Abstract
Due to the widespread use of abdominal imaging, the number of incidentally detected renal cortical neoplasms has significantly increased. There are multiple treatment modalities in the management of renal masses, including active surveillance for indolent and slow-growing tumors and extirpation, which remains the gold standard treatment. Thermal ablation, which in contemporary practice is currently largely defined as including cryoablation and radiofrequency ablation, has emerged as an alternative treatment option in selected patients with a small renal cortical neoplasm. With the continuous improvements in contemporary imaging modalities such as computer tomography, magnetic resonance imaging, and facilitated ultrasound technology, the role of ablation in the management of renal cortical neoplasms will almost certainly expand. Additionally, the truly minimally invasive nature of percutaneous image-guided ablation will further increase the appeal of ablative technologies. Emerging long-term follow-up data after cryoablation and radiofrequency ablation is promising with 5- and 10-year cancer-specific survival rates approaching extirpation treatment modalities. Repeat ablation after primary failure of cryoablation is feasible and safe with excellent medium-term follow-up outcomes. In this chapter, we will discuss the current state of thermal ablation including cryoablation and radiofrequency ablation. We will provide details on indications for thermal ablation and patient selection, surgical techniques, and perioperative outcomes. Finally, we will summarize the long-term oncologic outcomes of thermal ablation and the results of salvage cryoablation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Kidney cancer
- Renal cell carcinoma
- Cryoablation
- Radiofrequency ablation
- Radiation
- Minimally invasive
- Ultrasonography
- Computed tomography
- Laparoscopy
- Renal cortical neoplasm
- Percutaneous cryoablation
- Laparoscopic cryoablation
Introduction
Kidney cancer is among the ten most common cancers in men and women, accounting for approximately 62,700 of new cases and 14,240 deaths per year in the United States [1]. Due to the increased use of cross-sectional imaging for abdominal imaging in recent decades, there has been a significant rise in the incidental detection and subsequent treatment of renal cortical neoplasms (RCN) [2]. The majority of RCN are discovered in early stages resulting in a paradigm shift in the management of small renal mass (SRM) (T1a). Historically, the standard treatment for all RCN, including SRM, was radical nephrectomy, although the management of RCN has evolved with the advancement of minimally invasive technology . The development of laparoscopic nephrectomy (LRN) in the 1990s—a technique first described by Clayman, Kavoussi, and colleagues—commenced a new era in treatment of RCN [3]. Consequently, LRN became the preferred treatment option for RCN. The pervasive use of LRN, however, led to two major sequelae. First, radical nephrectomy (RN) by any technique resulted in diminished renal function, which has been associated with poor cardiovascular outcomes, and decreased survival [4–7]. Second, insight into the natural history and progression of SRM was impeded due to the extensive use of RN.
Partial nephrectomy (PN) gradually emerged as a viable alternative to RN in the treatment of RCN, representing a nephron-sparing approach capable of averting chronic kidney disease (CKD) and cardiovascular sequelae associated with RN. Evidence of excellent outcomes following partial nephrectomy for SRM led the American Urological Association (AUA) to recommend that partial nephrectomy become the gold standard for all T1 (≤7 cm) lesions when surgically feasible [8].
Recently, further advances in minimally invasive technology have expanded the spectrum of available treatment modalities for SRM. Treatments now include laparoscopic PN (LPN) and robot-assisted PN (RAPN), in addition to ablative modalities and active surveillance. While PN remains the current gold standard treatment for RCN [9], thermal ablation (TA) has emerged as a viable, less-invasive alternative to surgical extirpation, for patients who are poor surgical candidates, those with bilateral tumors or functioning solitary kidney. Cryoablation (CA) , which may be delivered both laparoscopically (LCA) or percutaneously (PCA), and radiofrequency ablation (RFA) represent two TA modalities that have been best studied. Long-term retrospective studies regarding the efficacy of CA and RFA are emerging, allowing assessment of their viability as alternatives to PN. Several other TA technologies have also been developed, including high-intensity focused ultrasound (HIFU) , laser interstitial thermal ablation, radiosurgery, and microwave ablation. In contrast to CA and RFA, few studies have addressed the efficacy of these approaches. This chapter will focus on LCA, PCA, and RFA, highlighting their indications, surgical approaches, and long-term oncological outcomes as therapeutic options for SRM.
The Small Renal Mass Dilemma
With the advancement of minimally invasive techniques , several treatment modalities are now available to patients for the treatment of RCN. While large RCN (> 4 cm lesions) are frequently extirpated by either PN or RN, determining the optimal approach for SRM (≤ 4 cm lesions) is more complex. In addition, considering factors such as patient age, patient preference, tumor size, and physician preference, among others, there may be a role for renal mass biopsy in influencing treatment decisions. In current AUA guidelines (updated 2011), active surveillance (AS) , TA approaches (e.g., CA and RFA), and PN are all considered viable treatment options for T1a and T1b tumors, although PN remains the gold standard treatment.
As the literature on SRM has matured, the natural history of SRM is gradually being elucidated. It is now known that approximately 20 % of SRMs are benign, while another 50–60 % display low-grade features, and the remaining 20–30 % display aggressive features [10–12]. Given that a significant percentage of SRMs are benign or relatively indolent, surgical intervention may now be delayed or avoided following appropriate diagnostic workup.
A large series recently published by our group indicated that most SRMs grow slowly, with a growth rate of 0.34 cm/year and low metastatic rate (1.9 %), suggesting that AS is a reasonable treatment option for RCN in older patients [13]. Similarly, another study reported comparable findings with SRM having an annual tumor growth rate of 0.31 cm/year and 1.4 % metastatic rate [14]. The AUA guidelines panel concluded these rates of metastasis were sufficiently low and concluded that AS is a reasonable option in certain patient populations. Recent studies now point to expanding the use of AS. A study by Patel and colleagues suggested that AS appears to provide oncological efficacy equivalent to surgery , at least in the short- and intermediate-term management of SRM—a finding that requires confirmation in further studies [15]. It is now also thought that T1b and T2 renal tumors demonstrate similar growth rates compared to smaller T1a tumors. Growth rates for these tumors were found to be 0.58 cm/year, and this suggests AS may represent a viable treatment option even in larger renal tumors and should be considered in patients presenting with significant competing risks or limited life expectancy [16, 17].
Partial nephrectomy remains the current gold standard treatment of T1a renal cell carcinoma (RCC) , although long-term follow-up data on CA and RFA and results from emerging studies involving AS may warrant reassessment of treatment indications. As data continues to emerge and the role of renal biopsy has been expanding, the algorithm of directing the urologist toward immediate nephron-sparing surgical extirpation may continue to be amended to support increased use of AS and ablative therapy. Patients seeking to avoid surgical resection can now be directed toward ablative therapy, given promising long-term data supporting its routine use. With the guidance of renal biopsy , AS also must be considered a viable alternative both as a strategy in initial management of nonaggressive SRM and in management of small recurrences following ablative or extirpative therapy. In the elderly, given the morbidity of active treatment , it is recommended that AS should be instituted followed by a minimalistic approach, such as ablation in those patients who progress or do not tolerate AS [18].
Renal Biopsy
Traditionally, most solid RCN were presumed to be malignant and treated with surgical extirpation. However, contemporary series have demonstrated that only 80 % of tumors less than 4 cm are malignant and that only a minority are high grade with potentially aggressive features [12]. Given the knowledge that at least 20 % of tumors are benign or relatively indolent, with proper diagnostic workup, there are many instances in which surgical intervention can be delayed or avoided completely [19]. The prediction of histopathology based on preoperative imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) is limited and contemporary imaging modalities do not provide sufficient, reliable, and reproducible information for differential diagnosis of benign tumors, except for angiomyolipoma [20]. Percutaneous renal mass biopsy (RMB) has emerged as a reliable and safe diagnostic procedure to preoperatively characterize the histology and grade of SRMs. With a variety of treatment modalities available for SRMs, including AS, extirpative surgery, and TA technologies, indications for a RMB are expanding. Several studies have used RMB as a guide in treatment decisions in the management of patients with RCN [21–23].
The overview of contemporary series of RMB is provided in Table 2.1 [23–34]. Diagnostic rate and accuracy of SRM biopsy have steadily improved, and in contemporary series, it may be greater than 90 %. This is related to accumulating and growing experience with the procedure, continuous improvement in biopsy techniques, and facilitating technology [24]. Contemporary technology now allows the assessment of the histopathology of renal masses to properly counsel the patients and select the optimal treatment strategy [35]. Although minimally invasive treatment options for RCC have expanded, preoperative diagnosis is crucial for their proper use. According to meta-analysis performed by Kutikov and colleagues, only 75.8 % of patients who underwent CA had proven malignancy during intraoperative biopsy [36]. More than 20 % of patients who have had a benign histopathology with no potential threat to the patient still underwent ablative procedures. This and many other reports again raise a concern of an overtreatment of many indolent SRMs. Surgical resections or ablation may not be necessary for benign and certain indolent malignant RCN. In a study by Hu and colleagues, who evaluated the role of biopsy in the management of 206 patients with SRM, the diagnostic rate was 89 %. Of these, 84 % of patients who had biopsy-proven benign disease avoided any surgical intervention and were actively surveyed [35]. The consequences of indeterminate biopsy results are unknown and challenging to define. It is impossible to determine the relationship between the indeterminate and negative biopsy results if the patient did not undergo surgical extirpation. This topic is increasingly becoming one of concern [37]. Jewett and colleagues performed a repeat biopsy on patients with initially non-diagnostic biopsy results and demonstrated a malignancy diagnostic rate of 80 %, which was similar to initial biopsy rate [25]. This study has demonstrated that repeat RMB is feasible, safe, and can be expected to identify tumors with a similar success rate as the initial overall biopsy cohort.
Additionally, percutaneous RMB has been reported to be safe with the overall mean rate of minor and major complications of 5 % and 0.02 %, respectively (Table 2.1). In experienced centers, most complications are limited to local hematoma with minimal morbidity. With the contemporary facilitated ultrasound (US) technology and properly selected patients, the procedure can be performed in less than 15 minutes in an outpatient office setting [35].
Preoperative histopathological diagnosis of SRM with percutaneous biopsy along with other patient-related factors such as age, tumor size, and existing patient comorbidities is crucial in the decision-making process and selecting the most optimal treatment modality for patients with SRM. Beyond the fact that many SRMs are benign, there are major biologic differences between RCC subtypes, which may impact management strategies. As such, pretreatment biopsy should be considered for all RCN.
Tumor seeding along the biopsy needle tract is exceedingly rare. There are only three documented events in the last three decades [38–41]. Akhavein and colleagues reported a case of an 84-year-old man with an asymptomatic 2.7 cm enhancing lower pole renal masses. Preoperative radiological evaluation demonstrated no evidence of metastases, and preoperative biopsy confirmed histopathological diagnosis of a clear cell renal cell carcinoma [41]. Due to the patient’s comorbidities, percutaneous cryoablation was recommended. The patient underwent an uneventful percutaneous cryoablation with no evidence of residual disease at the termination of the procedure and at 5 and 12 months of follow-up. However, the patient underwent surveillance imaging at 15 months post-ablation, and while there was no evidence of local recurrence , he had numerous soft tissue nodules in the retroperitoneal fat posterior to the kidney, consistent with seeding in the cryoablation probe tract. Histopathological confirmation with biopsy was not possible due to an intraoperative complication, and the patient was managed with systemic therapy and close imaging surveillance. Sainani and colleagues reported another event of RCC seeding along the cryoablation probe tract [39]. A 61-year-old man with three bilateral masses on each side with a biopsy-proven RCC and oncocytoma underwent MRI-guided percutaneous cryoablation of three tumors and extirpative procedure for the remaining tumors that were not deemed amenable for an ablative procedure. In 4 years after the initial procedure, imaging revealed new enhancing soft tissue nodules up to 1.2 cm in the right retroperitoneum and paraspinal musculature. CT-guided biopsies revealed papillary RCC, and all enhancing lesions were managed with CT-guided cryoablation. At the follow-up imaging, there was no evidence of tumor. Mullins and Rodriguez reported a third case of RCC seeding of a percutaneous biopsy tract [38]. They reported a case of a 68-year-old man with papillary-type RCC who underwent a percutaneous biopsy. Local extension was detected at the time of partial nephrectomy, and biopsy confirmed papillary-type RCC. The patient underwent successful surgical excision of the tumor with no evidence of tumor recurrence on subsequent imaging surveillance.
These reports are very rare and should not discourage the use of percutaneous image-guided procedures such as biopsy or ablation . Proper actions can be taken to prevent these events [42].
Role, Indications, and Contraindications of Ablative Therapy
Given that most patients with SRM are elderly (>70 years) and present with significant comorbidities, it is important to balance the risks of surgical treatment with less-invasive ablative approaches and active surveillance.
The rationale for AT is to treat asymptomatic SRM in patients at high surgical risk with potentially reduced morbidity. Other potential indications include renal insufficiency, solitary kidney, transplant patients, and multiple or bilateral masses. With contemporary technology and techniques, these procedures can be performed in outpatient setting using image guidance with significantly reduced morbidity. The limitation for treating a renal lesion with CA is largely dependent upon obtaining an adequate ablation zone with the current technology. The larger the renal lesion, the more challenging it becomes to completely cover the lesion with the iceball while avoiding complications such as tumor cracking and bleeding. Patient preference plays an important role in selecting the choice of treatment. The less-invasive nature of renal ablation makes this modality very attractive for elderly patients with serious medical comorbidities who desire active treatment.
Contraindications to AT are tumors with a low chance of successful treatment, including tumor size greater than 3.5 cm. Location is another important factor to consider; posteriorly and laterally located tumors are more amenable for image-guided PCA . More anterior tumors are treated via laparoscopic approach. Hilar tumors close to the renal vasculature, ureter, and collecting system should avoid AT due to an increased risk of major complications and risk of recurrence.
Surgical Approach
CA and RFA are the most extensively characterized TA modalities. Both can be pursued laparoscopically or percutaneously under image guidance. The surgical approach is largely dependent on the location of the renal mass (Fig. 2.1). Lesions located on the anterior aspect of the kidney are more suitably approached laparoscopically via a transperitoneal approach , while posteriorly and laterally located tumors are best approached either percutaneously (CT or MRI guided) or via a retroperitoneal laparoscopic technique . Given the difficulties in approaching lateral tumors, they present a small challenge with the approach being based on surgeon preference. The majority of RFA is performed percutaneously, while CA has been well described both laparoscopically (trans- and retroperitoneally) and percutaneously. Another significant factor is the availability of CT ablation suites and having a good working relationship with an interventional radiology team. Interventional radiologists have extensive knowledge of image-guided ablation and can be outstanding partners for achieving optimal treatment outcome.
Tumors located on the posterior aspect of the kidney (red) are ideally approached either percutaneously or via retroperitoneal laparoscopy . Tumors located on the anterior aspect of the kidney (green) are ideally approached by transperitoneal laparoscopy. Tumors located on the lateral aspect of the kidney (blue) can be approached by any technique
Patient Preparation
Preoperatively, patients should undergo a history and physical examination that includes a complete set of vitals, careful review of the past medical and surgical history, social history including smoking history, and a review of their medications. Laboratory examination should include a complete metabolic panel, complete blood count, and, when appropriate, a coagulation panel. All patients over the age of 40 should undergo a preoperative electrocardiogram and a chest X-ray. Elevated liver enzymes may suggest either Stauffer’s syndrome or, perhaps more ominous, metastasis to the liver. Careful reevaluation of the liver with axial imaging is warranted. Abnormal neurological findings or recent onset of headaches or blurred vision should prompt the surgeon to investigate the possibility of brain metastasis with a head CT or MRI . Similarly, complaints of bony pain, especially with concomitant elevations in serum alkaline phosphatase and/or calcium, could be indicative of bony metastasis, which should be evaluated with a nuclear bone scan. Finally, anticoagulants , including aspirin products, should be discontinued for an appropriate amount of time prior to treatment, and these patients should often be managed in conjunction with a medical team. The goal of this extensive preoperative routine is to identify potential obstacles that may affect surgical outcome. For example, vital signs may identify poorly controlled or previously unidentified hypertension, which places the patient at risk for intraoperative and postoperative bleeding, or labs that reveal a coagulopathy may increase bleeding diathesis. A thorough preoperative workup will stratify individual patients into the various management strategies mentioned earlier.
Recent high-quality axial imaging via CT or MRI with and without intravenous contrast is a key component to every preoperative routine. Poor quality or inadequate imaging may compromise surgical outcomes and should therefore be repeated prior to discussing management strategies. The surgeon should take special note of tumor characteristics such as size; location, especially in relation to the upper, lower, and interpolar regions, hilum and the collecting system (especially the ureter and ureteropelvic junction); and enhancement properties. Additionally, renal landmarks should be identified to aid in intraoperative location of the mass. Other metrics that should be recorded include whether the mass is exophytic (≥ 50 % of mass extending beyond renal contour), mesophytic (20–50 % of mass beyond renal contour), cystic or solid, enhancement qualities, and abnormalities of shape or contour that may have to be accounted for during TA [43]. Additionally, recent evidence supports the use of the RENAL nephrometry score (Radius, Exophytic/endophytic properties, Nearness of the tumor to the collecting system or sinus, Anterior/posterior, Location relative to the polar lines) as a preoperative metric capable of predicting PN, LCA, and PCA complexity, complication rates, and outcomes [44–50]. Okhunov and colleagues demonstrated that tumors with RENAL nephrometry of higher than eight have significant risks for complications and local tumor recurrences after LCA [44]. Blute and colleagues also confirmed these findings in patients undergoing PCA. With each increase in RENAL nephrometry score , the risk of complications and recurrence increases 1.5-fold [51]. Additionally, skin-to-tumor distance has been shown to be an important factor in patients undergoing PCA. While the RENAL nephrometry score does not appear to be predictive of complications in RFA [52, 53], a modified RENAL score, using an adjusted size variable, R, may allow more accurate prediction and stratification of outcomes [54].
Occasionally, despite the use of high-quality axial imaging, the renal mass is difficult to discern from the surrounding normal renal parenchyma. This can be especially true with endophytic lesions. A preoperative ultrasound of the kidney may help characterize and further delineate the lesion. This may also prove useful since ultrasonography is the primary intraoperative imaging modality utilized in LCA. If the lesion is isoechoic on preoperative ultrasound, it may be difficult to accurately locate at the time of LCA, and options should be preoperatively discussed with the patient.
Principles of Ablation
As new technologies continue to shape the surgical landscape, it is the responsibility of the surgeon to fully understand the method of action, capabilities, and limitations of each new advancement in order to optimize outcomes. This is especially true of TA, which utilizes unique energy delivery systems, different methods of action for tissue destruction, and different targeting and monitoring systems. A proper appreciation for the various treatment modalities improves efficacy and decreases the complication rate.
Cryoablation
Cryoablation was first described in 1995 by Uchida and colleagues [55], and it is currently the most studied of all ablative modalities in the treatment of SRM. CA exploits the Joule-Thomson principle to produce rapid temperature decreases at the probe tip [56]. At room temperature, with the exceptions of hydrogen, helium, and neon, all gases cool upon expansion. As gas molecules expand, collision rates between molecules decrease, thereby increasing potential energy and decreasing kinetic energy and therefore temperature. Specifically, the modern system utilizes highly pressurized liquid state argon gas that is allowed to expand into the gaseous state near the tip of the probe. The resulting expansion and phase change causes extreme drops in temperature, which induces iceball formation. Iceball dimensions and ablation zones are largely affected by the probe’s design (at what point the gas is allowed to expand and changes in insulation) along with local tissue properties. The iceball does not extend appreciably beyond the tip of the probe but instead extends radially and proximally along the shaft of the probe.
There are several mechanisms that are ultimately responsible for cell death. The rapid cooling initially produces extracellular ice crystal formation followed by intracellular ice crystal formation. The intracellular crystals mechanically disrupt the cell membrane causing dramatic changes in intracellular pH and ionic composition, ultimately leading to protein denaturation. The extreme temperatures bring about local microcirculatory failure , which causes thrombosis, coagulation necrosis, and apoptosis. The dramatic fall in temperature additionally amplifies the extracellular osmotic force resulting in cellular crenation and dehydration. The sum of these effects is uniform cellular death within the ablation zone.
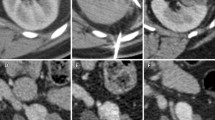
There is not one consistent temperature within the iceball but actually a gradient that extends from −140 to −190 °C at the cryoprobe tip to −3 °C at the edge of the iceball [57]. The phenomenon known as freezing point depression necessitates a temperature below 0 °C at the edge of the iceball. When solutes are added to a solvent, in this case the saline environment of tissue, these ions interfere with ice formation requiring a temperature below freezing in the periphery. This important property of the iceball is the main determinant in CA success and failures. While there is extracellular ice crystal formation at the iceball edge, there are no intracellular ice crystals, and it is the intracellular ice that causes cell lysis. Cellular death begins to occur at temperatures below −20 °C but is somewhat inconsistent [58]. Uniform and consistent cellular necrosis does not occur until temperatures fall below −40 °C. When the iceball temperature gradient is combined with the temperature requirements for cell death , three “zones” with different ablation properties are created within the iceball (Fig. 2.2). The central zone extends from the cryoprobe tip to the points within the iceball that are consistently below −40 °C. This central zone is characterized by consistent and uniform cellular necrosis. The intermediate zone comprises the iceball area that has reached temperatures between −40 °C and −20 °C and is characterized by both necrotic and viable tissue elements. The outer zone extends from −20 °C to the warmer iceball edge and is characterized by mostly viable tissue. It has been determined that temperatures of >−20 °C can be measured within 3.1 mm of the iceball edge [59]. Therefore, the standard practice in CA is to extend the iceball to 1 cm beyond the tumor edge to ensure uniform tissue ablation. One of the advantages of LCA is the ability to monitor iceball formation in real time using a laparoscopic ultrasound probe. The expanding iceball creates a readily visualized hyperechoic expanse that delineates the iceball edge (Fig. 2.3). After the freeze cycle is complete, helium is used to actively thaw the cryoprobe followed by a repeat freeze-thaw cycle to ensure complete ablation.
Iceball ablation zones : The central/necrosis zone is characterized by uniform ablation and temperatures < −40 °C. Surrounding the central zone is the indeterminate zone, which has areas of cell death intermixed with viable cells and temperatures between −40 °C and −20 °C. The outermost zone is comprised of mostly viable cells with little to no necrosis and temperatures > −20 °C
(a) At the start of the freeze cycle , the iceball appears as an expanding hyperechoic region extending radially from the cryoprobes. (b) The hyperechoic regions begin to coalesce as the iceball expands. (c) At the completion of the freeze cycle, the iceball appears as a single hyperechoic mass that extends beyond the margin of the mass
Radiofrequency Ablation
The first report of RFA in the human kidney was in 1997 by Zlotta and colleagues, who utilized RFA in the treatment of exophytic renal masses in three patients [60]. After studies demonstrating safety and short- and intermediate-term efficacy, RFA has since gained significant popularity as a technique to ablate SRM. RFA induces thermal injury through a high-frequency, alternating electric current with a wavelength of 460–500 kHz that exploits the resistive properties of the kidney [61–63]. Probes introduced into the ablation zone deliver the electrical current to the target area, inducing the resistive heating of tissues adjacent to the electrode (Joule effect ). The local tissue’s high resistance allows dramatic increases in temperature as the electrical current is transformed to heat, resulting in ionic agitation, denaturation of proteins, membrane damage, and vascular congestion [64]. Cellular injury does not typically occur until temperatures reach 50 °C for 4 to 6 min [65]. Instantaneous coagulative necrosis occurs as temperatures climb over 60 °C [66]. Given that temperatures over 105 °C induce tissue vaporization and ineffective ablation, RFA is optimally performed at temperatures 60–100 °C. To ensure adequate treatment, the ablation zone is extended to 1 cm beyond the tumor periphery. Because the ablation zone cannot be monitored in real time in RFA, temperature or impedance probes are placed near the area of interest to determine the extent of the effect.
Cryoablation Techniques
Maximizing the success of CA involves a combination of appropriate patient selection, understanding, and appropriately applying cryosurgical technology, adhering to the “imaging trifecta,” precise initial probe placement, and accurate iceball management with a willingness to make intraoperative adjustments to any inconsistencies. Patient selection has been discussed elsewhere in this chapter, but in brief, the ideal patient has a mass ≤3.5 cm in size and has been preoperatively evaluated and counseled appropriately, and the approach has been tailored to the tumor location. The imaging trifecta refers mostly to the laparoscopic approach but certainly pertains to all TA modalities. The first part is the preoperative, high-quality imaging that allows the surgeon to accurately characterize the mass. The second is the liberal use of intraoperative imaging including laparoscopic ultrasound (LCA) . Laparoscopic approach for renal ablation is used infrequently but still remains an option in selected patients with anteriorly located tumors. For image-guided PCA, US and CT or a combination of both is used during the tumor evaluation and probe placement. The final aspect is careful iceball monitoring during the freeze-thaw cycles to ensure that the iceball forms as expected with all of the expected margins extending beyond the mass. Correct initial probe placement might be among the most important determinants in success. Once the iceball begins to form, the probe cannot be repositioned, and furthermore, the expanding iceball creates a large acoustic shadow that makes targeting of the deep tissues difficult (Fig. 2.4). Occasionally, local tissue properties and/or poor initial probe placement creates an iceball that does not completely ablate the tumor. When this occurs, the surgeon should allow the probes to thaw, reassess, and reposition the probes and perform a repeat cycle to ensure complete tissue destruction.
Laparoscopic Cryoablation
After the patient is repositioned, trocars are placed in a standard nephrectomy template. The colon is reflected medially, and if on the right side, the duodenum is kocherized. The psoas muscle is identified as it courses posteromedial to the lower pole of the kidney. At this point, we usually place a laparoscopic retractor (Jarit® Padron Endoscopic Exposing Retractor (P.E.E.R.) , Integra, Plainsboro, NJ) through a 5-mm port positioned in the midaxillary line or just anterior to it. This not only allows the kidney to be elevated for the remainder of the dissection but also for it to be positioned and stabilized in a manner that optimizes the renal mass’ position during the actual ablation.
For lesions that are >3.5 cm or are exophytic , there is an increased risk for iceball cracking with subsequent major bleeding. In patients in whom this is a concern, the routine practice is to prepare the kidney as if a partial nephrectomy was going to be performed. The renal artery and vein are completely exposed, and Gerota’s fascia is dissected away from the mass and the surrounding normal renal parenchyma. In this manner, should iceball cracking occur, clamping the renal artery can rapidly attain hemostasis, and the surgeon can proceed with partial nephrectomy without delay.
In order to maximize ablation efficacy, the cryoprobes should enter the intended ablation zone perpendicular to the mass. Tangentially placed probes are difficult to accurately position and often lead to viable residual tumor. First the kidney is manipulated to expose the renal mass to the anterolateral flank using the PEER to stabilize it. A BD™ Spinal Needle (BD Medical, Franklin Lakes, NJ) is used as a “finder needle ” by passing it percutaneously until an ideal perpendicular trajectory is identified. A skin incision is then made adjacent to the spinal needle and several biopsies of the mass are taken using a Bard® MaxCore Disposable Core Biopsy Instrument (18G × 25 cm, Bard Peripheral Vascular, Inc./Bard Biopsy Systems, Tempe, AZ). The cryoprobes are then deployed at the predefined trajectory to sit at right angles to the mass. There are a variety of probes that are currently available; however, we prefer the IceRod cryoprobe (Galil Medical, Minneapolis, MN) due to its small size (1.47 mm) and consistently large ablation zone.
Of all the steps in renal ablation , accurate cryoprobe deployment ranks among the most important. It should be recognized that the iceball extends radially along the shaft of the probe, but does not extend appreciably beyond the tip [67]. To avoid deep margin recurrence, the probes should therefore be positioned 5 mm beyond the tumor. For solid masses, the probes are placed just within the tumor’s margin. If the mass has cystic components, the cryoprobes are placed just outside the margin to avoid rupture and subsequent tumor spillage. Once the freeze cycle begins, the expanding iceball obscures the margins, making subsequent probe placement more challenging.
Tumor identification , especially endophytic tumors , probe deployment, and active iceball monitoring are all facilitated by the use of a laparoscopic ultrasound probe. Typically two freeze-thaw cycles are performed to ensure complete ablation , during which active ultrasonography ensures that the iceball extends 1 cm beyond the margins. In this manner, cryoablation is unique among other TA techniques in that the direct visualization of the growing iceball verifies complete ablation of the intended target. Following the second thaw cycle, the probes are removed, and the kidney is observed for a short period of time.
Percutaneous Cryoablation
Our team has found that optimizing successful outcomes with PCA requires close collaboration between interventional radiology (IR) and urology . The interventionist provides experience with percutaneous targeting and imaging modalities, while the urologist provides expertise and insight into the treatment of renal malignancies. As mentioned previously, PCA is usually reserved for tumors on the posterior and lateral aspect of the kidney. Because the probes are passed from the posterolateral flank into the kidney, performing PCA on an anterior renal mass requires traversing a significant portion of the kidney and is not recommended.
The “as low as reasonably achievable” (ALARA) principle states that the lowest dosage of ionizing radiation necessary should be used to achieve the desired therapeutic or diagnostic goal, without compromising quality of care. At University of California, Irvine we developed a technique combining the use of US in conjunction with CT imaging for PCA of renal masses. This technique is used in an effort to reduce the total radiation dose per procedure.
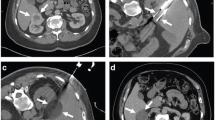
The patient is placed prone on a CT scanner or, if the probes are MRI compatible, on an MRI scanner . Intravenous sedation utilizing midazolam and fentanyl is initiated with monitoring in accordance with the UC Irvine Moderate Sedation Policy . Local lidocaine 1 % is used for local anesthesia. In CT-guided cases, US is used to localize the tumor (Fig. 2.5a), and the intended initial access location is identified. Initial probe placement is performed under US (Fig. 2.5b). A focused non-contrast axial image is then obtained through the area of the kidney and compared to the preoperative contrast image (Fig. 2.5c, d). Based upon CT findings, the initial probe placement is optimized if necessary. If the tumor margins cannot be clearly identified, a repeat scan with a half bolus of intravenous contrast can be performed. Additional cryoprobes (up to a total of 3) are placed under US guidance, with limited axial CT acquisition used for final confirmation of optimal probe position. Probes are positioned one at a time, ensuring that the tips extend at least 5 mm beyond the deep margin. Careful attention should be paid when deploying multiple probes to avoid confusion in matching the intracorporeal cryoprobes as seen on axial imaging to the extracorporeal shafts as seen by the surgeon. Once desired cryoprobe deployment is achieved, a standard double freeze-thaw cycle is performed with the goal of extending the iceball approximately 1 cm beyond the tumor margins in all directions. Toward the end of the first freeze cycle, limited axial CT images without contrast are obtained to assess iceball geometry and to ensure iceball extension beyond the margins of the tumor in all dimensions. Limited axial CT images without contrast are again obtained at the midpoint of the second freeze cycle to reassess adequacy of the iceball. Although seldom employed, active iceball formation can also be monitored using US. After completion of the double freeze-thaw cycles and removal of cryoprobes, a half-dose contrast-enhanced limited CT is obtained to confirm complete ablation of the SRM and to confirm the presence of a surrounding therapeutic margin and to identify possible viable tumor. Enhancement within or near the margin of the expected ablation zone is suggestive of residual tumor, which can be treated with the deployment of an additional cryoprobe and repeat ablation.
Imaging of ultrasound-facilitated computed tomography-guided percutaneous cryoablation . (a) Long axis ultrasound image of the left kidney. Xs indicate superior and inferior poles of kidney. Solid arrowheads delineate borders of the cortical neoplasm. (b) Long axis ultrasound image of the left kidney. Solid arrows mark the cryoprobe traversing subcutaneous fat, muscle, and perirenal fat with tip of the first cryoprobe within the superior aspect of the neoplasm. Solid arrowheads mark the cortical neoplasm. (c) Scout computed tomography image demonstrates the presence of initial cryoprobe, placed under ultrasound guidance. Solid arrows mark cryoprobe. (d) Initial low-dose limited axial computed tomography images confirming cryoprobe location within the cortical neoplasm after initial ultrasound-guided placement. Solid arrow identifies cryoprobe tip within the neoplasm
Oncological Outcomes and Follow-up
Successful outcomes of cryoablated tumors are characterized on CT imaging by significant shrinkage and loss of contrast enhancement [68]. Tumors successfully treated with RFA demonstrate no contrast enhancement with minimal shrinkage on CT [69]. On MRI, the imaging hallmark of successful renal tumor ablation is lack of tumor enhancement at gadolinium-enhanced imaging. Rim enhancement, believed to represent reactive change, may occasionally be seen at early post-procedural MR scanning after RFA or cryoablation, which later resolves.
Efficacy of Cryoablation
Longer-term follow-up studies (>60 months) assessing the efficacy of CA are beginning to emerge. Multiple long-term studies of LCA have demonstrated that this technique provides excellent oncological outcomes [70]. Although, there are fewer reports of longer-term follow-up after PCA in the literature, the limited data is similarly promising.
In a recent study of 138 patients undergoing LCA with mean follow-up of 98.8 months, Caputo and colleagues determined 5-year disease-free survival (DFS) , cancer-specific survival (CSS) , and overall survival (OS) of 86.5 %, 96.8 %, and 79.1 %, respectively. Ten-year DFS, CSS, and OS were 86.5 %, 92.6 %, and 53.8 %, while mean time to recurrence was 2.3 years post-ablation [71]. In another study of 112 T1 tumors including 92 RCC-confirmed tumors with the mean follow-up of 97.9 months post LCA, Johnson et al. determined OS, progression-free survival (PFS) , and CSS of 98.5 %, 91.0 %, and 98.5 %, respectively [72]. Similarly, Tanagho and co-workers reported 76 months of follow-up data from 35 RCC-confirmed tumors treated with LCA, noting 6-year DFS, CSS, and OS of 80 %, 100 %, and 76.2 %, respectively, and excellent renal functional outcomes. The study demonstrated six patients (17 %) who experienced local recurrences after LCA [73]. Aron and colleagues reported their data on 80 patients who underwent LCA with median follow-up of 93 months [74]. The study reported 5-year OS, disease-specific survival (DSS ), and RFS of 84 %, 92 %, and 81 %, respectively, and 10-year OS, DSS, and RFS of 51 %, 83 %, and 78 %, respectively. In this study, however, the ablation was performed using a single, large 4.8-mm probe; as this single probe technique has been largely supplanted by the use of multiple ultrathin (1.47 mm) probes, the continual evolution of technology and technique and its effect on outcomes remains to be seen.
Recent studies comparing CA and PN are also emerging. While perioperative outcomes in CA are superior, the data is unclear whether CA represents an increased risk of recurrence. Thompson and colleagues recently showed that recurrence rate was similar in patients who underwent PN and PCA for cT1 renal masses [75]. Overall survival was superior after PN, likely resulting from selection bias. A meta-analysis by Klatte and colleagues compared laparoscopic PN and LCA, combining 13 studies. They found that patients treated with LCA demonstrated a shorter length of stay, less blood loss, and lower risk of complications, but that LCA was associated with an increased risk of recurrence (relative risk = 9.39) and metastatic progression (RR = 4.68) [76].
Studies comparing LCA vs PCA have found no difference in overall mortality or recurrence rates. Kim and colleagues compared 145 LCA and 118 PCA cases with mean follow-up 71.4 months for LCA and 38.6 months for PCA. The reported 5-year OS and RFS for LCA were 79.3 % and 85.5 %, respectively. Five-year OS and RFS for PCA were 86.3 % and 86.3 %, respectively. Cryoablation approach (LCA vs PCA) was not predictive of overall mortality or disease recurrence, although mean length of stay was shorter for PCA [77]. Similarly, Zargar and colleagues determined no significant difference in OS or RFS at 5 years between the patients undergoing LCA (n = 275) and PCA (n = 137). Tumor size and anterior location were predictive of higher local recurrence rates, while RENAL nephrometry score or type of cryoablation was not associated with tumor recurrence [78].
CA represents an alternative approach to the treatment of renal masses. The long-term oncological outcomes are promising, as are the improved renal functional outcomes , compared to PN. These findings make CA an ideal minimally invasive modality and support its use in a wider population. However, there is still an unclear risk of increased recurrence in CA , which balances the improved perioperative outcomes.
Salvage Cryoablation in the Setting of Recurrence Following Primary Cryoablation
Recurrence rate after focal TA is relatively higher when compared to extirpation. This increased oncological failure rate incites the potential need for salvage procedure. Currently there are no guidelines or recommendations regarding the management of recurrences following TA. This has led to controversy regarding the most appropriate salvage treatment therapy. The management of recurrent disease after PCA poses a great challenge to urologists and interventional radiologists. Extirpative management of locally recurrent RCC can be challenging due to the local fibrosis and eradication of anatomical surgical planes [79]. As thermal ablation of SRM emerges as a viable alternative to surgical extirpation, many patients are now treated with repeat PCA after recurrence following primary PCA. According to a literature review performed between 2000 and 2006 by Long and colleagues, repeat cryoablation is the most common treatment modality following failed prior cryoablation. Approximately 66 % to 73 % of patients who fail thermal ablation are managed by repeat focal therapy; overall, 0.9 % of all renal masses that underwent CA and fail receive salvage TA treatment [80]. The data is very limited regarding patient’s characteristics, perioperative complications, and oncologic outcomes in those undergoing repeat ablation. Overall, repeat PCA has demonstrated an improved safety and convalescence profile compared to salvage LPN . Despite the technical challenges of the procedure, repeat PCA has gained increased popularity among urologists [81]. The advantages of using PCA in this patient population include faster convalescence, significantly shorter operative times, less pain, and the ability to perform the procedure under moderate sedation thus providing a viable treatment option for patients with significant comorbidities avoiding the risks associated with general anesthesia. Hegg and colleagues reported a major complication rate of 5.7 % in patients who underwent repeat PCA after local recurrence following LPN [82]. In our recent series, 8 % of 250 patients who underwent PCA for SRM underwent secondary ablation for biopsy-proven RCC recurrence. Our data demonstrated 86 % success rate with the mean follow-up of 30 months (Fig. 2.6). Only three patients were identified to have a second episode of local recurrence following PCA. All three patients were found to have biopsy-confirmed chromophobe-type RCC. One patient was reablated for a third time and two patients underwent laparoscopic partial nephrectomy . All three patients had no evidence of local or distant tumor progression at later follow-up visits. There were no complications, and no patients needed blood transfusions. All procedures were performed in less than 2 h with no patients needing general anesthesia [83]. Repeat PCA for locally recurrent disease is technically feasible, has a low complication rate, and demonstrates acceptable short-term oncologic outcomes in this challenging population.
Efficacy of Radiofrequency Ablation
The recent emergence of longer-term follow-up reports has demonstrated that RFA provides durable oncologic outcomes comparable to those reported following partial nephrectomy, in addition to improved renal functional outcomes. Multiple studies with follow-up > 60 months have demonstrated that RFA as a treatment of T1a RCCs provides long-term oncological control with survival rates comparable to those in PN [84–88].
A report by Olweny and colleagues compared patients with histologically confirmed T1a RCC treated by percutaneous RFA (n = 37) and PN (n = 37) with a median follow-up of 6.5 years. There were no significant differences in any of the survival rates between the two treatment groups: for RFA vs PN, the 5-year OS was 97.2 % vs 100 % (p = 0.31); CSS was 97.2 % vs 100 % (p = 0.31); DFS was 89.2 % vs 89.2 % (p = 0.78); local RFS was 91.7 % vs 94.6 % (p = 0.96); and metastasis-free survival (MFS) was 97.2 % vs 91.8 % (p = 0.35), respectively [89]. Chang and co-workers compared a propensity-matched cohort of T1a patients treated with RFA (n = 45) and LPN (n = 45), with a median follow-up 67.6 months. For RFA, the 5-year OS, CSS, DFS, RFS, and MFS were 90.2 %, 95.6 %, 86.7 %, 95.4 %, and 95.5 %, respectively. For LPN, these rates were 93.2 %, 97.7 %, 88.5 %, 97.7 %, and 95.5 %, respectively. The authors also found that RFA provided better renal functional preservation than PN [90].
Additional studies have similarly shown improved renal functional outcomes in RFA when compared to PN. A study of patients undergoing RFA (n = 21) and RN (n = 39) for T1b cancer determined that although OS was significantly lower in RFA vs RN, the RCC-related survival rate and disease-free survival rates were comparable between the two groups, and RFA was associated with less renal function decrease (12.5 %) compared to PN (32.5 %) [91]. Recently, Ji and colleagues found that for treatments of cT1a renal tumors, laparoscopic RFA provided excellent perioperative results, long-term functional and oncological outcomes. The decrease in glomerular filtration rate (GFR) was significantly lower in the LRFA group than the LPN group (p = 0.021) [92]. Faddegon and colleagues also determined that 5-year freedom from CKD stage progression for radiofrequency ablation and partial nephrectomy was 85.4 % vs 82.1 % (p = 0.06), concluding that RFA provides similar long-term renal function preservation benefit as partial nephrectomy. [93].
In a study of 1424 cT1a patients comparing PN (n = 1057), CA (n = 187), and RFA (n = 180), Thompson and colleagues determined that while RFS was similar among the three treatments, metastasis-free survival (MFS) was superior for PN and CA patients when compared with RFA for cT1a patients (p = 0.005 and p = 0.021, respectively) [75].
Recent literature is promising and suggests that both CA and RFA are effective and durable treatment options for SRM. Like CA, RFA has undergone technological advancements that may continue to improve upon the emerging data . Additional prospective randomized studies may help further evaluate the efficacy and safety in relation to PN and CA.
Conclusion
The armamentarium in the treatment of the SRM continues to expand. Outcomes data on TA continue to mature, and recent longer-term follow-up results are very promising. These results suggest that CA and RFA may have a wider indication in the treatment of renal tumors. In order to effectively utilize the newer TA technologies, it is paramount to understand the technology being employed. The role of biopsy has been expanding and plays an important role in the decision-making process and patient counseling. Considering the myriad of options now afforded in the treatment of the SRM, a detailed discussion should be held with the patient prior to rendering any treatment, especially as the role of TA continues to expand.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–4.
Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Merety KS, Darcy MD, et al. Laparoscopic nephrectomy. N Engl J Med. 1991;324(19):1370–1.
Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–40.
McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59(6):816–20.
Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pt1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179(2):468–71. discussion 472-463
Kim SP, Thompson RH, Boorjian SA, Weight CJ, Han LC, Murad MH, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. 2012;188(1):51–7.
Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical t1 renal mass. J Urol. 2009;182(4):1271–9.
Kutikov A, Kunkle DA, Uzzo RG. Focal therapy for kidney cancer: a systematic review. Curr Opin Urol. 2009;19(2):148–53.
Lane BR, Babineau D, Kattan MW, Novick AC, Gill IS, Zhou M, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178(2):429–34.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: An analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–20.
Volpe A, Mattar K, Finelli A, Kachura JR, Evans AJ, Geddie WR, et al. Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. J Urol. 2008;180(6):2333–7.
Rosales JC, Haramis G, Moreno J, Badani K, Benson MC, McKiernan J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183(5):1698–702.
Graversen JA, Mues AC, de Lorca A P-L, Landman J. Active surveillance of renal cortical neoplasms: a contemporary review. Postgrad Med. 2011;123(1):105–13.
Patel N, Cranston D, Akhtar MZ, George C, Jones A, Leiblich A, et al. Active surveillance of small renal masses offers short-term oncological efficacy equivalent to radical and partial nephrectomy. BJU Int. 2012;110(9):1270–5.
Mehrazin R, Smaldone MC, Kutikov A, Li T, Tomaszewski JJ, Canter DJ, et al. Growth kinetics and short-term outcomes of ct1b and ct2 renal masses under active surveillance. J Urol. 2014;192(3):659–64.
Mues AC, Haramis G, Badani K, Gupta M, Benson MC, McKiernan JM, et al. Active surveillance for larger (ct1bn0m0 and ct2n0m0) renal cortical neoplasms. Urology. 2010;76(3):620–3.
Lane BR, Abouassaly R, Gao T, Weight CJ, Hernandez AV, Larson BT, et al. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer. 2010;116(13):3119–26.
Ordon M, Landman J. Renal mass biopsy: “Just do it”. J Urol. 2013;190(5):1638–40.
Lienert AR, Nicol D. Renal angiomyolipoma. BJU Int. 2012;110(Suppl 4):25–7.
Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60(1):39–44.
Tsivian M, Rampersaud Jr EN, Del Pilar Laguna Pes M, Joniau S, RJ L, WB S, et al. Small renal mass biopsy – how, what and when: report from an international consensus panel. BJU Int. 2014;113(6):854–63.
Halverson SJ, Kunju LP, Bhalla R, Gadzinski AJ, Alderman M, Miller DC, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol. 2013;189(2):441–6.
Hu R, Montemayor-Garcia C, Das K. Role of percutaneous needle core biopsy in diagnosis and clinical management of renal masses. Hum Pathol. 2015;46(4):570–6.
Leveridge MJ, Finelli A, Kachura JR, Evans A, Chung H, Shiff DA, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60(3):578–84.
Eshed I, Elias S, Sidi AA. Diagnostic value of CT-guided biopsy of indeterminate renal masses. Clin Radiol. 2004;59(3):262–7.
Neuzillet Y, Lechevallier E, Andre M, Daniel L, Coulange C. Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. J Urol. 2004;171(5):1802–5.
Shannon BA, Cohen RJ, de Bruto H, Davies RJ. The value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. J Urol. 2008;180(4):1257–61. discussion 1261
Schmidbauer J, Remzi M, Memarsadeghi M, Haitel A, Klingler HC, Katzenbeisser D, et al. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol. 2008;53(5):1003–11.
Wang R, Wolf Jr JS, Wood Jr DP, Higgins EJ, Hafez KS. Accuracy of percutaneous core biopsy in management of small renal masses. Urology. 2009;73(3):586–90. discussion 590-581
Tan HJ, Jacobs BL, Hafez KS, Montgomery JS, Weizer AZ, Wood Jr DP, et al. Understanding the role of percutaneous biopsy in the management of patients with a small renal mass. Urology. 2012;79(2):372–7.
Park SY, Park BK, Kim CK, Kwon GY. Ultrasound-guided core biopsy of small renal masses: diagnostic rate and limitations. J Vasc Interv Radiol. 2013;24(1):90–6.
Menogue SR, O’Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: A diagnostic tool to better stratify patients for surgical intervention. BJU Int. 2013;111(4 Pt B):E146–151.
Jaff A, Molinie V, Mellot F, Guth A, Lebret T, Scherrer A. Evaluation of imaging-guided fine-needle percutaneous biopsy of renal masses. Eur Radiol. 2005;15(8):1721–6.
Menhadji AD, Nguyen V, Okhunov Z, Bucur P, Chu WH, Cho J, et al. Technique for office-based, ultrasound-guided percutaneous biopsy of renal cortical neoplasms using a novel transducer for facilitated ultrasound targeting. BJU Int. 2016;117(6):948–53.
Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma–a meta-analysis and review. J Urol. 2008;179(4):1227–33. discussion 1233-1224
Babaian KN, Okhunov Z, Juncal S, Ordon M, Lusch A, Zand T, et al. Clinical outcomes of patients with nondiagnostic biopsy during cryoablation of small renal masses. Urology. 2015;85(3):605–9.
Mullins JK, Rodriguez R. Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J. 2013;7(3–4):E176–9.
Sainani NI, Tatli S, Anthony SG, Shyn PB, Tuncali K, Silverman SG. Successful percutaneous radiologic management of renal cell carcinoma tumor seeding caused by percutaneous biopsy performed before ablation. J Vasc Interv Radiol. 2013;24(9):1404–8.
Slywotzky C, Maya M. Needle tract seeding of transitional cell carcinoma following fine-needle aspiration of a renal mass. Abdom Imaging. 1994;19(2):174–6.
Akhavein A, Neuberger MM, Dahm P. Tumour-seeding: a rare complication of ablative therapy for clinically localised renal cell carcinoma. BMJ Case Rep. doi:10.1136/bcr-2012-006948.
Richard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68:1007–13.
Kutikov A, Uzzo RG. The R.E.N.A.L. Nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–53.
Okhunov Z, Shapiro EY, Moreira DM, Lipsky MJ, Hillelsohn J, Badani K, et al. R.E.N.A.L. Nephrometry score accurately predicts complications following laparoscopic renal cryoablation. J Urol. 2012;188(5):1796–800.
Sisul DM, Liss MA, Palazzi KL, Briles K, Mehrazin R, Gold RE, et al. Renal nephrometry score is associated with complications after renal cryoablation: a multicenter analysis. Urology. 2013;81(4):775–80.
Schmit GD, Thompson RH, Kurup AN, Weisbrod AJ, Boorjian SA, Carter RE, et al. Usefulness of R.E.N.A.L. Nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol. 2013;189(1):30–5.
Hayn MH, Schwaab T, Underwood W, Kim HL. Renal nephrometry score predicts surgical outcomes of laparoscopic partial nephrectomy. BJU Int. 2011;108(6):876–81.
Gupta GN, Boris R, Chung P, Linehan WM, Pinto PA, Bratslavsky G. Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up. Urol Oncol. 2013;31(1):51–6.
Hew MN, Baseskioglu B, Barwari K, Axwijk PH, Can C, Horenblas S, et al. Critical appraisal of the padua classification and assessment of the R.E.N.A.L. Nephrometry score in patients undergoing partial nephrectomy. J Urol. 2011;186(1):42–6.
Camacho JC, Kokabi N, Xing M, Master VA, Pattaras JG, Mittal PK, et al. R.E.N.A.L. (radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol. 2015;26(5):686–93.
Blute ML, Jr., Okhunov Z, Moreira DM, George AK, Sunday S, Lobko II, et al. Image-guided percutaneous renal cryoablation: Preoperative risk factors for recurrence and complications. BJU Int. 2013;111(4 Pt B):E181–185.
Seideman CA, Gahan J, Weaver M, Olweny EO, Richter M, Chan D, et al. Renal tumour nephrometry score does not correlate with the risk of radiofrequency ablation complications. BJU Int. 2013;112(8):1121–4.
Chang X, Liu T, Zhang F, Qian C, Ji C, Zhao X, et al. The comparison of r.E.N.A.L., padua and centrality index score in predicting perioperative outcomes and complications after laparoscopic radio frequency ablation of renal tumors. J Urol. 2015;194(4):897–902.
Gahan JC, Richter MD, Seideman CA, Trimmer C, Chan D, Weaver M, et al. The performance of a modified renal nephrometry score in predicting renal mass radiofrequency ablation success. Urology. 2015;85(1):125–9.
Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H. Percutaneous cryosurgery for renal tumours. Br J Urol. 1995;75(2):132–6. discussion 136-137
Rewcastle JC, Sandison GA, Saliken JC, Donnelly BJ, McKinnon JG. Considerations during clinical operation of two commercially available cryomachines. J Surg Oncol. 1999;71(2):106–11.
Gill W, Fraser J, Carter DC. Repeated freeze-thaw cycles in cryosurgery. Nature. 1968;219(5152):410–3.
Gill IS, Novick AC. Renal cryosurgery. Urology. 1999;54(2):215–9.
Campbell SC, Krishnamurthi V, Chow G, Hale J, Myles J, Novick AC. Renal cryosurgery: experimental evaluation of treatment parameters. Urology. 1998;52(1):29–33. discussion 33-24
Zlotta AR, Wildschutz T, Raviv G, Peny MO, van Gansbeke D, Noel JC, et al. Radiofrequency interstitial tumor ablation (rita) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11(4):251–8.
De Filippo M, Bozzetti F, Martora R, Zagaria R, Ferretti S, Macarini L, et al. Radiofrequency thermal ablation of renal tumors. La Radiol Med. 2014;119(7):499–511.
Ramanathan R, Leveillee RJ. Ablative therapies for renal tumors. Ther Adv Urol. 2010;2(2):51–68.
Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of renal cell carcinoma. Eur Radiol. 2005;15(5):960–7.
Aron M, Gill IS. Minimally invasive nephron-sparing surgery (minss) for renal tumours. Part II: probe ablative therapy. Eur Urol. 2007;51(2):348–57.
Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(2):323–31.
Zagoria RJ, Hawkins AD, Clark PE, Hall MC, Matlaga BR, Dyer RB, et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol. 2004;183(1):201–7.
Young JL, Kolla SB, Pick DL, Sountoulides P, Kaufmann OG, Ortiz-Vanderdys CG, et al. In vitro, ex vivo and in vivo isotherms for renal cryotherapy. J Urol. 2010;183(2):752–8.
Tsivian M, Kim CY, Caso JR, Rosenberg MD, Nelson RC, Polascik TJ. Contrast enhancement on computed tomography after renal cryoablation: an evidence of treatment failure? J Endourol. 2012;26(4):330–5.
Matsumoto ED, Watumull L, Johnson DB, Ogan K, Taylor GD, Josephs S, et al. The radiographic evolution of radio frequency ablated renal tumors. J Urol. 2004;172(1):45–8.
Rodriguez Faba O, Akdogan B, Marszalek M, Langenhuijsen JF, Brookman-May S, Stewart GD, et al. Current status of focal cryoablation for small renal masses. Urology. 2016;90:9–15.
Caputo PA, Ramirez D, Zargar H, Akca O, Andrade HS, O'Malley C, et al. Laparoscopic cryoablation for renal cell carcinoma: 100-month oncologic outcomes. J Urol. 2015;194(4):892–6.
Johnson S, Pham KN, See W, Begun FP, Langenstroer P. Laparoscopic cryoablation for clinical stage t1 renal masses: long-term oncologic outcomes at the medical college of wisconsin. Urology. 2014;84(3):613–8.
Tanagho YS, Roytman TM, Bhayani SB, Kim EH, Benway BM, Gardner MW, et al. Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology. 2012;80(2):307–14.
Aron M, Kamoi K, Remer E, Berger A, Desai M, Gill I. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. 2010;183(3):889–95.
Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for ct1 renal masses. Eur Urol. 2015;67(2):252–9.
Klatte T, Shariat SF, Remzi M. Systematic review and meta-analysis of perioperative and oncologic outcomes of laparoscopic cryoablation versus laparoscopic partial nephrectomy for the treatment of small renal tumors. J Urol. 2014;191(5):1209–17.
Kim EH, Tanagho YS, Saad NE, Bhayani SB, Figenshau RS. Comparison of laparoscopic and percutaneous cryoablation for treatment of renal masses. Urology. 2014;83(5):1081–7.
Zargar H, Samarasekera D, Khalifeh A, Remer EM, O’Malley C, Akca O, et al. Laparoscopic vs percutaneous cryoablation for the small renal mass: 15-year experience at a single center. Urology. 2015;85(4):850–5.
Breda A, Anterasian C, Belldegrun A. Management and outcomes of tumor recurrence after focal ablation renal therapy. J Endourol. 2010;24(5):749–52.
Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113(10):2671–80.
Patel SR, Abel EJ, Hedican SP, Nakada SY. Ablation of small renal masses: practice patterns at academic institutions in the United States. J Endourol. 2013;27(2):158–61.
Hegg RM, Schmit GD, Boorjian SA, McDonald RJ, Kurup AN, Weisbrod AJ, et al. Percutaneous renal cryoablation after partial nephrectomy: technical feasibility, complications and outcomes. J Urol. 2013;189(4):1243–8.
Okhunov Z, Chamberlin J, Moreira DM, George A, Babaian K, Shah P, et al. Salvage percutaneous cryoablation for locally recurrent renal-cell carcinoma after primary cryoablation. J Endourol. 2016;30(6):632–7.
Lorber G, Glamore M, Doshi M, Jorda M, Morillo-Burgos G, Leveillee RJ. Long-term oncologic outcomes following radiofrequency ablation with real-time temperature monitoring for t1a renal cell cancer. Urol Oncol. 2014;32(7):1017–23.
Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for t1 renal cell carcinoma. Eur Urol. 2013;63(3):486–92.
Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77(6):1393–7.
Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010;116(13):3135–42.
Ma Y, Bedir S, Cadeddu JA, Gahan JC. Long-term outcomes in healthy adults after radiofrequency ablation of t1a renal tumours. BJU Int. 2014;113(1):51–5.
Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical t1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156–61.
Chang X, Liu T, Zhang F, Ji C, Zhao X, Wang W, et al. Radiofrequency ablation versus partial nephrectomy for clinical t1a renal-cell carcinoma: long-term clinical and oncologic outcomes based on a propensity score analysis. J Endourol. 2015;29(5):518–25.
Takaki H, Soga N, Kanda H, Nakatsuka A, Uraki J, Fujimori M, et al. Radiofrequency ablation versus radical nephrectomy: clinical outcomes for stage t1b renal cell carcinoma. Radiology. 2014;270(1):292–9.
Ji C, Zhao X, Zhang S, Liu G, Li X, Zhang G, et al. Laparoscopic radiofrequency ablation versus partial nephrectomy for ct1a renal tumors: long-term outcome of 179 patients. Urol Int. 2016;96(3):345–53.
Faddegon S, Ju T, Olweny EO, Liu Z, Han WK, Yin G, et al. A comparison of long term renal functional outcomes following partial nephrectomy and radiofrequency ablation. Can J Urol. 2013;20(3):6785–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Okhunov, Z., Patel, R.M., Landman, J. (2017). Targeted Therapy for Localized Kidney Cancer. In: Polascik, T. (eds) Imaging and Focal Therapy of Early Prostate Cancer. Current Clinical Urology. Springer, Cham. https://doi.org/10.1007/978-3-319-49911-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-49911-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49910-9
Online ISBN: 978-3-319-49911-6
eBook Packages: MedicineMedicine (R0)