Abstract
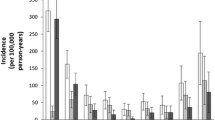
The incidence of proximal humerus fractures rises rapidly with age. More than 70 % of fractures of the proximal humerus can be assigned to the age above 60 years showing the highest age-specific peak in 80–89 year old women [10]. Between the age of 30 and 60 years the distribution in men and women is equal. Afterwards the incidence for proximal humeral fractures in women compared to men increases by 4 times leading to an estimated overall male to female ratio of 3:7 [10]. This strong effect of advanced age and female sex underlines the significant association between proximal humerus fractures and osteoporosis. Due to this fracture and concomitant disability promoting effect osteoporosis has gained vast clinical and public health importance over the last decades [3]. Multicenter studies have demonstrated the major impact of osteoporotic proximal humeral fractures on reduction of subjective patient-perceived health and functional disability [1].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bone Mineral Density
- Fragility Fracture
- Proximal Humeral Fracture
- Proximal Humerus Fracture
- Local Bone Mineral Density
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The incidence of proximal humerus fractures rises rapidly with age. More than 70 % of fractures of the proximal humerus can be assigned to the age above 60 years showing the highest age-specific peak in 80–89 year old women [10]. Between the age of 30 and 60 years the distribution in men and women is equal. Afterwards the incidence for proximal humeral fractures in women compared to men increases by 4 times leading to an estimated overall male to female ratio of 3:7 [10]. This strong effect of advanced age and female sex underlines the significant association between proximal humerus fractures and osteoporosis. Due to this fracture and concomitant disability promoting effect osteoporosis has gained vast clinical and public health importance over the last decades [3]. Multicenter studies have demonstrated the major impact of osteoporotic proximal humeral fractures on reduction of subjective patient-perceived health and functional disability [1].
Bone Mineral Density and Osteoporosis
Bone mass decline with in consequence low bone mineral density (BMD) and neuromuscular function diminishment with increased risk to fall are the major predisposing factors for occurrence of proximal humerus fractures [2, 10]. In consequence after the menopause in women and, to a lesser extent, with advancing age in men low-energy traumas as a fall from standing height are sufficient as mechanism of injury for three-quarters of all proximal humerus fractures [3].
In the Western world osteoporosis, a chronic progressive disease with multifactorial etiology and silent course is the most common metabolic bone disease [3]. The main clinical manifestations are fragility fractures of the distal radius, proximal femur, thoracolumbar spine and proximal humerus. Significant increase of proximal humerus fracture incidence in osteoporotic bone has been shown in several studies [3, 10].
Osteoporosis and Proximal Humerus Fracture Surgery
Osteoporosis not only raises the frequency of proximal humerus fractures. A lower trabecular bone density also leads to a reduced biomechanical stability of the fractured bone [13] with in consequence a diminished mechanical stability of internal (and external) fixation. Difficulty in surgical treatment and intra- as well as postoperative complications significantly increase [6]. In combination with local BMD additional risk factors as age, nonanatomic reduction and insufficient restoration of the medial cortical support further significantly rise the failure rate of internal fixation in unstable proximal humerus fractures [6].
Various studies have shown that pullout strength of screws is highly dependent on BMD [12]. Conventional plate fixations with spherical head screws are highly sensible for low BMD due to predominantly axial pullout forces. In contrast internal fixators with fixed-angle or polyaxial locking screws interact with axial and bending loads (Fig. 12.1). This is advantageous particularly in low BMD due to the greater resistance against shear forces [12]. Screw positioning itself can influence the bone-to-implant biomechanical behavior as well, which is of special importance in low BMD [4].
Delayed healing, non-union or simply implant cut out of the osteopenic bone (Fig. 12.2) mostly result due to prevention of dynamic bone contact caused by too rigid implants [11]. The high initial stiffness of rather rigid implants such as intramedullary nails and conventional plates leads to an early loosening and failure of the implant-bone interface under biomechanical cyclic loading [8]. In contrast implants with low stiffness and flexible characteristics such as the newer precontoured locking plates with suture augmentation (Fig. 12.1) minimize the peak stresses at the bone-implant interface [9]. This rather dynamic fixation construct makes them favorable especially for osteoporotic bone fractures in the elderly population [8]. However despite the experimentally shown strong evidence of local osteoporosis on fracture fixation implant anchorage in clinical studies this impact could not be directly reproduced yet. Lack of missing complication definitions, correct osteoporosis assessment and unclear inclusion criteria are thought to be responsible for this. Prospective studies directly examining the correlation between local BMD and the fixation failure risk are needed [5].
In order to manage surgical difficulties and avoid intra- and postoperative complications associated with osteoporosis sufficient preoperative assessment of the local bone quality is of utmost importance. This facilitates decision-making in the surgical treatment of patients sustaining proximal humerus fractures leading to better results.
Diagnostical Workup
The cornerstones of the preoperative fragility fracture workup are strictly based on a clinical setting where the time-span between initial radiological diagnosis of a proximal humerus fracture and its surgical treatment should be kept as short as possible in terms of morbidity and outcome [10].
Trauma Mechanism
A history of low-energy trauma, especially in the elderly population is highly suspicious for an underlying osteoporotic fracture genesis.
Evaluation of Osteoporosis Risks
Beside age and gender, in the medical history individual risk factors as diseases or medications, alcohol usage or smoking contributing to a low BMD should be questioned. As osteoporosis itself has no symptoms, one should focus on consequences of osteoporosis like an increased risk of fragility fractures. The skeletal history should include fractures and their healing in the past. Also chronic pain may be attributed to chronic fragility fractures.
In the physical examination signs of fragility fractures like a vertebral collapse, possibly presenting with sudden back pain or radicular pain, hump or loss of height as well as deformities of the extremities or an impaired mobility could serve as a warning signal. As osteoporosis is a recognized complication in specific diseases and disorders also external signs of these co-morbidities such as malnutrition, endocrine disorders like Cushing’s syndrome or hypogonadal states should be assessed.
Blood evaluation should be performed routinely for serum electrolytes, calcium, total protein, albumin, kidney and liver parameters and thyroid-stimulating hormone. For detection of potentially underlying causes of a low BMD in patients with a suspicious history it may be tailored enlarged with additional parameters such as phosphorus, magnesium, intact parathyroid hormone, 25-hydroxy vitamin D, serum testosterone and complete blood count. However in a clinical setting these additional parameters should not be routinely assessed.
Dual-energy X-ray absorptiometry (DXA) for quantitative assessment of BMD plays no role in a preoperative setting. Nevertheless DXA is considered the gold standard for osteoporosis diagnosis and should be employed postoperatively for further diagnosis and therapy.
Conventional Radiography
For preoperative assessment of osteoporotic changes in the proximal humerus plain radiography can be helpful. Prediction of local BMD via radiographs provides the most technically uncomplicated and cost-effective process for clinicians [10]. Thereby in anteroposterior radiographs the cortical thickness of the proximal humeral diaphysis may serve as a reliable predictor of local bone quality at the level of humeral head, surgical neck, greater and lesser tuberosity [14]. In general patients over 70 years show significantly lower cortical thickness and local BMD than those under 70 years [14]. However for decision making regarding operative and non-operative treatment (spiral) computed tomography imaging (CT) is more valuable [10].
Computed Tomography (CT)
Spiral CT is an established diagnostic tool for assessing local BMD in the spine. As CT scans display the preoperative imaging of choice in complex and/or low BMD proximal humeral fracture repairs they could be easily used at the same time for preoperative determination of local humeral BMD [10]. By calculating the average Hounsfield unit values in standardized regions of the proximal humerus and linear calibration equation to calculate from the obtained Hounsfield units to BMD, assessment of cancellous BMD of the proximal humerus is by principle possible with high intraobserver and interobserver reliability (intraclass correlation coefficient >0.9) [7]. In a clinical investigation with this method low local BMD has been shown to correlate with fracture fixation failure [6]. However it still remains to be determined whether CT based local BMD assessment can be easily reproduced and efficiently applied by clinicians in daily routine [10].
Preoperative Planning
When an underlying low BMD or osteoporosis in proximal humerus fracture is reasonably suspected or confirmed the thinking about correct treatment should deviate from standardized concepts. Especially in osteoporotic three- and four-part fractures of the proximal humerus treatment is difficult lacking a common consensus of the best technique leading to the best outcome in elderly patients. The preexisting reduced biological fracture healing potential (diminished periosteal blood supply, osteogenic activity and immune defense) should not be further harmed by the approach, surgical exposition, reduction technique or choice of implant. Secondly a more stable fixation is needed. (Low profile) locking plates with routinely performed suture fixation in the rotator cuff became more and more the treatment of choice even in cases with uncomplicated proximal humerus fracture (Fig. 12.3). However in osteoporosis minimally invasive reduction and implanting techniques should be favored. If percutaneous techniques with internal reduction are applied stability of fracture should not be neglected. Thirdly in the case of structural defects (biological) augmenting fixation may be indicated. Various tailored therapeutic augmentation concepts to fill the void in the humeral head are available from iliac crest bone graft, injectable resorbable calcium sulfate or phosphate and hydroxyapatite cement (Fig. 12.4), crushed cancellous allograft bone chips and intramedullary fibular grafts [10]. Finally in severely displaced three- and four-part fractures or even comminuted fractures with high risk of humeral head necrosis due to an underlying low BMD and other comorbidities primary arthroplasty should be considered as well. If preoperative planning already involves organization of postoperative treatment range-of-motion exercises producing a bending stress and avoiding axial stress should be favored early after operation [4].
Summary
The osteoporotic proximal humeral fracture is challenging and the risk of insufficient fixation with in consequence poor outcome evident. Fragility fracture workup with local BMD assessment is of utmost importance for the choice of the best patient tailored fracture management. Preoperative determination of local BMD may be helpful especially regarding the need of additional (biological) augmentation. However if successful surgical fixation of the osteoporotic proximal humerus fracture is highly questioned or even not possible due to lacking anatomic reduction without medial cortical support repair primary arthroplasty should be chosen.
References
Calvo E, Morcillo D, Foruria AM, et al. Nondisplaced proximal humeral fractures: high incidence among outpatient-treated osteoporotic fractures and severe impact on upper extremity. J Shoulder Elbow Surg. 2011;20(5):795–801.
Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37:691–7.
Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–7.
Fankhauser F, Schippinger G, Weber K, et al. Cadaveric-biomechanical evaluation of bone-implant construct of proximal humerus fractures (Neer type 3). J Trauma. 2003;55:345–9.
Goldhahn J, Suhm N, Goldhahn S, et al. Influence of osteoporosis on fracture fixation – a systematic literature review. Osteoporos Int. 2008;19(6):761–72.
Krappinger D, Bizzotto N, Riedmann S, et al. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283–8.
Krappinger D, Roth T, Gschwentner M, et al. Preoperative assessment of the cancellous bone mineral density of the proximal humerus using CT data. Skeletal Radiol. 2012;41:299–304.
Lill H, Hepp P, Korner J, et al. Proximal humeral fractures: how stiff should an implant be? A comparative mechanical study with new implants in human specimens. Arch Orthop Trauma Surg. 2003;123(2–3):74–81.
Namdari S, Lipman AJ, Ricchetti ET, et al. Fixation strategies to prevent screw cut-out and malreduction in proximal humeral fracture fixation. Clin Orthop Surg. 2012;4:321–4.
Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787–95.
Sadowski C, Riand N, Stern R, et al. Fixation of fractures of the proximal humerus with the PlantTan Humerus Fixator Plate: early experience with a new implant. J Shoulder Elbow Surg. 2003;12(2):148–51.
Seebeck J, Goldhahn J, Stadele H, et al. Effect of cortical thickness and cancellous bone density on the holding strength of internal fixator screws. J Orthop Res. 2004;22:1237–42.
Silva MJ. Biomechanics of osteoporotic fractures. Injury. 2007;38 Suppl 3:S69–76.
Tingart MJ, Apreleva M, von Stechow D. The cortical thickness of the proximal humeral diaphysis predicts bone mineral density of the proximal humerus. J Bone Joint Surg Br. 2003;85(4):611–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Banke, I.J. (2015). Osteoporosis and BMD of the Proximal Humerus. In: Biberthaler, P., Kirchhoff, C., Waddell, J. (eds) Fractures of the Proximal Humerus. Strategies in Fracture Treatments. Springer, Cham. https://doi.org/10.1007/978-3-319-20300-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-20300-3_12
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20299-0
Online ISBN: 978-3-319-20300-3
eBook Packages: MedicineMedicine (R0)