Abstract
Development of the porcine corpus luteum (CL) requires the initial preovulatory LH surge and support of many biologically active agents including tonic secretion of LH, ovarian steroids, growth factors, and prostaglandins. A lack of embryo presence in the uterus leads to CL regression, characterized by disrupted progesterone production (functional luteolysis) and further degeneration of luteal and endothelial cells (structural luteolysis) triggered by prostaglandin F2α (PGF2α). The porcine CL expresses abundant levels of PGF2α receptors in the early and mid-luteal phase of the estrous cycle but remains insensitive to a single treatment of exogenous PGF2α until about day 12 of the estrous cycle. The nature of porcine CL resistance to PGF2α remains unknown, and the mechanism of luteolytic sensitivity acquisition involves infiltration of immune cells into the CL. Former theories of luteolysis inhibition and maternal recognition of pregnancy in the pig have proposed that possible mechanism for prevention of luteal regression is connected with a limited PGF2α supply to CL, evoked by its sequestering in the uterus. Later studies besides the increased synthesis of prostaglandin E2 (PGE2) by the conceptus and endometrium revealed simultaneously decreased expression of PGF2α synthesis enzymes. This chapter summarizes available knowledge on the porcine CL maintenance and regression and present our recent studies leading to a novel ‘two signal-switch’ hypothesis, based on the interplay of both PGF2α and PGE2 postreceptor signaling pathways. Several practical aspects of how to prolong and enhance CL function and improve pregnancy maintenance are also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Corpus luteum
- Luteolytic sensitivity
- Luteolysis inhibition
- Pregnancy establishment
- Embryo signals
- Prostaglandins
- Two-switches hypothesis
- Pig
12.1 Pro-Luteal Environment in the Reproductive Tract in Advance of Maternal Recognition of Pregnancy
In sexual reproduction , specific organs have been developed to allow the introduction and passage of egg and sperm to reach each other before fertilization. These organs support the physiological demands of gametes and later developing embryo(s); however, nurturing factors may not only originate from local reserves. The maternal reproductive tract hosts a critical crosstalk with the embryo that starts at the very early stages of pregnancy. Although the response of the reproductive tract toward embryos at the very early stages of pregnancy is poorly understood, several investigators suggested the presence of early communication among gametes, embryos, and the female reproductive tract before the main maternal recognition of pregnancy signal occurs in pigs. Some early gametes or embryos and mother communication pathways can also be involved in luteal function.
Before embryo signals are systemically recognized by the mother and luteal function is maintained through pregnancy, mating or insemination affects several local processes in the porcine reproductive tract. Studies performed in many species, including pigs [1], suggests that embryo–maternal communication exists at the very early stages of pregnancy, long before the well-known embryonic signals can be detected. In pigs , as in other mammals, deposition of semen into the female reproductive tract triggers a cascade of cellular and molecular events that in many respects resembles a classic inflammatory response [2, 3]. Within hours after mating, neutrophils are recruited into the uterine lumen [4–6]. In endometrial stroma , however, an accumulation of macrophages and dendritic cells, granulocytes, and lymphocytes occurs [2, 7]. It was shown that inseminate constituents, such as seminal plasma , modulate the endometrial influx of polymorphonuclear leukocytes after insemination [8, 9]. Leukocyte recruitment is elicited after seminal factors signal uterine epithelial cells to induce expression of a number of proinflammatory factors, including granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-6 [10]. Furthermore, prostaglandin synthesis and angiogenesis pathways were also affected in a transient as well as a more prolonged manner in the porcine oviduct and endometrium [11–13]. On the other hand, the effects of intrauterine seminal fluid on the ovary were manifested as clear changes in the development and steroidogenic competence of the corpus luteum (CL) [14].
The effects of uterine exposure to seminal plasma on prostaglandins synthesis and secretion persist over the course of the prereceptive period and are of considerable interest for achievement of the pro-luteal embryotrophic milieu in the reproductive tract. Altered expression of prostaglandin endoperoxide synthase 2 (PTGS2, or cyclooxygenase-2, COX-2), and PGF2α synthase (PTGFS) in the endometrium on days 5 or 10 after seminal plasma exposure was accompanied by an increased PGE2 level on day 10, this being crucial for modulation of the PGE2:PGF2α ratio shortly before the maternal recognition of pregnancy [12]. Thus, it seems likely that seminal plasma constituents can sensitize the endometrium for forthcoming pregnancy by amplifying the uterine synthesis of crucial antiluteolytic/luteoprotective PGE2 and supporting key events occurring during early pregnancy, such as embryo development and maternal recognition of pregnancy. Whether the increased number of viable embryos and improved embryo growth observed by O’Leary and coworkers [10] 9 days after seminal plasma exposure might be linked with increased PGE2 levels and the PGE2:PGF2α ratio in the endometrium needs further investigation.
Interestingly, the effect of intrauterine seminal plasma exposure on CL development and ovarian steroidogenesis was also observed [14]. It was shown that plasma progesterone levels are higher and peaked earlier in gilts treated with seminal plasma. Concomitantly, this was associated with an increase in average weight of CL, without a concurrent increase in ovulation rate, suggesting that the number and output of steroidogenic luteal cells are greater in animals exposed to seminal components. Some authors proposed that the effect of seminal plasma presence in the uterine horns persisting over the course of early pregnancy might be partly the consequence of elevated local progesterone synthesis, which could act to differentially regulate several progesterone-responsive uterine parameters [15], including the observed altered cytokine, angiogenic factors, and prostaglandin synthesis pathway gene expression [10–13].
Moreover, significant elevation in the abundance of activated macrophages in the thecal and perifollicular stromal tissue 34 h after seminal plasma treatment suggests that these cells and their secretory products influence the architecture and functionality of the vascular stroma and theca tissues of the ovary with direct or indirect effects on granulosa cells, showing accelerated progesterone synthesis when cultured in the presence of human chorionic gonadotropin (hCG) [14]. Recently, the CL of macrophage-depleted mice have been shown to produce substantially less progesterone, have disrupted blood vasculature, and exhibit changes in the local expression of genes encoding angiogenic regulators [16]. The reduced progesterone production was fully responsible for the infertility defect in mice because pregnancy was restored and supported to term through exogenous progesterone administration. On the other hand, our findings showed clearly that seminal plasma can alter vascular endothelial growth factor A (VEGFA) ligand–receptor system expression and vascular density in the porcine endometrium and oviduct [12, 13]. These findings indicate the substantial function of semen in controlling macrophage luteal populations and its paramount function at this time to provide trophic support for formation of the vascular network pivotal to CL development, progesterone synthesis, and the establishment of viable pregnancy.
Recently, downregulation of a set of immune-related genes expressed in the presence of a 6-day-old blastocyst were observed in the porcine endometrium [1]. Additionally, changes observed in the uterine horn while the embryo was still in the oviduct imply that there is a local effect of the embryo on the oviduct that is extended to the uterine horn. These changes have been suggested to help prepare the uterus for the acceptance of the embryo, a semi-allograft in the maternal organism.
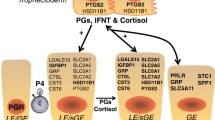
Taking into account the aforementioned facts, it seems likely that uterine response to the presence of semen and embryos could directly and indirectly influence the milieu of the reproductive tract. If we take under consideration the potential involvement of lymphatic pathways and countercurrent transfer of ‘programming information’ from uterine lymphatics into ovarian arterial blood [17], a hypothesis involving the indirect effects of semen and embryos on the female reproductive tract seems more likely to be accurate. Using this route, cytokines, PGs, and other biologically active molecules (of uterine, seminal plasma, or embryonic origin) may reach the oviduct and ovarian tissues via the arteries, having entered the utero-ovarian artery from the uterine lymphatics or veins by means of countercurrent exchange [18, 19], and in consequence affect several pathways in the female reproductive tract, including progesterone and PG synthesis, as well as angiogenesis, leading to successful pregnancy outcomes (Fig. 12.1).
The proposed potential mechanism of seminal plasma-mediated increase in early embryo survival and quality: involvement of steroidogenesis and prostaglandin pathways as well as immune cell infiltration. Seminal plasma initiates the immune cell infiltration (e.g., T and NK cells) and de novo protein synthesis in the endometrium. For instance, the prostaglandin synthesis pathway is affected, as PGE2 levels are higher and consequently the PGE2 to PGF2α ratio is increased in the endometrium. The biologically active molecules of uterine or seminal plasma origin can also reach the ovarian and oviduct tissues directly or via the arteries, having entered the uterine (UA) and ovarian arteries (OA) from uterine lymphatics or veins (UV) by means of countercurrent exchange. These sequences of events change prostaglandin synthesis in the oviduct as well as immune cell infiltration and progesterone synthesis in the ovary. P4 progesterone, T reg T-regulatory cells, T h T-helper cells, NK natural killer cells, AA arterio-arterial anastomoses connecting uterine and ovarian arteries, SLA-II swine leukocyte antigen class II. (Modified from Ziecik et al. [20])
12.2 Porcine CL Development, Regression, and Maintenance
12.2.1 Development
According to the old paradigm, formation of porcine CL requires only an initial surge of the preovulatory LH triggered by the pituitary, and then its further existence is independent on LH until day 12 of the estrous cycle. Such an opinion was drawn from the experiment when pigs hypophysectomized on the first day of estrus developed apparently normal CL up to day 12 of the estrous cycle, which then regressed by day 16 [21]. Conclusion on the maintenance of LH dependence of CL was justified by the fact that the majority of mature gilts and postpartum sows exhibit the maximal LH levels at the first observance of estrus [22]. The question whether the porcine CL is fully ‘autonomous,’ as was believed earlier [23], still remains open. Evidence that (1) the passive immunization of the gilt with anti-pLH serum on day 8 of the estrous cycle dramatically decreased progesterone level in the blood [24] and (2) LH in a time- and dose-dependent manner increased secretion of progesterone by cultured luteal slices collected at mid-luteal phase [25] indicates that this pituitary gonadotropin may still have an important if not decisive function in the maintenance of porcine CL function. The supportive role of many biologically active agents including ovarian steroids [26–28], growth factors (e.g., IGF-I) [29], and prostaglandins [25, 30, 31] in luteal function maintenance was also well documented.
12.2.2 Regression
It is believed that regression of porcine CL occurring on days 15–16 of the estrous cycle results from an increase in pulsatile endometrial secretion of PGF2α [32]. However, the highest pulses of PGF2α occur after a decline of progesterone level in the blood plasma, that is, when the functional luteolysis of CL is completed [33]. So far, oxytocin [34], TNFα [31, 35, 36], and LH [37, 38] are considered as the potential modulators of endometrial prostaglandin production. A strong relationship between oxytocin, oxytocin receptors, and PGF2α release in vitro was reported for the cultured explants of porcine endometrium collected on days 15–16 of the estrous cycle [39, 40]. However, the agreement between peaks of oxytocin and inactive metabolite of PGF2α (PGFM) peaks in the blood of gilts reached only about 30 %, whereas blocking oxytocin receptors neither prevented luteolysis nor changed the duration of the estrous cycle [41]. Furthermore, a much higher agreement was found between peaks of LH and PGFM (75.5 %); thus, the “luteolytic” role of LH can be limited only to the period of the late luteal phase of the porcine estrous cycle [33].
It is believed that in pigs, as in many species, luteolysis is triggered mainly by PGF2α. Although porcine CL express abundant levels of PGF2α receptors also in the early luteal phase [42, 43], a luteal tissue remains refractory to a single treatment with exogenous PGF2α for the first 12–13 days of the estrous cycle. Furthermore, experiments employing the in vivo microdialysis system [31] and in vitro incubation of luteal slices of porcine CL [25] indicate an increased progesterone secretion after PGF2α treatment during the mid-luteal phase of the estrous cycle, that is, before acquisition of luteolytic sensitivity (LS). An acquisition of LS to PGF2α is still not a fully determined phenomenon in pigs. Moreover, it does not depend on a number of PGF2α-binding sites in luteal cells as suggested earlier [42, 43].It is a very complex process, but it seems likely that PGF2α induces different molecular pathways in porcine CL with and without acquired LS [44–48]. For example, PGF2α affects the signaling pathway and its own synthesis [44, 49], as well as estradiol-17β [45], progesterone [46], endothelin-1 (EDN1) [50], chemokine CCL2 and its receptor (CCR2) [51] levels, but only in porcine CL being already sensitive to the luteolytic action of PGF2α.
Wuttke and colleagues [31] suggested that PGF2α-induced estradiol-17β secretion is stimulatory to progesterone production in young and middle-aged CL. The macrophage-delivered TNFα stimulates progesterone secretion in the early and middle-aged CL. Lack of estradiol-17β supply causes functional luteolysis triggered by PGF2α and TNFα [31]. It is interesting that the significant decrease of luteal progesterone content begins after day 12 of the estrous cycle contrary to the parallel day of pregnancy (Fig. 12.2). Factors inducing apoptosis (Bax and Bcl-2; TNF family) are also involved in the process of LS acquisition in pigs [48, 54].
It has become more generally accepted that elevated macrophage infiltration into porcine CL throughout the estrous cycle [55, 56], similarly to other species, coincides with the development of LS during the estrous cycle [29]. Macrophages are the major source of TNFα in the porcine corpus luteum [56]. A decreased luteal concentration of progesterone on day 14 of the estrous cycle is proceeded by an expression of TNFA and IFNG mRNA on day 12, suggesting that those cytokines are required for LS acquisition in pigs [52]. Recently, Przygrodzka and colleagues [53] identified TNFα receptor-1 signaling, apoptosis signaling, and production of nitric oxide (NO) and reactive oxygen species (ROS) among the canonical pathways activated in CL collected as early as on day 12 of the estrous cycle.
12.2.3 Maintenance
Establishment of pregnancy requires the maintenance of a functional CL beyond its normal cyclic lifespan to sustain production of progesterone. Progesterone stimulates secretory activity of the endometrium that is crucial for embryonic development and implantation. The first described embryonic signal in the pig is estrogen (mainly estradiol-17β) secreted by the conceptus on days 11 and 12 of pregnancy [57], that is, 2 days before CL begins to regress in nonpregnant gilts/sows.
The maternal recognition of pregnancy coincides with a rapid transformation of the conceptus from the spherical to tubular and then filamentous forms between days 10 and 12 after fertilization, when the first estradiol-17β peak secretion of conceptus origin is noted. The second peak of estradiol-17β secretion by conceptus appears in the maternal circulation on days 15–30 of pregnancy [28], when the lifespan of the CL is already extended. It is not clear whether the second elevation of embryo-originated estrogens in the maternal blood is related to CL maintenance during this period of pregnancy. In the pig, embryo implantation (days 14–18) and placentation take place during days 14–30 of pregnancy. It is believed that this second peak of estrogen conceptus secretion is rather needed for early embryo development. Moreover, estrogen was shown to affect the porcine CL in two ways, acting as a luteotropic or antiluteolytic agent. The luteotropic action of estradiol-17β depends on its direct action on CL by enhancing production of progesterone, as previously found in in vivo [26] and in vitro studies [25].
The indirect effects of estradiol-17β on porcine CL function range from an increase of luteal LH receptor concentration [58], and a decrease in PGF2α release from the uterus into the peripheral circulation [59], to the control of prostaglandin synthesis in the endometrium [60] and conceptus [61]. The period of estrogen secretion is also correlated with an increase of estrogen receptor expression in the luminal and glandular epithelium of the endometrium [62] and the conceptus itself [63].
12.2.4 Theories of Maternal Recognition of Pregnancy
The demonstration of aromatase activity in the preimplantation pig blastocyst [57] was confirmed and extended later by others [42], leading to the nomination of estradiol-17β as the embryonic signal necessary for the maternal recognition of pregnancy in the pig [64]. Observation of higher PGF2α concentrations in the utero-ovarian vein between days 12 and 18 of the estrous cycle than in pregnant animals suggested that PGF2α is directed primary toward the uterine vessel drainage (endocrine direction) and to CL in nonpregnant animals [65]. After reaching the CL, PGF2α initiates a cascade of events leading to luteolysis. A low PGF2α accumulation in the uterine lumen (exocrine direction) during the estrous cycle [23] seemed to confirm the foregoing supposition.
According to the original Bazer and Thatcher [59] concept of maternal recognition of pregnancy, estrogens produced by the pig blastocyst as early as on day 11 (tubular and filamentous blastocysts) of pregnancy alter the direction of PGF2α secretion in pregnant pigs toward the uterine lumen, preventing PGF2α entrance to the uterine venous drainage and weakening its luteolytic effect on the CL. The authors suggested that the luteostatic effect of estrogens originating from the blastocyst (or exogenous estrogen) is mediated on the uterine endometrium level.
Another explanation for the abundance of PGF2α in the uterus is its retrograde transfer from the venous blood and uterine lymph into the uterine lumen, as well as accumulation of PGF2α by the uterine veins and arterial walls [18, 66]. The high PGF2α level found in the uterine lumen during early pregnancy would be a consequence of PGF2α uptake from the arterial blood, supporting the uterus, and its removal into the uterine lumen. Similarly, as suggested by Bazer and Thatcher [59], transfer of PGF2α to the uterine lumen may strongly reduce the peak of its concentration in the peripheral blood during pulsatile release of PGF2α from the uterus. Nevertheless, Hunter and Poyser [15] suggested that the exocrine redirection of the uterine PGF2α secretion may not provide a full explanation for maintenance of the CL in pregnant pigs and pointed out that this route of delivery for the luteolytic agent may not always be effective.
The aforementioned theories of maternal recognition of pregnancy of both ‘endocrine versus exocrine’ and ‘retrograde’ transfer of PGF2α in the porcine reproductive tract were proposed in the last decades of the twentieth century, before the “omics era” had dawned, and were focused on the sequestering of PGF2α in the uterus (early pregnancy) or its redirection toward the ovary (late luteal phase of the estrous cycle) . Nevertheless, many researchers were encouraged to undertake new studies in the next decades using state-of-the-art methods.
12.3 The Roles of PGF2α and PGE2 and Their Receptors in Porcine CL Function
A part of the potential mechanism by which the conceptus prevents luteolysis is changing prostaglandin synthesis in favor of the luteo-protective PGE2. The porcine conceptus and endometrium synthesize large amounts of PGE2 before implantation [61, 67]. Additionally, the porcine myometrium secretes more PGE2 than PGF2α during early pregnancy [68]. The PGE2:PGF2α ratio is increased in the uterine lumen and vein [30, 69] as well as in the trophoblastic tissue on days 10–13 of gestation [61].
Evidence for a luteotropic/antiluteolytic effect of PGE2 in the pig was demonstrated by Akinlosotu and coworkers [70]. Moreover, a direct effect of exogenous PGE2 delivered in implants to luteal tissue in protecting porcine CL from the luteolytic dose of PGF2α was shown by Ford and Christenson [27]. However, the direct intrauterine application of PGE2 was incapable of extending luteal function in nonpregnant gilts [71] and simultaneously caused an elevation of PGF2α concentration in the utero-ovarian venous blood [72], probably overcoming the luteotropic effect of PGE2. On the other hand, an infusion of PGE2 into the ovarian artery elevated the concentration of progesterone in the ovarian venous blood on days 13 and 14 of pregnancy [73]. During early pregnancy, the expression of microsomal PGE2 synthase (mPGES1) is intermediate in the porcine endometrium on days 10–11, low on days 14–17, and increases after day 22 [67]. Its mRNA and protein levels were significantly elevated (28 fold versus days 14–15) on days 10–13 in spherical/tubular and filamentous conceptuses [61]. mPGES1 leads to the higher PGE2:PGF2α ratio in spherical/tubular day 10–13 conceptuses at the time of maternal recognition of pregnancy. Also, the PGFM:PGF2α ratio, which is an index of 15-hydroxyprostaglandin dehydrogenase activity, was very low in spherical/tubular and filamentous conceptuses and markedly enhanced after day 14 of pregnancy [74]. These results suggest an increased metabolism of PGF2α during implantation to prevent the luteolytic effect of native PGF2α. The pattern of mPGES1 expression in conceptuses maximizes, in such a way, the biological effect of luteotropic PGE2 and overlaps with the occurrence of the biphasic profile of estrogen synthesis and secretion by blastocysts [75].
In contrast, the low expression of carbonyl reductase , PG 9-ketoreductase (CBR1), an important enzyme catalyzing nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reversible conversion of PGE2 into PGF2α in conceptus on days 10–13 of pregnancy, also indicates a significant contribution of the preimplantation conceptus to the synthesis of PGE2 during the maternal recognition of pregnancy in the pig [61]. Simultaneously, moderate changes in levels of PGF2α and PGE2 synthases occur in the porcine CL [76] and endometrium [67]. In addition to the possible endocrine role of conceptus-delivered PGs in porcine CL function, it can be important in altering gene expression in the endometrium before pregnancy recognition [77].
Another explanation for a mechanism preventing luteolysis can be distribution of PGF2α and PGE2 transporters , that is, ABCC4 (ATP-binding cassette subfamily C member 4 out of the cell, at the cell surface) and SLCO2A1 (solute carrier organic anion transporter family member 2A1, into the cell), respectively, which were abundant in the porcine endometrium on day 12 of pregnancy when conceptuses elongate and initiate implantation in pigs [78]. However, estradiol-17β did not increase ABCC4 and SLCO2A1 mRNA levels in cultured explants of porcine endometrium [78]. On the other hand, a high content of both PGs transporters is in agreement with the elevated concentrations of PGF2α and PGE2 in the uterus of pregnant gilts [79].
Earlier pioneering studies by Gadsby and coworkers [42, 43] could suggest that the level of PGF2α receptors (PTGFR) is fundamental in sensitizing the porcine CL to the luteolytic action of PGF2α, because a decreased expression of this receptor was found in luteal cells of pregnant and pseudo-pregnant pigs . In contrast, Przygrodzka and coworkers [53] did not observe a significant decrease of PTGFR at either mRNA or protein levels (Fig. 12.3) in the CL of early pregnant gilts in comparison to CL of cyclic gilts. The present results support a similar distribution of PTGFR mRNA in cyclic and early pregnant sheep [80]. It is worth noting that Gadbsy and colleagues [42, 43] used a different technique, investigating the capacity of [3H]PGF2α-binding sites in isolated porcine luteal cells. It is unclear whether a high number of binding sites reflects their ability to activate postreceptor signaling mechanisms because recent studies suggested new, alternatively spliced PTGFR, leading to stimulation or inhibition of CL function [81].
Concentrations of PGE2 (a) and PGF2α (b) and the content of PTGER4 (c) and PTGFR protein (d) in corpus luteum on days 8–14 of the estrous cycle and pregnancy in pigs. (Adapted from Przygrodzka et al. [52])
On the other hand, expression of PTGS2 mRNA and protein [53], as well as the content of PGF2α, was elevated in the porcine CL at the time of luteolysis (Fig. 12.3). It brings evidence that confirms the concept of possible increase of PGF2α synthesis in CL with acquired LS [44].
Surprisingly, PTGFR mRNA [53] and protein levels (Fig. 12.3) were more abundant in the CL of pregnant gilts than the cyclic counterparts. Because the expression of PTGFR is prominent in endothelial cells of porcine CL [82], PGF2α may be involved also in luteal function maintenance after overcoming luteolysis during early pregnancy through its participation in angiogenesis [83, 84]. Interestingly, treatment with PGF2α elevated the synthesis of progesterone and content of cAMP-response element-binding protein (CREB) in cultured luteal slices from pregnant pigs [53].
Simultaneously, a decreased level of PTGFR (Fig. 12.3) in CL collected on day 14 of the estrous cycle could be an effect of negative feedback between increasing concentrations of intraluteal PGF2α and PTGFR expression, as previously described in porcine luteal tissue [44]. The earlier report of Zorilla and coworkers [49] suggested that activation of different post-PTGFR signaling pathways , for example, an increase of the specific protein kinase C (PKC) ε expression, is more important for acquisition of luteolytic sensitivity in porcine CL than just a precise level of PTGFR.
Przygrodzka and coworkers [53] showed a fourfold higher concentration of PGE2 in the porcine CL on day 14 of pregnancy than on the parallel day of the estrous cycle (Fig. 12.3). Similarly, PGE2 content was higher only in CL ipsilateral to the gravid horn of unilateral pregnant gilts [85]. Because PGE2 content did not correspond to the mPGES1 expression in the luteal tissue, analogical as in ovine CL [86], the synthesis of PGE2 in the conceptus and endometrium, rather than in CL, seems to contribute to the process of luteal function rescue during the maternal recognition of pregnancy in the pig. A local transfer of PGE2 from the uterus to the ovary [73] may be involved in this mechanism. Moreover, a significantly increased content of one isoform of PGE2 receptors (PTGER4) was found in porcine CL collected on days 12 and 14 of pregnancy (Fig. 12.3). The presence of a second isoform of PGE2 receptor (PTGER2) was also documented earlier [36], and both receptors were shown to participate in cAMP production in cultured luteal slices [53]. It is worth emphasizing that secretion of luteal progesterone is stimulated by PGE2 through a cAMP-mediated pathway in many species [85]. As PGF2α increased the content of cAMP response element-binding protein (CREB) in CL of early pregnant pigs [53], it is possible that ‘luteolytic’ PGF2α can enhance accumulation of cAMP, already stimulated by PGE2, via both Ca2+ and PKC activation [87] as well as increase availability of CREB for its further activation by luteotropic hormones. Our recent in vitro studies [25] showed that PGF2α enhanced progesterone secretion by precision-cut luteal slices obtained from the mid-luteal phase CL, but diminished progesterone secretion by luteal slices obtained at the late luteal phase. The observed effects were consistent with results of in vivo experiments employing the microdialysis system in pigs [31].
The aforementioned studies clearly indicate a stronger role of conceptus- and uterus-delivered PGE2 in the rescue of porcine CL. Figure 12.4 presents the ‘two signal-switch’ hypothesis on the role of post PGF2α and PGE2 signaling pathways in CL regression and its overcoming during maternal recognition of pregnancy in the pig. It seems likely that the PTGFR level is less responsible for events leading to regression of CL than a sudden shift in the post-PTGFR signaling pathways occurring in CL after acquisition of luteolytic sensitivity, most probably under the influence of cytokines and endothelin-1. At the time when cells within the CL are ‘sensitive’ to PGF2α (“the LS switch”), its postreceptor signaling pathway leads to activation of the PKC pathway via diacyloglicerol (DAG) and inositol (1,4,5)-triphosphate (IP3) formation as well as elevated concentration of Ca2+-activating protein-serine threonine kinase (RAF1) and initiating signaling cascades RAF1/MAPK1/ERK1/2 . Activated proteins ERK1/2 can be translocated to the nucleus, where they phosphorylate transcription factors such as ETS domain-containing protein (ELK-1) and affect transcription of early response genes, that is, cellular oncogene FOS and JUN. In contrast, the aforementioned post-PTGFR pathway, leading finally to functional and structural destruction of CL, is blocked during the period of pregnancy establishment or CL rescue. By means of an embryonic signal (“the RESCUE switch ”), PGE2 through PTGER2 and PTGER4 may activate protein kinase A, leading to inhibition of main downstream elements of the post-MAPK signaling pathway, most likely via direct blockage of MAPK activator–RAF [88, 89] and turning of “the LS switch.” In consequence. instead of apoptotic genes induction, the expression of CREB is enhanced to support steroidogenesis, angiogenesis, and cell survival.
‘Two signal-switch’ hypothesis of PGF2α and PGE2 involvement in regression or rescue of porcine CL. Before acquisition of luteolytic sensitivity (LS), PGF2α acts on luteal cells through its specific transmembrane G protein-coupled receptor (PTGFR) amplifying LH-stimulated cAMP accumulation (dotted green arrows) and supports porcine CL function until day 12 of the estrous cycle, i.e., until acquisition of LS. Turning the ‘LS switch’ on by mediators of luteal regression (red pathway) changes the post-PTGFR sequence of events leading to inhibition of cAMP accumulation and to luteolysis. However, during pregnancy the embryo(s) signals estradiol-17β and PGE2, produced mainly by conceptuses and the endometrium, to turn the ‘RESCUE switch’ on (green solid arrows) and induce the post-PTGER2/4 pathway leading to protein kinase A (PKA) activation. The letter green pathway leads to inhibition RAF, blockage, or turning off the ‘LS switch’ and activation of CREB for maintenance of steroidogenesis, angiogenesis, and cell survival. PLC phospholipase C. (Adapted from Przygrodzka et al. [52, 53])
Additionally, our previous studies suggest that the luteoprotective action of PGE2 may involve a stimulation of VEGF expression in luteal cells on day 10–12 of pregnancy [90]. Interestingly, downregulation of a strong endogenous antagonist of VEGF soluble receptor (sFLT1) in the CL on day 12 of pregnancy may increase the amount of bioavailable VEGF in the porcine CL [91]. As a result, prolonged progesterone production is enhanced by increasing luteal capillary permeability and delivery of cholesterol to the luteal cells as well as facilitated PG transport from the circulation.
12.4 Genes Involved in Rescue of Corpus Luteum
Beside modulation of post-PGF2α and -PGE2 receptor signaling pathways, as well as an increase of intraluteal concentration of progesterone, the presence of live embryos in the uterus can enhance the expression of crucial genes involved in steroidogenesis [i.e., scavenger receptor class B, member 1 (SCARB1), steroidogenic acute regulatory protein (STAR), hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (HSD3B1), and luteinizing hormone/choriogonadotropin receptor (LHCGR)] in porcine CL collected on day 14 of pregnancy [53]. Found in the same study, the elevated levels of nuclear receptor subfamily 5, group A, member 1 (NR5A1 = SF-1), an activator of steroidogenic genes transcription, suggest that its presence can be important to sustain progesterone production as suggested for bovine CL [92]. Similarly, the high abundance of progesterone receptor membrane component 1 (PGRMC1) in porcine CL collected on day 14 of pregnancy [53] could be essential to enhance steroidogenesis [93] and can be the gateway of antiapoptotic action mediated by progesterone in luteal cells [74].
Recently, Przygrodzka and coworkers [52, 53] examined the expression of 50 genes associated with synthesis and action of steroids, PGs, angiogenesis, and apoptosis in the porcine CL collected at the mid- and late luteal phases of the estrous cycle and parallel days of early pregnancy. Venn diagrams revealed that EDN1, cytochrome P450 19A1 (CYP19A1), estrogen receptor 2 (ESR2), PTGS2, JUN, and FOS were downregulated on day 14 of pregnancy, whereas among upregulated genes kinase insert domain receptor (KDR), angiopoietin 2 (ANGPT2), pentraxin 3 (PTX3), HSD3B1, low density lipoprotein receptor (LDLR), STAR, estrogen receptor 1 (ESR1), LHCGR, progesterone receptor (PGR), PGRMC1, progesterone receptor membrane component 2 (PGRMC2), NR5A1, nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (NFKB1), prostaglandin F synthase (PTGFS), and hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD) were identified. For example, Fig. 12.5 presents the relatively constant expression of 13 genes connected to steroidogenesis, angiogenesis, and PG metabolism on day 12 of pregnancy and the estrous cycle but their up- or downregulation 2 days later.
Moreover, in silico analysis revealed that T-cell migration , activation of leukocytes, and infiltration of lymphocytes were already inhibited in CL obtained on day 12 of pregnancy. Also, the production of NO and ROS in macrophages was among decreased ingenuity pathways analysis of biological functions and pathways in CL collected on days 12 and 14 of pregnancy.
Although the involvement of immune cells in the regulation of regression and rescue of porcine CL remains practically unknown [55], the data presented here suggest the potential role of immune system cells in the control of luteal lifespan in the pig. It seems likely that crosstalk between immune cells products, that is, cytokines and factors involved in post-PG receptor-signaling pathways are crucial for CL lifespan during the estrous cycle and pregnancy in pigs and other mammals.
12.5 The Effect of hCG Administration on Luteal Function Maintenance During the Estrous Cycle and Pregnancy
Because the majority of embryonic losses (20–30 %) in pigs occurs between days 12 and 30 of gestation [94] and the level of progesterone is positively correlated with embryonic survival during the first week of pregnancy [95], many attempts with progesterone supplementation were performed to support pregnancy. Intriguingly, some studies showed improved embryonic rates [95], whereas others indicated its negative influence on fertilization [96] and embryo survival rates [97]. A single injection of hCG on day 12 of the estrous cycle prolonged the lifespan of CL; consequently, extended progesterone production and delayed luteolysis in the pig were observed [98, 99]. Except increased progesterone concentration on days 15–17 of the estrous cycle, elevated amounts of estradiol-17β in the blood plasma were indicated on days 14 and 15 of the estrous cycle. Because estradiol-17β downregulates endometrial PTGFS and CBR1 protein concentration [76], both estradiol-17β alone and the increased ratio of PGE2:PGFM could be responsible for prolonged luteal function in hCG-treated cyclic gilts.
A sufficient supply of progesterone and continuous maintenance of CL are necessary for the establishment of pregnancy in the pig [20]. A minimum of 4 ng/ml progesterone in the blood plasma has been found to be crucial to maintain pregnancy in pigs [100]. Moreover, the concentration of progesterone is positively correlated with embryonic survival during the first month of gestation [95]. However, a single administration of hCG did not affect progesterone content in the systemic circulation of pregnant gilts [98, 101]. Similarly, injection of hCG during the first 8 days of pregnancy has not affected the concentration of progesterone in the blood plasma [102]; this may be caused by an increased metabolism of progesterone and its active transport into the uterus. In contrast, administration of hCG on day 12 of pregnancy led to elevated amounts of progesterone caused by an increased number of additional CL in ruminants [103, 104]. In pregnant gilts, injections of 500 or 1000 IU hCG did not affect the number of CL [101], but elevated amounts of estradiol-17β on days 14 and 15 of pregnancy were observed [98, 101]. A similar effect was revealed in pregnant sheep [103].
On the other hand, a single intramuscular injection of 750 IU hCG increased embryonic viability on day 30 of pregnancy in the pig [98, 101]. Moreover, in the luteal tissue of pregnant gilts given 750 IU hCG, augmented expression of STAR and LH/hCG receptors was found, with simultaneously increased angiogenesis, a reduced percentage of CL cells in the stage of early and late apoptosis, and elevated percentage of viable cells [98].
The majority of studies performed so far on the effect of various hormones on embryo survival in pigs were concentrated exclusively on the period up to day 30 of pregnancy [98, 101, 105–107]; thus, it was still not clear whether this effect can be maintained until the end of pregnancy. Recently, studies have clearly showed that hCG does not have a negative effect on the pregnancy rate, but administration of hCG on day 12 or 20 of pregnancy results in an elevated litter size and significant increase in the number of total piglets born, respectively [108]. Additionally, the number of piglets weaned tended to be increased in sows treated with hCG on day 20 of pregnancy. This study revealed that hCG administration during early pregnancy does not have a negative effect on pregnancy performance in gilts and sows and can be even beneficial for pregnancy outcome.
12.6 Other Attempts to Prolong CL Function in Pigs
Early studies showed that the luteal function in pigs is continued until day 60 of pregnancy after removal of all fetuses on day 30 of gestation [107], which may indicate that intrauterine stimulus is not needed to maintain the porcine CL between days 30 and 60 of gestation. Similar observations were made in pigs during pseudo-pregnancy caused by injections of pharmacological doses of estrogens between days 11 and 15 of the estrous cycle [109, 110]. Estrogen treatment on days 12–15 of the estrous cycle sufficiently suppresses the luteolytic effects of the uterus and allows continuation of the luteal function for a period similar to that observed after hysterectomy [111]. However, we have to bear in mind that estrogen-induced pseudo-pregnancy does not fully mimic the endocrine events associated with early pregnancy [112].
The possibility of using a vaginal route delivery of biologically active factors applying low doses of PGE2 and estradiol-17β to affect luteal function in cyclic gilts was also studied. Prolonged luteal function and extended synthesis of progesterone were observed in two of five gilts simultaneously receiving PGE2 and estradiol-17β on days 11–16 of the estrous cycle [113]. Intravaginal application of PGE2 and 17β-estradiol in pregnant primiparous sows revealed their possible supporting effects on luteal function when administered in the second crucial period adjusted to a natural increase of embryonic estrogens in the blood, that is, on days 16–25 of gestation [28], the time when the establishment of pregnancy occurred and embryo implantation ends in pigs. Although there were no significant differences in the number of total piglets born, a clear tendency to increased numbers of live-born and weaned piglets was noticed [108]. Summarizing, the intravaginal application of estradiol-17β and PGE2 on days 17–23 of pregnancy seems to be a promising approach to improve embryo survival, but a practical treatment protocol should be elaborated in the future.
12.7 Concluding Remarks
The pro-luteal environment in the reproductive tract in advance of maternal recognition of pregnancy caused by gametes, embryos, and seminal plasma is beneficial but not sufficient for prolonged CL lifespan. In pigs, pregnancy recognition is the result of conceptus secretion of estrogens on day 11 and 12, which affects PG synthesis and transport in favor of luteoprotective PGE2.
The development of advanced ‘omics’ tools in the past 25 years has revolutionized research methods also in the reproductive biology of pigs. The former basic theories of the maternal recognition of pregnancy in pigs are valuable but seem to be insufficient to fully understand the process of CL rescue, dependent on cooperation of many pleiotropic factors at systemic, local, and intracellular molecular levels.
Further studies are needed to explain how conceptuses and endometrial factors regulate differential PGs synthesis, the way of their release on days 11–14 of estrous cycle and pregnancy, or differential response of CL to PGF2α and PGE2 in those periods. Perhaps the first time presentation of the ‘two signal-switch’ hypothesis of PGs involvement in CL rescue will be a small contribution to understanding the complexity of CL control and function and give impetus to further large-scale investigation. One of the most important challenges in understanding the mechanism of CL function in the pig and other species is to establish a hierarchy and timing of molecular relationships between numerous mediators of luteal regression (cytokines, chemokines, endothelin-1) and rescue (embryo signals, angiogenic factors, gonadotropins), as well as post-PG receptor intracellular pathway elements.
References
Alminana C, Heath PR, Wilikson S, Sanchez-Osorio J, Cuello C, Parrilla I, Gil MA, Vazguez JL, Vazguez JM, Roca J, Martinez EA, Fazeli A. Early developing pig embryos mediate their own environment in the maternal tract. PLoS One. 2012;7, e33625.
Bischof RJ, Brandon MR, Lee CS. Cellular immune responses in the pig uterus during pregnancy. J Reprod Immunol. 1995;29:161–78.
Robertson SA, Mau VJ, Tremellen KP, Seamark RF. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil. 1996;107:265–77.
Claus R. Physiological role of seminal components in the reproductive tract of the female pig. J Reprod Fertil Suppl. 1990;40:117–31.
Lovell JW, Getty R. Fate of semen in the uterus of the sow: histologic study of endometrium during the 27 hours after natural service. Am J Vet Res. 1968;29:609–25.
Rozeboom K, Troedsson MH, Crabo BG. Characterization of uterine leukocyte infiltration in gilts after artificial insemination. J Reprod Fertil. 1998;14:195–9.
Bischof RJ, Lee CS, Brandon MR, Meeusen E. Inflammatory response in the pig uterus induced by seminal plasma. J Reprod Immunol. 1994;26:131–46.
Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci. 2007;85E(suppl):E36–44.
Taylor U, Schuberth HJ, Rath D, Michelmann HW, Sauter-Louis C, Zerbe H. Influence of inseminate components on porcine leucocyte migration in vitro and in vivo after pre and post-ovulatory insemination. Reprod Domestic Anim. 2009;44:180–8.
O’Leary S, Jasper MJ, Warnes GM, Armstrong DT, Robertson SA. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction. 2004;128:237–47.
Kaczmarek MM, Krawczynski K, Blitek A, Kiewisz J, Schams D, Ziecik AJ. Seminal plasma affects prostaglandin synthesis in the porcine oviduct. Theriogenology. 2010;74:1207–20.
Kaczmarek MM, Krawczynski K, Filant J. Seminal plasma affects prostaglandin synthesis and angiogenesis in the porcine uterus. Biol Reprod. 2013;88:72.
Krawczynski K, Kaczmarek MM. Does seminal plasma affect angiogenesis in the porcine oviduct? Reprod Biol. 2012;12:347–54.
O’Leary S, Jasper MJ, Robertson SA, Armstrong DT. Seminal plasma regulates ovarian progesterone production, leukocyte recruitment and follicular cell responses in the pig. Reproduction. 2006;132:147–58.
Hunter RH, Poyser NL. Uterine secretion of prostaglandin F2a in anaesthetized pigs during the oestrous cycle and early pregnancy. Reprod Nutr Dev. 1982;22:1013–23.
Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest. 2013;123:3472–87.
Waberski D, Dohring A, Ardon F, Ritter N, Zerbe H, Schuberth H-J, Hewicker-Trautwein M, Weitze KF, Hunter RHF. Physiological routes from intra-uterine seminal contents to advancement of ovulation. Acta Vet Scand. 2006;48:13.
Krzymowski T, Stefańczyk-Krzymowska S. The oestrous cycle and early pregnancy-a new concept of local endocrine regulation. Vet J. 2004;168:285–96.
Stefańczyk-Krzymowska S, Krzymowski T. Local adjustment of blood and lymph circulation in the hormonal regulation of reproduction in female pigs: facts, conclusions and suggestions for future research. Reprod Biol. 2002;2:115–32.
Ziecik AJ, Wacławik A, Kaczmarek MM, Blitek A, Moza Jalali B, Andronowska A. Mechanisms for the establishment of pregnancy in the pig. Reprod Domest Anim. 2011;46(S3):31–41.
du Du Mesnil Buisson F, Leglise PC. Effet de l’hypophysectomie sue les corps jaunes de la truie. Resultatas preliminaires. C R Hebd Seanc Acad Sci Paris. 1963;257:261–3.
Tilton JE, Foxcroft GR, Ziecik AJ, Coombs SL, Williams GL. Time of the preovulatory LH surge in the gilts and sow relative to the onset of behavioral estrus. Theriogeneology. 1982;18:227–36.
Bazer FW, Geisert RD, Thatcher WW, Roberts RM. The establishment and maintenance of pregnancy. In: Cole DJA, Foxcroft GR, editors. Control of pig reproduction. London: Butterworth; 1982. p. 227–53.
Szafranska B, Ziecik A. Active and passive immunization against luteinizing hormone in pigs. Acta Physiol Hung. 1989;74:253–8.
Przygrodzka E, Lopinska M, Ziecik AJ. Precision-cut luteal slices: a promising approach for studying luteal function in pigs. Reprod Biol. 2014;14:243–7.
Conley AJ, Ford SP. Direct luteotrophic effect of oestradiol-17β on pig corpora lutea. J Reprod Fertil. 1989;87:125–31.
Ford SP, Christenson LK. Direct effects of oestradiol-17β and prostaglandin E2 in protecting pig corpora lutea from a luteolytic dose of prostaglandin F2α. J Reprod Fertil. 1991;93:203–9.
Geisert RD, Zavy MT, Moffatt RJ, Blair RM, Yellin T. Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil. 1990;40:293–305.
Gadsby J, Rose L, Sriperumbudur R, Ge Z. The role of intra-luteal factors in the control of the porcine corpus luteum. In: Ashworth CJ, Kraeling RR, editors. Control of pig reproduction, vol VII, Reproduction. Supplement 62. UK: Nottingham University Press; 2006. p. 69–83.
Christenson LK, Farley DB, Anderson LH, Ford SP. Luteal maintenance during early pregnancy in the pig: role for prostaglandin E2. Prostaglandins. 1994;47:61–75.
Wuttke W, Spiess S, Knoke I, Pitzel L, Leonhardt S, Jarry H. Synergistic effects of prostaglandin F2alpha and tumor necrosis factor to induce luteolysis in the pig. Biol Reprod. 1998;58:1310–5.
Moeljono MP, Thatcher WW, Bazer FW, Frank M, Owens LJ, Wilcom CJ. A study of prostaglandin F2alpha as the luteolysin in swine: II. Characterization and comparison of prostaglandin F, estrogens and progestin concentrations in utero-ovarian vein plasma of nonpregnant and pregnant gilts. Prostaglandins. 1977;14:543–55.
Ziecik AJ, Kotwica G. Involvement of gonadotropins in induction of luteolysis in pigs. Reprod Biol. 2001;2001(1):33–50.
Carnahan KG, Prince BC, Mirando MA. Exogenous oxytocin stimulates uterine secretion of prostaglandin F2 alpha in cyclic and early pregnant swine. Biol Reprod. 1996;55:838–43.
Ludwig TE, Sun BC, Carnahan KG, Uzumcu M, Yelich JV, Geisert RD, Mirando MA. Endometrial responsiveness to oxytocin during diestrus and early pregnancy in pigs is not controlled solely by changes in oxytocin receptor population density. Biol Reprod. 1998;58:769–77.
Waclawik A, Blitek A, Ziecik AJ. Oxytocin and tumor necrosis factor α stimulate expression of prostaglandin E2 synthase and secretion of prostaglandin E2 by luminal epithelial cells of the porcine endometrium during early pregnancy. Reproduction. 2010;140:613–22.
Blitek A, Ziecik AJ. Role of tumour necrosis factor alpha in stimulation of prostaglandins F(2alpha) and E(2) release by cultured porcine endometrial cells. Reprod Domestic Anim. 2006;41:562–7.
Blitek A, Mendrzycka AU, Bieganska MK, Waclawik A, Ziecik AJ. Effect of steroids on basal and LH-stimulated prostaglandins F(2alpha) and E(2) release and cyclooxygenase-2 expression in cultured porcine endometrial stromal cells. Reprod Biol. 2007;7:73–88.
Whiteaker SS, Mirando MA, Becker WC, Hostetler CE. Detection of functional oxytocin receptors on endometrium of pigs. Biol Reprod. 1994;51:92–8.
Whiteaker SS, Mirando MA, Becker WC, Peters DN. Relationship between phosphoinositide hydrolysis and prostaglandin F2 alpha secretion in vitro from endometrium of cyclic pigs on day 15 postestrus. Domestic Anim Endocrinol. 1995;12:95–104.
Kotwica G, Franczak A, Okrasa S, Kotwica J. Effect of an oxytocin antagonist on prostaglandin F2 alpha secretion and the course of luteolysis in sows. Acta Vet Hung. 1999;47:249–62.
Gadsby JE, Balapure AK, Britt JH, Fitz TA. Prostaglandin F2 alpha receptors on enzyme-dissociated pig luteal cells throughout the estrous cycle. Endocrinology. 1990;126:787–95.
Gadsby JE, Lovdal JA, Britt JH, Fitz TA. Prostaglandin F2 alpha receptor concentrations in corpora lutea of cycling, pregnant, and pseudopregnant pigs. Biol Reprod. 1993;49:604–8.
Diaz FJ, Crenshaw TD, Wiltbank MC. Prostaglandin F(2alpha) induces distinct physiological responses in porcine corpora lutea after acquisition of luteolytic capacity. Biol Reprod. 2000;63:1504–12.
Diaz FJ, Wiltbank MC. Acquisition of luteolytic capacity: changes in prostaglandin F2alpha regulation of steroid hormone receptors and estradiol biosynthesis in pig corpora lutea. Biol Reprod. 2004;70:1333–9.
Diaz FJ, Wiltbank MC. Acquisition of luteolytic capacity involves differential regulation by prostaglandin F2alpha of genes involved in progesterone biosynthesis in the porcine corpus luteum. Domestic Anim Endocrinol. 2005;28:172–89.
Diaz FJ, Luo W, Wiltbank MC. Effect of decreasing intraluteal progesterone on sensitivity of the early porcine corpus luteum to the luteolytic actions of prostaglandin F2alpha. Biol Reprod. 2011;841:26–33.
Diaz FJ, Luo W, Wiltbank MC. Prostaglandin F2a regulation of mRNA for activating protein 1 transcriptional factors in porcine corpora lutea (CL): lack of induction of JUN and JUND in CL without luteolytic capacity. Domestic Anim Endocrinol. 2013;44:98–108.
Zorrilla LM, Irvin MS, Gadsby JE. Protein kinase C isoforms in the porcine corpus luteum: temporal and spatial expression patterns. Domestic Anim Endocrinol. 2009;36:173–85.
Zorrilla LM, Sriperumbudur R, Gadsby JE. Endothelin-1, endothelin converting enzyme-1 and endothelin receptors in the porcine corpus luteum. Domestic Anim Endocrinol. 2010;38:75–85.
Luo W, Diaz FJ, Wiltbank MC. Induction of mRNA for chemokines and chemokine receptors by prostaglandin F2a is dependent upon stage of the porcine corpus luteum and intraluteal progesterone. Endocrinology. 2011;152:2797–805.
Przygrodzka E, Witek KJ, Kaczmarek MM, Andronowska A, Ziecik AJ. Expression of factors associated with apoptosis in the porcine 1 corpus luteum throughout the luteal phase of the estrous cycle and early pregnancy: their possible involvement in acquisition of luteolytic sensitivity. Theriogenology. 2015;83:535–45.
Przygrodzka E, Kaczmarek MM, Kaczyński P, Zięcik AJ. Steroid hormones, prostanoids and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction. 2016;151:135–47.
Zorrilla LM, D’Annibale MA, Swing SE, Gadsby JE. Expression of genes associated with apoptosis in the porcine corpus luteum during the oestrous cycle. Reprod Domestic Anim. 2013;48:755–61.
Hehnke KE, Christenson LK, Ford SP, Taylor M. Macrophage infiltration into to porcine corpus luteum during prostaglandin F2α-induced luteolysis. Biol Reprod. 1994;50:10–5.
Zhao Y, Burbach JA, Roby KF, Terranova PF, Brannian JD. Macrophages are the major source of tumor necrosis factor alpha in the porcine corpus luteum. Biol Reprod. 1998;59:1385–91.
Perry JS, Heap RB, Amoroso EC. Steroid hormone production by pig blastocysts. Nature (Lond). 1973;245:45–7.
Garverick HA, Polge C, Flint AP. Oestradiol administration raises luteal LH receptor levels in intact and hysterectomized pigs. J Reprod Fertil. 1982;66:371–7.
Bazer FW, Thatcher WW. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2alpha by the uterine endometrium. Prostaglandins. 1977;14:397–400.
Waclawik A, Jabbour HN, Blitek A, Ziecik AJ. Estradiol-17-beta, prostaglandin E2 (PGE2) and the prostaglandin E2 receptor are involved in PGE2 positive feedback loop in the porcine endometrium. Endocrinology. 2009;150:3823–32.
Waclawik A, Ziecik AJ. Differential expression of prostaglandin synthesis enzymes in conceptus during periimplantation period and endometrial expression of carbonyl reductase/prostaglandin 9-ketoreductase in the pig. J Endocrinol. 2007;194:499–510.
Geisert RD, Brenner RM, Moffatt J, Harney JP, Yellin T, Bazer FW. Changes in oestrogen receptor protein, mRNA expression and localization in the endometrium of cyclic and pregnant gilts. Reprod Fertil Dev. 1993;5:247–60.
Kautz E, Gram A, Aslan S, Ay SS, Selçuk M, Kanca H, Koldaş E, Akal E, Karakaş K, Findik M, Boos A, Kowalewski MP. Expression of genes involved in the embryo-maternal interaction in the early-pregnant canine uterus. Reproduction. 2014;8:703–17.
Heap RB, Flint APF, Hartman PE, Gadsby JE, Staples LD, Ackalnd N, Hamon N. Oestrogen production in early pregnancy. J Endocrinol Suppl. 1981;89:77P–94.
Frank M, Bazer FW, Thatcher WW, Wilcox CJ. A study of prostaglandin F2alpha as the luteolysin in swine: III effects of estradiol valerate on prostaglandin F, progestins, estrone and estradiol concentrations in the utero-ovarian vein of nonpregnant gilts. Prostaglandins. 1977;14:1183–96.
Krzymowski T, Czarnocki J, Koziorowski M, Stefańczyk-Krzymowska S. Counter current transfer of 3H-PGF2α in the mesometrium: a possible mechanism for prevention of luteal regression. Anim Reprod Sci. 1986;11:259–72.
Waclawik A, Rivero-Muller A, Blitek A, Kaczmarek MM, Brokken LJ, Watanabe K, Rahman NA, Ziecik AJ. Molecular cloning and spatio-temporal expression of prostaglandin F synthase and microsomal prostaglandin E synthase-1 in porcine endometrium. Endocrinology. 2006;147:210–21.
Franczak A, Kotwica G, Kurowicka B, Oponowicz A, Wocławek-Potocka I, Petroff BK. Expression of enzymes of cyclooxygenase pathway and secretion of prostaglandin E2 and F2α by porcine myometrium during luteolysis and early pregnancy. Theriogenology. 2006;66:1049–56.
Davis DL, Blair RM. Studies of uterine secretions and products of primary cultures of endometrial cell in pigs. J Reprod Fertil Suppl. 1993;48:143–55.
Akinlosotu BA, Diehl JR, Gimenez T. Sparing effects of intrauterine treatment with prostaglandin E2 on luteal function in cycling gilts. Prostaglandins. 1986;32:291–9.
Schneider TM, Tilton JE, Okrasa S, Mah J, Weigl RM, Williams GL. The effect of intrauterine infusions of prostaglandin E2 on luteal function in nonpregnant gilts. Theriogenology. 1983;20:509–20.
Okrasa S, Tilton JE, Weigl RM. Utero-ovarian venous concentrations of prostaglandin E2 (PGE2) and prostaglandin F2a (PGF2a) following PGE2 intrauterine infusions. Prostaglandins. 1985;30:851–6.
Stefanczyk-Krzymowska S, Wasowska B, Chłopek J, Gilun P, Grzegorzewski W, Radomski M. Retrograde and local destination transfer of uterine prostaglandin E2 in early pregnant sow and its physiological consequences. Prostaglandins Other Lipid Mediat. 2006;81:71–9.
Engmann L, Losel R, Wehling M, Peluso JJ. Progesterone regulation of human granulose/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab. 2006;91:4962–8.
Geisert RD, Yelich JV. Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl. 1997;52:133–49.
Waclawik A, Kaczmarek MM, Kowalczyk AE, Bogacki M, Ziecik AJ. Expression of prostaglandin synthesis pathway enzymes in the porcine corpus luteum during the oestrous cycle and early pregnancy. Theriogenology. 2008;70:145–52.
Spencer TE, Forde N, Dorniak P, Hansen TR, Romero JJ, Lonergan P. Conceptus-derived prostaglandins regulate gene expression in the endometrium prior to pregnancy recognition in ruminants. Reproduction. 2013;146:377–87.
Seo H, Choi Y, Shim J, Yoo I, Ka H. Prostaglandin transporters ABCC4 and SLCO2A1 in the uterine endometrium and conceptus during pregnancy in pigs. Biol Reprod. 2014;90:1–10.
Wasielak M, Kaminska K, Bogacki M. Effect of the conceptus on uterine prostaglandin-F2α and prostaglandin-E2 release and synthesis during the periimplantation period in the pig. Reprod Fertil Dev. 2009;21:1–9.
Wiepz GL, Wiltbank MC, Nett TM, Niswender GD, Sawyer HR. Receptors for prostaglandin F2 alpha and E2 in ovine corpora lutea during maternal recognition of pregnancy. Biol Reprod. 1992;47:984–91.
Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci. 2002;7:1949–78.
Zannoni A, Bernardini C, Rada T, Ribeiro LA, Forni M, Bacci ML. Prostaglandin F2-alpha receptor (FPr) expression on porcine corpus luteum microvascular endothelial cells (pCL-MVECs). Reprod Biol Endocrinol. 2007;5:31.
Shirasuna K, Akabane Y, Beindorff N, Nagai K, Sasaki M, Shimizu T, Bollwein H, Meidan R, Miyamoto A. Expression of prostaglandin F2α (PGF2α) receptor and its isoforms in the bovine corpus luteum during the estrous cycle and PGF2α-induced luteolysis. Domestic Anim Endocrinol. 2012;43:227–38.
Zalman Y, Klipper E, Farberov S, Mondal M, Wee G, Folger JK, Smith GW, Meidan R. Regulation of angiogenesis-related prostaglandin F2alpha-induced genes in the bovine corpus luteum. Biol Reprod. 2012;86:1–10.
Waclawik A. Novel insights into the mechanisms of pregnancy establishment: regulation of prostaglandin synthesis and signaling in the pig. Reproduction. 2011;142:389–99.
Lee JH, McCracken JA, Stanley JA, Nithy TK, Banu SK, Arosh JA. Intraluteal prostaglandin biosynthesis and signaling are selectively directed towards PGF2α during luteolysis but towards PGE2 during the establishment of pregnancy in sheep. Biol Reprod. 2012;87:1–14.
Mamluk R, Defer N, Hanoune J, Meidan R. Molecular identification of adenyl cyclase 3 in bovine corpus luteum and its regulation by prostaglandin F2α-induced signaling pathways. Endocrinology. 1999;140:4601–8.
Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–205.
Hsi LC, Eling TE. Inhibition of EGF-dependent mitogenesis by prostaglandin E2 in Syrian hamster embryo fibroblasts. Prostag Leukotr Essent Fatty Acids. 1998;58:271–81.
Kowalczyk AE, Kaczmarek MM, Schams D, Ziecik AJ. Effect of prostaglandin E(2) and tumor necrosis factor alpha on the VEGF-receptor system expression in cultured porcine luteal cells. Mol Reprod Dev. 2008;75:1558–66.
Kaczmarek MM, Kiewisz J, Schams D, Ziecik AJ. Expression of VEGF-receptor system in conceptus during peri-implantation period and endometrial and luteal expression of soluble VEGFR-1 in the pig. Theriogenology. 2009;71:1298–306.
Taniguchi H, Komiyama J, Viger RS, Okuda K. The expression of the nuclear receptors NR5A1 and NR5A2 and transcription factor GATA6 correlates with steroidogenic gene expression in the bovine corpus luteum. Mol Reprod Dev. 2009;76:873–80.
Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5:143–9.
Lambert E, Williams DH, Lynch PB, Hanrahan TJ, McGeady TA, Austin FH, Boland MP, Roche JF. The extent and timing of prenatal loss in gilts. Theriogenology. 1991;36:655–65.
Jindal R, Cosgrove JR, Foxcroft GR. Progesterone mediates nutritionally induced effects on embryonic survival in gilts. J Anim Sci. 1997;75:1063–70.
Day BN, Pologe C. Effects of progesterone on fertilization and egg transport in the pig. J Reprod Fertil. 1968;17:227–30.
Mao J, Foxcroft GR. Progesterone therapy during early pregnancy and embryonal survival in primiparous weaned sows. J Anim Sci. 1998;76:1922–8.
Bolzan E, Andronowska A, Bodek G, Morawska-Pucinska E, Krawczynski K, Dabrowski A, Ziecik AJ. The novel effect of hCG administration on luteal function maintenance during the estrous cycle/pregnancy and early embryo development in the pig. Pol J Vet Sci. 2013;116:323–32.
Guthrie HD, Bolt DJ. Changes in plasma estrogen, luteinizing hormone, follicle stimulating hormone and 13,14-dihydro-15-ketoprostaglandin F2α during blockade of luteolysis in pigs after human chorionic gonadotropin treatment. J Anim Sci. 1983;52:993–1000.
Ellicott AR, Dziuk PJ. Minimum daily dose of progesterone and plasma concentration for maintenance of pregnancy in ovariectomized gilts. Biol Reprod. 1973;9:300–4.
Tilton JE, Schmidt AE, Weigl RM, Ziecik AJ. Ovarian steroid secretion changes after hCG stimulation in early pregnant pigs. Theriogenology. 1989;32:623–31.
Stone BA, Heap PA, Seamark RF. Changes in peripheral progestagen levels in early pregnant gilts following injection of human chorionic gonadotrophin. J Endocrinol. 1987;115:161–7.
Khan TH, Beck NF, Khalid M. The effects of GnRH analogue (buserelin) or hCG (Chorulon) on day 12 of pregnancy on ovarian function, plasma hormone concentrations, conceptus growth and placentation in ewes and ewe lambs. Anim Reprod Sci. 2007;102:247–57.
Rajamahendran R, Sianangama PC. Effect of human chorionic gonadotrophin on dominant follicles in cows: formation of accessory corpora lutea, progesterone production and pregnancy rates. J Reprod Fertil. 1992;95:577–84.
Chłopek J, Gilun P, Tabęcka Łonczyńska A, Koziorowski M, Stefańczyk-Krzymowska S. The effect of intravaginal application of estradiol and progesterone on porcine embryo development. Pol J Vet Sci. 2008;11(4):287–93.
Pope WF, Lawyer MS, Butler WR, Foote RH, First NL. Dose-response shift in the ability of gilts to remain pregnant following exogenous estradiol-17beta exposure. J Anim Sci. 1986;63:1208–10.
Webel SK, Reimers TJ, Dziuk PJ. The lack of relationship between plasma progesterone levels and number of embryos and their survival in the pig. Biol Reprod. 1975;13:177–86.
Ziecik AJ, Lopinska M, Przygrodzka E, Wasielak M, Kempa W. Effect of hCG and intravaginal application of estradiol and prostaglandin E2 on pregnancy rate and litter size in gilts and sows. Anim Sci Pap Rep. 2014;32:5–13.
Geisert RD, Zavy MT, Wettemenn RP, Biggers BG. Length of pseudopregnancy and pattern of uterine protein release and influenced by time and duration of oestrogen administration in the pig. J Reprod Fertil. 1987;79:163–72.
Kidder HE, Casida LE, Grummer RH. Some effects of estrogen injections on estrual cycle of gilts. J Anim Sci. 1995;14:470–4.
Pusateri AE, Wilson ME, Diekman MA. Maternal recognition of pregnancy in swine. II. Plasma concentrations of progesterone and 13,14-dihydro-15-keto-prostaglandin F2 alpha during the estrous cycle and during short and long pseudopregnancy in gilts. Biol Reprod. 1996;55(3):590–7.
Ziecik A, Doboszynska T, Dusza L. Concentrations of LH, prolactin and progesterone in early-pregnant and oestradiol treated pigs. Anim Reprod Sci. 1986;10:215–24.
Przygrodzka E, Andronowska A, Janowski T, Zięcik AJ. The effect of vaginal administration of prostaglandin (PG) E2 and/or 17β-estradiol (E2) 1 on luteal function and histological characteristics of the cervix in cyclic pigs. Pol J Vet Sci. 2014;17:123–30.
Acknowledgments
The recent authors’ studies (2014–2015) described in this chapter were supported by the State Committee for Scientific Research (grant no. 2011/01/B/NZ4/04970) and the National Centre for Research and Development (NR 12-0039-10) in Poland. We thank J. Murawska-Kempa for her cheerful assistance in typing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ziecik, A.J., Przygrodzka, E., Kaczmarek, M.M. (2017). Corpus Luteum Regression and Early Pregnancy Maintenance in Pigs. In: Meidan, R. (eds) The Life Cycle of the Corpus Luteum. Springer, Cham. https://doi.org/10.1007/978-3-319-43238-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-43238-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43236-6
Online ISBN: 978-3-319-43238-0
eBook Packages: MedicineMedicine (R0)