Abstract
Expression and increased activity of receptor and nonreceptor tyrosine kinases is a characteristic of many cancers. Receptor tyrosine kinase epidermal growth factor (EGFR) is one of the key molecules associated with cancer. Mutant alleles of EGFR L858R and Del E746-A750, which are common in human lung adenocarcinomas, result in increased phosphorylation of signaling molecules. Receptor tyrosine kinases and EGFR inhibitor MIG6 are more phosphorylated in human bronchial epithelial cells expressing mutant compared to those expressing wild type EGFR. Activation of EGFR results from the formation of a homodimer or heterodimer. EGFR and its active form resembles inactive conformations of Src and Hck. In this chapter, active and inactive conformations of EGFR and its oncogenic and drug-resistant mutant forms are described together with various modes of EGFR interactions with substrates and inhibitors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Protein tyrosine kinases (PTKs) proved important role in transduction and amplification of exogenous signals received by cells. Since the discovery of src family of genes, the product of Rous sarcoma virus oncogene, v-src, and the product of the cellular proto-oncogene, c-src, PTKs were shown to be essential components of many cell signaling pathways (Hunter and Sefton 1980; Kefalas et al. 1995; Parsons and Parsons 2004).
Receptor and non-receptor protein tyrosine kinases form two large groups. Among non-receptor PTKs, the most abundant are src and tec protein tyrosine kinases which together comprise approximately 40–45 % of cytoplasmic PTKs (Nore et al. 2003). Ten members of c-src family (blk, c-fgr, fyn, hck, lck, Lyn, c-src, c-yes, yak, and frk) (Amata et al. 2014) encode cytoplasmic proteins that function in T-lymphocyte maturation and activation, bone maintenance, learning and memory. Five members of tec family include Bruton’s tyrosine kinase, btk, itk, tec, bmx, and txk/rlk (Nore et al. 2003). PTK contains six domains: (1) N-terminal domain that contains sites of lipid modifications important for PTK targeting to the plasma or intracellular membrane (SH4) (2) unique domain (UD), (3) catalytic domain (SH1), (4) phosphotyrosine recognition domain (SH2), (5) polyproline sequence specific (SH3) domain, and (6) C-terminal tail with a regulatory tyrosine (Gmeiner and Horita 2001). SH2 and SH3 domains can also be found in adaptor proteins that lack enzymatic activity, such as Grb-2 and α-spectrin, and in proteins that possess other than tyrosine kinase activity: phospholipase C-γ1 (PLC-γ1), ras GTPase-activating protein (ras GAP), phosphatidyl-inositol 3-kinase (PI3-kinase) (Kefalas et al. 1995). Depending on definition of a domain we can also say that there are three domains and three additional regions. We will call all of them domains throughout this chapter.
Some PTKs (Fyn, Src, and Yes) are expressed in most cell types. Others exhibit specific expression: Hck in myeloid cells, Blk in B cells, Lck in T lymphocytes (Arold et al. 2001).
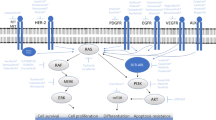
Large group of receptor protein tyrosine kinases (RTKs) include epidermal growth factor, nerve growth factor, fibroblast growth factor and other receptors that contain extracellular ligand binding domain, membrane anchoring domain, cytoplasmic protein tyrosine kinase domain, regulatory domains, and C-terminal tail. Growth factor binds to the extracellular portion of the receptor and induces dimerization that triggers formation of signaling complexes and turns on signaling cascades on the cytoplasmic side of the membrane. Receptors are specifically regulated by multiple adaptor proteins (Kalman et al. 2013).
2 Growth Factors and Receptors
Nerve growth factors (NGFs), fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs), stem cell factor (SCF), transforming growth factors (TGFs), vascular endothelial growth factors (VEGFs), and epidermal growth factors (EGFs) each specifically bind to their receptors located at the cell surface and activate the receptor.
Each type of neurotrophins, NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3/7 (NT-3–NT-7) and other, promote the survival of certain sets of neurons. Two classes of cell surface receptors, the Trk receptors and the p75 neurotrophin receptors, are activated by the specific ligand. TrkA/NGF, TrkB/(BDNF or NT-4/5), TrkC/NT-3 and p75NTR/NGF complexes are main components that regulate development and support of the nervous system (Ultsch et al. 1999).
Target cell derived neurotrophic factor, NGF, is essential for survival of developing sympathetic and cutaneous sensory neurons; tyrosine kinase TrkA complex with NGF travels retrogradely from the axon to the cell body and induces transcriptional and signaling events necessary for survival (Deppmann et al. 2008).
In response to neurotrophins, β-actin mRNA is both targeted to axons and locally translated there. Local protein synthesis of β-actin allows rapid response that is independent of cell body. Impa1 mRNA is targeted to sympathetic neuron axons and locally translated in response to nerve growth factor (NGF) stimulation of distal axons.
Neurotrophin stimulation of distal axons elevates production of proteins responsible for axon growth and maintenance and depends on retrograde pathways. For example, neurotrophin induces transcription of bclw mRNA in cell bodies that involves Trk-Erk5 pathway. Neurotrophins regulate Bclw at various stages: transcription, transport, and local translation. Neurotrophic regulation of local, axonal synthesis of Bclw is necessary for supporting axonal survival. Bclw/Bax suppresses cascade 6 apoptotic activity. A bidirectional mechanism is employed: retrograde neurotrophin signaling from the axon activates transcription of response genes and leads to increased expression of axon-targeted mRNA and protein necessary for axon survival. These events contribute to our understanding of regulatory mechanisms of normal development of neuronal circuitry and axonal degeneration (Cosker et al. 2013).
FGFs activate intracellular signaling by binding to cell surface tyrosine kinase receptors FGFR1-4. FGFRL1, member of FGFR family, lacks a kinase domain and was proposed to act as a decoy receptor to inhibit FGFR ligand-induced signaling. Expressed in pancreatic islet β-cells, FGFR1 regulates insulin processing via canonical ligand binding. FGFRL1 is expressed in plasma membrane and insulin secretory granules of β-cells. Its intracellular domain contains a pY SH2-binding motif that binds phosphatase SHP-1 and stimulates ERK1/2 pathway. SH2-domain of SHP-1 when not bound to FGFRL1, autoinhibits the phosphatase. Its unique intracellular domain contains a tandem tyrosine-based motif involved in endocytosis and a histidine-rich region, site of metals binding. FGF/FGFR signaling is known to regulate development of the embryonic and neonatal pancreas (Silva et al. 2013). Many tyrosine phosphatases act as tumor suppressors. Shp2, was found to act as tumor suppressor but also as a positive signal transducer (Li et al. 2012).
PDGF and EGF are signaling molecules that bind to their cell surface receptors, PDGFR and EGFR, and induce receptor activation. Functionally significant differences in mitogenic signaling for these growth factors involves stimulation by PDGF of sphingosine kinase activity in fibroblasts and increase intracellular levels of sphingolipid metabolites that lead to cell proliferation (Rani et al. 1997). Cancer associated fibroblasts secreted soluble factors affect not only cancer cells but also many other types of cells (Räsänen and Vaheri 2010). For both growth factors, receptor and MAPK activation, mediated by adaptor proteins, leads to the activation of transcription factors. Networks of transcription factors in healthy and transformed cells play key role in cell growth, differentiation and development being determinants of cell states (Sive and Göttgens 2014).
Expression and increased activity of receptor and nonreceptor tyrosine kinases is characteristic of many cancers (Brueggemeier et al. 2005). EGFR is one of the key molecules associated with cancer. Approximately 60–65 % of all adenocarcinomas are linked to mutations in one of oncogenes KRAS, EGFR, ALK fusion, BRAF, HER2, NRAS, or MEK1 (Suehara et al. 2014) and many inhibitors of pathways controlled by these genes are protein protein interactions modules carrying SH2, SH3, and proline rich domains (Fiorentino et al. 2000; Frosi et al. 2010; Guvakova et al. 2014; Xu et al. 2005; Wendt et al. 2015). Mutant alleles of EGFR L858R and Del E746-A750, which are common in human lung adenocarcinomas, result in increased phosphorylation of signaling molecules. Receptor tyrosine kinases and EGFR inhibitor MIG6 are more phosphorylated in human bronchial epithelial cells expressing mutant compared to those expressing wild type EGFR (Guha et al. 2008). New data suggest that Mig6, phosphorylated at Y394/395 in EGFR-mutant human lung adenocarcinoma cell lines, stabilizes EGFR since its interaction with EGFR increases; MIG6 also does not promote degradation of mutant EGFR (Maity et al. 2015). EGF, TGFα, and amphiregulin are EFFR ligands. Activation of EGFR results from the formation of a homodimer or heterodimer paired with another family member such as HER2-4 (Kalman et al. 2013). EGFR asymmetric dimer activation (Zhang et al. 2006, 2007) does not require autophosphorylation of the activation loop (A-loop). Some mutations such as L858R can activate the receptor (Yoshikawa et al. 2012). As a result, C-terminal tyrosines are autophosphorylated and via involvement of SH2 domain bind downstream signaling molecules initiating signal transduction, i.e. MAPK, PI3 K/Akt, PLC, STAT, and SRC/FAK pathways (Kalman et al. 2013). Crystallographic structures of the active conformation of unphosphorylated EGFR alone and in complex with the inhibitor erlotinib show that EGFR kinase activation loop adopts a conformation similar to that of the phosphorylated form of insulin receptor kinase (Stamos et al. 2002). Recently determined structures of EGFR mutant forms alone and in complexes with MIG6, gefitinib, and dacomitinib demonstrate various conformational states of the molecule (Gajiwala et al. 2013). Activity in Src and Hck enzymes is regulated by phosphorylation of a specific tyrosine at the C-terminus. Intramolecular binding of SH2 domain to phosphotyrosine keeps the receptor in the inactive state. The regulatory mechanism involves SH2 and SH3 domains that affect relative orientation of the N-lobe and C-lobe of the kinase. SH3 domains of tyrosine kinases Src, c-Abl, and Bcr-Abl autoinhibit their kinase activities (Review: Kristensen et al. 2006). EGFR bound to Lapatinib (GW572016) exhibits conformation similar to that of Src or Hck in inactive state. EGFR active form resembles inactive conformations of Src and Hck (Gajiwala et al. 2013). Activation mutant L858R and double mutant L858R/T790M (Fig. 1) retain conformation similar to inactive state and were proposed to possess greater propensity to form asymmetric diners that explains their unregulated activity. Inhibitor gefitinib binds to the inactive kinase V948R/L858R/T790M mutant (Fig. 2).
Phosphotyrosine residues that are required for signaling mediated by receptor tyrosine kinases, such as EGFR, are unique binding sites of proteins containing SH2 domains (Koch et al. 1991). SH2 domain of the Src protein binds EGFR. Several phosphopeptides corresponding to five major autophosphorylation sites of EGFR Y1173, Y1148, Y1086, Y1068, and Y992 and putative phosphorylation sites Y1114, Y1101, and Y1045 were used to show Src SH2/SH3 interaction with EGFR. SH2/SH3 construct is better compared to SH2 alone. Therefore, SH3 facilitates association of SH2 with EGFR phosphotyrosine sites (Luttrell et al. 1994). Role of phosphorylation within SH3 domain was studied for CAS, a tyrosine phosphorylated protein in cells transformed by v-crk and v-src oncogenes that are responsible for invasiveness of Src-transformed cells. Using phosphomimicking Y12E and non-phosphorylatable Y12F mutants (Janoštiak et al. 2011) it was shown that the Y12E mutation leads to decreased interaction of CAS SH3 with FAK and PTP-PEST and reduced tyrosine phosphorylation of FAK whereas Y12F mutation results in hyperphosphorylation of CAS substrate domain, slower turnover of focal adhesions, decreased cell migration, and decreased invasiveness. GEF for Rap1 and R-Ras, C3G, transduces signal to c-Jun kinase (JNK). R-Ras activates JNK-dependent transcription and cell transformation (Mochizuki et al. 2010). Cas contains multiple sites of interactions with SH2 and SH3 domains: polyproline regions, NxxY motifs, and SH3 domain. Tyrosine phosphorylation mediates Cas binding to SH2/SH3 of Lck (Nasertorabi et al. 2006).
Signaling pathways of wild type EGFR and its mutant forms differ in regulation and trafficking (Hampton and Craven 2014). Tyrosine phosphorylation very often abolishes binding and results in dissociation of the modified molecule from the enzyme (Zhao et al. 1998; Wang et al. 2013; Tatarova et al. 2013).
Epithelial lumen surface glycoproteins, mucins, which function to protect mucous epithelium, contain several domains including protein tyrosine kinase domain homologous to EGFR, transmembrane domain, MIG6 domain, and multiple O-glycosylation sites (Nollet et al. 1998). Some mucins were shown to mediate breast cancer cell migration through interaction with intracellular adhesion molecule 1 that depends on mucin cytoplasmic domain activation by c-src with involvement of competitive SH3 binding (Gunasekara et al. 2012).
3 Cytoplasmic PTK
Cytoplasmic PTKs exhibit many similarities to RTKs. Their kinase domains are regulated by SH2/SH3 and the C-terminal domains. Kinase domains of EGFR and Lck, for instance, and their mode of regulation carry many important conserved features. C-terminus of EGFR contains several tyrosine phosphorylation sites that regulate binding of SH2-containing molecules to EGFR upon phosphorylation. Peptides with phosphotyrosine bind to EGFR interdomain lobe (Stamos et al. 2002; Wood et al. 2004).
Src family kinases, including Lck, are regulated by phosphorylation of its C-terminal tyrosine in a ‘tail’ peptide that binds to SH2/SH3 domains and inhibits the kinase activity. If the ‘tail’ peptide is dephosphorylated or SH3/SH2 domains bind to competitive ligands, kinase is activated by autophosphorylation of a tyrosine in its activation loop (Eck et al. 1994; Pisabarro et al. 1998; Sicheri et al. 1997; Schweimer et al. 2002; Cowan-Jacob et al. 2005). SH3 and SH2 domains are connected by a linker that contains polyproline sequences involved in regulation process. In some kinases, such as C-terminal Src kinase, Csk, not activation loop phosphorylation but peripheral motifs and SH2/SH3 are required for its activation/deactivation. Mutational studies in the regions of the interface between the kinase domain and regulatory domains help to identify key residues and understand how peripheral regions relay signals to the active site. SH2-kinase linker, disulfide bridge, and SH2 loops located at a distance more than 45 Angstrom from the active site, particularly CD and DE loops, are important allosteric sites (Barkho et al. 2009).
4 SH4 Domain
5 Unique Domain
Domain between the N-terminal SH4 and regulatory SH3 domains is named Unique (UD) since it is less conserved in its amino sequence within src family tyrosine kinases. However, strong conservation of the UD of each SFK member among different organisms suggests some important function. Conservation of residues of the UD in Src, Fyn, and Yes is associated with its binding to various targets: acidic lipids, SH3, and calmodulin. This region is phosphorylated in Lck (Ser 59 in proline rich region), Lyn (Y32), Yes, Fgr, Fyn (S21 in RxxS site of PKA), Src (S17, T37, S75), and autophosphorylated in Hck (Y29). For Hck, autophosphorylation of the UD domain along with autophosphorylation in the activation loop of the catalytic domain contributes to its activation (Amata et al. 2014).
6 SH3 Domain-Containing Proteins
SH3 domains mediate protein-protein interactions (Gmeiner and Horita 2001; Kurochkina and Guha 2013). They bind proline-rich sequences, particularly those carrying PxxP motif, in left-handed polyproline 2 (PPII) conformation (Musi et al. 2006). For some, important function in recognition and signaling was assigned, but for others it has to be determined (Foth et al. 2005). Posttranslational modifications of SH3 s play important role in regulation of cellular processes. Synapsin I, through SH3- or SH2-mediated interactions, activates Src. Src-mediated tyrosine phosphorylation of synapsin I increases binding to synaptic vesicles/actin and formation of synapsin dimers, whereas serine phosphorylation increases synaptic vesicles availability for exocytosis by impairing synapsin association with synaptic vesicles and/or actin (Messa et al. 2010). Adaptor protein Crk (Matsuda et al. 1996; Kobashigawa et al. 2007) for which two isoforms are known, CrkI (SH2-SH3) and CrkII (SH2-SH3-SH3), regulates cytoskeletal reorganization and motility involved in cell growth by facilitating protein-protein interactions. CrkII regulates NWASP and Cdc42 activation, actin polymerization, and development of tension in muscle. CrkII SH3 N mutants inhibit tension development upon stimulation of smooth muscle with acetylcholine possibly due to disruption of Ca2+ signaling pathways (Tang et al. 2005). The linker region between two SH3 domains contains a regulatory tyrosine Y222. Regulation of Abl kinase by Crk is proposed to occur via intermolecular and intramolecular PxxY sequences (Reichman et al. 2005). Some SH3 binding sequences are phosphorylated such as RKXXY294XXY297 motif in SKAP-55 bound to ADAP SH3c. Interestingly, SH3 domain of SKAP-55 binds a proline-rich region of ADAP (Duke-Cohan et al. 2006). This sequence, represented as RxxYxxY or RxxYxxF, is reminiscent of Class I motif RxxPxxP (Kang et al. 2000). There can be drawn a parallel with SKAP-55 and ADAP mode of binding: whereas the FYB SH3 binds tyrosine-based site in SKAP-55, the SKAP-55 recognizes proline-rich site in FYB. Adaptor protein Nck associates with a number of target proteins through a preferred motif. The phosphorylatable serine in the motif is a regulatory site that mediates binding to PAK (Zhao et al. 2000). Nck interaction with CD3ε regulates T cell receptor activity with involvement of noncanonical binding of Nck2 first SH3 motif to PxxDY sequence (Takeuchi et al. 2008). Phosphorylation of tyrosine in this motif abolishes binding of Nck and EGFR substrate, Eps8 (Kesti et al. 2007). Recently, synergetic binding of Cdc42 and EPS8 SH3 to Crib domain and adjacent proline-rich region of IRSp53 have shown possible mechanisms of regulation of membrane and GTPase function (Kast et al. 2014).
Regulation of GTP-binding proteins is important function of SH3 domains that extend their role as mediators of protein-protein interactions. Dynamin binds to microtubules via its C-terminus. This binding induces GTPase activity. For example, GST-Grb2 protein and GST-SH3 domains of c-src, fgr, and fyn stimulate dynamin GTPase activity, whereas C-terminal Grb2 SH3 domain, PLCγ, and p85α bind efficiently but do not stimulate GTPase activity (Gout et al. 1993).
Hydrophobic and electrostatic interactions are important for recognition of polyproline sequences by SH3 domains. Class I sequence RXLPPXP and class II sequence XPPLPXR include PxxP hydrophobic motif that adopts polyproline type II conformation. These sequences are flanked by charged residues. Although class I and class II motifs contain charged residues, flanking polyprolines from one side, and this arrangement is linked to ability of these motifs to bind SH3 domain in two different orientations, sequences with polyprolines only were identified as class III ligands. Besides, noncanonical sequences were identified: PxxDY in EPS8, RKxxYxxY in SKAP55, RxxK in GADS, PXXXPR in βPIX and CIN85/CNS, RxxPxxxP in BK channels. Hydrophobic and charged residues flanking PxxP motif were shown to enhance interaction. Arginines and lysines, in addition to charge, contain large hydrophobic chain that very often is important for association. Some sequences bind without proline (Mayer 2001; Kang et al. 2000; Gushchina et al. 2011; He et al. 2014; Teyra et al. 2012; Kim et al. 2008; Pires et al. 2003; Tian et al. 2006; Tonikian et al. 2007).
Regulation by association of SH3 domains also occurs via formation of homodimers (IB1 protein; Kristensen et al. 2006) or heterodimers (VAV N-terminal and GRB2 C-terminal SH3 domains complex; Nishida et al. 2001).
Very important functions of SH3 are emphasized in giving rise to emerging actin filaments controlled through specific assembly of SH3 regulated multiprotein complexes by phosphorylation and guanine exchange, regulation of cytoskeletal organization and gene expression during development (Mayer 2001; Kawauchia et al. 2001).
SH3 domains are also regulated by phosphorylation. Tyrosine phosphorylation in SH2, SH3, and WW domains of adaptor proteins is more frequently observed than serine/threonine phosphorylation indicating its important function. Tyrosine phosphorylated SH3 domains can reduce, disrupt, or increase binding, affect protein localization, stimulate or inhibit signaling, regulate gene expression. The most frequently phosphorylated position within SH3 domain corresponds to c-Src Tyr-90 and is characterized by a consensus sequence ALYD(Y/F) (Nore et al. 2003; Tatarova et al. 2013). In tertiary structure, this sequence belongs to the loop between the first two β-strands named RT that is involved in recognition of binding partners. Second frequently phosphorylated site corresponds to Tyr-131 and is located in the distal loop, also peptide binding pocket (Review: Kurochkina and Guha 2013). Autophosphorylation of the RT loop of SH3 domain in Itk, Btk, and other TEC family members of non-receptor tyrosine kinases results in the kinase activation (Joseph et al. 2007; Park et al. 1996). Activation of Btk involves phosphorylation of one tyrosine in activation loop followed by autophosphorylation of another tyrosine in SH3 domain. Tec family PTKs exhibit preference for phosphorylation of their own SH3 domains. Transphosphorylation can be specific not only for SH3 domains but for joint SH3/SH2 domain. One of the proposed regulation mechanisms suggests that when coexpressed in a single cell type (Btk and Tec in B-cells or Itk and Txk in T-cells) these kinases may use transphosphorylation to mediate activity of each other (Nore et al. 2003). Phosphorylation of key residues in SH3 domains affects their function in many ways (Table 1).
SH3 domain has been implicated in important processes in cell biology. SH3 domains play critical roles in migration and invasiveness (Yamada et al. 2011), actin reorganization induced by extracellular signals (Antoku and Mayer 2009), and shaping spines in neurons (Sheng and Kim 2000; Ehlers 2002). The role of SH3 domain in these biological processes has implication in cellular homeostasis, as well as disease states.
7 SH2
SH2 domains bind phosphotyrosine sequences and contribute to the assembly of signaling complexes that are formed as a result of the growth receptor stimulation. EGF, PDGF or other receptors contain tyrosines that are phosphorylated and bind to SH2 domains of the downstream signaling molecule such as p85 subunit of PI3 kinase, the GTPase-activating protein, growth factor receptor-bound protein 2, and PLCγ. Specificity of binding is determined by several amino acids surrounding phosphotyrosine. Several regulatory events are important for the signaling: (1) kinases are phosphorylated at their C-terminal tyrosine and phosphotyrosine binding to SH2 domain inhibits kinase; (2) the kinase binds to the activated receptor via SH2 domain (Bibbins et al. 1993).
Downstream molecules signaling also involves specific interactions of their SH2 and SH3 domains. One of the important signaling molecules, PLCγ, activates receptor tyrosine kinase receptors (EGFR, PDGFR). SH2 domain of PLCγ also interacts with adaptor proteins (GRB2, SOS1, AP180). When cells are stimulated by growth factors, PLCγ is recruited to the plasma membrane where its substrates are located. Its SH3 domain interacts with βPix-a, GEF for small G-proteins RAC1/Cdc42 important for actin cytoskeleton reorganization. This interaction contributes to tumor growth in breast cancer cells. PLCγ expression was also increased in colorectal cancer. Polyproline region at the C-terminus of PLCγ can bind SH3 domains of PLCγ, PAK, and GIT1. PLCγ induces RAC activation and Iba1-dependent membrane ruffling (Bae et al. 2005). Competition takes place between PLCγ and Grb2 for binding to FGFR. Low concentrations of Grb2 elevates metastatic potential of FGFR–expressing cells (Timsah et al. 2014). PLCγ/Grb2 dimer binding to 2 molecules of FGFR occurs via Grb2 SH3 domain and results in the formation of an active heterotetramer even without a growth factor stimulation. The two SH2 domains of PLCγ have distinct roles: the N-terminal SH2 domain regulates binding of PLCγ to receptor protein tyrosine kinases which then phosphorylate PLCγ; The C-terminal SH2 domain binds this phosphorylated tyrosine and activates phospholipase activity (Poulin et al. 2005).
Although SH2 domain mainly binds phosphorylated tyrosine, binding can also occur to phosphorylated serine/threonine as demonstrated by binding of Abl SH2 to BCR protein or binding of Fyn/Src SH2 to Raf1 (Pendergast et al. 1991). Raf1 also was found to interact with the SH2 domain of Src and this intéractíon depends on arginine. Interaction of Raf1 with Src SH2 is weaker but can be enhanced by the presence of SH3 and N-terminus. Another example of deviation from the specific pattern is binding of Src SH2 to nonphosphorylated PDGFR peptide (Cleghon and Morrison 1994). In some proteins, high affinity binding can be achieved with contribution of two SH2 domains as demonstrated by GAP or p85 binding to PDGF receptor, whereas binding of one SH2 domain does not result in stable complex (Cooper and Kashishian 1993). SH2 domains are also regulated by phosphorylation (Jin et al. 2015).
8 SH1
The first tyrosine kinase discovered, a product of the viral gene v-src, lacks autoinhibition and is constitutively active resulting in cell-transforming activity, whereas its cellular counterpart, c-src, is subject to regulation (Hunter and Sefton 1980; Brown and Cooper 1996; Sicheri and Kuriyan 1997).
The structure of the catalytic domain, SH1, of many tyrosine kinases was extensively studied by crystallography, NMR, and other methods in active and inactive states and bears common architecture of other kinases, such as protein kinase A (PKA). Two lobes, N-terminal (N-lobe) and C-terminal (C-lobe) form a cleft at the interface that hosts ATP molecule. N-lobe comprises a five-stranded β-sheet and regulatory α-helix (αC). C-lobe is predominantly α-helical. Important segments include ATP-binding glycine-rich strand-beta-strand motif, metal-binding catalytic segment, and subject to phosphorylation activation segment. Complexes of the kinase with nonhydrolizable ATP analogs and peptides substrate analogs reveal protein groups responsible for interactions with nucleotide, phosphate groups and substrate. Comparison of the crystal structures of human c-Src and human Hck in inactive state shows high similarity of the two kinases. SH2 and SH3 domains are bound intramolecularly: SH2 to the regulatory C-terminal tyrosine, SH3 to the polyproline type II helix of the linker between the SH2 and catalytic domains. These interactions can be replaced by intermolecular upon kinase involvement in interactions with other proteins. SH2 and SH3 domains do not impede substrate access to the active site or change orientation of the kinase lobes. The regulatory role of SH2 and SH3 domains to inhibit kinase activity is linked to the displacement of helix αC and influence of flexibility of the kinase lobes. In the inactive state, αC is displaced and essential catalytic residues are removed from the active site. Phosphorylation of the activation segment results in active state in which catalytic residues are positioned in conformation required for the catalysis.
9 Adaptor Proteins
Adaptor and scaffold proteins lack enzymatic activity. Their major role is to bring together numerous components of the signaling pathway. This role is critical for the function of signaling complexes. Disregulation of this processes results in many pathologies including cancer development. Multidomain CAS (SH3-containing) and NSP (SH2-containing) families that interact with each other, contain regulatory phosphorylation sites and are important for transmembrane receptors signaling including integrin receptor for extracellular matrix proteins, growth factor receptor tyrosine kinases, and cytokine receptors (Wallez et al. 2012). Adaptor proteins of the GAB family are essential for embryo survival (Wang et al. 2015). Gab1/SHP complex is associated with linking stimulation to proliferation and was recognized as a key participant of the liver regeneration process (Pagano et al. 2012). SH2B1 is an adaptor protein that contains SH2 and PH domains. It enhances insulin regulation of glucose metabolism. Impaired function of SH2B1 results in obesity and type II diabetes. SH2B1 regulates TAG biosynthesis, lipolysis, and VLDL secretion (Sheng et al. 2013).
Adaptor protein Grb2 (Seem-5 in C. elegance, DRK in Drosophila) (Mayer 2001) links RTKs to ras pathway. Grb2 SH2 binds to phophorylated tyrosines of EGFR, Erbb2, PDGFR, Shc, Insulin receptor substrate 1, and focal adhesion kinase. Grb2 SH3 binds to ras-guanine nucleotide exchange factor Sos1, mainly its C-terminal proline-rich regions, that leads to activation of Ras/MEK/MAPK (extracellular signal-regulated kinase, ERK) (Sastry et al. 1997; Qu et al. 2014a). Grb2 SH2/SH3 roles were established as mediator of actin polymerization (Bisson et al. 2012), proangiogenic events (Soriano et al. 2004) and coupling of PAK1 to activated Grb2 (Puto et al. 2003). Grb2 carries out another important function—negative regulation of cell signaling through receptor degradation that involves ubiquitin ligases and inhibitory molecules. The assembly of kinases, such as Cbl, and inhibitory molecules, such as Sprouty, into multiprogein complex lines out the following events: Grb2-SH2 binds RTK phosphotyrosine, Grb2-SH3 binds RTK proline rich region, and Cbl binds inhibitory molecules, such as SHIP. Formed complex binds Ubc E2 enzymes initiating RTK degradation (Reebye et al. 2012). Some SH2 domains bind pY-containing peptides in extended conformation; other, such as GRB2 and GRB7, in β-turn conformation. The SH2 domain of Grb7 specifically binds phosphotyrosine 1139 of the ERB2 receptor; the SH2 domain of GRB14 binds phosphotyrosine 766 of the FGFR. The SH2–GRB10 binds many different proteins: insulin, IGF1 and PDGFb receptors, RET, KIT, Raf1, NED4, and MEK1. Peptides inhibitors that bind to pY binding site were developed and used as combinations treatments against cancer. Phosphotyrosine as part of a drug peptide has drawbacks such as strong charge of the phosphogroup that impairs its ability to cross cellular membrane (Spuches et al. 2007). Some peptides are designed to bind pY + 1 position and exhibit high affinity toward Grb2 (Kang et al. 2007). GRB2 SH2 phosphotyrosine binding site is also used to inhibit GRB2 action by pharmacological agents, such as bicyclic peptide BC1 and cyclic peptide HT1, mimicking phosphorylation. Constrained peptides despite net negative charge have better penetration, metabolic stability, affinity and selectivity (Quartararo et al. 2014).
Grb2 C-terminal SH3 (CSH3) domain binds the N-terminal SH3 domain of VaV, a nucleotide exchange factor for the Rho/Rac family of proteins expressed in hematopoietic cells. VaV SH3 binding site for proline sequence is blocked by its own RT loop and has a regulatory role. VaV forms multiprotein complexes with involvement of Grb2 and PI3 k, Slp76, and SBC in T cells, Slp65 in B cells, and Rac1, and mitogen activated kinase in mast cells (Nishida and Hirano 2003). N-terminal and C-terminal SH3 GRB2 domains possess different specificity toward their ligands (Paster et al. 2013). Grb7-10-14 proteins exhibit common architecture: N-terminus polyproline region binding SH3 domains followed by RA, PH, and SH2 domains (Holt and Siddle 2005). RA-PH domains bind small GTPases and phosphoinositide lipids (Qamra and Hubbard 2013).
Linker for activation of T-cells (LAT), integral membrane adaptor protein upon TCR stimulation and phosphorylation on several tyrosines binds to SH2 of PLCγ, Grb2, or Gads. Association of Grb2 with SOS and GADS with SLP76 brings Sos, Cbl, SLP76 and LAT together (Houtman et al. 2004).
Adaptor proteins regulation of RTKs that involves ubiquitination is important for downregulation of their activity. Many SH3 domains are identified as ubiquitin-binding domains that can direct proteins to degradation/recycling pathways. C-terminal (third) domain, one of three SH3 domains, of both adaptor proteins CIN85 and CD2AP, exhibit various modes of interactions with ubiquitin. Mechanism of EGF-dependent CD2AP/CIN85 monoubiquination allows selective recognition by ubiquitin of various molecules (Roldan et al. 2013).
CRM-like (CrkL), Nck (Ngoenkam et al. 2014; Li et al. 2014), Stap -2 (Sekine 2014), Gab (Wang et al. 2015), Kindlin (Qu et al. 2014b), APS (Xu et al. 2003) and many other adaptor molecules are regulated by phosphorylation and influence cellular pathways by extensive network of effector molecules.
10 Multiprotein Complexes and Signaling Pathways
Multiprotein complexes that are formed with receptors and adapter proteins are important for activation of signaling pathways. Cell surface receptor signaling, initiated with activation of the receptor in the cellular membrane, and intracellular receptor signaling that involves molecules in the cytoplasm, the nuclear membrane, and the nucleus, are two major routes of signal transduction. More and more data confirm that intracellular adaptor proteins play important role to relay growth factor signals for subsequent transcriptional and translational regulation and cell fate determination. They are able to interact simultaneously with several other molecules bringing them together in a multiprotein complex and amplify signaling cascades (Kebache et al. 2002; Semela and Dufour 2004; Huizing et al. 2008; Reebye et al. 2012; Au et al. 2013; Di Fiore et al. 2002; Shelby et al. 2013).
Fyn-mediated signaling pathway in bone marrow-derived mast cells induced in response to allergic inflammation involves binding of Fyn/SH2 to vimentin, pyruvate kinase, p62 ras-GAP associated phosphoprotein, SLP-76, HS-1, and FYB (Nahm et al. 2003). Fyn SH3 interacts with Liver kinase β1 (LKB1) proline rich domain and this interaction affects LKB1 subcellular localization and ability of LKB1 regulate phosphorylation of AMPK, its downstream signaling molecule. Fyn/peptide interaction shows to reproduce features of kinase inhibition and AMPK activation (Yamada and Bastie 2014). Neutrophils, responsible for host defense against microorganisms, are mediated by various cell surface receptors, such as G-protein-coupled receptors, Fc receptors, cytokine receptors, lectins, NOD-like receptors, and many others. Signal transduction pathways activated by the receptors involve Src-family kinase signaling. G-protein-coupled receptors signaling, for instance, occurs via several pathways: PLCβ2/3, PI3 Kγ, and Src-family kinase (Futosi et al. 2013). B cell receptor (BCR) pathway regulation involves interaction between the phosphorylated ITIM of the IgG receptor FcγRIIB and SH2 of inositol 5’-phosphatase (SHIP), proline rich motifs binding to SH3 domains of Grb2 and PLCγ, and NPXY motif of SHIP interaction with phosphotyrosine base domain of Shc (Tridandapani et al. 1999; Leung et al. 2008).
Signaling complexes assemble via specific interactions of proteins with multiple ligands. Membrane associated guanylate kinases (MAGUK) cluster and anchor glutamate receptors and other proteins at synapses. The MAGUK family of proteins includes PSD95, PSD93, SAP102, SAP97, essential components of postsynaptic density, PSD. Stabilization of SAP102 at the PSD depends on SH3/GK domain. Actin, a core skeletal component in spines, interacts with multiple proteins of PSD (Zheng et al. 2010). MAGUKs indirectly bind to actin via complexes SAP97—MyosinVI—actin, PSD-95—SPAR—actin, PSD-95——GKAP—Shank—cortactin—actin, and PSD-95—NMDAR—actinin—actin (Petralia et al. 2008).
SH2 and SH3 domains are important components of assembly of signaling complexes. These and other protein interactions domains, such as helical toroids and beta-helices, are highly selective toward their ligands. Protein-protein and protein-ligand interactions to a large extent depend on specific interactions of amino acids at secondary structure interfaces that determine parameters characterizing angles, distances, chirality and shape of the assemblies (Kurochkina 2008; Kurochkina and Iadarola 2015).
11 Modes of Regulation
SH2 and SH3 were shown to exhibit various ways of regulation of the kinase activity. In ARMS/Kidins220, for instance, mutually exclusive events are observed: binding of polyproline sequence to CrkL/SH3 domain or binding of the adjacent phosphorylated tyrosine sequence to CrkL/SH2 domain (Arevalo et al. 2006; Akiva et al. 2012). Similarly, coupling of phosphorylation and binding with alternation of binding events happens in CD3e-Eps8L1/SH3-Zap70/SH2, growth hormone receptor-Nck1/SH3-STATS-SH2, and Cbl-Src/SH3-Fyn/SH2 complexes. Some alternatively binding pairs involve PDZ/PDZ and WW/SH2 and there exist many potential double switches in Grb2, Ptp2, Stat3/5, Crk and other proteins.
SH2/SH3 regulation of the kinase activity also involves an interdomain linker regions SH2/SH3 and SH2-kinase. In inactive (or closed) conformation of the kinase demonstrated by crystallographic structures of Src and Hck, SH2 domain binds to the phosphotyrosine pY527 in the C-terminal tale of the kinase and SH3 domain binds to the N-terminal lobe via polyproline sequence of the SH2/kinase linker leading to movement of the regulatory helix C. Dephosphoryation of Y527 or binding of ligand, such as viral NEF/SH3, activates the kinase. Intramolecular interaction can be replaced by intermolecular interaction as is observed upon NEF, HIV accessory protein, binding to Hck SH3 via polyproline region and displacing Hck PxxP site (Jung et al. 2011). Another example is provided by the focal adhesion kinase (FAK) activation and deactivation via SH2/SH3 interplay without requirement for phosphorylation of corresponding Y416. Fyn, Abl, Grb2, and Lck contain tandem SH2/SH3 domains. SH2/SH3 domains of Fyn retain conformation reminiscent of that in complex with inactive kinase even in the absence of the kinase domain. This conformation (310 helix) is stabilized by SH2/SH3 polyproline sequence and represents an independent fragment (Arold et al. 2001).
Regulation by tyrosine phosphorylation is not unique to animals. For example, genome of unicellular protist Monosiga brevicollis contains tyrosine phosphatase (PTP) with two SH2 domains just like in animals (Zhao and Zhao 2014).
12 Diseases and Therapies
Field of drug development has tremendous advancements as more and more new inhibitors and mediators of catalytic activity of PTKs and protein-protein interactions become available for treatments. Many more emerging therapies come from new area of stem cell—based therapies. PTKs, protein tyrosine phosphatases, and SH2 domains comprise a module that coordinates intracellular phosphotyrosine-based signals as a response to extracellular ligands. Intracellular pathways regulated by tyrosine phosphorylation play a critical role in biological processes that maintain and restore cell life functions. One example is the process of liver regeneration. Involvement of not only reserve progenitor cell population but to a large extent mature hepatocytes which are stimulated to re-enter the cell cycle and to replicate as a result of complex regulation of metabolic pathways is remarkable demonstration of new applications (Pagano et al. 2012).
Stem cell factor receptor, c-Kit, which is mainly expressed in early hematopoetic stem cells and detected after differentiation only in mast cells and dendritic cells, represents attractive source of new drug development. Its mutant forms are associated with small cell lung carcinoma, malignant melanomas, colorectal cancer, and gastrointestinal stromal tumors. Imatinib, tyrosine kinase inhibitor of BCR/Abl and platelet-derived growth factor, is also effective against c-Kit in GIST treatment. Since c-Kit is involved in asthma and allergy progression, its inhibitor masitinib is used in treatment of asthmatics (Lennartsson and Rönnstrand 2012).
Antibodies against RTKs are good for treatments that handle overexpressed receptors. Both selective inhibitors that has fewer side effects and broad selectivity inhibitors that target multiple pathways were designed that act in tyrosine kinase pathways (Lennartsson and Rönnstrand 2012). Significant progress was made in applying tyrosine kinase inhibitors in treatment of cancers including non-small-cell lung cancer (Nguyen and Neal 2012).
Ephrin-mediated Eph receptors signaling undergoes modifications in epithelial cancers and forms another important group of pharmacological targets, particularly regarding nonmelanoma skin cancer and psoriasis. Abnormal EphB receptors and Ephrin B function is linked to malignancies in the gut. EphA receptors are known as tumor suppressors in the skin (White and Getsios 2015).
SH2 regulated pathways play an important role in most cancers including breast and lung cancer. Targeting SH2 domains presents problems for some groups of compounds since phosphotyrosine negative charge is an obstacle that prevents molecules from crossing the membrane and being delivered. Nevertheless, peptidomimetic substances, hydroxysalycilates, overcome this problem and inhibit STAT3 SH2. GRB2 peptidomimetic inhibitors based on phosphanates are less charged, are able to cross cell membrane and can be used for treatments. GRB7 cyclic peptides represent another successful alternative. STAT3 pathway inhibitors, STA-21 and cryptotanshinone, act not directly on SH2 domain of STAT3 but via off-target sites (Brábek et al. 2005; Morlacchi et al. 2014).
Receptor Patched 1 contains polyproline sequences at its C-terminus that bind SH3 or WW domains. This part of the molecule is important for its interaction with c-src and signaling via Hh pathway critical for mammary gland development in verterbrates: the results produced by studies of mesenchymal dysplasia gene variant (Harvey et al. 2014).
Chonic myeloic leukemia is associated with BCR-ABL tyrosine kinase activity that affects Ras, PI3 K, Jak/Stat, and NFkB pathways stimulating proliferation and inhibiting apoptosis. Existing therapies using tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib face resistance due to mutations in BCR-ABL gene and stem/progenitor cells unresponsiveness. Drug resistance problem can be addressed by introducing other candidate molecules such as adaptor protein Abi1 that contains SH3 and WW40 domains which can be targeted for the disruption of AH1 interaction with BCR-ABL and JAK2 (Liu et al. 2012).
Stem cell based therapies find more and more applications in the development of new treatments. Human umbilical cord perivascular cells (HUCPV), for example, are used to produce morphologically homogenious population of fibroblastic cells that expresses α-actin, desmin, vimentin, and 3G5 for cardiovascular tissue engineering. Subpopulations of these cells do not express class I/II major histocompatibility antigens and can be valuable source of compatible tissues (Sarugaser et al. 2005). Hematopoietic stem cell transcription regulation and understanding changes associated with aging or disease provide basis for possible new treatments (Sive and Göttgens 2014; Babovic and Eaves 2014).
Stem cell therapies have wide range of promising applications to treatments of neuronal injuries and orthopedics medicine (Law and Chaudhuri 2013). New cell lines are explored for their potential in promoting adult neurogenesis, particularly transcriptional events that accompany signaling by growth factors and cytokines leading to cell proliferation and migration (Williams et al. 2013).
References
Akiva, E., Friedlander, G., Itzhaki, Z., & Margalit, H. (2012). A dynamic view of domain-motif interactions. PLoS Computational Biology, 8, e1002341.
Amata, I., Maffei, M., & Pons, M. (2014). Phosphorylation of unique domains of Src family kinases. Frontiers in genetics, 5, 1–6.
Anselmi, F., Orlandini, M., Rocchigiani, M., Clemente, C. D., Salameh, A., Lentucci, C., et al. (2012). c-ABL modulates MAP kinases activation downstream of VEGFR-2 signaling by direct phosphorylation of the adaptor proteins GRB2 and NCK1. Angiogenesis, 15, 187–197.
Antoku, S., & Mayer, B. J. (2009). Distinct roles for Crk adaptor isoforms in actin reorganization induced by extracellular signals. Journal of Cell Science, 122, 4228–4238.
Arevalo, J. C., Pereira, D. B., Yano, H., Teng, K. K., & Chao, M. V. (2006). Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation. Journal of Biological Chemistry, 281, 1001–1007.
Arold, S. T., Ulmer, T. S., Mulhern, T. D., Werner, J. M., Ladbury, J. E., Campbell, I. D., & Noble, M. E. M. (2001). Src kinases Homology 2 interface in the regulation of the role of the src homology 3-Src. Journal of Biological Chemistry, 276, 17199–17205.
Au, E., Ahmed, T., Karayannis, T., Shiona, B., Gan, L., & Fishell, G. (2013). A modular gain-of-function approach to generate cortical interneuron subtypes from ES cells. Neuron, 80, 1145–1158.
Babovic, S., & Eaves, C. J. (2014). Hierarchical organizationoffetalandadult hematopoietic stemcells. Experimental Cell Research, 329, 185–191.
Bae, J.-Y., Ahn, S.-J., Lee, J. E., Kim, J.-E., Han, M.-R., Han, W., et al. (2005). bPix-a enhances the activity of phospholipase Cg1 by binding SH3 domain in breast cancer. Journal of Cellular Biochemistry, 94, 1010–1016.
Barkho, S., Pierce, L. C. T., McGlone, M. L., Li, S., Woods, V. L. Jr., Walker, R. C., et al. (2009). Distal loop flexibility of a regulatory domain modulates dynamics and activity of C-terminal Src kinase (Csk). PLOS Computational Biology, 9, e1003188.
Bibbins, K. B., Boeuf, H., & Varmus, H. E. (1993). Binding ov theSrc SH2 domain to phosphopeptides is determined byrdsidues in both the SH2 domain and the phosphopeptides. Molecular and Cellular Biology, 13, 7278–7287.
Bisson, N., Ruston, J., Jeansson, M., Vanderlaan, R., Hardy, W. R., Du, J., et al. (2012). The adaptor protein Grb2 is not essential for the establishment of the glomerular filtration barrier. PLoS ONE, 7, e50996.
Brábek, J., Constancio, S. S., Siesser, P. F., Shin, N.-Y., Pozzi, A., & Hanks, S. K. (2005). Crk-associated substrate tyrosine phosphorylationsites are critical for invasion and metastasis of Src-transformed cells. Molecular Cancer Research, 3, 307–315.
Broome, M. A., & Hunter, T. (1997). The PDGF receptor phosphorylates Tyr 138 in the c-Src SH3 domain in vivo reducing peptide ligand binding. Oncogene, 14, 17–34.
Brown, M. T., & Cooper, J. A. (1996). Regulation, substrates and functions of Src~. Biochimica et Biophysica Acta, 1287, 121–149.
Brueggemeier, S. B., Wu, D., Kron, S. J., & Palecek, S. P. (2005). Protein-acrylamide copolymer hydrogels for array-based detection of tyrosine kinase activity from cell lysates. Biomacromolecules, 6, 2765–2775.
Cleghon, V., & Morrison, D. K. (1994). Raf-1 interacts with Fyn and Src in a non-phosphotyrosine-dependent manner. Journal of Biological Chemistry, 269, 17749–17755.
Cooper, J. A., & Kashishian, A. (1993). In vivo binding properties of SH2 domains from GTPase-activating protein and phosphatidylinositol 3-kinase. Molecular and Cellular Biology, 13, 1737–1745.
Cosker, K. E., Pazyra-Murphy, M. F., Fenstermacher, S. J., & Segal, R. A. (2013). Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. Journal of Neuroscience, 33, 5195–5207.
Cowan-Jacob, S. W., Fendrich, G., Manley, P. W., Jahnke, W., Fabbro, D., Liebetanz, J., & Meyer, T. (2005). The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure, 13, 861–871.
Deppmann, C. D., Mihalas, S., Sharma, N., Lonze, B. E., Niebur, E., & Ginty, D. D. (2008). A model for neuronal competition during development. Science, 320, 369–373.
Duke-Cohan, J. S., Kang, H., Liu, H., & Rudd, C. E. (2006). Regulation and function of SKAP-55 non-canonical motif binding to the SH3c domain of adhesion and degranulation-promoting adaptor protein. Journal of Biological Chemistry, 281, 13743–13750.
Eck, M. J., AttwellSK, Shoelson S. E., & Harrison, S. C. (1994). Struture of the regulatory domains of the Src family tyrosine kinase Lck. Nature, 368, 764–769.
Ehlers, M. D. (2002). Molecular morphogens for dendritic spines. Trends in Neurosciences, 25, 64-67
Fiorentino, L., Pertica, C., Fiorina, M., Talora, C., Crescenz, M., Castellani, L., et al. (2000). Inhibition of ErbB-2 mitogenic and transforming activity by RALT, a mitogen-induced signal transducer which binds to the ErbB-2 kinase domain. Molecular and Cellular Biology, 20, 7735–7750.
Foth, B. J., Goedecke, M. C., & Soldati, D. (2005). New insights into myosin evolution and classification. Proceedings of the National Academy of Sciences of the United States of America, 103, 3681–3686.
Frosi, Y., Anastasi, S., Ballarò, C., Varsano, G., Castellani, L., Maspero, E., et al. (2010). A two-tiered mechanism of EGFR inhibition by RALT/MIG6 via kinase suppression and receptor degradation. Journal of Cell Biology, 189, 557–571.
Futosi, K., Fodor, S., & Mócsai, A. (2013). Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. International Immunopharmacology, 17, 1185–1197.
Gajiwala, K. S., Feng, J., Ferre, R., Ryan, K., Brodsky, O., Weinrich, S., et al. (2013). Insights into the aberrant activity of mutant EGFR kinase domain and drug recognition. Structure, 21, 209–219.
Gmeiner, W. H., & Horita, D. A. (2001). Implications of SH3 domain structure and dynamics for protein regulation and drug design. Cell Biochemistry and Biophysics, 35, 127–140.
Gout, I., Dhand, R., Hiles, L. D., Fry, M. J., Panayotou, G., Das, P., et al. (1993). The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell, 75, 25–36.
Guha, U., Chaerkady, R., Marimuthu, A., Patterson, A. S., Kashyap, M. K., Harsha, H. C., et al. (2008). Comparisons of tyrosine phosphorylated proteins in cells expressing lung cancer-specific alleles of EGFR and KRAS. Proceedings of the National Academy of Sciences of the United States of America, 105, 14112–14117.
Gunasekara, N., Sykes, B., & Hugh, J. (2012). Characterization of a novel weak interaction between MUC1 and Src-SH3 using nuclear magnetic resonance spectroscopy. Biochemical and Biophysical Research Communications, 421, 832–6.
Gushchina, L. V., Gabdulkhakov, A. G., Nikonov, S. V., & Filimonov, V. V. (2011). High-resolution crystal structure of spectrin SH3 domain fused with a proline-rich peptide. Journal of Biomolecular Structure & Dynamics, 29, 485–495.
Guvakova, M. A., Lee, W. S. Y., Furstenau, D. K., Prabakaran, I., Li, D. C., Hung. R., et al. (2014). The small GTPase Rap1 promotes cell movement rather than stabilizes adhesion in epithelial cells responding to insulin-like growth factor I. Biochemical Journal, 463, 257–270
Hampton, K. K., & Craven, R. J. (2014). Pathways driving the endocytosis of mutant and wild-type EGFR in cancer. Oncoscience, 1, 504–512.
Harvey, M. C., Fleet, A., Okolowsky, N., & Hamel, P. A. (2014). Distinct Effects of the mesenchymal dysplasia gene variant of murine patched-1 protein on canonical and non-canonical Hedgehog signaling pathways. Journal of Biological Chemistry, 289, 10939–10949.
He, P., Wu, W., Wang, H.-D., Liao, K. L., Zhang, W., Lv, F.-L., & Yang, K. (2014). Why ligand cross-reactivity is high within peptide recognition domain families? A case study on human c-Src SH3 domain. Journal of Theoretical Biology, 340, 30–37.
Holt, L. J. (2005). Siddle, K. (2005). Grb10 and Grb14: enigmatic regulators of insulin action—and more? Biochemical Journal, 388, 393–406.
Houtman, J. C. D., Higashimoto, Y., Dimasi, N., Cho, S., Yamaguchi, H., Bowden, B., et al. (2004). Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry, 43, 4170–4178.
Huizing, M., Helip-Wooley, A., Westbroek, W., Gunay-Aygun, M., & Gahl, W. A. (2008). Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annual Review of Genomics and Human Genetics, 9, 359–386.
Hunter, T., & Sefton, B. M. (1980). Transforming gene product of Roussarcoma virus phosphorylates tyrosine. Proceedings of the National Academy of Sciences of the United States of America, 77, 1311–1315.
Janoštiak, R., Tolde, O., Brůhová, Z., Novotný, M., Hanks, S. K., Rösel, D., & Brábek, J. (2011). Tyrosine phosphorylation within the SH3 domain regulates CAS subcellular localization. Cell Migration, and Invasiveness, 22, 4256–4267.
Jin, L. L., Wybenga-Groot, L. E., Tong, J., Taylor, P., Minden, M. D., Trudel, S., et al. (2015). Tyrosine phosphorylation of the Lyn SH2 domain modulates its binding affinity and specificity. Mol Cell Proteomic, 14, 695–706.
Joseph, R. E., Fulton, D. B., & Andreotti, A. H. (2007). Mechanism and functional significance of Itk autophosphorylation. Journal of Molecular Biology, 373, 1281–1292.
Jung, J., Byeon, I.-J. L., Ahn, J., & Gronenborn, A. M. (2011). Structure, dynamics and Hck interaction of full-length HIV-1 Nef. Proteins, 79, 1609–1622.
Kalman, B., Szep, E., Garzuly, F., & Post, D. E. (2013). Epidermal Growth Factor Receptor as a Therapeutic Target in Glioblastoma. NeuroMolecular Medicine, 15, 420–434.
Kang, S.-U., Choi, W. J., Oishi, S., Lee, K., Karki, R. G., Worthy, K. M., et al. (2007). Examination of acylated 4-aminopiperidine-4-carboxylic acid residues in the phosphotyrosyl + 1 position of Grb2 SH2 domain-binding tripeptides. Journal of Medicinal Chemistry, 50, 1978–1982.
Kang, H., Freund, C., Duke-Cohan, J. S., Musacchio, A., Wagner, G., & Rudd, C. E. (2000). SH3 domain recognition of a proline-independent tyrosine-based RKxxYxx motif in immune cell adaptor SKAP55. EMBO Journal, 11, 2889–2899.
Kast, D. J., Yang, C., Disanza, A., Boczkowska, M., Madasu, Y., Scita, G., et al. (2014). Mechanism of IRSp53 inhibition and combinatorial activation by Cdc42 and downstream effectors. Nature Structural & Molecular Biology, 1, 413–22.
Kawauchia, T., Ikeyab, M., Takadab, S., Uedaa, K., Shiraic, M., Takiharac, Y., et al. (2001). Expression of vinexin a in the dorsal half of the eye and in the cardiac out¯ow tract and atrioventricular canal. Mechanisms of Development, 106, 147–150.
Kebache, S., Zuo, D., Chevet, E., & Larose, L. (2002). Modulation of protein translation by Nck-1. Proceedings of the National Academy of Sciences of the United States of America, 99, 5406–5411.
Kefalas, T., Brown, T. R. P., & Bicknell, P. M. (1995). Signaling by the p-60 c-src family of the protein- tyrosine kinases. International Journal of Biochemistry & Cell Biology, 27, 531–63.
Kesti, T., Ruppelt, A., Wang, J.-H., Liss, M., Wagner, R., Taskén, K., & Saksela, K. (2007). Reciprocal Regulation of SH3 and SH2 Domain Binding via Tyrosine phosphorylation of a common Site in CD3ε. The Journal of Immunology, 179, 878–885.
Kim, J. M., Lee, C. D., Rath, A., & Davidson, A. R. (2008). Recognition of non-canonical peptides by the yeast Fus1p SH3 domain: elucidation of a common mechanism for diverse sh3 domain specificities. Journal of Molecular Biology, 377, 889–901.
Kobashigawa, Y., Sakai, M., Naito, M., Yokochi, M., Kumeta, H., Makino, Y., et al. (2007). Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Oncogene, 14, 503–510.
Koch, C. A., Anderson, D., Moran, M. F., Ellis, C., & Pawson, T. (1991). SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science, 252, 668–674.
Kristensen, O., Guenat, S., Dar, I., Allaman-Pillet, N., Abderrahmani, A., Ferdaoussi, M., et al. (2006). A unique set of SH3–SH3 interactions controls IB1 homodimerization. EMBO Journal, 25, 785–797.
Kurochkina, N. (2008). Specific sequence combinations at parallel and antiparallel helix-helix interfaces. Journal of Theoretical Biology, 255, 188–198.
Kurochkina, N., & Guha, U. (2013). SH3 domains: modules of protein-protein interactions. Biophysical Reviews, 5, 29–39. doi:10.1007/s12551-012-0081-z.
Kurochkina, N., & Iadarola, M. (2015). Helical assemblies: Structure determinants. Journal of Theoretical Biology, 369C, 80–84.
Law, S., & Chaudhuri, S. (2013). Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. American Journal of Stem Cells, 2, 22–38.
Lennartsson, J., & Rönnstrand, L. (2012). Stem cell factor receptor/c-kit: from basic science to clinical implications. Physiological Reviews, 92, 1619–1649.
Leung, W.-H., Tarasenko, T., & Bolland, S. (2008). Differential roles for the inositol phosphatase SHIP in the regulation of macrophages and lymphocytes. Immunologic Research, 43, 243–251.
Li, S., Couvillon, A. D., Brasher, B. B., & Van Etten, R. A. (2001). Tyrosine phosphorylation of Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling. EMBO Journal, 20, 6793–6804.
Li, H., Dusseault, J., & Larose, L. (2014). Nck1 depletion induces activation of the PI3 K/Akt pathway by attenuating PTP1B protein expression. Cell Communication and Signaling, 12, 71–86.
Li, S., Hsu, D. D., Wang, H., & Feng, G.-S. (2012). Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis. Frontiers in Medicine, 6, 275–279.
Liu, X., Chen, M., Lobo, P., An, J., Cheng, S. W. G., Moradian, A., et al. (2012). Molecular and structural characterization of the SH3 domain of AHI-1 in regulation of cellular resistance of BCR-ABL + chronic myeloid leukemia cells to tyrosine kinase inhibitors. Proteomics, 12, 2094–2106.
Luttrell, D. K., Lee, A., Lansing, T. J., Crosby, R. M., Jung, K. D., Willard, D., et al. (1994). Involvement of pp6Oc-src with two major signaling pathways in human breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 91, 83–87.
Maity, T. K., Venugopalan, A., Linnoila, I., Cultraro, C. M., Giannakou, A., Nemati, R., et al. (2015). Loss of Mig6 accelerates initiation and progression of mutant epidermal growth factor receptor-driven lung carcinoma. Cancer Discovery, in press
Marles, J. A., Dahesh, S., Haynes, J., Andrews, B. J., & Davidson, A. R. (2004). Protein-protein interaction affinity plays a crucial role in controlling the Sho1p-mediated signal transduction pathway in yeast. Molecular Cell, 14, 813–823.
Matsuda, M., Ota, S., Tanimurai, R., Nakamurai, H., Matuoka, H., Takenawa, T., et al. (1996). Interaction between the Amino-terminal SH3 domain of CRK and its natural target proteins. Journal of Biological Chemistry, 271, 14468–14472.
Mayer, B. J. (2001). SH3 domains: complexity in moderation. Journal of Cell Science, 114, 1253–63.
Messa, M., Congia, S., Defranchi, E., Valtorta, F., Fassio, A., Onofri, F., & Benfenati, F. (2010). Tyrosine phosphorylation of synapsyn I by Src regulates synaptic vesicle trafficking. Journal of Cell Science, 123, 2256–2265.
Mochizuki, N., Ohba, Y., Kobayashi, S., Otsuka, N., Graybiel, A. M., & Tanaka, S. (2000). Matsuda M (2000) Crk activation of JNK via C3G and R-Ras. Journal of Biological Chemistry, 275, 12667–12671.
Morlacchi, P., Robertson, F. M., Klostergaard, J., & McMurray, J. S. (2014). Targeting SH2 domains in breast cancer. Future Medicinal Chemistry, 6, 1909–1905.
Musi, V., Birdsall, B., Fernandez-Ballester, G., Guerrini, R., Salvatori, S., Serrano, L., & Pastore, A. (2006). New approaches to high-throughput structure characterization of SH3 complexes: The example of Myosin-3 and Myosin-5 SH3 domains from S. cerevisiae. Protein Science, 15, 795–807.
Nahm, D.-H., Tkaczyk, C., Fukuishi, N., Colucci-Guyon, E., Gilfillan, A. M., & Metcalfe, D. D. (2003). Identification of Fyn-binding proteins in MC/9 mast cells using mass spectrometry. Biochemical and Biophysical Research Communications, 310, 202–208.
Nasertorabi, F., Tars, K., Becherer, K., Kodandapani, R., Liljas, L., Vuori, K., & Ely, K. R. (2006). Molecular basis for regulation of Src by the docking protein p130Cas. Journal of Molecular Recognition, 19, 30–38.
Ngoenkam, J., Paensuwan, P., Preechanukul, K., Khamsri, B., Yiemwattana, I., Beck-García, E., et al. (2014). Non-overlapping functions of Nck1 and Nck2 adaptor proteins in T cell activation. Cell Communication and Signaling, 12, 21–33.
Nguyen, K-.S. H., Neal, J. W. (2012). First-line treatment of EGFR-mutant non-small-cell lung cancer: the role of rrlotinib and other tyrosine kinase inhibitors. Biologics: Targets and Therapy, 6, 337–345.
Nishida, M., Nagata, K., Hachimory, Y., Horiuchi, M., Ogura, K., Mandiyan, V., et al. (2001). Novel recognition mode between VAV and GRB2 SH3 domains. EMBO Journal, 20, 2995–3007.
Nishida, K., & Hirano, T. (2003). The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Science, 94, 1029–1033.
Nollet, S., Moniaux, N., Maury, J., Petitprez, D., Degand, P., Laine, A., et al. (1998). Human mucin gene MUC4: organization of its 5 « -region and polymorphism of its central tandem repeat array. Biochemical Journal, 332, 739–748.
Nore, B. F., Mattsson, P. T., Antonsson Per, Ba¨ckesjöC.-M., Westlund, A., Lennartsson, J., et al. (2003). Identification of phosphorylation sites within the SH3 domains of Tec family tyrosine kinases. Biochimica et Biophysica Acta, 1645, 123–132
Pagano, M. A., Tibaldi, E., Gringeri, E., & Brunati, A. M. (2012). Tyrosine phosphorylation and liver regeneration: A glance at intracellular transducers. Life, 64, 27–351.
Park, H., Wahl, M. I., Afar, D. E. H., Turck, C. W., Rawlings, D. J., Tam, C., et al. (1996). Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity, 4, 515–525.
Parsons, S. J., & Parsons, J. T. (2004). Src family kinases, key regulators of signal transduction. Oncogene, 23, 7906–7909.
Paster, W., Brockmeyer, C., Fu, G., Simister, P. C., de Wet, B., Martinez-Rian˜o, A., et al. (2013). GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. The Journal of Immunology, 190, 3749–3756.
Pendergast, A. M., Muller, A. J., Havlik, M. H., Maru, Y., Witte, O. N., Ullrich, A., et al. (1991). BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL SH2 regulatory domain in a non-phosphotyrosine-dependent manner. Embo J, 9, 4375–4380.
Petralia, R. S., Al-Hallaq, R. A., & Wenthold, R. J. (2008). Trafficking and targeting of NMDA receptors. In VanDongen, A. M. (Ed.), Biology of the NMDA receptor (Vol. 1, pp. 149–200). Taylor and Francis Group.
Pisabarro, M. T., Serrano, L., & Wilmanns, M. (1998). Crystal structure of the Abl-SH3 domain complexed with a designed high-affinity peptide ligand: implications for SH3-ligand interactions. Journal of Molecular Biology, 281, 513–521.
Pires, J. R., Hong, X., Brockmann, C., Volkmer-Engert, R., Schneider-Mergener, J., Oschkinat, H., & Erdmann, R. (2003). The ScPex13p SH3 domain exposes two distinct binding sites for Pex5p and Pex14p. Journal of Molecular Biology, 326, 1427–1435.
Poulin, B., Sekiya, F., & Rhee, S. G. (2005). Intramolecular interaction between phosphorylatedtyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-_1. Proceedings of the National Academy of Sciences of the United States of America, 102, 4276–4281.
Puto, L. A., Pestonjamasp, K., King, C. C., & Bokoch, G. M. (2003). P21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. Journal of Biological Chemistry, 278, 9388–9393.
Qamra, R., & Hubbard, S. R. (2013). Structural basis for the interaction of the adaptor protein Grb14 with activated Ras. PLoS ONE, 8, e72473.
Qu, Y., Chen, Q., Lai, X., Zhu, C., Chen, C., Zhao, X., et al. (2014a). SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1. Molecular Cancer, 13, 95–106.
Qu, H., Tu, Y., Guan, J.-L., Xiao, G., & Wu, C. (2014b). Kindlin-2 tyrosine phosphorylation and interaction with Src serve as a regulatable switch in the integrin outside-in signaling circuit. Journal of Biological Chemistry, 289, 31001–31013.
Quartararo, J. S., Eshelman, M. R., Peraro, L., Yu, H., Baleja, J. D., Lin, Y.-S., & Kritzer, J. A. (2014). A bicyclic peptide scaffold promotes phosphotyrosine mimicry and cellular uptake. Bioorganic & Medicinal Chemistry, 22, 6387–6391.
Rani, C. S. S., Wang, F., Fuior, E., Berger, A., Wu, J., Sturgill, T. W., et al. (1997). Divergence in signal transduction pathways of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors: Involvement of sphingosine 1-phosphate In PDGF but not EGF signaling. Journal of Biological Chemistry, 272, 10777–10783.
Räsänen, K., & Vaheri, A. (2010). Activation of fibroblasts in cancer stroma. Experimental Cell Research, 316, 2713–2722.
Reebye, V., Frilling, A., Hajitou, A., Nichols, J. P., Habib, N. A., & Mintz, P. J. (2012). Cell Signaling, 24, 388–392.
Reichman, C., Singh, K., Liu, Y., Singh, S., Li, H., Fajardo, J. E., et al. (2005). Transactivation of Abl by the Crk II adapter protein requires a PNAY sequence in the Crk C-terminal SH3domain. Oncogene, 24, 8187–8199.
Roldan, J. L. O., Casares, S., Jensen, M. R., Cárdenes, N., Bravo, J., Blackledge, M., et al. (2013). Distinct ubiquitin binding modes exhibited by SH3 domains: Molecular determinants and functional implications. PlosOne, 8, 1–14.
Sarugaser, R., Lickorish, D., Baksh, D., Hosseini, M. M., & Davies, J. E. (2005). Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells, 23, 220–229.
Sastry, L., Cao, T., & King, C. R. (1997). Multiple Grb2–protein complexes in human cancer cells. International Journal of Cancer, 70, 208–213.
Schweimer, K., Hoffmann, S., Bauer, F., Friedrich, U., Kardinal, C., Feller, S. M., et al. (2002). Structural investigation of the binding of a herpesviral protein to the SH3 domain of tyrosine kinase Lck. Biochemi, 41, 5120–5130.
Semela, D., & Dufour, J.-F. (2004). Angiogenesis and hepatocellular carcinoma. Journal of Hepatology, 41, 864–880.
Sekine, Y. (2014). Adaptor Protein STAP-2 modulates cellular signaling in immune systems. Biological & Pharmaceutical Bulletin, 37, 185–194.
Shelby, S. J., Colwill, K., Dhe-Paganon, S., Pawson, T., & Thompson, D. A. (2013). MERTK interactions with SH2-domain proteins in the retinal pigment epithelium. PLoS ONE, 8, e53964.
Sheng, M., & Kim, F. (2000). The Shank family of scaffold proteins. Journal of Cell Science, 113, 1851–1856.
Sheng, L., Liu, Y., Jiang, L., Chen, Z., Zhou, Y., Cho, K. W., & Rui, L. (2013). Hepatic SH2B1 and SH2B2 regulate liver lipid metabolism and VLDL secretion in mice. PlosOne, 8, e83269.
Sicheri, F., Moarefi, I., & Kuriyan, J. (1997). Crystal structure of the Src family kinase Hck. Nature, 385, 602–609.
Sicheri, F., & Kuriyan, J. (1997). Structures of Src-family kinases. Current opinions in structural biology, 7, 777–785.
Silva, P. N., Altamentova, S. M., Kilkenny, D. M., & Rocheleau, J. V. (2013). Fibroblast growth factor receptor Like-1 (FGFRL1) interacts with SHP-1 phosphatase at insulin secretory granules and induces beta-cell ERK1/2 protein activation. Journal of Biological Chemistry, 288, 17859–17870.
Silverman, R., & Resh, M. D. (1992). Lysine residues form an integral component of a novel NH2-terminal membrane targeting moti for myristylated pp60 v’src. Journal of Cell Biology, 119, 415–425.
Sive, J. I., & Göttgens, B. (2014). Transcriptional network control of normal and leukaemic haematopoiesis. Experimental Cell Research, 329, 255–264.
Soriano, J. V., Liu, N., Gao, Y., Yao, Z.-J., Ishibashi, T., Underhill, C., et al. (2004). Inhibition of angiogenesis by growth factor receptor bound protein 2-Src homology 2 domain bound antagonists. Molecular Cancer Therapeutics, 3, 1289–1299.
Spuches, A. M., Argiros, H. J., Lee, K. H., Haas, L. L., Pero, S. C., Krag, D. N., et al. (2007). Calorimetric investigation of phosphorylated and non-phosphorylated peptide ligand binding to the human Grb7-SH2 domain. Journal of Molecular Recognition, 20, 245–252.
Sriram, G., Reichman, C., Tunceroglu, A., Kausha, N., Saleh, T., Machida, K., et al. (2011). Phosphorylation of Crk on tyrosine 251 in the RT loop of the SH3C domain promotes Abl kinase transactivation. Oncogene, 30, 4645–4655.
Stamos, J., Sliwkowski, M. X., & Eigenbrot, C. (2002). Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-Anilinoquinazoline inhibitor. Journal of Biological Chemistry, 277, 46265–46272.
Suehara, Y., Maria, A., Lu, W., Adnan, H., Daphne, A., Tatsuo, I., et al., (2014). Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions. Clinical Cancer Research, 18, 6599–6608.
Takeuchi, K., Yang, H., Ng, E., S-y, Park, Sun, Z.-Y. J., Reinherz, E. L., & Wagner, G. (2008). Structural and functional evidence that Nck interaction with CD3ε regulates T cell receptor activity. Journal of Molecular Biology, 380, 704–716.
Tang, D. D., Zhang, W., & Gunst, S. J. (2005). The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. Journal of Biological Chemistry, 280, 23380–23389.
Tata´rova´ Z, Bra´bek J, Rösel D, Novotny M (2013) SH3 domain tyrosine phosphorylation—Sites, role and evolution.Plos ONE, 7, e36310
Teyra, J., Sachdev, S., Sidhu, S. S., & Kim, P. M. (2012). Elucidation of the binding preferences of peptide recognition modules: SH3 and PDZ domains. FEBS Letters, 586, 2631–2637.
Tian, L., Chen, L., McClafferty, H., Sailer, C. A., Ruth, P., Knaus, H.-G., & Shipston, M. J. (2006). A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB Journal, 20, E2048.
Timsah, Z., Ahmed, Z., Lin, C.-C., Melo, F. A., Stagg, L. J., Leonard, P. G., et al. (2014). Competition between Grb2 and Plcγ1 for FGFR2 regulates basal phospholipase activity and invasion. Nature Structural & Molecular Biology, 21, 180–191.
Tonikian, R., Zhang, Y., Boone, C., & Sidhu, S. S. (2007). Identifying specificity profiles for peptide recognition modules from phage-displayed peptide libraries. Nature Protocols, 2, 1368–1386.
Tridandapani, S., Pradhan, M., LaDine, J. R., Garber, S., Anderson, C. L., & Coggeshall, K. M. (1999). Protein interactions of Src homology 2 (SH2) from Fc gRIIb in B Cells (SHIP): association with Shc displaces SHIP domain-containing inositol phosphatase. Journal Immunol, 162, 1408–1414.
Ultsch, M. H., Wiesmann, C., Simmons, L. C., Henrich, J., Maria, Y., Dorothea, R., et al. (1999). Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. Journal of Molecular Biology, 290, 149–159.
Wallez, Y., Mace, P. D., Pasquale, E. B., & Riedl, S. J. (2012). NSP-CAS Protein complexes: Emerging signaling modules in cancer. Genes & Cancer, 3, 382–393.
Wang, Z., Raines, L. L., Hooy, R. M., Roberson, H., Leahy, D. J., & Cole, P. A. (2013). Tyrosine phosphorylation of Mig6 reduces its inhibition of the epidermal growth factor receptor. ACS Chemical Biology, 8, 2372–2376.
Wang, W., Xu, S., Yin, M., & Jin, Z. G. (2015). Essential roles of Gab1 tyrosine phosphorylation in growth factor-mediated signaling and angiogenesis. International Journal of Cardiology, 181, 180–184.
Wendt, M. K., Williams, W. K., Pascuzzi, P. E., Nikolas, G., ,Schiemann, B. J., et al. (2015). The Antitumorigenic function of EGFR in metastatic breast cancer is regulated by expression of Mig6. Neoplasia, 1, 124–133.
White, B. E. P., & Getsios, S. (2015). Eph receptor and ephrin function in breast, gut, and skin epithelia. Cell Adhesion & Migration, 8, 327–338.
Williams, G., Zentar, M. P., Gajendra, S., Sonego, M., Doherty, P., & Lalli, G. (2013). Transcriptional basis for the inhibition of neural stemcell proliferation and migration by the TGFb-family member GDF11. PLoS ONE, 8, e78478.
Wood, E. R., Truesdale, A. T., McDonald, O. B., Yuan, D., Hassell, A., Dickerson, S. H., et al. (2004). A unique structure for epidermal growth factor receptor bound to gw572016 (LAPATINIB): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Research, 64, 6652–6659.
Xu, J., Liu, J., Ghirlando, R., Saltiel, A. R., & Hubbard, S. R. (2003). Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Molecular Cell, 12, 1379–1389.
Xu, D., Makkinje, A., & Kyriakis, J. M. (2005). Gene 33 is an endogenous inhibitor of epidermal growth factor (EGF) receptor signaling and mediates dexamethasone-induced suppression of EGF function. Journal of Biological Chemistry, 280, 2924–2933.
Yamada, E., & Bastie, C. C. (2014). Disruption of Fyn SH3 domain interaction with a proline-rich motif in liver kinase B1 results in activation of AMP-activated protein kinase. Plos ONE, 9, e89604.
Yamada, S., Yanamoto, S., Kawasaki, G., Rokutanda, S., Yonezawa, H., Kawakita, A., & Nemoto, T. K. (2011). Overexpression of CRKII increases migration and invasive potential in oral squamous cell carcinoma. Cancer Letters, 303, 84–91.
Yang, W., & Desiderio, S. (1997). BAP-135, a target for Bruton’s tyrosine kinase in response to B cell receptor engagement. Proceedings of the National Academy of Sciences of the United States of America, 94, 604–609.
Yoshikawa, S., Kukimoto-Niino, M., Parker, L., Handa, N., Terada, T., Fujimoto, T., et al. (2012). Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene, 32, 27–38.
Zhang, X., Gureasko, J., Shen, K., Cole, P. A., & Kurian, J. (2006). An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Molecular Cell, 125, 1137–1149.
Zhang, X., Pickin, K. A., BoseR, Jura N, Cole, P. A., & Kuriyan, J. (2007). Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature, 450, 741–744.
Zhao, B. F., & Zhao, Z. J. (2014). Molecular cloning and characterization of a tyrosine phosphatase from Monosiga brevicollis. Biochemical and Biophysical Research Communications, 453, 761–766.
Zhao, H., Okada, S., Pessin, J. E., & Koretzky, G. A. (1998). Insulin receptor-mediated dissociation of Grb2 from Sos involves phosphorylation of Sos by kinase(s) other than extracellular signal- regulated kinase. Journal of Biological Chemistry, 273, 12061–12067.
Zhao, Z.-S., Manser, E., & Lim, L. (2000). Interaction between PAK and Nck: A template for Nck targets and role of PAK autophosphorylation. Molecular and Cellular Biology, 20, 3906–3917.
Zheng, C.-Y., Petralia, R. S., Wang, Y.-X., Kachar, B., & Wenthold, R. J. (2010). SAP102 is a highly mobile MAGUK in spines. Journal of Neuroscience, 30, 4757–4766.
Acknowledgments
Funding. The Intramural Research programs of the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA and Research Funds of The School of Theoretical Modeling, Washington, DC, USA.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kurochkina, N., Guha, U., Lu, Z. (2015). SH Domains and Epidermal Growth Factor Receptors. In: Kurochkina, N. (eds) SH Domains. Springer, Cham. https://doi.org/10.1007/978-3-319-20098-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-20098-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20097-2
Online ISBN: 978-3-319-20098-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)