Abstract
Ventilator-associated pneumonias (VAP) are the most common complication in the course of intubated patients and are the leading cause of death in critical care settings worldwide, as well as being the first cause of antibiotic prescription in intensive care units (ICUs). As an important cause of increased morbidity and mortality in hospitalized pediatric patients, healthcare systems are now required to report VAPs, among other healthcare-associated infections (HAIs) through the CDC National Healthcare Safety Network (NHSN). Here we will review the revised 2013 CDC/NHSN definitions and surveillance guidelines for ventilator-associated events (VAE) in adult inpatient locations and examine how these guidelines can be applied or adapted in pediatric patients. Additionally, this chapter will provide an overview of VAPs by reviewing the recent data describing the pathogenesis, diagnosis, prevention, and treatment strategies of VAPs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ventilator-associated pneumonias
- Pediatric ventilator-associated pneumonia
- Ventilator-associated complications
- Ventilator-associated events
- Nosocomial infections
- Healthcare-associated infections
- Mechanical ventilation
- Ventilator-associated tracheobronchitis
Introduction
Despite providing quality care for hospitalized children, nosocomial infections remain a challenge, particularly in critical care settings. Among these infections, hospital-acquired pneumonias, and specifically ventilator-associated pneumonias (VAP), are the most common complication in the course of intubated patients. VAPs are the leading cause of death in critical care settings worldwide, in addition to being the first cause of antibiotic prescription in intensive care units (ICUs). Recent surveys using the National Healthcare Safety Network (NHSN) criteria indicate that in the adult population in 2011, an approximated 157,000 healthcare-associated pneumonias occurred in US hospitals and an estimated 39% of these were VAPs [1]. Additionally, the suspicion of VAPs in pediatric ICU (PICU) patients increases the overall exposure rate to antibiotics by over twofold, and the high rate of prescribed antibiotics for presumed VAP supersedes even that for suspected bloodstream infections [2,3,4], increasing overall PICU length of stay significantly in some studies. Patients with confirmed VAPs experience an increased length of mechanical ventilation by more than 11 days, and a recent multicenter study of pediatric VAP showed a three-fold increase in severity-adjusted PICU mortality [5] as well as additional direct cost of more than $50,000 [6, 7]. Healthcare systems are now required to report VAPs, among other healthcare-associated infections (HAIs) through the Centers for Disease Control and Prevention (CDC) NHSN, focused on quality, infection tracking, and prevention nationwide, as well as for Medicaid and Medicare reimbursement [8].
In the past, there has been great variability in both the surveillance and reporting of VAPs in both the adult and pediatric population, leading to a redefining of VAPs by the CDC in 2012. The new paradigm provided more objective documentation and recognized ventilator-associated complications other than infection. It also redefined the language to describe and classify VAP. In this chapter, these definitions and the newly revised criteria, as well as the pathogenesis, diagnosis, and treatment strategies for VAPs, will be discussed.
VAPs: Defined
Hospital-acquired pneumonias are considered any nosocomial pneumonia, while ventilator-associated pneumonias (VAP) are specific to patients who are mechanically ventilated and are of great consequence to quality patient care in critical care settings. National surveillance for VAP has long been a challenge because of the lack of objective, reliable definitions. The incidence of VAPs is also used as a quality benchmark indicator, though data shows that there is great variability in the accuracy of reporting VAPs due to a lack of objective, reliable definitions [8,9,10]. The standardization of clinical criteria for VAPs was first developed in 2002 by the CDC and National Nosocomial Infections Surveillance (NNIS) to promote consistent diagnosis and reporting. These criteria consisted of radiographic, clinical, and laboratory evidence supporting the diagnosis. However, after several years of data collection, analyses revealed that application of these criteria resulted in poor outcomes with inconsistent and imprecise reporting due to these initial criteria being poorly defined, time-consuming, and subjective in this complicated patient cohort [11,12,13,14,15].
To address these issues, the National Healthcare Safety Network (NHSN) replaced surveillance for VAPs in adult inpatient locations with surveillance for ventilator-associated events (VAE) in January of 2013 [1]. In this updated criterion, patients on mechanical ventilation must meet a minimum threshold of worsening oxygenation as evidenced by increased positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FiO2), must have evidence for infection (fever or abnormal serum leukocyte count), and must be treated with a new antibiotic for 4 or more days. To date, these new criteria have yet to be validated in pediatric populations.
In attempts to include more objective and inclusive guidelines, several new definitions were added to the 2013 NHSN criteria, comprising different “levels” of ventilator-associated events (Fig. 7.1) (https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf):
-
1.
Ventilator-associated conditions (VACs) : occurs on or after 3 days of mechanical ventilation and within 2 days before or after the onset of worsening oxygenation, where the patient has (a) temperature >38 °C or <36 °C or (b) has a white blood cell count ≥12,000 cell/mm3 or ≤4000/mm3 and (c) a new antimicrobial agent(s) added to the patient’s treatment regimen
-
2.
Infection-related VACs (IVACs) : which aim to identify the subset of VAC cases potentially caused by infection through culture or other forms of laboratory identification of infectious organisms
-
3.
Possible VAP (PVAP): which aims to identify the subset of IVAC cases caused by pneumonia
CDC/NHSN ventilator-associated events surveillance algorithm [16]
The current NHSN definition for adults specifically identifies a VAC when, after a period of stability or improvement on the ventilator, a patient has one or both of the following indicators of worsening oxygenation: (1) the minimum daily fraction of inspired oxygen (Fio2) increases at least 0.20 over the daily minimum Fio2 in the preceding two calendar days and the increase is sustained for at least 2 days or (2) the minimum daily positive end-expiratory pressure (PEEP) values increase at least 3 cm H2O over the daily minimum PEEP in the preceding two calendar days and the increase is sustained for at least 2 days [17, 18] (https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf). Defining VAP and its prevalence in the pediatric population is challenging. VAP surveillance using the criteria found in the CDC/NHSN 2014 guidelines excludes neonates and is only available in pediatric inpatient locations where denominator data can be collected (consisting of device days and patient days), including pediatric critical care units and specialty care areas, step-down units, wards, and long-term care units [18].
In September 2012, the CDC convened the Pediatric and Neonatal Ventilator-Associated Event Working Group, comprised of 20 representatives from pediatric, pediatric critical care, neonatal critical care, and infectious disease societies, to evaluate and adapt the new VAE surveillance methods for use in pediatric and neonatal ICUs. While this work is ongoing, the group elucidated important data that pediatric patients with ventilator-associated conditions are at substantially higher risk for mortality and morbidity and concluded that further studies are needed to identify risk factors, etiologies, and preventative measures in the pediatric population.
Importantly, this group identified key differences in adult and pediatric populations and considered 12 alternative definitions for pediatric VAC. All patients evaluated required worsening oxygenation be sustained for at least 2 days after ≥2 days of stability on mechanical ventilation. Two criteria were found to best capture neonatal and pediatric VACs in all ICUs: an increase in minimum daily Fio2 by ≥0.25 and increase in mean airway pressure (MAP) by ≥4 cm H2O for 2 or more days after a period of stability. These criteria helped to identify patients at significantly increased risk for adverse outcomes. The authors modified the adult definition and used MAP instead of positive end-expiratory pressure (PEEP) to define pediatric VAC, since different modes of mechanical ventilation (e.g., high-frequency oscillatory ventilation) are used in pediatric acute respiratory failure, where PEEP is difficult to measure. Additionally, the pediatric ICU population is quite diverse, as is their oxygenation requirements: patients may range from infants with cyanotic heart disease with lower SpO2 goals, to neonates with persistent pulmonary hypertension which may require a higher FIO2 or MAP than other patients [19].
VAP Pathogenesis
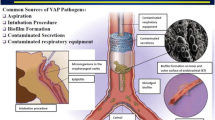
The etiology of nosocomial pneumonias in ICU patients is likely complicated and multifactorial and results from a breakdown in the host immune system on several levels in the mechanically ventilated patient. Traditionally, it was assumed that these infections were likely caused by the direct inoculation of bacteria into a sterile lung via the endotracheal tube or the bloodstream in critically ill patients. It is well accepted that the aspiration of colonized oropharyngeal secretions across the endotracheal tube (ETT) cuff plays a significant role in the pathogenesis of VAP. The ETT and its placement and care are also independent factors contributing to VAP; the ETT is a direct conduit for bacteria to reach the airways and acts as a substrate that allows the adherence of various microorganisms (biofilm).
As VAP infrequently occurs as a direct consequence of bacteremia, the majority of these infections appear to result from aspiration of potential pathogens that have colonized the mucosal surfaces of the oropharyngeal airways. Introduction of a foreign body into the airway of a critically ill patient compromises the natural barrier between the oropharynx and trachea and may facilitate the entry of bacteria into the lung by pooling and leakage of contaminated secretions around the endotracheal tube cuff. Additionally, the longer a patient has been hospitalized, the more risk they are at for colonization of the upper airway with pathogens including gram-negative bacteria and Staphylococcus aureus [20]. Also, according to the gastro-pulmonary hypothesis of colonization, pathogens may translocate from the stomach into the oropharynx, especially in patients in the supine, fully horizontal position or in patients who have aspirated [21].
Newer research suggests that the host lung and airway possess an incredibly complex microbiome, which is altered in mechanically ventilated critically and chronically ill patients. Endogenous airway bacteria maintain a balance between host immune activation and suppression, but shifts in the airway environment likely contribute to VAP. Factors including bacteria, viruses, and critical illness itself may cause this imbalance, leading to a suppression of host immune responses and infectious consequences [22].
Host Risk Factors
Second only to bloodstream infections, VAPs are among the most frequently occurring healthcare-associated infection in PICUs and account for up to 20% of infections in this patient population, increasing length of stay and cost, as well as morbidity and mortality [23, 24].
In a recent case-control study of 600 pediatric ICU patients, Guess et al. found an association between VAEs and several new risk factors in critically ill children. This study found that in univariate analysis acute kidney injury (AKI) (defined as a greater than 50% change in creatinine clearance per pediatric RIFLE {acronym for risk for renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage renal disease} guidelines), increased peak cumulative fluid overload, mean peak inspiratory pressure (PIP), mean airway pressure, using neuromuscular blockade (NMB), and steroids were associated with VAC. Subsequently, following multivariable analyses, the authors concluded that AKI and mean PIP were independent risk factors for VAC and that AKI and NMB infusion were independent risk factors for IVAC [25].
In a separate pediatric retrospective cohort study, researchers identified children with pediatric VACs and matched them to children without VACs (n = 192). This cohort of children was taken from pediatric, cardiac, and neonatal ICUs of six different US hospitals. Similar to other studies, several possible risk factors were identified for pediatric patients, including neuromuscular blockade (odds ratio, 2.29; 95% CI, 1.08–4.87), positive fluid balance (highest quartile compared with the lowest, odds ratio, 7.76; 95% CI, 2.10–28.6), and blood product use (odds ratio, 1.52; 95% CI, 0.70–3.28). Additionally, potential protective factors were identified, including reduced sedation or interruption of sedation (odds ratio, 0.44; 95% CI, 0.18–1.11). In neonatal patients, risk factors included blood product use (odds ratio, 2.99; 95% CI, 1.02–8.78), neuromuscular blockade (odds ratio, 3.96; 95% CI, 0.93–16.9), and recent surgical procedures (odds ratio, 2.19; 95% CI, 0.77–6.28). Weaning or interrupting sedation was also found to be potentially protective (odds ratio, 0.07; 95% CI, 0.01–0.79) in neonates [26]. Additionally, in premature infants, a birth weight less than 750 g has been shown to be an independent risk factor for the development of VAP. Patients with VACs had longer length of stays and incurred higher hospital costs as well [27].
A recent study in PICU patients using the framework for VAEs adapted for critically ill pediatric patients by Cocoros et al. specifically utilized an escalation in mean airway pressure (MAP, ≥4 cm H2O) and a larger increase in inspired oxygen as criteria to identify “ventilator-associated conditions” [17, 28, 29]. Patients were further diagnosed with a pediatric VAP (PVAP) if they had a positive respiratory culture. Two hundred seventy-seven children representing a diverse group of PICU patients who had been initially diagnosed with VAPs were included in the study. This study revealed that few children with a ventilator-associated pneumonia diagnosis met the proposed PVAP criteria (16%, n = 45), with only 18% (n = 49) having any ventilator-associated condition using the proposed revised pediatric guidelines. Failure to fulfill new definitions was based largely on inadequate increase in mean airway pressure in 90% or Fio2 in 92%, suggesting that additional study is needed before new definitions for VAP are introduced for children.
In adults, the incidence of VAP in mechanically ventilated trauma patients has been reported to be as high as 29%, and a subset of these patients (13%) develop bacteremia as well. Perhaps the most significant risk factor for bloodstream infections in this subset of patients has been found to be transfusion with packed red blood cells (pRBCs). Bochicchio et al. demonstrated that blood product transfusion is an independent risk factor for the development of VAP in trauma patients and that this risk increased as more units were transfused [30]. Moreover, all blood products were shown to have an increase in VAPs, including pRBCs, fresh frozen plasma, and platelets.
VAPs have also been associated with inappropriate empiric antimicrobial therapy. In the study done by Kunac et al., 32% of patients with VAP treated with ineffective therapy became bacteremic compared with only 11% of those treated with appropriate initial antimicrobial agents (p < 0.05) [31]. Additional studies in adult ICU patients have demonstrated more than twice the mortality rate in patients who received inappropriate early antibiotic coverage compared to those who received appropriate empiric coverage [32].
Prolonged mechanical ventilation for more than 48 h is perhaps the most important risk factor associated with healthcare-associated pneumonia. However, VAP may occur within the first 48 h after intubation. It is also important to consider the timing of the infection, as “early-onset VAP,” occurring within the first 4 days of ventilation, is typically less severe as compared to “late-onset VAP” and often has different causative agents [33, 34].
VAP is thought to be a common complication in critically ill patients with acute respiratory distress syndrome (ARDS), though the diagnosis of pulmonary infections in patients with ARDS can be difficult, and studies vary on the incidence of VAP in ARDS patients, ranging from 15% to 60% [33, 36,37,38,38]. A common risk factor associated with both ARDS and VAP is hyperoxemia, which is common in critically ill mechanically ventilated patients. Hyperoxemia leads to acute lung injury, inhibition of surfactant production, reduced bacterial clearance in the lung by impairing mucociliary clearance, and the impaired antimicrobial action capacity of macrophages and immune cells, increasing the risk for VAPs [39].
Lastly, additional studies have also evaluated various other risk factors for mechanically ventilated patients, such as patient positioning, endotracheal tube material, and open versus closed suction systems for ventilated patients. A 2016 Cochrane review evaluating semi-recumbent positioning versus supine positioning in mechanically ventilated patients found that a semi-recumbent position of ≥30° may reduce clinically suspected VAP compared to a 0°–10° supine position [40]. Additional risk factors which should be considered in the diagnosis and treatment of VAP include admission from long-term care facilities, patients on dialysis, immunosuppression, gastric acid suppression, recent hospital admissions, and patients with greater severities of illness [42,43,44,44].
Diagnosis
Following CDC/NHSN guidelines, there is a tiered approach to diagnosing patients with ventilator-associated conditions, ranging from VAC to IVAC, to possible ventilator-associated pneumonias (PVAP) (see section “VAPs: Defined”). To differentiate these conditions and accurately diagnose VAPs in mechanically ventilated patients, specific criteria must be met. These diagnostic criteria are detailed in Fig. 7.1.
Next-generation diagnostic tools are up and coming and offer promising advances in both the rapid and specific identification of VAPs. Several studies have also looked at the use of biomarkers in the diagnosis and management of VAPs. The BioVAP study (biomarkers in the diagnosis and management of ventilator-associated pneumonia) is a prospective, multicenter, observational study evaluating the kinetics of procalcitonin (PCT) and C-reactive protein (CRP) in patients with VAP following initiation of antibiotic therapy in order to recognize patients with poor outcome early in their clinical course as well as to identify the individual patterns of CRP and PCT kinetics following antibiotics. This study found that CRP rate of decline significantly differed between survivors and non-survivors (p = 0.026 and p = 0.005, respectively). On day 4 of antibiotic therapy, CRP of survivors was 47% of the initial value, while it was 96% in non-survivors. These data suggest that perhaps C-reactive protein kinetics can be used to identify VAP patients with poor outcome as soon as 4 days after the initiation of treatment [45]. Additionally, multiplex PCR allows for not only pathogen identification (bacterial and non-bacterial) but can also reveal the presence of the most frequently encountered drug-resistance genes. As such, this modality may serve as a useful adjunct in the rapid identification of VAP pathogens . Lastly, exhalome analysis is an up and coming, noninvasive methodology of diagnosing respiratory infections in patients, based on the detection of ethanol in exhaled breath using mass spectrometry, and has shown promising results in diagnosing patients with VAPs [46].
Microbiology
In a study describing pathogen distribution and antimicrobial resistance patterns for healthcare-associated infections (HAIs) reported to the NHSN from pediatric locations during 2011–2014, a total of 1366 VAP pathogens were reported. Among these, 63% were reported from NICUs and 37% from PICUs. S. aureus, Pseudomonas aeruginosa, Klebsiella spp., and Enterobacter spp. were the four most common pathogens in both location types. Streptococcus pneumoniae ranked fifth in PICUs, and E. coli ranked fifth in NICUs [47]. Interestingly, in previous reporting from 2006 to 2008, the most common pathogen was P. aeruginosa (16.1% of 830 reported pathogens) followed closely by S. aureus (15.8%). Additional data from the 2011 to 2014 cohort showed that for pediatric patients with VAPs due to K. pneumoniae/K. oxytoca and P. aeruginosa, resistance was higher overall in PICUs than in NICUs. In PICUs, >10% of K. pneumoniae/K. oxytoca and P. aeruginosa were resistant to carbapenems [47].
In adult populations, Acinetobacter baumannii, P. aeruginosa, and methicillin-resistant S. aureus (MRSA) were the most common causative microorganisms for VAP. Information on the causative organisms of VAP at one’s own institution and their antibiotic susceptibility profiles, along with the regular monitoring of resistance patterns, is important to make effective empiric antibiotic choices. Thus, every hospital should monitor its own microbial flora and rates of resistance to antibiotics [34]. While resistant bacteria may be present even in early-onset VAP, taking length of stay (LOS) into consideration may improve estimates of the presence of resistant bacteria. One study reported that patients admitted for longer than 48 h are more likely to be colonized with Pseudomonas spp. and MRSA [48]. Conversely, Karakuzu et al. found A. baumannii, P. aeruginosa, K. pneumoniae, and MRSA to be the most common bacteria seen in early-onset VAP. Important factors associated with these organisms were patients who had been in the hospital for longer than 48 h before admission to the ICU (and prior to mechanical ventilation) and those having received prior antimicrobial therapy [34]. Chastre et al. also demonstrated that LOS and prior antibiotic treatment were the major risk factors for resistant bacteria in VAP [33]. A summary of the most common bacteria found in VAPs is shown in Table 7.1. Lastly, viral pathogens, including influenza, RSV, adenovirus, human metapneumovirus, and parainfluenza must be considered as common causes of VAP in the pediatric population.
Management
An important determinant of the morbidity and mortality of VAP is the timing of appropriate antibiotic therapy administered for its treatment. Delay in the initiation of empiric antibiotic therapy until culture results are available is associated with negative clinical outcomes [50, 51]. With the increasing development of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) organisms, it is crucial that each organization is aware of their own microbial ecology, antibiotic resistance profiles, and frequency of specific pathogens causing VAP in their ICU patient populations to help tailor empiric antibiotic coverage. Additionally, in choosing empiric antibiotics, it is important to consider certain features present on admission, such as severe hypoxemia, bilateral infiltrates, and presence of pleural effusion, as these factors could point toward a more virulent or resistant pathogen [52].
A recent prospective, observational cohort study conducted in 47 PICUs in the United States, Canada, and Australia evaluated the clinical variables associated with continuing antibiotics after initial evaluation for suspected ventilator-associated infection to determine whether clinical variables or antibiotic treatment influenced outcomes. Positive respiratory cultures were the primary determinant of continued antibiotic treatment in children with suspected VAPs. Positive cultures were, however, not associated with worse outcomes regardless of antibiotic therapy [53].
Antibiotic Therapy and Antimicrobial Resistance
In adults, published guidelines for the diagnosis and management of hospital- and ventilator-acquired pneumonias are available and well delineated by the Infectious Diseases Society of America and the American Thoracic Society [54]. These guidelines include the following recommendations and can be adapted to pediatric patients:
-
All hospitals should regularly summarize and report a local antibiogram, and empiric therapy regimen should be guided by local distribution of pathogens associated with VAP and their antimicrobial susceptibilities.
-
Early, appropriate, broad-spectrum, antibiotic therapy should be prescribed with adequate doses to optimize antimicrobial efficacy.
-
Combination empiric therapy (two antipseudomonal antibiotics from different classes) may be considered in instances of suspected P. aeruginosa pneumonia, in patients in septic shock, those at risk for resistant isolates or until susceptibility data are available.
-
Linezolid is an alternative to vancomycin, and unconfirmed, preliminary data suggest it may have an advantage for proven VAP due to MRSA.
-
Colistin should be considered as therapy for patients with VAP due to a carbapenem-resistant Acinetobacter species.
-
Aerosolized antibiotics may have value as adjunctive therapy in patients with VAP due to some MDR pathogens. For organisms only susceptible to aminoglycosides or colistin, both inhaled and systemic antimicrobials should be considered.
-
Antibiotic therapy should be de-escalated once susceptibility data are available based on the results of lower respiratory tract cultures and the patient’s clincial response.
-
A shorter duration of antibiotic therapy (7 days) is recommended for patients with uncomplicated VAP who have received initially appropriate therapy with good clinical response.
Of note, in the largest meta-analysis to date, a Cochrane review in 2016 found no difference in outcome between monotherapy and combination therapy for the treatment of adult patients with VAP [55].
In choosing appropriate antibiotic coverage in the pediatric ICU, the value of understanding common bacterial infections and resistance patterns cannot be overstated. Typically for ICU patients, S. aureus coverage along with a third-generation cephalosporin, and possibly Pseudomonas coverage, is necessary depending upon the patient population. Risk factors for multidrug resistance in VAP include prior intravenous antibiotic use within 90 days, septic shock at the time of VAP, ARDS preceding the diagnosis of VAP, 5 or more days of hospitalization prior to the occurrence of VAP, and acute renal replacement therapy prior to VAP onset [54]. These factors should be considered in the choice of antimicrobial therapies for critically ill patients.
Duration of Treatment
Meta-analyses demonstrate comparable patient outcomes in those receiving 7 days of antimicrobial therapy versus 10–14 days. Therefore, 7 days of therapy is recommended for most VAP patients, with the caveat that longer course may be necessary for patients who respond slowly. Prior recommendations have suggested longer treatment in patients with VAP secondary to non-lactose fermenting gram-negative bacteria, including Pseudomonas spp. and Acinetobacter spp., because of a perception that there is a higher rate of recurrent VAP if such patients are treated with 7–8 days of therapy [54], which was supported by a 2015 Cochrane review [56]. However, additional meta-analyses evaluating short versus long courses in patients with VAP due to these organisms showed no differences in mortality, recovery, disease recurrence, and duration of mechanical ventilation. Therefore, 7 days of therapy are recommended as the standard for most patients with VAP though, depending upon unique patient responses, longer courses may be appropriate, and clinical judgment is indicated [57].
New Agents
New antibiotics with activity against MRSA such as tedizolid, ceftaroline, and eravacycline represent promising options for the treatment of VAP in patients with risk factors for this organism. Tedizolid, a new oxazolidinone approved by Food and Drug Administration for the treatment of acute bacterial skin and soft tissue infections in adults, demonstrated potent in vitro activity against MRSA and vancomycin-resistant enterococci (VRE), including some linezolid-resistant strains. While still in phase III trials for patients with VAPs, this drug has advantages to linezolid with an improved side effect profile [58]. Ceftaroline is a cephalosporin with activity against MRSA and drug-resistant pneumococci and has been approved to treat community-acquired pneumonia and skin and soft tissue infection in children >2 years and could conceivably have a place in the treatment of MRSA VAP. Additionally, new antimicrobials such as ceftolozane-tazobactam and ceftazidime-avibactam with broad-spectrum activity against MDR gram-negative bacteria will enhance the available armamentarium for VAP. Notably, however a limited number of new compounds have shown activity against MDR Acinetobacter baumannii [59].
Nebulized antibiotics are also advantageous in the treatment of VAPs, with the key advantage of being able to deliver high concentrations of drug directly to the site of infection without the systemic side effects [60]. New inhaled antibiotic options are currently under development for the treatment of VAP including inhaled ciprofloxacin, arbekacin, murepavadin, and amikacin [62,63,63]. As many of these drugs are still in phase II and III trials, it is unclear if/when they will be available for use in the pediatric population.
Prevention
Many evidence-based clinical practice guidelines exist for preventing VAP, although data show only an estimated 50% of patients receive such evidence-based recommended care in hospital settings [64, 65].
VAP bundles
In an effort to reduce the number of VAPs in intensive care units, ventilator “bundles” were developed by the Institute for Healthcare Improvement (IHI). These bundles are defined as a set of evidence-based practices that when each element is executed individually, it improves the patient recovery process and outcomes; when all of the practices are executed together, however, they provide better outcomes than when implemented individually [66]. The ventilator care bundle consists of five interventions: head of bed elevation, daily sedative holidays and assessment of readiness to extubate, peptic ulcer prophylaxis, deep vein thrombosis prophylaxis, and daily oral care with chlorhexidine. Additionally, some institutions have added additional evidence-based interventions to the IHI VAP bundle, creating customized VAP bundles for decreasing VAP rates, and data have shown that the implementation of these tailored VAP bundles results in improved patient outcomes [67, 68]. Additional interventions added to VAP bundles commonly include hand hygiene and endotracheal tube cuff pressure monitoring.
Probiotics
Several published studies have demonstrated that probiotics are safe for patients in the ICU setting and much interest exists in the utilization of probiotics for the prevention of HAIs. Some studies suggest that the use of probiotics is associated with a reduction in the incidence of VAP; however, the quality of the evidence is low. Furthermore, the available evidence is not clear regarding any decrease in ICU or in hospital mortality associated with probiotic use [69].
Respiratory Infections in the Chronically Ventilated Child
When considering the pediatric population most at risk for ventilator-associated conditions, one must also include patients with tracheostomies who are ventilator- dependent due to chronic respiratory failure. This unique population of children is at risk for increased hospital admissions, morbidity, and mortality. In a 2008 study examining 70 hospitalized patients who received tracheostomies at a large tertiary pediatric hospital, 81% of children were discharged home; 63% of these children were readmitted within 6 months, with 11% of those patients requiring four or more admissions [70]. It is estimated that hospitalizations in pediatric patients with pre-existing tracheostomy resulted in an estimated $1.4 billion in hospital charges in 2012 alone [71]. A majority of pediatric patients (up to 90%) with tracheostomies will have respiratory cultures positive for P. aeruginosa at some point in time, with limited oral treatment options, resulting in repeated and often frequent hospitalizations. Likewise, over 70% of chronically ventilated pediatric patients hospitalized with respiratory tract infections receive empiric antibiotics that target P. aeruginosa.
In a study describing the respiratory pathogens in a cohort of pediatric patients with tracheostomies, McCaleb et al. found the most common organism isolated to be P. aeruginosa (90.3%), with gram-negative organisms as a whole predominating. However, 55.9% of the study population also had isolation of MRSA from a respiratory culture, underscoring the often polymicrobial nature of such infections. The organism found to be isolated earliest after tracheostomy placement was methicillin-sensitive S. aureus (MSSA) [72].
Furthermore, it is important to differentiate between ventilator-associated tracheobronchitis (VAT) and ventilator-associated pneumonia (VAP). Key differences include a new and persistent infiltrate on chest radiograph as well as at least moderate bacterial growth in cultures from bronchoscopic bronchoalveolar lavage in VAPs as opposed to VATs [73]. Findings of recent study of VAT by Nseir et al. and a systematic review by Agrafiotis et al. of randomized controlled trials demonstrated that patients treated for VAT were less likely to progress to VAP [74, 75], emphasizing that it is critical to adequately and properly treat identified VATs in tracheostomized children to prevent the progression to worsening disease. Simpson et al. reported 3.4% VAT incidence in pediatric ventilated patients and a significant association between VAT and tracheostomy and between VAT and chronic ventilator dependence. In addition, patients with VAT experienced significantly more ventilator days and a longer ICU length of stay [76].
Conclusions
The definition, pathogenesis, and treatment of VAP in pediatric patients are complex, and VAP remains the most common healthcare-associated infection in critically ill, mechanically ventilated patients. A continued need for improved methods of diagnosis and treatment remains, and surveillance definitions for ventilator-associated pneumonia in children are currently in the process of being updated. A critical piece in the quality of care of these patients includes recognizing those children most at risk and diagnosing these patients early by utilizing the CDC guidelines provided in this chapter. Additionally, the adherence to evidence-based ventilator bundles has been proven to significantly improve outcomes in this patient population, particularly when combined with tailoring appropriate antimicrobial therapy based on cultures and regional antimicrobial resistance profiles. With these interventions and preventative steps, the rate of VAPs can be reduced, and long-term outcomes can be optimized in pediatric patients.
References
Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care- associated infections, 2011. N Engl J Med. 2014;370:1198–208.
Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med. 2000;26(7):959–66.
Fayon MJ, Tucci M, Lacroix J, Farrell CA, Gauthier M, Lafleur L, et al. Nosocomial pneumonia and tracheitis in a pediatric intensive care unit: a prospective study. Am J Respir Crit Care Med. 1997;155:162–9.
Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275(11):866–9.
Gupta S, Boville BM, Blanton R, et al. A multicentered prospective analysis of diagnosis, risk factors, and outcomes associated with pediatric ventilator- associated pneumonia. Pediatr Crit Care Med. 2015;16(3):e65–73.
Bigham MT, Amato R, Bondurrant P, et al. Ventilator-associated pneumonia in the pediatric intensive care unit: Characterizing the problem and implementing a sustainable solution. J Pediatr. 2009;154:582–587.e2.
Brilli RJ, Sparling KW, Lake MR, et al. The business case for preventing ventilator-associated pneumonia in pediatric intensive care unit patients. Jt Comm J Qual Patient Saf. 2008;34:629–38.
Klompas M. Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control. 2010;38:237–9.
Dudeck MA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am J Infect Control. 2013;41:1148–66.
Klompas M, et al. Risk of misleading ventilator-associated pneumonia rates with use of standard clinical and microbiological criteria. Clin Infect Dis. 2008;46:1443–6.
Beyersmann J, Gastmeier P, Grundmann H, et al. Use of multistate models to assess prolongation of intensive care unit stay due to nosocomial infection. Infect Control Hosp Epidemiol. 2006;27(5):493–9.
Safdar N, Dezfulian C, Collard HR, et al. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–93.
Suka M, Yoshida K, Uno H, et al. Incidence and outcomes of ventilator- associated pneumonia in Japanese intensive care units: the Japanese nosocomial infection surveillance system. Infect Control Hosp Epidemiol. 2007;28(3):307–13.
Cordero L, Ayers LW, Miller RR, et al. Surveillance of ventilator-associated pneumonia in very-low-birth-weight infants. Am J Infect Control. 2002;30(1):32–9.
Emori TG, Edwards JR, Culver DH, et al. Accuracy of reporting nosocomial infections in intensive-care-unit patients to the National Nosocomial Infections Surveillance system: a pilot study. Infect Control Hosp Epidemiol. 1998;19(5):308–16.
https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf. Accessed 17 Apr 2018.
Cocoros NM, et al. Ventilator-associated events in neonates and children – a new paradigm. Crit Care Med. 2016;44(1):14–22.
https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf. Accessed 17 Apr 2018.
Mhanna MJ. Ventilator-associated events in neonates and children: a single definition for a heterogeneous population. Crit Care Med. 2016;44(1):233–4.
American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med. 1996;153:1711–25.
Pinciroli R, et al. Respiratory therapy device modifications to prevent ventilator-associated pneumonia. Curr Opin Infect Dis. 2013;26:175–83.
Mourani PM, Sontag MK. Ventilator-associated pneumonia in critically ill children: a new paradigm. Pediatr Clin N Am. 2017;64:1039–56.
Foglia E, Meier MD, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev. 2007;20(3):409–25.
Elward AM. Pediatric ventilator-associated pneumonia. Pediatr Infect Dis J. 2003;22(5):445–6.
Guess R, et al. Risk factors for ventilator-associated events in a PICU. Pediatr Crit Care Med. 2018;19:e7–e13.
Cocoros NM. Factors associated with pediatric ventilator-associated conditions in 6 US hospitals: a nested case-control study. Pediatr Crit Care Med. 2017;18(11):e536–45.
Thatrimontrichai A, et al. Outcomes and risk factors of ventilator-associated pneumonia in neonates. World J Pediatr. 2017;13(4):328–34.
Cocoros NM, Priebe GP, Logan LK, et al. A pediatric approach to ventilator-associated events surveillance. Infect Control Hosp Epidemiol. 2017;38:327–33.
Gionfriddo A, et al. Retrospective application of new pediatric ventilator-associated pneumonia criteria identifies a high-risk population. Pediatr Crit Care Med. 2018;19:507. https://doi.org/10.1097/PCC.0000000000001522.
Bochicchio GV, Napolitano L, Joshi M, et al. Blood product transfusion and ventilator-associated pneumonia in trauma patients. Surg Infect. 2008;9:415–22.
Kunac A, Sifri ZC, Mohr AM, Horng H, Lavery RF, Livingston DH. Bacteremia and ventilator-associated pneumonia: a marker for contemporaneous extra-pulmonic infection. Surg Infect. 2014;15(2):77–83.
Luna CM, et al. Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator-associated pneumonia. Chest. 1999;116:1075–84.
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903.
Karakuzu Z, et al. Prognostic risk factors in ventilator-associated pneumonia. Med Sci Monit. 2018;24:1321–8.
Sutherland KR, Steinberg KP, Maunder RJ, Milberg JA, Allen DL, Hudson LD. Pulmonary infection during the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:550–6.
Chastre J, Trouillet JL, Vuagnat A, Joly-Guillou ML, Clavier H, Dombret MC, Gibert C. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1165–72.
Delclaux C, Roupie E, Blot F, Brochard L, Lemaire F, Brun-Buisson C. Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome: incidence and diagnosis. Am J Respir Crit Care Med. 1997;156:1092–8.
Markowitz P, Wolff M, Djedaini K, Cohen Y, Chastre J, Delclaux C, Merrer J, Herman B, Veber B, Fontaine A, et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med. 2000;161:1942–8.
Six S, et al. Hyperoxemia as a risk factor for ventilator-associated pneumonia. Crit Care. 2016;20:195.
Wang L, et al. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst Rev. 2016;(1):CD009946.
Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for healthcare-associated pneumonia. Arch Intern Med. 2008;168:2205–10.
Aliberti S, Di Pasquale M, Zanaboni AM, et al. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;54:470–8.
Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188:985–95.
Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare- associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57:1373–83.
Povoa P, et al. Biomarkers kinetics in the assessment of ventilator-associated pneumonia response to antibiotics – results from the BioVAP study. J Crit Care. 2017;41:91–7.
Bos LD, Martin-Loeches I, Kastelijn JB, et al. The volatile metabolic fingerprint of ventilator-associated pneumonia. Intensive Care Med. 2014;40:761–2.
Lake JG, et al. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011-2014. Infect Control Hosp Epidemiol. 2018;39:1–11.
Olsen B, Weinstein RA, Nathan C, et al. Epidemiology of endemic Pseudomonas aeruginosa: why infection control efforts have failed. J Infect Dis. 1984;150:808–16.
Oliveira J, et al. Prevention of ventilator-associated pneumonias. Rev Port Pneumol. 2014;20(3):152–61.
Baker AM, Meredith JW, Chang M, et al. Bronchoscopically guided management of ventilator-associated pneumonia in trauma patients. J Bronchology. 2003;10:7–16.
Muscedere JC, et al. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: an important determinant of outcome. J Crit Care. 2012;27:322.e7–322.e14.
Falcone M, Russo A, Giannella M, et al. Individualizing risk of multidrug- && resistant pathogens in community-onset pneumonia. PLoS One. 2015;10:e0119528.
Wilson DF, et al. Pediatric ventilator-associated infections: the ventilator-associated infection study. Pediatr Crit Care Med. 2017;18:e24–34.
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111.
Arthur LE et al. Antibiotics for ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;(10):CD004267.
Pugh R1, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospitalacquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015;8:1–43.
Metersky ML, Kalil AC. New guidelines for nosocomial pneumonia. Curr Opin Pulm Med. 2017;23(3):211–7.
Lodise TP, Drusano GL. Use of pharmacokinetic/pharmacodynamic systems analyses to inform dose selection of tedizolid phosphate. Clin Infect Dis. 2014;58(Suppl 1):S28–34.
Bassetti M, et al. New antibiotics for ventilator-associated pneumonia. Curr Opin Infect Dis. 2018;31:177–86.
Sole-Lleonart C, Rouby JJ, Blot S, et al. Nebulization of antiinfective agents in invasively mechanically ventilated adults: a systematic review and meta analysis. Anesthesiology. 2017;126:890–908.
Niederman MS, Chastre J, Corkery K, et al. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med. 2012;38:263–71.
Kaku N, Morinaga Y, Takeda K, et al. Efficacy and pharmacokinetics of ME1100, a novel optimized formulation of arbekacin for inhalation, com- pared with amikacin in a murine model of ventilator-associated pneumonia caused by Pseudomonas aeruginosa. J Antimicrob Chemother. 2017;72:1123–8.
Falco V, Burgos J, Papiol E, et al. Investigational drugs in phase I and phase II & clinical trials for the treatment of hospital-acquired pneumonia. Expert Opin Investig Drugs. 2016;25:653–65.
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PC, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65.
Marwick C, Davey P. Care bundles: the holy grail of ınfectious risk management in hospital? Curr Opin Infect Dis. 2009;22:364–9.
Institute for Healthcare Improvement. How to guide: prevent ventilator- associated pneumonia. Cambridge, MA. Available from: http://www.ihi.org.
Alcan AO, Korkmaz FD, Uyar M. Prevention of ventilator-associated pneumonia: Use of the care bundle approach. Am J Infect Control. 2016;44:e173–6.
Eom JS, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: a multicenter study. Am J Infect Control. 2014;42:34–7.
Bo L, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014;(10):CD009066.
Graf JM, Montagnino BA, Hueckel R. McPherson ML. Pediatric tracheostomies: a recent experience from one academic center. 2008;9:96–100.
AHRQ. Agency for healthcare research and quality. HCUP KID database 2012.
McCaleb R, et al. Description of respiratory microbiology of children with long-term tracheostomies. Respir Care. 2016;61(4):447–52.
Craven DE, Hudcova J, Rashid J. Antibiotic therapy for ventilator-associated tracheobronchitis: a standard of care to reduce pneumonia, morbidity and costs? Curr Opin Pulm Med. 2015;21(3):250–9.
Nseir S, Martin-Loeches I, Makris D, et al. Impact of appropriate antimicrobial treatment on transition from ventilator-associated tracheobronchitis to ventilator-associated pneumonia. Crit Care. 2014;18:R129.
Agrafiotis M, Siempos II, Falagas ME. Frequency, prevention, outcome and treatment of ventilator-associated tracheobronchitis: systematic review and meta-analysis. Respir Med. 2010;104:325–36.
Simpson VS, Bailey A, Higgerson RA, Christie LM. Ventilator-associated tracheobronchitis in a mixed medical/surgical pediatric ICU. Chest. 2013;144:32–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Arrington, A.S. (2019). Ventilator-Associated Pneumonias. In: McNeil, J., Campbell, J., Crews, J. (eds) Healthcare-Associated Infections in Children. Springer, Cham. https://doi.org/10.1007/978-3-319-98122-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-98122-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98121-5
Online ISBN: 978-3-319-98122-2
eBook Packages: MedicineMedicine (R0)