Abstract
In the last few years, several studies have shown a strong association among obesity, altered redox state and inflammation; these studies have also shown that such alterations may be the link between obesity and obesity-related diseases (including Type 2 diabetes, cardiovascular disease, non-alcoholic fatty liver disease and cancer). Obese subjects usually show high levels of reactive oxygen or nitrogen species, impaired antioxidant defences and increased levels of inflammatory adipokines. Oxidative stress is certainly a result of excessive fat accumulation, but it has also been shown that oxidative stress, per se, leads to weight gain; therefore, it is not easy to establish the correct cause-effect relationship between obesity and oxidative stress.
This chapter analyses the main aspects linking oxidative stress to obesity, describing human studies in support of the association between alterations of redox homeostasis and obesity, the molecular mechanisms underlying these modifications and potential non-pharmacological strategies (including weight loss, physical activity, diet, dietary supplementation and microbiota modulation) aimed at reducing oxidative stress in obese individuals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Obesity is a nutritional disorder, characterized by abnormal or excessive fat accumulation, as a result of adipocyte hypertrophy (increase in size) and/or hyperplasia (increase in cell number). It represents a serious risk to health, as it increases the likelihood of several pathologies (including metabolic syndrome, Type 2 diabetes, cardiovascular diseases, non-alcoholic fatty liver disease, cancer, sleep apnoea and gynaecological pathologies), thus reducing quality of life and life expectancy [1].
A lot of evidence has shown that obesity is a state of chronic oxidative stress, although it is not completely understood if alteration in redox balance is a trigger rather than a result of obesity [2–19]. Overfeeding, high-fat (especially rich in saturated and trans-fatty acids) and high-carbohydrate meals stimulate specific signalling pathways, promoting oxidative stress and inflammation in different cell types [20–23]. On the other hand, oxidative unbalance alters food intake [24, 25] and stimulates adipocyte proliferation and differentiation, thus playing a crucial role in controlling body weight [26–28].
Systemic oxidative stress is achieved through multiple biochemical mechanisms, including superoxide generation, endoplasmic reticulum stress, glyceraldehyde autoxidation, enhanced flux in the polyol and hexosamine pathways, and activation of redox-sensitive kinases and transcription factors [29, 30]. Noticeably, in obese individuals, oxidative stress is so closely interlinked with inflammation as to trigger a vicious circle: oxidants activate specific redox-sensitive transcription factors [including nuclear factor-kB (NF-kB) and activator protein-1 (AP-1)], which drive the expression of pro-inflammatory cytokines; these mediators, in turn, enhance production of reactive oxygen species (ROS), thus contributing to the onset and maintenance of oxidative stress [31].
Besides pharmacological approach, several strategies (weight reduction, physical activity and antioxidant-rich diet) may be taken to lower oxidative stress in obesity. Firstly, weight loss and/or physical activity increase antioxidant defences, thus decreasing oxidative markers and risks for obesity co-morbidities [32–36]. Secondly, diet rich in fruits, vegetables, fish and olive oil helps to maintain the right weight and reduce the risk of metabolic diseases [37–39]. Certain nutrients (including monounsaturated fatty acids, ω-3 polyunsaturated fatty acids, vitamins C and E, phytochemicals and probiotics) contained in such food may account for reduced oxidative stress and inflammation observed in obese subjects [40–44]. Although underlining healthy effects, nonetheless observational and human intervention studies failed to demonstrate the efficacy of a single dietary component [45]; rather, it is likely that the beneficial effects on reduction of oxidative stress observed in obese subjects have to be ascribed to cumulative effects of multiple nutrients.
Oxidative Stress: An Overview
In the last 40 years, vast array of data have tried to elucidate the mechanisms involved in the maintenance of redox state in humans; however, some critical issues, related to interpretation of data obtained on in vitro experimental models, have been raised as well [46–48]. In addition, this research field often experiences confusion about the correct meaning of some terms. Below, a brief description of concepts commonly used in studies on redox balance is given.
Free Radical
Any molecule that contains one or more unpaired electrons, with a half-life varying from a few nanoseconds (very reactive radicals) to seconds and hours (rather stable radicals).
Antioxidant
A molecule with the ability to scavenge free radicals and hence able to protect biological targets (DNA, proteins, and lipids) against oxidative damage.
Reactive Oxygen Species (ROS)
A collective term that includes radical (hydroxyl radical .OH, or superoxide O2 −.) and non-radical (hydrogen peroxide H2O2) derivatives of oxygen.
Reactive Nitrogen Species (RNS)
A term including derivatives of nitrogen, such as nitric oxide (NO), nitrogen dioxide (NO2 .), dinitrogen trioxide (N2O3) and peroxynitrite (ONOO−).
Oxidative Damage
Injury caused by RO(N)S to cells and tissues.
Oxidative Stress
A term formulated by Sies in 1985, referring to a significant imbalance between RO(N)S generation and antioxidant protection (in favour of the former), causing excessive oxidative damage [48], depending on the cellular source and/or the major ROS produced. It can be sub-classified in metabolic, environmental, drug-dependent or nutritional oxidative stress [20]. Main ROS and RNS, together with their biochemical characteristics, are summarized in Table 6.1.
All these reactive species, RO(N)S, are continuously produced through cellular metabolism and also act as signalling molecules. Indeed these reactive species, if maintained below a critical threshold value, may play regulatory roles in a range of biological phenomena [49, 50]. Mitochondria are the major site of intracellular ROS production, due to electron leakage along the respiratory chain; other sources include plasma membrane systems, endoplasmic reticulum (ER), lysosomes, peroxisomes and cytosolic enzymes.
At low concentrations, RO(N)S act as secondary messengers, modulating specific signal transduction pathways that, in turn, regulate cell homeostasis, proliferation, differentiation and cell death. Moreover, RO(N)S can directly or indirectly be involved in immune-mediated defence against pathogenic microorganisms [46]. These physiological effects are mainly mediated by changes in the redox state of crucial intracellular and/or surface thiols. Indeed, the RO(N)S-triggered reversible modification of sulphur-containing amino acids represents a common post-translational mechanism for regulating the activity of enzymes, transporters, receptors and transcription factors. Spatial and temporal regulation includes covalent modification of cysteine thiols within the active and allosteric sites of enzymes, oxidation of iron-sulphur clusters, S-glutathionylation (disulfide link between protein thiols and glutathione), S-nitrosylation (reaction between NO and thiol radical), and S-nitrosation (reaction between nitrosonium ion and protein thiolates). Conversely, RO(N)S over-production (often coupled with impaired antioxidant defences) can damage DNA, lipids and proteins; thus potentially being harmful to living organisms and being causative of several pathologies, including metabolic alterations, cardiovascular and neurodegenerative diseases, and cancer [51].
To keep RO(N)S at correct levels, tissues possess antioxidant molecules working in synergy to minimize free radical cytotoxicity. The main endogenous antioxidant compounds include: (i) enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase, glutathione S-transferase, catalase, thioredoxin reductase, peroxiredoxins (Prx), NAD(P)H:ubiquinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1) and paraoxonase-1 (PON-1); (ii) low molecular weight molecules, such as urate, glutathione, ubiquinone and thioredoxin; and (iii) some proteins (ferritin, transferrin, lactoferrin, caeruloplasmin) able to bind and sequester transition metals that may trigger oxidative reactions. Exogenous antioxidants, coming from diet, include: vitamin C, vitamin E and a broad spectra of bioactive compounds (such as phytochemicals); other important nutrients are certain minerals (zinc, manganese, copper and selenium) that are crucial for the activity of antioxidant enzymes [50].
Biomarkers of Redox State
Oxidative stress can be evaluated by direct assessment of free radical production or by indirect methods that assess end-products of oxidative damage to proteins, lipids and nucleic acids in blood and urine (Table 6.2).
Direct measures of free radicals, carried out by electron spin resonance (ESR) or by immuno spin-trapping methods are difficult and expensive; therefore, even if promising, they mostly are inapplicable in human research [52]. Oxidative damage to proteins is normally assessed by measuring plasma protein carbonyls, 3-nitrotyrosine, advanced glycosylation end products (AGEs) and advanced oxidation protein products (AOPPs). Indicators of lipid peroxidation are F2-isoprostanes, malondialdehyde (MDA), oxidized LDL (oxLDL), thiobarbituric acid reactive substances (TBARs) and 4-hydroxynonenal (4-HNE). Some biomarkers (such as F2-isoprostanes) are highly sensitive, while others (such as TBARs) are much less sensitive and specific. Furthermore, F2-isoprostanes reflect both acute and chronic oxidative stress. DNA oxidative damage is usually evaluated by measuring urinary 8-hydroxy-2′-deoxyguaine [53–56]. Several studies also employed NADPH oxidase or myeloperoxidase activities in neutrophils. The first enzyme is a plasma membrane enzyme catalysing the mono-electronic reduction of exogenous oxygen using NADPH as an internal electron donor (thus producing O2 −.), while the second enzyme is heme-containing protein catalysing the reaction between chloride and H2O2 (thus generating the potent oxidant hypochlorous acid) [57].
Another method, widely used to investigate oxidative stress in various diseases and in post-prandial responses, is ROS generation by isolated mononuclear cells [22]. Other promising biomarkers of oxidative stress include urinary levels of allantoin (produced by the oxidative breakdown of urate), acrolein-lysine and dityrosine [56]. Finally, a stable, sensitive and inexpensive method to assess changes in oxidant levels is the measurement of total oxidant status (TOS), which is based on the oxidation of ferrous ion to ferric ion in the presence of oxidative species in the acidic medium. By this method, additive effects exerted by different oxidant molecules can easily be determined at the same time [58].
Plasma antioxidant profiles may be useful in conjunction with other biomarkers to measure the levels of oxidative stress. The most widely used biomarkers of the antioxidant state are serum concentrations of antioxidant molecules (retinol, carotenoids, vitamin E, vitamin C, glutathione, uric acid), minerals (selenium and zinc), as well as antioxidant enzyme (SOD, catalase, glutathione reductase, PON1). Another useful biomarker is the total antioxidant capacity (TAC), which evaluates the integrated action of some plasma antioxidants (uric acid, protein thiols and vitamin C) [20]. Finally, quantitative proteomic is emerging as an additional approach to simultaneously evaluate any change taking place in different members of the antioxidant enzyme network [59].
Evidence for Obesity-Related Oxidative Stress in Humans
Substantial cross-sectional studies have outlined the presence of altered redox state in obese subjects and redox imbalance has been demonstrated by the way of increased oxidative stress biomarkers and/or decreased antioxidant defences, both in obese children and adults.
Impairment of body defences in obese subjects may arise either from inadequate intake of antioxidant micronutrients and phytochemicals, from metabolic alterations leading to modifications in the endogenous antioxidant machinery, or from enhanced requirements due to RO(N)S over-production. Currently, the relationship between body mass index (BMI), body fat, and antioxidant defences is still an open question, especially concerning the expression and activity of antioxidant enzymes [34, 60–63]. Controversial data, however, may be explained in terms of time-window, as tissues, at the onset of obesity, increase the activity of antioxidant enzymes in order to counteract oxidative stress, but, as obesity goes on, the antioxidant apparatus is progressively depleted.
In recent years, particular attention has been given to the assessment of redox state in obese children and adolescents, with or without the simultaneous presence of metabolic alterations (insulin resistance and steatosis), since these patients show higher risk to early develop obesity-associated chronic diseases. Moreover, children and adolescents often have poor intakes of vegetables and fruit, as well as micronutrient deficiencies (iron, zinc, vitamins A, E and C), that may contribute to oxidative stress [4].
A significant inverse relation has been found between adiposity and serum concentrations of carotenoids and vitamin E, in Mexican-American children (8–15 years of age), included in the 2001–2004 U.S. National Health And Nutrition Examination Survey (NHANES). The finding has been confirmed in NHANES III (as well as in separate studies carried out in the United States, Brazil, France, and Italy) that pointed out lower serum levels of lipophilic vitamins in overweight or obese children and adolescents with respect to normal-weight counterparts(despite similar daily intakes of fruit and/or vegetables) [11, 64]. These differences seem to be not gender-specific, since similar results have been obtained in boys and girls [11]. Also vitamin E and C levels appear to be inversely proportional with body fat, abdominal fat and waist/height ratio, in 197 Mexican school-aged children. In addition, low concentrations of zinc, vitamins A and E appear to be positively correlated with insulin resistance and inflammation in overweight or obese children [8]. Deficiencies in selenium and zinc have also been reported in children with central adiposity [65, 66]. Finally, a relationship between glutathione homeostasis and obesity has been proven: a significant reduction in total, reduced and oxidized glutathione as well as in glutathionylated proteins has been found in 30 obese children [5].
As mentioned before, less clear are investigations carried on antioxidant enzymatic activities. Sfar and colleagues reported increased SOD activity in 54 obese healthy children (aged 6–12 years), while GPx and CAT activities appeared unaffected [9]. Conversely, Sun’s group documented a decrease in SOD activity, associated with increased MDA levels and NADPH oxidase activity, in 93 overweight adolescents [10]. Similarly, lower thiol content and SOD activity (paralleled by enhanced GPx1 and catalase activities) have been found to be related to central obesity, in 156 children and adolescents (47 lean, 27 overweight and 82 obese subjects). This cohort also showed inverse relation between PON1 activity and central obesity. All these associations were gender dependent: PON1 catalytic activity appeared to be sensitive to oxidative stress in girls, while being modulated by inflammation in boys [16]. Accordingly, Agirbasli reported a negative correlation between BMI and PON1 catalytic activity, while Torun found that PON1 activity significantly increased in 109 obese children and adolescents (either with or without steatosis) [12, 17].
Juvenile overweight and obesity is also linked to high levels of oxidative stress and inflammation markers [67, 68]. Pirgon and co-workers found decreased TAC values in obese adolescents (either with or without steatosis), increased oxidative stress index (TOS/TAC ratio) and insulin resistance [69]. Decreased TAC and increased oxidative stress was also found in 25 Caucasian obese children [70]. Conversely, Torun’s group reported comparable oxidative stress indexes in obese and lean children, and increased TAC measurements in obese children with non-alcoholic fatty liver disease (NAFLD) [12].
Central obesity, triglycerides and insulin correlated also with oxidative stress markers. A positive correlation between AOPPs and obesity has been found in children and adolescent; this marker also correlated with glucose/insulin ratio and HDL-cholesterol [71, 72]. In 112 overweight and obese children (7–11 years old), F2-isoprostane concentrations were positively linked to body mass index (BMI), waist circumference, insulin resistance, and dyslipidemia, while being inversely associated with fitness (peak of oxygen consumption: VO2max) [73]. Similar results have been obtained in a study enrolling 82 African American and 76 White American youth (8–17 years old) [68]. In a cross-sectional study, severely obese Caucasian children (7–14 years old) showed enhanced levels of AOPPs and inflammatory markers [72].
Mineral and vitamin deficiencies are often detected in obese adults [74–76]. Studies on Coronary Artery Risk Development in Young Adults (CARDIA) showed a strong inverse relationship between BMI and serum carotenoids (α-carotene, β-carotene, β-cryptoxanthin, zeaxanthin/lutein) [77]. The multicentre prospective population study of diet and cancer in Europe (EPIC) reported inverse correlation between plasma vitamin C concentrations and central fat distribution [78]. As obesity goes on, a linear rate of deficiency of vitamins A, B6, C, D and E has been noted [79].
Several experimental and clinical trials have shown that the catalytic activity of antioxidant enzymes is often reduced in adult obese subjects, although available data remain ambiguous and unconfirmed. Indeed, obese individuals with insulin resistance appear to have lower plasma SOD activity and higher GPx activity than healthy, lean controls [80]. In addition, serum GPx activity seems to be positively correlated with weight reduction [63]. Accordingly, several studies underlined an inverse relationship between BMI and antioxidant enzyme activities (especially SOD, catalase, PON1 and GPx) [13, 61, 62, 81, 82].
In a population-based (3042 Greek adults) study, an inverse relationship was found between waist circumference and TAC, regardless of variations in, sex, age, physical activity, smoking, and dietary habits [83] Comparable reduction in TAC measurements (together with low levels of vitamins C and E and high levels of hydroperoxides and carbonyl proteins) was observed in young and old obese patients, and signs of oxidative stress were aggravated in older adults [84].
Concerning biomarkers of oxidative stress, levels of F2-isoprostane, protein carbonyl, TOS, oxLDL and TBARs have been shown to be positively associated with BMI and waist circumference in adults [2, 3, 14, 82, 85, 86]. In addition, endothelial dysfunction and endothelial NADPH oxidase activity were found to be associated with central adiposity markers (waist circumference or waist-to-hip ratio) [87]. Interestingly, association of central adiposity markers with oxLDL and TAC is more evident in women, thus suggesting that changing the gynoid to android phenotype may lead to an unfavourable redox state in young women rather than in men [88].
Recently, several studies have focused on the relationship between obesity and redox state during pregnancy. In a prospective case-control study, obese pregnant women showed increased inflammation and oxidative stress (low levels of vitamins B6, C, E, and folate, high levels of GSSG/GSH ratio, C-reactive protein and IL-6), with respect to lean pregnant women. Newborns from these obese mothers, however, did not show significant changes in oxidative stress and inflammation [89]. On the other hand, dysregulation of redox balance in the mother-placenta-fetus axis has been reported by Malti’s group; maternal, foetal and placental tissues coming from obese women (pre-pregnancy BMI > 30 kg/m2) showed higher oxidative stress (MDA, carbonyl proteins, O2 −, NO) and lower antioxidant defences (GSH and SOD activities), if compared to control pregnant women, and variations of redox balance were also observed in newborns from these obese mothers [90].
In obese women, oxidative stress may also be associated with gynaecological disorders. A systematic meta-analysis showed increased SOD activity, decreased GSH levels and decreased PON1 activity in patients with polycystic ovary syndrome [91]. Also localised oxidative stress and insulin resistance (in abdominal adipose tissue) seem to play a crucial role in the pathogenesis of this syndrome [15].
In conclusion, conflicting results emerging from cross-sectional and intervention studies in obesity field are difficult to interpret. However, they could be explained in terms of the use, in each investigation, of single or only few, insensitive, non-specific markers of redox state [53]. The best and unambiguous results demonstrating the relationship between oxidative stress and obesity have been obtained when several oxidant and antioxidant molecules were considered together or when urinary isoprostanes (the best available biomarker of lipid peroxidation) were measured.
Mechanisms Underlying Oxidative Stress in Obesity and Oxidative Stress-Induced Diseases
Mechanisms Underlying Oxidative Stress
Oxidative stress is clearly connected with obesity, although it is not fully understood the real cause-effect relationship. Nutritional overload (especially after consumption of high-fat high-carbohydrate meals, as well as of high dietary saturated fatty acids and trans-fatty acids) leads to oxidative stress by different mechanisms [23]. On the other hand, oxidative stress could play a causative role in the development of obesity, by altering food intake and stimulating adipocyte proliferation and differentiation [24–28]. Several factors contribute to obesity-associated oxidative stress, including abnormal post-prandial ROS generation, low antioxidant defences, hyperleptinemia, tissue dysfunction and chronic inflammation [2–22, 92, 93]. In this context, it should be recalled that a vicious circle can be established: ROS activate redox sensitive transcription factors [including nuclear factor-kB (NF-kB) and activator protein-1 (AP-1)], which in turn promote over-expression of inflammatory cytokines that lead to exacerbation of ROS production [31] (Table 6.3).
In obese subjects, the adipocytes surpass to non-physiological limits and become unable to function as an energy storage organ; therefore, fat is improperly accumulated in heart, muscle, liver and pancreas, where it can trigger these organs’ dysfunction. In particular, adipose tissue dysfunction contributes to the onset of oxidative stress, by increasing expression of adipokines (MCP-1, -2, -4, and macrophage inflammatory protein (MIP) -1α, -1β, -2α) that trigger macrophage infiltration and subsequent overproduction of ROS and inflammatory cytokines [94, 95]. Simultaneously, the activity of the redox-sensitive transcription factor, nuclear factor E2-related factor 2 (Nrf2) becomes impaired. As a result, the expression of Nrf2 downstream targets (antioxidant and phase II detoxifying enzymes) is inhibited, leading to the weakening of the body antioxidant defences [96]. Also bioavailability of antioxidant molecules may be impaired, as is the case with vitamin C. Also, sodium-dependent vitamin C transporters expression has been shown to be modulated by metabolic and/or oxidative stress, thus affecting cellular uptake and the overall homeostasis of this vitamin [97].
Additionally, intracellular triglyceride accumulation triggers lipotoxicity, by inhibiting the adenosine nucleotide translocator (ANT) leading to ATP accumulation into mitochondria. Mitochondrial ADP fall reduces the speed of oxidative phosphorylation and mitochondrial uncoupling promotes electron leakage and ROS generation. High fat diet-induced mitochondrial dysfunction also triggers the endoplasmic reticulum (ER) stress. The ER is a cytosolic organelle that participates in the regulation of lipid, glucose and protein metabolism, apart from being at the site where protein folding occurs. ER stress observed in obesity basically results in impairment of protein folding leading to production of misfolded proteins which activate abnormal “unfolded protein response” (UPR). In turn this stimulates ROS production, with subsequent systemic release of free fatty acids and inflammatory mediators, lipid droplet creation and hepatic cholesterol accumulation [98, 99].
Ectopic-fat deposition also inhibits glucose transport and insulin signalling in skeletal muscle, thus promoting insulin resistance [62, 100]. Hyperglycaemia is another condition promoting oxidative stress, by enhancing oxidative degradation of glucose; the resulting increase in proton gradient across the mitochondrial inner membrane leads to electron leakage and O2 −. production. As a result, glycolytic metabolites are shifted to four alternative pathways: (i) glucose is redirected into the polyol pathway; (ii) fructose-6-phosphate is redirected into the hexosamine pathway; (iii) triose phosphates produce methylglyoxal, the main precursor of advanced glycosylation end products (AGEs), and (iv) dihydroxyacetone phosphate is converted to diacylglycerol, thus activating protein kinase C (PKC) [101]. These four pathways induce oxidative/nitrosative stress, by different mechanism. Activation of the polyol pathway leads to NADPH depletion, glucosamine-6-phosphate derived from the hexosamine pathway induces oxidative and ER stress, and AGEs and PKC stimulate ROS/RNS production by activating NADPH oxidases and NF-kB [102, 103]. In particular, NF-kB is a transcription factor whose downstream targets are pro-inflammatory cytokines (TNF-α and IL-6), inducible nitric oxide synthase (iNOS), adhesion molecules (E-selectin, intercellular adhesion molecule-1 and endothelin-1), and some microRNAs (miR). Aberrant expression of these NF-kB-responsive genes may account for increased oxidative/nitrosative stress and inflammation, as well as for enhanced adipogenesis (for example, modulation of miR-103, miR-143, miR-27a and miR-27b has been recognized as condition leading to adipose hypertrophy and hyperplasia) observed in obese individuals [104–106]. Finally, oxidative stress is exacerbated by inflammatory mediators that worsen insulin signalling, thus intensifying hyperglycaemia [6].
A relevant source of ROS can also be represented by endothelial dysfunction, commonly found in obesity, due to chronic inflammation and dysregulation of adipocyte-derived factors. Activation of endothelial NADPH oxidase (triggered by cytokines, hormones, elevated intraluminal pressure and hypertension) increases ROS levels, thus aggravating vascular injury [107].
Among adipocyte-derived factors, the hormone leptin plays a crucial role in obesity-associated oxidative stress. Hyperleptinemia increases mitochondrial and peroxisomal fatty acid oxidation, with subsequent stimulation of ROS production via the mitochondrial respiratory chain [92, 108]. In addition, it stimulates activation of monocytes/macrophages, with production of pro-inflammatory cytokines (IL-6 and TNF-α) that intensify oxidative stress [109].
Genetic variants, such as single nucleotide polymorphisms (SNPs) in genes encoding for mediators of redox balance, represent an emerging research field in obesity. Recently, Rupérez and colleagues reviewed the current knowledge about the impact of specific SNPs on the risk of obesity or obesity-associated co-morbidities [110]. In particular, they focused on SNPs of antioxidant enzymes (GPx, PON1, catalase, peroxiredoxins, SOD), ROS-producing enzymes (NADPH oxidases) and transcription factors involved in ROS response mechanisms (PPARγ, PGC1α, Nrf2). Other genetic loci relevant for body weight regulation and oxidative stress have also been identified, including protective and susceptible SNPs in the mitochondrial control region [111], in fat mass and obesity associated (FTO) gene [112], and in the gene encoding for uncoupling protein 2 (UCP2) that leads to heat dissipation (without synthesis of ATP) and controls mitochondrial free radical production [113]. However, the expression of metabolic phenotypes is markedly affected also by environmental and epigenetic factors, thus making difficult to assess the real impact of genetically controlled heritability in human obesity.
Oxidative Stress-Induced Diseases
All the above mentioned ROS-generating conditions may play a causative role in obesity-related co-morbidities [114]. In Non-alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic Steatohepatitis (NASH), mitochondrial dysfunction, ER stress and hyperglycaemia cause excessive electron flux in the electron transport chain and ROS overproduction. As a result, fatty acid catabolism is impaired, while lipogenesis is stimulated, and, therefore, lipids abnormally accumulate in hepatocytes [115]. ROS accumulation also plays a key role in the development of metabolic syndrome, characterized by central obesity associated with two or more complications (hyperglycaemia, hypertension, dyslipidaemia), representing elevated risk factors for cardiovascular pathologies. Subsequently redox-inflammatory processes, together with visceral adiposity, disrupts downstream events of the insulin signalling pathway, thus triggering insulin resistance, which in turn promotes endothelial dysfunction, decreased vasodilatation and increased blood pressure [116]. Type 2 diabetes develops in obese individuals as a result of insulin-resistance. Impaired glucose tolerance is achieved through multiple mechanisms, all involving oxidative stress: (i) β-cells (possessing low scavenging ability) die because of chronic oxidative stress and adipokine secretion, (ii) adipocyte oxidative stress leads to production of glutathionylated products of lipid peroxidation that results in insulin resistance and inflammation, and (iii) protein oxidation and/or misfolding, resulting in proteasomal dysfunction, contribute to the onset of insulin-resistant and obese phenotype [117–119].
Circulating free fatty acids, insulin resistance, oxidative stress, mitochondrial and endothelial dysfunction are also key pathogenic factors of obesity-associated cardiovascular pathologies (including coronary and peripheral artery disease, stroke, cardiomyopathy and congestive heart failure) [95]. Elderly subjects appear to be more susceptible to obesity-associated vascular complications than younger individuals, may be because aging worsens obesity-triggered inflammation in perivascular adipose tissue, thus increasing oxidative stress and inflammation in a paracrine manner [120]. A significant correlation has been found between BMI and tumour susceptibility: obesity accounts for 14–20 % of deaths due to gastrointestinal, breast, prostate, endometrium, uterus and ovary tumours in both males and females [121].
It is now well recognized that ROS overproduction triggers DNA damage, thus leading to genomic instability associated with activation of oncogenes and/or inactivation of tumour suppressor genes [122–124]. A recent systematic meta-analysis documented that oxidative stress, together with visceral adipose tissue, is one of the pathogenic mechanisms accounting for polycystic ovary syndrome. Indeed, abdominal adipocytes coming from patients affected by polycystic ovary syndrome show enhanced oxidative stress and impaired insulin signalling [15, 91]. Finally, oxidative stress and inflammation are involved in the pathogenesis of obstructive sleep apnea, a breathing disorder often associated with central obesity. In this disorder repeated breathing arrests lead to an ischemia/reperfusion condition, resulting in ROS overproduction that is enhanced by high levels of pro-inflammatory cytokines found in neutrophils and monocytes of sleep apnea patients [125].
Lifestyle and Nutritional Intervention to Reduce Oxidative Stress in Obesity
Natural strategies designed to increase antioxidant defences in obese subjects could be useful to prevent and treat obesity and co-morbidities. Herein, we will report recent experimental evidences concerning the effects of physical activity and diet on modulation of redox state; we will also describe potential mechanisms through which weight loss, overall diet composition and single diet components (macronutrients, micronutrients, and phytochemicals) modulate redox homeostasis.
Independent of weight reduction, physical activity exerts positive effects on oxidant/antioxidant balance. For example exercise reduces NADPH oxidase activity and ROS generation, while increasing blood levels of anti-inflammatory cytokines (interleukins 1 and 10) [126, 127]. In a retrospective analysis enrolling 108 obese, middle-aged men, exercise training without dietary restriction has been shown to improve hepatic inflammation and related oxidative stress [128]. A cross-sectional study carried out on overweight/obese postmenopausal women (45–64 years old) showed that active lifestyle (aerobic exercise for at least 30 min, three times per week) was associated with increased antioxidant enzyme activities (catalase and SOD) in peripheral blood mononuclear cells [36]. Krause and colleagues reported that aerobic exercise (at moderate intensity, three times per week) did not change body composition or aerobic fitness, but ameliorated oxidative markers in obese subjects with or without Type 2 diabetes [129]. A retrospective analysis have been conducted to determine whether exercise without dietary restriction can influence the pathophysiology of abnormal liver function in obese, middle-age men. Results showed that physical activity benefits the management of obesity-related liver diseases regardless of detectable weight reduction; in particular, these effects seem to be acquired through an improvement in hepatic oxidative stress levels and related inflammatory conditions [128]. Besides direct effects on oxidative stress, exercise is also able to reduce abnormal conditions such as inflammation and insulin resistance that underpin obesity-associated diseases. Aerobic and resistance exercise (Nordic walking) for 12 weeks without dietary intervention does not influence oxidative stress, but decreases atherogenic index in overweight or obese males (40–65 years) with impaired glucose regulation [130]. Therefore, regular exercise acts as a natural antioxidant and anti-inflammatory strategy for preventing obesity-associated complications.
Combination of regular exercise with caloric restriction potentiates the beneficial effects on redox balance. Physical activity associated with weight loss has been found to be the most efficacious approach to prevent dyslipidemia, hypertension, Type 2 diabetes, cardiovascular diseases, NAFLD and colorectal cancer, even though it is difficult to determine if the observed effects are due to exercise, weight loss or specific diet components [131, 132]. Gutierrez-Lopez et al. showed that regular and moderate aerobic exercise plus hypocaloric diet was more effective than hypocaloric diet alone in decreasing oxidative stress markers and insulin polymerization, in 32 obese subjects [34]. Lifestyle intervention (including both exercise and diet) has been shown to be a successful approach for ameliorating endothelial dysfunction, inflammation and oxidative stress, in obese children [133].
Weight reduction obtained via caloric restriction alone has been proven to reduce the levels of protein carbonylation, AOPPs, lipid peroxidation, oxidized lipoproteins and F2-isoprostanes, as well as of inflammatory markers [33, 35, 134, 135]. In overweight and obese women, a modest reduction in caloric intake (25 % caloric restriction) is sufficient to rapidly decrease oxidative stress [33]. This finding has been confirmed by Chae’s study showing that a daily 100-kcal calorie reduction was able to revert the elevated oxidative stress observed in overweight and obese individuals (3-year follow-up leading to only 5.4 % weight loss). Noticeably, lifestyle intervention has to be long-lasting, as resuming a habitual diet brings back the levels of oxidative markers to the baseline values within 3 months, in ∼80 % of women [33]. The mechanisms by which lowering energy supply ameliorates metabolic functions mainly include activation of sirtuins, NAD+-dependent deacetylases that regulate metabolism, improve antioxidant defences and dampen inflammatory activities [136]. Also the transcription factor FoxO (Forkhead box, sub-group O), which up-modulates the expression of genes involved in energy homeostasis, induces cell survival and inflammatory responses [137].
Besides weight reduction, diet quality is a key factor for redox homeostasis. Western diets (increased intake of artificial energy-dense foods and reduced intake of complex carbohydrates, fibres, fruits and vegetables) deeply contribute to oxidative stress and metabolic alterations promoting lipotoxicity [20–23, 40, 138]. Conversely, antioxidant-rich diets are effective in both reducing oxidative stress and speeding up weight loss. Indeed, Mediterranean diet (rich in whole-grains, legumes, nuts, fruit, vegetables, fish, low-fat dairy products, and olive oil as principal source of fat) and Okinawan diet (rich in unprocessed foods, vegetables, sweet potatoes with small amount of fish and lean meat) exerts protective effects against obesity and obesity-related pathologies [37, 139–141]. In particular, the Mediterranean diet (even without weight reduction) is able to reduce oxidative stress and inflammation, as well as to improve insulin sensitivity. For example, abdominally overweight men and women showed lower concentrations of pro-inflammatory cytokines after 8 weeks of a Mediterranean diet [142]. In addition, among high cardiovascular risk subjects (carrying the genetic variants rs9939609 for FTO and rs17782313 for MC4R, conferring genetic susceptibility to obesity and diabetes), those adhering to Mediterranean diet had lower rate of Type 2 diabetes [143].
Specific food or nutrients also exert positive effect on redox balance, inflammatory biomarkers and metabolic alterations associated with obesity. Animal and human studies have highlighted the protective role of mono-unsaturated fatty acids (abundant in olive oil) and ω-3 poly-unsaturated fatty acids (abundant in fish and nuts) on cardiovascular risk in overweight and obese subjects, via different mechanisms: (i) reduction of oxidative stress and inflammation, (ii) increase of antioxidant defences via the Nrf2/HO-1 pathway, (iii) prevention of endothelial dysfunction, (iv) improvement of hyperglycaemia and hyperinsulinemia [142, 144–147]. Other subsidiary dietary compounds (such as antioxidant vitamins and phytochemicals) contribute to a well redox balance, by directly scavenging ROS or indirectly modulating the activity of redox-sensitive transcription factors and enzymes, as well as by exerting anti-inflammatory actions. A diet containing food with high antioxidant capacity (such as fruits, vegetables, and legumes) is negatively associated with adiposity, oxidative stress markers, and obesity-related co-morbidities (Type 2 diabetes and cardiovascular diseases) [148, 149].
From the NHANES (2003–2006) studies, it has emerged that consumption of orange juice lowers about 21 % the risk of obesity and of about 36 % the risk of metabolic syndrome. On the other hand mandarin juice consumption up-modulates antioxidant defences in obese children, tomato juice consumption improves plasma TAC and erythrocyte antioxidant enzymes in overweight females [150–152]. Daily consumption of grapefruit (fruit or juice) ameliorates oxidative stress in overweight and obese adults with metabolic syndrome [153]. A 24-weeks trial with unsalted pistachio nuts (20 % of total energy) leads to beneficial effects on redox state and cardio-metabolic profile of Asian Indians with metabolic syndrome [154]. The same beneficial effects on serum TAC and oxidative stress indexes have also been obtained with consumption of broccoli sprouts powder and carrot juice, in overweight and Type-2 diabetes patients [155, 156].
Antioxidant supplements (vitamins C and E, carotenoids, lipoic acid) may also be useful in primary and secondary prevention of ROS-induced health problems. Several short-term studies have shown that vitamin E supplementation resulted in significant reduction of oxidative stress and improvement of lipid state and cardio-metabolic alterations, in children and adults [157–159]. However, adverse effects have been reported as well, especially in long-term clinical trials, so that vitamin E supplementation should be carefully evaluated [160, 161]. Obese individuals and diabetic subjects often experience high rate of vitamin C deficiency and, therefore, regular consumption of vitamin C-rich foods has to be recommended [7]. Indeed, observational and interventional studies have suggested a beneficial role of vitamin C on prevention of diabetes, hypertension, stroke and heart failure, but supplementation data do not allow to univocally establish the role played by vitamin C on health outcomes [162–165]. Another vitamin deficiency commonly found in obese individuals are carotenoids (both pro-vitamin A and not pro-vitamin A carotenoids), so that these individuals may benefit from their supplementation [166]. Acting as antioxidant and anti-inflammatory agents, they modulate markers of inflammation, oxidative stress, and endothelial dysfunction, thus exerting a protective role against obesity-associated diseases [167, 168]. However, both positive and negative effects have been reported by in vivo supplementation studies with β-carotene, astaxanthin, β-cryptoxanthin and lycopene [169]. Interestingly, the potential of α-Lipoic acid (LA) in human therapeutics was found to bring strength in several human supplementation studies. LA increases the Nrf2-mediated anti-oxidant responses and prevents obesity-induced oxidative stress and lipoapoptosis in rat liver [170]. Administration of LA to patients with Type 2 diabetes decreases plasma oxidative products and improves insulin sensitivity [171]. Oral LA supplementation promotes body weight loss in healthy overweight/obese subjects [172, 173]. The mechanisms accounting for these positive effects not only mainly rely on modulation of redox homeostasis, but also on increased insulin sensitivity, mitochondrial biogenesis and promotion of browning process in white adipose tissue [174].
Among phytochemicals provided by food of plant origin, polyphenols constitute the most abundant and heterogeneous class and indeed, different categories (phenolic acids, stilbenes, flavonoids, chalcones, lignans and curcuminoids) can be identified on the basis of their structures. Depending on chemical structure, bioavailability and metabolism, each phytochemical exerts distinct physiological effects through multiple (sometimes overlapping) mechanisms. Referring to a more comprehensive review about the best characterized biological properties of each bioactive compound [7], here we only emphasize that polyphenols may modulate inflammation and redox state, as well as adipocyte differentiation and lipid metabolism [175]. In this way they exert protective effects on oxidatively triggered pathologies [41, 176]. Short-term clinical trials have indeed pointed out a positive role of specific compounds on obesity, glucose tolerance and cardiovascular risk factors [177–180]. A particular class deserves to be mentioned is isoflavones (genistein, daidzein and glycitein), because they are analogues of estrogens, therefore, their ability to exert anti-adipogenic and anti-lipogenic effects mainly rely on binding to estrogen receptors, thus modulating the expression of genes involved in adipose development, insulin sensitivity and fatty acid metabolism rather than only on antioxidant activity [181, 182]. However, it should be recalled that many of the positive effects ascribed to polyphenols have been demonstrated by in vitro studies and in vivo biological relevance has not been established yet, especially considering the low bioavailability and rapid body metabolism of these bioactive phytochemicals.
Another interesting finding is that the interaction of polyphenol-gut microbiota represents an additional modulator of oxidative stress-mediated pathologies. Indeed, studies have shown that microbiota modulates the activity of different polyphenols (thus explaining the inter-individual variability observed in polyphenol supplementation studies), and, meanwhile, polyphenols change intestinal redox state (thus modulating quantitative and qualitative features of gut-microbiota) [183].
Microbial activities and gut ‘dysbiosis’ are involved in controlling the body weight and insulin-resistance [184, 185]. On the other hand, probiotics (healthy micro-organisms, including Bifidobacteria and Lactobacilli) and prebiotics (non-viable food components, such as inulin-type fructans, able to modulate microbiota composition) may confer health benefits for obese individuals, lowering oxidative unbalance [43, 185]. Indeed, daily consumption of probiotics has been shown to improve antioxidant parameters (TAC, enzymatic activities of SOD and GPx) and to decrease oxidative markers (MDA, oxLDL and 8-isoprostanes), both in healthy subjects and type 2 diabetic patients [186, 187].
Conclusions
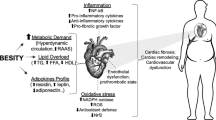
Numerous investigations indicate that obesity is strictly linked to changes in redox state. Abnormal production of ROS and nitrogen species, due to unhealthy lifestyle (chronic hyper-nutrition, low quality diet and sedentary life), affects white adipose tissue, endothelium and muscle biology, thus leading to obesity-associated pathologies (including NAFLD, diabetes, hypertension, cardiovascular diseases and cancer) (Fig. 6.1).
Relationship among oxidative stress, obesity and obesity-associated diseases. Excessive caloric intake, low-quality diet and sedentary lifestyle, even before weight gain, are suggested to be primary triggers of systemic oxidative stress and inflammation; genetic variants are involved as well. A vicious circle is established: by stimulating white adipose tissue deposition and altering food intake, oxidative stress contributes to the onset and progression of obesity, as well as to development of obesity-associated diseases. Both obesity and oxidative stress trigger inflammatory conditions that, in turn, lead inexorably to a worsening of the situation (see Text for further details)
Governmental and non-governmental organizations are developing new strategies for prevention and control of obesity, especially concerning lifestyle intervention, in order to limit morbidity and mortality rates, as well as health care costs. Approaches aimed at modulating redox homeostasis are emerging as novel tools for preventing or slowing down progression of obesity-associated pathologies. Studies on humans claim that the first goal to be achieved should be to reduce oxidative stress by combination of weight loss, physical activity and high quality diet. Obese individuals might also benefit from regular consumption of foods with high antioxidant natural compounds rather than from supplementation with antioxidant compounds. This is because of paucity of data (often controversial and not conclusive) concerning clinical trials with specific nutrients. More promising appears to be a diet rich in polyphenols, widely distributed in fruits, vegetables and some plant-derived beverages (such as coffee and tea), which are effective in counteracting weight gain and oxidative stress. Moreover, their biological activity may be enhanced by modulating composition of gut microbiota that represents a novel way of dealing with redox unbalance in overweight or obese individuals. In conclusion, the winning strategy for lowering risk factors of obesity-associated complications remains weight loss through physical activity and diet rich in fruits, vegetables and spices (containing antioxidant vitamins and phytochemicals), fish (containing ω-3 poly-unsaturated fatty acids) and low-fat, fermented dairy products (especially those containing probiotics).
References
WHO: World Health Organization obesity and overweight, Fact sheet N°311. Updated Jan 2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/.
Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
Dorjgochoo T, Gao YT, Chow WH, et al. Obesity, age, and oxidative stress in middle-aged and older women. Antioxid Redox Signal. 2011;4:2453–60.
Gates A, Hanning RM, Gates M, et al. Vegetable and fruit intakes of on-reserve first nations schoolchildren compared to Canadian averages and current recommendations. Int J Environ Res Public Health. 2012;9:1379–97.
Pastore A, Ciampalini P, Tozzi G, et al. All glutathione forms are depleted in blood of obese and type 1 diabetic children. Pediatr Diabetes. 2012;13:272–7.
Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68:701–11.
Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10497–538.
García OP, Ronquillo D, del Carmen Caamaño M, et al. Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients. 2013;5:5012–30.
Sfar S, Boussoffara R, Sfar MT, et al. Antioxidant enzymes activities in obese Tunisian children. Nutr J. 2013;12:18.
Sun M, Huang X, Yan Y, et al. Rac1 is a possible link between obesity and oxidative stress in Chinese overweight adolescents. Obesity (Silver Spring). 2012;20:2233–40.
Gunanti IR, Marks GC, Al-Mamun A, et al. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. J Nutr. 2014;144:489–95.
Torun E, Gökçe S, Ozgen İT, et al. Serum paraoxonase activity and oxidative stress and their relationship with obesity-related metabolic syndrome and non-alcoholic fatty liver disease in obese children and adolescents. J Pediatr Endocrinol Metab. 2014;27:667–75.
Amirkhizi F, Siassi F, Djalali M, et al. Impaired enzymatic antioxidant defense in erythrocytes of women with general and abdominal obesity. Obes Res Clin Pract. 2014;8:e26–34.
Cervellati C, Bonaccorsi G, Cremonini E, et al. Waist circumference and dual-energy X-ray absorptiometry measures of overall and central obesity are similarly associated with systemic oxidative stress in women. Scand J Clin Lab Invest. 2014;74:102–7.
Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102:1167–74.
Krzystek-Korpacka M, Patryn E, Hotowy K, et al. Paraoxonase (PON)-1 activity in overweight and obese children and adolescents: association with obesity-related inflammation and oxidative stress. Adv Clin Exp Med. 2013;22:229–36.
Agirbasli M, Tanrikulu A, Erkus E, et al. Serum paraoxonase-1 activity in children: the effects of obesity and insulin resistance. Acta Cardiol. 2014;69:679–85.
Marseglia L, Manti S, D’Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16:378–400.
Youn JY, Siu KL, Lob HE, et al. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63:2344–55.
Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–72.
Patel C, Ghanim H, Ravishankar S, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. 2007;92:4476–9.
Dandona P, Ghanim H, Chaudhuri A, et al. Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Exp Mol Med. 2010;42:245–53.
Muñoz A, Costa M. Nutritionally mediated oxidative stress and inflammation. Oxid Med Cell Longev. 2013;2013:610950.
Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab. 2009;20:78–87.
Drougard A, Fournel A, Valet P, et al. Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake. Front Neurosci. 2015;9:56.
Lee H, Lee YJ, Choi H, et al. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284:10601–9.
Higuchi M, Dusting GJ, Peshavariya H, et al. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box o1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878–88.
Aroor AR, De Marco VG. Oxidative stress and obesity: the chicken or the egg? Diabetes. 2014;63:2216–8.
Serra D, Mera P, Malandrino MI, et al. Mitochondrial fatty acid oxidation in obesity. Antioxid Redox Signal. 2013;19:269–84.
Le Lay S, Simard G, Martinez MC, et al. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. 2014;2014:908539.
Bryan S, Baregzay B, Spicer D, et al. Redox-inflammatory synergy in the metabolic syndrome. Can J Physiol Pharmacol. 2013;91:22–30.
Bigornia SJ, Mott MM, Hess DT, et al. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring). 2010;18:754–9.
Buchowski MS, Hongu N, Acra S, et al. Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS One. 2012;7:e47079.
Gutierrez-Lopez L, Garcia-Sanchez JR, Rincon-Viquez Mde J, et al. Hypocaloric diet and regular moderate aerobic exercise is an effective strategy to reduce anthropometric parameters and oxidative stress in obese patients. Obes Facts. 2012;5:12–22.
Chae JS, Paik JK, Kang R, et al. Mild weight loss reduces inflammatory cytokines, leukocyte count, and oxidative stress in overweight and moderately obese participants treated for 3 years with dietary modification. Nutr Res. 2013;33:195–203.
Farinha JB, De Carvalho NR, Steckling FM, et al. An active lifestyle induces positive antioxidant enzyme modulation in peripheral blood mononuclear cells of overweight/obese postmenopausal women. Life Sci. 2015;121:152–7.
Sofi F, Abbate R, Gensini GF, et al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–96.
Kwan HY, Chao X, Su T, et al. The anti-cancer and anti-obesity effects of mediterranean diet. Crit Rev Food Sci Nutr. 2015:0. doi: 10.1080/10408398.2013.852510.
Widmer RJ, Flammer AJ, Lerman LO, et al. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229–38.
Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106:S5–78.
González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: a review. Pharmacol Res. 2011;64:438–55.
Sies H, Hollman PC, Grune T, et al. Protection by flavanol-rich foods against vascular dysfunction and oxidative damage: 27th Hohenheim Consensus Conference. Adv Nutr. 2012;3:217–21.
Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition. 2013;29:591–6.
Khor A, Grant R, Tung C, et al. Postprandial oxidative stress is increased after a phytonutrient-poor food but not after a kilojoule-matched phytonutrient-rich food. Nutr Res. 2014;34:391–400.
Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176.
Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–65.
Jones DP, Radi R. Redox pioneer: professor Helmut Sies. Antioxid Redox Signal. 2014;21:2459–68.
Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4C:180–3.
Murphy MP, Holmgren A, Larsson NG, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–6.
Ye ZW, Zhang J, Townsend DM. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim Biophys Acta. 2015;1850(8):1607–21.
Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224C:164–75.
Lee MC. Assessment of oxidative stress and antioxidant property using electron spin resonance (ESR) spectroscopy. J Clin Biochem Nutr. 2013;52:1–8.
Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55.
Komosinska-Vassev K, Olczyk P, Winsz-Szczotka K, et al. Plasma biomarkers of oxidative and AGE-mediated damage of proteins and glycosaminoglycans during healthy ageing: a possible association with ECM metabolism. Mech Ageing Dev. 2012;133:538–48.
Dorjgochoo T, Gao YT, Chow WH, et al. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. 2012;96:405–14.
Il’yasova D, Wang F, Spasojevic I, et al. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity. 2012;20:1915–21.
Olza J, Aguilera CM, Gil-Campos M, et al. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care. 2012;35:2373–6.
Jansen EH, Ruskovska T. Comparative analysis of serum (anti)oxidative status parаmeters in healthy persons. Int J Mol Sci. 2013;14:6106–15.
Rindler PM, Plafker SM, Szweda LI, et al. High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem. 2013;288:1979–90.
Brown LA, Kerr CJ, Whiting P, et al. Oxidant stress in healthy normal-weight, overweight, and obese individuals. Obesity (Silver Spring). 2009;17:460–6.
Mittal PC, Kant R. Correlation of increased oxidative stress to body weight in disease-free post menopausal women. Clin Biochem. 2009;42:1007–11.
Olivares-Corichi IM, Viquez MJ, Gutierrez-Lopez L, et al. Oxidative stress present in the blood from obese patients modifies the structure and function of insulin. Horm Metab Res. 2011;43:748–53.
Bougoulia M, Triantos A, Koliakos G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Hormones (Athens). 2006;5:192–9.
Strauss RS. Comparison of serum concentrations of -tocopherol and -carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr. 1999;134:160–5.
Weisstaub G, Hertrampf E, López de Romaña D, et al. Plasma zinc concentration, body composition and physical activity in obese preschool children. Biol Trace Elem Res. 2007;118:167–74.
Ortega RM, Rodríguez-Rodríguez E, Aparicio A, et al. Young children with excess of weight show an impaired selenium status. Int J Vitam Nutr Res. 2012;82:121–9.
Tran B, Oliver S, Rosa J, et al. Aspects of inflammation and oxidative stress in pediatric obesity and type 1 diabetes: an overview of ten years of studies. Exp Diabetes Res. 2012;2012:683680.
Warolin J, Coenen KR, Kantor JL, et al. The relationship of oxidative stress, adiposity and metabolic risk factors in healthy Black and White American youth. Pediatr Obes. 2014;9:43–52.
Pirgon Ö, Bilgin H, Çekmez F, et al. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2013;5:33–9.
Faienza MF, Francavilla R, Goffredo R, et al. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78:158–64.
Krzystek-Korpacka M, Patryn E, Boehm D, et al. Advanced oxidation protein products (AOPPs) in juvenile overweight and obesity prior to and following weight reduction. Clin Biochem. 2008;41:943–9.
Codoñer-Franch P, Tavárez-Alonso S, Murria-Estal R, et al. Elevated advanced oxidation protein products (AOPPs) indicate metabolic risk in severely obese children. Nutr Metab Cardiovasc Dis. 2012;22:237–43.
Dennis BA, Ergul A, Gower BA, et al. Oxidative stress and cardiovascular risk in overweight children in an exercise intervention program. Child Obes. 2013;9:15–21.
Via M. The malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012;2012:103472.
Kaidar-Person O, Person B, Szomstein S, et al. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–6.
Kaidar-Person O, Person B, Szomstein S, et al. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B: minerals. Obes Surg. 2008;18:1028–34.
Andersen LF, Jacobs Jr DR, Gross MD, et al. Longitudinal associations between body mass index and serum carotenoids: the CARDIA study. Br J Nutr. 2006;95:358–65.
Canoy D, Wareham N, Welch A, et al. Plasma ascorbic acid concentrations and fat distribution in 19 068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am J Clin Nutr. 2005;82:1203–9.
Aasheim ET, Bøhmer T. Low preoperative vitamin levels in morbidly obese patients: a role of systemic inflammation. Surg Obes Relat Dis. 2008;4:779–80.
Tinahones FJ, Murri-Pierri M, Garrido-Sánchez L, et al. Oxidative stress in severely obese persons is greater in those with insulin resistance. Obesity (Silver Spring). 2009;17:240–6.
Viroonudomphol D, Pongpaew P, Tungtrongchitr R, et al. Erythrocyte antioxidant enzymes and blood pressure in relation to overweight and obese Thai in Bangkok. Southeast Asian J Trop Med Public Health. 2000;31:325–34.
Aslan M, Horoz M, Sabuncu T, et al. Serum paraoxonase enzyme activity and oxidative stress in obese subjects. Pol Arch Med Wewn. 2011;121:181–6.
Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: the ATTICA study. Nutr Metab Cardiovasc Dis. 2007;17:590–7.
Karaouzene N, Merzouk H, Aribi M, et al. Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: a comparison of older with young men. Nutr Metab Cardiovasc Dis. 2011;21:792–9.
Rajappa M, Tagirasa R, Nandeesha H, et al. Synergy of iron, high sensitivity C-reactive protein and ceruloplasmin with oxidative stress in non-diabetic normo-tensive South Indian obese men. Diabetes Metab Syndr. 2013;7:214–7.
Ferretti G, Bacchetti T, Masciangelo S, et al. HDL-paraoxonase and membrane lipid peroxidation: a comparison between healthy and obese subjects. Obesity (Silver Spring). 2010;18:1079–84.
Li Y, Mouche S, Sajic T, et al. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int J Obes (Lond). 2012;36:1503–13.
Hermsdorff HH, Barbosa KB, Volp AC, et al. Gender-specific relationships between plasma oxidized low-density lipoprotein cholesterol, total antioxidant capacity, and central adiposity indicators. Eur J Prev Cardiol. 2014;21:884–91.
Sen S, Iyer C, Meydani SN. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol. 2014;34:105–11.
Malti N, Merzouk H, Merzouk SA, et al. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–6.
Murri M, Luque-Ramírez M, Insenser M, et al. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–88.
Bełtowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol. 2012;39:168–78.
Jones DA, Prior SL, Barry JD, et al. Changes in markers of oxidative stress and DNA damage in human visceral adipose tissue from subjects with obesity and type 2 diabetes. Diabetes Res Clin Pract. 2014;106:627–33.
Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36.
Santilli F, Guagnano MT, Vazzana N, et al. Oxidative stress drivers and modulators in obesity and cardiovascular disease: from biomarkers to therapeutic approach. Curr Med Chem. 2015;22:582–95.
Xue P, Hou Y, Chen Y, et al. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes. 2013;62:845–54.
Hierro C, Monte MJ, Lozano E, et al. Liver metabolic/oxidative stress induces hepatic and extrahepatic changes in the expression of the vitamin C transporters SVCT1 and SVCT2. Eur J Nutr. 2014;53:401–12.
Yuzefovych LV, Musiyenko SI, Wilson GL, et al. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059.
Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–67.
Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;23:391–8.
Amati F. Revisiting the diacylglycerol-induced insulin resistance hypothesis. Obes Rev. 2012;13:40–50.
Diaz-Meco MT, Moscat J. The atypical PKCs in inflammation: NF-kB and beyond. Immunol Rev. 2012;246:154–67.
Piperi C, Adamopoulos C, Dalagiorgou G, et al. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012;97:2231–42.
Boldin MP, Baltimore D. MicroRNAs, new effectors and regulators of NF-kB. Immunol Rev. 2012;246:205–20.
Williams MD, Mitchell GM. MicroRNAs in insulin resistance and obesity. Exp Diabetes Res. 2012;2012:484696.
Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25:2515–27.
Kang YS. Obesity associated hypertension: new insights into mechanism. Electrolyte Blood Press. 2013;11:46–52.
Ceci R, Sabatini S, Duranti G, et al. Acute, but not chronic, leptin treatment induces acyl-CoA oxidase in C2C12 myotubes. Eur J Nutr. 2007;46:364–8.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Rupérez AI, Gil A, Aguilera CM. Genetics of oxidative stress in obesity. Int J Mol Sci. 2014;15:3118–44.
Weng SW, Lin TK, Wang PW, et al. Single nucleotide polymorphisms in the mitochondrial control region are associated with metabolic phenotypes and oxidative stress. Gene. 2013;531:370–6.
Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61.
Donadelli M, Dando I, Fiorini C, et al. UCP2, a mitochondrial protein regulated at multiple levels. Cell Mol Life Sci. 2014;71:1171–90.
Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–41.
Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69.
Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–20.
Chetboun M, Abitbol G, Rozenberg K, et al. Maintenance of redox state and pancreatic beta-cell function: role of leptin and adiponectin. J Cell Biochem. 2012;113:1966–76.
Frohnert BI, Long EK, Hahn WS, Bernlohr DA. Glutathionylated lipid aldehydes are products of adipocyte oxidative stress and activators of macrophage inflammation. Diabetes. 2014;63:89–100.
Diaz-Ruiz A, Guzman Ruiz R, Moreno Castellanos N, et al. Proteasome dysfunction associated to oxidative stress and proteotoxicity in adipocytes compromise insulin sensitivity in human obesity. Antioxid Redox Signal. 2015;00:1–16. doi: 10.1089/ars.2014.5939.
Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–92.
Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43.
Donmez-Altuntas H, Sahin F, Bayram F, et al. Evaluation of chromosomal damage, cytostasis, cytotoxicity, oxidative DNA damage and their association with body-mass index in obese subjects. Mutat Res Genet Toxicol Environ Mutagen. 2014;771:30–6.
Cerdá C, Sánchez C, Climent B, et al. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv Exp Med Biol. 2014;824:5–17.
Booth A, Magnuson A, Fouts J, et al. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21:57–74.
Lee SD, Ju G, Choi JA, et al. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath. 2012;16:511–7.
Feairheller DL, Brown MD, Park JY, et al. Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. Med Sci Sports Exerc. 2009;41:1421–8.
De Lemos ET, Oliveira J, Pinheiro JP, et al. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev. 2012;2012:741545.
Oh S, Tanaka K, Warabi E, et al. Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med Sci Sports Exerc. 2013;45:2214–22.
Krause M, Rodrigues-Krause J, O’Hagan C, et al. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur J Appl Physiol. 2014;114:251–60.
Venojärvi M, Korkmaz A, Wasenius N, et al. 12 weeks’ aerobic and resistance training without dietary intervention did not influence oxidative stress but aerobic training decreased atherogenic index in middle-aged men with impaired glucose regulation. Food Chem Toxicol. 2013;61:127–35.
Rahimi RS, Landaverde C. Nonalcoholic fatty liver disease and the metabolic syndrome: clinical implications and treatment. Nutr Clin Pract. 2013;28:40–51.
Pendyala S, Neff LM, Suárez-Fariñas M, et al. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–42.
Montero D, Walther G, Perez-Martin A, et al. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: markers and effect of lifestyle intervention. Obes Rev. 2012;13:441–55.
Tumova E, Sun W, Jones PH, et al. The impact of rapid weight loss on oxidative stress markers and the expression of the metabolic syndrome in obese individuals. J Obes. 2013;2013:729515.
Crujeiraseiras AB, Parra D, Milagro FI, et al. Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low-calorie diet: a nutrigenomics study. OMICS. 2008;12:251–61.
Gallí M, Van Gool F, Leo O. Sirtuins and inflammation: friends or foes? Biochem Pharmacol. 2011;81:569–76.
Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl). 2011;89:667–76.
Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–74.
Agnoli C, Grioni S, Sieri S, et al. Italian mediterranean index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int J Cancer. 2013;132:1404–11.
Samieri C, Okereke OI, Devore E E, et al. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. 2013;143:493–9.
Willcox DC, Willcox BJ, Todoriki H, et al. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;28:500S–16.
van Dijk SJ, Feskens EJ, Bos MB, et al. Consumption of a high monounsaturated fat diet reduces oxidative phosphorylation gene expression in peripheral blood mononuclear cells of abdominally overweight men and women. J Nutr. 2012;142:1219–25.
Ortega-Azorín C, Sorlí JV, Asensio EM, et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc Diabetol. 2012;11:137.
Balakumar P, Taneja G. Fish oil and vascular endothelial protection: bench to bedside. Free Radic Biol Med. 2012;53:271–9.
Sjoberg NJ, Milte CM, Buckley JD, et al. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br J Nutr. 2010;103:243–8.
Rhee Y, Brunt A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: a randomized crossover design. Nutr J. 2011;10:44.
Kusunoki C, Yang L, Yoshizaki T, et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2013;430:225–30.
Rendo-Urteaga T, Puchau B, Chueca M, et al. Total antioxidant capacity and oxidative stress after a 10-week dietary intervention program in obese children. Eur J Pediatr. 2014;173:609–16.
Annuzzi G, Bozzetto L, Costabile G, et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: a randomized controlled trial. Am J Clin Nutr. 2014;99:463–71.
O’Neil CE, Nicklas TA, Rampersaud GC, et al. 100% Orange juice consumption is associated with better diet quality, improved nutrient adequacy, decreased risk for obesity, and improved biomarkers of health in adults. Nutr J. 2012;11:107.
Codoñer-Franch P, López-Jaén AB, De La Mano-Hernández A, et al. Oxidative markers in children with severe obesity following low-calorie diets supplemented with mandarin juice. Acta Paediatr. 2010;99:1841–6.
Ghavipour M, Sotoudeh G, Ghorbani M. Tomato juice consumption improves blood antioxidative biomarkers in overweight and obese females. Clin Nutr. 2014. pii: S0261-5614(14)00265-9. doi:10.1016/j.clnu.2014.10.012.
Dow CA, Wertheim BC, Patil BS, et al. Daily consumption of grapefruit for 6 weeks reduces urine F2-isoprostanes in overweight adults with high baseline values but has no effect on plasma high-sensitivity C-reactive protein or soluble vascular cellular adhesion molecule 1. J Nutr. 2013;143:1586–92.
Gulati S, Misra A, Pandey RM, et al. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. 2014;30:192–7.
Bahadoran Z, Mirmiran P, Hosseinpanah F, et al. Broccoli sprouts reduce oxidative stress in type 2 diabetes: a randomized double-blind clinical trial. Eur J Clin Nutr. 2011;65:972–7.
Potter AS, Foroudi S, Stamatikos A, et al. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr J. 2011;10:96.
Wang Q, Sun Y, Ma A, et al. Effects of vitamin E on plasma lipid status and oxidative stress in Chinese women with metabolic syndrome. Int J Vitam Nutr Res. 2010;80:178–87.
D’Adamo E, Marcovecchio ML, Giannini C, et al. Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E. Free Radic Res. 2013;47:146–53.
Murer SB, Aeberli I, Braegger CP, et al. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr. 2014;144:193–201.
Klein EA, Thompson Jr IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549–56.
Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;10:14–23.
Song Y, Xu Q, Park Y, et al. Chen, H. Multivitamins, individual vitamin and mineral supplements, and risk of diabetes among older U.S. adults. Diabetes Care. 2011;34:108–14.
Juraschek SP, Guallar E, Appel LJ, Miller 3rd ER. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079–88.
Myint PK, Luben RN, Wareham NJ, et al. Association between plasma vitamin C concentrations and blood pressure in the European prospective investigation into cancer-Norfolk population-based study. Hypertension. 2011;58:372–9.
Pfister R, Sharp SJ, Luben R, et al. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition-Norfolk prospective study. Am Heart J. 2011;162:246–53.
Suzuki K, Inoue T, Hioki R, et al. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin Nutr. 2006;25:780–9.
Hozawa A, Jacobs Jr DR, Steffes MW, et al. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53:447–55.
Hozawa A, Jacobs Jr DR, Steffes MW, et al. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Hypertens. 2009;27:237–42.
Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57.
Pilar Valdecantos M, Prieto-Hontoria PL, Pardo V, et al. Essential role of NRF2 in the protective effect of lipoic acid against lipoapoptosis in hepatocytes. Free Radic Biol Med. 2015. pii: S0891-5849(15)00143-4. doi:10.1016/j.freeradbiomed.2015.03.019.
Gianturco V, Bellomo A, D’Ottavio E, et al. Impact of therapy with alpha-lipoic acid (ALA) on the oxidative stress in the controlled NIDDM: a possible preventive way against the organ dysfunction? Arch Gerontol Geriatr. 2009;49:129–33.
Yan W, Li N, Hu X, et al. Effect of oral ALA supplementation on oxidative stress and insulin sensitivity among overweight/obese adults: a double-blinded, randomized, controlled, cross-over intervention trial. Int J Cardiol. 2013;167:602–3.
Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, et al. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity (Silver Spring). 2015;23:313–21.
Fernández-Galilea M, Pérez-Matute P, Prieto-Hontoria PL, et al. α-Lipoic acid treatment increases mitochondrial biogenesis and promotes beige adipose features in subcutaneous adipocytes from overweight/obese subjects. Biochim Biophys Acta. 2015;1851:273–81.
Baret P, Septembre-Malaterre A, Rigoulet M, et al. Dietary polyphenols preconditioning protects 3T3-L1 preadipocytes from mitochondrial alterations induced by oxidative stress. Int J Biochem Cell Biol. 2013;45:167–74.
Leiherer A, Mündlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol. 2013;58:3–20.
Tomé-Carneiro J, Gonzálvez M, Larrosa M, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48.
Nagao T, Meguro S, Hase T, et al. Catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring). 2009;17:310–7.
Bogdanski P, Suliburska J, Szulinska M, et al. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–7.
Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013;39:101–21.
Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698:31–8.
Hurt RT, Wilson T. Geriatric obesity: evaluating the evidence for the use of flavonoids to promote weight loss. J Nutr Gerontol Geriatr. 2012;31:269–89.
Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24:220–5.
Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.
Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12:272–81.
Kullisaar T, Songisepp E, Mikelsaar M, et al. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr. 2003;90:449–56.
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–43.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Savini, I., Gasperi, V., Catani, M.V. (2016). Oxidative Stress and Obesity. In: Ahmad, S., Imam, S. (eds) Obesity. Springer, Cham. https://doi.org/10.1007/978-3-319-19821-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-19821-7_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19820-0
Online ISBN: 978-3-319-19821-7
eBook Packages: MedicineMedicine (R0)