Abstract
Few, if any, biological processes are as diverse among domestic species as establishment of early pregnancy, in particular maternal recognition of pregnancy. Following fertilization and initial development in the mare oviduct, selective transport of the embryo through the uterotubal junction driven by embryo-derived PGE2 occurs. Upon arrival in the uterus, an acellular glycoprotein capsule is formed that covers the embryo, blastocyst, and conceptus (embryo and associated extraembryonic membranes) between the second and third weeks of pregnancy. Between Days 9 and 15/16 of pregnancy, the conceptus undergoes an extended phase of mobility. Conceptus mobility is driven by conceptus-derived PGF2α and PGE2 that stimulate uterine contractions which in turn propel migration of the conceptus within the uterine lumen. Cessation of conceptus mobility is referred to as fixation and appears to be attributable to increasing size of the conceptus, preferential thickening of the endometrium near the mesometrial attachment referred to as encroachment, and a reduction in sialic acid content of the capsule. During maternal recognition of pregnancy, endometrial PGF2α release is attenuated, a consequence of reduced expression of key enzymes involved in prostaglandin production. Oxytocin responsiveness is altered during early pregnancy, and reduced expression of the oxytocin receptor appears to be regulated at the posttranscriptional level rather than the transcriptional level. Prostaglandin release is attenuated temporarily only during early pregnancy; during the third week of pregnancy, the endometrium resumes the ability to secrete PGF2α. The equine conceptus initiates steroidogenesis as early as Day 6 and synthesizes estrogens, androgens, and progesterone. Estrogens are metabolized locally, presumably regulating their bioavailability and actions. Results of experiments attempting to prove that conceptus-derived estrogens are responsible for extension of corpus luteum function have been inconclusive. By the fourth week of pregnancy, the chorionic girdle becomes visible on the trophoblast. Subsequent invasion of chorionic girdle cells leads to formation of endometrial cups which secrete equine chorionic gonadotropin. Equine chorionic gonadotropin has luteinizing hormone functions in the mare, causing luteinization of follicles resulting in the formation of secondary corpora lutea essential to production of progesterone and maintenance of pregnancy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Few, if any, biological processes are as diverse among domestic species as establishment of pregnancy. The mare has several unique features, including selective transport of embryos through the uterotubal junction, an extended phase of conceptus mobility throughout the two uterine horns, and an acellular glycoprotein capsule covering the conceptus between the second and third weeks of pregnancy. Furthermore, the horse is the mammal with the longest known preimplantation phase of pregnancy; implantation does not occur until Day 40 (Allen and Stewart 2001). Of particular interest are events leading to prolongation of luteal lifespan, collectively termed “maternal recognition of pregnancy” (Short 1969). The maternal recognition of pregnancy factor secreted by the conceptus to signal its presence to the maternal system varies among species. These processes are best understood in domestic ruminants and other ungulates, such as the pig; interferon tau has been identified as a conceptus-derived paracrine factor exhibiting antiluteolytic properties in ruminants, whereas conceptus-derived estrogens are the primary pregnancy recognition signal in pigs (Bazer et al. 1997; Geisert et al. 1990). Although the horse is one of the few domestic species in which the conceptus-derived pregnancy recognition signal has not been definitively identified, equids appear to be distinct from ruminants and pigs in the signal(s) used for maternal recognition of pregnancy. Contrasting with the bovine conceptus, equine conceptuses express interferons at negligible levels only; interferons delta 1 and 2 and interferon alpha 1 are expressed at low levels, but only after the critical time of maternal recognition of pregnancy only (Budik et al. 2010; Cochet et al. 2009; Klein 2015). Similar to the porcine conceptus, the equine conceptus synthesizes large amounts of estrogen during the critical time of maternal recognition of pregnancy; unlike in the pig though, a role for conceptus-derived estrogens in maternal recognition of pregnancy remains to be proven (Vanderwall et al. 1994; Woodley et al. 1979). The signal/signals released by the equine conceptus appear to have a molecular weight between 1 and 6 kDa, and its ability to reduce release of prostaglandin F2alpha by endometrial explants in culture is eliminated through proteinase K and charcoal stripping (Ababneh et al. 2000; Sharp et al. 1989).
The earliest evidence for systemic recognition of the conceptus in mares is the presence of an immunosuppressive protein termed “early pregnancy factor” which can be detected (rosette inhibition test) in serum as early as 2 days after ovulation (Ohnuma et al. 2000; Takagi et al. 1998). This early pregnancy factor has been characterized as an extracellular form of heat shock protein 10 (Cavanagh 1996); Day-25 conceptuses have higher transcript abundance than Day-8 blastocysts and protein expression was localized to trophectoderm cells (Hatzel et al. 2014).
9.2 Selective Transport of Equine Embryos
Following fertilization and initial development of the equine embryo in the oviduct, there is selective transport of equine embryos through the uterotubal junction. In that regard, unfertilized oocytes are retained in the oviduct (Betteridge and Mitchell 1972, 1974; Flood et al. 1979), whereas embryos are transported through the oviduct and pass the uterotubal junction to reach the uterine lumen 6.0–6.5 days after fertilization (Battut et al. 1997). This selective transport is a unique feature among domestic animals. Coinciding with transport through the oviduct, the equine embryo secretes considerable amounts of prostaglandin E2 (PGE2; Weber et al. 1991b), and intraoviductal application of PGE2 not only hastens oviductal transport of embryos but also results in recovery of unfertilized oocytes from the uterine lumen (Weber et al. 1991a). Taken together with the observation that the equine oviduct contains receptors for PGE2 (Weber et al. 1992), it appears that the equine conceptus facilitates its own transport and passage through the uterotubal junction via secretion of PGE2. Furthermore, laparoscopic application of PGE2 on the serosal surface of the oviduct in mares has been reported to overcome unexplained infertility (Arnold and Love 2013).
9.3 Prostaglandin F2α Release Is Attenuated during Maternal Recognition of Pregnancy in the Mare
Mares have an estrous cycle of 21 days and, as in other large animal domestic species, prostaglandin F2alpha (PGF2α) is the endogenous luteolysin (Allen and Rowson 1973; Douglas and Ginther 1972). In the absence of a conceptus, luteal regression is initiated approximately 14 days after ovulation, as evident by high PGF2α concentrations in uterine flushings (Stout and Allen 2002), endometrial tissue (Vernon et al. 1981), and uterine vein blood (Douglas and Ginther 1976). A recent study elucidated an auto-amplification system of prostaglandin F2α production in the equine endometrium. Mares given a synthetic analog of prostaglandin F2α during the mid-luteal phase responded with a two-phase increase in circulating concentrations of PGFM (13,14-dihydro-15-keto-PGF2α, the main metabolite of PGF2α); the first peak within 45 min, attributed to administration of exogenous PGF2α, and the second peak at 16 h, with concentrations of PGFM remaining elevated for 56 h. The second increase in PGFM was likely due to PGF2α production by the endometrium triggered by exogenous PGF2α. In vitro studies using endometrial explant cultures and cultured epithelial and stromal cells confirmed that exposure to PGF2α increased PTGS2 expression and stimulated release of PGF2α (Okuda et al. 2014).
A hallmark of maternal recognition of pregnancy in the mare is reduced endometrial secretion of PGF2α in the presence of a conceptus during the expected time of luteolysis. In this regard, concentrations of PGF2α in the blood from uterine veins are lower in pregnant than nonpregnant mares on Days 10 and 14 after ovulation (Douglas and Ginther 1976). Likewise, PGF2α is undetectable in uterine flushings collected from the non-gravid horns of pregnant mares before Day 18 after ovulation (Berglund et al. 1982; Stout and Allen 2002). Co-incubation of endometrium with embryos in vitro reduces the amount of PGF2α released by the endometrium, reflecting the inhibitory effect of the conceptus on prostaglandin synthesis and/or release (Berglund et al. 1982; Ealy et al. 2010; Watson and Sertich 1989).
Oxytocin plays a central role in the release of PGF2α from the endometrium at the time of luteolysis. There is accumulating evidence that oxytocin responsiveness is altered during early pregnancy in the mare. In nonpregnant mares, endogenous oxytocin, provoked through repeated transcervical endometrial biopsies on Days 12 and 14 after ovulation, induces an increase in circulating concentrations of PGFM, whereas pregnant mares lack a corresponding increase in PGFM following cervical stimulation (Sharp et al. 1997). Furthermore, exogenous oxytocin provokes release of PGFM in nonpregnant mares, whereas this response is attenuated in pregnant mares (Goff et al. 1987; Starbuck et al. 1998). Nonpregnant mares respond to exogenous oxytocin with the greatest increase in circulating concentrations of PGFM around the time of luteolysis, i.e., between Days 13 and 16 after ovulation, whereas pregnant mares show no increase in circulating PGFM concentrations in response to exogenous oxytocin given around the time of expected luteolysis (Goff et al. 1987; Starbuck et al. 1998). In line with the differential response to oxytocin depending on pregnancy status, endometrial oxytocin receptor concentrations differ between pregnant and nonpregnant mares around the time of luteolysis; concentrations of oxytocin receptors increase in nonpregnant mares, whereas there is no corresponding increase in pregnant mares (Sharp et al. 1997; Starbuck et al. 1998). There are indications that expression of oxytocin receptors is regulated at the posttranscriptional level, rather than the transcriptional level, during maternal recognition of pregnancy in the mare; transcript levels remain unchanged throughout early pregnancy (de Ruijter-Villani et al. 2014; Klein et al. 2010), whereas protein levels are decreased (de Ruijter-Villani et al. 2014; Sharp et al. 1997; Starbuck et al. 1998). In addition to reduced expression of oxytocin receptors, their function is altered during early pregnancy, manifested as lower affinity of the receptor for binding oxytocin in pregnant versus nonpregnant mares (Sharp et al. 1997). Further supporting evidence for involvement of oxytocin in regulation of luteolysis and its alteration during maternal recognition of pregnancy comes from the observation that repeated administration of oxytocin prolongs luteal function in mares (Stout et al. 1999; Vanderwall et al. 1994, 2007). Oxytocin receptor transcript levels remain unaltered in mares displaying prolonged luteal function following repeated oxytocin exposure, whereas prostaglandin-endoperoxide synthase 2 expression is reduced (Keith et al. 2013).
In ruminants, the corpus luteum and the posterior pituitary are sources of oxytocin that stimulate luteolytic pulses of PGF2α from the endometrium at the end of diestrus (Flint and Sheldrick 1986). Unlike in ruminants, the corpus luteum of the mare does not contain oxytocin (Stevenson et al. 1991; Stock et al. 1995), whereas the endometrium expresses oxytocin (Bae and Watson 2003; Behrendt-Adam et al. 1999). Oxytocin-neurophysin I transcript abundance is highest during estrus and is negatively correlated with circulating concentrations of progesterone in pregnant and nonpregnant mares (Behrendt-Adam et al. 1999). Within the endometrium, oxytocin mRNA and protein localize to luminal epithelial cells and superficial glandular epithelial cells, in addition to being secreted into the uterine lumen (Bae and Watson 2003). Recently, leucyl-cystinyl aminopeptidase (LNPEP), an enzyme that cleaves oxytocin, has been reconsidered for its potential role in regulating levels of oxytocin expression during maternal recognition of pregnancy. Albeit more work is needed, it appears that oxytocinase levels in serum are below the detection limit during diestrus, whereas the highest concentrations of oxytocinase in serum are during early pregnancy (Diel de Amorim et al. 2014).
Interestingly, attenuation of endometrial release of PGF2α seems to be a temporary event during maternal recognition of pregnancy in the mare; during the third week of pregnancy, the endometrium resumes the capability to secrete PGF2α. Although uterine flushings of pregnant mares contain no detectable amounts of PGF2α on Days 12, 14, and 16 after ovulation, the abundance of PGF2α in uterine flushings from pregnant mares starts to increase on Day 18 of pregnancy, peaks on Day 20, and decreases thereafter to negligible levels by Day 30 (Stout and Allen 2002). Likewise, by Day 18 of pregnancy, the uterus has regained the ability to respond to oxytocin with an increase in circulating concentrations of PGFM, although this is apparently not associated with an increase in expression of oxytocin receptors in the endometrium (Starbuck et al. 1998). Resumption of PGF2α production during early pregnancy is puzzling, given that the mare relies on luteal progesterone production throughout the first trimester of gestation. One way for the corpus luteum to escape the luteolytic action of PGF2α would be to reduce binding sites for PGF2α. During early pregnancy, binding capacity of the corpus luteum for PGF2α is high until Day 18. However, as of Day 20 of pregnancy-binding capacity of the CL for PGF2α begins to decline (Vernon et al. 1979). The hypothesis that reduced affinity of the corpus luteum to bind PGF2α prevents luteolysis after the second week of pregnancy has not been tested.
9.4 Regulation of the Pathway for Prostaglandin Synthesis
Enzymes involved in prostaglandin production are regulated with respect to level of expression throughout the estrous cycle and during early pregnancy. The first step in prostaglandin production is release of arachidonic acid from membrane phospholipids through the action of phospholipase A2 (PLA2). PLA2 has several isoforms that differ with regard to substrate specificity, dependence on calcium, and lipid modification: (1) cytosolic PLA2 (cPLA2), (2) calcium-dependent secretory PLA2 (sPLA2), and (3) calcium-independent intracellular PLA2 (iPLA2) (Chakraborti 2003). Phospholipase A2 activity is highest in equine endometria on Day 14 after ovulation (compared to estrus and Days 3 and 8 after ovulation), whereas the presence of a conceptus decreases activity at Day 14 of pregnancy (Ababneh and Troedsson 2013a). PLA2 kinetics in endometrial tissue obtained from pregnant mares indicates the presence of an unidentified competitive inhibitor of PLA2. Since uteroglobin is a known inhibitor of PLA2, uteroglobin may function as the inhibitor of PLA2 during pregnancy (Ababneh and Troedsson 2013a). However, uteroglobin expression decreases during pregnancy (Hayes et al. 2012), necessitating more investigations to clarify the identity of this inhibitor. Endometrial expression of cytosolic PLA2 is reduced in the presence of a conceptus, despite uncertainty regarding timing and progesterone dependency. There is decreased expression of cytosolic PLA2 in pregnant mares although concentrations of progesterone are high on Day 14 and low on Day 18 (Ozel et al. 2014). Conversely, Ababneh and coworkers reported attenuated expression of cytosolic PLA2 at Day 15 of pregnancy, but only if circulating concentrations of progesterone were low. Nonpregnant mares with high circulating concentration progesterone had similar expression of PLA2 as pregnant mares on Day 15 after ovulation (Ababneh et al. 2011). Treating ovariectomized mares with estrogen and/or progesterone revealed that cytosolic PLA2 transcript expression is inversely correlated to concentrations of progesterone, whereas estrogen alone has no effect on its mRNA abundance (Ababneh and Troedsson 2013b). Taken together, expression of cytosolic PLA2 is attenuated during early pregnancy, consistent with reduced production of PGF2α by the endometrium. Likewise, endometrial expression of secretory PLA2 is decreased during early pregnancy, both at the transcript (Ozel et al. 2014) and protein levels (Hayes et al. 2012). Expression of PLA2 mRNA is inversely correlated with concentrations of progesterone, whereas estrogen alone has no effect on PLA2 mRNA abundance in ovariectomized mares treated with estrogen and progesterone (Ababneh and Troedsson 2013b). The contribution of secretory PLA2 to endometrial production of PGF2α that causes luteolysis is unknown. Secretory PLA2 functions as an innate immune protein, exerting antibacterial properties and enhancing the removal of cell debris (Beers et al. 2002; Birts et al. 2008), perhaps by contributing to functions of cells that form the innate immune defense system to maintain a normal uterine environment during estrus. Although endometrial expression of calcium-independent intracellular PLA2 is attenuated on Day 14 after ovulation in pregnant mares, its expression is upregulated on Day 22 after ovulation (Ozel et al. 2014). Increased expression of calcium-independent intracellular PLA2 during the third week of pregnancy may explain resumption of secretion of PGF2α at that stage of pregnancy. Estrogen and progesterone concentrations do not affect expression of intracellular PLA2 transcript abundance in endometria of mares (Ababneh and Troedsson 2013b).
Following its liberation from membrane phospholipids, arachidonic acid is converted to prostaglandin-endoperoxide H2 (PGH2) through the actions of prostaglandin-endoperoxide synthase 1 and 2 (PTGS1 and PTGS2). Expression of PTGS2 in endometria of nonpregnant mares is highest during the expected time of luteolysis, whereas expression is significantly reduced on corresponding days of pregnancy (Atli et al. 2010; Boerboom et al. 2004; de Ruijter-Villani et al. 2014; Ealy et al. 2010). Using endometrial explant cultures, Ealy and coworkers (2010) demonstrated that conceptus’ secretions downregulate expression of PTGS2 and reduce the amount of PGF2α released by endometrial explants, indicating that altered expression of PTGS2 is a central mechanism of maternal recognition of pregnancy in mares. However, PTGS1 expression was unaltered around the time of luteolysis (Atli et al. 2010; de Ruijter-Villani et al. 2014). PGH2 is metabolized to PGF2α or PGE2 through the action of prostaglandin F synthase (PGFS) or prostaglandin E synthase (PGES). In addition, expression of PGFS and PEGS does not differ between nonpregnant and pregnant mares on Day 15 after ovulation (Atli et al. 2010; Boerboom et al. 2004), whereas on Day 14 after ovulation, expression of endometrial PGFS mRNA is higher in nonpregnant versus pregnant mares (Atli et al. 2010). The prostaglandin receptor F (PTGFR) mediates the action of PGF2α, and its expression is attenuated in pregnant mares around the time of luteolysis (Atli et al. 2010; de Ruijter-Villani et al. 2014). Actions of PGE2 are mediated via four receptors; expression of prostaglandin receptor E2 (PTGER2) and PTGER4 is unaltered around the time of expected luteolysis (Atli et al. 2010). Coinciding with the ability of the endometrium to secrete PGF2α during the third week of pregnancy, upregulation of expression of PTGS1 occurs in pregnant mares between Days 18 and 21 compared to Days 14 and 15 of pregnancy (Atli et al. 2010). However, there are conflicting reports regarding expression of PTGS2 during the third week of pregnancy. Atli and coworkers (2010) detected low expression of PTGS2 between Days 18 and 22 of pregnancy, whereas de Ruijter-Villani (2014) reported increased expression of PTGS2 on Day 21 of pregnancy. Expression of PGFS and PGES was unchanged on Day 22 of pregnancy (Atli et al. 2010). Expression of solute carrier organic anion transporter family, member 2A1 (SLCO2A1), also known as prostaglandin transporter, increases during the third week of pregnancy, with expression being significantly greater at Day 22 of pregnancy than at estrus or on Days 14, 15, or 18 of the estrous cycle or pregnancy. No effect of day or pregnancy status on expression of SLCO2A1 at 14, 15, or 18 days after ovulation was reported for pregnant or nonpregnant mares (Atli et al. 2010). In cattle, SLCO2A1 expression is downregulated at the time of maternal recognition of pregnancy (Banu et al. 2003). The increased expression of SLCO2A1 during the third week of pregnancy in mares could contribute to the resumption of secretion of PGF2α by endometria of mares.
In addition to altered expression of key enzymes for prostaglandin synthesis, endometrial cytosol contains an inhibitor of prostaglandin synthesis during early pregnancy in the mare (Watson 1991). In cattle, early pregnancy is likewise associated with an increase of an intracellular inhibitor of prostaglandin synthesis in the endometrium (Gross et al. 1988) which has been identified as linoleic acid (Thatcher et al. 1994). The nature of the inhibitor of prostaglandin synthesis in the mare is unknown.
Alternatively, arachidonic acid can be converted to leukotriene A4 (LTA4) through the action of arachidonate 5-lipoxygenase, which is then either converted to leukotriene B4 or cysteinyl LTC4 through the action of LTC4 synthase and LTA4 hydrolase, respectively. In ruminants, local production of leukotrienes in the corpus luteum contributes to regulation of luteal function, whereas intrauterine administration of an arachidonate 5-lipoxygenase inhibitor delays luteolysis (Cooke and Ahmad 1998; Korzekwa et al. 2010; Milvae et al. 1986). Endometrial expression of arachidonate 5-lipoxygenase, LTC4 synthase, and LTA4 hydrolase is attenuated during early pregnancy in the mare, suggesting that regulation of the lipoxygenase pathway contributes to maintenance of pregnancy (Guzeloglu et al. 2013).
Recently, the effect of cytokines on endometrial prostaglandin production has been assessed. Interleukin-1 alpha, interleukin-1 beta, and interleukin-6 stimulate the production of PGF2α and PGE2 by epithelial and stromal cells in vitro, with estrogen and progesterone modulating the response (Szostek et al. 2014). Tumor necrosis factor alpha stimulates prostaglandin production by mixed epithelial and stromal cells in vitro obtained from mare during the follicular phase but not when cells were obtained from mares during the mid-luteal phase (Galvao et al. 2013). How these findings relate to events during early pregnancy in the mare is unknown to date.
9.5 Spatial and Temporal Regulation of Endometrial Receptors for Estrogen and Progesterone
The actions of estrogen and progesterone are mediated via their respective receptors, of which estrogen receptor alpha (ESR1) and progesterone receptor (PGR) have been most extensively studied in the context of early pregnancy. Steroid hormones regulate the expression of their own receptors in endometrial cells, with estrogen and progesterone displaying differing effects: estrogens enhance expression of both ESR1 and PGR, whereas progesterone downregulates the expression of both receptors (Spencer and Bazer 2002). In ruminants, particularly ovine, spatial and temporal regulations of endometrial receptors for estrogen and progesterone during the estrous cycle and early pregnancy are well documented. Expression for both receptors is highest during estrus; however, continued progesterone exposure during diestrus results in the loss of PGR and ESR1 expression. This loss is most notable in luminal and superficial glandular epithelium, while deep glandular epithelium and stromal cells retain their already low levels of expression. Cyclic loss of PGR expression permits the reappearance of ESR1 expression, which is closely followed by an increase in PGR expression (Spencer and Bazer 1995). During early pregnancy, ESR1 and PGR expression in luminal and shallow glandular epithelium remains low; yet, the deep glandular epithelium and stroma maintain expression of both receptors (Spencer and Bazer 1995). A pregnancy-dependent block in receptor expression is due to the actions of interferon tau, which blocks expression of ESR1 (Spencer et al. 1995). It has been postulated that progesterone acts on the PGR-positive stroma during pregnancy, which in turn produces paracrine factors that act upon the PGR negative luminal epithelium (Spencer and Bazer 1995). During early pregnancy in the mare, a similar pattern of temporal and spatial expression of PGR and ESR1 in the endometrium can be observed, as expression of both receptors is highest during estrus and declines under the influence of progesterone following ovulation (de Ruijter-Villani et al. 2014; Hartt et al. 2005; McDowell et al. 1999; Tomanelli et al. 1991; Watson et al. 1992). Similar to sheep, expression of these receptors is primarily downregulated in the luminal epithelium, whereas the deep glandular epithelium and stroma retain low expression levels (de Ruijter-Villani et al. 2014; Hartt et al. 2005). By Day 15 after ovulation, expression patterns differ dependent on pregnancy status of the mare. While endometrial expression levels of ESR1 and PGR are upregulated in nonpregnant mares, expression levels remain low in pregnant mares (de Ruijter-Villani et al. 2014; Hartt et al. 2005; McDowell et al. 1999). Low levels of expression are confined to the deep glandular epithelium and stroma, whereas the luminal epithelium remains receptor negative during early pregnancy (de Ruijter-Villani et al. 2014; Hartt et al. 2005; Wilsher et al. 2011). In all of the above-cited studies, receptor expression was localized to the nuclei of endometrial cells, whereas little to no cytoplasmic expression was observed. A recent study presents contrary results with respect to ESR1 expression during early pregnancy (Wilsher et al. 2011): minimal to moderate cytoplasmic expression of ESR1 was observed in luminal epithelium, in addition to more intense cytoplasmic expression in glandular epithelium throughout early pregnancy (Days 20–68). Only very occasional nuclear expression was observed in epithelial cells (Wilsher et al. 2011). The apparent discrepancy to reports that describe the absence of ESR1 expression in epithelial cells during early pregnancy may be the result of different antibodies being used. Subcellular staining patterns of ESR1 can depend on the primary antibody used for localization studies; for instance, a particular antibody can show a preference for cytoplasmic staining versus nuclear staining and vice versa (Schuler et al. 2002; Sierralta and Thole 1996).
It should be noted that during early pregnancy in the pig, luminal and glandular epithelial cells maintain ESR1 expression, albeit with immunoreactivity confined to nuclei and not the cytoplasm (Geisert et al. 1993; Knapczyk-Stwora et al. 2011). Similar to the equine conceptus, porcine conceptuses secrete significant amounts of estrogen during the time of MRP (Perry et al. 1973), which raises the likelihood of continued ESR1 expression by epithelial cells to be a unique feature to species in which conceptuses secrete large quantities of estrogen. It appears that ESR1 expression by luminal and glandular epithelial cells is retained during early pregnancy in the mare, but, in contrast to porcine, expression is retained in the cytoplasm, rather than in the nucleus. Even though the precise function of ESR1 found in cytoplasm is unknown, these receptors are likely to contribute to non-genomic actions of estrogen (Levin 2001, 2005). Continued expression of cytoplasmic ESR1 allows conceptus-derived estrogens to act upon epithelial cells and trigger events required for pregnancy maintenance in the mare. One could hypothesize that the different subcellular localizations of ESR1 in the epithelia of pregnant pigs and mares could explain the ability of conceptus-derived estrogens to block luteolysis in the pig (Geisert et al. 1990), whereas there is no clear evidence for the latter in mares.
9.6 Conceptus Mobility Is Essential to Maternal Recognition of Pregnancy in the Mare
Conceptus mobility is integral to maternal recognition of pregnancy in mares and can be observed as early as Day 9 after ovulation, with a marked increase in mobility on Day 10, and maximum mobility between Days 11 and 14. Restriction of conceptus mobility through experimental ligation of uterine horns results in a decline in circulating concentrations of progesterone and return to estrus (McDowell et al. 1988). However, administration of exogenous progesterone to pregnant mares with experimentally ligated uterine horns prevents pregnancy loss, indicating that luteolysis is the cause of embryonic loss when conceptus mobility is restricted (McDowell et al. 1988). The period of maximum conceptus mobility coincides with the time of maternal recognition of pregnancy and the period of attenuated prostaglandin release by the endometrium. Conceptus mobility likely serves to distribute conceptus-derived factors over the entire surface of the endometrium. The utero-ovarian vein and the ovarian artery in the mare are clearly separate, resulting in a systemic pathway of luteolysis, i.e., PGF2α released by the endometrium reaches the ovaries via the systemic circulation (Ginther 1974). Consequently, PGF2α production during maternal recognition of pregnancy has to be attenuated from the entire endometrium, explaining why conceptus mobility is integral to maintenance of pregnancy.
Coinciding with the end of conceptus migration, there is a marked decline in uterine contractility, indicating that uterine contractions are the driving force of conceptus mobility and that the embryo seems to be a direct stimulator thereof (Gastal et al. 1996). The independent migration of twin conceptuses is another indicator that the conceptus itself stimulates uterine contractility (Ginther 1985). De novo synthesis of prostaglandins is required for conceptus mobility, as administration of flunixin meglumine, a nonsteroidal anti-inflammatory drug, to pregnant mares on Days 12 and 14 after ovulation results in the immediate and marked reduction of conceptus migration (Stout and Allen 2001). Although it is generally accepted that prostaglandins stimulate uterine contractions that propel the conceptus within the uterine lumen, it does not define the site of prostaglandin production, i.e., conceptus versus endometrium. Given that the uterine lumen contains no prostaglandins during this time of pregnancy, whereas the conceptus secretes both PGF2α and PGE2 (Stout and Allen 2002), it is likely that the conceptus is the source of prostaglandins that stimulate uterine contractions. Alternatively, but less likely, localized release of endometrial PGF2α could be caused by conceptus-derived estrogens (Stout and Allen 2001), since estrogens can stimulate secretion of PGF2α by equine endometrium in vitro (Vernon et al. 1981) and in vivo (Goff et al. 1993).
Interestingly, flunixin meglumine does not reduce conceptus mobility on Day 10 of pregnancy (Stout and Allen 2001). However, Day-10 conceptuses produce much higher amounts of PGF2α and PGE2 per mg of tissue (Stout and Allen 2002) compared to later stages of conceptus development; therefore, flunixin meglumine concentrations may not have been sufficient to fully inhibit de novo prostaglandin synthesis. To date, no studies have addressed which of the two conceptus-derived prostaglandins, PGF2α or PGE2, is the stimulator of uterine contractions. The addition of flunixin meglumine to Day-14 conceptuses in culture significantly reduced secretion of PGE2, but not PGF2α, indicating that Day-14 conceptuses do not produce PGF2α de novo. However, since de novo synthesis of prostaglandins is required for conceptus mobility on Day 14 (Stout and Allen 2001), it seems likely that PGE2 is the conceptus-derived prostaglandin stimulating uterine contractility required for conceptus mobility. The actions of PGE2 are mediated via four receptors, of which PTGRE1 and PTGRE3 mediate smooth muscle contractility (Coleman et al. 1994). Unfortunately, expression of PTGRE1 and PTGRE3 in equine myometrium has not been reported. Everything considered, it appears that during the initial phase of conceptus mobility, conceptus-derived PGF2α and PGE2 contribute to conceptus mobility, whereas during the final phase of conceptus mobility, conceptus-derived PGE2 appears to be sole driver of uterine contractions. In pigs, estrogen-induced release of histamine from the endometrium has been suggested to contribute to conceptus migration (Pope et al. 1982). In mice, lysophosphatidic acid drives spacing of blastocysts, and in mice lacking the receptor for lysophosphatidic acid, blastocysts fail to distribute throughout the uterine lumen (Hama et al. 2007). Neither one of these concepts has been investigated in the mare.
Cessation of conceptus mobility, referred to as “fixation,” occurs on average 15–16 days after ovulation (Ginther 1983b; Leith and Ginther 1984). During the last day of the mobile phase, the conceptus spends significantly more time in the uterine horn in which it will become fixed, with fixation occurring predominantly in the caudal segments of uterine horns (Silva and Ginther 2006). Interestingly, the site of future fixation can be predicted 1–4 days before fixation occurs, as endometrial thickness at the mesometrial aspect at the site of future fixation increases significantly (Silva and Ginther 2006). This noteworthy predictability reflects a conceptus-maternal interaction crucial to maternal recognition of pregnancy and implantation. Cessation of conceptus migration appears to be a multifactorial event. The diameter of the developing conceptus and time of fixation are negatively correlated (Gastal et al. 1996), indicating that physical impediments contribute to fixation, i.e., the conceptus becomes too large to move through the uterine lumen. An increase in uterine tone in conjunction with encroaching endometrial folds has been hypothesized to contribute to fixation (Ginther 1983a). Coinciding with cessation of conceptus mobility, the sialic acid content of the mucin-like capsular glycoproteins decreases, which has been suggested to be “a unique developmentally regulated mechanism for the control of embryo mobility” (Oriol et al. 1993b); the equine conceptus expresses NEU2, an enzyme also known as sialidase 2, which cleaves sialic acid from polysaccharide chains. Expression of NEU2 increases from Days 8 to 16 of conceptus development, and conceptus-conditioned medium contains functional sialidase, whereas the endometrium was not identified as a major source of NEU2. Therefore, developmentally regulated expression of NEU2 provides a mechanism whereby the conceptus controls sialic acid content of its own capsule (Klein and Troedsson 2012).
9.7 The Embryonic Capsule of the Equine Conceptus
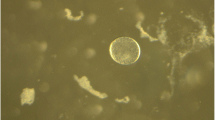
The equine conceptus is surrounded by an acellular glycoprotein capsule during the second and third week of gestation. It is composed of mucin-like glycoproteins; galactose, N-acetylglucosamine, sulfated sugars, and sialic acid represent the majority of the carbohydrates (Oriol et al. 1993a). Capsule formation starts as the embryo enters the uterus, and it is surrounded by the zona pellucida (Betteridge et al. 1982). Within 24 h after entry into the uterus, the zona pellucida is shed and the hatched blastocyst is covered completely by the capsule (Flood et al. 1982). The trophoblast and not the endometrium is the source of capsular material, as evident through xenogeneic transplantation of trophoblast and endometrium into immunodeficient mice (Albihn et al. 2003). Indeed, transcriptional profiling of equine conceptuses revealed developmentally regulated expression of a sialic acid transporter and sialyltransferases (Klein and Troedsson 2011). By Day 22, the capsule disappears, but the responsible mechanism remains unknown (Oriol et al. 1993b). Nevertheless, shedding of the capsule allows first intimate contact between trophectoderm and uterine luminal epithelium to initiate attachment and adhesion phases of implantation.
The capsule ensures that the equine conceptus maintains its spherical shape, a prerequisite to conceptus mobility. In addition, the capsule provides mechanical resilience, so the conceptus can withstand forces exerted on it during its mobility phase. Furthermore, the high sialic acid content of the capsule may confer anti-adhesive properties that facilitate conceptus mobility (Oriol et al. 1993b). Removal of the capsule between Days 6 and 7 after ovulation followed by transfer of the blastocyst to a synchronized recipient mare results in failure to establish pregnancy, indicating that the capsule is essential for pregnancy recognition and/or maintenance of pregnancy in the horse (Stout et al. 2005).
9.8 Estrogen Synthesis by the Equine Conceptus
Biosynthesis of steroid hormones such as estrogens by trophectoderm/chorion is common among conceptuses of domestic animals. The equine conceptus initiates steroidogenesis as early as Day 6 and synthesizes estrogens, androgens, and progesterone in measurable quantities by Day 8 of development (Paulo and Tischner 1985). After Day 12, a large increase in estrogen content occurs in yolk sac and uterine luminal fluid (Zavy et al. 1984). The increased production of free and conjugated estrogens with increasing age of the conceptus is due to an increase in cell number and not due to an increase in estrogen production on a cellular level (Choi et al. 1997b). No increase in concentrations of free estrogens in the systemic circulation has been reported, indicating the conceptus is the sole source of estrogens at the conceptus-maternal interface during early pregnancy (Zavy et al. 1984).
By Days 18–20 of pregnancy, estrone is the main estrogen present in yolk sac fluid (Raeside et al. 2009). Local metabolism of estrogens plays a role in mediating the actions of estrogen and also contributes to regulating bioavailability as estrone is much weaker estrogen than estradiol (Zhu and Conney 1998). Estradiol is metabolized to estrone by extraembryonic tissues and to a lesser extent by the embryo proper. Estrone-to-estradiol conversion only occurs to a small extent in the wall of the bilaminar yolk sac. Both the embryo proper and extraembryonic tissues conjugate estrone and estradiol with sulfoconjugation dominating over glucuronidation (Raeside et al. 2009). The endometrium also contributes to conjugation of estrone and estradiol, with levels of conjugation being higher than trophoblast tissue (Raeside et al. 2004). Taken together, it seems that the conceptus regulates the bioavailability of the massive amounts of estrogen synthesizes through conversion of estradiol to estrone and through extensive sulfoconjugation of both estrogen and estrone. Therefore, one must likely measure estrone sulfate in blood of mares to assess intrauterine production and metabolism of estrogens.
The conceptus expresses a number of enzymes involved in the synthesis and metabolism of steroid hormones. The rate-limiting step in the production of steroid hormones is the transfer of cholesterol within mitochondria to the inner mitochondrial membrane by steroidogenic acute regulatory protein (STAR), and equine conceptuses increase expression of STAR with advancing stage of development (Klein and Troedsson 2011). Similar to the expression pattern of STAR, cytochrome P450 cholesterol side-chain cleavage enzyme (P450SCC) transcript abundance increases with age of conceptus; P450SCC catalyzes the conversion of cholesterol to pregnenolone (Klein and Troedsson 2011). Then 3-beta-hydroxysteroid dehydrogenase (HSD3B1), a key enzyme in the production of progesterone, androgen, and estrogen, is expressed by cells of the trophectoderm/chorion (Flood and Marrable 1975), and, like STAR and HSD3B1, expression increases with stage of conceptus development (Klein and Troedsson 2011). CYP19A1, commonly known as aromatase, catalyzes the last steps of estrogen biosynthesis and is expressed by trophectoderm and extraembryonic endoderm just beneath the embryonic disk (Walters et al. 2000). Stage-specific expressions of 17β-hydroxysteroid dehydrogenases occur during equine conceptus development and are proposed to contribute to conversion of estrone to estradiol and vice versa (Klein and Troedsson 2011).
The estrogens synthesized by the equine conceptus have been explored in the search for the pregnancy recognition signal; however, experiments attempting to prove that embryo-derived estrogens are responsible for extension of corpus luteum function through systemic administration of estrogen to cycling mares have been inconclusive (Vanderwall et al. 1994; Woodley et al. 1979). The prolonged production of estrogens far past the time of maternal recognition of pregnancy suggests roles for estrogens and their metabolites in development of the conceptus well beyond the time of maternal recognition of pregnancy.
High concentrations of 19-norandrorstenedione and its sulfoconjugate are present in yolk sac fluid, the biological significance of which is unclear (Raeside and Christie 2008). The high concentration of androgens is likely attributable to the expression of a blastocyst-specific isoform of aromatase that favors production of 19-nortestosterone over estradiol (Choi et al. 1997a).
9.9 Expression of Proteins Related to Uterine Receptivity to Implantation
Regulation of several endometrial proteins has been associated with endometrial receptivity to implantation across species. Mucin 1 (MUC1), a glycoprotein with an extensive extracellular domain, is expressed by epithelial cells and acts as an anti-adhesive molecule. In several species, there is a decrease in MUC1 expression by uterine luminal epithelia at the time of implantation, either generalized (rodents and pigs) or localized at the site of implantation (rabbits) (Bowen et al. 1996; Hoffman et al. 1998; Johnson et al. 2001). Wilsher and coworkers demonstrated the presence of a MUC1 protein at the conceptus-maternal interface at varying stages of pregnancy (20–309 days after ovulation) in the mare and concluded that implantation and placentation in the mare occur despite persistence of expression of MUC1. Notwithstanding, this should be interpreted with care, as the antibody used likely recognized an isoform of MUC1, MUC1/Y, which has a smaller extracellular domain than MUC1, reflected by its smaller molecular weight (<58 kDa versus >120 kDa for full-length MUC1) (Levitin et al. 2005). Owing to the smaller extracellular domain, MUC1/Y does not confer anti-adhesive properties. Perhaps, expression of full-length MUC1 is downregulated at fixation/implantation in the mare, either through proteolytic cleavage of its extracellular domain (Parry et al. 2001) or by preferential transcription of the splice variant MUC1/Y (Obermair et al. 2001). However, expression of MUC1 in the nonpregnant mare has not been reported.
Secreted phosphoprotein 1 (SPP1), also known as osteopontin, is an extracellular matrix protein whose differential regulation of expression during early pregnancy has been implicated in uterine receptivity in humans, pigs, mice, and sheep (Johnson et al. 2014; Liu et al. 2013; Qu et al. 2008); in that regard, SPP1 is an extracellular matrix protein that mediates conceptus adhesion by bridging trophoblast and endometrial integrins. In the mare, there are limited studies characterizing expression and localization of SPP1 at the conceptus-maternal interface. No difference in expression of SPP1 by the endometrium during early pregnancy and diestrus was reported for mares (Hitit et al. 2014). Furthermore, SPP1 transcript abundance decreases markedly from Days 8 to 14 of conceptus development, which has been hypothesized to contribute to the prolonged preimplantation phase of conceptus development in the horse (Klein and Troedsson 2011).
Leukemia inhibitory factor (LIF) is an indispensable cytokine for murine implantation, as blastocysts fail to implant in LIF null mice (Stewart 1994). In the equine endometrium, there is one report that there is no significant upregulation of LIF mRNA expression within the first 22 days of pregnancy (Hitit et al. 2014), whereas another report indicated increases in expression of LIF mRNA between Days 14 and 21 of pregnancy (Villani et al. 2010). Therefore, the functional relevance of LIF expression at the conceptus-maternal interface in the mare remains to be determined. Macrophage migration inhibitory factor (MIF) is another cytokine implicated in reproductive processes at the fetal-maternal interface during pregnancy. In that regard, MIF is expressed by equine conceptuses and endometria, and it was postulated that “MIF is part of the molecular repertoire that contributes to normal endometrial function” (Klein and Troedsson 2013).
Fibroblast growth factor 2 (FGF2), along with the corresponding receptors FGFR1-4, is expressed at the conceptus-maternal interface in the mare (de Ruijter-Villani et al. 2013). Endometrial expression increases as early pregnancy advances, with FGF2 localizing to luminal and glandular epithelial cells of the endometrium. Although the role of FGF2 during early pregnancy in the mare is unknown, its pregnancy-specific increase in expression suggests that it contributes to pregnancy maintenance, in particular during the fourth week of pregnancy.
During the extended pre-attachment period, the conceptus relies on nutrients delivered through protein-rich uterine secretions termed histotroph (Zavy et al. 1982). The predominant protein of uterine histotroph in mares is uterocalin (P19), a member of the lipocalin family of proteins which transport small hydrophobic molecules (Flower et al. 1993). Large amounts of P19 are present in uterine flushings during the first 23 days of pregnancy (Stewart et al. 1995), and P19 is the most abundant endometrial transcript at Day 16 of pregnancy (Klein 2015). Within the endometrium, expression of P19 mRNA and protein is restricted to the luminal and glandular epithelia (Crossett et al. 1996, 1998). Endogenous and exogenous progesterone stimulate P19 expression; however, disappearance of expression of P19 in the third week of pregnancy suggests that factors other than progesterone regulate its expression. The capsule of the developing conceptus contains large amount of P19 protein although there is the absence or low expression of P19 mRNA, indicating that the capsule takes up or binds P19 from uterine secretions (Crossett et al. 1998). Equine uterocalin binds fatty acids and retinol; therefore, it seems likely that its main function is to deliver nutrients to the developing conceptus (Suire et al. 2001). Exposure of in vitro-produced equine embryos to P19 improves capsule formation, indicating a likely function of P19 (Smits et al. 2012).
9.10 The Endometrial Cup Reaction
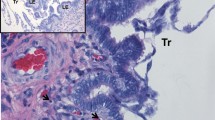
By the fourth week of pregnancy, a distinct, temporary structure appears on the trophoblast at the interface between the expanding allantochorion and the regressing yolk sac, the chorionic girdle. Following a phase of rapid proliferation and transformation into binucleate cells, the cells of the chorionic girdle gain an invasive phenotype and penetrate through the luminal endometrial epithelium, resulting in the disappearance of the chorionic girdle from the surface of the conceptus (Enders and Liu 1991). Soon thereafter, the endometrial cups become visible as slightly raised pale white plaques on the endometrial surface (Yamauchi 1975). The horse is unique among domestic animals in the production of a chorionic gonadotropin (eCG, equine chorionic gonadotropin), which is the result of the endometrial cup reaction. eCG can be detected as early as Days 37–41, peaks between Days 60 and 75, after which time it starts to decline and disappears by Days 120–150 of gestation (Evans et al. 1933). eCG displays a remarkably long biological half-life (Catchpole et al. 1935), and its LH function induces luteinization of theca and granulosa cells of follicles resulting in the development of secondary corpora lutea which can be found between Days 40 and 150 of gestation (Amoroso et al. 1948; Cole et al. 1931). Formation of these secondary corpora lutea is essential to maintenance of pregnancy; they are the sole source of progesterone as progesterone production by the primary CL wanes until onset of production of progestins by the chorion (Squires and Ginther 1975).
Invasion of chorionic girdle cells provokes an immune reaction evident through the accumulation of lymphocytes around the endometrial cups (Grunig et al. 1995). Invasive chorionic girdle cells express high levels of paternal major histocompatibility complex class I (MHCI) antigens (Donaldson et al. 1990), and an immunological mechanism leading to the temporary lifespan of the endometrial cups has been suspected for a long time. This hypothesis had to be revised, however, given that MHC-compatible pregnancies (Antczak et al. 1982) and prior immunological sensitization of mares to paternal MHC antigens (Adams et al. 2007) do not alter lymphocyte accumulation or lifespan of the endometrial cups. It seems that a mechanism intrinsic to the cells of endometrial cups determines their life cycle (Fig. 9.1).
9.11 Conclusions
Indirect conclusions can be drawn only about the time frame during which maternal recognition of pregnancy occurs. The conceptus has to be recognized before Day 14 after ovulation, as this is the time that luteolysis is initiated in the mare (Ginther et al. 2011). Although expression of oxytocin receptors is similar for nonpregnant and pregnant mares on Days 10 and 12 after ovulation, expression of oxytocin receptors is less for pregnant than cyclic mares on Day 14 after ovulation (de Ruijter-Villani et al. 2014; Sharp et al. 1997; Starbuck et al. 1998). Continuous infusions of oxytocin from 8 days after ovulation prolong luteal phase, whereas continuous infusions of oxytocin from 10 days after ovulation initiate luteolysis (Stout et al. 1999), indicating that maternal recognition of pregnancy is initiated by Day 10 postovulation. Wilsher and coworkers (2010) recently suggested reevaluation of the concept that maternal recognition of pregnancy occurs on or before Day 10. Surprisingly, all six asynchronous transfers of 10-day-old blastocysts into recipient mares on Day 12 of diestrus resulted in establishment of pregnancy and survival of the conceptus to the “heartbeat stage” of development of the embryo (end of observation period). Notwithstanding, various mechanisms lead to luteal maintenance when using repeated/continuous administration of oxytocin versus transfer of a blastocyst as in vitro factors secreted by the conceptus lead to a rapid (within 24 h) reduction in secretion of PGF2α by endometrial explant cultures (Ealy et al. 2010). It seems likely that the mechanism leading to luteal maintenance via repeated or continuous treatments with oxytocin must be initiated well before maternal recognition of pregnancy occurs.
References
Ababneh MM, Troedsson MH (2013a) Endometrial phospholipase A2 activity during the oestrous cycle and early pregnancy in mares. Reprod Domest Anim 48:46–52. doi:10.1111/j.1439-0531.2012.02023.x
Ababneh MM, Troedsson MH (2013b) Ovarian steroid regulation of endometrial phospholipase A2 isoforms in horses. Reprod Domest Anim 48:311–316. doi:10.1111/j.1439-0531.2012.02151.x
Ababneh MM, Troedsson MH, Michelson JR, Seguin BE (2000) Partial characterization of an equine conceptus prostaglandin inhibitory factor. J Reprod Fertil Suppl (56):607–613
Ababneh M, Ababneh H, Shidaifat F (2011) Expression of cytosolic phospholipase A2 in equine endometrium during the oestrous cycle and early pregnancy. Reprod Domest Anim 46:268–274. doi:10.1111/j.1439-0531.2010.01657.x
Adams AP, Oriol JG, Campbell RE, Oppenheim YC, Allen WR, Antczak DF (2007) The effect of skin allografting on the equine endometrial cup reaction. Theriogenology 68:237–247. doi:10.1016/j.theriogenology.2007.04.058
Albihn A, Waelchli RO, Samper J, Oriol JG, Croy BA, Betteridge KJ (2003) Production of capsular material by equine trophoblast transplanted into immunodeficient mice. Reproduction 125:855–863
Allen WR, Rowson LE (1973) Control of the mare’s oestrous cycle by prostaglandins. J Reprod Fertil 33:539–543
Allen WR, Stewart F (2001) Equine placentation. Reprod Fertil Dev 13:623–634
Amoroso EC, Hancock JL, Rowlands IW (1948) Ovarian activity in the pregnant mare. Nature 161:355
Antczak DF, Bright SM, Remick LH, Bauman BE (1982) Lymphocyte alloantigens of the horse. I. Serologic and genetic studies. Tissue Antigens 20:172–187
Arnold CE, Love CC (2013) Laparoscopic evaluation of oviductal patency in the standing mare. Theriogenology 79:905–910. doi:10.1016/j.theriogenology.2012.12.004
Atli MO, Kurar E, Kayis SA, Aslan S, Semacan A, Celik S, Guzeloglu A (2010) Evaluation of genes involved in prostaglandin action in equine endometrium during estrous cycle and early pregnancy. Anim Reprod Sci 122:124–132. doi:10.1016/j.anireprosci.2010.08.007
Bae SE, Watson ED (2003) A light microscopic and ultrastructural study on the presence and location of oxytocin in the equine endometrium. Theriogenology 60:909–921
Banu SK, Arosh JA, Chapdelaine P, Fortier MA (2003) Molecular cloning and spatio-temporal expression of the prostaglandin transporter: a basis for the action of prostaglandins in the bovine reproductive system. Proc Natl Acad Sci U S A 100:11747–11752. doi:10.1073/pnas.1833330100
Battut I, Colchen S, Fieni F, Tainturier D, Bruyas JF (1997) Success rates when attempting to nonsurgically collect equine embryos at 144, 156 or 168 hours after ovulation. Equine Vet J Suppl (25):60–62
Bazer FW, Spencer TE, Ott TL (1997) Interferon tau: a novel pregnancy recognition signal. Am J Reprod Immunol 37:412–420
Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, Wilton DC (2002) The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem 277:1788–1793. doi:10.1074/jbc.M109777200
Behrendt-Adam CY, Adams MH, Simpson KS, McDowell KJ (1999) Oxytocin-neurophysin I mRNA abundance in equine uterine endometrium. Domest Anim Endocrinol 16:183–192
Berglund LA, Sharp DC, Vernon MW, Thatcher WW (1982) Effect of pregnancy and collection technique on prostaglandin F in the uterine lumen of Pony mares. J Reprod Fertil Suppl 32:335–341
Betteridge KJ, Mitchell D (1972) Retention of ova by the Fallopian tube in mares. J Reprod Fertil 31:515
Betteridge KJ, Mitchell D (1974) Direct evidence of retention of unfertilized ova in the oviduct of the mare. J Reprod Fertil 39:145–148
Betteridge KJ, Eaglesome MD, Mitchell D, Flood PF, Beriault R (1982) Development of horse embryos up to twenty two days after ovulation: observations on fresh specimens. J Anat 135:191–209
Birts CN, Barton CH, Wilton DC (2008) A catalytically independent physiological function for human acute phase protein group IIA phospholipase A2: cellular uptake facilitates cell debris removal. J Biol Chem 283:5034–5045. doi:10.1074/jbc.M708844200
Boerboom D, Brown KA, Vaillancourt D, Poitras P, Goff AK, Watanabe K, Dore M, Sirois J (2004) Expression of key prostaglandin synthases in equine endometrium during late diestrus and early pregnancy. Biol Reprod 70:391–399. doi:10.1095/biolreprod.103.020800
Bowen JA, Bazer FW, Burghardt RC (1996) Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 55:1098–1106
Budik S, Lussy H, Aurich C (2010) Quantification of different type I interferon transcripts in equine embryos at days 10 to 16 of gestation. Anim Reprod Sci 121:307–308. doi: http://dx.doi.org/10.1016/j.anireprosci.2010.04.058
Catchpole HR, Cole HH, Pearson PB (1935) Studies on the rate of disappearance and fate of mare gonadotropic hormone following intravenous injection. Amer. J. Phy8iol. 112: 21–26
Cavanagh AC (1996) Identification of early pregnancy factor as chaperonin 10: implications for understanding its role. Rev Reprod 1:28–32
Chakraborti S (2003) Phospholipase A(2) isoforms: a perspective. Cell Signal 15:637–665
Choi I, Collante WR, Simmen RC, Simmen FA (1997a) A developmental switch in expression from blastocyst to endometrial/placental-type cytochrome P450 aromatase genes in the pig and horse. Biol Reprod 56:688–696
Choi SJ, Anderson GB, Roser JF (1997b) Production of free estrogens and estrogen conjugates by the preimplantation equine embryo. Theriogenology 47:457–466
Cochet M, Vaiman D, Lefevre F (2009) Novel interferon delta genes in mammals: cloning of one gene from the sheep, two genes expressed by the horse conceptus and discovery of related sequences in several taxa by genomic database screening. Gene 433:88–99. doi:10.1016/j.gene.2008.11.026
Cole HH, Howell CE, Hart GH (1931) The changes occurring in the ovary of the mare during pregnancy. Anat Rec 49:199–209. doi:10.1002/ar.1090490305
Coleman RA, Smith WL, Narumiya S (1994) International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46:205–229
Cooke RG, Ahmad N (1998) Delayed luteolysis after intra-uterine infusions of nordihydroguaiaretic acid in the ewe. Anim Reprod Sci 52:113–121
Crossett B, Allen WR, Stewart F (1996) A 19 kDa protein secreted by the endometrium of the mare is a novel member of the lipocalin family. Biochem J 320(Pt 1):137–143
Crossett B, Suire S, Herrler A, Allen WR, Stewart F (1998) Transfer of a uterine lipocalin from the endometrium of the mare to the developing equine conceptus. Biol Reprod 59:483–490
de Ruijter-Villani M, van Boxtel PR, Stout TA (2013) Fibroblast growth factor-2 expression in the preimplantation equine conceptus and endometrium of pregnant and cyclic mares. Theriogenology 80:979–989. doi:10.1016/j.theriogenology.2013.07.024
de Ruijter-Villani M, van Tol HT, Stout TA (2014) Effect of pregnancy on endometrial expression of luteolytic pathway components in the mare. Reprod Fertil Dev. doi:10.1071/RD13381
Diel de Amorim M, Nielsen K, Card C (2014) 113 Preliminary characterization of oxytocinase in equine serum. Reprod Fertil Dev 27:149. doi: http://dx.doi.org/10.1071/RDv27n1Ab113
Donaldson WL, Zhang CH, Oriol JG, Antczak DF (1990) Invasive equine trophoblast expresses conventional class I major histocompatibility complex antigens. Development 110:63–71
Douglas RH, Ginther OJ (1972) Effect of prostaglandin F2alpha on length of diestrus in mares. Prostaglandins 2:265–268
Douglas RH, Ginther OJ (1976) Concentration of prostaglandins F in uterine venous plasma of anesthetized mares during the estrous cycle and early pregnancy. Prostaglandins 11:251–260
Ealy AD, Eroh ML, Sharp DC 3rd (2010) Prostaglandin H synthase Type 2 is differentially expressed in endometrium based on pregnancy status in pony mares and responds to oxytocin and conceptus secretions in explant culture. Anim Reprod Sci 117:99–105. doi:10.1016/j.anireprosci.2009.03.014
Enders AC, Liu IK (1991) Trophoblast-uterine interactions during equine chorionic girdle cell maturation, migration, and transformation. Am J Anat 192:366–381. doi:10.1002/aja.1001920405
Evans HM, Gustus EL, Simpson ME (1933) Concentration of the gonadotropic hormone in pregnant mare’s serum. J Exp Med 58:569–574
Flint AP, Sheldrick EL (1986) Ovarian oxytocin and the maternal recognition of pregnancy. J Reprod Fertil 76:831–839
Flood PF, Marrable AW (1975) A histochemical study of steroid metabolism in the equine fetus and placenta. J Reprod Fertil Suppl (23):569–573
Flood PF, Jong A, Betteridge KJ (1979) The location of eggs retained in the oviducts of mares. J Reprod Fertil 57:291–294
Flood PF, Betteridge KJ, Diocee MS (1982) Transmission electron microscopy of horse embryos 3–16 days after ovulation. J Reprod Fertil Suppl 32:319–327
Flower DR, North AC, Attwood TK (1993) Structure and sequence relationships in the lipocalins and related proteins. Protein Sci 2:753–761. doi:10.1002/pro.5560020507
Galvao A, Valente L, Skarzynski DJ, Szostek A, Piotrowska-Tomala K, Rebordao MR, Mateus L, Ferreira-Dias G (2013) Effect of cytokines and ovarian steroids on equine endometrial function: an in vitro study. Reprod Fertil Dev 25:985–997. doi:10.1071/RD12153
Gastal MO, Gastal EL, Kot K, Ginther OJ (1996) Factors related to the time of fixation of the conceptus in mares. Theriogenology 46:1171–1180
Geisert RD, Zavy MT, Moffatt RJ, Blair RM, Yellin T (1990) Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil Suppl 40:293–305
Geisert RD, Brenner RM, Moffatt RJ, Harney JP, Yellin T, Bazer FW (1993) Changes in oestrogen receptor protein, mRNA expression and localization in the endometrium of cyclic and pregnant gilts. Reprod Fertil Dev 5:247–260
Ginther OJ (1974) Internal regulation of physiological processes through local venoarterial pathways: a review. J Anim Sci 39:550–564
Ginther OJ (1983a) Fixation and orientation of the early equine conceptus. Theriogenology 19:613–623
Ginther OJ (1983b) Mobility of the early equine conceptus. Theriogenology 19:603–611
Ginther OJ (1985) Dynamic physical interactions between the equine embryo and uterus. Equine Vet J 17:41–47. doi:10.1111/j.2042-3306.1985.tb04592.x
Ginther OJ, Hannan MA, Beg MA (2011) Luteolysis and associated interrelationships among circulating PGF2alpha, progesterone, LH, and estradiol in mares. Domest Anim Endocrinol 41:174–184. doi:10.1016/j.domaniend.2011.06.003
Goff AK, Pontbriand D, Sirois J (1987) Oxytocin stimulation of plasma 15-keto-13,14-dihydro prostaglandin F-2 alpha during the oestrous cycle and early pregnancy in the mare. J Reprod Fertil Suppl 35:253–260
Goff AK, Sirois J, Pontbriand D (1993) Effect of oestradiol on oxytocin-stimulated prostaglandin F2 alpha release in mares. J Reprod Fertil 98:107–112
Gross TS, Thatcher WW, Hansen PJ, Johnson JW, Helmer SD (1988) Presence of an intracellular endometrial inhibitor of prostaglandin synthesis during early pregnancy in the cow. Prostaglandins 35:359–378
Grunig G, Triplett L, Canady LK, Allen WR, Antczak DF (1995) The maternal leucocyte response to the endometrial cups in horses is correlated with the developmental stages of the invasive trophoblast cells. Placenta 16:539–559
Guzeloglu A, Atli MO, Kurar E, Kayis SA, Handler J, Semacan A, Aslan S (2013) Expression of enzymes and receptors of leukotriene pathway genes in equine endometrium during the estrous cycle and early pregnancy. Theriogenology 80:145–152. doi:10.1016/j.theriogenology.2013.03.025
Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, Arai H (2007) Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod 77:954–959. doi:10.1095/biolreprod.107.060293
Hartt LS, Carling SJ, Joyce MM, Johnson GA, Vanderwall DK, Ott TL (2005) Temporal and spatial associations of oestrogen receptor alpha and progesterone receptor in the endometrium of cyclic and early pregnant mares. Reproduction 130:241–250. doi:10.1530/rep.1.00596
Hatzel JN, Bouma GJ, Cleys ER, Bemis LT, Ehrhart EJ, McCue PM (2014) Identification of heat shock protein 10 within the equine embryo, endometrium, and maternal peripheral blood mononuclear cells. Theriogenology. doi:10.1016/j.theriogenology.2014.11.020
Hayes MA, Quinn BA, Cote O, Bienzle D, Waelchli RO, Betteridge KJ (2012) Changes in various endometrial proteins during cloprostenol-induced failure of early pregnancy in mares. Anim Reprod 9:723–741
Hitit M, Guzeloglu A, Ozel C, Atli MO, Kurar E, Kayis SA (2014) 109 Expression of genes related to endometrial receptivity in equine endometrium during the estrous cycle and early pregnancy. Reprod Fertil Dev 27:147. doi: http://dx.doi.org/10.1071/RDv27n1Ab109
Hoffman LH, Olson GE, Carson DD, Chilton BS (1998) Progesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epithelium. Endocrinology 139:266–271. doi:10.1210/endo.139.1.5750
Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC (2001) Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod 65:820–828
Johnson GA, Burghardt RC, Bazer FW (2014) Osteopontin: a leading candidate adhesion molecule for implantation in pigs and sheep. J Anim Sci Biotechnol 5:56. doi:10.1186/2049-1891-5-56
Keith L, Ball BA, Scoggin K, Esteller-Vico A, Woodward EM, Troedsson MH, Squires EL (2013) Diestrus administration of oxytocin prolongs luteal maintenance and reduces plasma PGFM concentrations and endometrial COX-2 expression in mares. Theriogenology 79:616–624. doi:10.1016/j.theriogenology.2012.11.015
Klein C (2015) Novel equine conceptus-endometrial interactions on Day 16 of pregnancy based on RNA sequencing. Reprod Fertil Devel (in press)
Klein C, Troedsson MH (2011) Transcriptional profiling of equine conceptuses reveals new aspects of embryo-maternal communication in the horse. Biol Reprod 84:872–885. doi:10.1095/biolreprod.110.088732
Klein C, Troedsson M (2012) Equine pre-implantation conceptuses express neuraminidase 2–a potential mechanism for desialylation of the equine capsule. Reprod Domest Anim 47:449–454. doi:10.1111/j.1439-0531.2011.01901.x
Klein C, Troedsson MH (2013) Macrophage migration inhibitory factor is expressed by equine conceptuses and endometrium. Reprod Domest Anim 48:297–304. doi:10.1111/j.1439-0531.2012.02148.x
Klein C, Scoggin KE, Ealy AD, Troedsson MH (2010) Transcriptional profiling of equine endometrium during the time of maternal recognition of pregnancy. Biol Reprod 83:102–113. doi:10.1095/biolreprod.109.081612
Knapczyk-Stwora K, Durlej M, Duda M, Czernichowska-Ferreira K, Tabecka-Lonczynska A, Slomczynska M (2011) Expression of oestrogen receptor alpha and oestrogen receptor beta in the uterus of the pregnant swine. Reprod Domest Anim 46:1–7. doi:10.1111/j.1439-0531.2009.01505.x
Korzekwa AJ, Bah MM, Kurzynowski A, Lukasik K, Groblewska A, Skarzynski DJ (2010) Leukotrienes modulate secretion of progesterone and prostaglandins during the estrous cycle and early pregnancy in cattle: an in vivo study. Reproduction 140:767–776. doi:10.1530/REP-10-0202
Leith GS, Ginther OJ (1984) Characterization of intrauterine mobility of the early equine conceptus. Theriogenology 22:401–408
Levin ER (2001) Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol (1985) 91:1860–1867
Levin ER (2005) Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959. doi:10.1210/me.2004-0390
Levitin F, Stern O, Weiss M, Gil-Henn C, Ziv R, Prokocimer Z, Smorodinsky NI, Rubinstein DB, Wreschner DH (2005) The MUC1 SEA module is a self-cleaving domain. J Biol Chem 280:33374–33386. doi:10.1074/jbc.M506047200
Liu N, Zhou C, Chen Y, Zhao J (2013) The involvement of osteopontin and beta3 integrin in implantation and endometrial receptivity in an early mouse pregnancy model. Eur J Obstet Gynecol Reprod Biol 170:171–176. doi:10.1016/j.ejogrb.2013.06.019
McDowell KJ, Sharp DC, Grubaugh W, Thatcher WW, Wilcox CJ (1988) Restricted conceptus mobility results in failure of pregnancy maintenance in mares. Biol Reprod 39:340–348
McDowell KJ, Adams MH, Adam CY, Simpson KS (1999) Changes in equine endometrial oestrogen receptor alpha and progesterone receptor mRNAs during the oestrous cycle, early pregnancy and after treatment with exogenous steroids. J Reprod Fertil 117:135–142
Milvae RA, Alila HW, Hansel W (1986) Involvement of lipoxygenase products of arachidonic acid metabolism in bovine luteal function. Biol Reprod 35:1210–1215
Obermair A, Schmid BC, Stimpfl M, Fasching B, Preyer O, Leodolter S, Crandon AJ, Zeillinger R (2001) Novel MUC1 splice variants are expressed in cervical carcinoma. Gynecol Oncol 83:343–347. doi:10.1006/gyno.2001.6396
Ohnuma K, Yokoo M, Ito K, Nambo Y, Miyake YI, Komatsu M, Takahashi J (2000) Study of early pregnancy factor (EPF) in equine (Equus caballus). Am J Reprod Immunol 43:174–179
Okuda K, Tokuyama S, Kozai K, Toishi Y, Tsunoda N, Taya K, Sakatani M, Takahashi M, Nambo Y (2014) Auto-amplification system in prostaglandin F2α production by endometrium for initiating and progressing luteolysis in mares. J Equine Vet Sci 34:139. doi:10.1016/j.jevs.2013.10.095
Oriol JG, Betteridge KJ, Clarke AJ, Sharom FJ (1993a) Mucin-like glycoproteins in the equine embryonic capsule. Mol Reprod Dev 34:255–265. doi:10.1002/mrd.1080340305
Oriol JG, Sharom FJ, Betteridge KJ (1993b) Developmentally regulated changes in the glycoproteins of the equine embryonic capsule. J Reprod Fertil 99:653–664
Ozel C, Guzeloglu A, Hitit M, Atli MO, Kurar E, Kayis SA (2014) 111 Expression of phospholipase a2 isoforms in equine endometrium during the estrous cycle and early pregnancy. Reprod Fertil Dev 27:148. doi: http://dx.doi.org/10.1071/RDv27n1Ab111
Parry S, Silverman HS, McDermott K, Willis A, Hollingsworth MA, Harris A (2001) Identification of MUC1 proteolytic cleavage sites in vivo. Biochem Biophys Res Commun 283:715–720. doi:10.1006/bbrc.2001.4775
Paulo E, Tischner M (1985) Activity of delta(5)3beta-hydroxysteroid dehydrogenase and steroid hormones content in early preimplantation horse embryos. Folia Histochem Cytobiol 23:81–84
Perry JS, Heap RB, Amoroso EC (1973) Steroid hormone production by pig blastocysts. Nature 245:45–47
Pope WF, Maurer RR, Stormshak F (1982) Intrauterine migration of the porcine embryo: influence of estradiol-17 beta and histamine. Biol Reprod 27:575–579
Qu X, Yang M, Zhang W, Liang L, Yang Y, Zhang Y, Deng B, Gao W, Liu J, Yang Q, Kong B, Gong F (2008) Osteopontin expression in human decidua is associated with decidual natural killer cells recruitment and regulated by progesterone. In Vivo 22:55–61
Raeside JI, Christie HL (2008) The presence of 19-norandrostenedione and its sulphate form in yolk-sac fluid of the early equine conceptus. J Steroid Biochem Mol Biol 108:149–154. doi:10.1016/j.jsbmb.2007.09.021
Raeside JI, Christie HL, Renaud RL, Waelchli RO, Betteridge KJ (2004) Estrogen metabolism in the equine conceptus and endometrium during early pregnancy in relation to estrogen concentrations in yolk-sac fluid. Biol Reprod 71:1120–1127. doi:10.1095/biolreprod.104.028712
Raeside JI, Christie HL, Waelchli RO, Betteridge KJ (2009) Estrogen metabolism by the equine embryo proper during the fourth week of pregnancy. Reproduction 138:953–960. doi:10.1530/REP-09-0235
Schuler G, Wirth C, Teichmann U, Failing K, Leiser R, Thole H, Hoffmann B (2002) Occurrence of estrogen receptor alpha in bovine placentomes throughout mid and late gestation and at parturition. Biol Reprod 66:976–982
Sharp DC, McDowell KJ, Weithenauer J, Thatcher WW (1989) The continuum of events leading to maternal recognition of pregnancy in mares. J Reprod Fertil Suppl 37:101–107
Sharp DC, Thatcher MJ, Salute ME, Fuchs AR (1997) Relationship between endometrial oxytocin receptors and oxytocin-induced prostaglandin F2 alpha release during the oestrous cycle and early pregnancy in pony mares. J Reprod Fertil 109:137–144
Short RV (1969) Implantation and the maternal recognition of pregnancy. In: Wolstenholme GEW, O’Connor M (eds) Ciba foundation symposium on foetal autonomy. Churchill, London, pp 2–26
Sierralta WD, Thole HH (1996) Retrieval of estradiol receptor in paraffin sections of resting porcine uteri by microwave treatment. Immunostaining patterns obtained with different primary antibodies. Histochem Cell Biol 105:357–363
Silva LA, Ginther OJ (2006) An early endometrial vascular indicator of completed orientation of the embryo and the role of dorsal endometrial encroachment in mares. Biol Reprod 74:337–343. doi:10.1095/biolreprod.105.047621
Smits K, Govaere J, Peelman LJ, Goossens K, de Graaf DC, Vercauteren D, Vandaele L, Hoogewijs M, Wydooghe E, Stout T, Van Soom A (2012) Influence of the uterine environment on the development of in vitro-produced equine embryos. Reproduction 143:173–181. doi:10.1530/REP-11-0217
Spencer TE, Bazer FW (1995) Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod 53:1527–1543
Spencer TE, Bazer FW (2002) Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci 7:d1879–d1898
Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW (1995) Ovine interferon-tau regulates expression of endometrial receptors for estrogen and oxytocin but not progesterone. Biol Reprod 53:732–745
Squires EL, Ginther OJ (1975) Follicular and luteal development in pregnant mares. J Reprod Fertil Suppl (23):429–433
Starbuck GR, Stout TA, Lamming GE, Allen WR, Flint AP (1998) Endometrial oxytocin receptor and uterine prostaglandin secretion in mares during the oestrous cycle and early pregnancy. J Reprod Fertil 113:173–179
Stevenson KR, Parkinson TJ, Wathes DC (1991) Measurement of oxytocin concentrations in plasma and ovarian extracts during the oestrous cycle of mares. J Reprod Fertil 93:437–441
Stewart CL (1994) Leukaemia inhibitory factor and the regulation of pre-implantation development of the mammalian embryo. Mol Reprod Dev 39:233–238. doi:10.1002/mrd.1080390217
Stewart F, Charleston B, Crossett B, Barker PJ, Allen WR (1995) A novel uterine protein that associates with the embryonic capsule in equids. J Reprod Fertil 105:65–70
Stock AE, Emeny RT, Sirois J, Fortune JE (1995) Oxytocin in mares: lack of evidence for oxytocin production by or action on preovulatory follicles. Domest Anim Endocrinol 12:133–142
Stout TA, Allen WR (2001) Role of prostaglandins in intrauterine migration of the equine conceptus. Reproduction 121:771–775
Stout TA, Allen WR (2002) Prostaglandin E(2) and F(2 alpha) production by equine conceptuses and concentrations in conceptus fluids and uterine flushings recovered from early pregnant and dioestrous mares. Reproduction 123:261–268
Stout TA, Lamming GE, Allen WR (1999) Oxytocin administration prolongs luteal function in cyclic mares. J Reprod Fertil 116:315–320
Stout TA, Meadows S, Allen WR (2005) Stage-specific formation of the equine blastocyst capsule is instrumental to hatching and to embryonic survival in vivo. Anim Reprod Sci 87:269–281. doi:10.1016/j.anireprosci.2004.11.009
Suire S, Stewart F, Beauchamp J, Kennedy MW (2001) Uterocalin, a lipocalin provisioning the preattachment equine conceptus: fatty acid and retinol binding properties, and structural characterization. Biochem J 356:369–376
Szostek AZ, Galvao AM, Hojo T, Okuda K, Skarzynski DJ (2014) Interleukins affect equine endometrial cell function: modulatory action of ovarian steroids. Mediators Inflamm 2014:208103. doi:10.1155/2014/208103
Takagi M, Nishimura K, Oguri N, Ohnuma K, Ito K, Takahashi J, Yasuda Y, Miyazawa K, Sato K (1998) Measurement of early pregnancy factor activity for monitoring the viability of the equine embryo. Theriogenology 50:255–262
Thatcher WW, Staples CR, Danet-Desnoyers G, Oldick B, Schmitt EP (1994) Embryo health and mortality in sheep and cattle. J Anim Sci 72:16–30. doi:/1994.72suppl_316x
Tomanelli RN, Sertich PL, Watson ED (1991) Soluble oestrogen and progesterone receptors in the endometrium of the mare. J Reprod Fertil Suppl 44:267–273
Vanderwall DK, Woods GL, Weber JA, Lichtenwalner AB (1994) Corpus luteal function in nonpregnant mares following intrauterine administration of prostaglandin E(2) or estradiol-17beta. Theriogenology 42:1069–1083
Vanderwall DK, Rasmussen DM, Woods GL (2007) Effect of repeated administration of oxytocin during diestrus on duration of function of corpora lutea in mares. J Am Vet Med Assoc 231:1864–1867. doi:10.2460/javma.231.12.1864
Vernon MW, Strauss S, Simonelli M, Zavy MT, Sharp DC (1979) Specific PGF-2 alpha binding by the corpus luteum of the pregnant and non-pregnant mare. J Reprod Fertil Suppl (27):421–429
Vernon MW, Zavy MT, Asquith RL, Sharp DC (1981) Prostaglandin F2alpha in the equine endometrium: steroid modulation and production capacities during the estrous cycle and early pregnancy. Biol Reprod 25:581–589
Villani MV, Vriend BAM, Paris DBBP, Stout TAE (2010) A role for Leukemia Inhibitory Factor (LIF) during implantation in the mare? Anim Reprod Sci 121:309. doi: http://dx.doi.org/10.1016/j.anireprosci.2010.04.060
Walters KW, Corbin CJ, Anderson GB, Roser JF, Conley AJ (2000) Tissue-specific localization of cytochrome P450 aromatase in the equine embryo by in situ hybridization and immunocytochemistry. Biol Reprod 62:1141–1145
Watson ED (1991) Do mares possess an intracellular endometrial inhibitor of prostaglandin synthesis during early pregnancy? Theriogenology 36:67–71
Watson ED, Sertich PL (1989) Prostaglandin production by horse embryos and the effect of co-culture of embryos with endometrium from pregnant mares. J Reprod Fertil 87:331–336
Watson ED, Skolnik SB, Zanecosky HG (1992) Progesterone and estrogen receptor distribution in the endometrium of the mare. Theriogenology 38:575–580
Weber JA, Freeman DA, Vanderwall DK, Woods GL (1991a) Prostaglandin E2 hastens oviductal transport of equine embryos. Biol Reprod 45:544–546
Weber JA, Freeman DA, Vanderwall DK, Woods GL (1991b) Prostaglandin E2 secretion by oviductal transport-stage equine embryos. Biol Reprod 45:540–543
Weber JA, Woods GL, Freeman DA, Vanderwall DK (1992) Prostaglandin E2-specific binding to the equine oviduct. Prostaglandins 43:61–65
Wilsher S, Clutton-Brock A, Allen WR (2010) Successful transfer of day 10 horse embryos: influence of donor-recipient asynchrony on embryo development. Reproduction 139:575–585. doi:10.1530/REP-09-0306
Wilsher S, Gower S, Allen WR (2011) Immunohistochemical localisation of progesterone and oestrogen receptors at the placental interface in mares during early pregnancy. Anim Reprod Sci 129:200–208. doi:10.1016/j.anireprosci.2011.11.004
Woodley SL, Burns PJ, Douglas RH, Oxender WD (1979) Prolonged interovulatory interval after oestradiol treatment in mares. J Reprod Fertil Suppl (27):205–209
Yamauchi S (1975) Morphology and histochemistry of the endometrial cup. J Reprod Fertil Suppl (23):397–400
Zavy MT, Sharp DC, Bazer FW, Fazleabas A, Sessions F, Roberts RM (1982) Identification of stage-specific and hormonally induced polypeptides in the uterine protein secretions of the mare during the oestrous cycle and pregnancy. J Reprod Fertil 64:199–207
Zavy MT, Vernon MW, Sharp DC 3rd, Bazer FW (1984) Endocrine aspects of early pregnancy in pony mares: a comparison of uterine luminal and peripheral plasma levels of steroids during the estrous cycle and early pregnancy. Endocrinology 115:214–219
Zhu BT, Conney AH (1998) Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 19:1–27
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Klein, C. (2015). Pregnancy Recognition and Implantation of the Conceptus in the Mare. In: Geisert, R., Bazer, F. (eds) Regulation of Implantation and Establishment of Pregnancy in Mammals. Advances in Anatomy, Embryology and Cell Biology, vol 216. Springer, Cham. https://doi.org/10.1007/978-3-319-15856-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-15856-3_9
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15855-6
Online ISBN: 978-3-319-15856-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)