontol Scand. 2014;72:1–6.
Arbabzadeh-Zavareh F, Gibbs T, Meyers IA, Bouzari M, Mortazavi S, Walsh LJ. Recharge pattern of contemporary glass ionomer restoratives. Cord Conf Proc. 2012;9:139–45.
Atmeh AR, Chong EZ, Richard G, Festy F, Watson TF. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res. 2012;91:454–9.
Bakis C, Bank LC, Brown V, Cosenza E, Davalos J, Lesko J, Machida A, Rizkalla S, Triantafillou T. Fiber-reinforced polymer composites for construction-state-of-the-art review. J Compos Constr. 2002;6:73–87.
Bala O, Arisu HD, Yikilgan I, Arslan S, Gullu A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dent. 2012;6:79–86.
Bate CS. The pathology of dental caries. Odontological Society of Great Britain London: Cox & Wyman; 1864.
Benelli EM, Serra MC, Rodrigues Jr AL, Cury JA. In situ anticariogenic potential of glass ionomer cement. Caries Res. 1993;27:280–4.
Blossey R. Self-cleaning surfaces – virtual realities. Nat Mater. 2003;2:301–6.
Boehm AJR, Peuker M, Walter A, Broyles BR, Oxman JD, Dubbe JW, Hartung MG, Guggenmos S. Mixer for mixing a dental composition. United States Patent Application 20110189059. 2011.
Burke FJ. Dental materials – what goes where? The current status of glass ionomer as a material for loadbearing restorations in posterior teeth. Dent Update. 2013;40:840–4.
Burke FJ. Reinforced glass-ionomer restorations. Dent Abstr. 2014;59, E79.
Busscher HJ, Rinastiti M, Siswomihardjo W, Van Der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89:657–65.
Carlén A, Nikdel K, Wennerberg A, Holmberg K, Olsson J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials. 2001;22:481–7.
Cheetham JJW. Dental capsule. United States Patent Application 20140305816. 2014.
Cheetham JJ, Palamara JE, Tyas MJ, Burrow MF. A comparison of resin-modified glass-ionomer and resin composite polymerisation shrinkage stress in a wet environment. J Mech Behav Biomed Mater. 2014a;29:33–41.
Cheetham JJ, Palamara JEA, Tyas MJ, Burrow MF. Evaluation of the interfacial work of fracture of glass-ionomer cements bonded to dentin. J Mech Behav Biomed Mater. 2014b;29:427–37.
Colquhoun H, Klumperman B. Self-healing polymers. Polym Chem. 2013;4:4832–3.
Culbertson BM. Glass-ionomer dental restoratives. Prog Polym Sci. 2001;26:577–604.
Dabsie F, Gregoire G, Sixou M, Sharrock P. Does strontium play a role in the cariostatic activity of glass ionomer? Strontium diffusion and antibacterial activity. J Dent. 2009;37:554–9.
De Munck J, Mine A, Poitevin A, Van Ende A, Cardoso MV, Van Landuyt KL, Peumans M, Van Meerbeek B. Meta-analytical review of parameters involved in dentin bonding. J Dent Res. 2012;91:351–7.
Diem VT, Tyas MJ, Ngo HC, Phuong LH, Khanh ND. The effect of a nano-filled resin coating on the 3-year clinical performance of a conventional high-viscosity glass-ionomer cement. Clin Oral Investig. 2014;18:753–9.
Dimkov A, Nicholson JW, Gjorgievska E. On the possibility of incorporating antimicrobial components into glass-ionomer cements. Prilozi. 2009;30:219–37.
Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, Kotsanos N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent Mater J. 2013;32:296–304.
El-Askary FS, Nassif MS. The effect of the pre-conditioning step on the shear bond strength of nano-filled resin-modified glass-ionomer to dentin. Eur J Dent. 2011;5:150–6.
El-Baky RMA, Hussien SM. Comparative antimicrobial activity and durability of different glass ionomer restorative materials with and without chlorohexidine. J Adv Biotech Bioeng. 2013;1:14–21.
Fareed MA, Stamboulis A. Effect of nanoclay dispersion on the properties of a commercial glass ionomer cement. Int J Biomater. 2014a.
Fareed MA, Stamboulis A. Nanoclays reinforced glass ionomer cements: dispersion and interaction of polymer grade (Pg) montmorillonite with poly(acrylic acid). J Mater Sci Mater Med. 2014;25:91–9.
Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20.
Ferracane JL. Resin composite—state of the art. Dent Mater. 2011;27:29–38.
Ferreira JM, Pinheiro SL, Sampaio FC, Menezes VA. Use of glass ionomer cement containing antibiotics to seal off infected dentin: a randomized clinical trial. Braz Dent J. 2013;24:68–73.
Fischer H. Polymer nanocomposites: from fundamental research to specific applications. Mater Sci Eng C. 2003;23:763–72.
Forsten L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials. 1998;19:503–8.
Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90:1847–68.
Fukuda R, Yoshida Y, Nakayama Y, Okazaki M, Inoue S, Sano H, Suzuki K, Shintani H, Van Meerbeek B. Bonding efficacy of polyalkenoic acids to hydroxyapatite, enamel and dentin. Biomaterials. 2003;24:1861–7.
Garcia SJ. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur Polym J. 2014;53:118–25.
Gerdolle DA, Mortier E, Droz D. Microleakage and polymerization shrinkage of various polymer restorative materials. J Dent Child. 2008;75:125–33.
Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–15.
Giray F, Peker S, Durmus B, Kargül B. Microleakage of new glass ionomer restorative materials in permanent teeth. Eur J Paediatr Dent. 2014;15:122–6.
Gu YW, Yap AU, Cheang P, Kumar R. Spheroidization of glass powders for glass ionomer cements. Biomaterials. 2004;25:4029–35.
Guan K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of Tio2/Sio2 films. Surf Coat Technol. 2005;191:155–60.
Guggenberger R, May R, Stefan K. New trends in glass-ionomer chemistry. Biomaterials. 1998;19:479–83.
Guida A, Hill RG, Towler MR, Eramo S. Fluoride release from model glass ionomer cements. J Mater Sci Mater Med. 2002;13:645–9.
Hamama HH, Burrow MF, Yiu C. Effect of dentine conditioning on adhesion of resin-modified glass ionomer adhesives. Aust Dent J. 2014;59:193–200.
Hammouda IM. Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech Behav Biomed Mater. 2009;2:73–81.
Han L, Okiji T. Evaluation of the ions release / incorporation of the prototype S-Prg filler-containing endodontic sealer. Dent Mater J. 2011.
Hatanaka K, Irie M, Tjandrawinata R, Suzuki K. Effect of spherical silica filler addition on immediate interfacial Gap-formation in class V cavity and mechanical properties of resin-modified glass-ionomer cement. Dent Mater J. 2006;25:415–22.
Hengtrakool C, Pearson GJ, Wilson M. Interaction between GIC and S. sanguis biofilms: antibacterial properties and changes of surface hardness. J Dent. 2006;34:588–97.
Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007.
Hokii Y, Yoshimitsu R, Yamamoto K, Fukushima S, Fusejima F, Kumagai T. Protection of enamel-glass ionomer restorative margins by resin-coatings. Dent Mater. 2014;30 Suppl 1:E107.
Hook ER, Owen OJ, Bellis CA, Holder JA, O’sullivan DJ, Barbour ME. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J Nanobiotechnol. 2014;12:3.
Huo X, Torres V, Elsner O, Pfefferkorn F. Texture analysis of glass ionomers. IADR 89th General Session San Diego, USA, 2011.
Irie M, Nagaoka N, Tamada Y, Maruo Y, Nishigawa G, Minagi S, Finger WJ. Effect of spherical silica additions on marginal gaps and compressive strength of experimental glass-ionomer cements. Am J Dent. 2011;24:310–4.
ISO 2008. ISO 7405:2008 Dentistry-evaluation of biocompatibility of medical devices used in dentistry.
ISO 2009. International Standards Organisation 10993 – biological evaluation of medical devices part 1: evaluation and testing.
Jayabal J, Mahesh R. Current state of topical antimicrobial therapy in management of early childhood caries. ISRN Dent. 2014;2014:5.
Jones FH, Hutton BM, Hadley PC, Eccles AJ, Steele TA, Billington RW, Pearson GJ. Fluoride uptake by glass ionomer cements: − a surface analysis approach. Biomaterials. 2003;24:107–19.
Kamijo K, Mukai Y, Tominaga T, Iwaya I, Fujino F, Hirata Y, Teranaka T. Fluoride release and recharge characteristics of denture base resins containing surface pre-reacted glass-ionomer filler. Dent Mater J. 2009;28:227–33.
Kawano F, Kon M, Kobayashi M, Miyai K. Reinforcement effect of short glass fibers with Cao–P2o5–Sio2–Al2o3 glass on strength of glass-ionomer cement. J Dent. 2001;29:377–80.
Kazunori K, Glenn SK, Masayuki Y, Teruo O, Yasuhisa S. Block copolymer micelles as vehicles for drug delivery. J Control Release. 1993;24:119–32.
Knight GM. The pharmacological management of caries. Dental Asia, September/October 2007.
Knight GM. The pharmacological management of dentine to protect against plaque microorganism degradation. PhD thesis, University of Adelaide; 2008.
Knight GM, Mcintyre JM, Craig G, Zilm PS, Gully N. Inability to form a biofilm of Streptococcus mutans on silver fluoride-and potassium iodide-treated demineralized dentin. Quin Int. 2009;40:155.
Korkmaz Y, Ozel E, Attar N, Ozge Bicer C. Influence of different conditioning methods on the shear bond strength of novel light-curing nano-ionomer restorative to enamel and dentin. Lasers Med Sci. 2010;25:861–6.
Latta M, Gross SM, Mchale WA. Microencapsulated compositions and methods for tissue mineralization. United States Patent Application 8889161. 2014.
Lazaridou D, Belli R, Kramer N, Petschelt A, Lohbauer U. Dental materials for primary dentition: are they suitable for occlusal restorations? A two-body wear study. Eur Arch Paediatr Dent. 2015;16(2):165–72.
Lloyd CH, Adamson M. The development of fracture toughness and fracture strength in posterior restorative materials. Dent Mater. 1987;3:225–31.
Lohani A, Singh G, Bhattacharya SS, Verma A. Interpenetrating polymer networks as innovative drug delivery systems. J Drug Deliv. 2014;2014:583612.
Lohbauer U. Dental glass ionomer cements as permanent filling materials?–Properties, limitations and future trends. Materials. 2009;3:76–96.
Lohbauer U, Walker J, Nikolaenko S, Werner J, Clare A, Petschelt A, Greil P. Reactive fibre reinforced glass ionomer cements. Biomaterials. 2003;24:2901–7.
Lohbauer U, Frankenberger R, Clare A, Petschelt A, Greil P. Toughening of dental glass ionomer cements with reactive glass fibres. Biomaterials. 2004;25:5217–25.
Mackey TK, Contreras JT, Liang BA. The Minamata convention on mercury: attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci Total Environ. 2014;472:125–9.
Manuja N, Nagpal R, Pandit IK. Dental adhesion: mechanism, techniques and durability. J Clin Pediatr Dent. 2012;36:223–34.
Mckinney JE, Antonucci JM, Rupp NW. Wear and microhardness of glass-ionomer cements. J Dent Res. 1987;66:1134–9.
Mckinney JE, Antonucci JM, Rupp NW. Wear and microhardness of a silver-sintered glass-ionomer cement. J Dent Res. 1988;67:831–5.
Mei ML, Li Q-L, Chu C-H, Lo EC-M, Samaranayake LP. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12:4.
Mitsuhashi A, Hanaoka K, Teranaka T. Fracture toughness of resin-modified glass ionomer restorative materials: effect of powder/liquid ratio and powder particle size reduction on fracture toughness. Dent Mater. 2003;19:747–57.
Mohiti-Asli M, Pourdeyhimi B, Loboa EG. Novel, silver-ion-releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater. 2014;10:2096–104.
Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008;4:432–40.
Moshaverinia A, Brantley WA, Chee WWL, Rohpour N, Ansari S, Zheng F, Heshmati RH, Darr JA, Schricker SR, Rehman IU. Measure of microhardness, fracture toughness and flexural strength of N-vinylcaprolactam (NVC)-containing glass-ionomer dental cements. Dent Mater. 2010;26:1137–43.
Moshaverinia A, Roohpour N, Chee WW, Schricker SR. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011;21:1319–28.
Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–94.
Ogledzki M, Perry RD, Kugel G. Translucency of resin modified glass ionomer restoratives. AADR annual meeting, Tampa; 21–24 Mar 2012.
Osorio R, Osorio E, Medina-Castillo AL, Toledano M. Polymer nanocarriers for dentin adhesion. J Dent Res. 2014;93(12):1258–63.
Perdigão J. Dentin bonding – variables related to the clinical situation and the substrate treatment. Dent Mater. 2010;26:E24–37.
Peterson AM, Kotthapalli H, Rahmathullah MAM, Palmese GR. Investigation of interpenetrating polymer networks for self-healing applications. Compos Sci Technol. 2012;72:330–6.
Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–81.
Powis D, Follerås T, Merson S, Wilson A. Materials science improved adhesion of a glass ionomer cement to dentin and enamel. J Dent Res. 1982;61:1416–22.
Prabhakar AR, Prahlad D, Kumar SR. Antibacterial activity, fluoride release, and physical properties of an antibiotic-modified glass ionomer cement. Pediatr Dent. 2013;35:411–5.
Priebe M, Fromm KM. One-pot synthesis and catalytic properties of encapsulated silver nanoparticles in silica nanocontainers. Part Part Syst Char. 2014;31(6):645–51.
Qizheng C, Xiangting D, Weili Y, Jinxian W, Huiru W, Xiaofeng Y, Xiaohui Y. New developments of inorganic nanofibers fabricated by electrospinning. Rare Met Mater Eng. 2006;35:1167.
Roche KJ, Stanton KT. Precipitation of biomimetic fluorhydroxyapatite/polyacrylic acid nanostructures. J Cryst Growth. 2015;409:80–8.
Samadzadeh M, Boura SH, Peikari M, Kasiriha S, Ashrafi A. A review on self-healing coatings based on micro/nanocapsules. Prog Org Coat. 2010;68:159–64.
Seemann R, Flury S, Pfefferkorn F, Lussi A, Noack MJ. Restorative dentistry and restorative materials over the next 20 years: a Delphi survey. Dent Mater. 2014;30(4):442–8.
Sennou HE, Lebugle AA, Gregoire GL. X-Ray photoelectron spectroscopy study of the dentin-glass ionomer cement interface. Dent Mater. 1999;15:229–37.
Seppa L, Forss H, Øgaard B. The effect of fluoride application on fluoride release and the antibacterial action of glass lonomers. J Dent Res. 1993;72:1310–4.
Setien VJ, Armstrong SR, Wefel JS. Interfacial fracture toughness between resin-modified glass ionomer and dentin using three different surface treatments. Dent Mater. 2005;21:498–504.
Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83–96.
Shimazu K, Ogata K, Karibe H. Caries-preventive effect of fissure sealant containing surface reaction-type pre-reacted glass ionomer filler and bonded by self-etching primer. J Clin Pediatr Dent. 2012;36:343–7.
Shiozawa M, Takahashi H, Iwasaki N. Fluoride release and mechanical properties after 1-year water storage of recent restorative glass ionomer cements. Clin Oral Investig. 2013;18:1–8.
Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467–78.
Steinberg D. Studying plaque biofilms on various dental surfaces. In: An Y, Friedman R, editors. Handbook of bacterial adhesion. Totowa, NJ: Humana Press; 2000.
Suzuki YI-K, Aoyagi S, Kaneko M, Mukasa Y. Vacuum assisted mixer for capsule of dental restoration material. United States Patent Application 6776516. 2004.
Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17:68–81.
Tezvergil-Mutluay A, Mutluay M, Seseogullari-Dirihan R, Agee KA, Key WO, Scheffel DL, Breschi L, Mazzoni A, Tjaderhane L, Nishitani Y, Tay FR, Pashley DH. Effect of phosphoric acid on the degradation of human dentin matrix. J Dent Res. 2013;92:87–91.
Tyas MJ. Cariostatic effect of glass ionomer cement: a five-year clinical study. Aust Dent J. 1991;36:236–9.
Tyas MJ. Milestones in adhesion: glass-ionomer cements. J Adhes Dent. 2003;5:259–66.
Van Amerongen WE. Dental caries under glass ionomer restorations. J Public Health Dent. 1996;56:150–4; discussion 161–3.
Van Duinen RNB, Kleverlaan CJ, De Gee AJ, Werner A, Feilzer AJ. Early and long-term wear of ‘fast-Set’ conventional glass–ionomer cements. Dent Mater. 2005;21:716–20.
Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, Van Landuyt K, Lambrechts P, Vanherle G. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215–35.
Van Meerbeek B, Peumans M, Poitevin A, Mine A, Van Ende A, Neves A, De Munck J. Relationship between bond-strength tests and clinical outcomes. Dent Mater. 2010;26:E100–21.
Walls A. Glass polyalkenoate (glass-ionomer) cements: a review. J Dent. 1986;14:231–46.
Weng Y. Advanced antibacterial glass ionomer cements for improved dental restoratives. PhD 3481168, Purdue University; 2011.
Weng Y, Howard L, Xie D. A novel star-shaped poly (carboxylic acid) for resin-modified glass-ionomer restoratives. J Biomater Sci Polym Ed. 2014;18:1–15.
Wessel C, Ostermann R, Dersch R, Smarsly BM. Formation of inorganic nanofibers from preformed TiO2 nanoparticles via electrospinning. J Phys Chem C. 2010;115:362–72.
Williams JA, Billington RW, Pearson GJ. Comparison of Ion release from a glass ionomer cement as a function of the method of incorporation of added ions. Biomaterials. 1999;20:589–94.
Williams JA, Billington RW, Pearson GJ. The glass ionomer cement: the sources of soluble fluoride. Biomaterials. 2002;23:2191–200.
Wilson AD. Acidobasicity of oxide glasses used in glass ionomer cements. Dent Mater. 1996;12:25–9.
Wilson AD, Nicholson JW. Acid–base cements: their biomedical and industrial applications. New York, NY, USA: Cambridge University Press; 2005.
Wilson GO, Andersson HM, White SR, Sottos NR, Moore JS, Braun PV. Self‐healing polymers. In: Encyclopedia of polymer science and technology. Hoboken, NJ: Wiley-Interscience. 2010.
Wu N, Xia X, Wei Q, Huang F. Preparation and properties of organic/inorganic hybrid nanofibres. Fibres and Textiles in Eastern Europe. 2010;18(78):21–3.
Wu W, Xie D, Puckett A, Mays JW. Synthesis and formulation of vinyl-containing polyacids for improved light-cured glass-ionomer cements. Eur Polym J. 2003;39:663–70.
Wu DY, Meure S, Solomon D. Self-healing polymeric materials: a review of recent developments. Prog Polym Sci. 2008;33:479–522.
Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000;16:129–38.
Xie D, Zhao J, Weng Y. Synthesis and application of novel multi-arm poly(carboxylic acid)s for glass-ionomer restoratives. J Biomater Appl. 2010;24:419–36.
Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–96.
Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9.
Yamazaki T, Schricker SR, Brantley WA, Culbertson BM, Johnston W. Viscoelastic behavior and fracture toughness of six glass-ionomer cements. J Prosthet Dent. 2006;96:266–72.
Yelamanchili A, Darvell BW. Network competition in a resin-modified glass-ionomer cement. Dent Mater. 2008;24:1065–9.
Yiu CK, Tay FR, King NM, Pashley DH, Carvalho RM, Carrilho MR. Interaction of resin-modified glass-ionomer cements with moist dentine. J Dent. 2004a;32:521–30.
Yiu CK, Tay FR, King NM, Pashley DH, Sidhu SK, Neo JC, Toledano M, Wong SL. Interaction of glass-ionomer cements with moist dentin. J Dent Res. 2004b;83:283–9.
Yli-Urpo H, Narhi T, Soderling E. Antimicrobial effects of glass ionomer cements containing bioactive glass (S53p4) on oral micro-organisms in vitro. Acta Odontol Scand. 2003;61:241–6.
Yoshida Y, Van Meerbeek B, Nakayama Y, Snauwaert J, Hellemans L, Lambrechts P, Vanherle G, Wakasa K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res. 2000;79:709–14.
Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–74.
Yuan Y, Yin T, Rong M, Zhang M. Self healing in polymers and polymer composites. Concepts, realization and outlook: a review. Exp Polym Lett. 2008;2:238–50.
Zalizniak I, Palamara JE, Wong RH, Cochrane NJ, Burrow MF, Reynolds EC. Ion release and physical properties of CPP-ACP modified GIC in acid solutions. J Dent. 2013;41:449–54.
Zhang M, Bando Y, Wada K, Kurashima K. Synthesis of nanotubes and nanowires of silicon oxide. J Mater Sci Lett. 1999;18:1911–3.
Zhang L, Tang T, Zhang ZL, Liang B, Wang XM, Fu BP. Improvement of enamel bond strengths for conventional and resin-modified glass ionomers: acid-etching vs. conditioning. J Zhejiang Univ Sci B. 2013;14:1013–24.
Zhao J, Weng Y, Xie D. In vitro wear and fracture toughness of an experimental light-cured glass–ionomer cement. Dent Mater. 2009;25:526–34.
Zhou F-L, Gong R-H. Manufacturing technologies of polymeric nanofibres and nanofibre yarns. Polym Int. 2008;57:837–45.
Zoergiebel J, Ilie N. Evaluation of a conventional glass ionomer cement with new zinc formulation: effect of coating, aging and storage agents. Clin Oral Investig. 2013;17:619–26.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cheetham, J.J. (2016). The Future of Glass-ionomers. In: Sidhu, S. (eds) Glass-Ionomers in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-319-22626-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-22626-2_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22625-5
Online ISBN: 978-3-319-22626-2
eBook Packages: MedicineMedicine (R0)