Abstract
This chapter focuses on hospital-acquired pneumonias (HAP) which are the second most common nosocomial infections after urinary tract infections, but account for the majority of deaths and costs due to hospital-acquired infections. Highlights of the chapter include the changing epidemiology, pathogenesis, and treatment of HAP, including ventilator-associated pneumonia (VAP), and ventilator-associated tracheobronchitis (VAT). Our primary focus is on the common bacterial pathogens causing HAP and VAP in immunocompetent adults and multidrug-resistant pathogens that have emerged over the past decade. We emphasize evidence-based clinical management and current emerging prevention strategies aimed at improving patient outcomes, decreasing health expenditures and reducing chronic impairments following critical illness.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hospital-acquired pneumonia
- Ventilator-associated pneumonia
- Epidemiology
- Diagnosis
- Surveillance definitions
- Multidrug-resistant pathogens
- Antibiotic treatment
- Prevention strategies
- Outcomes
Introduction
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) cover pneumonias occurring in hospital setting. HAP requires at least 48 h of hospital stay and condition must not be present on admission. VAP refers to pneumonia arising 48 h or more after endotracheal intubation and mechanical ventilation. Healthcare-associated pneumonia (HCAP) is part of the continuum of pneumonia, which includes patients who were hospitalized in an acute-care hospital for ≥2 days within 90 days of the infection; resided in a long-term care facility; received recent intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days of the current infection; or attended a hospital or hemodialysis clinic [1, 2]. Importantly, pathogens in HCAP are often multidrug resistant and more closely resembles HAP and VAP rather than community acquired pneumonia (CAP) [3]. HAP is the second most common nosocomial infections after urinary tract infections (UTIs), but it is the leading cause of mortality due to hospital-acquired infections [4, 5].
This chapter highlights the changing epidemiology, pathogenesis, and treatment of HAP and VAP. Our primary focus is on bacterial pathogens causing HAP in immunocompetent adults with emphasis on evidence-based patient management and prevention strategies to improve patient outcomes.
Epidemiology
Each year there are five to ten episodes of HAP per 1000 hospital admissions, which vary by hospital size and type [1, 2, 6]. HAP accounts for 15 % of all healthcare-associated infections and approximately 25 % of all intensive care unit (ICU) infections. Rates of HAP tend to be higher in university versus non-teaching hospitals. In the ICU, almost 90 % of HAP occurs in mechanically ventilated patients [2]. Previous VAP rates in the Centers for Disease Control and Prevention’s (CDC) National Nosocomial Infections Surveillance (NNIS) system varied by the type of ICU with a pooled mean of 4.9/1000 ventilator days for medical versus 9.3 for surgical ICUs [7].
Crude and attributable mortality rates for HAP and VAP vary by patient population and method of diagnosis [2, 6, 8]. In two major studies of VAP, the mortality rate varied between 4 % in patients without prior antibiotic exposure to 73 % in those with VAP due to multidrug-resistant (MDR) pathogens (e.g., Pseudomonas aeruginosa or Acinetobacter baumannii), and attributable mortality ranged from 6 to 14 % [9].
Several studies have demonstrated that rates of VAP increase with the duration of mechanical ventilation and attack rates have been estimated to be approximately 3 %/ day during the first 5 days, 2 %/day during days 5–10, and 1 %/day thereafter [10]. Early-onset HAP and VAP occur within first 4 days of hospital stay. Late-onset HAP and VAP, defined as pneumonia occurring ≥5 days of hospital stay, portends worse outcome since it is mostly caused by MDR pathogens [2]. An average episode of VAP increased hospitalization by 12 days, mechanical ventilation by 10 days, ICU stay by 6 days, and mean hospital charges by $40,000 per patient [8, 11].
Pathogenesis
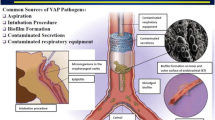
Pathogenesis of HAP involves the direct interaction between the pathogen(s) and the host (Fig. 30.1). Variables which play a role in the pathogenesis of pneumonia include medications, type of nutrition, airway instrumentation, and patients’ position [1, 2, 6]. Microorganisms causing HAP and VAP may originate either from the host’s endogenous flora or from the environment that includes hands or apparel of the hospital staff, visitors, and contaminated surfaces or devices.
Microaspiration in non-ventilated patients and leakage of bacteria around the endotracheal tube cuff in intubated patients are the primary route of bacterial entry into the lower respiratory tract (Fig. 30.2) [1, 2]. Patients who are sedated, postoperative, trauma, or have abnormal swallowing are at higher risk [1, 2]. The gastric cavity, which is sterile under normal circumstances, may become an important reservoir of pathogens causing VAP in patients receiving antacids, histamine type 2 receptor antagonists (H2RA), and proton pump inhibitors (PPI) for stress bleeding prophylaxis. Supine position and the presence of a nasogastric tube further promote orogastric reflux of colonized gastric contents [1, 12–15].
An intubated patient with oropharyngeal colonization . Subglottic secretions pooled above the endotracheal tube (ETT) cuff may leak around the cuff or be introduced directly into the trachea, resulting in either colonization. Depending on the level of bacterial colonization, using semiquantitative samples of endotracheal aspirates (SQ-ETA) or quantitative-ETA, a diagnosis of ventilator-associated tracheobronchitis (VAT) and ventilator-associated pneumonia (VAP) can be made. Quantitative diagnostic sampling of the alveolar space by bronchoscopic or non-bronchoscopic, bronchoalveolar lavage (BAL), or protected specimen brush (PSB) may also be used to diagnose VAP
In the mechanically ventilated patient, inhalation of contaminated bacterial aerosols, or tubing condensate, are also possible routes for bacterial entry into the lower respiratory tract (Fig. 30.2) [16, 17]. Local trauma and inflammation from the endotracheal tube (ETT) increase tracheal colonization and reduce clearance of organisms and secretions from the lower respiratory tract. Prolonged intubation may be complicated by the formation of a biofilm, or a thin layer of polymer-encased bacteria on the inner surface of the ETT (Fig. 30.3). Biofilm may be embolized into the lower airway following suctioning or bronchoscopy [18].
The pathogenesis of lower respiratory tract infections often begins with tracheal colonization with rare or few colonies (+ or ++) of pathogen in semiquantitative (SQ) endotracheal aspirate (ETA) cultures which may progress to heavy colonization characterized by moderate or heavy growth of pathogen (+++ or ++++) or quantitative (Q) ETA culture with ≥ 10 cfu/ml. If patient with heavy colonization displays clinical signs of infection such as fever, leukocytosis, and/or purulent sputum, infection in form of VAT (ventilator-associated tracheobronchitis) or VAP is suspected. Chest radiographs help to discriminate both conditions. While VAT spares lung parenchyma, VAP is defined as a new or progressive infiltrate on the chest X-ray [19, 20].
Immune Defenses in the Lung
The response of pulmonary host defenses to invading microorganisms plays an integral part in the pathogenesis and outcome of infection (Fig. 30.1) [2, 6, 21, 22]. Mucociliary and mechanical clearances in the upper airway are important defense mechanisms. Bacterial antigens and cytokines that alter the activity and efficacy of ciliary cells in clearing bacteria from the lower airway need further study. The ability of macrophages and polymorphonuclear leukocytes to eliminate bacterial pathogens, and the interaction of these cells with inflammatory cytokines, probably play important roles in the pathogenesis of pneumonia. Cell-mediated immune responses are controlled by a complex array of lipids, peptides, and cytokines, including interleukin-1 and -2; interferons, growth factors, and chemotactic factors are also important. Leukotrienes, complement components, and platelet-activating factor also assist in the inflammatory response and contribute to the pathogenesis of pneumonia.
Etiologic Agents
The spectrum of etiologic agents causing HAP/VAP varies by hospital, type of ICU, and patient population studied (Table 30.1) [1, 2, 6, 8, 23–26]. Prior hospitalization, exposure to chronic care facilities, and antibiotic therapy are important predisposing factors for MDR pathogens [27–30]. Early onset HAP is usually caused by Streptococcus pneumoniae, Moraxella catarrhalis, Haemophilus influenzae, or anaerobic bacteria (Table 30.1). By comparison, late-onset HAP is more commonly caused by MDR Gram-negative bacilli (GNB), such as Klebsiella pneumoniae with extended-spectrum beta-lactamases (ESBL+), A. baumannii, P. aeruginosa, or methicillin-resistant Staphylococcus aureus (MRSA) [31].
GNB have been implicated in more than 60 % of reported episodes of HAP, and S. aureus (often MRSA) accounts for 20–40 % of episodes in the USA [1, 5, 23]. Overall rates of MDR pathogen infections are increasing in the USA and many other countries [5, 32, 33]. Most episodes of pneumonia are caused by more than one species of bacteria [1, 2, 6, 8].
Diagnosis
There is no current gold standard for diagnosis of VAP. Clinical signs and microbiologic criteria, together with radiographic findings, are commonly used for VAP diagnosis. However, portable chest radiographs are often of poor quality and it may be difficult to differentiate VAP from VAT or other pulmonary pathologies, such as atelectases, acute respiratory distress syndrome (ARDS), or pulmonary edema (Fig. 30.4). The use of a computerized tomographic (CT) scan improves imaging but quality of sputum samples for Gram stain and culture are of paramount importance for providing clues to possible pathogens. For mechanically ventilated patients, there has been considerable controversy regarding the use of SQ-ETA or Q-ETA samples versus quantitative cultures obtained from bronchoscopic bronchoalveolar lavage (B-BAL) or protective specimen brush (B-PSB) or non-bronchoscopic BAL/PSB (NB-BAL or NB-PSB) [2].
Clinical Diagnosis
The clinical diagnosis of HAP and VAP is defined as the presence of a new or persistent radiographic infiltrate plus at least two of three clinical features (fever >38 °C, leukocytosis or leukopenia, and purulent secretions). While sensitivity for the presence of pneumonia is increased if only one criterion is used, specificity is reduced, leading to significantly increased use of antibiotics. Requiring all three clinical criteria is too insensitive, resulting in under-prescribing for patients with HAP.
Occasionally, the clinical pulmonary infection score (CPIS) , is used for VAP diagnosis. CPIS is a clinical score of 0–12, based on the following six variables: body temperature, leukocyte count, volume and character of tracheal secretions, arterial oxygenation expressed as PaO2/FiO2 ratio, chest radiographic findings, and results of tracheal aspirate cultures [31]. Each variable may be assigned a score of 0, 1, or 2. When the CPIS score was >6, good correlation was found with the presence of pneumonia [34]. However, numerous studies found CPIS to have limited value to diagnose VAP with low sensitivity and specificity and great interobserver variability [35].
Microbiologic Diagnosis
Microbiologic criteria are of utmost importance for diagnosis and management of pneumonia. Samples obtained by invasive techniques, such as B-BAL, NB-BAL, and B-PSB, are evaluated by Gram stain (smear) and by quantitative cultures. Less invasive suctioning of trachea yields valuable ETAs that are assessed by Gram stain and bacterial culture, either semiquantitative or quantitative. Gram stain provides rapid information about the quality of the specimen and the number and morphology of infecting pathogens. Oral contamination of specimen is reflected by the presence of many squamous epithelial cells. The morphology of the bacteria is a clue to the offending bacteria (i.e., Gram-positive cocci in clusters suggest S. aureus and GNB may suggest Klebsiella spp., E. coli, or P. aeruginosa). Infection is suspected if many polymorphonuclear cells and many bacteria are detected on Gram stain. The presence of many bacteria on Gram stain correlates with a culture >105 bacteria/ml on a Q-ETA. A Gram stain of sputum or ETA without bacteria or inflammatory cells has a strong negative predictive value for HAP or VAP and may suggest another cause for the patient’s fever, leukocytosis, and infiltrate on chest X-ray.
Standardized criteria for the microbiologic diagnosis of VAP exist for invasive techniques represented by B-BAL and NB-BAL (104 cfu/ml), and B-PSB (103 cfu/ml) (Fig. 30.2). Quantitative cultures less than those thresholds suggest colonization or contamination with some exceptions. Assessment of noninvasive ETAs is less standardized. Most laboratories use semiquantitative methods. Moderate (+++) or heavy (++++) bacterial growth is a usual threshold to diagnose HAP or VAP (Fig. 30.2). There is no definitive value to diagnose infection by quantitative techniques and most providers use the threshold of ≥105 cfu/ml (Fig. 30.2). Growth of pathogen/s below this limit is most likely colonization.
A recent Cochrane review compared effect of quantitative versus qualitative cultures of respiratory secretions on outcomes in patients with VAP. Five RCTs (1376 patients) were included in meta-analysis. There was no difference in mortality, duration of ICU stay, duration of mechanical ventilation, and rates of antibiotic change when comparing quantitative and qualitative diagnostic techniques and invasive versus noninvasive strategies in patients with VAP [37]. More recently, some hospital laboratories have transitioned to real-time PCR and mass spectroscopy to identify pathogens and antibiotic sensitivity within hours rather than days [36].
Antimicrobial Management
Current management principles for HAP and VAP summarized in the 2005 American Thoracic Society & Infectious Diseases Society (ATS/IDSA) Guidelines include early, appropriate, and adequately dosed antibiotic therapy, followed by de-escalating antibiotics based on clinical response and available microbiologic data and reducing duration of therapy to 7–8 days in responders [2]. An alternative management strategy has been suggested that focuses on treating VAT before the development of VAP using empiric or targeted antibiotic therapy in a patient with fever, purulent sputum, leukocytosis, plus a Q-ETA with a pathogen(s) growth ≥105 cfu/ml, without evidence of a new infiltrate on chest X-ray or computer tomographic scan. Treatment of VAT has been demonstrated to reduce ventilator days and ICU stay and to prevent progression to VAP [19, 20].
Early, Appropriate, and Adequate Initial Empiric Antibiotic Therapy
As soon as HAP/VAP is suspected, the collection of respiratory samples and the prompt initiation of appropriate antibiotics, in adequate doses, are suggested (Fig. 30.5 and Table 30.2) [2]. It has been shown that the shorter the time between diagnosis and initiation of treatment the better patient outcome [38–41]. Appropriate therapy means that the pathogen is susceptible to the chosen regimen, whereas adequate therapy means that appropriate drugs, with good lung penetration, are given in optimal doses via the correct route. Choosing an initial, appropriate intravenous antibiotic regimen depends on the likelihood of infection with MDR pathogens, such as P. aeruginosa, A. baumannii, ESBL+ K. pneumoniae and other GNB, or MRSA.
Approach to initial antibiotic therapy and management of HAP /VAP. Based in part on Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. American Thoracic Documents. Approved by the ATS Board of Directors and the IDSA Guideline Committee. Am J Respir Crit Care Med. 2005, 2005;17:388–416 [2]
Risk factors for MDR pathogens include prior hospitalization, late-onset infection, prior antibiotic therapy, and chronic dialysis, and are more for residents of chronic care facilities and for immunocompromised patients. Patients without MDR risk factors and early onset HAP or VAP usually can be treated with a more limited spectrum of antibiotics, such as ceftriaxone plus azithromycin, a third- or fourth-generation quinolone (i.e., levofloxacin), or ampicillin–sulbactam (Table 30.2). Broader initial antibiotic therapy is suggested if patients are at risk for MDR pathogens (Table 30.3) [2]. Finally, it is important to use doses of antibiotics that will achieve adequate concentrations in the lung parenchyma, which are outlined in the ATS/IDSA Guideline [2].
Assessing Clinical Response, Cultures, and Antibiotic De-escalation
While initial antibiotic coverage should be liberal and broad enough to cover all suspected pathogens, de-escalation or streamlining antibiotic therapy, based on the patient’s clinical response and microbiologic data, is of critical importance to improve patient outcomes and minimize antibiotic use (Fig. 30.5) [2, 42].
Limiting Duration of Therapy
Effectiveness of short versus prolonged antibiotic administration for HAP in critically ill adults, including patients with VAP was evaluated in the recent Cochrane meta-analysis that included six studies [43]. For patients with VAP, a short 7- to 8-day course of antibiotics compared with prolonged 10–15 days of antibiotics (two studies, n = 431) increased 28-days antibiotic free days (mean difference 4.02; 95 %CI 2.26–5.78) and reduced recurrence of VAP due to MDR organisms (OR 0.44; 95 %CI 0.21–0.95) without adversely affecting other outcomes. However, for cases of VAP due to non-fermenting GNB (NF-GNB) recurrence was greater after short-course therapy (OR 2.18, 95 %CI 1.14–4.16). Based on those results short (7–8 days) course of antibiotics should be sufficient if VAP is not caused by NF-GNB. For VAP due to NF-GNB (e.g., P. aeruginosa) prolonged treatment (12–15 days) may be considered to alleviate risk of recurrence.
Management of Selected MDR Pathogens
MRSA
Vancomycin has been considered the standard therapy for MRSA pneumonia . However, clinical trials and studies from different centers have reported clinical failure rates >40 % with a standard low dose of 1 g every 12 h, which is likely related to inadequate dosing [44–46]. Initial vancomycin dosages are calculated from actual body weight, including for obese patients. Subsequent doses should be adjusted based on actual serum concentrations. Trough serum vancomycin concentrations are the most accurate method for monitoring vancomycin effectiveness and should be obtained just before the next dose at steady-state conditions, which is usually the fourth dose. The target level for trough in HAP is 15–20 mcg/ml. However, this level of trough may not be reached by the recommended dose of 15 mg/kg every 12 h in an adult with normal kidney function and higher loading doses (25 mg/kg) might be necessary [47]. Lower trough levels have been associated with development of resistant strains [48]. Determination of minimum inhibitory concentration (MIC) is important parameter for successful dosing regimen. Vancomycin treatment failure in MRSA infections has been documented with MIC value of 4 mg/ml and recently at 2 mg/ml [48]. The use of continuous vancomycin infusions has not been proved to be more advantageous compared with twice-daily dosing in severe MRSA infections [47, 49]. Vancomycin administration may be associated with dose-independent hypersensitivity reactions. The most common reaction peculiar to vancomycin is red man syndrome manifested as tingling and flushing of head, neck, and upper torso. It has been associated with rapid infusion of the first dose and is likely related to histamine release [50]. It can be prevented by slower administration of vancomycin [47]. The syndrome is treated by discontinuation of infusion and antihistamines [50]. After syndrome dissipates vancomycin can be resumed at a slower infusion rate. Nephrotoxicity has been reported with vancomycin and is more likely to occur in the presence of other nephrotoxic agents such as aminoglycosides. Monitoring of trough levels is recommended in patients with unstable renal function (either deteriorating or rapidly improving) and in patients receiving prolonged course of therapy [47].
In contrast to vancomycin, linezolid 600 mg IV/PO every 12 h is another agent that has been used for the treatment of patients with suspected HAP or VAP due to MRSA. No dose adjustment is needed for abnormal renal function. Four large multicenter trials have demonstrated at least equivalence to vancomycin in the treatment of these patients [51–54]. In the most recent study of patients with nosocomial pneumonia by Wunderink et al., patients randomized to linezolid had a better clinical response, eradication of MRSA and reduced renal toxicity than the vancomycin group, but no difference was found in mortality [53]. Also, linezolid has higher lung penetration as measured by epithelial lining fluid concentration, when compared with vancomycin. Linezolid suppresses S. aureus toxin production and should be used in patients infected with S. aureus isolates having a vancomycin MIC ≥ 2, those with lack of response to vancomycin and patients with renal failure [55, 56]. The presence of renal insufficiency was a significant predictor of vancomycin failure in a multivariate analysis of patients with VAP [51]. There is also concern about increased nephrotoxicity in patients receiving vancomycin and when receiving other nephrotoxic medications, such as aminoglycosides, patient with hemodynamic instability, heart failure (ejection fraction < 30–40 %, and obesity (BMI > 30)) [49, 57, 58].
Other approved new agents for nosocomial MRSA infections include quinupristin/dalfopristin, daptomycin, and tigecycline. Daptomycin is not used to treat MRSA pneumonia since its antimicrobial activity is inhibited by surfactant [59]. Tigecycline has not been approved for HAP/VAP treatment by FDA [60]. Ceftobiprole and dalbavancin also have in vitro activity against MRSA, but are not currently approved for use in the USA [61–64].
Pseudomonas aeruginosa
This pathogen is distinguished by its capacity to develop resistance to all known classes of antibiotics even while the patient is still on therapy. It is unclear if this problem could be avoided with the use of combination therapy [65, 66]. Potential advantage of combination therapy is synergy, prevention of resistance, and adequacy of empiric therapy [67].
A recent meta-analysis of eight retrospective and two prospective studies involving 1239 patients examined combination of antibiotic therapy versus monotherapy in P. aeruginosa bacteremia [68]. Authors found no significant difference in all cause mortality between combination therapy and monotherapy in all patients and also after adjustment for study design and type of therapy.
Only few studies examined combination therapy with monotherapy in P. aeruginosa VAP. Garnacho-Montero et al. compared combination therapy with monotherapy in 183 episodes of monobacterial P. aeruginosa VAP. Rates of appropriate empirical therapy were significantly higher in the combination group versus monotherapy (90.5 % vs. 56.7 %, p < 0.001) and patients treated initially with inappropriate antibiotics had significantly higher mortality (72.5 % vs. 23.1 %, p < 0.05). When monotherapy was compared to combination therapy in the definitive regimen, there was no difference in mortality, length of stay, development of resistance, and recurrences [69]. Also, study by Heyland et al. showed that patients in the combination group were more likely to receive appropriate antibiotics and to achieve microbiologic eradication of infecting organisms but there was no difference in clinical outcomes [70]. Based on those results, it seems appropriate to use combination therapy in critically ill patients when suspicion for Pseudomonas infection is high until antibiotic sensitivity is available.
In a more recent study of confirmed VAP due to P. aeruginosa (PA) Planquette et al. reported predictive factors of treatment failure in 314 ICU patients who had 393 episodes of PA-VAP confirmed by culture [71]. MDR pathogens were defined as resistance to at least two of the following antibiotics: fluoroquinolones (FQ), piperacillin, ceftazidime, imipenem, and colistin. Treatment failure was defined as recurrence of PA-VAP or by death. Factors associated with treatment failure were: age (p < 0.02), at least one chronic illness (p < 0.02), limitation of life support (p = 0.0004), a high SOFA score (p < 0.0001), PA-bacteremia (p < 0.003), and prior treatment with a FQ before the 1st PA-VAP (p < 0.0007). Failure risk decreased in case of VAP treatment that included FQ. Of note is that neither antibiotic resistance profile nor bi-antibiotic therapy decreased the risk of PA-VAP treatment failure.
Acinetobacter Species
The choices of treatment of Acinetobacter species pneumonia are limited because of its native resistance to many classes of antibiotics. Carbapenems, polymyxins, and the sulbactam component of ampicillin–sulbactam are considered the most effective antibiotic classes. Wood and coworkers demonstrated equivalent rates of clinical cure in a population with trauma surgery with ampicillin–sulbactam, compared with imipenem, including patients with imipenem-resistant isolates [72]. The emergence of carbapenem-resistant clones suggests the need for use of optimal doses of carbapenem. In a recent meta-analysis by Chu et al., sulbactam-based therapy seems similarly efficacious to alternative antimicrobial therapy for A. baumannii infections [73].
Polymyxins are significantly nephrotoxic, limiting their widespread intravenous use; there may be some benefit from aerosolized polymyxin [55, 74]. A recent study of colistin versus combination therapy for VAP due to a carbapenem resistant strain of A. baumannii was reported by Aydemir et al. [75]. There were 43 patients randomly assigned to colistin therapy alone versus colistin plus rifampicin. Although clinical, laboratory, radiologic, and microbiologic responses were better in the combination group, these differences were not statistically significant. However, time to microbiologic clearance was significantly shorter (p < 0.03) in the combination group. VAP-related mortality was lower in the combination therapy group versus the colistin therapy group, but not statistically significant (31 % vs. 64 %, p < 0.17).
Gram-Negative Bacteria with Extended-Spectrum Β-lactamase Producers and Stenotrophomonas maltophilia
The hallmark of ESBL-producing Enterobacteriaceae, such as K. pneumoniae, Escherichia coli, and Enterobacter species, is a variable response to cephalosporins , and therefore third- and fourth-generation agents should be avoided as monotherapy when these pathogens are suspected or isolated [76]. Third-generation cephalosporins (e.g., cefotaxime) should not be used for treatment of Enterobacter spp. because of the high frequency of resistance of this pathogen to this therapy [77]. The use of the fourth-generation cephalosporin (e.g., cefepime) is also not recommended [76, 78]. A most reliable empiric choice is a carbapenem, such as imipenem, meropenem, doripenem, or ertapenem [79]. None of the carbapenems are active against Stenotrophomonas maltophilia and these patients should be treated with either fluoroquinolone or trimetoprim-sulfamethoxazole, depending on the antibiotic sensitivity pattern.
Aerosolized and Parenteral Antibiotics
In the past 5 years, there has been increased interest in the use of aerosolized antibiotic therapy for the treatment of VAT and VAP caused by MDR Gram-negative pathogens, such as Acinetobacter species and P. aeruginosa [80]. Several studies have had variable results. However, improved aerosol delivery systems for colistin, polymyxin, aminoglycosides, cephalosporins, and more recently fosfomycin are used in conjunction with intravenous antibiotics to increase the concentration of antibiotics in lung parenchyma. Recent interest in fosfomycin, which has both Gram-negative and Gram-positive activity, has been used in combination with tobramycin to successfully treat chronic endobronchial infections due to P. aeruginosa and MRSA in cystic fibrosis patients. Palmer and coworkers had investigated treatment with aerosolized antibiotics (AA) vs placebo in two double-blind (DB) placebo-controlled randomized trials (RCTs). In the study from 2008, patients treated with AA had decreased rate of VAP and other signs of respiratory infections, and weaning from the ventilator was facilitated. Bacterial resistance and use of systemic antibiotics were also reduced [81]. In the second DB RCT, treatment with AA vs. placebo was associated with eradication of multiple-drug resistant organisms present on admission (p <.001) and decreased CPIS (p =0.0008). Resistance to systemic antibiotics was significantly increased in the placebo group (p =0.03) [82]. Several large multicenter clinical trials for VAP are in progress to assess the impact of combinations of aerosolized antibiotics with intravenous antibiotics for the treatment of VAP due to GNB, in hope of reducing antimicrobial resistance and improving patient outcomes.
Lack of Response to Initial Therapy
In most patients, clinical improvement takes 24–48 h. Therefore, the selected antimicrobial regimen should not be changed during this time unless there is evidence of progressive deterioration.
Possible causes of rapid deterioration or failure to improve include three possibilities.
-
1.
Wrong diagnosis—pulmonary embolism, atelectasis, pulmonary hemorrhage, neoplastic or connective tissue disease, chemical pneumonitis, acute respiratory distress syndrome (ARDS) with diffuse alveolar damage, other source of infection.
-
2.
Wrong antimicrobial therapy—drug-resistant pathogen, inadequate dosing, wrong antimicrobial agent.
-
3.
Wrong pathogen—tuberculosis, fungal or viral infection, opportunistic infection, Legionella infection—or complication of pneumonia (empyema or lung abscess, Clostridium difficile colitis, MDR pathogen, Herpes simplex or Candida albicans or drug fever).
Changes in the CDC Surveillance Definitions for VAP
VAP has become a reportable event. However, institutions differ in surveillance techniques and use different criteria to diagnose VAP. This affects validity of publicly reported hospital acquired infections (HAI) data. To standardize reports, the Centers for Disease Control and Prevention (CDC) convened a multidisciplinary working group and developed a new surveillance definition for Ventilator-associated Events (VAE) that was implemented in 2013. Of note, the VAE definition is not a clinical definition, but serves as a surveillance algorithm that is critical for assessing and implementing better VAP prevention strategies and comparing rates between hospitals. This algorithm applies to different pathologies, infectious or non-infectious in nature, that are associated with increasing oxygen requirements and that are classified as Ventilator-associated Conditions (VAC), Infection-related Ventilator-associated Complications (IVAC), and Possible or Probable VAP.
These new CDC surveillance definitions are also designed to study and identify trends in the population and to be used in internal quality improvement projects. Details are available on the CDC Web site [83].
General Prevention Strategies
Most hospitals are using the Institute for Healthcare Improvement (IHI) bundles to reduce VAP. This quality improvement effort, coupled with other measures regarding reduced reimbursement for healthcare-associated infections, has decreased rates of reported VAP in the USA and Europe.
Staff education is needed for all clinicians and staff who manage HAP and VAP. Zack et al. used successfully a self-study module, in-service teaching programs that were coordinated with ICU staff meetings, along with fact sheets and posters, which were placed in the ICU and respiratory care departments [84]. Rates of VAP dropped nearly 58 %, and the cost savings were estimated to be between $425,606 and >$4,000,000. Babcock et al., using an extension of this program in an Integrated Health Care System, reported a 46 % reduction in VAP over an 18-month period [85]. Staffing in the ICU is important, which is underappreciated, and must be sufficient for patient care and compliance with infection control practices [1, 4, 85–87].
Use of proper isolation techniques and effective infection control practices are cornerstones for prevention of HAP [1, 86, 88]. Infection control programs have repeatedly demonstrated efficacy in reducing infection and colonization due to MDR organisms [1, 4, 89–92]. Unfortunately, staff compliance with proven infection control measures, such as hand hygiene, remains inconsistent in many hospitals. Also, surveillance of ICU infections to identify and quantify endemic and new MDR organisms with timely feedback of data is critical [90, 93–96]. Timely communication of current data among clinical, laboratory, pharmacy, and infection control staff is essential. Organism-specific strategies may need to be complemented by more aggressive eradication methods [97–99].
Studies are beginning to implicate the inanimate environment as an indirect contributor to pathogen acquisition [86]. Special interventions, including targeted environmental sampling and aggressive routine environmental disinfection, and techniques evaluating adequacy of cleaning activities and disinfection have been investigated [100]. Those are especially indicated during outbreaks involving MDR pathogens or organisms that are more resistant to routine cleaning, such as C. difficile.
Antibiotic stewardship programs play an extremely important role in the overall effort to control and reduce healthcare-associated infections, the emergence of MDR pathogens, and to control spiraling healthcare costs [101]. Antibiotic stewardship should be focused, dynamic, and carefully monitored regarding appropriate choice, dose, especially in patients with hepatic failure, renal failure, or hemodialysis in addition to management of specific MDR pathogens [15, 102]. An infectious disease pharmacist in the ICU, or a computerized decision support program to optimize drug regimens and de-escalation of antibiotics has clearly demonstrated decrease in inappropriate antibiotic use [1, 2]. By comparison, antibiotic cycling or rotation programs are more difficult to evaluate because of study design issues [2, 102–105].
Modifiable Risk Factors for HAP and VAP
Risk factors for the development of HAP can be differentiated into modifiable and non-modifiable conditions. Aspiration, the primary route of bacterial entry into the lung, may be modified by following interventions [1, 106–110].
Positioning
Supine patient positioning may facilitate aspiration, which can be decreased by maintaining a semirecumbent patient position. Although maintaining mechanically ventilated and/or enterally fed patients in a 30–45° position continues to be strongly recommended, recent studies have suggested that this may not be practical, at least at the levels currently recommended [1, 2, 111]. A study by van Nieuwenhoven et al. in ventilated patients who were randomly assigned to backrest elevation of 45° versus 10°, demonstrated barriers to implementing this strategy [112]. The targeted level of 45° was not reached for 85 % of the study time and the actual achieved difference was 28° versus 10°, which did not reduce VAP. Leng et al. performed a meta-analysis of five RCTs (427 patients) comparing the effect of different head of bed elevation on the outcomes of mechanical ventilated patients. The risks of developing clinically diagnosed VAP were significantly lower among the patients in 45° semirecumbent angle position compared to the patients in lower position (15.96 % vs. 26.64 %, RR = 0.57, 95 %CI 0.39–0.83, p = 0.003). Sub-analysis of two trials involving 91 patients failed to show benefit of 45° over 25° to 30° angle group on patient outcomes [113].
Modulation of Bacterial Colonization
Two different strategies might be implemented to decrease oral bacterial load: oral care with antiseptics and/or antibiotics and selective decontamination of the digestive tract (SDD).
Oral Care
Oropharyngeal colonization is the primary source of pathogens causing HAP and VAP, and therefore reducing levels of colonization or eliminating potential pathogens is an obvious risk reduction [114–117]. Mori et al. compared rates of VAP in a nonrandomized group compared to historic controls [118]. The oral care consisted of rinsing oral cavity with diluted povidone-iodine and using toothbrush. The incidence of VAP in the oral care group was 3.9 episodes/1000 days versus 10.4/1000 days in the control group. A study by Koeman et al. provides important data from a multicenter, double-blind, RCT investigating oral decontamination with chlorhexidine (CHX) on VAP rates [119]. Subjects were randomized into three groups: 2 % CHX, 2 % CHX + 2 % colistin (COL), and placebo. Compared to the placebo group, the daily risk of VAP was reduced by 65 % in the CHX group (p = 0.012) and 55 % in the CHX-COL group (p = 0.030). CHX/COL provided reduction in oropharyngeal colonization with both, the gram-negative and gram-positive microorganisms, whereas CHX mostly affected gram-positive microorganisms. This impressive result of an inexpensive, nontoxic, topically applied modality warrants further attention, but is difficult to reconcile with the absence of effect on ventilator days, length of stay, or mortality.
Chan et al. performed systematic review and meta-analysis examining oral decontamination for prevention of pneumonia in mechanically ventilated patient. Eleven trials with 3242 patients were included. Among four trials with 1098 patients oral antibiotic decontamination did not significantly reduce the incidence of VAP (RR 0.69, 95 %CI 0.41–1.18). In seven trials with 2144 patients oral application of antiseptics significantly reduced the incidence of VAP (RR 0.56; 95 %CI 0.39–0.81). When the results of all 11 trials were pooled, rates of VAP were significantly lower with either method of oral decontamination [120]. These findings are comparable to those of another meta-analysis of seven trials involving 1650 patients that evaluated the topical CHX application on VAP prevention [121]. Incidence of VAP was significantly reduced with topical CHX application when compared with placebo or standard oral care. The benefit was more pronounced in patients who had undergone cardiac surgery. However, both reviews found that oropharyngeal antiseptics had no impact on mortality or length of stay in the ICU.
Selective Decontamination of the Digestive Tract
Modulation of oropharyngeal colonization by combinations of oral antibiotics, with or without systemic therapy, or selective decontamination of the digestive tract (SDD) is effective in preventing HAP/VAP. The usual protocol consists of short course of systemic antibiotics such as cefotaxime combined with enteral antimicrobials in the form of polymyxin E, tobramycin, and amphotericin B applied to oral cavity in form of paste and suspension into stomach. The goal of SDD is to eradicate oropharyngeal and intestinal carriage of potentially pathogenic microorganisms. Studies investigating SDD differ in methodology, specific regimens used, study populations, and clinical impact [2, 116, 122, 123].
In two meta-analyses and one additional study, decreased mortality was demonstrated in critically ill surgical patients receiving SDD, including both systemic and local prophylactic antibiotics, raising questions about the relative importance of systemic rather than non-absorbed antibiotics [122, 124, 125].
Preventive effects of intravenous antibiotics were evaluated in only one RCT: Administration of cefuroxime for 24 h at the time of intubation reduced the incidence of early onset HAP in patients with closed head injury [126]. The clinical evidence for the efficacy of SDD was reviewed by Kallet and Quinn and in a Cochrane review by Liberati et al. [127, 128]. In the latter study analyzing 33 RCTs and 5697 patients, the frequency of respiratory tract infections was 19 % among treated patients and 40 % among controls using a combination of topical plus systemic antibiotic and 20 % and 31 %, testing the effectiveness of topical antibiotics. The results indicate that four or seven patients need to be treated to prevent one infection, depending on whether combinations of topical and systemic treatment or topical antimicrobial alone were tested. Eighteen patients would need to be treated to prevent one death.
Antimicrobial resistance with use of SDD was reviewed in the recent review by Silvestri and van Saene [129]. Four articles analyzed resistance to GNB as a primary endpoint. SDD reduced GNB resistance and showed that SDD was superior to selective oropharyngeal decontamination and standard care. Despite of those findings selection of highly resistant microorganisms still exists and SDD should be considered only for carefully chosen patients [2, 130].
Endotracheal Tube and Mechanical Ventilation
Several devices have been identified as risk factors for HAP. Many of these devices are used in mechanically ventilated patients and increase the risk of VAP; intervention strategies are summarized in several review articles [2, 131].
Subglottic Secretion Drainage
Endotracheal tubes (ETTs) with subglottic secretion drainage (SSD) are specially designed tubes that allow drainage of accumulated secretions via a separate lumen that opens just above the ETT cuff. Two meta-analyses of the published RCTs compared the use of SSD tubes with standard ETTs on VAP rates and other outcomes. In a meta-analysis by Dezfulian et al., use of SSD tubes showed reduction of VAP by 50 % (RR 0.51; 95 %CI 0.37–0.71), primarily by reducing early-onset VAP, shortened duration of mechanical ventilation by 2 days (95 %CI 1.7–2.3), and ICU stay by 3 days (95 %CI 2.1–3.9). The onset of VAP was delayed by 6.8 days (95 %CI 5.5–8.1) [132]. The recent review by Muscedere et al. demonstrated benefit of SSD in 12 of the 13 included studies [133]. The risk ratio for VAP was 0.55 (95 %CI 0.46–0.66, p < 0.00001). The number needed to treat to prevent one case of VAP was 11. Patients with SSD tubes demonstrated −1.52 ICU days (p < 0.03), −1.08 ventilator days (p < 0.03), and increased time to first episode of VAP by 2.66 days (p < 0.001) when compared with patients treated with conventional ETTs.
Silver-Coated ETT Tubes
Biofilm-encased bacteria on the inner surface of ETT may act as a reservoir of pathogens that can be dislodged to lower airways after instrumentation. Antimicrobial activity of silver in vitro led to the development of a silver-coated tube with silver ions dispersed in polymer. Polymer may enhance antimicrobial activity of silver by blocking bacterial adhesion to the ETT [134]. Large, randomized study of 1509 patients intubated for more than 24 h compared the use of colloidal silver-coated ETT (Bard Pharmaceuticals) to a conventional ETT [134]. The silver-ETT group had a lower incidence of VAP (4.8 % vs. 7.5 %, p = 0.03), with a relative risk reduction of 35.9 % and an absolute reduction of 2.7 %, but did not reduce mortality rates, duration of intubation, ICU stay, or hospital stay. The silver-ETT delayed the onset of VAP, had its greatest effect in patients ventilated for more than 48 h, and was highly active against pathogens, such as P. aeruginosa and MRSA. Cost of the tube and identifying patients that would need prolonged mechanical ventilation have been limits to more widespread use.
Noninvasive Positive Pressure Ventilation
Noninvasive positive pressure ventilation (NPPV) provides ventilatory support without the need for intubation and for earlier removal of the endotracheal tube to reduce complications related to prolonged intubation. NPPV using a face mask is an attractive alternative for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) or acute hypoxemic respiratory failure, and for some immunosuppressed patients with pulmonary infiltrates and respiratory failure [2]. Burns et al. reported significant benefits: decreased mortality (RR 0.41, 95 %CI 0.22–0.76), lower rates of VAP (RR 0.28, 95 %CI 0.90–0.85), decreased length of ICU stay and shorter hospital stays, and lower duration of mechanical support [135]. The impact of NPPV is greater in patients with COPD exacerbations or congestive heart failure than for patients with VAP. Data also indicate that NPPV may not be a good strategy to avoid re-intubation after initial extubation and is recommended for hospitals with staff who are experienced in this technique [136].
Sedation Vacation and Walk to Wean
Efforts to reduce the likelihood of aspiration of oropharyngeal bacteria around the endotracheal tube cuff into the lower respiratory tract include limiting the use of sedative and paralytic agents that depress cough and other host-protective mechanisms, and maintaining endotracheal cuff pressure at >20 cm H2O [137]. Re-intubation should be avoided, if possible, as it increases the risk of VAP [138]. Efforts to reduce acute lung injury by using smaller tidal volumes and lower pressures have been suggested [139].
Other strategies to reduce the duration of mechanical ventilation include improved methods of sedation and the use of protocols to facilitate and accelerate weaning [140]. These interventions clearly are dependent on adequate ICU staffing [141]. Dries et al. compared rates of VAP, ventilator days, and ICU days between patients before and after institution of the ventilator weaning protocol [142]. Protocol driven weaning reduced the ratio of days of mechanical ventilation to total ICU days from 0.47 to 0.33, number of patients failing extubation, and the rates of VAP [142].
Schweickert et al. randomized 128 mechanically ventilated patients to daily interruption of sedative infusions (N = 66) versus sedation directed by the MICU team without this strategy (N = 60) [143]. Daily interrupted sedative infusions reduced the ICU length of stay (6.2 vs. 9.9 days, p < 0.01), duration of mechanical ventilation (4.8 vs. 7.3 days, p < 0.003), and the incidence of complications (13/12 patients vs. 26/19 patients, p < 0.04). In a follow-up study, Schweickert et al. randomized 104 ventilated patients to physical and occupational therapy during periods of daily sedation interruption (“walk to wean”) versus their standard ICU care [144]. Return to independent functional status at hospital discharge occurred in 59 % patients in the intervention group compared with 35 % patients in the control group (OR 2.7, 95 %CI 1.2–6.1; p = 0.02). The “walk-to-wean” patients had significantly reduced delirium days (2 vs. 4, p = 0.03) and more ventilator free days (23.5 days vs. 21.1 days, p = 0.02) during the 28 days follow up period. The intervention group also scored higher on activities of daily living at hospital discharge such, as transfer from bed to chair, using the toilet, eating, bathing, dressing, grooming, and walking.
Sedation vacation and readiness to wean paired with spontaneous breathing trial were evaluated in the Awakening and Breathing Controlled trial [145]. Results demonstrated a significant decrease in ICU and ventilator days in study patients who were managed with a protocol of daily interruption of sedation followed by spontaneous breathing trial (SBT) as compared with the control group. Self-extubation rates were higher in the intervention group, but total reintubation rates were similar in both groups. The patients in the intervention group had less ventilator days and were discharged 4 days earlier from the ICU and the hospital on average. One-year survival rates were higher in the intervention group. For every seven patients treated with the daily awakening plus SBT protocol, one life was saved.
Miscellaneous Strategies
Nutrition
Enteral nutrition has been considered a risk factor for the development of HAP, mainly secondary to the increased risk of aspiration of gastric contents. Parenteral nutrition is associated with a higher risk of intravascular-device-associated infection and complications from central venous catheter insertion, higher costs, and loss of intestinal villous architecture, which may facilitate enteral microbial translocation. Accurate assessment of the patient’s nutritional status and the use of enteral feeding, rather than parenteral nutrition, appear to reduce the risk of HAP [146]. Early initiation of enteral feeding may help maintain the gastrointestinal epithelium and prevent bacterial translocation, but it is not without risk. Enteral feeding protocols have been suggested to reduce complications [4, 147]. Early gastrostomy for enteral feedings has been considered as a strategy to reduce VAP in patients with head injury and stroke.
Use of Probiotics
Several studies have examined the effect of probiotics for reducing VAP. In a randomized, double-blind study, 146 patients were given Lactobacillus rhamnosus GG via nasogastric tube or placebo [148]. Patients receiving the probiotic has significantly reduced rates of VAP (40 % vs. 19 %, p < 0.007). Patients treated with probiotics had significantly less C. difficile-associated diarrhea than patients treated with placebo (18.6 % vs. 5.8 %; p = 0.02), and fewer days of antibiotics prescribed for VAP (8.6 ± 10.3 vs. 5.6 ± 7.8; p = 0.05) and for C. difficile-associated diarrhea (2.1 ± 4.8 vs. 0.5 ± 2.3; p = 0.02). No adverse events related to probiotic administration were identified.
Stress Bleeding Prophylaxis
Randomized trials using different doses and various study populations, have provided controversial results on the benefits of specific stress bleeding prophylaxis agents in relation to the increased risk of VAP and bleeding [13, 149].
An association of acid-suppressive drugs and risk of pneumonia as a primary outcome was systematically reviewed by Eom et al. [150]. Thirty one studies were included in this meta-analysis: 8 observational studies and 23 RCTs. Significant positive association existed between PPIs and risk of pneumonia in observational studies. In the RCTs, use of H2RA was associated with elevated risk of HAP (RR 1.22, 95 %CI 1.01–1.48). Both, the H2RAs and PPIs, demonstrated higher risk of pneumonia within the first 7 days of therapy.
Another meta-analysis comparing efficacy of PPIs and H2RAs for stress-related mucosal disease reported no difference in the rate of pneumonia between patients treated with either agent (OR 1.02, 95 %CI 0.59–1.75) [151].
Concerns have been raised over reports of increased rates of C. difficile infections among persons receiving PPIs [152]. Patients started on PPI for stress bleeding prophylaxis should have them discontinued at discharge, as older patients are at greater risk for 1-year mortality [153].
Transfusion Risk
Multiple studies have identified exposure to allogeneic blood products as a risk factor for postoperative infection and postoperative pneumonia, and the length of time of blood storage as another factor modulating risk [2]. In one prospective randomized control trial, the use of leukocyte-depleted red blood cell transfusions resulted in a lower incidence of postoperative infections and, specifically, a reduced incidence of pneumonia in patients undergoing colorectal surgery [154]. Routine red blood cell transfusion should therefore be conducted with a restricted transfusion trigger policy.
Treatment of VAT to Prevent VAP
Over the past decade there has been interest in patients who were intubated and mechanically ventilated >48 h who developed fever (>38 °C), and leukocytosis >10,000/mm3 without other recognizable cause, plus purulent ETAs with polymorphonuclear leukocytes, and significant bacterial growth in ETAs (SQ culture ≥ +++ or Q culture of a pathogen(s) with ≥105–6 cfu/ml, and no radiologic signs of pneumonia [155]. Several studies demonstrated that VAT patients experienced longer ICU stays and increased mechanical ventilation days, and greater risk of progressing to VAP [19, 156].
Since VAT may be a precursor to VAP, there has been interest in preemptive, targeted antibiotic treatment for VAT to prevent VAP and improved patient outcomes. In the only randomized study, 58 VAT patients were randomly assigned (1:1) to treatment with antibiotics (n = 22) versus no antibiotic (n = 36). The antibiotic-treated group had more mechanical ventilator-free days (12 vs. 2 days, p < 0.001), reduced progression to VAP (13 % vs. 47 %, p < 0.01), and reduced mortality (18 % vs. 47 %, p = 0.05) [19]. In the study by Palmer et al. treatment of patients with VAT with AA significantly decreased the rate of VAP (p =0.007) vs. placebo (p =0.28) [81].
Long-Term Outcomes
Long-term outcomes in patients recovered from VAP have not been systematically studied. However, Unroe and coworkers presented very interesting data regarding the 1-year trajectories of care and resource utilization for survivors of prolonged mechanical ventilation (Fig. 30.6) [157]. This study analyzed outcomes of 126 intensive care unit patients who received long-term ventilation in five ICUs at Duke University (NC, USA).
Summary of outcomes and transitions of care for critically ill and mechanically ventilated patients treated in five intensive care units (ICUs) at Duke Medical Center, Durham, NC. Ninety-nine survivors were followed for 1 year after discharge. Note that there were 150 hospital readmissions, 457 transitions of care, involving long-term care associated facilities (LTAC), rehabilitation facilities (REHAB), skilled nursing facilities (SNF), outside hospitals (Hospital) or Home (adapted from ref. 154 with permission from the American College of Physicians)
At 1 year, 70 patients (56 %) were alive and only 11 (9 %) were independently functioning. Ninety-nine hospital survivors had 150 hospital readmissions and there were numerous transitions among long-term facilities, rehabilitation, home, and hospitals. Only 3 of 54 previously employed patients had returned to work. The total cohort cost was $38.1 million with the estimated cost of $3.5 million per survivor. These data also underscore the importance of primary prevention as well as the critical need for reducing transitions of care by better support and education of informal supports and utilization of effective transitions of care models.
Preventing Readmission
The focus of prevention has been on ICU patients while in the ICU, but these patients are also at increased risk for relapse or reinfection during their rehabilitation. In general, readmissions are common, with 20 % of hospitalized patients readmitted within 30 days and 56 % within a year, but rates vary considerably [158]. In a study of 11,855,702 Medicare beneficiaries who had been discharged from a hospital between 2003 and 2004, 147,185 were re-hospitalized with a diagnosis of pneumonia and pulmonary infections [159]. Efforts should be directed at available risk reduction strategies at discharge, such as routine vaccinations and patient education aimed at health promotion, such as smoking cessation, exercise, and weight control. For any type of readmission, inadequate coordination between the different health care providers and subsequently poor discharge planning have been identified as a major component of care lacking at time of discharge [160].
Conclusions
In spite of the progress in the diagnosis, prevention, and management of HAP/VAP, these diseases still have a significant effect on patient outcomes. Immediate administration of adequate antimicrobials is now considered a critical element in the effort to improve survival for patients with HAP, VAT, and VAP. The choice of the initial antibiotic regimen should be patient oriented and guided by directed staining of respiratory samples. Prior hospitalization, presence of comorbidities, and antibiotic treatments increase the risk for MDR pathogens. Local surveillance data and prior exposure to specific antibiotics (which should be avoided in the initial regimen) help in the choice of the initial antibiotic treatment. Antimicrobial therapy should be adjusted 48–72 h after the onset of pneumonia, based on a combination of quantitative respiratory cultures and resolution assessment. The duration of treatment should also be individualized; however, courses longer than 1 week are rarely justified. Investing in primary prevention can pay great dividends, improve quality of life and reduce morbidity and mortality.
References
Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care—associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1–36.
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Kollef MH, Morrow LE, Baughman RP, Craven DE, McGowan JE Jr, Micek ST, et al. Health care-associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes—proceedings of the HCAP Summit. Clin Infect Dis. 2008;46 Suppl 4:S296–334.
Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32:1396–405.
Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–92.
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903.
Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Pollock DA, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011;39:798–816.
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21.
Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159:1249–56.
Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–40.
Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312–7.
Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, et al. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164:382–8.
Niederman MS, Craven DE. Devising strategies for preventing nosocomial pneumonia—should we ignore the stomach? Clin Infect Dis. 1997;24:320–3.
Bonten MJ, Gaillard CA. Ventilator-associated pneumonia: do the bacteria come from the stomach? Neth J Med. 1995;46:1–3.
Prod’hom G, Leuenberger P, Koerfer J, Blum A, Chiolero R, Schaller MD, et al. Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. A randomized controlled trial. Ann Intern Med. 1994;120:653–62.
Craven DE, Lichtenberg DA, Goularte TA, Make BJ, McCabe WR. Contaminated medication nebulizers in mechanical ventilator circuits. Source of bacterial aerosols. Am J Med. 1984;77:834–8.
Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect. 1996;11:32–53.
Inglis TJ, Lim EW, Lee GS, Cheong KF, Ng KS. Endogenous source of bacteria in tracheal tube and proximal ventilator breathing system in intensive care patients. Br J Anaesth. 1998;80:41–5.
Nseir S, Favory R, Jozefowicz E, Decamps F, Dewavrin F, Brunin G, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care. 2008;12:R62.
Craven DE, Chroneou A, Zias N, Hjalmarson KI. Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135:521–8.
Determann RM, Millo JL, Gibot S, Korevaar JC, Vroom MB, van der Poll T, et al. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med. 2005;31:1495–500.
Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–8.
National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85.
Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–8.
Rello J, Lorente C, Diaz E, Bodi M, Boque C, Sandiumenge A, et al. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest. 2003;124:2239–43.
Craven DE, Grgurich P, Steger Craven K, Balaguera H. Hospital-acquired and ventilator-associated pneumonia (Chapter 32). In: Jarvis WR, editor. Bennett and Brashman’s hospital infections. Philadelphia: Kluwer; 2013. p. 485–500. ISBN-13:978-1451175929; ISBN-10:1451175922.
Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–9.
Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–62.
Craven DE. What is healthcare-associated pneumonia, and how should it be treated? Curr Opin Infect Dis. 2006;19:153–60.
El-Solh AA, Aquilina AT, Dhillon RS, Ramadan F, Nowak P, Davies J. Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med. 2002;166:1038–43.
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–9.
Fridkin SK. Increasing prevalence of antimicrobial resistance in intensive care units. Crit Care Med. 2001;29:N64–8.
Mylotte JM. Nursing home-acquired pneumonia. Clin Infect Dis. 2002;35:1205–11.
Fartoukh M, Maitre B, Honore S, Cerf C, Zahar JR, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168:173–9.
Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51 Suppl 1:S131–5.
Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256.
Berton DC, Kalil AC, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev. 2012;1:CD006482.
Kollef MH, Ward S. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest. 1998;113:412–20.
Clec’h C, Timsit JF, De Lassence A, Azoulay E, Alberti C, Garrouste-Orgeas M, et al. Efficacy of adequate early antibiotic therapy in ventilator-associated pneumonia: influence of disease severity. Intensive Care Med. 2004;30:1327–33.
Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–8.
Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001;27:355–62.
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11.
Pugh R, Grant C, Cooke RPD, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital acquired pneumonia in critically ill adults (Review). Cochrane Database Syst Rev. 2015;(8). Art. No.: CD007577.
Moise PA, Forrest A, Bhavnani SM, Birmingham MC, Schentag JJ. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm. 2000;57 Suppl 2:S4–9.
Fagon J, Patrick H, Haas DW, Torres A, Gibert C, Cheadle WG, et al. Treatment of gram-positive nosocomial pneumonia. Prospective randomized comparison of quinupristin/dalfopristin versus vancomycin. Nosocomial Pneumonia Group. Am J Respir Crit Care Med. 2000;161:753–62.
Malangoni MA, Crafton R, Mocek FC. Pneumonia in the surgical intensive care unit: factors determining successful outcome. Am J Surg. 1994;167:250–5.
Rybak M, Lomaestro B, Rotschafer JC, Moellering Jr R, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98.
Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–7.
Wysocki M, Thomas F, Wolff MA, Pean Y, Ravaud Y, Herman B. Comparison of continuous with discontinuous intravenous infusion of vancomycin in severe MRSA infections. J Antimicrob Chemother. 1995;35:352–4.
Sivagnanam S, Deleu D. Red man syndrome. Crit Care. 2003;7:119–20.
Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–97.
Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32:402–12.
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–9.
Torres A. Antibiotic treatment against methicillin-resistant Staphylococcus aureus hospital- and ventilator-acquired pneumonia: a step forward but the battle continues. Clin Infect Dis. 2012;54:630–2.
Hamer DH. Treatment of nosocomial pneumonia and tracheobronchitis caused by multidrug-resistant Pseudomonas aeruginosa with aerosolized colistin. Am J Respir Crit Care Med. 2000;162:328–30.
Conte Jr JE, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46:1475–80.
Goetz MB, Sayers J. Nephrotoxicity of vancomycin and aminoglycoside therapy separately and in combination. J Antimicrob Chemother. 1993;32(2):325–34.
Elting LS, Rubenstein EB, Kurtin D, Rolston KV, Fangtang J, Martin CG, et al. Mississippi mud in the 1990s: risks and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer. 1998;83:2597–607.
Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149–52.
Maclayton DO, Hall 2nd RG. Pharmacologic treatment options for nosocomial pneumonia involving methicillin-resistant Staphylococcus aureus. Ann Pharmacother. 2007;41:235–44.
Drew RH. Emerging options for treatment of invasive, multidrug-resistant Staphylococcus aureus infections. Pharmacotherapy. 2007;27:227–49.
Bush K, Heep M, Macielag MJ, Noel GJ. Anti-MRSA beta-lactams in development, with a focus on ceftobiprole: the first anti-MRSA beta-lactam to demonstrate clinical efficacy. Expert Opin Investig Drugs. 2007;16:419–29.
Salem AH, Zhanel GG, Ibrahim SA, Noreddin AM. Monte Carlo simulation analysis of ceftobiprole, dalbavancin, daptomycin, tigecycline, linezolid and vancomycin pharmacodynamics against intensive care unit-isolated methicillin-resistant Staphylococcus aureus. Clin Exp Pharmacol Physiol. 2014;41:437–43.
Fink MP, Snydman DR, Niederman MS, Leeper Jr KV, Johnson RH, Heard SO, et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother. 1994;38:547–57.
Cometta A, Baumgartner JD, Lew D, Zimmerli W, Pittet D, Chopart P, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother. 1994;38:1309–13.
Traugott KA, Echevarria K, Maxwell P, Green K, Lewis 2nd JS. Monotherapy or combination therapy? The Pseudomonas aeruginosa conundrum. Pharmacotherapy. 2011;31:598–608.
Hu Y, Li L, Li W, Xu H, He P, Yan X, et al. Combination antibiotic therapy versus monotherapy for Pseudomonas aeruginosa bacteraemia: a meta-analysis of retrospective and prospective studies. Int J Antimicrob Agents. 2013;42:492–6.
Garnacho-Montero J, Sa-Borges M, Sole-Violan J, Barcenilla F, Escoresca-Ortega A, Ochoa M, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888–95.
Heyland DK, Dodek P, Muscedere J, Day A, Cook D. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737–44.
Planquette B, Timsit JF, Misset BY, Schwebel C, Azoulay E, Adrie C, et al. Pseudomonas aeruginosa ventilator-associated pneumonia. predictive factors of treatment failure. Am J Respir Crit Care Med. 2013;188:69–76.
Wood GC, Hanes SD, Croce MA, Fabian TC, Boucher BA. Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of acinetobacter ventilator-associated pneumonia. Clin Infect Dis. 2002;34:1425–30.
Chu ML, Rowe D, Nicholls AC, Pope FM, Prockop DJ. Presence of translatable mRNA for pro alpha 2(I) chains in fibroblasts from a patient with osteogenesis imperfecta whose type I collagen does not contain alpha 2(I) chains. Coll Relat Res. 1984;4:389–94.
Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar AE, Garcia-Garmendia JL, Bernabeu-Wittel IM, et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111–8.
Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect. 2013;141:1214–22.
Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39:2206–12.
Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–90.
Queenan AM, Foleno B, Gownley C, Wira E, Bush K. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J Clin Microbiol. 2004;42:269–75.
Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–7.
Kollef MH, Hamilton CW, Montgomery AB. Aerosolized antibiotics: do they add to the treatment of pneumonia? Curr Opin Infect Dis. 2013;26:538–44.
Palmer LB et al. Crit Care Med. 2008;36:2008–13.
Palmer LB, Smaldone GC. Am J Respir Crit Care Med. 2014;189:1225–33.
Zack JE, Garrison T, Trovillion E, Clinkscale D, Coopersmith CM, Fraser VJ, et al. Effect of an education program aimed at reducing the occurrence of ventilator-associated pneumonia. Crit Care Med. 2002;30:2407–12.
Babcock HM, Zack JE, Garrison T, Trovillion E, Kollef MH, Fraser VJ. Ventilator-associated pneumonia in a multi-hospital system: differences in microbiology by location. Infect Control Hosp Epidemiol. 2003;24:853–8.
Crnich CJ, Safdar N, Maki DG. The role of the intensive care unit environment in the pathogenesis and prevention of ventilator-associated pneumonia. Respir Care. 2005;50:813–36.
Dang D, Johantgen ME, Pronovost PJ, Jenckes MW, Bass EB. Postoperative complications: does intensive care unit staff nursing make a difference? Heart Lung. 2002;31:219–28.
Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005;50:725–39.
Bonten MJ, Weinstein RA. Infection control in intensive care units and prevention of ventilator-associated pneumonia. Semin Respir Infect. 2000;15:327–35.
Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059–93.
Rosenthal VD, Guzman S, Crnich C. Impact of an infection control program on rates of ventilator-associated pneumonia in intensive care units in 2 Argentinean hospitals. Am J Infect Control. 2006;34:58–63.
Crnich CJ, Proctor RA. Ventilator-associated pneumonia: does surveillance have a role in its management? Crit Care Med. 2003;31:2411–2.
Eggimann P, Hugonnet S, Sax H, Touveneau S, Chevrolet JC, Pittet D. Ventilator-associated pneumonia: caveats for benchmarking. Intensive Care Med. 2003;29:2086–9.
Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med. 2001;29:1109–15.
L'Heriteau F, Alberti C, Cohen Y, Troche G, Moine P, Timsit JF. Nosocomial infection and multidrug-resistant bacteria surveillance in intensive care units: a survey in France. Infect Control Hosp Epidemiol. 2005;26:13–20.
Vandenbroucke-Grauls C, Schultsz C. Surveillance in infection control: are we making progress? Curr Opin Infect Dis. 2002;15:415–9.
Muto CA. Methicillin-resistant Staphylococcus aureus control: we didn’t start the fire, but it’s time to put it out. Infect Control Hosp Epidemiol. 2006;27:111–5.
de Lassence A, Hidri N, Timsit JF, Joly-Guillou ML, Thiery G, Boyer A, et al. Control and outcome of a large outbreak of colonization and infection with glycopeptide-intermediate Staphylococcus aureus in an intensive care unit. Clin Infect Dis. 2006;42:170–8.
Vos MC, Ott A, Verbrugh HA. Successful search-and-destroy policy for methicillin-resistant Staphylococcus aureus in The Netherlands. J Clin Microbiol. 2005;43:2034.
Carling PC, Briggs JL, Perkins J, Highlander D. Improved cleaning of patient rooms using a new targeting method. Clin Infect Dis. 2006;42:385–8.
Madaras-Kelly KJ, Remington RE, Lewis PG, Stevens DL. Evaluation of an intervention designed to decrease the rate of nosocomial methicillin-resistant Staphylococcus aureus infection by encouraging decreased fluoroquinolone use. Infect Control Hosp Epidemiol. 2006;27:155–69.
Rahal JJ, Urban C, Segal-Maurer S. Nosocomial antibiotic resistance in multiple gram-negative species: experience at one hospital with squeezing the resistance balloon at multiple sites. Clin Infect Dis. 2002;34:499–503.
Warren DK, Hill HA, Merz LR, Kollef MH, Hayden MK, Fraser VJ, et al. Cycling empirical antimicrobial agents to prevent emergence of antimicrobial-resistant Gram-negative bacteria among intensive care unit patients. Crit Care Med. 2004;32:2450–6.
Isakow W, Kollef MH. Preventing ventilator-associated pneumonia: an evidence-based approach of modifiable risk factors. Semin Respir Crit Care Med. 2006;27:5–17.
Kollef MH, Vlasnik J, Sharpless L, Pasque C, Murphy D, Fraser V. Scheduled change of antibiotic classes: a strategy to decrease the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997;156:1040–8.
Parker CM, Heyland DK. Aspiration and the risk of ventilator-associated pneumonia. Nutr Clin Pract. 2004;19:597–609.
Pneumatikos J, Koulouras B, Frangides C, Goe D, Nakos G. Cisapride decreases gastric content aspiration in mechanically ventilated patients. Crit Care. 1999;3:39–43.
Cook D, Mandell L. Endotracheal aspiration in the diagnosis of ventilator-associated pneumonia. Chest. 2000;117:195S–7.
Smith G, Ng A. Gastric reflux and pulmonary aspiration in anaesthesia. Minerva Anestesiol. 2003;69:402–6.
Kallel H, Chelly H, Bahloul M, Ksibi H, Dammak H, Chaari A, et al. The effect of ventilator-associated pneumonia on the prognosis of head trauma patients. J Trauma. 2005;59:705–10.
Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851–8.
van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, Joore HC, van Schijndel RJ, van der Tweel I, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006;34:396–402.
Leng YX, Song YH, Yao ZY, Zhu X. Effect of 45 degree angle semirecumbent position on ventilator-associated pneumonia in mechanical ventilated patients: a meta-analysis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012;24:587–91.
van Nieuwenhoven CA, Buskens E, Bergmans DC, van Tiel FH, Ramsay G, Bonten MJ. Oral decontamination is cost-saving in the prevention of ventilator-associated pneumonia in intensive care units. Crit Care Med. 2004;32:126–30.
Munro CL, Grap MJ. Oral health and care in the intensive care unit: state of the science. Am J Crit Care. 2004;13:25–33.
Brennan MT, Bahrani-Mougeot F, Fox PC, Kennedy TP, Hopkins S, Boucher RC, et al. The role of oral microbial colonization in ventilator-associated pneumonia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:665–72.
Cutler CJ, Davis N. Improving oral care in patients receiving mechanical ventilation. Am J Crit Care. 2005;14:389–94.
Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006;32:230–6.
Koeman M, van der Ven AJ, Hak E, Joore HC, Kaasjager K, de Smet AG, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173:1348–55.
Chan EY, Ruest A, Meade MO, Cook DJ. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ. 2007;334:889.
Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2007;35:595–602.
Liberati A, D’Amico R, Pifferi, Torri V, Brazzi L. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2004;(1):CD000022.
Silvestri L, Petros AJ, Viviani M, Rommes JH, van Saene HK. Selective decontamination of the digestive tract and ventilator-associated pneumonia (part 1). Respir Care. 2006;51:67–9.
Krueger WA, Unertl KE. Selective decontamination of the digestive tract. Curr Opin Crit Care. 2002;8:139–44.
Nathens AB, Marshall JC. Selective decontamination of the digestive tract in surgical patients: a systematic review of the evidence. Arch Surg. 1999;134:170–6.
Sirvent JM, Torres A, El-Ebiary M, Castro P, de Batlle J, Bonet A. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med. 1997;155:1729–34.
Kallet RH, Quinn TE. The gastrointestinal tract and ventilator-associated pneumonia. Respir Care. 2005;50:910–21.
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2009;(4):CD000022.
Silvestri L, van Saene HK. Selective decontamination of the digestive tract: an update of the evidence. HSR Proc Intensive Care Cardiovasc Anesth. 2013;4:21–9.
Kollef MH. Selective digestive decontamination should not be routinely employed. Chest. 2003;123:464S–8.
Hess DR, Kallstrom TJ, Mottram CD, Myers TR, Sorenson HM, Vines DL. Care of the ventilator circuit and its relation to ventilator-associated pneumonia. Respir Care. 2003;48:869–79.
Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118:11–8.
Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011;39:1985–91.
Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805–13.
Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth. 2006;53:305–15.
Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguia C, Gonzalez M, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60.
De Jonghe B, Cook D, Sharshar T, Lefaucheur JP, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Groupe de Reflexion et d’Etude sur les Neuromyopathies En Reanimation. Intensive Care Med. 1998;24:1242–50.
Torres A, Gatell JM, Aznar E, el-Ebiary M, Puig de la Bellacasa J, Gonzalez J, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–41.
Dreyfuss D, Ricard JD. Acute lung injury and bacterial infection. Clin Chest Med. 2005;26:105–12.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7.
Thorens JB, Kaelin RM, Jolliet P, Chevrolet JC. Influence of the quality of nursing on the duration of weaning from mechanical ventilation in patients with chronic obstructive pulmonary disease. Crit Care Med. 1995;23:1807–15.
Dries DJ, McGonigal MD, Malian MS, Bor BJ, Sullivan C. Protocol-driven ventilator weaning reduces use of mechanical ventilation, rate of early reintubation, and ventilator-associated pneumonia. J Trauma. 2004;56:943–51.
Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272–6.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82.
Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–34.
Heyland DK, Drover JW, Dhaliwal R, Greenwood J. Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: role of small bowel feeding. JPEN J Parenter Enteral Nutr. 2002;26:S51–5.
Bowman A, Greiner JE, Doerschug KC, Little SB, Bombei CL, Comried LM. Implementation of an evidence-based feeding protocol and aspiration risk reduction algorithm. Crit Care Nurs Q. 2005;28:324–33.
Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–64.
Cook D, Guyatt G, Marshall J, Leasa D, Fuller H, Hall R, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791–7.
Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183:310–9.
Pongprasobchai S, Kridkratoke S, Nopmaneejumruslers C. Proton pump inhibitors for the prevention of stress-related mucosal disease in critically-ill patients: a meta-analysis. J Med Assoc Thai. 2009;92:632–7.
Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772–8.
Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, Butto V, et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173:518–23.
Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet. 1996;348:841–5.
Nseir S, Ader F, Marquette CH. Nosocomial tracheobronchitis. Curr Opin Infect Dis. 2009;22:148–53.
Craven DE, Lei Y, Ruthazer R, Sarwar A, Hudcova J. Incidence and outcomes of ventilator-associated tracheobronchitis and pneumonia. Am J Med. 2013;126:542–9.
Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–75.
Epstein AM. Revisiting readmissions—changing the incentives for shared accountability. N Engl J Med. 2009;360:1457–9.
Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28.
Bodenheimer T. Coordinating care—a perilous journey through the health care system. N Engl J Med. 2008;358:1064–71.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hudcova, J., Craven, K.A., Craven, D.E. (2016). Pneumonia. In: O'Donnell, J., Nácul, F. (eds) Surgical Intensive Care Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-19668-8_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-19668-8_30
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19667-1
Online ISBN: 978-3-319-19668-8
eBook Packages: MedicineMedicine (R0)