Abstract
Hydrogen sulfide (H2S) is a biologically active gas that is synthesized naturally by three enzymes, cystathionine γ-lyase (CSE), cystathionine β-synthetase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST). These enzymes are constitutively present in a wide array of biological cells and tissues and their expression can be induced by a number of disease states. It is becoming increasingly clear that H2S is an important mediator of a wide range of cell functions in health and in disease. This review therefore provides an overview of the biochemical and molecular regulation of H2S synthesizing enzymes both in physiological conditions and their modulation in disease states with particular focus on their regulation in asthma, atherosclerosis and diabetes. The importance of small molecule inhibitors in the study of molecular pathways, the current use of common H2S synthesizing enzyme inhibitors and the relevant characteristics of mice in which these enzymes have been genetically deleted will also be summarized. With a greater understanding of the molecular regulation of these enzymes in disease states, as well as the availability of novel small molecules with high specificity targeted towards H2S producing enzymes, the potential to regulate the biological functions of this intriguing gas H2S for therapeutic effect can perhaps be brought one step closer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Research into the biology of hydrogen sulfide (H2S) over the last decade has exponentially increased our understanding of the way in which this gasotransmitter influences physiological and pathophysiological (i.e. disease) processes in a wide range of biological systems. With the characteristic and undoubtedly obnoxious odour of rotten eggs and an early history which appeared to mark H2S as nothing more than a poisonous gas (Beauchamp et al. 1984), it is perhaps surprising that H2S is synthesized endogenously in mammalian cells by both enzymatic and non-enzymatic mechanisms and that, once formed, H2S produces the wide array of biological effects which are now being reported (Li et al. 2011). H2S has therefore followed an interesting ‘development curve’ in the minds of researchers starting as an agent which was of toxicological (sometime devastatingly so) impact, via a gas of interest to pharmacologists to a molecule which now occupies a very much more central position in gas physiology and with growing therapeutic potential.
The last decade has seen multiple advances in our knowledge of the mechanisms by which H2S is synthesized, how these are regulated, the tissue and cellular distribution of H2S synthesizing enzymes in tissues and how this distribution changes in disease. These various areas form the central themes of this present review.
H2S can be produced naturally in the body by at least three enzymes. Cystathionine γ-lyase (CSE), cystathionine β-synthetase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST) are known to be significant producers of endogenous H2S. Collectively they have been termed ‘H2S synthesizing enzymes’. Both CSE and CBS are pyridoxal-5′-phosphate (PLP) dependent and utilize l-cysteine as substrate whilst 3-MST is non-PLP dependent and uses 3-mercaptopyruvate as substrate (Shibuya et al. 2009a; Steegborn et al. 1999). Endogenous H2S can also be produced non-enzymatically. For example, erythrocytes, supplemented with glucose and sulfur and containing electron carriers such as NADH, NADPH and reduced glutathione (GSH), spontaneously react with sulfur to produce H2S (Searcy and Lee 1998). Iron-sulfur cluster containing proteins carrying Fe2S2, Fe3S4 or Fe4S4 clusters are another source of non-enzymatically generated H2S. These include proteins such as ferredoxins and ‘Rieske’ proteins among others (Beinert et al. 1997). In the presence of reducing agents such as glutathione, H2S can also be released from bound sulfur in, for example, persulfides in neurons and astrocytes (Ishigami et al. 2009). Although non-enzymatic routes for H2S generation have been identified, it seems likely that the bulk, if not all, of the H2S which is generated for the purpose of carrying out biological functions in the body is derived enzymatically. This no doubt explains the huge emphasis in recent years in understanding as much as possible about the biochemistry, molecular biology and physiological relevance of the H2S synthesizing enzymes.
The list of reported biological effects of both endogenous and exogenously administered H2S is far reaching and expanding on an almost weekly basis. Numerous molecular targets for this gas have also been identified. The biological effects of H2S are outside the scope of this present review and are dealt with elsewhere in this volume. Rather, we will provide here an overview of the H2S synthesizing enzymes. This will encompass a discussion of the way in which H2S synthesizing enzymes are regulated in health and in disease, as well as a consideration of the limitations of the currently available H2S synthesizing enzyme inhibitors which have been widely used to probe the biological roles of this gas.
2 H2S Synthesizing Enzymes: The Beginnings
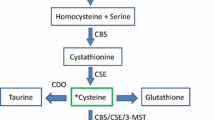
The detection of sulfide in brain tissue in 1989 prompted the suggestion that H2S, produced endogenously, may have a role to play in physiology (Goodwin et al. 1989). The subsequent demonstration that H2S produced by the enzyme CBS in the mammalian brain selectively enhanced NMDA receptor-mediated responses and facilitated the induction of hippocampal long-term potentiation led to the first proposed biological role of this gas as a neuromodulator (Abe and Kimura 1996). Shortly thereafter, the second H2S producing enzyme, CSE, was found to be expressed in guinea pig ileum, rat portal vein and thoracic aorta, and the smooth muscle relaxant effect of H2S was noted for the first time (Hosoki et al. 1997). This seminal work led to a number of detailed investigations which rapidly established the vascular (Zhao et al. 2001a, b) and gastrointestinal (Teague et al. 2002) muscle relaxant properties of this gas. Several years later, the third H2S synthesizing enzyme (3-MST) was discovered when brain homogenates of CBS knockout mice were shown to produce H2S at levels similar to that of wild-type mice in the absence of PLP, suggesting redundancy of CBS and the existence of yet another H2S producing enzyme. This new enzyme, 3-MST, produced H2S from 3-mercaptopyruvate, which is derived from cysteine in the presence of α-ketoglutarate. This latter reaction is catalysed by the enzyme cysteine aminotransferase (CAT), which is identical with aspartate aminotransferase (AAT) (Fig. 1) (Shibuya et al. 2009b).
Pathways for H2S biosynthesis in the cell. The endogenous production of H2S occurs via two main pathways—reverse transsulfuration and cysteine oxidation which take place partly inside mitochondria. 3-MST, 3-mercaptopyruvate sulfurtransferase; CBS, cystathionine β-synthetase; CSE, cystathionine γ-lyase; H2S, hydrogen sulfide; NH3, ammonia
Over recent years, considerable attention has been focused on the biochemistry and molecular biology of the H2S synthesizing enzymes. Much is now known about the mechanics of H2S biosynthesis in terms of substrate specificity, cofactor requirement and tissue and cell distribution, and the molecular topology of these enzymes has also been described. Interested readers are referred to several excellent reviews on this topic (e.g. Kabil and Banerjee 2014; Kabil et al. 2014; Nagahara et al. 2007; Singh and Banerjee 2011). In contrast, there have been few concerted attempts to review changes in the expression or activity of the H2S synthesizing enzymes with disease states and this review will thence, in part, concentrate on this aspect.
3 Endogenous Synthesis of H2S and Regulation of Its Synthesizing Enzymes Under Physiological Conditions
CBS catalyses the formation of cystathionine via two separate pathways. The first pathway involves linking homocysteine with serine (Stipanuk 1986) and the second pathway links homocysteine with cysteine (Chen et al. 2004). H2S is produced only from the second reaction. Besides generating cystathionine, CBS also converts cysteine to serine and lanthionine, and in the process yields H2S. These reactions are part of the reverse transsulfuration pathway of H2S biosynthesis (See Fig. 1).
Although found in many organs including brain, kidney, liver, ileum, uterus, placenta (Patel et al. 2009) and pancreatic islets (Kaneko et al. 2006), mouse tissue-specific quantification of CBS revealed that this enzyme is predominantly expressed in the brain and kidney (Kabil et al. 2011a).
The regulation of CBS activity is dependent on the presence of several cofactors and activators. Besides requiring PLP to function, the NH2 terminal end of CBS contains the binding site of a heme cofactor group that further regulates the activity of CBS by functioning as a redox-sensitive gas sensor (Banerjee and Zou 2005). Because CBS contains iron, the oxidation state of CBS can be toggled, viz. ferric (Fe3+) to ferrous (Fe2+) CBS by reducing agents such as NADPH in the presence of diflavin methionine synthase reductase (Kabil et al. 2011b). In the ferrous (Fe2+) state, CBS can be bound by other gasotransmitters like CO (Puranik et al. 2006) and NO (Taoka and Banerjee 2001), resulting in downregulation of the activity of CBS. Subsequently, the oxidation of the ligand-bound CBS (i.e. CO-CBS or NO-CBS) by air to the ferric (Fe3+) state restores the catalytic activity of CBS. Such a regulatory mechanism suggests a complex interplay between the different gasotransmitters in controlling endogenous H2S biosynthesis at least via the CBS route. This would further imply that in conditions such as inflammation or hypoxia in which cellular NO and CO levels may be either increased or decreased, the precise effect on CBS activity and hence H2S generation by this enzyme will be difficult to predict.
CBS activity is also regulated by allosteric binding of S-adenosylmethionine (SAM) (Finkelstein et al. 1975), which is a co-substrate that is involved in a multitude of other metabolic pathways including transmethylation, transsulfuration and aminopropylation (Cantoni 1952). Using mice in which the MAT1A gene (which encodes for SAM) has been disrupted and human hepatocellular carcinoma cells which have the MAT1A gene silenced, it was demonstrated that the binding of SAM to CBS stabilizes the protein against proteolysis (Prudova et al. 2006). In vitro, SAM increased CBS enzyme activity and thence H2S production (Finkelstein et al. 1975).
CBS activity is not only regulated by cofactors and activators but also by its location within the cell. Using yeast cells as a model system, human CBS has been shown to be post-translationally modified by the small ubiquitin-like modifier-1 (SUMO-1) protein at its C-terminal regulatory domain. SUMOylation enables CBS to be shuttled into the nucleus where it associates with the nuclear scaffold (Kabil et al. 2006). SUMOylation of CBS was enhanced by human polycomb group protein 2 (hPc2). It has been proposed that nuclear localization of CBS occurs when local glutathione demand is high, since CBS generates cysteine, which is the rate-limiting amino acid in glutathione synthesis (Agrawal and Banerjee 2008). Coupled with the observation that regulation of CBS was correlated with the rate of proliferation in human and yeast cells through a redox-sensitive mechanism (Maclean et al. 2002), it is possible that the presence of CBS in the nucleus provides a source of an antioxidant (i.e. H2S) in the cell when, and if, required. This possibility warrants further investigation to determine whether such a mechanism does indeed exist in cells either in vitro or in vivo.
Like CBS, cystathionine γ-lyase (CSE) uses homocysteine as substrate to produce H2S, α-ketobutyrate, ammonia and homolanthionine. CSE can also generate H2S from cysteine, forming cystathionine and pyruvate (See Fig. 1). Although both homocysteine and cysteine are substrates of CSE, at physiological concentrations of cysteine and homocysteine, kinetic simulations employing physiologically relevant concentrations of homocysteine and cysteine using the dithiobisnitrobenzene (DTNB) assay showed that α- and β-elimination of cysteine catalysed by CSE accounts for approximately 70 % of the H2S produced while the α,γ-elimination of homocysteine contributes about 29 %. However, once homocysteine levels are elevated to those apparent in hyperhomocysteinemia, then about 90 % of the H2S generated by CSE occurs via α,γ-elimination and γ-replacement reactions of homocysteine (See Fig. 1) (Chiku et al. 2009). This indicates that the identity of the main substrate for CSE-dependent generation of H2S in cells is likely to depend on the relative availability of cysteine and homocysteine.
CSE is located in a wider range of tissues than CBS. CSE activity is several-fold higher in human brain than in mouse brain (Diwakar and Ravindranath 2007). The mouse small intestine, stomach (Ishii et al. 2004), rat portal vein and thoracic aorta (Hosoki et al. 1997), mouse pancreas (Kaneko et al. 2006), rat uterus, human placenta, myometrium, amnion and chorion (Patel et al. 2009) all express CSE. As compared to CBS, CSE is more abundant in mouse liver where it is the principal enzyme responsible for H2S generation (Kabil et al. 2011a).
Like CBS, the activity of CSE is also regulated by a number of cofactors. CSE was initially reported to be activated physiologically by calcium-calmodulin after stimulation of muscarinic cholinoceptors in vascular endothelial cells in culture, as determined by an increase in CSE activity and H2S production. Through co-immunoprecipitation experiments, recombinant CSE was found to bind directly to calmodulin (Yang et al. 2008). Although Ca2+ has been suggested to activate CSE, the precise concentration of Ca2+ is critical as too much Ca2+ inhibits purified rat CSE-mediated H2S production in the presence of PLP. At physiologically relevant Ca2+ concentrations (i.e. ~100 nM) H2S was efficiently produced in the presence of PLP. However, H2S production was decreased at 300 nM Ca2+ and reduced even further at concentrations up to 3 μM. The involvement of calmodulin in regulating CSE activity however is not yet clear since a recent study showed that neither calmodulin nor a calmodulin antagonist, W-7, affected the production of H2S by purified rat liver CSE (Mikami et al. 2013).
SUMO, besides decreasing CBS activity, also targets CSE in vitro, in that recombinant human CSE can also be SUMOylated (Agrawal and Banerjee 2008). However, whether the SUMOylation of CSE occurs within the cell and has a physiological effect in consequence is not clear. If this is indeed the case then the re-localization of both CBS and CSE into the nucleus may be a strategy to increase cysteine levels inside the nucleus when the demand for glutathione is increased such as during early phases of cell proliferation (Markovic et al. 2007) or when telomerase activity is high as observed in 3T3 fibroblast cells (Borrás et al. 2004).
As an important intracellular antioxidant protecting the cell against oxidative stress, physiological glutathione levels are well controlled via glutathione synthesis in the transsulfuration pathway. Without glutathione, numerous cell types including lymphocytes (Franco and Cidlowski 2009), embryos (Dalton et al. 2004) and hepatocytes (Ghibelli et al. 1999) undergo apoptosis due to excess reactive oxygen species (ROS) generation. Since activation transcription factor 4 (ATF4) controls expression of genes responsible for amino acid metabolism and redox status, ATF4 might, by increasing glutathione synthesis through the transsulfuration pathway, be a key cellular survival factor. Indeed, the absence of ATF4 in mouse embryonic fibroblasts resulted in a substantial fall in glutathione levels due to downregulation of CSE expression (Dickhout et al. 2012). Thus, if the amount of H2S synthesizing enzymes in the nucleus are reduced, the levels of glutathione would subsequently be reduced, thereby affecting the fate of the cell.
The most recently discovered H2S synthesizing enzyme 3-MST, unlike CBS and CSE, is PLP independent. 3-MST catalyses the conversion of 3-mercaptopyruvate to pyruvate by degrading cysteine. In this pathway, cysteine is first converted by cysteine aminotransferase (CAT), also known as aspartate aminotransferase (AAT), to 3-mercaptopyruvate via the incorporation of α-ketoglutarate into the reaction. 3-MST then forms a persulfide by transferring a sulfur from 3-mercaptopyruvate onto itself which in the presence of a reductant like thioredoxin produces pyruvate and H2S (Nagahara et al. 2007; Yadav et al. 2013). Dihydrolipoic acid has also been identified to associate with 3-MST to release H2S (Mikami et al. 2011a).
Like CBS and CSE, 3-MST is found in many tissues with high activity in the proximal tubular epithelium of the kidney, pericentral hepatocytes in the liver, cardiac cells and neuroglial cells (Shibuya et al. 2009b). Within the cell, 3-MST is located both in the cytoplasm as well as the mitochondria. However, because the concentration of cysteine is much higher in the mitochondria, as compared to the cytoplasm, it is plausible that most of the H2S generated by 3-MST occurs in mitochondria (Nagahara et al. 1998).
H2S production by 3-MST can be significantly inhibited by aspartate which is a potent inhibitor of CAT (Akagi 1982). H2S produced by 3-MST and CAT in retinal neurons is also regulated by Ca2+. A negative feedback mechanism has been proposed whereby H2S produced after activation by Ca2+ in turn suppresses Ca2+ influx into photoreceptor cells by activating the vacuolar-type H(+)-ATPase (V-ATPase). The activated V-ATPase then releases protons into the synaptic cleft thereby suppressing Ca2+ channels found in the photoreceptor cells (Mikami et al. 2011b).
Unlike CBS and CSE, the activity of which is regulated for the most part by binding of other factors, 3-MST activity is probably regulated intrinsically by its redox state. Structural studies have shown that 3-MST contains catalytic site cysteines (Cys247, Cys154 and Cys263) each of which is redox active (Nagahara 2013). Cys154 is unique to rat 3-MST whilst Cys263 is conserved in mammalian 3-MST (Nagahara et al. 2007). The activity of 3-MST is regulated by both intermolecular and intramolecular redox-sensing switches as reviewed elsewhere (Nagahara 2013). Nevertheless, it is important to note that the redox-dependent modulation of 3-MST may not be relevant in human MST since the cysteine residues (i.e. Cys154) which are involved in the cross-linking in rat MST are not conserved in the human enzyme (Nagahara et al. 2007).
4 Molecular Regulation of H2S Synthesizing Enzymes
Hydrogen sulfide contributes to cell signalling by regulating the function of a number of molecular targets including ion channels (Sun et al. 2008; Zhao et al. 2001b), kinases (Hu et al. 2007) and transcription factors such as signal transducer and activator of transcription (STAT) (Calvert et al. 2009; Li et al. 2009a) as well as nuclear factor-kappaB (NFκB) (Li et al. 2011; Oh et al. 2006). However, the expression of the enzymes that are responsible for producing H2S (i.e. CSE, CBS and 3-MST) are also frequently regulated at a transcriptional and subsequently at a protein level by multiple molecular factors. In many disease states, the expression of these enzymes is both inducible and dynamic. With a better understanding of how the H2S synthesizing enzymes are regulated in disease states, a more targeted effort could be developed to identify drugs to affect endogenous production of H2S in disease states with a view to restoring physiological H2S levels.
To date, changes in expression (mRNA and/or protein) of H2S synthesizing enzymes have been identified in a number of cells and tissues in a variety of different disease states and involving the intermediacy of a range of different signalling pathways (summarized in Table 1). Most evidence collected to date suggests a pivotal role for NFκB in the control of cellular CSE and CBS expression, and this interaction will therefore be described in some detail below.
In an early study seeking to identify the molecular targets of H2S, it was shown that H2S inhibited the activation of NFκB in lipopolysaccharide (LPS)-stimulated mouse macrophages. The mechanism is believed to be via increased heme-oxygenase-1 (HO-1) expression under the control of an ERK1/2-dependent pathway. Increased HO-1 expression then led to reduced IκB phosphorylation and degradation and impaired translocation of NFκB into the nucleus (Oh et al. 2006). Later studies confirmed that H2S affects NFκB but by a different mechanism. In this case, H2S was shown to sulfhydrate NFκB on the p65 subunit at the cysteine-38 residue. This molecular event then increased NFκB binding to a co-activator ribosomal protein, S3 (RPS3), which triggers the transcription of antiapoptotic genes (Sen et al. 2012). Interestingly, recent work has shown that NFκB also regulates CSE expression by binding to its promoter region in macrophages challenged with LPS (Wang et al. 2014). In a separate study, rats with chronic visceral hyperalgesia displayed upregulated colonic CBS expression. An inhibitor of the p65 subunit of NFκB, pyrrolidine dithiocarbamate (PDTC), reduced the expression of CBS, suggesting that activation of the p65 subunit of NFκB upregulates CBS expression (Li et al. 2012). Thus, it appears that H2S may act to maintain the levels of CSE and CBS inside the cell in check during an inflammatory response by regulating the levels of NFκB.
Drugs have also been shown to regulate CSE expression. In the case of atherosclerosis, statins, a class of drugs used to lower cholesterol levels by inhibiting the enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase), were recently reported to upregulate CSE transcription and subsequent H2S generation. This effect was mediated by activation of Akt signalling (Xu et al. 2014b). In inflammation, dexamethasone, a glucocorticoid steroid, reduced CSE expression in neutrophils isolated from LPS-challenged rats. Dexamethasone, in this model, was proposed to inhibit the formation of pro-inflammatory H2S, most probably through reduced NFκB-mediated CSE expression (Li et al. 2009b).
In certain disease conditions, the effect of the disease itself could also have a role to play in directly regulating the expression of H2S synthesizing enzymes. For example in diabetes, high glucose significantly reduced CSE mRNA and CSE activity levels in freshly isolated rat pancreatic islets and in the rat pancreatic cell line, INS-1E. This was mediated by stimulating phosphorylation of the transcription factor SP1 via p38 MAPK activation which subsequently led to decreased CSE promoter activity (Zhang et al. 2011).
5 H2S Synthesizing Enzymes in Diseases States
5.1 Asthma
Asthma is a chronic inflammatory disease characterized by shortness of breath, wheezing and recurring cough. It is caused by a combination of excess airway mucus production and reversible airflow obstruction. Immune cells such as eosinophils and neutrophils play a major role in this inflammatory state although different patients with severe asthma may exhibit different phenotypes that need to be properly defined if the most effective treatment is to be provided (Gibeon and Chung 2012). Current methods for distinguishing different phenotypes of asthma involve detecting sputum eosinophils (eosinophilic asthma), serum periostin levels which is a biomarker for eosinophilic asthma (Jia et al. 2012) and breath nitric oxide (NO) levels (Dweik et al. 2011). H2S is one such potential biomarker of asthma given that it was found to be elevated in the sputum of asthmatics (Chung 2014; Saito et al. 2013).
Endogenous serum H2S levels were found to be altered in chronic obstructive pulmonary disease (COPD) in man almost a decade ago (Chen et al. 2005). This group reported that endogenous serum H2S levels were higher in patients with COPD as compared to patients with acute exacerbation of COPD (AECOPD). Serum levels decreased in patients with stable COPD as the condition became clinically more severe. Subsequently, serum H2S levels in asthmatic children were reportedly decreased compared with a matched healthy control group (Tian et al. 2012).
A more recent clinical study examined levels of H2S in serum and sputum from 40 patients with varying degrees of severity of asthma and compared the data obtained with that of 15 healthy subjects (Saito et al. 2013). H2S levels in sputum of asthma patients were significantly higher than those in sputum from healthy subjects. Serum H2S concentration in asthmatics was also 10 times higher than that in sputum. Sputum measurements are more likely to be indicative of asthma as measurement of serum H2S will be confounded by the presence of H2S generated by non-respiratory tissues. Moreover, a positive correlation was identified between sputum H2S levels and sputum neutrophil number. These observations raise the possibility that sputum H2S concentration may perhaps be a biomarker of neutrophilic asthma.
Since endogenous H2S levels are modulated during asthma, it is conceivable that the activity or expression of H2S synthesizing enzymes may also be altered at one or other time in the course of the disease (See Table 1). To date, there have been few studies to investigate this possibility, although there are clues in the literature that this may be the case. For example, it has recently been shown, using mouse lung slices, that exogenous H2S impeded airway contraction of smooth muscle cells by inhibiting intracellular Ca2+ release evoked by inositol-1,4,5-triphosphate (InsP3). This effect was also apparent when murine lung tissues was treated with l-cysteine (an endogenous H2S precursor) which effect was reversed by the irreversible CSE inhibitor, DL-propargylglycine (PAG) (Castro-Piedras and Perez-Zoghbi 2013). More direct evidence comes from experiments using an ovalbumin (OVA)-induced mouse model of acute asthma. In this case, CSE expression was downregulated in the lungs of wild-type mice challenged with OVA, whilst mice in which CSE was knocked out (CSE−/−) displayed augmented airway inflammation following ovalbumin treatment as indicated by higher levels of Th2 cytokines such as IL-5, IL-13 and eotaxin-1. Exogenous H2S administration, in the form of NaHS, effectively ‘rescued’ CSE−/− mice from the exacerbated symptoms of asthma strongly implying that an upregulated CSE/H2S system has a protective role during the development, or perhaps the maintenance, of asthma in this model (Zhang et al. 2013). Whether such a mechanism also occurs in man is not yet known. Considerable evidence now exists, reviewed elsewhere in this volume, that H2S exhibits both pro- and anti-inflammatory effects in a range of animal models. Thus, whether an H2S donor administered may prove beneficial or deleterious in asthma remains an open question.
5.2 Atherosclerosis
H2S exhibits pronounced vasodilator activity both in vitro and in vivo, and it is hence no surprise that deranged H2S biosynthesis has been implicated in a number of cardiovascular diseases including atherosclerosis. H2S dilates blood vessels by various mechanisms, including the opening of potassium-activated ATP channels (KATP channels) in vascular smooth muscle (Zhao et al. 2001a) as well as potentially via intracellular acidification by activating of the Cl−/HCO3 − exchanger (Lee et al. 2007). Atherosclerosis is a chronic and complex inflammatory condition. Its pathogenesis involves an intricate tapestry of prolonged immune cell recruitment and cytokine/chemokine secretion coupled with the presence of reactive oxygen species (ROS), as well as vascular smooth muscle cell proliferation and migration (Ross 1999). Intriguingly, each of these separate events has been reported to be regulated by H2S (Du et al. 2004; Yan et al. 2006). However based on the evidence available thus far, the link between H2S and atherosclerosis and the involvement of the H2S synthesizing enzymes in the process is far from clear.
In a recent study, Peh and colleagues reported reduced expression of CSE and CBE protein in liver, kidneys and lungs of mice fed a high-fat diet. 3-MST expression was also reduced in liver. In addition, CSE/CBS and 3-MST enzyme activity was also diminished as determined by ex vivo H2S synthesis using either cysteine or 3-mercaptopyruvate as substrates. It should be noted that these animals showed no evidence of frank atherosclerosis in that plasma levels of serum amyloid A (SAA) and C-reactive protein (CRP) were normal and the histological appearance of blood vessels revealed no ongoing disease. Changes in the expression of these enzymes were therefore likely to be a consequence of fat feeding and not a reaction to vascular disease. If this is the case, then changes in H2S synthesis may be presumed to occur before the onset of atherosclerosis (Peh et al. 2014). Whether measurement of these enzymes in susceptible individuals might be a surrogate biomarker of impeding vascular damage due to atherosclerosis remains to be determined. Intriguingly, in a separate study, mice fed a high-fat diet leading to the development of fatty liver exhibited higher levels of CSE and CBS in the liver (Hwang et al. 2013). The mechanisms which control CSE, CBS and 3-MST expression in animals consuming fat in their diet warrant further study.
Other researchers have studied H2S synthesizing enzyme expression and activity in apolipoprotein E (ApoE) knockout mice. These animals have increased plasma levels of cholesterol, triglyceride and low-density lipoprotein cholesterol, develop a frank atherosclerotic state and also show evidence of defective H2S biosynthesis including lower production of H2S in aorta and reduced plasma H2S concentration. Interestingly, CSE mRNA expression in the aorta was actually increased. Administration of exogenous H2S (NaHS) elevated plasma H2S and reduced aortic CSE mRNA thereby suggesting an inverse correlation between CSE expression and H2S levels in the aorta which may be a consequence of a positive feedback mechanism in which decreased H2S production (due to vascular disease) is compensated for by increased CSE gene expression (Wang et al. 2009). Interestingly, elevated plasma H2S has also been reported in patients with atherosclerosis (Peter et al. 2013), whilst diminished production of endogenous H2S which is apparent in CSE−/− mice is associated with accelerated atherosclerosis (Mani et al. 2013). In this latter work, the rate of development of atherosclerosis in these animals could be reduced by replacement therapy with NaHS. The current consensus is therefore that lack of H2S contributes to accelerated progression of atherosclerosis (Szabó et al. 2011; Xu et al. 2014a; Zhang et al. 2012).
5.3 Diabetes
Altered expression of H2S synthesizing enzymes as well as endogenous H2S levels has been observed in diabetic animals. In an early study, plasma H2S and the expression of CSE and CBS were elevated in the pancreas of streptozotocin (STZ)-induced diabetic rats (Yusuf et al. 2005) which suggests a possible correlation between Type 1 diabetes (Schnedl et al. 1994) and increased pancreatic and liver H2S synthesis. To evaluate more precisely the role of H2S in diabetes, a subsequent study made use of CSE−/− mice injected with STZ to provoke a diabetic state. Since the pathogenesis of diabetes is associated with reduced number, but increased activity, of KATP channels in β-pancreatic cells, these authors sought to compare the mass and KATP channel activity of β-pancreatic cells in CSE−/− mice with that of wild-type mice (Yang et al. 2011). CSE deficiency protected mice from STZ-induced diabetes as well as the damage and dysfunction of pancreatic islets—as determined by measurement of the changes in insulin secretion and electrophysiological recording of KATP channel currents after induction of a diabetic state.
Curiously, other studies have reported diametrically opposite results. For example, it was previously noted that circulating H2S levels were lower (c.f. control, non-diabetic animals) in both STZ-treated rats and non-obese diabetic (NOD) models of Type 1 diabetic mice (Brancaleone et al. 2008; Jain et al. 2010). A similar conclusion was reached from the examination of blood H2S levels in humans with diabetes (Jain et al. 2010). CSE enzyme activity was also reduced in the liver of Type 1 diabetic rats as well as in peripheral blood mononuclear cells from Type 1 diabetic patients (Manna et al. 2014).
In contrast to Type 1 diabetes which is caused by the death of β-pancreatic cells, Type 2 diabetes arises, usually later in life, with the development of insulin resistance. To study the possible role of H2S in this process, 6-month-old CSE−/− mice were fed for 8 weeks with a high-fat diet in order to induce hyperglycaemia (Okamoto et al. 2013). The mice developed impaired glucose tolerance and decreased insulin content in their pancreatic cells as compared to their wild-type counterparts. It was noted that the lack of CSE in these animals promoted thioredoxin-binding protein-2 (TBP-2) gene expression which increases insulin insensitivity and causes glucose intolerance (Yoshihara et al. 2010). These results indicate that changes in expression or activity of CSE (and perhaps other H2S synthesizing enzymes) are implicated in the outcome and severity of both Type 1 and 2 diabetes.
6 Frequently Used Inhibitors and Mouse Knockout Models of H2S Synthesizing Enzymes
To study the biological effects of endogenously synthesized H2S, as well as to study the characteristics and behaviour of the H2S synthesizing enzymes, the use of selective inhibitors of H2S synthesizing enzymes is crucial. Currently, several inhibitors of CSE and CBS have been used (See Fig. 2) although to date, pharmacological inhibitors of the enzyme 3-MST have yet to be identified. In this context, l-aspartate inhibits the production of H2S by 3-MST indirectly by inhibiting CAT/AAT (Akagi 1982). Mice with genetic deletion of one or other of the H2S synthesizing enzymes are also beginning to show their value to the field as well. Of the three known H2S synthesizing enzymes only CSE−/− mice have been widely used as 90 % of CBS−/− mice died during the first 2 weeks of neonatal life because of growth retardation and severe hepatopathy (Watanabe et al. 1995) and only lately has it been possible to produce 3-MST knockout mice (Nagahara 2013). However, recently a non-neonatal fatal mouse model with the cbs gene inactivated but instead expresses low levels of the human CBS transgene and exhibits classical homocystinuria could provide a useful, alternative to a CBS−/− mouse model (Maclean et al. 2010).
Some of the commonly used pharmacological inhibitors of CSE are DL-propargylglycine (PAG) (Marcotte and Walsh 1975) which irreversibly inhibits CSE by physically obstructing the access of the substrate to the active site of the enzyme (Sun et al. 2009) and the reversible inhibitor β-cyanoalanine (BCA) which has also been proposed as a competitive inhibitor of this enzyme (Pfeffer and Ressler 1967). However, these compounds have frequently been claimed to exhibit poor selectivity, require high concentrations and have limited ability to permeate the cell membrane (Szabó 2007). For example, PAG was reported many years ago to cause an irreversible inactivation of both aspartate aminotransferase (Tanase and Morino 1976) and alanine transaminase (Burnett et al. 1980). Aminooxyacetic acid (AOAA) is also very commonly used and is often stated to be a selective inhibitor of CBS (d’Emmanuele di Villa Bianca et al. 2009; Oh et al. 2006; Roy et al. 2012). However, experiments using recombinant human CSE and CBS enzymes have revealed that AOAA inhibits both CSE and CBS. Indeed, AOAA appeared to be an even more potent inhibitor of CSE than CBS at least under these experimental conditions (Asimakopoulou et al. 2013). Moreover, AOAA, like PAG, is a general inhibitor of pyridoxal phosphate-dependent enzymes and has been reported to inhibit enzymes such as 4-aminobutyrate aminotransferase (GABA-T) (WALLACH 1961) and aspartate aminotransferase (Kauppinen et al. 1987).
7 Conclusion
Much has been learnt about the physiological and pathophysiological implications of H2S since it was realized that this evanescent gas is formed naturally in cells and tissues in the 1990s. Indeed, research in the last two decades has thrown H2S very much into the spotlight and it is now widely referred to as the so-called third gasotransmitter after CO and NO. Many mechanisms governing the regulation of H2S synthesizing enzymes both in homeostatic and disease conditions have been elucidated. However, many questions still remain to be answered. For example, the quantitative contribution that each of the three H2S synthesizing enzymes makes to the levels of H2S found naturally in cells in health and disease remains unclear. This is important since, as detailed in this review, the presence and/or activity of such enzymes may be useful biomarkers of disease progression in some cases and it is critical to understand which H2S synthesizing enzyme is important in which cell at which time point and how this is affected by disease. Moreover, whilst the biological significance of H2S has been under intense scrutiny for more than two decades, there are still no reliably potent and selective inhibitors of any of the three H2S synthesizing enzymes. For comparison, potent and selective inhibitors of both cyclooxygenase and nitric oxide synthase are available and have played crucial roles in understanding the complex biological roles of prostanoids and nitric oxide, respectively. Potent and targeted inhibitors of CSE, CBS and 3-MST could be expected to fill a similar niche. Finally, the precise intracellular signalling pathways which determine the degree of expression of H2S synthesizing enzymes in healthy and in diseased cells have yet to be fully elucidated.
While many advances have been made, more selective and potent H2S synthesizing enzyme inhibitors as well as cells in which the H2S synthesizing enzymes (both alone and in combination) have been knocked out would certainly provide powerful tools to aid the understanding of the role of endogenous H2S in biology. With the recent advent of high-throughput tandem-microwell assays that can screen enormous libraries of potential H2S producing enzyme inhibitors (Zhou et al. 2013) as well as the introduction of 3-MST−/− mice and novel transgenic mouse models of CBS-deficient homocystinuria, there is certainly much to look forward to in moving the field of H2S biology forward towards its therapeutic potential.
References
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071
Agrawal N, Banerjee R (2008) Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine β-synthase sumoylation. PLoS ONE 3:e4032
Akagi R (1982) Purification and characterization of cysteine aminotransferase from rat liver cytosol. Acta Med Okayama 36:187–197
Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A (2013) Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol 169:922–932. doi:10.1111/bph.12171
Banerjee R, Zou C-G (2005) Redox regulation and reaction mechanism of human cystathionine-β-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys 433:144–156
Beauchamp R, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA, Leber P (1984) A critical review of the literature on hydrogen sulfide toxicity. CRC Crit Rev Toxicol 13:25–97
Beinert H, Holm RH, Münck E (1997) Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 277:653–659
Borrás C, Esteve JM, Viña JR, Sastre J, Viña J, Pallardó FV (2004) Glutathione regulates telomerase activity in 3T3 fibroblasts. J Biol Chem 279:34332–34335. doi:10.1074/jbc.M402425200
Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G (2008) Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol 155:673–680. doi:10.1038/bjp.2008.296
Burnett G, Marcotte P, Walsh C (1980) Mechanism-based inactivation of pig heart L-alanine transaminase by L-propargylglycine. Half-site reactivity. J Biol Chem 255:3487–3491
Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ (2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105:365–374. doi:10.1161/CIRCRESAHA.109.199919
Cantoni G (1952) The nature of the active methyl donor formed enzymatically from l-methionine and adenosinetriphosphate1, 2. J Am Chem Soc 74:2942–2943
Castro-Piedras I, Perez-Zoghbi JF (2013) Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol 591:5999–6015. doi:10.1113/jphysiol.2013.257790
Chen X, Jhee K-H, Kruger WD (2004) Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279:52082–52086
Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, Tang CS (2005) Endogenous hydrogen sulfide in patients with COPD. Chest 128:3205–3211. doi:10.1378/chest.128.5.3205
Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS (2009) Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 45:117–123. doi:10.1016/j.cyto.2008.11.009
Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R (2009) H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284:11601–11612
Chung KF (2014) Hydrogen sulfide as a potential biomarker of asthma. Expert Rev Respir Med 8:5–13. doi:10.1586/17476348.2014.856267
Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG (2004) Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med 37:1511–1526. doi:10.1016/j.freeradbiomed.2004.06.040
D’Emmanuele di Villa Bianca R, Sorrentino R, Maffia P, Mirone V, Imbimbo C, Fusco F, De Palma R, Ignarro LJ, Cirino G (2009) Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci USA 106:4513–4518. doi:10.1073/pnas.0807974105
Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, Austin RC (2012) Integrated stress response modulates cellular redox state via induction of cystathionine γ-lyase: cross-talk between integrated stress response and thiol metabolism. J Biol Chem 287:7603–7614. doi:10.1074/jbc.M111.304576
Diwakar L, Ravindranath V (2007) Inhibition of cystathionine-gamma-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem Int 50:418–426. doi:10.1016/j.neuint.2006.09.014
Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, Tang C (2004) The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels 19:75–80. doi:10.1007/s00380-003-0743-7
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR, Applications ATSCoIoENOLFfC (2011) An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184:602–615. doi:10.1164/rccm.9120-11ST
Finkelstein JD, Kyle WE, Martin JJ, Pick A-M (1975) Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem Biophys Res Commun 66:81–87
Franco R, Cidlowski JA (2009) Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16:1303–1314. doi:10.1038/cdd.2009.107
Ge Y, Jensen TL, Matherly LH, Taub JW (2003) Transcriptional regulation of the cystathionine-beta -synthase gene in Down syndrome and non-Down syndrome megakaryocytic leukemia cell lines. Blood 101:1551–1557. doi:10.1182/blood-2002-07-2337
Ghibelli L, Coppola S, Fanelli C, Rotilio G, Civitareale P, Scovassi AI, Ciriolo MR (1999) Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J 13:2031–2036
Gibeon D, Chung KF (2012) The investigation of severe asthma to define phenotypes. Clin Exp Allergy 42:678–692. doi:10.1111/j.1365-2222.2012.03959.x
Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein R, Dowling G (1989) Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol 13:105–109
Hassan MI, Boosen M, Schaefer L, Kozlowska J, Eisel F, von Knethen A, Beck M, Hemeida RA, El-Moselhy MA, Hamada FM, Beck KF, Pfeilschifter J (2012) Platelet-derived growth factor-BB induces cystathionine γ-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br J Pharmacol 166:2231–2242. doi:10.1111/j.1476-5381.2012.01949.x
Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237:527–531
Hu LF, Wong PT, Moore PK, Bian JS (2007) Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100:1121–1128. doi:10.1111/j.1471-4159.2006.04283.x
Hwang SY, Sarna LK, Siow YL, O K (2013) High-fat diet stimulates hepatic cystathionine β-synthase and cystathionine γ-lyase expression. Can J Physiol Pharmacol 91:913–919. doi:10.1139/cjpp-2013-0106
Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H (2005) Cystathionine beta-synthase is enriched in the brains of Down’s patients. Biochem Biophys Res Commun 338:1547–1550. doi:10.1016/j.bbrc.2005.10.118
Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H (2009) A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11:205–214
Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H (2004) Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J 381:113–123. doi:10.1042/BJ20040243
Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA (2010) Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 12:1333–1337. doi:10.1089/ars.2009.2956
Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, Bradding P, Fahy JV, Woodruff PG, Harris JM, Arron JR, Group BERSoBiC-rABS (2012) Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 130:647–654.e10. doi:10.1016/j.jaci.2012.06.025
Kabil O, Banerjee R (2014) Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20:770–782. doi:10.1089/ars.2013.5339
Kabil O, Zhou Y, Banerjee R (2006) Human cystathionine β-synthase is a target for sumoylation. Biochemistry 45:13528–13536
Kabil O, Vitvitsky V, Xie P, Banerjee R (2011a) The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15:363–372
Kabil O, Weeks CL, Carballal S, Gherasim C, Alvarez B, Spiro TG, Banerjee R (2011b) Reversible heme-dependent regulation of human cystathionine β-synthase by a flavoprotein oxidoreductase. Biochemistry 50:8261–8263
Kabil O, Motl N, Banerjee R (2014) HS and its role in redox signaling. Biochim Biophys Acta 1844:1355–1366. doi:10.1016/j.bbapap.2014.01.002
Kaneko Y, Kimura Y, Kimura H, Niki I (2006) L-cysteine inhibits insulin release from the pancreatic β-cell possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes 55:1391–1397
Kauppinen RA, Sihra TS, Nicholls DG (1987) Aminooxyacetic acid inhibits the malate-aspartate shuttle in isolated nerve terminals and prevents the mitochondria from utilizing glycolytic substrates. Biochim Biophys Acta 930:173–178
Lee SW, Cheng Y, Moore PK, Bian JS (2007) Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun 358:1142–1147. doi:10.1016/j.bbrc.2007.05.063
Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK (2005) Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19:1196–1198. doi:10.1096/fj.04-3583fje
Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK (2009a) GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med 47:103–113. doi:10.1016/j.freeradbiomed.2009.04.014
Li L, Whiteman M, Moore PK (2009b) Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J Cell Mol Med 13:2684–2692. doi:10.1111/j.1582-4934.2008.00610.x
Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187. doi:10.1146/annurev-pharmtox-010510-100505
Li L, Xie R, Hu S, Wang Y, Yu T, Xiao Y, Jiang X, Gu J, Hu CY, Xu GY (2012) Upregulation of cystathionine beta-synthetase expression by nuclear factor-kappa B activation contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Mol Pain 8:89. doi:10.1186/1744-8069-8-89
Maclean KN, Janošík M, Kraus E, Kožich V, Allen RH, Raab BK, Kraus JP (2002) Cystathionine β-synthase is coordinately regulated with proliferation through a redox‐sensitive mechanism in cultured human cells and Saccharomyces cerevisiae. J Cell Physiol 192:81–92
Maclean KN, Sikora J, Kožich V, Jiang H, Greiner LS, Kraus E, Krijt J, Overdier KH, Collard R, Brodsky GL, Meltesen L, Crnic LS, Allen RH, Stabler SP, Elleder M, Rozen R, Patterson D, Kraus JP (2010) A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol Genet Metab 101:153–162. doi:10.1016/j.ymgme.2010.06.010
Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R (2013) Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 127:2523–2534. doi:10.1161/CIRCULATIONAHA.113.002208
Manna P, Gungor N, McVie R, Jain SK (2014) Decreased cystathionine-γ-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. J Biol Chem 289:11767–11778. doi:10.1074/jbc.M113.524645
Marcotte P, Walsh C (1975) Active site-directed inactivation of cystathionine gamma-synthetase and glutamic pyruvic transaminase by propargylglycine. Biochem Biophys Res Commun 62:677–682
Markovic J, Borrás C, Ortega A, Sastre J, Viña J, Pallardó FV (2007) Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem 282:20416–20424. doi:10.1074/jbc.M609582200
Miao X, Meng X, Wu G, Ju Z, Zhang HH, Hu S, Xu GY (2014) Upregulation of cystathionine-β-synthetase expression contributes to inflammatory pain in rat temporomandibular joint. Mol Pain 10:9. doi:10.1186/1744-8069-10-9
Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H (2011a) Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 439:479–485. doi:10.1042/BJ20110841
Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H (2011b) Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 286:39379–39386. doi:10.1074/jbc.M111.298208
Mikami Y, Shibuya N, Ogasawara Y, Kimura H (2013) Hydrogen sulfide is produced by cystathionine γ-lyase at the steady-state low intracellular Ca(2+) concentrations. Biochem Biophys Res Commun 431:131–135. doi:10.1016/j.bbrc.2013.01.010
Nagahara N (2013) Regulation of mercaptopyruvate sulfurtransferase activity via intrasubunit and intersubunit redox-sensing switches. Antioxid Redox Signal 19:1792–1802. doi:10.1089/ars.2012.5031
Nagahara N, Ito T, Kitamura H, Nishino T (1998) Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cell Biol 110:243–250
Nagahara N, Yoshii T, Abe Y, Matsumura T (2007) Thioredoxin-dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. J Biol Chem 282:1561–1569. doi:10.1074/jbc.M605931200
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT (2006) Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41:106–119. doi:10.1016/j.freeradbiomed.2006.03.021
Okamoto M, Yamaoka M, Takei M, Ando T, Taniguchi S, Ishii I, Tohya K, Ishizaki T, Niki I, Kimura T (2013) Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochem Biophys Res Commun 442:227–233. doi:10.1016/j.bbrc.2013.11.023
Patel P, Vatish M, Heptinstall J, Wang R, Carson RJ (2009) The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod Biol Endocrinol 7:10. doi:10.1186/1477-7827-7-10
Peh MT, Anwar AB, Ng DS, Atan MS, Kumar SD, Moore PK (2014) Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide 41:138–145. doi:10.1016/j.niox.2014.03.002
Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, Kevil CG (2013) Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc 2:e000387. doi:10.1161/JAHA.113.000387
Pfeffer M, Ressler C (1967) Beta-cyanoalanine, an inhibitor of rat liver cystathionase. Biochem Pharmacol 16:2299–2308
Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R (2006) S-adenosylmethionine stabilizes cystathionine β-synthase and modulates redox capacity. Proc Natl Acad Sci USA 103:6489–6494
Puranik M, Weeks CL, Lahaye D, Kabil Ö, Taoka S, Nielsen SB, Groves JT, Banerjee R, Spiro TG (2006) Dynamics of carbon monoxide binding to cystathionine β-synthase. J Biol Chem 281:13433–13438
Ramasamy S, Singh S, Taniere P, Langman MJ, Eggo MC (2006) Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol Gastrointest Liver Physiol 291:G288–G296. doi:10.1152/ajpgi.00324.2005
Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT (2002) Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem 277:42912–42918. doi:10.1074/jbc.M206588200
Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/NEJM199901143400207
Roy A, Khan AH, Islam MT, Prieto MC, Majid DS (2012) Interdependency of cystathione γ-lyase and cystathione β-synthase in hydrogen sulfide-induced blood pressure regulation in rats. Am J Hypertens 25:74–81. doi:10.1038/ajh.2011.149
Saito J, Zhang Q, Hui C, Macedo P, Gibeon D, Menzies-Gow A, Bhavsar PK, Chung KF (2013) Sputum hydrogen sulfide as a novel biomarker of obstructive neutrophilic asthma. J Allergy Clin Immunol 131:232–234.e1-3. doi:10.1016/j.jaci.2012.10.005
Schnedl WJ, Ferber S, Johnson JH, Newgard CB (1994) STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 43:1326–1333
Searcy DG, Lee SH (1998) Sulfur reduction by human erythrocytes. J Exp Zool 282:310–322
Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH (2012) Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45:13–24. doi:10.1016/j.molcel.2011.10.021
Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H (2009a) Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 146:623–626. doi:10.1093/jb/mvp111
Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H (2009b) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11:703–714
Singh S, Banerjee R (2011) PLP-dependent H(2)S biogenesis. Biochim Biophys Acta 1814:1518–1527. doi:10.1016/j.bbapap.2011.02.004
Steegborn C, Clausen T, Sondermann P, Jacob U, Worbs M, Marinkovic S, Huber R, Wahl MC (1999) Kinetics and inhibition of recombinant human cystathionine gamma-lyase. Toward the rational control of transsulfuration. J Biol Chem 274:12675–12684
Stipanuk MH (1986) Metabolism of sulfur-containing amino acids. Annu Rev Nutr 6:179–209
Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC (2008) Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79:632–641. doi:10.1093/cvr/cvn140
Sun Q, Collins R, Huang S, Holmberg-Schiavone L, Anand GS, Tan CH, van-den-Berg S, Deng LW, Moore PK, Karlberg T, Sivaraman J (2009) Structural basis for the inhibition mechanism of human cystathionine gamma-lyase, an enzyme responsible for the production of H(2)S. J Biol Chem 284:3076–3085. doi:10.1074/jbc.M805459200
Szabó C (2007) Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6:917–935. doi:10.1038/nrd2425
Szabó G, Veres G, Radovits T, Gero D, Módis K, Miesel-Gröschel C, Horkay F, Karck M, Szabó C (2011) Cardioprotective effects of hydrogen sulfide. Nitric Oxide 25:201–210. doi:10.1016/j.niox.2010.11.001
Takano N, Peng YJ, Kumar GK, Luo W, Hu H, Shimoda LA, Suematsu M, Prabhakar NR, Semenza GL (2014) Hypoxia-inducible factors regulate human and rat cystathionine β-synthase gene expression. Biochem J 458:203–211. doi:10.1042/BJ20131350
Tanase S, Morino Y (1976) Irreversible inactivation of aspartate aminotransferases during transamination with L-propargylglycine. Biochem Biophys Res Commun 68:1301–1308
Taoka S, Banerjee R (2001) Characterization of NO binding to human cystathionine β-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87:245–251
Teague B, Asiedu S, Moore PK (2002) The smooth muscle relaxant effect of hydrogen sulfide in vitro: evidence for a pysiological role to control intestinal contractility. Br J Pharmacol 137:139–145
Tian M, Wang Y, Lu YQ, Yan M, Jiang YH, Zhao DY (2012) Correlation between serum H2S and pulmonary function in children with bronchial asthma. Mol Med Rep 6:335–338. doi:10.3892/mmr.2012.904
Wallace JL, Dicay M, McKnight W, Martin GR (2007) Hydrogen sulfide enhances ulcer healing in rats. FASEB J 21:4070–4076. doi:10.1096/fj.07-8669com
Wallach DP (1961) Studies on the GABA pathway. I. The inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase in vitro and in vivo by U-7524 (amino-oxyacetic acid). Biochem Pharmacol 5:323–331
Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J (2009) Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 29:173–179. doi:10.1161/ATVBAHA.108.179333
Wang M, Guo Z, Wang S (2012a) Cystathionine gamma-lyase expression is regulated by exogenous hydrogen peroxide in the mammalian cells. Gene Expr 15:235–241
Wang Y, Qu R, Hu S, Xiao Y, Jiang X, Xu GY (2012b) Upregulation of cystathionine β-synthetase expression contributes to visceral hyperalgesia induced by heterotypic intermittent stress in rats. PLoS ONE 7:e53165. doi:10.1371/journal.pone.0053165
Wang M, Guo Z, Wang S (2014) The binding site for the transcription factor, NF-κB, on the cystathionine γ-lyase promoter is critical for LPS-induced cystathionine γ-lyase expression. Int J Mol Med 34:639–645. doi:10.3892/ijmm.2014.1788
Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N (1995) Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci 92:1585–1589
Wu N, Siow YL, O K (2010) Ischemia/reperfusion reduces transcription factor Sp1-mediated cystathionine beta-synthase expression in the kidney. J Biol Chem 285:18225–18233. doi:10.1074/jbc.M110.132142
Xu S, Liu Z, Liu P (2014a) Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis. Int J Cardiol 172:313–317. doi:10.1016/j.ijcard.2014.01.068
Xu Y, Du HP, Li J, Xu R, Wang YL, You SJ, Liu H, Wang F, Cao YJ, Liu CF, Hu LF (2014b) Statins upregulate cystathionine γ-lyase transcription and H2S generation via activating Akt signaling in macrophage. Pharmacol Res 87C:18–25. doi:10.1016/j.phrs.2014.06.006
Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R (2013) Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem 288:20002–20013. doi:10.1074/jbc.M113.466177
Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH (2006) Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun 351:485–491. doi:10.1016/j.bbrc.2006.10.058
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322:587–590. doi:10.1126/science.1162667
Yang G, Tang G, Zhang L, Wu L, Wang R (2011) The pathogenic role of cystathionine γ-lyase/hydrogen sulfide in streptozotocin-induced diabetes in mice. Am J Pathol 179:869–879. doi:10.1016/j.ajpath.2011.04.028
Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y, Li N, Qi J, Wang L, Shi Y, Qiu S, Fan J, Zha X (2012) Sp1 is involved in regulation of cystathionine γ-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal 24:1229–1240. doi:10.1016/j.cellsig.2012.02.003
Yoshihara E, Fujimoto S, Inagaki N, Okawa K, Masaki S, Yodoi J, Masutani H (2010) Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat Commun 1:127. doi:10.1038/ncomms1127
Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, Moore PK (2005) Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun 333:1146–1152. doi:10.1016/j.bbrc.2005.06.021
Zhang L, Yang G, Tang G, Wu L, Wang R (2011) Rat pancreatic level of cystathionine γ-lyase is regulated by glucose level via specificity protein 1 (SP1) phosphorylation. Diabetologia 54:2615–2625. doi:10.1007/s00125-011-2187-4
Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang L, Wang C (2012) Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS ONE 7:e41147. doi:10.1371/journal.pone.0041147
Zhang G, Wang P, Yang G, Cao Q, Wang R (2013) The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol 182:1188–1195. doi:10.1016/j.ajpath.2012.12.008
Zhao W, Zhang J, Lu Y, Wang R (2001a) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20:6008–6016
Zhao W, Zhang J, Lu Y, Wang R (2001b) The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20:6008–6016. doi:10.1093/emboj/20.21.6008
Zhou Y, Yu J, Lei X, Wu J, Niu Q, Zhang Y, Liu H, Christen P, Gehring H, Wu F (2013) High-throughput tandem-microwell assay identifies inhibitors of the hydrogen sulfide signaling pathway. Chem Commun (Camb) 49:11782–11784. doi:10.1039/c3cc46719h
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Huang, C.W., Moore, P.K. (2015). H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. In: Moore, P., Whiteman, M. (eds) Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. Handbook of Experimental Pharmacology, vol 230. Springer, Cham. https://doi.org/10.1007/978-3-319-18144-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-18144-8_1
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18143-1
Online ISBN: 978-3-319-18144-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)