Abstract
Angiotensin II (Ang II) was described as a peripheral hormone; its synthesis and metabolism were characterized and it is currently known as the renin-angiotensin system (RAS). All the components of the RAS, including the receptors, have been found in brain tissue, indicating a role as a hormone or neuromodulator in the central nervous system. Ang II exerts its principal known actions at the AT1 receptor. Its functions related to AT2 receptors are controversial and associated with AT1 opposite effects, although there is evidence showing cross-talk between both receptors. The metabolism of Ang II generates other active peptides, such as Angiotensin 1–7 and Angiotensin IV, which will not be discussed. Neurobiological research has explained many of the different neuroendocrine and behavioral responses to stressors. Stress is a complex phenomenon in response to physical, environmental, or psychological stimulus. Stress triggers important adaptive functions improving health and survival. Meanwhile, excessive stress can be deleterious, therefore, individuals unable to cope with stress are highly vulnerable to a variety of diseases. Stress is a major contributor of cardiovascular disorders and psychiatric illness such as anxiety and depression. Many studies have confirmed that stress also increases the vulnerability to drug abuse.
The role of Ang II at the periphery and in the central nervous system is vast and complex. For this reason, in this chapter we will focus on the role of brain RAS in stress responses and related pathologies from many other important aspects of Ang II research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ptsd Symptom
- Corticotrophin Release Hormone
- Renin Angiotensin System
- Nucleus Tractus Solitarius
- Chronic Unpredictable Stress
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The Renin-Angiotensin System
Angiotensin II (Ang II) was discovered in 1940 and described as a peripheral hormone. Later its synthesis and metabolism were characterized, currently known as the renin angiotensin system (RAS) [1]. The precursor molecule is the angiotensinogen synthesized in the liver and cleaved by a renal protease, giving an inactive decapeptide, angiotensin I. This is converted into the active octapeptide ANG II, by action of a circulating enzyme called angiotensin converting enzyme (ACE), which is also responsible for inactivating bradykinin. The octapeptide hormone was subsequently found to be produced in numerous tissues, including the adrenal glands, heart, kidney, vasculature, adipose tissue, gonads, pancreas, prostate, eye, placenta. and brain [2].
The principal actions related to Ang II are vasoconstriction, aldosterone release, sodium retention, and a key role in blood pressure control and fluid homeostasis.
All the components of the RAS, including the receptors, have been found in brain tissue, indicating a role as a hormone or neuromodulator in the central nervous system [2, 3]. Ang II exerts its principal known effects acting through the AT1 receptor. The actions of Ang II related to AT2 receptors are controversial and associated with AT1 opposite effects, although there is evidence showing cross-talk between both receptors.
Angiotensin II Receptors
It has been initially described that there are two principal subtypes of Ang II receptors named AT1 and AT2. The characterization was first made based on their affinity to specific ligands and later by molecular cloning. The AT1 receptors were designed AT1A after the discovery of other subtype of receptor designed as AT1B cloned in rats [4, 5], mice [6], and humans [7]. Although the isoform AT1A is responsible for the associated functions of the brain Ang II system [8, 9], we will refer to it as AT1 receptor. The AT1 receptor is a typical heptahelical G protein-coupled receptor and is made up of 359 amino acids with an unmodified molecular weight of 41,000 [10]. AT1 receptor stimulation induces multiple cellular responses, predominantly via coupling to Gq/11 stimulates phospholipases A2, C, and D and activates inositol trisphosphate/Ca2+ signalling, protein quinase C isoforms, and mitogen-activated protein kinases (MAPKs), as well as several tyrosine kinases (Pyk2, Src, Tyks, Fak), scaffold proteins (G protein-coupled receptor kinase-interacting protein 1, p130Cas, paxillin, vinculin), receptor tyrosine kynases, and the nuclear factor-κB pathway. The AT1 receptor also signals via G12/13 proteins, and Gi/o in rodents and stimulates G protein-independent signalling pathways, such as β arrestin-mediated MAPK activation and the janus kinase/signal transducer and activator of transcription [11, 12]. The AT1 receptor is responsible for most of the known biologic effects of Ang II, including those of the central nervous system [13, 14]. Alterations in homo- or heterodimerization of the AT1 receptor may also contribute to its pathophysiological roles. Many of the deleterious actions of AT1 receptors are initiated by locally generated, rather than circulating Ang II [12]. The AT1 and AT2 receptors have a similar binding affinity for Ang II although they only share a 32–34 % identity at the amino acid level [15, 16].
Brain Ang II
It is generally accepted that the Ang II from the periphery does not cross the blood–brain barrier (BBB) but stimulates the AT1 receptors located in the circumventricular organ outside the BBB [8]. This stimulation increases the intake of fluids and salt. The brain RAS generates the Ang II which stimulates receptors inside the BBB [17]. The neuroanatomical localization of Ang II, AT1, and AT2 receptors has been precisely described in different brain areas placed on neurons, astrocytes, and oligodendrocytes [8, 18, 19]. It has also described the presence of components of RAS in glial cells suggesting a more important role for these that was previously postulated [20].
Both subtypes of receptors were found to have a similar, but not identical, distribution in all the mammalian species studied, including humans [8]. While AT1 receptors predominate in adult animals, AT2 are expressed in the developing brain [8]. AT1 receptors are located in brain areas related with the control of neuroendocrine functions and the autonomic regulation of limbic and cardiovascular systems, while AT2 receptors are involved in organogenesis and in the functions of motor and sensorial systems [21].
The role of brain Ang II is complex and is related by control of the autonomic, hormonal system, and sensorial and cognitive processes including regulation of cerebral blood flow [16].
AT1 and AT2 Receptors in Stress-Involved Brain Areas
The AT1 receptors are distributed throughout the brain, including the key areas regulating stress response such as the hypothalamus–pituitary–adrenal (HPA) axis [22]. The medial, basomedial, lateral, and basolateral nuclei of amygdala control the emotional responses such as fear conditioning and adaptation to danger, and these nuclei are enriched with AT1 receptors [23, 24]. The other stress responsive brain regions include the cortex, hippocampus, locus coerulus (LC), median eminence (ME), subfornical organ (SFO), dorsomedial hypothalamus (DMH), and nucleus tractus solitarius (NTS), and are also enriched with Ang II and its receptors. The different sections of cortex such as the prefrontal cortex, entorhinal, piriform cortex and neocortex control cognition and emotional behavior [22, 25]. The hippocampus is an important structure for storing memory processes during stress. The LC region is located in the pons (a part of the brainstem). It is actively involved in controlling the physiological response to stress. Additionally, LC is a site of origin of sympathetic innervations to the cortex involved in stress-induced central sympathetic stimulation. The DMH is a nucleus of the hypothalamus that regulates panic-like responses [26, 27]. The paraventricular nucleus (PVN) of the hypothalamus is also an important stress-involved brain region; it is activated by a variety of stressful and/or physiological changes. The SFO is a sensory circumventricular organ and is also involved in regulating the stress response. NTS is a group of cells in the brainstem that receive viscera sensory information and send it to the basal forebrain, actively involved in regulating cortical processing of anxiogenic stimuli. Moreover, additional nerve projections from the NTS to the LC, the bed nucleus of stria terminalis and the amygdaloid structures, influence the processing of anxiogenic stimuli [28]. The ME is an integral part of the hypophyseal portal system, which connects the hypothalamus to the pituitary gland. The projections of neurons from median preoptic hypothalamic nucleus to the ME regulate the stress response by controlling the release of stress hormones [29–31].
Functional and anatomical studies have shown the abundant presence of AT2 receptors in neonates, where these are involved in the development of central nervous structures [8, 22]. Although, their number is significantly reduced in the adult tissues, including the brain [32], and is restricted to the inferior olivary complex, thalamic nuclei and LC to control sensory, motor, and behavioral functions [22]. Interestingly, it has been proposed that the AT2 receptors exhibit their functional role only after their up-regulation under pathologic conditions [33], but it has been found a physiological role in mice lacking AT2 receptors (knock out), suggesting that these receptors are also functional during normal nonpathological conditions [28, 34, 35].
Ang II as a Stress Mediator
There is a large body of evidence at pharmacological, neuroanatomical, and physiological levels, supporting a key role for Ang II in the stress response, including regulation of the sympathetic and neuroendocrine systems [16, 36–38]. The presence of AT1 receptors has been shown at all levels of the HPA axis, with a higher concentration in key areas for the control of stress response, such as the PVN [39], ME, anterior pituitary, zona glomerulosa, and adrenal medulla [40]. Exposure to stress induces an increase in circulating and brain Ang II levels [41, 42]. Brain Ang II stimulates local receptors in the PVN and LC, among other nuclei, while circulating Ang II also stimulates the AT1 receptors in the subfornical organ, to which it is connected through the ME and PVN [40, 43].
Castren and Saavedra found that exposure to acute stress induced an increase in AT1 receptor density in the anterior pituitary, although exposure to repeated stress sessions increased AT1 receptor density in the PVN [44], expressed in the cellular body of neurons that synthesize corticotrophin releasing hormone (CRH) [40, 43]. In agreement with this, it has been found that AT1 receptor stimulation by Ang II induced an increase in the production of CRH [45, 46]. CRH is a hormone released to the circulation that increases adrenocorticotropic hormone (ACTH) release from the pituitary. It has been found that due to stress, high levels of adrenal glucocorticoids induced an increase in the expression of AT1 receptors in the PVN [45]. Moreover, there is local production of Ang II in the anterior pituitary which, acting together with circulating Ang II, induces an increase in ACTH secretion [38].
The PVN is an important area in the processing and integration of many different stress signals [47]. This nucleus receives noradrenergic input from the LC and serotoninergic input from the dorsal raphe nucleus, and there are reciprocal interactions between these two regions and the PVN [48, 49]. In addition, there are reciprocal neural connections between CRH neurons from the PVN and noradrenergic neurons from the LC. It has been shown that both adrenergic receptor subtypes regulate the ACTH secretion, and CRH controls central noradrenergic activity. Most of the available evidence suggests that CRH acts as a neurotransmitter in the LC modulating the noradrenergic activation in response to stress [50].
Exposure to one session of social isolation for 24 or 2 h of cold restraint induced an increase in the enzyme tyrosine hydroxylase (TH) mRNA in the LC and, in both cases, this increase was prevented by previous administration of an AT1 receptor blocker (ARB) [31, 51]. This evidence suggests that AT1 receptors are involved in the control of central sympathetic activity through the regulation of TH transcription. However, it should be taken in consideration that influences of AT2 receptor activation in the LC respect the TH regulation. This last is based on results showing dual control by AT1 and AT2 receptors in TH transcription and in the synthesis of catecholamine at the adrenal medulla [52].
The evidence obtained using ARBs gives support for a key role for Ang II in the stress response. It is important to highlight that their actions may not be limited to the HPA axis only [16, 30, 31, 51, 53]. Supporting the extra-hypothalamic influence of Ang II in the stress response are the results obtained with candesartan, an ARB, showing a prevention of isolation-induced decrease in CRH1 receptors and the GABAA complex in the brain cortex [51]. Moreover, when the animals were tested in the plus maze they exhibited an increase in the parameters associated with anxiolytic effects. Altogether, this strongly suggests a role for AT1 receptors not only in autonomic and hormonal response, but also in behavioral response to stress [51, 54].
Ang II and Stress-Related Disorders
Anxiety
The HPA axis has a major role in stress response being mediated by CRH, although the behavioral stress response mediated by CRH occurs in a manner independent of the HPA axis. This is based, among other evidence, on the fact that hypophysectomy and the blockade of the HPA axis response with dexametaxone do not alter the stress response induced by brain CRH administration. This strongly suggests a central action responsible for the coordination of stress-related behaviors [55].
It has been found that isolation stress [51], electric shock [56], and chronic unpredictable stress [57] produce, among others, a decrease in brain CRH1 density. This can be reproduced in the prefrontal cortex by intracerebral CRH administration, and a decrease in mRNA levels of CRH1 receptors has been shown in vitro in a cell line derived from CRH neurons incubated with CRH. These results support the idea of down-regulation induced by the CRH increase [58]. Interestingly, candesartan, an ARB that crosses the BBB was found to prevent a decrease in brain cortex CRH1 induced by isolation stress [51], indicating that cortical CRH activation is positively regulated by AT1 receptors, similar to what occurs at the hypothalamic level. Even so, the presence of AT1 receptors has been determined in the piriform and entorhinal cortex [22] but only of the mRNA of AT1 receptors in the neocortex [25]. This suggests that AT1 receptor blockade could reduce the decrease in cortical CRH1 receptors [51]. There is reciprocity between CRH and noradrenergic systems during stress: the LC is activated by CRH [27] and stress induces an increase in CRH in this brain area [59]. In this sense, it has been found that CRH injected into the LC induced behavioral activation and noradrenaline release in the prefrontal cortex and these two responses were blocked by a CRH receptor antagonist [60]. However, the AT1 receptors seem to modulate only the CRH1 receptors because there is no evidence showing any action on the CRH2 receptors [51].
The CRH is also related to the GABA system because GABAA receptors are located in CRH neurons. In this respect, it has been shown that the exposure to different kinds of stressors induced a decrease in the benzodiazepine binding in the frontal cortex, increasing the anxiety behavior [61, 62]. The administration of ARBs decreased the CRH release, and this could explain the prevention of the GABAA binding decrease in animals exposed to isolation stress [51].
There is much evidence showing the anxiolytic effect of ARBs described by different authors, administered orally or intracerebrally [16, 24, 63, 64].
Gastric Ulcerations
Gastric ulcerations are largely associated with stress exposure through the development of gastric ulcers or ulcerations [65] as a result of complex psychological factors influencing individual vulnerability, the stimulation of brain specific pathways regulating autonomic function, decreased blood flow to the mucosa, increase in muscular contractility, mast cell degranulation, leukocyte activation, and increased free radical generation, resulting in increased lipid peroxidation [65–68].
The events association with gastric lesion formation are sudden blood flow reduction to the gastric mucosa and increased free radical formation [67]. For this reason, the maintenance of gastric blood flow is important to protect the mucosa from endogenous and exogenous damage factors. It has been described that Ang II levels increase during stress in plasma and tissues, including the stomach tissue [41]. The role of Ang II in the stomach is the regulation of gastric vascular tone though AT1 receptor stimulation [69]. It is already known that Ang II generates reactive oxygen species with cellular damage and inflammation [70]. The mucosal vasoconstriction and proinflammatory effects of Ang II could contribute to the production of stress-induced gastric ulcers.
An experimental model commonly used to induce acute gastric damage is cold-restraint stress, which is also clinically relevant [71]. Using this stress model in male spontaneously hypertensive rats (SHRs), it was found that AT1 receptor inhibition prevented gastric lesions by combined local and systemic mechanisms, including gastric blood flow maintenance, inhibition of proinflammatory cascade activation, preventing the gastric ischemia and inflammation characteristic of a major stress response, and resulting in the protection of the gastric mucosa from stress-induced ulcerations [53]. The experiment was carried out in SHRs because these animals have an increased expression of the brain RAS components and have been described as stress-prone animals. More specifically it was found that blood flow to the stomach was significantly increased in animals treated with the ARB compared with vehicle-treated controls [53]. Interestingly, the maintenance of a normal pituitary–adrenal response to stress is a phenomenon that runs parallel with protection from gastric injury. This view is supported by the fact that endogenous corticoids contribute to protect the gastric mucosa from ulceration during stress, probably by contributing to an increase in blood flow and bicarbonate secretion [72].
The presence and density of Ang II receptors in the stomach was analyzed by autoradiography and showed the presence of AT1 receptors and a lower number of AT2 receptors, in all layers of the stomach and furthermore, the ARB treatment decreased the AT1 receptor binding [53]. This last could be the result of receptor occupancy with the insurmountable antagonist candesartan or of receptor down regulation [73]. Therefore, the decreased number of gastric AT1 receptors after stress may be related to receptor occupancy by the increased Ang II levels or to a receptor compensatory mechanism due to Ang II increased stimulation [37].
The exposure to cold restraint in rats induced a marked increase in the expression of the intercellular adhesion molecule 1 (ICAM-1), the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), and the number of infiltrating neutrophils in the gastric mucosa [53] and it is known that these components play crucial roles in the progression of gastric injury [74]. It is also known that activated neutrophils release inflammatory mediators, capable of damaging endothelial cells and inhibition of neutrophil infiltration prevents the stress-induced reduction of mucosal blood flow and the production of gastric lesions [75]. Likewise, it has been described that Ang II promotes tissue inflammation through AT1 receptor stimulation, enhancing neutrophil infiltration [76, 77], increasing the expression of TNF-α [76, 78, 79] and ICAM-1 [80]. It was reported that TNF-α down regulated AT1 receptors [81, 82], and that TNF-α, acting with other proinflammatory cytokines, up regulated AT1 receptors and increased the profibrotic effects induced by Ang II [83, 84]. The increase of TNF-α and neutrophil infiltration in the gastric mucosa induced by cold restraint was prevented by ARB [53].
The increased ICAM-1 expression induced by cold restraint exposure in the endothelium of arteries of the gastric mucosa and submucosa, and in venules of the submucosa (where AT1 receptors are located), was also prevented by the AT1 receptor blockade [53]. The anti-inflammatory effects of AT1 receptor blockade could thus be important for protection against stress-induced gastric ulcers.
Posttraumatic Stress Disorder
Posttraumatic stress disorder (PTSD) is a debilitating stress-related illness associated with exposure to trauma. The peripheral and central mechanisms mediating stress response in PTSD are incompletely understood. The renin-angiotensin pathway is essential to cardiovascular regulation but as it was described above is also involved in mediating stress and anxiety. Based on these data, Khoury et al. examined the relationship between active treatment with blood pressure medication, including ACE inhibitors and ARBs, and PTSD symptom severity within a highly traumatized civilian medical population [85]. This study was a larger study performed in patients recruited from Grady Memorial Hospital’s outpatient population from 2006 to November 2010. Multivariable linear regression models were fit to statistically evaluate the independent association of being prescribed an ACE inhibitor or ARB with PTSD symptoms, using a subset of patients for whom medical information was available (n = 505). Categorical PTSD diagnosis was assessed using the modified PTSD Symptom Scale (PSS) based on Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria, and PTSD symptom severity (the primary outcome of interest) was measured using the PSS and Clinician-Administered PTSD Scale [85]. The authors found a significant association between presence of an ACE inhibitor/ARB medication and decreased PTSD symptoms. Meanwhile, other blood pressure medications, including β-blockers, calcium channel blockers, and diuretics were not significantly associated with reduced PTSD symptoms. The authors provide the first clinical evidence supporting a role for the renin-angiotensin system in the regulation of stress response in patients diagnosed with PTSD. Further studies need to examine whether available medications targeting this pathway should be considered for future treatment and potential protection against PTSD symptoms.
Drug Abuse
The hormonal changes, involving increased peripheral glucocorticoid levels and CRH release in different brain sites, initiate a cascade of biological responses to counteract the altered homeostatic balance of the organism in response to stress. The modification in the brain physiology induced by stress triggers the release of neuroactive hormones such as biogenic amines and adrenal steroids, which activate the same neuronal circuit as the psychostimulant drugs, cocaine or amphetamine. Many years ago, clinical studies of methadone-treated heroin addicts [86] showed atypical stress response in both active and long-term abstinent heroin addicts, similar to the atypical stress response of the HPA axis that has been found in abstinent cocaine addicts [87]. Thus, it has been hypothesized that an atypical response to stressors may contribute to compulsive drug use [88]. Furthermore, it have been demonstrated in a series of studies that rats with higher levels of behavioral and neuroendocrine response to stress develop psychostimulant drug self-administration more rapidly than low responders [89, 90]. In conjunction with other evidence, this supports a major role for stress in individual vulnerability to self-administer drugs of choice for abuse. In addition, corticosterone, the major glucocorticoid end-product of HPA axis activation in rodents, was shown to be self-administered in rats [90], and pharmacological manipulation of the circulating corticosterone levels altered cocaine self-administration behavior [91]. These results and many others suggest that the activity of the HPA axis may play a role in different phases of drug addiction.
Brain Ang II was found to regulate some responses induced by drugs of choice for abuse such as cocaine and amphetamine, among others [92–95]. The presence of Ang II AT1 receptors has been described in pre- and postsynaptic CPu dopaminergic neurons [96], which are involved in the motor and behavioral responses induced by psychostimulants, as well as their modulatory action on noradrenergic [97], serotoninergic [98], glutamatergic, and gabaergic neurotransmission [99, 100]. It has been described that Ang II modulates the neuronal response to glutamate via both AT1 and AT2 receptors possibly at the postsynaptic level in the superior colliculus, locus coerulus, and dorsal lateral nucleus in addition to other areas [101–103].
There is indirect evidence of RAS involvement in neuroadaptative changes induced by psychostimulant drugs. In this sense, a history of sodium depletion, which activates RAS and Ang II synthesis, was found to develop cross-sensitization effects leading to enhanced locomotor activity responses to amphetamine or cocaine [104, 105]. Evidence was recently found in our laboratory involving the activation of brain AT1 receptors in the development [93, 94] and expression [106] of behavioral and neurochemical neuroadaptations induced by amphetamine in rats.
Hypertension
It has been found that repeated stress in rats significantly increased blood pressure and noradrenaline and adrenaline levels, and these effects were attenuated by adrenalectomy [107].
The stress response increases sympathetic nervous activity, which can adversely affect the cardiovascular system [108]. Cardiovascular disease is in part a result of stress-induced mechanisms mediated primarily through increased adrenergic stimulation. These stress-induced mechanisms include elevation in serum lipid levels, alterations in blood coagulation, atherogenesis, vascular changes in hypertension, and myocardial ischemia. Stress management interventions for hypertension are controversial; however, interventions for coronary heart disease-prone behavior patterns have proved successful. Stress management interventions have also reduced cardiovascular events, mortality, and coronary atherosclerosis. Assessment of stress includes individual interviews which can be complemented by information derived from questionnaires and mental stress testing. Educational and relaxation strategies can prepare patients to understand and cope with stress. These approaches will hopefully decrease the occurrence of stress and, ultimately, the risk for cardiovascular disease [109].
Circulating and locally formed Ang II controls cerebral blood flow by AT1 receptor stimulation in cerebral vessels and sympathetic nerves. Brain Ang II and sympathetic systems are stimulated in spontaneous hypertensive rats, producing increased vasoconstrictor tone and arterial thickness with smooth muscle proliferation, decreased vascular compliance, and decreased ability of cerebral vessels to dilate during hypoperfusion. Blockade of Ang II formation by ACE inhibitors inhibits cerebrovascular tone, and cerebral blood flow is maintained by compensatory small resistance artery vasoconstriction, improving tolerance to hypotension and increasing adaptation to the reduction in blood flow during stroke. In this sense, it was found the protective effect of chronic administration of candesartan during ischemia and the improvement of cerebral blood flow in spontaneous hipertensive rats by chronic pretreatment with candesartan [110]. Moreover, Ang II system inhibition protected against neuronal injury more effectively than other antihypertensive drugs such as calcium channel blockers. The protection after AT1 receptor blockade is not directly correlated with blood pressure reduction but with normalization of middle cerebral artery media thickness, leading to increased arterial compliance and reduced cerebral blood flow decrease during ischemia at the periphery of the lesion [110].
Conclusions
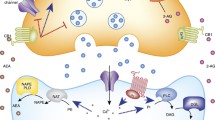
The results presented from studies on the physiological and pathological role of brain Ang II aim to encourage the study of this system in the context of the search for new pharmacological tools in the treatment of stress-related disorders (Fig. 8.1). Moreover, the advantage of the available compounds that interfere with the RAS, ACE inhibitors and ARBs, is that they are tolerated well and widely used in the treatment of hypertension. Interestingly, the ARBs do not modify the blood pressure in normotensive individuals.
References
Braun-Menéndez E, Fasciolo JC, Leloir LF, Muñoz JM. The substance causing renal hypertension. J Physiol. 1940;98:283–98.
Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology. 2003;144:2179–83.
Danser AH. Local renin-angiotensin systems: the unanswered questions. Int J Biochem Cell Biol. 2003;35:759–68.
Iwai N, Inagami T. Identification of two subtypes in the rat type 1 angiotensin II receptor. FEBS Lett. 1992;298:257–60.
Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Biochem Biophys Res Commun. 1992;31:1090–6.
Sadamura H, Hein L, Kriegger JE, Pratt RE, Kobilka BK, Dzau V. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem Biophys Res Commun. 1992;185:253–9.
Konoshi H, Kuroda S, Inada Y, Fujisawa Y. Novel subtype of human angiotensin II type 1 receptor: cDNA cloning and expression. Biochem Biophys Res Commun. 1994;199:467–74.
Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13(2):329–80.
Thomas WG, Mendelsohn FAO. Molecules in focus: angiotensin receptors form and function and distribution. Int J Biochem Cell Biol. 2003;35:774–9.
Rose JM, Audus KL. AT1 receptors mediate angiotensin II uptake and transport by bovine brain microvessel endothelial cells in primary culture. J Cardiovasc Pharmacol. 1999;33(1):30–5.
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–72.
Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20(5):953–70.
Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45(2):205–51.
Barnes JM, Steward LJ, Barber PC, Barnes NM. Identification and characterisation of angiotensin II receptor subtypes in human brain. Eur J Pharmacol. 1993;230(3):251–8.
Clauser E, Curnow KM, Davies E, Conchon S, Teutsch B, Vianello B, et al. Angiotensin II receptors: protein and gene structures, expression and potential pathological involvements. Eur J Endocrinol. 1996;134(4):403–11.
Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, et al. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept. 2005;128:237–8.
Mendelsohn FAO, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA. 1984;81:1575–9.
Daubert DL, Meadows GG, Wang JH, Sanchez PJ, Speth RC. Changes in angiotensin II receptors in dopamine-rich regions of the mouse brain with age and ethanol consumption. Brain Res. 1999;816:8–16.
Wright JW, Harding JW. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning and memory. Prog Neurobiol. 2004;72:263–93.
Fogarty DJ, Matute C. Angiotensin receptor-like immunoreactivity in adult brain white matter astrocytes and oligodendrocytes. Glia. 2001;35(2):131–46.
Phillips MI, Sumners C. Angiotensin II in the central nervous system physiology. Regul Pept. 1998;78:1–11.
Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991;261:R209–16.
Schulkin J. Angst and the amygdala. Dialogues Clin Neurosci. 2006;8(4):407–16.
Llano Lopez LH, Caif F, Garcia S, Fraile M, Landa AI, Baiardi G, et al. Anxiolytic-like effect of losartan injected into amygdala of the acutely stressed rats. Pharmacol Rep. 2012;64(1):54–63.
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type 1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82:827–41.
Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463(1–3):235–72.
Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84.
Bali A, Jaggi AS. Angiotensin as stress mediator: role of its receptor and interrelationships among other stress mediators and receptors. Pharmacol Res. 2013;76:49–57.
Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24(24):5516–24.
Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1–18.
Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM. Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress. 2008;11(6):457–66.
Baxter CR, Horvath JS, Duggin GG, Tiller DJ. Effect of age on specific angiotensin II-binding sites in rat brain. Endocrinology. 1980;106(3):995–9.
Israel A, Stromberg C, Tsutsumi K, Garrido MR, Torres M, Saavedra JM. Angiotensin II receptor subtypes and phosphoinositide hydrolysis in rat adrenal medulla. Brain Res Bull. 1995;38(5):441–6.
Voigt JP, Hortnagl H, Rex A, van Hove L, Bader M, Fink H. Brain angiotensin and anxiety-related behavior: the transgenic rat TGR(ASrAOGEN)680. Brain Res. 2005;1046(1–2):145–56.
Belcheva I, Georgiev V, Chobanova M, Hadjiivanova C. Behavioral effects of angiotensin II microinjected into CA1 hippocampal area. Neuropeptides. 1997;31(1):60–4.
Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin -releasing hormone neurons by angiotensin II. Neuroendocrinology. 1995;61:437–44.
Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, et al. Peripheral administration of an angiotensin II AT1 receptor decreases the hypothalamic-pituitary-adrenal response to stress. Endocrinology. 2001;142:3880–9.
Ganong WF, Murakami K. The role of angiotensin II in the regulation of ACTH secretion. Ann N Y Acad Sci. 1987;512:176–86.
Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 2007;1142:92–9.
Tsutsumi K, Saavedra JM. Angiotensin II receptor subtypes in median eminence and basal forebrain areas involved in the regulation of pituitary function. Endocrinology. 1991;129:3001–8.
Xang G, Xi ZX, Wan Y, Wang H, Bi G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals. 1993;2:166–72.
Yang G, Wan Y, Zhu Y. Angiotensin II an important stress hormone. Biol Signals. 1996;5:1–8.
Shigematsu K, Saavedra JM, Plunkett LM, Correa FMA. Angiotensin II binding site in the anteroventral-third ventricle (AV3V) area and related structures of the rat brain. Neurosci Lett. 1986;67:37–41.
Castrén E, Saavedra JM. Repeated stress increase the density of angiotensin II binding sites in the rat paraventricular nucleus and subfornical organ. Endocrinology. 1988;122:370–2.
Aguilera G, Kiss A, Luo X. Increased expression of type 1 of angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol. 1995;7:775–83.
Jezova D, Ochedalski T, Kiss A, Aguilera G. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol. 1998;10:67–72.
Makara GB, Antoni FA, Stark E, Karteszi M. Hypothalamic organization of corticotropin releasing factor (CRF) producing structures. In: Muller E, Macleod RM, editors. Endocrine perspective, vol. 4. Amsterdam: Elsevier; 1984. p. 71–120.
Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by retrograde tracing technique. J Comp Neurol. 1978;178:1–14.
Thiboliet E, Dreifuss JJ. Localization of neurons projecting to the hypothalamic paraventricular nucleus area of the rat : a horseradish peroxidase study. Neuroscience. 1981;6:1315–28.
Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80.
Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, et al. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 2006;31:1123–34.
Jezova M, Armando I, Bregonzio C, Yu Z-X, Quian S, Ferrans VJ, et al. Angiotensin II AT1 and AT2 receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase transcription. Endocrinology. 2003;144:2092–101.
Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol. 2003;285:G414–23.
Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci. 2003;985:308–25.
Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–89.
Anderson SM, Kant JG, De Souza EB. Effect of chronic stress on anterior pituitary and brain corticotropin-releasing factor receptors. Pharmacol Biochem Behav. 1993;44:755–61.
Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, Duman RS. Differential regulation of corticotrophin-releasing factor receptor 1 expression by stress and agonist treatments in brain and cultured cells. Mol Pharmacol. 1996;50:1103–10.
Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of immature rat brain. Exp Neurol. 2002;176:75–86.
Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C. Alterations in corticotropin-releasing factor like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–14.
Shimizu N, Nakane H, Hori T, Hayashi Y. CRF receptor antagonist attenuates stress-induced noradrenaline release in the medial prefrontal cortex of rats. Brain Res. 1994;654:145–8.
Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, Serra M. Gabaergic and dopaminergic transmission in the rat cerebral cortex: effects of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther. 1990;48:121–42.
Nutt DJ, Malizia AL. New insights into the role of the GABA (A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–4.
Barnes NM, Costall B, Kelly ME, Murphy DA, Naylor RJ. Anxiolytic-like action by DuP753, a non-peptide angiotensin II receptor antagonist. Neuroreport. 1990;1:20–1.
Kaiser FC, Palmer GC, Wallace AV, Carr RD, Fraser-Rae L, Hallam C. Antianxiety properties of the angiotensin II antagonist, Dup753, in the rat using the elevated plus-maze. Neuroreport. 1992;3:922–4.
Overmier JB, Murison R. Anxiety and helplessness in the face of stress predisposes, precipitates, and sustains gastric ulceration. Behav Brain Res. 2000;110:161–74.
Andrade TG, Graeff FG. Effect of electrolytic and neurotoxic lesions of the median raphe nucleus on anxiety and stress. Pharmacol Biochem Behav. 2001;70(1):1–14.
Tuncel N, Erkasap N, Sahinturk V, Ak DD, Tuncel M. The protective effect of vasoactive intestinal peptide (VIP) on stress-induced gastric ulceration in rats. Ann N Y Acad Sci. 1998;865:309–22.
Yelken B, Dorman T, Erkasap S, Dundar E, Tanriverdi B. Clonidine pretreatment inhibits stress-induced gastric ulcer in rats. Anesth Analg. 1999;89(1):159–62.
Heinemann A, Sattler V, Jocic M, Wienen W, Holzer P. Effect of angiotensin II and telmisartan, an angiotensin1 receptor antagonist, on rat gastric mucosal blood flow. Aliment Pharmacol Ther. 1999;13(3):347–55.
Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97(8):1916–23.
Senay CE, Levine RJ. Synergism between cold and restraint for rapid production of stress ulcer in rats. Proc Soc Exp Biol Med. 1967;124:1221–3.
Filaterova LP, Filaretov AA, Makara GB. Corticosterone increase inhibits stress-induced gastric erosions in rats. Am J Physiol. 1998;274:G1024–30.
Nishimura Y, Ito T, Hoe K, Saavedra JM. Chronic peripheral administration of the angiotensin II AT(1) receptor antagonist candesartan blocks brain AT(1) receptors. Brain Res. 2000;871(1):29–38.
Hamaguchi M, Watanabe T, Higuchi K, Tominaga K, Fujiwara Y, Arakawa T. Mechanisms and roles of neutrophil infiltration in stress-induced gastric injury in rats. Dig Dis Sci. 2001;46(12):2708–15.
Liu W, Okajima K, Murakami K, Harada N, Isobe H, Irie T. Role of neutrophil elastase in stress-induced gastric mucosal injury in rats. J Lab Clin Med. 1998;132(5):432–9.
Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:S12–22.
Tamarat R, Silvestre JS, Durie M, Levy BI. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Invest. 2002;82(6):747–56.
Nakamura A, Johns EJ, Imaizumi A, Niimi R, Yanagawa Y, Kohsaka T. Role of angiotensin II-induced cAMP in mesangial TNF-alpha production. Cytokine. 2002;19(1):47–51.
Kalra D, Sivasubramanian N, Mann DL. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation. 2002;105(18):2198–205.
Strawn WB, Dean RH, Ferrario CM. Novel mechanisms linking angiotensin II and early atherogenesis. J Renin Angiotensin Aldosterone Syst. 2000;1(1):11–7.
Sasamura H, Nakazato Y, Hayashida T, Kitamura Y, Hayashi M, Saruta T. Regulation of vascular type 1 angiotensin receptors by cytokines. Hypertension. 1997;30(1 Pt 1):35–41.
Bucher M, Ittner KP, Hobbhahn J, Taeger K, Kurtz A. Downregulation of angiotensin II type 1 receptors during sepsis. Hypertension. 2001;38(2):177–82.
Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH. Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem. 2002;277(8):5719–24.
Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002;91(12):1119–26.
Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849–55.
Dole VP, Nyswander ME. Rehabilitation of heroin addicts after blockade with methadone. N Y State J Med. 1966;66(15):2011–7.
Kreek MJ. Effects of opiates, opioid antagonists and cocaine on the endogenous opioid system: clinical and laboratory studies. NIDA Res Monogr. 1992;119:44–8.
Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47.
Piazza PV, Le Moal M. The role of stress in drug-self administration. Trends Pharmacol Sci. 1998;19:67–74.
Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90(24):11738–42.
Goeders NE. Stress, the hypothalamic-pituitary-adrenal axis, and vulnerability to drug abuse. NIDA Res Monogr. 1998;169:83–104.
Hosseini M, Sharifi MR, Alaei H, Shafei MN, Karimooy HA. Effects of angiotensin II and captopril on rewarding properties of morphine. Indian J Exp Biol. 2007;45(9):770–7.
Paz MC, Assis MA, Cabrera RJ, Cancela LM, Bregonzio C. The AT angiotensin II receptor blockade attenuates the development of amphetamine-induced behavioral sensitization in a two-injection protocol. Synapse. 2011;65(6):505–12.
Paz MC, Marchese NA, Cancela LM, Bregonzio C. Angiotensin II AT(1) receptors are involved in neuronal activation induced by amphetamine in a two-injection protocol. Biomed Res Int. 2013;2013:534817.
Watanabe MA, Kucenas S, Bowman TA, Ruhlman M, Knuepfer MM. Angiotensin II and CRF receptors in the central nucleus of the amygdala mediate hemodynamic response variability to cocaine in conscious rats. Brain Res. 2010;1309:53–65.
Brown DC, Steward LJ, Ge J, Barnes NM. Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br J Pharmacol. 1996;118(2):414–20.
Gelband CH, Sumners C, Lu D, Raizada MK. Angiotensin receptors and norepinephrine neuromodulation: implications of functional coupling. Regul Pept. 1998;73(3):141–7.
Nahmod VE, Finkielman S, Benarroch EE, Pirola CJ. Angiotensin regulates release and synthesis of serotonin in brain. Science. 1978;202(4372):1091–3.
Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1340–53.
Oz M, Yang KH, O’Donovan MJ, Renaud LP. Presynaptic angiotensin II AT1 receptors enhance inhibitory and excitatory synaptic neurotransmission to motoneurons and other ventral horn neurons in neonatal rat spinal cord. J Neurophysiol. 2005;94(2):1405–12.
Mooney RD, Zhang Y, Rhoades RW. Effects of angiotensin II on visual neurons in the superficial laminae of the hamster’s superior colliculus. Vis Neurosci. 1994;11(6):1163–73.
Xiong HG, Marshall KC. Angiotensin II modulation of glutamate excitation of locus coeruleus neurons. Neurosci Lett. 1990;118(2):261–4.
Albrecht D, Broser M, Kruger H, Bader M. Effects of angiotensin II and IV on geniculate activity in nontransgenic and transgenic rats. Eur J Pharmacol. 1997;332(1):53–63.
Clark JJ, Bernstein IL. Reciprocal cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav. 2004;78(4):691–8.
Acerbo MJ, Johnson AK. Behavioral cross-sensitization between DOCA-induced sodium appetite and cocaine-induced locomotor behavior. Pharmacol Biochem Behav. 2011;98(3):440–8.
Paz MC, Marchese NA, Stroppa MM, Gerez de Burgos NM, Imboden H, Baiardi G, et al. Involvement of the brain renin-angiotensin system (RAS) in the neuroadaptive responses induced by amphetamine in a two-injection protocol. Behav Brain Res. 2014;272C:314–23.
Dobrakovova M, Oprsalova Z, Mikulaj L, Kvetnansky R, Murgas K, Lichardus B. Hypertension induced by repeated stress: possible participation of sympathetic-adrenomedullary catecholamines. Endocrinol Exp. 1984;18(3):169–76.
Eliot RS. Stress and cardiovascular disease. Eur J Cardiol. 1977;5(2):97–104.
Engler MB, Engler MM. Assessment of the cardiovascular effects of stress. J Cardiovasc Nurs. 1995;10(1):51–63.
Ito T, Yamakawa H, Bregonzio C, Terron JA, Falcon-Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33(9):2297–303.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bregonzio, C., de los Angeles Marinzalda, M., Baiardi, G.C. (2015). Role of the Neuropeptide Angiotensin II in Stress and Related Disorders. In: Gargiulo, P., Arroyo, H. (eds) Psychiatry and Neuroscience Update. Springer, Cham. https://doi.org/10.1007/978-3-319-17103-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-17103-6_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17102-9
Online ISBN: 978-3-319-17103-6
eBook Packages: MedicineMedicine (R0)