Abstract
The increasing use of cross-sectional imaging has led to a significant increase in the detection of small renal masses. Given the heterogeneous nature of these tumors and the paucity of biomarkers for predicting disease progression, the optimal approach for managing these small renal masses is unclear. One of these possible approaches, minimally invasive nephron-sparing ablation therapy, has achieved oncologic outcomes comparable to surgical resection in the intermediate term and represents a paradigm shift in the management of this disease. Long-term follow-up data is now emerging that will help further define the role of ablation therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Take-Home Points-
Energy ablative therapies are used for treatment of small renal cell carcinomas in patients who are not suitable for surgical resection, who are at risk for multiple renal cell carcinomas, or who refuse surgery.
-
Radiofrequency ablation and cryoablation are safe and effective for treatment of small renal cell carcinomas.
-
A biopsy should be performed prior to ablation to confirm diagnosis of renal cell carcinoma.
-
Follow-up imaging should be performed regularly to evaluate for recurrent or metastatic disease.
1 Introduction
Thermal ablation is the destruction of tissue using either heat (radiofrequency, laser, microwave, or high-intensity focused ultrasound) or cold (cryoablation) [1]. Radiofrequency ablation (RFA) and cryoablation (CA) are the most commonly used thermal ablation techniques in the management of small renal cell carcinomas (RCC). Ablation can be performed by minimally invasive laparoscopic and percutaneous approaches. There are no prospective randomized data comparing ablation with the gold standard, nephrectomy. In the absence of long-term follow-up data, ablation is reserved for those patients who are unsuitable for surgical resection, are at risk for multiple RCC, or have minimal renal reserve (e.g., a solitary kidney).
This chapter reviews the range of ablation technologies used experimentally and clinically. The clinical approach to RFA and CA of small RCC is outlined. The merits, limitations, and controversies surrounding these two ablation modalities are discussed.
2 Energy Ablation Technology
The treatment of RCC is technically feasible using a range of ablation technologies. Clinical experience has been greatest with RFA and CA. The modality of choice often depends on local resources and expertise.
2.1 Radiofrequency Ablation Radiofrequency ablation Radio frequency ablation
RFA employs high-frequency (300–500 kHz) alternating electrical current transmission into the targeted tissue via needle electrodes to induce ionic agitation and friction resulting in the production of thermal energy that has both a direct cytotoxic effect and indirect ischemic effect on tissue microvasculature. Cell death happens in 4–6 min at tissue temperatures over 50 °C. Immediate cell death occurs at tissue temperatures over 60 °C. Tissue temperatures over 100 °C result in tissue vaporization, gas formation, tissue carbonization, and eschar formation around the electrode, which reduces the efficiency of the treatment. Thus, the goal of RFA is to maintain tissue temperature between 50 and 100 °C, producing coagulative necrosis while minimizing tissue vaporization and charring. Over time, the ablated tissue is replaced by fibrosis [2, 3].
RFA devices may be bipolar or monopolar. In bipolar RFA, a circuit is created with electrodes where the current flows from the generator to the active electrode, through the tumor, to the second electrode, and back to the generator. With monopolar RFA, a circuit is created with electrodes and grounding pads where current flows from an active electrode inserted into the tumor, via the patient’s body, to grounding pads on the patient’s skin. Monopolar systems are most widely used in the USA. Commercially available RFA systems use either temperature-based or impedance-based ablation algorithms. Temperature-based systems are designed to achieve a target temperature in the tissue surrounding the ablation probe for a determined duration. Impedance-based systems are designed to prevent excessive elevation in tissue impedance around the ablation probe allowing a determined duration of energy deposition while minimizing tissue charring.
The RF electrodes range in size from 14 to 17 gauge. Electrode design can vary from a multi-tined expandable electrode configuration to a simple straight probe in single or triple cluster configuration. Both the RITA StarBurst probe (Rita Medical Systems, Mountain View, CA) and the LeVeen probe (Boston Scientific, Natick, MA) are multi-tined expandable probes that produce teardrop- and discoid-shaped ablation zones, respectively. Probes of different diameters are available and may be deployed in a stepped fashion. The Cool-tip device (Covidien, Mansfield, MA) can be used as a single straight probe or a cluster probe in which three closely spaced straight electrodes are arranged in a triangular configuration. Internally cooled electrodes have chilled saline circulating through an internal lumen, thus minimizing charring at the electrode tip and optimizing energy transmission through the tissues. On the other hand, perfusion electrodes have an opening at the active tip that allows saline to be infused into the tissue during the ablation. This design has also been referred to as “wet RFA.” The saline alters the electrical and thermal conductivity of the tissue during ablation thus increasing the ablation zone. Studies have shown “wet” and “dry” RFA systems to be equally effective in achieving cell death [2, 3].
2.2 Cryoablation Cryoablation
CA employs alternating cycles of rapid tissue cooling and thawing to produce liquefactive necrosis. Cell death is induced by osmotic effect from extracellular ice crystal formation, direct injury to cell membranes from intracellular ice crystal formation, and ischemic injury to the microvasculature [4, 5]. Compressed gas, usually argon, is injected into the cryoprobe, and as the gas expands, it cools the shaft of the cryoprobe by the Joule-Thomson effect to as low as −190 °C. Thawing is achieved either by turning off the flow of argon and allowing the ice to passively melt or by introducing another compressed gas, usually helium, which when heats up as it expands by the Joule-Thomson effect and thus actively warms the cryoprobe [5]. Depending on the cell type, tumor temperatures between −19.4 and −40 °C are required to bring about cell death [4–7]. On imaging, the edge of the developing ice ball represents a 0 °C isotherm with the −20 °C isotherm several millimeters inside the edge of the ice ball [8, 9].
Cryoprobes come in different diameters (1.4–8 mm), and the ice balls produced vary in shape and size. As treatment efficacy drops off with increasing distance from the probe, a number of probes may be required to cover a tumor zone. Probes should be positioned within 1 cm from the tumor margin and no more than 1–2 cm from each other [10]. The use of multiple probes creates a synergistic effect that results in the formation of an even larger ice ball.
2.3 Laser Ablation
Traditionally, laser coagulation was based on Nd:YAG (Medilas Fibertom, Dornier MedTech, Germering, Germany) infrared laser light with a wavelength of 1,064 nm. More recently, diode-based systems (PhoTex 15; Visualase, Houston, Texas) have been introduced into clinical practice [11, 12]. These systems operate in the range of 805–980 nm, use smaller applicators, and create larger ablation zones in shorter periods of time. The energy is delivered via fibers with a flexible diffuser tip. The active length of the tip ranges from 2 to 4 cm. The radiant energy is absorbed by tissue and transformed into heat. Similar to RFA, cell death occurs by a process of coagulation necrosis. When several fibers are used simultaneously, a laser beam splitter can be applied to enable synchronous energy delivery to multiple fibers. Diode-based laser systems are smaller and lighter, and multiple devices can be used to operate several fibers. Newer devices are MRI compatible and consist of a cannulation needle, a sheath, and a laser irrigation catheter. The latter facilitates cooling of the laser tip and prevents direct contact between the laser applicator and the tissues [11]. There is limited experience with this technology for ablation of renal tumors [12–14].
2.4 Microwave Ablation
Microwave ablation relies on the emission of electromagnetic waves in the range of 30–30 GHz from applicators placed in tissue. These microwaves agitate water molecules in the vicinity of the applicator, producing friction and heat. As with RFA, once temperatures exceed 60 °C, cell death occurs via coagulative necrosis [15]. There are several systems approved for use in humans in the USA. Equipment consists of a generator and an applicator referred to as an “antenna.” The lack of electrical current being transmitted in the patient obviates the need for grounding pads. While there is limited clinical experience with microwave ablative technology, it does offer a number of theoretical advantages over other thermal ablation modalities [16]. Heating is not dependent on conduction from the antenna tip alone but occurs via a direct field effect in all tissues in the microwave field. This allows rapid and uniform heating of the tissues. The evidence for the application of microwave ablation in the kidney shows encouraging early and intermediate results [17–27].
2.5 Ultrasound Ablation
High-intensity focused ultrasound (HIFU) delivers targeted ultrasonic energy to tissue at a selected depth, and this energy is absorbed and converted to heat eventually resulting in coagulative necrosis. If the energy delivered is increased beyond a certain threshold, tissue cavitation, i.e., mechanical disruption, of the tissue occurs [28]. Thermal necrosis depends on ultrasound frequency, exposure time, absorption coefficient, acoustic reflection and refraction, and perfusion rate in the targeted tissue, while cavitation depends on energy pulse length, frequency, and tissue factors. HIFU may be performed laparoscopically or via extracorporeal approach [29, 30]. Some of the major limitations of HIFU when applied to renal tumor ablation are the difficulty of targeting in a mobile organ as well as overcoming the complex acoustic characteristics of intervening tissues when using extracorporeal approaches.
3 Selection Criteria
The primary indication for energy ablation of a primary RCC lesion is to eradicate a tumor with curative intent. In addition, ablation for palliation of intractable hematuria has been reported [31, 32].
3.1 Patient
Energy ablative therapy should be considered in patients who are poor surgical candidates, those at risk for multiple RCC, patients with limited renal functional reserve, and those who refuse surgical intervention. Poor surgical candidates include those with cardiovascular or respiratory comorbidities that result in an unacceptably high operative risk. The preservation of renal function is paramount in patients with renal insufficiency and those with a solitary anatomic or solitary functioning kidney, and since ablative therapy is nephron sparing, it may help minimize the need for dialysis in the long term [33–38]. A nonsurgical approach is also favored when residual or recurrent disease is identified in the nephrectomy bed.
A genetic predisposition to RCC is present with von Hippel-Lindau syndrome, Birt-Hogg-Dube syndrome, hereditary papillary cell carcinoma, and hereditary clear cell RCC. While many of these patients will ultimately require partial nephrectomy, ablative therapy may prolong the time to resection [39, 40]. In an effort to preserve renal function, synchronous RCCs (sporadic or genetic) may be treated with surgical resection of the larger lesion and energy ablation of the smaller lesion [41].
Given that many patients being considered for ablative therapy have multiple comorbidities, a risk-benefit evaluation should be performed. Patients should have an acceptable functional status. A coagulopathy that cannot be corrected is the only absolute contraindication to ablation therapy.
3.2 Tumor
The ideal renal tumor for therapeutic percutaneous ablation is small (≤3 cm), exophytic, and posteriorly located. If tumor eradication is the goal, the disease should be confined to the kidney (T1a). Extension into the adjacent nodes, the renal vein, or inferior vena cava is a relative contraindication to ablative therapy. In patients with an isolated metastasis that is amenable to treatment, energy ablation of the primary may still be considered [42]. Proximity to the central collecting system, bowel, pancreas, adrenal gland, liver, or gallbladder is a relative contraindication to percutaneous thermal ablation of a renal mass, but these structures can often be displaced or protected by various adjunctive techniques [43–46].
4 Pre-procedure Planning
Multiple issues need to be taken into consideration when planning an ablation procedure including patient factors, tumor characteristics, ablation modality, approach, and imaging guidance modality.
4.1 Patient
All patients should present for pre-procedure clinical assessment prior to intervention. Serum platelets and international normalized ratio (INR) should be determined. Commonly used laboratory criteria for ablation include a platelet count greater than 50,000/μL and an INR less than 1.5. Antiplatelet agents are withheld 5 days prior to the procedure. Patients receiving low-molecular-weight heparin have one dose held prior to the procedure. Baseline creatinine and glomerular filtration rate (GFR) should be recorded so that the impact of treatment on renal function can be established.
The patient’s ability to lie in the position planned for the procedure, usually prone, should be assessed. If the institutional criteria for conscious sedation are not met, anesthesia assistance should be sought. At our institution, general anesthesia is used because general anesthesia optimizes patient tolerance, allows greater control of respiratory motion when placing the probe, and may facilitate more accurate targeting of the lesion [47]. Renal ablation has been performed as an outpatient setting at many centers, but at our institution, we admit patients for a 23-h observation period [48].
4.2 Tumor
One of the ongoing controversies surrounding ablation of RCC is whether a biopsy should be acquired prior to treatment. Though an enhancing renal mass is most often a RCC, the differential diagnosis includes benign entities such as lipid-poor angiomyolipoma, oncocytoma, papillary adenoma, and metanephric adenoma. As the size of a renal mass decreases, the likelihood of a benign diagnosis increases with up to 25 % of renal tumors smaller than 4 cm found to be benign [49]. The Society of Interventional Radiology as well as a consensus panel of urologists recommended performing a biopsy prior to ablative therapy [50, 51]. A clearly negative result eliminates treatment of benign lesions. A positive result provides details of tumor subtype and grade, information that may become relevant should the patient ever require systemic therapy. A positive result is also important for the validation of ablative therapy and in defining the standard of care for small renal masses in the future. Ideally, the biopsy should be performed during a separate encounter so that sufficient time is given for a complete histological evaluation; however, at our institution, for the sake of patient convenience, most patients are biopsied and then ablated in the same session. The biopsy results are then used to personalize the follow-up regimen for each patient.
Once the ablation procedure has been deemed indicated and feasible, the factors affecting technical success should be assessed and optimized. Tumor size and location are the two most important predictors of technical success. Tumors smaller than 3 cm are ideal for ablative therapy [52–54], though larger tumors can also be successfully ablated [55, 56]. Tumor location may be described as exophytic, intraparenchymal, central, or mixed [57]. Exophytic tumors are defined as those with a component extending into the perirenal fat. Parenchymal tumors are defined as those limited to the renal parenchyma. Central tumors are defined as those with extension into the renal sinus fat. Mixed tumors have components extending into both the renal sinus fat and the perirenal fat. Noncentral, particularly exophytic, tumors have the best chance of complete ablation [52].
4.3 Cryoablation Versus Radiofrequency Ablation Radiofrequency ablation
The relative merits of CA include lower risk of ureteric injury for lesions close to the collecting system, less intra-procedural pain, and more accurate monitoring of treatment efficacy during the procedure [58, 59]. The ice balls created with cryoprobes are easily visualized using cross-sectional imaging. The zone of ablation can fairly predictably be calculated based on the width of the ice ball. While the ablation zone achieved with the RFA electrodes is relatively predictable, noninvasive monitoring of the ablation zone during the procedure is not currently clinically feasible. Hemostasis achieved by cauterizing vessels is the primary advantage of RFA over CA.
4.4 Surgical Versus Percutaneous
Both RF and CA have been successfully performed via open, laparoscopic, and percutaneous image-guided approaches. CA was first applied to RCC by urologists using an open surgical approach following the success achieved in treating prostatic tumors [60]. This has largely been replaced by laparoscopic ablation. A 2008 meta-analysis of 47 studies treating small renal masses using CA or RFA identified laparoscopy as the approach in almost two-thirds of CA cases, while 93 % of RFAs were performed percutaneously [61]. The introduction of lower-profile applicators have led to increased use of percutaneous CA among radiologists.
All patients undergoing open or laparoscopic ablation require general anesthesia. A percutaneous approach is less invasive and may be performed with moderate sedation. It allows faster recovery and is associated with fewer complications [62]. Percutaneous CA has been estimated to be 2.2–2.7 times less expensive than open or laparoscopic procedures [63]. As laparoscopic probes can be used to displace bowel and other structures out of the ablation zone or applicator trajectory, its use is often preferred for ablation of anterior and central lesions. This limitation of percutaneous ablation can often be circumvented with the use of hydrodissection and CO2 dissection techniques [43–46]. While the use of larger cryoprobes with a surgical approach can facilitate ablation of larger tumors, Lehman et al. reported a significantly higher complication rate of 62 % (13/21) for laparoscopic CA of tumors over 3 cm in size compared with 0 % (0/30) for tumors 3 cm and under (p = 0.0007) [64]. One of the advantages of a percutaneous approach is the visualization of the whole ablation probe as it is being placed and monitoring of deep structures during the ablation obtained with CT and MRI guidance. With laparoscopic sonography, echogenic shadowing behind the ice ball can limit visualization of the entire ablation zone and adjacent structures [65].
4.5 Imaging Modality
Guidance for thermal ablation of RCC may be provided by several imaging modalities. Ultrasound has many advantages. It is relatively low cost, is readily available, and enables real-time imaging. It does not expose the patient to ionizing radiation. The renal mass can be identified in multiple planes by simply angling the probe. Compression can help displace bowel loops out of the applicator trajectory and decrease the skin to target distance. However, ultrasound does have its limitations. Visualization may be limited by patient body habitus, small lesion size, overlying bowel gas, or intervening lung base. The tip of the applicators and the deep aspect of the ablation zone can be difficult to visualize once treatment has begun because of shadowing from microbubbles during RFA or the growing ice ball during CA; thus, some operators will use ultrasound for initial placement of the applicators but then use other modalities, usually CT, for treatment monitoring.
Computed tomography (CT) is the most commonly used imaging modality to guide ablation. It provides excellent visualization of the tumor, the applicators, and the surrounding anatomy. CT fluoroscopy enables real-time visualization of the applicator tip as it is being placed and facilitates precise targeting of the lesion. An initial contrast-enhanced study may be required if the lesion and surrounding normal renal parenchyma are isodense to the renal parenchyma. With CA, the hypodense ice ball is easily visualized. The main disadvantage of CT is the exposure to ionizing radiation for the patient. Radiation exposure also becomes a concern for the operator if CT fluoroscopy is used.
MRI offers superb soft tissue contrast. Multiplanar and real-time imaging can be performed. A combination of T1- and T2-weighted sequences can be used to accurately track the ice ball formed during CA [66]. MRI can also monitor treatment efficacy for RFA by tracking changes in tissue temperature [67]. The lack of ionizing radiation is a significant advantage. Disadvantages include lack of availability, small gantry size, lack of operator experience, the need for MRI-compatible equipment, longer procedure times, and greater cost.
5 Techniques
Renal mass ablation can be performed by both laparoscopic and percutaneous approaches. In addition, several adjunctive techniques have been developed to allow ablation of renal masses previously thought to be unamenable to ablation.
5.1 Laparoscopic Ablation
Laparoscopic ablation is performed via a retroperitoneal approach for posterior and posterolateral lesions. Anterior or anterolateral lesions are accessed using a transabdominal approach [68]. The ultrasound probe is placed on the side of the kidney opposite to the tumor. A percutaneous biopsy may be acquired using an 18-gauge biopsy needle and coaxial technique under ultrasound guidance. The size and number of applicators used depend on tumor shape and size. The probes are positioned and the treatment is monitored using ultrasound guidance. Most often, the laparoscopic approach is used for CA, and most often, a double freeze-thaw cycle is used [69]. To achieve a 5-mm margin of cell death around the tumor, an ice ball extending 10 mm beyond the tumor margin is desirable [8]. Hemostasis is achieved with direct pressure and hemostatic agents, e.g., Surgicel (Ethicon, San Angelo, TX). The cryoprobe tracks may be embolized with Gelfoam (Pfizer, New York City, NY) or fibrin glue (Tisseel VH, Baxter, Deerfield, IL). The site is observed for bleeding under low insufflation pressures. Gerota’s fascia is reapposed. The ports are removed and the port sites closed.
5.2 Percutaneous Ablation
Usually, the prone or prone-oblique positions are optimal, though the ideal position can vary depending on the location of the renal mass to be ablated. A pre-procedure study with or without contrast should be performed using CT or MRI. Ultrasound may be used in conjunction with other cross-sectional imaging modalities to target the lesion. Regular monitoring of the ablation zone is performed using intermittent imaging. An ablation margin of 5–10 mm around the tumor is desirable [2]. The zone of ablation covered will depend on the lesion, its proximity to vascular structures, the ablation modality used, and the number, size, and configuration of the applicators. Even with an array of single-tine RF probes, repositioning may be required and overlapping of ablation zones performed. Multiple cryoprobes can be used simultaneously to maximize the zone of ablation. The cryoprobes are placed up to 2 cm apart and up to 1 cm from the tumor margin [70]. An immediate post-ablation contrast-enhanced CT or MRI should be performed to assess the zone of ablation and rule out any complications. This is particularly relevant to RFA for which treatment efficacy is difficult to assess during the procedure [71]. However, care must be exercised in interpreting the immediate post RFA CT. As Javadi et al. showed, contrast medium can leak into the ablation zone immediately after RFA resulting in temporary homogeneous enhancement. The treated area can be better appreciated by identifying the relatively low-density sharply demarcated margins and comparing these with the pre-ablation studies [71].
5.3 Adjunctive Techniques
Occasionally, radiologists will perform trans-arterial embolization prior to percutaneous ablation when hemorrhage poses a significant complication risk [72–74]. In addition, embolization of larger tumors (>4 cm) prior to RFA decreases the perfusion-mediated cooling of the tissues and renders thermal ablation more effective [74].
To reduce the risk of thermal damage to the ureter and renal collecting system during RFA of an adjacent renal mass, retrograde pyeloperfusion with a cooled nonionic solution can be performed [44, 75, 76]. This requires transurethral placement of a 5–6-F ureteral catheter with the tip confirmed in the renal pelvis for infusion and a 14–16-F Foley catheter in the bladder for drainage. The ureteral catheter is removed at the end of the procedure. For CA, Froemming et al. described a probe retraction technique used to protect the ureter [77]. After positioning the cryoprobe, proximity to important structures is assessed using CT. Activation of the probe creates an initial small ice ball that fixes the probe in relation to the tumor and also acts as a point of fixation for manipulation. By manipulating the applicators, the tumor and kidney can be retracted away from the structures to be avoided, e.g., the ureter. CA can then be resumed with standard freeze-thaw cycles allowing the ice ball to extend distal to the probe tip.
If vital structures lie in the path of the applicator or are contiguous with the proposed ablation zone, noninvasive measures such as changing the patient position or levering the applicator against the skin to lift the tumor off the bowel or vascular structure may be performed. Applicator levering has been reported to increase the tumor to bowel distance by 3–4 mm [78]. The safety margin between the probe tines and the nearest adjacent bowel is 1–2 cm [79].
Hydrodissection or gas insufflation can be used to create a plane between the tumor and other structures [43, 45, 46, 80]. With hydrodissection, sterile liquid is instilled through an 18–21-gauge needle placed between the lesion and the bowel under CT or MRI guidance. For RFA, a relatively nonionic solution, e.g., D5W, should be used. With gas insufflation, gas can be delivered intraperitoneally via needle or laparoscopic port or directly into the perirenal space via needle puncture. Gas has a tendency to dissipate; thus, larger volumes are required compared with liquid. The adequacy of insufflation is best monitored with CT, as gas can obscure the view of the tumor when MRI or ultrasound guidance is used [46].
Interposing angioplasty balloons or esophageal dilator balloons between the tumor or applicator and the structure at risk can also decrease the risk of thermal injury [79]. For angioplasty balloons, an 18–19-gauge needle and 0.035″ wire access should be acquired in the plane in which the balloon is to be placed. The balloon should be placed through a sheath and advanced beyond its desired location. It is easier to retract the balloon into position rather than try to advance the balloon over a wire. Balloon expansion is completed once optimal position is obtained. One of the difficulties with balloon interposition is their tendency to slip away over time. Multiple balloons may be required for adequate tissue separation.
Thermosensors can be placed in cases of endophytic tumors and tumors larger than 3 cm to ensure adequate ablation and to prevent thermal injury to the normal renal parenchyma and adjacent structures. These fiber-optic nonconducting temperature probes should be arranged in a triangulated configuration at the deep and peripheral tumor margins and are advanced into position through a nonconducting sheath. A temperature probe may also be placed in a location where high temperatures are undesirable, e.g., periureteric tissue. Carey et al. reported 100 % primary effectiveness for RFA of 37 tumors 3–5 cm in diameter in which real-time temperature feedback of the ablation zone was used to determine the appropriate treatment endpoint [81]. These independent real-time thermosensors can also be used to determine if and where an electrode needs to be redeployed.
Oblique trajectories should be employed when accessing upper pole masses in an effort to minimize the risk of pneumothorax. Placing the patient in the ipsilateral decubitus position decreases lung excursion on the ipsilateral side and thus reduces the plane of contact between the tumor and the overlying lung. If an infradiaphragmatic approach to the tumor is not possible, another option is to use a technique described by Ahrar et al. whereby a transthoracic approach to upper pole renal masses is created by means of an intentional pneumothorax [82]. This involves placement of a 20- or 18-gauge needle and injecting gas into the pleural space. After completion of the ablation, the pneumothorax is treated with simple aspiration or placement of a small-bore (8–10 French) chest catheter under CT guidance. Alternatively, an iatrogenic pleural effusion may be created by injecting nonionic fluid. This technique allows for precise placement and repositioning of the RF electrode under CT guidance without repeated puncture of the visceral pleura.
6 Outcomes
Lack of histological evidence to confirm cell death has been one of the strongest criticisms of ablation therapy, particularly since positive biopsies have been reported in non-enhancing tumors. Currently, treatment success is based almost entirely on imaging findings. Furthermore, outcome data from many studies includes lesions for which no histological confirmation of malignancy was obtained. A meta-analysis of 47 RFA and CA studies found unknown pathology occurred in 33.5 % of ablated lesions [61]. To circumvent such criticism, we advocate performing biopsy before every renal mass ablation to assure accurate data and also to help in the appropriate management of patients [83].
In the published literature, residual or recurrent disease is usually defined as the presence of nodular or crescentic enhancement in the zone of ablation, especially if it is enlarging [84]. Thus, multiple ablations or reablations may be interpreted as an initial treatment failure. In the 2008 meta-analysis by Kunkle and colleagues, the outcomes of RFA (93.7 % performed percutaneously) were compared with CA (two-thirds performed laparoscopically). Any lesion with evidence of persistent local disease, radiographic or pathologic, was defined as local tumor progression, regardless of the time to reappearance. Repeat ablation was performed more frequently after RFA (8.5 % versus 1.3 %; p < 0.001), and the rates of local tumor progression were greater for RFA (12.9 % versus 5.2 %; p < 0.001) [61].
However, these results do not solely address the comparative effectiveness of RFA versus CA but rather also incorporate the results of the technique for ablation, percutaneous versus laparoscopic, as was shown by a meta-analysis of laparoscopic and percutaneous ablations conducted by Hui et al. Outcome measures were defined in terms of primary effectiveness (the percentage of tumors treated successfully by the initial procedure) and secondary effectiveness (the percentage of tumors treated successfully overall, including repeated procedures that followed identification of residual or recurrent tumor). A primary effectiveness of 87 % (95 % CI, 82–91 %) was achieved for percutaneous ablation compared to 94 % (95 % CI, 92–96 %) for a surgical approach (p < 0.05). The secondary effectiveness was not significantly different between the two groups (percutaneous 92 % versus surgical 95 %). The mean tumor size and the proportion of malignant lesions ablated were significantly greater for the percutaneous group (2.8 cm versus 2.5 cm and 84 % versus 64 %; p < 0.05) [62].
Thus, the apparent inferior results seen following RFA are due in part to patient selection bias, different approaches, and size, type, and number of applicators. In addition, when comparing the outcomes from percutaneous versus laparoscopic ablation, it should be remembered that these procedures are performed in very different settings. Percutaneous ablations are usually performed in an outpatient suite and most often with moderate sedation. Time constraints, patient tolerance, and respiratory motion may prevent treatment of the entire lesion during a single encounter. Given the minimally invasive nature of this approach and the relatively low risk of complications, some operators may choose to perform ablation in more than one session to treat the entire lesion. Laparoscopic ablations, on the other hand, are more invasive and require general anesthesia and in-hospital stay; thus, the aim is to treat the entire lesion during a single encounter. Performing repeat surgery in the same field is difficult and may have higher rates of complications [85].
Long-term follow-up data is now emerging for both RFA (Table 12.1) and CA (Table 12.2). Ma et al. reported on 52 patients who underwent both laparoscopic and percutaneous RFA with a median follow-up of 60 months. The reported 5-year disease-free survival (DFS) was 94.2 %, overall survival (OS) was 95.7 %, and cancer-specific survival (CSS) was 100 % [86]. Psutka et al. reported the results of 185 patients with biopsy-proven RCC treated with percutaneous RFA with a median follow-up of 6.4 years. The reported DFS, OS, and CSS at 5 years were 87.6 %, 73.3 %, and 99.4 %, respectively [87]. For laparoscopic RFA, Ramirez et al. demonstrated at a median of 4.9 years for 79 patients a 5-year DFS of 93.3 %, an OS of 72 %, and a CSS of 100 % [88]. Best and colleagues described the results of RFA performed for 142 patients with a median follow-up of 54 months. Seventy-two percent of the treated tumors were biopsy-proven RCC. Five-year DFS was 91 % overall and was dependent on tumor size. Tumors smaller than 3 cm had 5-year DFS of 95 %, and tumors 3 cm or larger had 5-year DFS of 79 % (p = 0.001) [89]. Olweny et al. performed a comparative study of outcomes for two cohorts of 37 patients each undergoing percutaneous RFA versus partial nephrectomy and showed that the 5-year DFS, OS, and CSS were very similar between the two cohorts [90]. Zagoria et al. reported their results for percutaneous RFA performed for 41 patients who were followed for a median of 4.7 years. The 5-year DFS was 85 %, OS was 66 %, and CSS was 97.5 % [91]. Levinson and colleagues related the results of their experience treating 31 patients with percutaneous RFA who were followed for an average of 5.1 years. They reported a 6.7-year DFS, OS, and CSS of 89.2 %, 62.7 %, and 100 %, respectively [92].
CA experience has been greatest using the laparoscopic approach, although long-term percutaneous CA series are also available. Johnson et al. reported on their experience with laparoscopic CA in 144 patients followed for an average of 8.2 years. They reported 5-year DFS of 95.4 %, OS 90.5 %, and CSS 100 % [93]. Georgiades and colleagues reported on their cohort of 134 patients treated with percutaneous CA followed for 5 years. The 5-year DFS was 97 %, OS was 97.8 %, and CSS was 100 % [94]. Tanagho et al. followed 62 patients for a mean of 76 months after laparoscopic CA and found a 6-year DFS of 80 %, OS 76.2 %, and CSS of 100 % [95]. Aron et al. reported 5-year disease-free survival of 81 % in 55 patients with biopsy-proven RCC at a median follow-up of 93 months [96].
Though these long-term data give one greater confidence in the efficacy of thermal ablation for RCC, continued follow-up of these cohorts is necessary because of the known indolent growth rates of small RCCs.
7 Post-procedure Follow-Up
Follow-up should encompass an assessment of the patient’s clinical status including renal function as well as a review of imaging looking for delayed complications and residual, recurrent, or metastatic disease. A clinic visit should be arranged in the weeks after the procedure to assess for pain, urinary symptoms, fever, or chills. The skin entry sites should be examined.
Given that ablative therapy is advocated in those with limited renal reserve, it is important that the impact of ablation on renal function if any be recorded. Lucas et al. examined the impact of RFA, partial nephrectomy, and radical nephrectomy on renal function in patients with small renal masses (<4 cm). The mean pretreatment GFR was 73.4, 70.9, and 74.8 mL/min/1.73 m2 in the RFA, partial nephrectomy, and radical nephrectomy groups. Following intervention, the 3-year freedom from stage 3 CKD was 95.2 % for RFA, 70.7 % for partial nephrectomy, and 39.9 % for radical nephrectomy (p < 0.001). Patients undergoing radical and partial nephrectomy were 34.3 (p = 0.001) and 10.9 (p = 0.024) times more likely, respectively, to develop stage 3 CKD compared to RFA counterparts [97]. In patients with a solitary kidney, Raman et al. examined the impact of RFA on renal function in 16 patients with 21 small renal masses (<=4 cm). In this series, the mean preoperative GFR of 54.2 mL/min/1.73 m2 declined only to 47.5 mL/min/1.73 m2 at the last follow-up (mean follow-up of 30.7 months). Patients treated with open partial nephrectomy had a greater decline in GFR compared with those who underwent RFA, at all post-procedure times evaluated: 15.8 % versus 7.1 % at 0–3 months, 24.5 % versus 10.4 % at 12 months, and 28.6 % versus 11.4 % at the last follow-up (p < 0.001 for all time periods) [34].
There is no standardized follow-up algorithm for ablated renal tumors. The follow-up imaging interval varies among institutions. Matin et al. detected 70 % of incomplete treatments within the first 3 months of treatment. They recommended at least three to four imaging studies in the first year after ablative therapy: months 1, 3, 6 (optional), and 12 [98]. Ideally, follow-up should be performed using the cross-sectional imaging modality used to perform the ablation. Persistent nodular enhancement in the ablation zone up to 3 months post treatment is worrisome for residual disease [99]. Differential diagnosis includes inflammation or volume averaging. Recurrent disease is suspected if the ablation zone is enlarging on serial scans and/or nodular contrast enhancement that was not present on the initial post-ablation study is identified [99]. The renal vein and IVC should be assessed for evidence of enlargement or abnormal enhancement. A search for a new primary tumor and metastatic disease should be performed. Classically, the RFA zone has a “bull’s-eye” appearance on surveillance imaging – non-enhancing soft tissue surrounded by enhancing normal renal parenchyma [99]. The ablation zone is usually T2 hypointense compared with the normal renal parenchyma and can have variable intensity on T1-weighted sequences [100, 101]. Subtraction of post-gadolinium and non-contrast T1-weighted data may enhance detection of subtle foci of residual or recurrent disease [102]. While hemorrhage can artificially increase the size of the ablation zone on the immediate post-procedure scan, the lesion should slowly involute to pre-RFA size on serial scans [103] (Figs. 12.1 and 12.2).
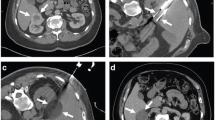
A 68-year-old man was found to have a 3.2-cm solid enhancing mass in the right kidney. Biopsy showed renal cell carcinoma, clear cell type. (a) Axial CT image of the abdomen without contrast medium shows a tumor (T) along the medial border of the right kidney. (b) After administration of iodinated contrast medium, the tumor (T) shows marked enhancement. (c) Axial CT image of the patient in prone position shows two radiofrequency electrodes (arrows) entering the tumor from a posterior approach. The tip of each electrode is carefully positioned at the anterior margin of the tumor. A retrograde ureteral catheter (arrowhead) was placed for continuous infusion of cold fluid to prevent heating injury to the ureteropelvic junction. Four overlapping ablations were performed to completely ablate the tumor. (d) Axial CT image of the abdomen without contrast medium 30 months after ablation shows a soft tissue density at the center of the ablation zone (A) surrounded by a fibrous capsule (arrowheads). The capsule has engulfed retroperitoneal fat into the ablation zone. (e) After administration of the contrast, there is no enhancement of the ablation zone (A). A biopsy of the ablation zone (not shown here) demonstrated necrotic tissue and no viable tumor
A 62-year-old man underwent CT examination for staging of prostate cancer. He was found to have a 2.7-cm enhancing mass at the upper pole of his left kidney. Biopsy showed renal cell carcinoma, papillary type 1 and Fuhrman nuclear grade 2. (a) Axial CT image of the abdomen after administration of contrast shows the tumor (T) involving the upper pole of the left kidney. (b) Axial CT image of the abdomen in prone position shows one of the three cryoprobes (arrow) placed into the tumor from a posterior approach under CT guidance. The ice ball has a lower density compared to the normal kidney. The edge of the ice ball is sharply demarcated at its boundary with normal renal parenchyma. Monitoring the size and extent of the ice ball with CT intermittent CT imaging helps avoid thermal injury to the adjacent structures such as the colon (C). (c) Axial CT image of the abdomen with iodinated contrast 17 months after ablation shows involution of the ablation zone (A) with minimal residual non-enhancing necrotic tissue
During CA, the tumor is frozen and is identified by a well-defined area of low attenuation on CT and is both T1 and T2 hypointense on MRI. While the cryoablated zone is typically non-enhancing on CT and MRI surveillance studies, residual contrast enhancement has been reported [104–106]. In a review of 32 lesions treated with laparoscopic CA, Stein et al. identified persistent ablation site enhancement in 15.6 % (5/32) at 3 months, three of which persisted at 6 months and one displayed enhancement at 9 months. The latter underwent partial nephrectomy that demonstrated no recurrent cancer [104]. The ablation zone is frequently isointense on T1-weighted sequences and hypointense on T2-weighted sequences relative to the renal parenchyma. Involution of the tumor mass on surveillance studies is more prominent following CA due to tissue resorption, than with RFA where the lesion is replaced by scar tissue [99]. Gill et al. reported that tumor size decreased an average of 75 % 3 years post ablation. A further 38 % of cryoablated tumors were not detectable by MR imaging at 3 years (Fig. 12.3) [107].
A 65-year-old woman underwent CT imaging for the workup of pancreatic cysts. She was found to have bilateral renal tumors. Biopsy showed renal cell carcinoma, clear cell type and Fuhrman nuclear grade 2 on the right and 1 on the left. Genetic analysis was negative for VHL. The left upper pole renal tumor (not shown here) was treated with percutaneous ablation. (a) Axial CT image of the abdomen after administration of IV contrast shows a solid mass (arrowhead) in the lateral mid-pole of the right kidney. The tumor was not easily seen on CT images without contrast. (b) Axial T2-weighted MRI shows the tumor as a bright, hyperintense lesion (arrowhead). She underwent MRI-guided cryoablation of her right renal tumor. (c) Axial T2-weighted MR image of the patient in prone position shows the ice ball (I) covering the entire tumor. (d) Axial contrast-enhanced CT of the abdomen 3 months after ablation shows the ablation zone (A) as non-enhancing soft tissue. (e) Follow-up CT study at 22 months shows complete resorption of the ablated tumor
When recurrence is suspected on follow-up imaging, further management options include active surveillance, repeated ablations, and surgical extirpation. Given that the mean growth rate of small renal masses is 0.13 cm per year, surveillance is reasonable [108]. The majority of recurrences are managed with repeat ablation. Between 7.4 % and 8.5 % of all RF lesions and 0.9 % and 1.3 % of all CA lesions are reablated [61, 109]. In a review of 337 CA patients and 283 RFA patients, Long et al. reported reablation rates of 2.5 % for those who underwent percutaneous CA, 8.8 % for those who underwent percutaneous RFA, and 0 % for those treated with laparoscopic RFA or CA [109]. The inferior results observed with RFA may relate to the inability to precisely monitor treatment efficacy during the procedure compared with CA and perhaps a lower threshold to repeat the percutaneous ablation in the presence of suspicious imaging results. In addition, larger applicators and their placement under direct vision are possible with a laparoscopic approach. Repeat ablations may be performed laparoscopically or percutaneously, although repeat laparoscopic intervention is more challenging. Matin et al. reported 4.2 % incidence of local disease progression after repeat ablations at 2-year follow-up [98]. Salvage nephrectomy is reserved for those in whom reablations have failed or the tumor is too large for reablation. While a surgical resection may be technically feasible, intraoperative and postoperative complications are greater [110].
8 Complications
Complications following energy ablation of a renal mass are infrequent and have an incidence of 3–12 % [52, 111–114]. Johnson et al. reviewed complications following 271 RF and CA procedures, both percutaneous and laparoscopic, performed at four institutions. A total of 30 complications (11.1 %) occurred including 5 major (1.8 %), 25 minor (9.2 %), and 1 death (0.4 %). Major and minor complication rates were 1.4 % and 12.2 % for CA and 2.2 % and 6 % for RFA [112]. Atwell and colleagues reported their single institution with 573 percutaneous RFA and CA procedures. They reported 63 overall complications (11 %) including 38 (6.6 %) major complications and no deaths. Major complication rates were 8.4 % for CA and 4.7 % for RFA, while minor complication rates were 4.8 % for CA and 5.1 % for RFA [114].
Ablation-related injuries are either mechanical or thermal. Structures that are at greatest risk of injury are nerves, vessels, the renal collecting system, and adjacent bowel. Hemorrhage is the most common major complication and is more commonly associated with CA [112, 114]. It usually arises from direct mechanical injury to a vessel by the applicator. The risk is greater with centrally located tumors in which the applicator may traverse numerous segmental vessels en route to the lesion. Bleeding requiring transfusion has been reported in <1 % of RFA and 4.9 % of CA cases. In a review from Lehman et al., major hemorrhage accounted for over 60 % of complications in lesions over 3 cm in size treated via laparoscopic CA [64]. In a retrospective review of 108 percutaneous CAs of lesions over 3 cm, Schmit et al. reported an 8 % major complication rate. Significant hemorrhage following removal of the cryoprobes from the ablated tumor occurred in four of the six patients who sustained a major complication [115]. Cracking of the ice ball with associated parenchymal injury is a recognized, albeit uncommon complication of CA that can result in significant hemorrhage [116, 117]. Potential risk factors include the use of larger-diameter and/or multiple CA probes, initiating a second adjacent ice ball after the primary ice ball had already been formed, and removal of the CA probes before the ice ball has completely thawed [116, 117]. If hemodynamic stability cannot be restored with conservative measures, trans-arterial embolization may be required. Massive hemorrhage due to an arteriovenous fistula is rare but has been described [118]. Bleeding may be avoided by ensuring that coagulopathies and thrombocytopenia are corrected in advance, antiplatelet and anticoagulant agents are held for an appropriate period prior to the procedure, and patient movement is minimized with adequate sedation. Continuous monitoring of the applicator during placement using ultrasound or CT fluoroscopy, ensuring the applicator position is stable before ablation is commenced, can help to minimize hemorrhage. In addition, pre-procedure arterial embolization might also help reduce hemorrhage after CA [73]. Ultrasound or CT imaging of the kidney should be performed at the end of the procedure to rule out bleeding. If ureteral or urethral obstruction with clots occurs, ureteric stenting and/or urinary catheter placement with bladder irrigation may be required.
The incidence of direct thermal injury to the ureter, usually with RFA, has been reported at 1–2 % [52, 111, 114]. Tumors located in the medial aspect of the lower pole are at greatest risk of injury due to their close proximity to the ureter. The risk of ureteral stricture is increased when the distance between the tumor and ureter is less than 2 cm [119]. Retrograde pyeloperfusion using a chilled dextrose solution can help avoid injury during ablation [44, 75, 76]. The trade-off may be suboptimal ablation due to heat sink from the adjacent fluid. CT urography should be performed following ablation if an injury is suspected. The injury can manifest radiologically as ureteral wall thickening, periureteral fat stranding, hydronephrosis, or urinoma. If not promptly identified, acute renal failure can ensue.
Perinephric fat thickness less than 5 mm between the tumor and the bowel is associated with increased risk of thermal injury to the bowel. The risk is greatest with lower pole anterior lesions. Bowel wall thickening is the most likely finding on immediate post-procedure CT. In the weeks after the procedure, the bowel may become adherent to the kidney. Long-term serious sequelae include stricture, obstruction, and perforation. Adjuvant techniques to avoid bowel injury are described in Sect. 12.5.
Pneumothorax has an incidence of 2 % [111]. The risk is greatest with upper pole RCC in which the lung base overlies the proposed electrode trajectory. The majority of cases can be managed conservatively. Moderate to severe pneumothoraces or those associated with new respiratory symptoms may require aspiration and possible chest tube placement. Seeding of the needle track is extremely rare, and enhancing nodules along the needle track often represent inflammatory nodules [120–122].
9 Conclusion
Partial nephrectomy remains the gold standard for the treatment of RCC. However, RFA and CA have been shown to be safe and effective treatment options in a select patient population. While the future of these minimally invasive therapies appears promising, the interpretation and validation of the data that exists are fraught with difficulty. Standardization of reporting criteria including clearly defined treatment outcomes and pretreatment histological proof of disease are required to better define the long-term oncologic efficacy of thermal ablation therapies.
Clinical Vignette
A 65-year-old man with history of diabetes mellitus, COPD, and coronary artery disease underwent CT urography without contrast for evaluation of left renal stone. CT study did not show any renal stones. However, an incidental 2.5-cm solid mass was identified in the lower pole of the right kidney. At the time of his presentation, his GFR was 65 mL/min/1.73 m2. He underwent a contrast-enhanced CT examination for better characterization of the right renal mass. Contrast-enhanced CT confirmed the presence of a 2.5-cm solid mass that showed rapid enhancement after administration of iodinated contrast. There were no suspicious nodes or metastases. Chest radiograph did not show any pulmonary nodules. A preoperative assessment placed him at moderate risk for surgery. He was then referred to interventional radiology for consideration of percutaneous thermal ablation.
A percutaneous CT-guided core biopsy showed renal cell carcinoma, clear cell type and Fuhrman nuclear grade 2. He had normal coagulation parameters including a normal INR and platelet count. He was treated with CT-guided radiofrequency ablation without complications. He was admitted for overnight observation and was discharged home on day 1 following the ablation. He returned to work on post-procedure day number 3.
His follow-up imaging for the first year consisted of CT (renal protocol) at 1, 6, and 12 months. For the second year after his ablation, he had CT scans at 18 and 24 months. These studies demonstrated a non-enhancing zone of ablation that showed some evidence of involution in the first year but remained stable thereafter. A biopsy of the ablation zone at 1 year after ablation showed necrotic tissue and no viable tumor. He will continue to have CT examination of abdomen and chest radiography once a year.
References
Goldberg SN et al (2009) Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 20(7 Suppl):S377–S390
Goldberg SN, Gazelle GS, Mueller PR (2000) Thermal ablation therapy for focal malignancy – a unified approach.pdf. AJR Am J Roentgenol 174:323–331
Hsu TH, Fidler ME, Gill IS (2000) Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology 56(5):872–875
Gage AA, Baust J (1998) Mechanisms of tissue injury in cryosurgery. Cryobiology 37(3):171–186
Gage AA, Baust JM, Baust JG (2009) Experimental cryosurgery investigations in vivo. Cryobiology 59(3):229–243
Hoffmann NE, Bischof JC (2002) The cryobiology of cryosurgical injury. Urology 60(2 Suppl 1):40–49
Silverman SG et al (2000) MR imaging-guided percutaneous cryotherapy of liver tumors: initial experience. Radiology 217(3):657–664
Campbell SC et al (1998) Renal cryosurgery: experimental evaluation of treatment parameters. Urology 52(1):29–33; discussion 33–34
Chosy SG et al (1998) Monitoring renal cryosurgery: predictors of tissue necrosis in swine. J Urol 159(4):1370–1374
Littrup PJ et al (2009) Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol 20(10):1343–1351
Ahrar K et al (2010) Preclinical assessment of a 980-nm diode laser ablation system in a large animal tumor model. J Vasc Interv Radiol 21(4):555–561
Kariniemi J et al (2010) MRI-guided percutaneous laser ablation of small renal cell carcinoma: initial clinical experience. Acta Radiol 51(4):467–472
Dick EA et al (2002) Magnetic resonance imaging-guided laser thermal ablation of renal tumours. BJU Int 90(9):814–822
Gettman MT et al (2002) Laparoscopic interstitial laser coagulation of renal tissue with and without hilar occlusion in the porcine model. J Endourol 16(8):565–570
Simon CJ, Dupuy DE, Mayo-Smith WW (2005) Microwave ablation: principles and applications. Radiographics 25(Suppl 1):S69–S83
Floridi C et al (2014) Microwave ablation of renal tumors: state of the art and development trends. Radiol Med 119(7):533–540
Carrafiello G et al (2010) Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol 33(2):367–374
Castle SM, Salas N, Leveillee RJ (2011) Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology 77(4):792–797
Clark PE et al (2007) Microwave ablation of renal parenchymal tumors before nephrectomy: phase I study. AJR Am J Roentgenol 188(5):1212–1214
Guan W et al (2012) Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol 106(3):316–321
Horn JC et al (2014) Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: technique and initial results. J Vasc Interv Radiol 25(3):448–453
Laeseke PF et al (2009) Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol 20(9):1224–1229
Liang P et al (2008) Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol 180(3):844–848; discussion 848
Lin Y et al (2014) Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology 83(2):357–363
Moore C et al (2010) Effects of microwave ablation of the kidney. J Endourol 24(3):439–444
Skonieczki BD et al (2011) Radiofrequency and microwave tumor ablation in patients with implanted cardiac devices: is it safe? Eur J Radiol 79(3):343–346
Zhang D et al (2009) Percutaneous microwave ablation or nephrectomy for VX-2 carcinoma in rabbit kidney. J Urol 182(4):1588–1593
Klatte T, Marberger M (2009) High-intensity focused ultrasound for the treatment of renal masses: current status and future potential. Curr Opin Urol 19(2):188–191
Marberger M et al (2005) Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int 95(Suppl 2):52–55
Margreiter M, Marberger M (2010) Focal therapy and imaging in prostate and kidney cancer: high-intensity focused ultrasound ablation of small renal tumors. J Endourol 24(5):745–748
Neeman Z et al (2005) Radiofrequency ablation for tumor-related massive hematuria. J Vasc Interv Radiol 16(3):417–421
Wood BJ, Grippo J, Pavlovich CP (2001) Percutaneous radio frequency ablation for hematuria. J Urol 166(6):2303–2304
Altunrende F et al (2011) Image guided percutaneous probe ablation for renal tumors in 65 solitary kidneys: functional and oncological outcomes. J Urol 186(1):35–41
Raman JD et al (2010) Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int 105(4):496–500
Shingleton WB, Sewell PE Jr (2003) Cryoablation of renal tumours in patients with solitary kidneys. BJU Int 92(3):237–239
Syvanthong C, Wile GE, Zagoria RJ (2007) Effect of radiofrequency ablation of renal tumors on renal function in patients with a solitary kidney. AJR Am J Roentgenol 188(6):1619–1621
Weisbrod AJ et al (2010) Percutaneous cryoablation of masses in a solitary kidney. AJR Am J Roentgenol 194(6):1620–1625
Hoffmann RT et al (2010) Renal cell carcinoma in patients with a solitary kidney after nephrectomy treated with radiofrequency ablation: mid term results. Eur J Radiol 73(3):652–656
Shingleton WB, Sewell PE Jr (2002) Percutaneous renal cryoablation of renal tumors in patients with von Hippel-Lindau disease. J Urol 167(3):1268–1270
Yang B et al (2013) Probe ablation as salvage therapy for renal tumors in von Hippel-Lindau patients: the Cleveland Clinic experience with 3 years follow-up. Urol Oncol 31(5):686–692
Matin SF et al (2008) Patterns of intervention for renal lesions in von Hippel-Lindau disease. BJU Int 102(8):940–945
Karam JA et al (2010) Radio frequency ablation of renal tumors in patients with metastatic renal cell carcinoma. J Urol 184(5):1882–1887
Bodily KD et al (2010) Hydrodisplacement in the percutaneous cryoablation of 50 renal tumors. AJR Am J Roentgenol 194(3):779–783
Cantwell CP et al (2008) Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol 19(7):1034–1040
Farrell MA et al (2003) Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol 181(5):1315–1317
Kam AW et al (2004) Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol 15(7):753–758
Gupta A et al (2009) General anesthesia and contrast-enhanced computed tomography to optimize renal percutaneous radiofrequency ablation: multi-institutional intermediate-term results. J Endourol 23(7):1099–1105
Ahrar K et al (2005) Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol 16(5):679–688
Frank I et al (2003) Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 170(6 Pt 1):2217–2220
Clark TW et al (2009) Reporting standards for percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol 20(7 Suppl):S409–S416
Tsivian M et al (2014) Small renal mass biopsy – how, what and when: report from an international consensus panel. BJU Int 113(6):854–863
Gervais DA et al (2005) Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 185(1):64–71
Atwell TD et al (2008) Percutaneous renal cryoablation: experience treating 115 tumors. J Urol 179(6):2136–2140; discussion 2140–2141
Zagoria RJ et al (2004) Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol 183(1):201–207
Takaki H et al (2014) Radiofrequency ablation versus radical nephrectomy: clinical outcomes for stage T1b renal cell carcinoma. Radiology 270(1):292–299
Atwell TD et al (2007) Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol 188(5):1195–1200
Gervais DA et al (2000) Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology 217(3):665–672
Brashears JH 3rd et al (2005) Renal cryoablation and radio frequency ablation: an evaluation of worst case scenarios in a porcine model. J Urol 173(6):2160–2165
Allaf ME et al (2005) Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation–initial observations. Radiology 237(1):366–370
Rukstalis DB et al (2001) Clinical experience with open renal cryoablation. Urology 57(1):34–39
Kunkle DA, Uzzo RG (2008) Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 113(10):2671–2680
Hui GC et al (2008) Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 19(9):1311–1320
Link RE et al (2006) Cost analysis of open, laparoscopic, and percutaneous treatment options for nephron-sparing surgery. J Endourol 20(10):782–789
Lehman DS et al (2008) First prize (tie): laparoscopic renal cryoablation: efficacy and complications for larger renal masses. J Endourol 22(6):1123–1127
Badger WJ et al (2009) Laparoscopic renal tumor cryoablation: appropriate application of real-time ultrasonographic monitoring. J Endourol 23(3):427–430
Caviezel A et al (2008) Percutaneous cryoablation of small kidney tumours under magnetic resonance imaging guidance: medium-term follow-up. Scand J Urol Nephrol 42(5):412–416
Lepetit-Coiffe M et al (2010) Real-time monitoring of radiofrequency ablation of liver tumors using thermal-dose calculation by MR temperature imaging: initial results in nine patients, including follow-up. Eur Radiol 20(1):193–201
Cestari A et al (2007) Laparoscopic cryoablation of small renal masses: technique and results after 6-year experience. Eur Urol Suppl 6(10):646–652
Woolley ML et al (2002) Effect of freezing parameters (freeze cycle and thaw process) on tissue destruction following renal cryoablation. J Endourol 16(7):519–522
Littrup PJ et al (2007) CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol 18(3):383–392
Javadi S et al (2010) Characterization of contrast enhancement in the ablation zone immediately after radiofrequency ablation of renal tumors. J Vasc Interv Radiol 21(5):690–695
Mondshine RT et al (2008) Combination embolization and radiofrequency ablation therapy for renal cell carcinoma in the setting of coexisting arterial disease. J Vasc Interv Radiol 19(4):616–620
Woodrum DA et al (2010) Role of intraarterial embolization before cryoablation of large renal tumors: a pilot study. J Vasc Interv Radiol 21(6):930–936
Yamakado K et al (2006) Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol 29(3):389–394
Rouviere O et al (2008) Radiofrequency ablation of renal tumors with an expandable multitined electrode: results, complications, and pilot evaluation of cooled pyeloperfusion for collecting system protection. Cardiovasc Intervent Radiol 31(3):595–603
Wah TM et al (2005) Radiofrequency ablation of a central renal tumor: protection of the collecting system with a retrograde cold dextrose pyeloperfusion technique. J Vasc Interv Radiol 16(11):1551–1555
Froemming A et al (2010) Probe retraction during renal tumor cryoablation: a technique to minimize direct ureteral injury. J Vasc Interv Radiol 21(1):148–151
Park BK, Kim CK (2008) Using an electrode as a lever to increase the distance between renal cell carcinoma and bowel during CT-guided radiofrequency ablation. Eur Radiol 18(4):743–746
Ginat DT, Saad WE (2010) Bowel displacement and protection techniques during percutaneous renal tumor thermal ablation. Tech Vasc Interv Radiol 13(2):66–74
Lee SJ et al (2006) Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol 17(12):1967–1969
Carey RI, Leveillee RJ (2007) First prize: direct real-time temperature monitoring for laparoscopic and CT-guided radiofrequency ablation of renal tumors between 3 and 5 cm. J Endourol 21(8):807–813
Ahrar K et al (2005) Percutaneous transthoracic radiofrequency ablation of renal tumors using an iatrogenic pneumothorax. AJR Am J Roentgenol 185(1):86–88
Halverson SJ et al (2013) Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol 189(2):441–446
Patel U, Sokhi H (2012) Imaging in the follow-up of renal cell carcinoma. AJR Am J Roentgenol 198(6):1266–1276
Breda A, Anterasian C, Belldegrun A (2010) Management and outcomes of tumor recurrence after focal ablation renal therapy. J Endourol 24(5):749–752
Ma Y et al (2014) Long-term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int 113(1):51–55
Psutka SP et al (2013) Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 63(3):486–492
Ramirez D et al (2014) Laparoscopic radiofrequency ablation of small renal tumors: long-term oncologic outcomes. J Endourol 28(3):330–334
Best SL et al (2012) Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol 187(4):1183–1189
Olweny EO et al (2012) Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 61(6):1156–1161
Zagoria RJ et al (2011) Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology 77(6):1393–1397
Levinson AW et al (2008) Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol 180(2):499–504; discussion 504
Johnson S et al (2014) Laparoscopic cryoablation for clinical stage T1 renal masses: long-term oncologic outcomes at the Medical College of Wisconsin. Urology 84(3):613–618
Georgiades CS, Rodriguez R (2014) Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol 37:1494
Tanagho YS et al (2012) Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology 80(2):307–314
Aron M et al (2010) Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol 183(3):889–895
Lucas SM et al (2008) Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 179(1):75–79; discussion 79–80
Matin SF et al (2006) Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol 176(5):1973–1977
Wile GE et al (2007) CT and MR imaging after imaging-guided thermal ablation of renal neoplasms. Radiographics 27(2):325–339; discussion 339–40
Boss A et al (2005) Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol 40(9):583–590
Merkle EM, Nour SG, Lewin JS (2005) MR imaging follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology 235(3):1065–1071
Merkle EM, Nour SG, Lewin JS (2005) MR imaging follow-up after radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology 235(3):1065–1071
Hegarty NJ et al (2006) Probe-ablative nephron-sparing surgery: cryoablation versus radiofrequency ablation. Urology 68(1 Suppl):7–13
Stein AJ et al (2008) Persistent contrast enhancement several months after laparoscopic cryoablation of the small renal mass may not indicate recurrent tumor. J Endourol 22(11):2433–2439
Schwartz BF et al (2006) Cryoablation of small peripheral renal masses: a retrospective analysis. Urology 68(1 Suppl):14–18
Shingleton WB, Sewell PE Jr (2001) Percutaneous renal tumor cryoablation with magnetic resonance imaging guidance. J Urol 165(3):773–776
Gill IS et al (2005) Renal cryoablation: outcome at 3 years. J Urol 173(6):1903–1907
Jewett MA et al (2011) Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 60(1):39–44
Long L, Park S (2009) Differences in patterns of care: reablation and nephrectomy rates after needle ablative therapy for renal masses stratified by medical specialty. J Endourol 23(3):421–426
Nguyen CT et al (2008) Surgical salvage of renal cell carcinoma recurrence after thermal ablative therapy. J Urol 180(1):104–109; discussion 109
Zagoria RJ et al (2007) Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol 189(2):429–436
Johnson DB et al (2004) Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol 172(3):874–877
Park BK, Kim CK (2009) Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol 19(9):2180–2190
Atwell TD et al (2012) Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 23(1):48–54
Schmit GD et al (2010) Percutaneous cryoablation of renal masses >or=3 cm: efficacy and safety in treatment of 108 patients. J Endourol 24(8):1255–1262
Hruby G et al (2008) Risk factors associated with renal parenchymal fracture during laparoscopic cryoablation. BJU Int 102(6):723–726
Schmit GD et al (2010) Ice ball fractures during percutaneous renal cryoablation: risk factors and potential implications. J Vasc Interv Radiol 21(8):1309–1312
Park BK, Kim CK, Moo HL (2007) Arteriovenous fistula after radiofrequency ablation of a renal tumor located within the renal sinus. J Vasc Interv Radiol 18(9):1183–1185
Gervais DA et al (2005) Radiofrequency ablation of renal cell carcinoma: part 2, lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 185(1):72–80
Durack JC et al (2014) Late emergence of contrast-enhancing fat necrosis mimicking tumor seeding after renal cryoablation. J Vasc Interv Radiol 25(1):133–137
Javadi S et al (2007) Unexpected atypical findings on CT after radiofrequency ablation for small renal-cell carcinoma and the role of percutaneous biopsy. J Vasc Interv Radiol 18(9):1186–1191
Lokken RP et al (2007) Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol 189(4):845–848
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing
About this chapter
Cite this chapter
Sabir, S.H., Shaw, C.M., Matin, S.F., Ahrar, K. (2015). Thermal Ablative Techniques in Renal Cell Carcinoma. In: Lara, P., Jonasch, E. (eds) Kidney Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-17903-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-17903-2_12
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17902-5
Online ISBN: 978-3-319-17903-2
eBook Packages: MedicineMedicine (R0)