Abstract
The mechanisms for pattern completion and pattern separation are described in the context of a theory of hippocampal function in which the hippocampal CA3 system operates as a single attractor or autoassociation network to enable rapid, one-trial associations between any spatial location (place in rodents, or spatial view in primates) and an object or reward, and to provide for completion of the whole memory during recall from any part. The factors important in the pattern completion in CA3 together with a large number of independent memories stored in CA3 include a sparse distributed representation which is enhanced by the graded firing rates of CA3 neurons, representations that are independent due to the randomizing effect of the mossy fibers, heterosynaptic long-term depression as well as long-term potentiation in the recurrent collateral synapses, and diluted connectivity to minimize the number of multiple synapses between any pair of CA3 neurons which otherwise distort the basins of attraction. Recall of information from CA3 is implemented by the entorhinal cortex perforant path synapses to CA3 cells, which in acting as a pattern associator allow some pattern generalization. Pattern separation is performed in the dentate granule cells using competitive learning to convert grid-like entorhinal cortex firing to place-like fields. Pattern separation in CA3, which is important for completion of any one of the stored patterns from a fragment, is provided for by the randomizing effect of the mossy fiber synapses to which neurogenesis may contribute, by the large number of dentate granule cells each with a sparse representation, and by the sparse independent representations in CA3. Recall to the neocortex is achieved by a reverse hierarchical series of pattern association networks implemented by the hippocampo-cortical backprojections, each one of which performs some pattern generalization to retrieve a complete pattern of cortical firing in higher-order cortical areas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hippocampus
- Attractor network
- Competitive network
- Pattern association network
- Episodic memory
- Recall
- Pattern separation
- Pattern completion
- Pattern generalization
Introduction
There is great interest in how pattern separation and pattern completion in the hippocampus contribute to its functions in memory and spatial function (Giocomo et al. 2011; Jezek et al. 2011; Leutgeb et al. 2007; McHugh et al. 2007; Nakashiba et al. 2012; Nakazawa et al. 2002, 2003; Wills et al. 2005), and among those who have made many contributions in this area are Ray Kesner and his colleagues (Hunsaker and Kesner 2008, 2013; Kesner 2007, 2013; Kesner et al. 2012; Rolls and Kesner 2006).
This chapter describes some of the different types of pattern separation and pattern completion in the hippocampal system, and the mechanisms that implement them. More comprehensive descriptions of my theory of hippocampal function, and of differences between the primate and rodent hippocampal neuronal representations and the implications for understanding human memory are provided elsewhere (Rolls 2008, 2010b, 2013; Rolls and Kesner 2006; Rolls and Xiang 2006; Kesner and Rolls 2015). The theory has been developed through many stages (Rolls 1987, 1989a, b, c, 1990a, b, 1991, 1995, 1996b, 2008, 2010b; Rolls and Deco 2010; Rolls and Kesner 2006; Rolls and Treves 1998; Treves and Rolls 1991, 1992, 1994), has as a predecessor developments made by David Marr (Marr 1971) (though he never identified the CA3 system as an autoassociation network), and has benefitted greatly from collaborations with many whose names appear below in the citations, including Alessandro Treves and Simon Stringer. The operation of pattern association networks, autoassociation networks, and competitive networks has been described elsewhere (Hertz et al. 1991; Rolls 2008; Rolls and Treves 1998; Rolls 2016).
Background to the Approach to Hippocampal Function
Event and Episodic Memory
The focus is on a fundamental property of episodic memory, the ability to store and retrieve the memory of a particular single event involving an association between items such as the place and the object or reward seen at that place. Episodic memory, in the sense of a series of linked events, requires this type of event memory, and could be implemented by linking together a series of events.
An event consists of a set of items that occur together, such as seeing a particular object or person’s face in a particular place. An everyday example might be remembering where one was for dinner, who was present, what was eaten, what was discussed, and the time at which it occurred. The spatial context is almost always an important part of an episodic memory (Dere et al. 2008), and it may be partly for this reason that episodic memory is linked to the functions of the hippocampal system which is involved in spatial processing and memory. The ability to recall a whole memory from a partial cue is an important property of episodic memory and is referred to as completion.
Systems-Level Functions and Connections of the Primate Hippocampus
Any theory of the hippocampus must state at the systems level what is computed by the hippocampus. Some of the relevant evidence about the functions of the hippocampus in memory comes from the effects of damage to the hippocampus, the responses of neurons in the hippocampus during behavior, and the systems-level connections of the hippocampus, as described in more detail elsewhere (Rolls 2008, 2010b; Kesner and Rolls 2015; Rolls and Xiang 2006). Many of the memory functions are important in event or episodic memory, in which the ability to remember what happened where on typically a single occasion (or trial in a learning experiment) is important. It is suggested that an autoassociation memory implemented by the CA3 neurons enables event or episodic memories to be formed by enabling associations to be formed between spatial and other including object or reward representations, and for completion to then occur in recall from any part. This is different from pattern association memory in which a visual stimulus might become associated with a taste by associative synaptic modification. Later presentation of the visual stimulus would retrieve the taste representation. However, presentation of the taste would not retrieve the visual representation, and this is an important and fundamental difference between autoassociation and pattern association, as described in detail elsewhere (Rolls 2008, 2014, 2016; Rolls and Treves 1998).
Information stored in the hippocampus will need to be retrieved and affect other parts of the brain in order to be used. The information about episodic events recalled from the hippocampus could be used to help form semantic memories (Rolls 1989b, c, 1990a; Treves and Rolls 1994). For example, remembering many particular journeys could help to build a geographic cognitive map in the neocortex. The hippocampus and neocortex would thus be complementary memory systems, with the hippocampus being used for rapid, “on the fly,” unstructured storage of information involving activity potentially arriving from many areas of the neocortex; while the neocortex would gradually build and adjust on the basis of much accumulating information, often recalled from the hippocampal unstructured store, the semantic representation (McClelland et al. 1995; Moscovitch et al. 2005; Rolls 1989b; Treves and Rolls 1994). The theory shows how information could be retrieved within the hippocampus, and how this retrieved information could enable the activity in neocortical areas that was present during the original storage of the episodic event to be reinstated, thus implementing recall, by using hippocampo-neocortical backprojections is described elsewhere (Rolls 1995, 1996b, 2008, 2010b; Treves and Rolls 1994; see Fig. 4.1).
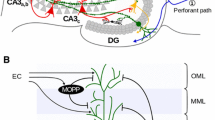
Forward connections (solid lines) from areas of cerebral association neocortex via the parahippocampal gyrus and perirhinal cortex, and entorhinal cortex, to the hippocampus; and backprojections (dashed lines) via the hippocampal CA1 pyramidal cells, subiculum, and parahippocampal gyrus to the neocortex. There is great convergence in the forward connections down to the single network implemented in the CA3 pyramidal cells; and great divergence again in the backprojections. Left: block diagram. Right: more detailed representation of some of the principal excitatory neurons in the pathways. D Deep pyramidal cells, DG Dentate granule cells, F Forward inputs to areas of the association cortex from preceding cortical areas in the hierarchy, mf mossy fibers, PHG parahippocampal gyrus and perirhinal cortex, pp perforant path, rc recurrent collateral of the CA3 hippocampal pyramidal cells, S Superficial pyramidal cells, 2 pyramidal cells in layer 2 of the entorhinal cortex, 3 pyramidal cells in layer 3 of the entorhinal cortex. The thick lines above the cell bodies represent the dendrites
To understand the functions of the primate hippocampus in event or episodic memory, it is necessary to understand from which other parts of the brain it receives information. Does it, for example, receive object as well as spatial information in terms of its connectivity? The primate hippocampus receives inputs via the entorhinal cortex (area 28) and the highly developed parahippocampal gyrus (areas TF and TH) as well as the perirhinal cortex from the ends of many processing streams of the cerebral association cortex, including the visual and auditory temporal lobe association cortical areas, the prefrontal cortex, and the parietal cortex (Amaral 1987; Amaral et al. 1992; Lavenex et al. 2004; Rolls 2008; Rolls and Kesner 2006; Suzuki and Amaral 1994b; Van Hoesen 1982; Witter et al. 2000b; see Fig. 4.1). The hippocampus is thus by its connections potentially able to associate together object and spatial representations. In addition, the entorhinal cortex receives inputs from the amygdala and the orbitofrontal cortex, which could provide reward-related information to the hippocampus (Carmichael and Price 1995; Pitkanen et al. 2002; Stefanacci et al. 1996; Suzuki and Amaral 1994a).
The primary output from the hippocampus to neocortex originates in CA1 and projects to subiculum, entorhinal cortex, and parahippocampal structures (areas TF-TH) as well as prefrontal cortex (Delatour and Witter 2002; van Haeften et al. 2003; Van Hoesen 1982; Witter 1993; see Fig. 4.1), though there are other outputs (Kesner and Rolls 2015). These are the pathways that are likely to be involved in the retrieval of information from the hippocampus back to the neocortex.
The theory is a quantitative theory and the numbers of synapses on the different types of neuron is an important feature of the circuitry emphasized next.
Hippocampal Circuitry
Hippocampal circuitry (Amaral 1993; Amaral and Witter 1989; Andersen et al. 2007; Kondo et al. 2009; Lavenex et al. 2004; Naber et al. 2001; Storm-Mathiesen et al. 1990; Witter 2007; Witter et al. 2000b) is illustrated in Fig. 4.1.
Projections from the entorhinal cortex layer 2 reach the granule cells (of which there are 106 in the rat) in the dentate gyrus (DG), via the perforant path (pp) (Witter 1993). The granule cells project to CA3 cells via the mossy fibers (mf), which provide a sparse but possibly powerful connection to the 3 × 105 CA3 pyramidal cells in the rat. Each CA3 cell receives approximately 46 mossy fiber inputs, so that the sparseness of this connectivity is thus 0.005 %. By contrast, there are many more—possibly weaker—direct perforant path inputs also from layer 2 of the entorhinal cortex onto each CA3 cell, in the rat of the order of 4 × 103. The largest number of synapses (about 1.2 × 104 in the rat) on the dendrites of CA3 pyramidal cells is, however, provided by the (recurrent) axon collaterals of CA3 cells themselves (rc) (see Fig. 4.2). It is remarkable that the recurrent collaterals are distributed to other CA3 cells largely throughout the hippocampus (Amaral et al. 1990; Amaral and Witter 1989, 1995; Ishizuka et al. 1990; Witter 2007), so that effectively the CA3 system provides a single network, with a connectivity of approximately 2 % between the different CA3 neurons given that the connections are bilateral. The CA3–CA3 recurrent collateral system is even more extensive in macaques than in rats (Kondo et al. 2009). The neurons that comprise CA3, in turn, project to CA1 neurons via the Schaffer collaterals. In addition, projections that terminate in the CA1 region originate in layer 3 of the entorhinal cortex (see Fig. 4.1).
CA3 as an Autoassociation or Attractor Memory: Pattern Completion
Arbitrary Associations and Pattern Completion in Recall
Many of the synapses in the hippocampus show associative modification as shown by long-term potentiation, and this synaptic modification appears to be involved in learning (see Andersen et al. 2007; Lynch 2004; Morris 1989, 2003; Morris et al. 2003; Nakazawa et al. 2004; Nakazawa et al. 2003; Wang and Morris 2010). On the basis of the evidence summarized above, Rolls (1987, 1989a, b, c, 1990a, b, 1991) and others (Levy 1989; McNaughton 1991; McNaughton and Morris 1987) have suggested that the CA3 stage acts as an autoassociation memory which enables episodic memories to be formed and stored in the CA3 network, and that subsequently the extensive recurrent collateral connectivity allows for the retrieval of a whole representation to be initiated by the activation of some small part of the same representation (the cue). The crucial synaptic modification for this is in the recurrent collateral synapses. (A description of the operation of autoassociative networks is provided in detail elsewhere (Amit 1989; Hertz et al. 1991; Rolls 2010a; Rolls and Deco 2002, 2010; Rolls and Treves 1998) including Memory, Attention, and Decision-Making (Rolls 2008)).
The architecture of an autoassociation network is effectively that of the recurrent collateral synapses shown in Fig. 4.2, and the learning rule for the change in the synaptic weights is as shown in Eq. (4.1) (Rolls 2008; Rolls and Treves 1998).
where k is a constant, r i is the activation of the dendrite (the postsynaptic term), r′ j is the presynaptic firing rate, and δw ij is the change in the synaptic weight w ij . (w ij refers to the j′th synapse onto the i′th neuron. An introduction to autoassociation, competitive, and pattern association networks is provided in the Appendices of Memory, Attention and Decision-Making: A Unifying Computational Neuroscience Approach (Rolls 2008).)
The hypothesis is that because the CA3 operates effectively as a single network, it can allow arbitrary associations between inputs originating from very different parts of the cerebral cortex to be formed. These might involve associations between information originating in the temporal visual cortex about the presence of an object, and information originating in the parietal cortex about where it is. I note that although there is some spatial gradient in the CA3 recurrent connections, so that the connectivity is not fully uniform (Ishizuka et al. 1990; Witter 2007), the network will still have the properties of a single interconnected autoassociation network allowing associations between arbitrary neurons to be formed, given the presence of many long-range connections which overlap from different CA3 cells, and the ability of attractor networks to operate with diluted connectivity shown in our computational studies prompted by this issue (Rolls 2012a; Rolls and Webb 2012; Treves 1990; Treves and Rolls 1991). It is very interesting indeed that in primates (macaques), the associational projections from CA3 to CA3 travel extensively along the longitudinal axis, and overall the radial, transverse, and longitudinal gradients of CA3 fiber distribution, clear in the rat, are much more subtle in the nonhuman primate brain (Kondo et al. 2009). The implication is that in primates, the CA3 network operates even more as a single network than in rodents.
Crucial issues include how many memories could be stored in this system (to determine whether the autoassociation hypothesis leads to a realistic estimate of the number of memories that the hippocampus could store); whether the whole of a memory could be completed from any part; whether the autoassociation memory can act as a short term memory, for which the architecture is inherently suited; and whether the system could operate with spatial representations, which are essentially continuous because of the continuous nature of space. These and related issues are considered in the remainder of “Storage Capacity” and in more detail elsewhere (Rolls 2008; Kesner and Rolls 2015).
Storage Capacity
We have performed quantitative analyses of the storage and retrieval processes in the CA3 network (Rolls 2012a; Rolls and Webb 2012; Treves and Rolls 1991, 1992; Webb et al. 2011). We have extended previous formal models of autoassociative memory (see Amit 1989) by analyzing a network with graded response units, so as to represent more realistically the continuously variable rates at which neurons fire, and with incomplete connectivity (Rolls et al. 1997b; Rolls and Webb 2012; Treves 1990; Treves and Rolls 1991; Webb et al. 2011). We have found that in general the maximum number p max of firing patterns that can be (individually) retrieved is proportional to the number C RC of (associatively) modifiable recurrent collateral (RC) synapses on to each neuron, by a factor that increases roughly with the inverse of the sparseness a of the neuronal representation. (Each memory is precisely defined in the theory: it is a set of firing rates of the population of neurons (which represent a memory) that can be stored and later retrieved, with retrieval being possible from a fraction of the originally stored set of neuronal firing rates.) The neuronal population sparseness a of the representation can be measured by extending the binary notion of the proportion of neurons that are firing to any one stimulus or event as
where r i is the firing rate of the i′th neuron in the set of N neurons. The sparseness ranges from 1/N, when only one of the neurons responds to a particular stimulus (a local or grandmother cell representation), to a value of 1.0, attained when all the neurons are responding to a given stimulus. Approximately,
where k is a factor that depends weakly on the detailed structure of the rate distribution, on the connectivity pattern, etc., but is roughly in the order of 0.2–0.3 (Treves and Rolls 1991). For example, for C RC = 12,000 and a = 0.02, p max is calculated to be approximately 36,000. This analysis emphasizes the utility of having a sparse representation in the hippocampus, for this enables many different memories to be stored. (The sparseness a in this equation is strictly the population sparseness (Franco et al. 2007; Treves and Rolls 1991). The population sparseness a p would be measured by measuring the distribution of firing rates of all neurons to a single stimulus at a single time. The single neuron sparseness or selectivity a s would be measured by the distribution of firing rates to a set of stimuli, which would take a long time. The selectivity or sparseness a s of a single neuron measured across a set of stimuli often takes a similar value to the population sparseness a p in the brain, and does so if the tuning profiles of the neurons to the set of stimuli are uncorrelated (Franco et al. 2007). These concepts are elucidated by Franco, Rolls et al. (2007).) (I note that the sparseness estimates obtained by measuring early gene changes, which are effectively population sparsenesses, would be expected to depend greatly on the range of environments or stimuli in which these were measured. If the environment was restricted to one stimulus, this would reflect the population sparseness. If the environment was changing, the measure from early gene changes would be rather undefined, as all the populations of neurons activated in an undefined number of testing situations would be likely to be activated.)
In order for most associative networks to store information efficiently, heterosynaptic long-term depression (as well as LTP) is required (Fazeli and Collingridge 1996; Rolls 2008; Rolls and Deco 2002; Rolls and Treves 1990, 1998; Treves and Rolls 1991). Simulations that are fully consistent with the analytic theory are provided by Rolls (1995, 2012a), Simmen et al. (1996), and Rolls et al. (1997b).
A number of points that arise, including measurement of the total amount of information (in bits per synapse) that can be retrieved from the network, the computational definition of a memory, the computational sense in which CA3 is an attractor network, and the possible computational utility of memory reconsolidation, are treated elsewhere (Rolls 2008; Rolls and Kesner 2006). Here I note that given that the memory capacity of the hippocampal CA3 system is limited, it is necessary to have some form of forgetting in this store, or other mechanism to ensure that its capacity is not exceeded. (Exceeding the capacity can lead to a loss of much of the information retrievable from the network.) Heterosynaptic LTD could help this forgetting, by enabling new memories to overwrite old memories (Rolls 1996a, 2008). The limited capacity of the CA3 system does also provide one of the arguments that some transfer of information from the hippocampus to neocortical memory stores may be useful (see Treves and Rolls 1994). Given its limited capacity, the hippocampus might be a useful store for only a limited period, which might be in the order of days, weeks, or months. This period may well depend on the acquisition rate of new episodic memories. If the animal were in a constant and limited environment, then as new information is not being added to the hippocampus, the representations in the hippocampus would remain stable and persistent. These hypotheses have clear experimental implications, both for recordings from single neurons and for the gradient of retrograde amnesia, both of which might be expected to depend on whether the environment is stable or frequently changing. They show that the conditions under which a gradient of retrograde amnesia might be demonstrable would be when large numbers of new memories are being acquired, not when only a few memories (few in the case of the hippocampus being less than a few hundred) are being learned.
Recall and Completion
A fundamental property of the autoassociation model of the CA3 recurrent collateral network is that the recall can be symmetric, that is, the whole of the memory can be retrieved and completed from any part (Rolls 2008; Rolls and Kesner 2006; Rolls and Treves 1998). For example, in an object–place autoassociation memory, an object could be recalled from a place retrieval cue, and vice versa. Kesner et al. (2008) tested this using an object-cued spatial location recall task, and a spatial location-cued object recall task (developed from an episodic flavor–place paired-associate task (Day et al. 2003)). After rats were trained to a criterion of 80 % correct on 1 of the 2 tasks, they received either a dorsal CA3 lesion or a vehicle control lesion. Control animals continued performing well on both tasks. Rats with lesions to dorsal CA3 were impaired on both tasks and performed at chance but were able to perform a non-episodic version of the task as a control. These data provide evidence that CA3 mediates episodic learning of arbitrary associations as tested in the 1-trial object cue with spatial location recall task, and the spatial location cue with object recall task (Kesner et al. 2008).
In an object–place task, rats were trained in a study phase to learn in one trial an association between two flavors of food and two spatial locations (Day et al. 2003). During a recall test phase they were presented with a flavor which served as a cue for the selection of the correct location. They found that injections of an N-methyl-D-aspartate (NMDA) receptor blocker (AP5) or AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/kainate receptor blocker (CNQX) to the dorsal hippocampus prior to the study phase impaired encoding, but injections of AP5 prior to the test phase did not impair the place recall, whereas injections of CNQX did impair the place recall. The interpretation is that somewhere in the hippocampus NMDA receptors are necessary for learning one-trial odor–place associations, and that recall can be performed without further involvement of NMDA receptors.
Evidence that the CA3 system is not necessarily required during recall in a reference memory spatial task, such as the water maze spatial navigation for a single spatial location task, is that CA3 lesioned rats are not impaired during recall of a previously learned water maze task (Brun et al. 2002; Florian and Roullet 2004). However, if completion from an incomplete cue is needed, then CA3 NMDA receptors are necessary (presumably to ensure satisfactory CA3–CA3 learning) even in a reference memory task (Gold and Kesner 2005; Nakazawa et al. 2002). Thus, the CA3 system appears to be especially needed in rapid, one-trial object–place recall, and when completion from an incomplete cue is required (see further “Pattern Separation Performed By Dentate Granule Cells”). Especially important though in assessing the implications of all such tests is that the theory sets out how the system operates when large numbers of memories, in the order of thousands, are to be stored and retrieved, and this is difficult to test adequately in behavioral experiments. Effects found when the storage and retrieval of just a few memories are tested may not reflect well the operation of the system when it is heavily loaded, as it is expected to be when operating in the natural environment.
Evidence for pattern completion has been observed using imaging with voltage-sensitive dye in the CA3 region of a rat hippocampal slice. Following the induction of long-term potentiation from two stimulation sites activated simultaneously, stimulation at either of the two sites produced the whole pattern of activation that could be produced from both stimulation sites before LTP, thus demonstrating pattern completion in CA3 (Jackson 2013).
Continuous, Spatial, Patterns, and CA3 Representations
The fact that spatial patterns, which imply continuous representations of space, are represented in the hippocampus has led to the application of continuous attractor models to help understand hippocampal function. This has been necessary, because space is inherently continuous, because the firing of place and spatial view cells is approximately Gaussian as a function of the distance away from the preferred spatial location, because these cells have spatially overlapping fields, and because the theory is that these cells in CA3 are connected by Hebb-modifiable synapses. This specification would inherently lead the system to operate as a continuous attractor network. Continuous attractor network models have been studied by Amari (1977), Zhang (1996), Taylor (1999), Samsonovich and McNaughton (1997), Battaglia and Treves (1998), Stringer et al. (2002a, b, 2004), Stringer and Rolls (2002) and Rolls and Stringer (2005) (see Rolls 2008; Rolls and Deco 2002), and are described briefly next.
A “continuous attractor” neural network (CANN) can maintain the firing of its neurons to represent any location along a continuous physical dimension such as spatial view, spatial position, head direction, etc. It uses excitatory recurrent collateral connections between the neurons (as are present in CA3) to reflect the distance between the neurons in the state space of the animal (e.g., place or head direction). These networks can maintain the bubble or packet of neural activity constant for long periods wherever it is started to represent the current state (head direction, position, etc) of the animal, and are likely to be involved in many aspects of spatial processing and memory, including spatial vision. Global inhibition is used to keep the number of neurons in a bubble or packet of actively firing neurons relatively constant, and to help to ensure that there is only one activity packet.
Continuous attractor networks can be thought of as very similar to autoassociation or discrete attractor networks (Rolls 2008), and have the same architecture, as illustrated in Fig. 4.3. The main difference is that the patterns stored in a CANN are continuous patterns, with each neuron having broadly tuned firing which decreases with, for example, a Gaussian function as the distance from the optimal firing location of the cell is varied, and with different neurons having tuning that overlaps throughout the space. Such tuning is illustrated elsewhere (Rolls 2008; Rolls et al. 2002). For comparison, autoassociation networks normally have discrete (separate) patterns (each pattern implemented by the firing of a particular subset of the neurons), with no continuous distribution of the patterns throughout the space. A consequent difference is that the CANN can maintain its firing at any location in the trained continuous space, whereas a discrete attractor or autoassociation network moves its population of active neurons toward one of the previously learned attractor states, and thus implements the recall of a particular previously learned pattern from an incomplete or noisy (distorted) version of one of the previously learned patterns.
Simulation of competitive learning in the dentate gyrus to produce place cells from the entorhinal cortex grid cell inputs. a and b Firing rate profiles of two entorhinal cortex (EC) grid cells with frequencies of 4 and 7 cycles. c and d Firing rate profiles of two dentate gyrus (DG) cells with no training using competitive learning. e and f Firing rate profiles of two dentate gyrus (DG) cells trained using competitive learning. (After Rolls et al. 2006.)
Space is continuous, and object representations are discrete. If these representations are to be combined in for example an object–place memory, then we need to understand the operation of networks that combine these representations. Rolls, Stringer, and Trappenberg (Rolls et al. 2002) have shown that attractor networks can store both continuous patterns and discrete patterns, and can thus be used to store for example the location in (continuous, physical) space (e.g., the place “out there” in a room represented by spatial view cells) where an object (a discrete item) is present. We showed this by storing associated continuous and discrete representations in the same single attractor network, and then showing that the representation in the continuous space could be retrieved by the discrete object that was associated with that spatial position; and that the representation of the discrete object could be retrieved by providing the position in the continuous representation of space.
If spatial representations are stored in the hippocampus, the important issue arises in terms of understanding memories that include a spatial component or context of how many such spatial representations could be stored in a continuous attractor network. The very interesting result is that because there are in general low correlations between the representations of places in different maps or charts (where each map or chart might be of one room or locale), very many different maps or charts can be simultaneously stored in a continuous attractor network (Battaglia and Treves 1998).
We have considered how spatial representations could be stored in continuous attractor networks, and how the activity can be maintained at any location in the state space in a form of short-term memory when the external (e.g., visual) input is removed. However, a property of some spatial representations is that they can be updated by self-motion, idiothetic, input, and mechanisms have been proposed for how this could be achieved (Rolls and Stringer 2005; Samsonovich and McNaughton 1997; Stringer and Rolls 2006; Stringer et al. 2005, 2002a, b; Walters et al. 2013), including in the entorhinal cortex grid cell system (Giocomo et al. 2011; Kropff and Treves 2008; Zilli 2012). The ways in which path integration could be implemented in recurrent networks such as the CA3 system in the hippocampus or in related systems are described elsewhere (McNaughton et al. 2006; Samsonovich and McNaughton 1997; Stringer et al. 2002a, b), and have been applied to primate spatial view cells by Rolls and colleagues (Rolls and Stringer 2005; Stringer et al. 2004, 2005). Cognitive maps (O’Keefe and Nadel 1978) can be understood by the operations of these attractor networks, and how they are updated by learning and by self-motion (Rolls 2008). It has been argued that the bumpiness of the CA3 representation of space is more consistent with episodic memory storage, as argued in this chapter, than with spatial path integration using the CA3 system as a continuous attractor network implementing path integration (Cerasti and Treves 2013; Stella et al. 2013).
Perforant Path Inputs to CA3 Cells Perform Completion and Initiate Recall in CA3
By calculating the amount of information that would end up being carried by a CA3 firing pattern produced solely by the perforant path input and by the effect of the recurrent connections, we have been able to show (Treves and Rolls 1992) that an input of the perforant path type, alone, is unable to direct efficient information storage. Such an input is too weak, it turns out, to drive the firing of the cells, as the “dynamics” of the network is dominated by the randomizing effect of the recurrent collaterals. On the other hand, an autoassociative memory network needs afferent inputs to apply the retrieval cue to the network. We have shown (Treves and Rolls 1992) that the perforant path system is likely to be the one involved in relaying the cues that initiate retrieval in CA3. The concept is that to initiate retrieval, a numerically large input (the perforant path system, see Fig. 4.2) is useful so that even a partial cue is sufficient (see Eq. 17 of Treves and Rolls (1992)); and that the retrieval cue need not be very strong, as the recurrent collaterals (in CA3) then take over in the retrieval process to produce good recall (Rolls 2008; Treves and Rolls 1992). In this scenario, the perforant path to CA3 synapses operate as a pattern associator, the quantitative properties of which are described elsewhere (Rolls 2008, 2016; Rolls and Treves 1990, 1998). If an incomplete recall cue is provided to a pattern association network using distributed input representations, then most of the output pattern will be retrieved, and in this sense pattern association networks do perform pattern generalization, and this generalization performed at the perforant path synapses to CA3 cells helps in the completion produced by the recurrent collateral CA3–CA3 autoassociation process.
In contrast, during storage, strong signals, in the order of mV for each synaptic connection, are provided by the mossy fiber inputs to dominate the recurrent collateral activations, so that the new pattern of CA3 cell firing can be stored in the CA3 recurrent collateral connections (Rolls 2008; Treves and Rolls 1992).
The Dilution of the CA3 Recurrent Collateral Connectivity Enhances Memory Storage Capacity and Pattern Completion
Figure 4.2 shows that in the rat, there are approximately 300,000 CA3 neurons, but only 12,000 recurrent collateral synapses per neuron. The dilution of the connectivity is thus 12,000/300,000 = 0.04. The connectivity is thus not complete, and complete connectivity in an autoassociation network would make it simple, for the connectivity between the neurons would then be symmetric (i.e., the connection strength from any one neuron to another is matched by a connection of the same strength in the opposite direction), and this guarantees energy minima for the basins of attraction that will be stable, and a memory capacity than can be calculated (Hopfield 1982). We have shown how this attractor type of network can be extended to have similar properties with diluted connectivity, and also with sparse representations with graded firing rates (Rolls and Treves 1990; Treves 1990, 1991; Treves and Rolls 1991).
However, the question has recently been asked about whether there are any advantages to diluted autoassociation or attractor networks compared to fully connected attractor networks (Rolls 2012a). One biological property that may be a limiting factor is the number of synaptic connections per neuron, which is 12,000 in the CA3–CA3 network just for the recurrent collaterals (see Fig. 4.2). The number may be higher in humans, allowing more memories to be stored in the hippocampus than order 12,000. I note that the storage of large number of memories may be facilitated in humans because the left and right hippocampus appear to be much less connected between the two hemispheres than in the rat, which effectively has a single hippocampus (Rolls 2008). In humans, with effectively two separate CA3 networks, one on each side of the brain, the memory storage capacity may be doubled, as the capacity is set by the number of recurrent collaterals per neuron in each attractor network (Eq. 4.3). In humans, the right hippocampus may be devoted to episodic memories with spatial and visual components, whereas the left hippocampus may be devoted to memories with verbal/linguistic components, that is, in which words may be the part of the episode (e.g., who said what to whom and when) (Barkas et al. 2010; Bonelli et al. 2010; Sidhu et al. 2013).
The answer that has been suggested to why the connectivity of the CA3 autoassociation network is diluted (and why neocortical recurrent networks are also diluted), is that this may help to reduce the probability of having two or more synapses between any pair of randomly connected neurons within the network, which it has been shown greatly impairs the number of memories that can be stored in an attractor network, because of the distortion that this produces in the energy landscape (Rolls 2012a). In more detail, the hypothesis proposed is that the diluted connectivity allows biological processes that set up synaptic connections between neurons to arrange for there to be only very rarely more than one synaptic connection between any pair of neurons. If probabilistically there were more than one connection between any two neurons, it was shown by simulation of an autoassociation attractor network that such connections would dominate the attractor states into which the network could enter and be stable, thus strongly reducing the memory capacity of the network (the number of memories that can be stored and correctly retrieved), below the normal large capacity for diluted connectivity. Diluted connectivity between neurons in the cortex thus has an important role in allowing high capacity of memory networks in the cortex, and helping to ensure that the critical capacity is not reached at which overloading occurs leading to an impairment in the ability to retrieve any memories from the network (Rolls 2012a). The diluted connectivity is thus seen as an adaptation that simplifies the genetic specification of the wiring of the brain, by enabling just simple attributes of the connectivity to be specified (e.g., from a CA3 to another CA3 neuron chosen at random to specify the CA3 to CA3 recurrent collateral connectivity), rather than which particular neuron should connect to which other particular neuron (Rolls 2012a; Rolls and Stringer 2000). Consistent with this hypothesis, there are NMDA receptors with the genetic specification that they are NMDA receptors on neurons of a particular type, CA3 neurons (as shown by the evidence from CA3-specific vs. CA1-specific NMDA receptor knockouts) (Nakazawa et al. 2002, 2003, 2004; Rondi-Reig et al. 2001). A consequence is that the vector of output neuronal firing in the CA3 regions, that is, the number of CA3 neurons, is quite large (300,000 neurons in the rat). The large number of elements in this vector may have consequences for the noise in the system, as we will see below.
The dilution of the CA3–CA3 recurrent collateral connectivity at 0.04 may be greater dilution than that in a local neocortical area, which is in the order of 0.1 (Rolls 2008, 2012a). This is consistent with the hypothesis that the storage capacity of the CA3 system is at a premium, and so the dilution is kept to a low value (i.e., great dilution), as then there is lower distortion of the basins of attraction and hence the memory capacity is maximized (Rolls 2012a).
Pattern Separation of CA3 Cell Populations Encoding Different Memories
For the CA3 to operate with high capacity as an autoassociation or attractor memory, the sets of CA3 neurons that represent each event to be stored and later recalled need to be as uncorrelated from each other as possible. Correlations between patterns reduce the memory capacity of an autoassociation network (Kohonen 1977, 1984; Kohonen et al. 1981; Marr 1971), and because storage capacity is at a premium in an episodic memory system, there are several mechanisms that reduce the correlations between the firing of the population vectors of CA3 neuron firing each one of which represents a different event to be stored in memory. In the theoretical physics approach to the capacity of attractor networks, it is indeed assumed that the different vectors of firing rates to be stored are well separated from each other, by drawing each vector of firing at random, and by assuming very large (infinite) numbers of neurons in each pattern.
We have proposed that there are several mechanisms that help to achieve this pattern separation, namely the mossy fiber pattern separation effect produced by the small number of connections received by a CA3 neuron from mossy fibers which dominate the CA3 cell firing; the expansion recoding, and the sparse representation provided by the dentate granule cells that form the mossy fiber synapses; and the sparseness of the CA3 cell representation. Neurogenesis of dentate granule cells is a fifth potential contributor to achieving pattern separation of CA3 cell firing. The five factors are described next. Before this, it is remarked that some of this architecture may be special to the hippocampus, and not found in the neocortex, because of the importance of storing and retrieving large numbers of (episodic) memories in the hippocampus. The neocortex in contrast is more concerned with building new representations for which competitive learning is more important, and thus neocortical circuitry does not use a mossy fiber system to produce new random sets of neurons activated (Rolls 2008, 2016).
Pattern Separation and the Sparse Connectivity of the Mossy Fiber Inputs to CA3 Cells
We hypothesize that the mossy fiber inputs force efficient information storage by virtue of their strong and sparse influence on the CA3 cell firing rates (Rolls 1987, 1989b, c; Treves and Rolls 1992). (The strong effects likely to be mediated by the mossy fibers were also emphasized by McNaughton and Morris (1987) and McNaughton and Nadel (1990)). We (Rolls and Treves) (Rolls 1987, 1989b, 1989c, 1990b, 2008; Rolls and Treves 1998; Treves and Rolls 1992) hypothesize that the mossy fiber input appears to be particularly appropriate in several ways. First, the fact that mossy fiber synapses are large and located very close to the soma makes them relatively powerful in activating the postsynaptic cell. Second, the firing activity of dentate granule cells appears to be very sparse (Jung and McNaughton 1993; Leutgeb et al. 2007) and this, together with the small number of connections on each CA3 cell, produces a sparse signal, which can then be transformed into sparse firing activity in CA3 by a threshold effect. The hypothesis is that the mossy fiber sparse connectivity solution performs the appropriate function to enable learning to operate correctly in CA3 (Cerasti and Treves 2010; Treves and Rolls 1992). The perforant path input would, the quantitative analysis shows, not produce a pattern of firing in CA3 that contains sufficient information for learning (Treves and Rolls 1992) (see further Section “Perforant Path Inputs to CA3 Cells Perform Completion and Initiate Recall in CA3”).
The particular property of the small number of mossy fiber connections onto a CA3 cell, approximately 46 (see Fig. 4.2), is that this has a randomizing effect on the representations set up in CA3, so that they are as different as possible from each other (Rolls 1989b, 1989c, 2008; Rolls and Kesner 2006; Rolls and Treves 1998; Treves and Rolls 1992). (This means, for example, that place cells in a given environment are well separated to cover the whole space.) The result is that any one event or episode will set up a representation that is very different from other events or episodes, because the set of CA3 neurons activated for each event is random. This is then the optimal situation for the CA3 recurrent collateral effect to operate, for it can then associate together the random set of neurons that are active for a particular event (e.g., an object in a particular place), and later recall the whole set from any part. It is because the representations in CA3 are unstructured, or random, in this way that large numbers of memories can be stored in the CA3 autoassociation system, and that interference between the different memories is kept as low as possible, in that they are maximally different from each other (Hopfield 1982; Rolls 2008; Rolls and Treves 1998; Treves and Rolls 1991).
The requirement for a small number of mossy fiber connections onto each CA3 neuron applies not only to discrete (Treves and Rolls 1992) but also to spatial representations, and some learning in these connections, whether associative or not, can help to select out the small number of mossy fibers that may be active at any one time to select a set of random neurons in the CA3 (Cerasti and Treves 2010). Any learning may help by reducing the accuracy required for a particular number of mossy fiber connections to be specified genetically onto each CA3 neuron. The optimal number of mossy fibers for the best information transfer from dentate granule cells to CA3 cells is in the order of 35–50 (Cerasti and Treves 2010; Treves and Rolls 1992). The mossy fibers also make connections useful for feedforward inhibition in CA3 (Acsady et al. 1998), which is likely to be useful to help in the sparse representations being formed in CA3.
On the basis of these and other points, we predicted that the mossy fibers may be necessary for new learning in the hippocampus, but may not be necessary for the recall of existing memories from the hippocampus (Rolls 2008; Rolls and Treves 1998; Treves and Rolls 1992). Experimental evidence consistent with this prediction about the role of the mossy fibers in learning has been found in rats with disruption of the dentate granule cells (Lassalle et al. 2000) (Pattern Separation Performed By Dentate Granule Cells).
We (Rolls and Kesner 2006) have hypothesized that nonassociative plasticity of mossy fibers (see Brown et al. 1989, 1990) might have a useful effect in enhancing the signal-to-noise ratio, in that a consistently firing mossy fiber would produce nonlinearly amplified currents in the postsynaptic cell, which would not happen with an occasionally firing fiber (Treves and Rolls 1992). This plasticity, and also learning in the dentate, would also have the effect that similar fragments of each episode (e.g., the same environmental location) recurring on subsequent occasions would be more likely to activate the same population of CA3 cells, which would have potential advantages in terms of economy of use of the CA3 cells in different memories, and in making some link between different episodic memories with a common feature, such as the same location in space. Consistent with this, dentate neurons that fire repeatedly are more effective in activating CA3 neurons (Henze et al. 2002).
As acetylcholine turns down the efficacy of the recurrent collateral synapses between CA3 neurons (Giocomo and Hasselmo 2007; Hasselmo et al. 1995), then cholinergic activation also might help to allow external inputs from the mossy fibers rather than the internal recurrent collateral inputs to dominate the firing of the CA3 neurons during learning, as the current theory proposes. If cholinergic activation at the same time facilitated LTP in the recurrent collaterals (as it appears to in the neocortex), then cholinergic activation could have a useful double role in facilitating new learning at times of behavioral activation (Giocomo and Hasselmo 2007; Hasselmo et al. 1995), when presumably it may be particularly relevant to allocate some of the limited memory capacity to new memories.
Pattern Separation and the Sparseness of the Firing of the Dentate Granule Cell Input Via the Mossy Fibers to CA3 Cells
The firing activity of dentate granule cells appears to be very sparse (Jung and McNaughton 1993; Leutgeb et al. 2007) and this, together with the small number of dentate mossy fiber connections on each CA3 cell, produces a sparse signal, which can then be transformed into sparse firing activity in CA3 by a threshold effect. The pattern separation mechanisms that enable the dentate to provide a sparse firing input to CA3 are described below.
Pattern Separation and the Large Number of Dentate Granule Cells Providing Inputs Via the Mossy Fibers to CA3 Cells
Expansion recoding can decorrelate input patterns, and this can be performed by a stage of competitive learning with a large number of neurons (Rolls 2008). A mechanism like this appears to be implemented by the dentate granule cells, which are numerous (1 × 106 in the rat, compared to 300,000 CA3 cells), have associatively modifiable synapses (required for a competitive network), and strong inhibition provided by the inhibitory interneurons. This may not represent expansion of numbers relative to the number of entorhinal cortex cells, but the principle of a large number of dentate granule cells, with competitive learning and strong inhibition through inhibitory interneurons, would produce a decorrelation of signals like that achieved by expansion recoding (Rolls 2008).
Sparseness of the CA3 Cell Representation and Pattern Separation
The firing of CA3 cells is relatively sparse, and this helps to decorrelate different population vectors of CA3 cell firing for different memories. (Sparse representations are more likely to be decorrelated with each other (Rolls 2008).) Evidence on the sparseness of the CA3 cell representation in rats includes evidence that CA3 cell ensembles may support the fast acquisition of detailed memories by providing a locally continuous, but globally orthogonal spatial representation, onto which new sensory inputs can rapidly be associated (Leutgeb and Leutgeb 2007). In the macaque hippocampus, in which spatial view cells are found (Georges-François et al. 1999; Robertson et al. 1998; Rolls et al. 1997a, 1998), for the representation of 64 locations around the walls of the room, the mean single cell sparseness a s was 0.34, and the mean population sparseness a pwas 0.33 (Rolls 2008; Rolls and Treves 2011; Rolls et al. 1998). For comparison, the corresponding values for inferior temporal cortex neurons tuned to objects and faces were 0.77 (Franco et al. 2007; Rolls 2008; Rolls and Treves 2011); for taste and oral texture neurons in the insular cortex the population sparseness was 0.71; for taste and oral texture neurons in the orbitofrontal cortex was 0.61; and for taste and oral texture neurons in the orbitofrontal cortex was 0.81 (Rolls 2008; Rolls and Treves 2011). Thus, the evidence is that the hippocampal CA3/pyramidal cell representation is more sparse in macaques than in neocortical areas and the amygdala, and this is consistent with the importance in hippocampal CA3 of using a sparse representation to produce a large memory capacity.
Representations in the neocortex and in the hippocampus are often distributed with graded firing rates in the neuronal populations (Rolls and Treves 2011). The firing rate probability distribution of each neuron to a set of stimuli is often exponential or gamma (Rolls and Treves 2011). These graded firing rate distributed representations are present in the hippocampus, both for place cells in rodents and for spatial view cells in the primate (Georges-François et al. 1999; McNaughton et al. 1983; O’ Keefe and Speakman 1987; O’Keefe 1979; Robertson et al. 1998; Rolls 2008; Rolls et al. 1997a, 1998; Rolls and Treves 2011). In processes in the brain such as memory recall in the hippocampus or decision-making in the cortex that are influenced by the noise produced by the close to random spike timings of each neuron for a given mean rate, the noise with this graded type of representation may be larger than with the binary firing rate distribution that is usually investigated (Rolls and Deco 2010). In integrate-and-fire simulations of an attractor decision-making network, we showed that the noise is indeed greater for a given sparseness of the representation for graded, exponential, than for binary firing rate distributions (Webb et al. 2011). The greater noise was measured by faster escaping times from the spontaneous firing rate state when the decision cues are applied, and this corresponds to faster decision or reaction times. The greater noise was also evident as less stability of the spontaneous firing state before the decision cues are applied. The implication is that spiking-related noise will continue to be a factor that influences processes such as decision-making, signal detection, short-term memory, and memory recall (including in the CA3 network) even with the quite large networks found in the cerebral cortex. In these networks there are several thousand recurrent collateral synapses onto each neuron. The greater noise with graded firing rate distributions has the advantage that it can increase the speed of operation of cortical circuitry (Webb et al. 2011). The graded firing rates also by operating in a nonlinear network effectively increase the sparseness of the representation, and this itself is a pattern separation effect (Webb et al. 2011).
Neurogenesis of Dentate Granule Cells to Provide New Representations in CA3 Uncorrelated with Previous CA3 Representations
If adult neurogenesis in the dentate gyrus does prove to be functionally relevant, its computational role could be to facilitate pattern separation for new patterns, by providing new dentate granule cells with new sets of random connections to CA3 neurons. Consistent with the dentate spatial pattern separation hypothesis (Rolls 1989b, c, 1996b; Treves and Rolls 1992, 1994), in mice with impaired dentate neurogenesis, spatial learning in a delayed non-matching-to-place task in the radial arm maze was impaired for arms that were presented with little separation, but no deficit was observed when the arms were presented farther apart (Clelland et al. 2009). Consistently, impaired neurogenesis in the dentate also produced a deficit for small spatial separations in an associative object-in-place task (Clelland et al. 2009).
The Direct Perforant Path to CA3 Cell Input: Poor at Pattern Separation and Forcing a New Memory Pattern into CA3 Cell Firing
It has been suggested that the feedforward connectivity from the entorhinal cortex via the perforant path to the CA3 neurons may act as a feedforward pattern association network that is more important than the CA3–CA3 recurrent collateral autoassociation system (Cheng 2013). The quantitative properties of pattern association networks are described elsewhere (Rolls 2008; Rolls and Treves 1990, 1998). If an incomplete recall cue is provided to a pattern association network using distributed input representations, then most of the output pattern will be retrieved, and in this sense pattern association networks do generalize. (As noted above, pattern association networks do not perform pattern completion, in that the unconditioned stimulus cannot recall the conditioned stimulus.) The analyses described in these sources shows that the capacity of pattern association networks (the maximum number of memories that can be stored and retrieved, here denoted by p max) is approximately
where C PA is the number of feedforward associatively modifiable connections per neuron, and a o is the sparseness of the representation in the output neurons of the pattern associator (Rolls 2008). Given that there are fewer feedforward (perforant path) synaptic connections onto CA3 neurons (3600) than recurrent synaptic connections between CA3 neurons (12,000 in the rat) (see Fig. 4.2), then the capacity of the feedforward system would be considerably smaller than that of the recurrent collateral CA3–CA3 system. (It is noted that the a o of Eq. (4) would be the same number as the a of Eq. (3), as that is just the sparseness of the firing of the population of CA3 neurons. The number of perforant path synapses is sufficiently large that it can act as a retrieval cue for even an incomplete pattern so that the CA3–CA3 connections can then complete the retrieval, given that the recall signal for the perforant path pattern associator is proportional to the square root of the number of perforant path synapses, as shown by Eq. 17 of Treves and Rolls (1992).) The feedforward hypothesis (Cheng 2013) thus has a strong argument against it of storage capacity, which would be much less (approximately 3600/12,000) than that of the CA3–CA3 recurrent collateral system operating as an autoassociation memory. Another disadvantage of the feedforward hypothesis is that the attractor properties of the CA3–CA3 connections would be lost, and these potentially contribute to holding one or more items simultaneously active in short-term memory (Rolls 2008; Rolls et al. 2013), and providing a basis for temporal order memory as described in “Dilution of the CA3 Recurrent Collateral Connectivity Enhances Memory Storage Capacity and Pattern Completion.” Another disadvantage is that we have been able to show (Treves and Rolls 1992) that an input of the perforant path type, alone, is unable to direct efficient information storage. Such an input is too weak, it turns out, to drive the firing of the cells, as the “dynamics” of the network is dominated by the randomizing effect of the recurrent collaterals. Another disadvantage of the feedforward hypothesis is that a pattern associator may not, with an incomplete cue, be able to recall the exact pattern that was stored, whereas an attractor network has the property that it can fall into an attractor basin that can reflect perfect retrieval of the memory (Rolls 2008; Rolls and Treves 1998).
Pattern Separation Performed by Dentate Granule Cells
The theory is that the dentate granule cell stage of hippocampal processing which precedes the CA3 stage acts as a competitive network in a number of ways to produce during learning the sparse yet efficient (i.e., nonredundant) representation in CA3 neurons that is required for the autoassociation implemented by CA3 to perform well (Rolls 1989b, c, 1990b; Kesner and Rolls 2015; Rolls et al. 2006; Treves and Rolls 1992). An important property for episodic memory is that the dentate by acting in this way would perform pattern separation (or orthogonalization) (Rolls 1989b; Kesner and Rolls 2015; Rolls et al. 2006; Treves and Rolls 1992), enabling the hippocampus to store different memories of even similar events, and this prediction has been confirmed (Gilbert et al. 2001; Goodrich-Hunsaker et al. 2008; Kesner et al. 2012; Leutgeb and Leutgeb 2007; McHugh et al. 2007; Rolls 2008; Rolls and Kesner 2006) (“Pattern Separation Performed By Dentate Granule Cells”). Consistently with this evidence for pattern separation by dentate granule cells, in rats small changes in the shape of the environment in which rats are exploring can substantially alter the activity patterns among place-modulated granule cells (Leutgeb et al. 2007).
As just described, the dentate granule cells could be important in helping to build and prepare spatial representations for the CA3 network. The actual representation of space in the primate hippocampus includes a representation of spatial view (Georges-François et al. 1999; Robertson et al. 1998; Rolls et al. 1997a, 1998; Rolls and Xiang 2006), whereas in the rat hippocampus it is of the place where the rat is. The representation in the rat may be related to the fact that with a much less developed visual system than the primate, the rat’s representation of space may be defined more by the olfactory and tactile as well as distant visual cues present, and may thus tend to reflect the place where the rat is. However, the spatial representations in the rat and primate could arise from essentially the same computational process as follows (de Araujo et al. 2001; Rolls 1999). The starting assumption is that in both the rat and the primate, the dentate granule cells (and the CA3 and CA1 pyramidal cells) respond to combinations of the inputs received. In the case of the primate, a combination of visual features in the environment will, because of the fovea providing high spatial resolution over a typical viewing angle of perhaps 10–20 °, result in the formation of a spatial view cell, the effective trigger for which will thus be a combination of visual features within a relatively small part of space. In contrast, in the rat, given the very extensive visual field subtended by the rodent retina, which may extend over 180–270 °, a combination of visual features formed over such a wide visual angle would effectively define a position in space that is a place (de Araujo et al. 2001).
The entorhinal cortex contains grid cells, which have high firing in the rat in a two-dimensional spatial grid as a rat traverses an environment, with larger grid spacings in the ventral entorhinal cortex (Fyhn et al. 2004; Hafting et al. 2005). This may be a system optimized for path integration (McNaughton et al. 2006) which may self-organize during locomotion with longer time constants producing more widely spaced grids in the ventral entorhinal cortex (Kropff and Treves 2008). How are the grid cell representations, which would not be suitable for association of an object or reward with a place to form an episodic memory, transformed into a place representation that would be appropriate for this type of episodic memory? I have proposed that this could be implemented by a competitive network (see Rolls 2008) in the dentate gyrus which operates to form place cells, implemented by each dentate granule cell learning to respond to particular combinations of entorhinal cortex cells firing, where each combination effectively specifies a place, and this has been shown to be feasible computationally (Rolls et al. 2006). The sparse representations in the dentate gyrus, implemented by the mutual inhibition through inhibitory interneurons and competitive learning, help to implement this “pattern separation” effect (Rolls 1989b, c, 2008; Rolls and Treves 1998). The investigations showed that learning in the perforant path to dentate granule cell representation, and the sparse representation in the dentate granule cells, are both important in the formation of place-like fields in dentate granule cells from the grid cells in the entorhinal cortex (Georges-François et al. 1999; Robertson et al. 1998; Rolls et al. 1997a; 1998). To illustrate this, Fig. 4.3 shows from these simulations the responses of the simulated grid cells (a, b), the dentate receptive fields formed by feedforward connections and a sparse representation in the dentate gyrus (c, d), and the dentate receptive fields formed when Hebbian synaptic modification and training is included in the feedforward connections to implement competitive learning (e, f). It is only with the full competitive learning that the dentate receptive fields self-organized to become small place-like receptive fields (Rolls et al. 2006) similar to those found in the rat dentate granule cells.
In primates, there is now evidence that there is a grid-cell like representation in the entorhinal cortex, with neurons having grid-like firing as the monkey moves the eyes across a spatial scene (Killian et al. 2012). Similar competitive learning processes may transform these entorhinal cortex “spatial view grid cells” into hippocampal spatial view cells, and may help with the idiothetic (produced in this case by movements of the eyes) update of spatial view cells (Robertson et al. 1998). The presence of spatial view grid cells in the entorhinal cortex of primates (Killian et al., 2012) is of course predicted from the presence of spatial view cells in the primate CA3 and CA1 regions (Georges-François et al. 1999; Robertson et al. 1998; Rolls 2008; Rolls et al. 1997a, 1998; Rolls and Xiang 2006). Further support of this type of representation of space being viewed “out there” rather than where one is located as for rat place cells is that cells in the human parahippocampal cortex with spatial view-like properties have now been described (Ekstrom et al. 2003).
CA1 Cells and Pattern Completion Prior to Hippocampo-Directed Recall to the Neocortex
The CA3 cells connect to the CA1 cells by the Schaeffer collateral synapses. The associative modifiability in this connection helps the full information present in CA3 to be retrieved in the CA1 neurons (Rolls 1995; Schultz and Rolls 1999; Treves 1995; Treves and Rolls 1994). Part of the hypothesis is that the separate subparts of an episodic memory, which must be represented separately in CA3 to allow for completion, can be combined together by competitive learning in CA1 to produce an efficient retrieval cue for the recall via the backprojection pathways to the neocortex of memories stored in the neocortex (Rolls 1989a, b, 1995, 1996b; Treves and Rolls 1994). Associative recall in the CA3 to CA1 feedforward connections is a prominent property which implements what amounts to pattern completion (Rolls 1995, 2008; Schultz et al. 2000), though for pattern associators this process is usually described as generalization (Rolls 2008).
Backprojections to the Neocortex, and Memory Retrieval from the Hippocampus Involving Pattern Completion
The need for information to be retrieved from the hippocampus to affect other brain areas was noted in the Introduction. The way in which this could be implemented via backprojections to the neocortex (Rolls 1995, 1996b, 2008; 2010b; Treves and Rolls 1994) is considered here in the context of recalling a complete memory representation in the complete set of cortical areas that provide inputs to the hippocampus (see Fig. 4.1).
It is suggested that the modifiable connections from the CA3 neurons to the CA1 neurons allow the whole episode in CA3 to be produced in CA1. The CA1 neurons would then activate, via their termination in the deep layers of the entorhinal cortex, at least the pyramidal cells in the deep layers of the entorhinal cortex (see Fig. 4.1). These entorhinal cortex layer 5 neurons would then, by virtue of their backprojections (Lavenex and Amaral 2000; Witter et al. 2000a) to the parts of cerebral cortex that originally provided the inputs to the hippocampus, terminate in the superficial layers (including layer 1) of those neocortical areas, where synapses would be made onto the distal parts of the dendrites of the (superficial and deep) cortical pyramidal cells (Rolls 1989a, b, c). The areas of cerebral neocortex in which this recall would be produced could include multimodal cortical areas (e.g., the cortex in the superior temporal sulcus which receives inputs from temporal, parietal, and occipital cortical areas, and from which it is thought that cortical areas such as 39 and 40 related to language developed), and also areas of unimodal association cortex (e.g., inferior temporal visual cortex). The backprojections, by recalling previous episodic events, could provide information useful to the neocortex in the building of new representations in the multimodal and unimodal association cortical areas, which by building new long-term and structured representations can be considered as a form of memory consolidation (Rolls 1989a, b, c; 1990a; b, 2008), or in organizing actions.
The hypothesis of the architecture with which this would be achieved is shown in Fig. 4.1. The feedforward connections from association areas of the cerebral neocortex (solid lines in Fig. 4.1), show major convergence as information is passed to CA3, with the CA3 autoassociation network having the smallest number of neurons at any stage of the processing. The backprojections allow for divergence back to neocortical areas. The way in which I suggest that the backprojection synapses are set up to have the appropriate strengths for recall is as follows (Rolls 1989a, b, c). During the setting up of a new episodic memory, there would be strong feedforward activity progressing toward the hippocampus. During the episode, the CA3 synapses would be modified, and via the CA1 neurons and the subiculum, a pattern of activity would be produced on the backprojecting synapses to the entorhinal cortex. Here, the backprojecting synapses from active backprojection axons onto pyramidal cells being activated by the forward inputs to entorhinal cortex would be associatively modified. A similar process would be implemented at preceding stages of neocortex, that is in the parahippocampal gyrus/perirhinal cortex stage, and in association cortical areas, as shown in Fig. 4.1.
The concept is that during the learning of an episodic memory, cortical pyramidal cells in at least one of the stages would be driven by forward inputs, but would simultaneously be receiving backprojected activity (indirectly) from the hippocampus which would by pattern association from the backprojecting synapses to the cortical pyramidal cells become associated with whichever cortical cells were being made to fire by the forward inputs. Then later on, during recall, a recall cue from perhaps another part of cortex might reach CA3, where the firing during the original episode would be completed. The resulting backprojecting activity would then, as a result of the pattern association learned previously, bring back the firing in any cortical area that was present during the original episode. Thus, retrieval involves reinstating the activity that was present in different cortical areas that was present during the learning of an episode. (The pattern association is also called heteroassociation, to contrast it with autoassociation. The pattern association operates at multiple stages in the backprojection pathway, as made evident in Fig. 4.1). If the recall cue was an object, this might result in recall of the neocortical firing that represented the place in which that object had been seen previously. As noted elsewhere in this chapter and by McClelland et al. (1995), that recall might be useful to the neocortex to help it build new semantic memories, which might inherently be a slow process and is not a part of the theory of recall.
A plausible requirement for a successful hippocampo-directed recall operation, is that the signal generated from the hippocampally retrieved pattern of activity, and carried backward toward neocortex, remain undegraded when compared to the noise due, at each stage, to the interference effects caused by the concurrent storage of other patterns of activity on the same backprojecting synaptic systems. That requirement is equivalent to that used in deriving the storage capacity of such a series of heteroassociative memories, and it was shown by Treves and Rolls (1991, 1994) that the maximum number of independently generated activity patterns that can be retrieved is given, essentially, by the same formula as (3) above where, however, a is now the sparseness of the representation at any given stage, and C is the average number of (back-)projections each cell of that stage receives from cells of the previous one. (k′ is a similar slowly varying factor to that introduced above.) If p is equal to the number of memories held in the hippocampal memory, it is limited by the retrieval capacity of the CA3 network, p max. Putting together the formula for the latter with that shown here, one concludes that, roughly, the requirement implies that the number of afferents of (indirect) hippocampal origin to a given neocortical stage (C HBP), must be C HBP = C RC a nc/a CA3, where C RC is the number of recurrent collaterals to any given cell in CA3, the average sparseness of a representation is a nc, and a CA3 is the sparseness of memory representations there in CA3.
The above requirement is very strong: even if representations were to remain as sparse as they are in CA3, which is unlikely, to avoid degrading the signal, C HBP should be as large as C RC, that is, 12,000 in the rat. If then C HBP has to be of the same order as C RC, one is led to a very definite conclusion: A mechanism of the type envisaged here could not possibly rely on a set of monosynaptic CA3-to-neocortex backprojections. This would imply that, to make a sufficient number of synapses on each of the vast number of neocortical cells, each cell in CA3 has to generate a disproportionate number of synapses (i.e., C HBP times the ratio between the number of neocortical and that of CA3 cells). The required divergence can be kept within reasonable limits only by assuming that the backprojecting system is polysynaptic, provided that the number of cells involved grows gradually at each stage, from CA3 back to neocortical association areas (Treves and Rolls 1994) (cf. Fig. 4.1).
The theory of recall by the backprojections thus provides a quantitative account of why the cerebral cortex has as many backprojection as forward projection connections.
These concepts show how the backprojection system to neocortex can be conceptualized in terms of pattern completion, as follows. First, the information that is present when a memory is formed may be present in different areas of the cerebral cortex, for example of a face in a temporal cortex face area (Rolls 2012b), of a spatial location in a neocortical location area, and of a reward received in the orbitofrontal cortex (Rolls 2014). To achieve detailed retrieval of the memory, reinstatement of the activity during recall of the neuronal activity during the original memory formation may be needed. This is what the backprojection system described could achieve, and is a form of completion of the information that was represented in the different cortical areas when the memory was formed. Because such a wide set of different neocortical areas must be content addressed, a multistage feedback system is required, to keep the number of synapses per neuron in the backprojection pathways down to reasonable numbers. (Having CA1 directly address neocortical areas would require each CA1 neuron to have tens of millions of synapses with cortical neurons. That is the part of the computational problem solved by the multistage backprojection system shown in Fig. 4.1.) Second, the backprojection system with its series of pattern associators can each be thought of as performing a type of pattern completion.
Further aspects of the operation of the backprojecting systems are described elsewhere (Rolls 2008, 2016).
Tests of Pattern Separation and Pattern Completion
There is now a large literature on tests of pattern separation and pattern completion in the hippocampus (Giocomo et al. 2011; Hunsaker and Kesner 2008, 2013; Jezek et al. 2011; Kesner 2007, 2013; Kesner et al. 2012; Leutgeb et al. 2007; McHugh et al. 2007; Nakashiba et al. 2012; Nakazawa et al. 2002, 2003; Rolls and Kesner 2006; Wills et al. 2005), and a brief summary of some of the findings is provided next. An important point is that the theory (Rolls 1987, 1989a, b, c, 1990a, b, 1991, 1995, 1996b, 2008, 2010b, 2013; Rolls and Deco 2010; Kesner and Rolls 2015; Rolls and Treves 1998; Treves and Rolls 1991, 1992, 1994) is a quantitative theory of hippocampal function, and addresses how pattern separation and pattern completion are important in enabling the hippocampal system to operate up to capacity, which is in the order of tens of thousands of different memories. Some predictions from the theory may only hold when the system is well loaded, that is tested when the system is operating with thousands of memories, for then the pattern separation will be important. It is possible to test the predictions in simulations, where the system can be trained up to capacity (Rolls 1995, 2012a; Rolls et al. 1997b). In vivo, it may be useful to test the storage and recall of as many memories as possible, and in addition testing animals kept in environments where memories of the hippocampal type are needed may also help to test hypotheses in situations where the hippocampus has been at least moderately well loaded with many different memories.
Dentate Granule Cells
The theory predicts that pattern separation is performed by competitive learning by the dentate granule cells. Evidence consistent with this has been found neurophysiologically in the small sparsely encoded place fields of dentate neurons (Jung and McNaughton 1993; Leutgeb and Leutgeb 2007) and their reflection in CA3 neurons (Leutgeb and Leutgeb 2007). Further, and consistent with the theory, it has been shown that selective dentate lesions in rats (Gilbert and Kesner 2003; Gilbert et al. 2001; Goodrich-Hunsaker et al. 2008; Hunsaker and Kesner 2013; Kesner 2013; Rolls 2008; Kesner and Rolls 2015) or dentate NMDA receptor knockouts in mice (McHugh et al. 2007) impair spatial, object–place (or reward–place: Remembering where to find a reward) association tasks especially when the places are close together and require pattern separation before storage in CA3.
Mossy Fiber Inputs to CA3 and Learning
The theory predicts that the dentate granule cell mossy fiber system of inputs to the CA3 neurons is necessary to store spatial memories, but not to recall them. Lassalle et al. (2000) have obtained evidence consistent with this in rats with damage to the mossy fiber system (Lassalle et al. 2000), and there is further evidence consistent with this (Daumas et al. 2009; Lee and Kesner 2004; Rolls and Kesner 2006).
Perforant Path Inputs to CA3 and Recall
The theory predicts that the direct perforant path input from the entorhinal cortex to the CA3 cells (which bypasses the dentate granule cells) is involved in the recall of memory from the CA3 system, and Lee and Kesner (2004) have obtained evidence consistent with this in a Hebb–Williams maze recall task (Lee and Kesner 2004).
CA3 and Pattern Completion
The theory predicts that the CA3 system is especially important in object–place or reward–place tasks in which associations must be formed between any spatial location and any object (referred to as arbitrary associations). There is much evidence from subregion analyses involving disruption of CA3 that CA3 is necessary for arbitrary associations between places and objects or rewards (Gilbert and Kesner 2003; Hunsaker and Kesner 2013; Rolls and Kesner 2006). Similar impairments were obtained following deletion of CA3 NMDA receptors in mice in the acquisition of an odor–context paired associate learning task (Rajji et al. 2006). If place or time is not a component, associative tasks such as odor–object association are not impaired (Rolls and Kesner 2006), underlining the fact that the hippocampus is especially involved in episodic types of associative memory which typically involve place and/or time.
The theory predicts that the CA3 is especially important in object–place or reward–place completion tasks, in which associations must be completed from a part of the whole. It has been shown that if completion from an incomplete cue is needed, then CA3 NMDA receptors are necessary (presumably to ensure satisfactory CA3–CA3 learning) even in a reference memory task (Gold and Kesner 2005; Hunsaker and Kesner 2013; Nakazawa et al. 2002).
The theory predicts that the CA3 system is especially needed in rapid, one-trial object–place, learning and recall. It has been shown that hippocampal NMDA receptors (necessary for long-term potentiation to occur) are needed for one-trial flavor–place association learning, and that hippocampal AMPA/kainate receptors are sufficient for the recall, though the hippocampal subregion involved was not tested (Day et al. 2003). In subregion studies, Kesner and colleagues have shown that CA3 lesions produce chance performance on a one-trial object–place recall task (Kesner et al. 2008) and other object–spatial tasks (Kesner and Rolls 2001; Rolls and Kesner 2006). For example, CA3 lesions produced chance performance on both a one-trial object–place recall and place–object recall task (Kesner et al. 2008). This is evidence that CA3 supports arbitrary associations as well as episodic memory based on 1-trial learning. A control fixed visual conditional to place task with the same delay was not impaired, showing that it is recall after one-trial (or rapid, episodic) learning that is impaired (Kesner et al. 2008). CA3 NMDA receptors are as predicted by the theory necessary for rapid/one-trial spatial learning, as shown by a mouse knockout study by Nakazawa, Tonegawa and colleagues (Nakazawa et al. 2004, 2003; Tonegawa et al. 2003). We have shown that hippocampal CA3 neurons reflect the computational processes necessary for one-trial object–place event memory, used as a model for episodic memory (Rolls and Xiang 2006).
Another type of test of the autoassociation (or attractor) hypothesis for CA3 has been to train rats in different environments, for example, a square and a circular environment, and then test the prediction of the hypothesis that when presented with an environment ambiguous between these, hippocampal neurons will fall into an attractor state that represents one of the two previously learned environments, but not a mixture of the two environments. Evidence consistent with the hypothesis has been found (Wills et al. 2005). In a particularly dramatic example, it has been found that within each theta cycle, hippocampal pyramidal neurons may represent one or other of the learned environments (Jezek et al. 2011). This is an indication, predicted by Rolls and Treves (1998), that autoassociative memory recall can take place sufficiently rapidly to be complete within one theta cycle (120 ms), and that theta cycles could provide a mechanism for a fresh retrieval process to occur after a reset caused by the inhibitory part of each theta cycle, so that the memory can be updated rapidly to reflect a continuously changing environment, and not remain too long in an attractor state.
Evidence that the firing of hippocampal pyramidal cells in macaques is more sparse than in neocortical areas is described in “Sparseness of the CA3 Cell Representation and Pattern Separation.” This is consistent with the premium placed in the hippocampus for storing and retrieving large numbers of independent memories.
The theory predicts that if primates including humans can form an episodic memory in which objects or people are seen at particular locations even though the observer viewing the space has never been to those locations “out there” in space, there should be a neural system in CA3 that can support such associations between places “out there” in a scene and objects. Exactly this is provided by the spatial view neurons Rolls and colleagues have discovered that are present in primate CA3 (Georges-François et al. 1999; Robertson et al. 1998; Rolls et al. 1997a, 1998; Rolls and Xiang 2005, 2006; Rolls et al. 2005). Place cells will not do for this type of episodic memory (Rolls 2010b, 2013).
Recall Via CA1 to Neocortex: A Reverse Hierarchy of Pattern Associators Each Performing Pattern Completion
The theory shows quantitatively, analytically, how memories could be retrieved from the hippocampus to the neocortex (Treves and Rolls 1994), and this has been shown by simulation of the multistage hippocampal system including the entorhinal cortex, dentate, CA3, CA1, and return to the entorhinal cortex to recall the memory to be quantitatively realistic (Rolls 1995).
It has been shown that after learning in hippocampal-dependent tasks, neocortical representations may change (Schwindel and McNaughton 2011). Although this has been interpreted as the transfer of memories from the hippocampus to the neocortex (Schwindel and McNaughton 2011), it should be noted that if the hippocampal representation changes as a result of learning, then the altered representation in CA1 will, even with fixed synaptic connections back to neocortex, alter neocortical firing, with no learning or actual “transfer” involved. (This occurs whenever one vector of neuronal firing changes and influences another vector of neuronal firing through fixed connections.) It has also been suggested that the transfer of information from the hippocampus to the neocortex occurs especially during sleep (Marr 1971; Schwindel and McNaughton 2011). My own view is that during waking would be the best time to retrieve a memory from the hippocampus to the neocortex by using the hippocampus to retrieve the complete episodic memory from a fragment. The retrieval would reinstate the neocortical activity present when the event was originally learned. The retrieved information now present in the neocortex could then be used to build new semantic memories, for example, a narrative account of all the events that took place on one’s fifth birthday party. During waking the building of semantic representations could be guided and organized by rational thought into useful semantic representations. To do this during sleep would run the risk of forming bizarre semantic representations of the type that we dream about during the unguided noise-driven stochastic firing during sleep (Rolls 2008; Rolls and Deco 2010). Further, the active recall during waking of memories from the hippocampus means that mainly relevant or useful memories would be retrieved from the hippocampus (not useless memories such as where one parked one’s bicycle two weeks ago), and only these memories would tend to become incorporated into useful long-term semantic representations, allowing memories not retrieved from the hippocampus to be overwritten by new memories in the process of forgetting that involves using CA3 sets of neurons chosen at random for new episodic memories (Rolls 2008).
Many further tests of the theory are described elsewhere (Hunsaker and Kesner 2013; Kesner et al. 2012; Rolls 2008, 2010b; Rolls and Kesner 2006). The theory has recently been extended to temporal order memory and temporal pattern separation (Rolls 2010b, 2013), which are also related to hippocampal function (Hoge and Kesner 2007; Kesner et al. 2002; Kesner and Rolls 2015).
References
Acsady, L., Kamondi, A., Sik, A., Freund, T., & Buzsaki, G. (1998). GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. The Journal of Neuroscience, 18(9), 3386–3403.
Amaral, D. G. (1987). Memory: Anatomical organization of candidate brain regions. In V. B. Mountcastle (Ed.), Handbook of physiology. section 1, the nervous system (Vol. V, higher functions of the brain, pp. 211–294). Washington DC: American Physiological Society.
Amaral, D. G. (1993). Emerging principles of intrinsic hippocampal organisation. Current Opinion in Neurobiology, 3, 225–229.
Amaral, D. G., & Witter, M. P. (1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience, 31, 571–591.
Amaral, D. G., & Witter, M. P. (1995). The hippocampal formation. In G. Paxinos (Ed.), The rat nervous system (pp. 443–493). San Diego: Academic.
Amaral, D. G., Ishizuka, N., & Claiborne, B. (1990). Neurons, numbers and the hippocampal network. Progress in Brain Research, 83, 1–11.
Amaral, D. G., Price, J. L., Pitkanen, A., & Carmichael, S. T. (1992). Anatomical organization of the primate amygdaloid complex. In J. P. Aggleton (Ed.), The amygdala (pp. 1–66). New York: Wiley-Liss.
Amari, S. (1977). Dynamics of pattern formation in lateral-inhibition type neural fields. Biological Cybernetics, 27, 77–87.
Amit, D. J. (1989). Modeling brain function. Cambridge: Cambridge University Press.
Andersen, P., Morris, R. G. M., Amaral, D. G., Bliss, T. V. P., & O’Keefe, J. (2007). The hippocampus book. London: Oxford University Press.
Barkas, L. J., Henderson, J. L., Hamilton, D. A., Redhead, E. S., & Gray, W. P. (2010). Selective temporal resections and spatial memory impairment: Cue dependent lateralization effects. Behavioural Brain Research, 208(2), 535–544. doi:10.1016/j.bbr.2009.12.035.
Battaglia, F. P., & Treves, A. (1998). Attractor neural networks storing multiple space representations: A model for hippocampal place fields. Physical Review E, 58, 7738–7753.
Bonelli, S. B., Powell, R. H., Yogarajah, M., Samson, R. S., Symms, M. R., Thompson, P. J., Duncan, J. S., et al. (2010). Imaging memory in temporal lobe epilepsy: Predicting the effects of temporal lobe resection. Brain, 133(Pt 4), 1186–1199. doi:10.1093/brain/awq006.
Brown, T. H., Ganong, A. H., Kairiss, E. W., Keenan, C. L., & Kelso, S. R. (Eds.). (1989). Long-term potentiation in two synaptic systems of the hippocampal brain slice. San Diego: Academic.
Brown, T. H., Kairiss, E. W., & Keenan, C. L. (1990). Hebbian synapses: Biophysical mechanisms and algorithms. Annual Review of Neuroscience, 13, 475–511.
Brun, V. H., Otnass, M. K., Molden, S., Steffenach, H. A., Witter, M. P., Moser, M. B., & Moser, E. I. (2002). Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science, 296, 2243–2246.
Carmichael, S. T., & Price, J. L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology, 346, 403–434.
Cerasti, E., & Treves, A. (2010). How informative are spatial CA3 representations established by the dentate gyrus? PLoS Computational Biology, 6(4), e1000759.
Cerasti, E., & Treves, A. (2013). The spatial representations acquired in CA3 by self-organizing recurrent connections. Frontiers in Cellular Neuroscience, 7, 112.
Cheng, S. (2013). The CRISP theory of hippocampal function in episodic memory. Frontiers in Neural Circuits, 7, 88.
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D., Jr., Fragniere, A., Tyers, P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325(5937), 210–213.
Daumas, S., Ceccom, J., Halley, H., Frances, B., & Lassalle, J. M. (2009). Activation of metabotropic glutamate receptor type 2/3 supports the involvement of the hippocampal mossy fiber pathway on contextual fear memory consolidation. Learning and Memory, 16(8), 504–507.
Day, M., Langston, R., & Morris, R. G. (2003). Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature, 424, 205–209.
de Araujo, I. E. T., Rolls, E. T., & Stringer, S. M. (2001). A view model which accounts for the spatial fields of hippocampal primate spatial view cells and rat place cells. Hippocampus, 11, 699–706.
Delatour, B., & Witter, M. P. (2002). Projections from the parahippocampal region to the prefrontal cortex in the rat: Evidence of multiple pathways. The European Journal of Neuroscience, 15, 1400–1407.
Dere, E., Easton, A., Nadel, L., & Huston, J. P. (Eds.). (2008). Handbook of episodic memory. Amsterdam: Elsevier.
Ekstrom, A. D., Kahana, M. J., Caplan, J. B., Fields, T. A., Isham, E. A., Newman, E. L., & Fried, I. (2003). Cellular networks underlying human spatial navigation. Nature, 425, 184–188.
Fazeli, M. S., & Collingridge, G. L. (Eds.). (1996). Cortical plasticity: LTP and LTD. Oxford: Bios.
Florian, C., & Roullet, P. (2004). Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behavioural Brain Research, 154, 365–374.
Franco, L., Rolls, E. T., Aggelopoulos, N. C., & Jerez, J. M. (2007). Neuronal selectivity, population sparseness, and ergodicity in the inferior temporal visual cortex. Biological Cybernetics, 96, 547–560.
Fyhn, M., Molden, S., Witter, M. P., Moser, E. I., & Moser, M. B. (2004). Spatial representation in the entorhinal cortex. Science, 305, 1258–1264.
Georges-François, P., Rolls, E. T., & Robertson, R. G. (1999). Spatial view cells in the primate hippocampus: Allocentric view not head direction or eye position or place. Cerebral Cortex, 9, 197–212.
Gilbert, P. E., & Kesner, R. P. (2003). Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behavioral Neuroscience, 117, 1385–1394.
Gilbert, P. E., Kesner, R. P., & Lee, I. (2001). Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus, 11, 626–636.
Giocomo, L. M., & Hasselmo, M. E. (2007). Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Molecular Neurobiology, 36(2), 184–200.
Giocomo, L. M., Moser, M. B., & Moser, E. I. (2011). Computational models of grid cells. Neuron, 71(4), 589–603. doi:S0896-6273(11)00650-7[pii]10.1016/j.neuron.2011.07.023.
Gold, A. E., & Kesner, R. P. (2005). The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus, 15, 808–814.
Goodrich-Hunsaker, N. J., Hunsaker, M. R., & Kesner, R. P. (2008). The interactions and dissociations of the dorsal hippocampus subregions: How the dentate gyrus, CA3, and CA1 process spatial information. Behavioral Neuroscience, 122(1), 16–26.
Hafting, T., Fyhn, M., Molden, S., Moser, M. B., & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436, 801–806.
Hasselmo, M. E., Schnell, E., & Barkai, E. (1995). Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. The Journal of Neurosciences, 15, 5249–5262.
Henze, D. A., Wittner, L., & Buzsaki, G. (2002). Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nature Neuroscience, 5(8), 790–795.
Hertz, J., Krogh, A., & Palmer, R. G. (1991). An introduction to the theory of neural computation. Wokingham: Addison-Wesley.
Hoge, J., & Kesner, R. P. (2007). Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiology of Learning and Memory, 88(2), 225–231.
Hopfield, J. J. (1982). Neural networks and physical systems with emergent collective computational abilities. Proceedings of the National Academy of Science U S A, 79, 2554–2558.
Hunsaker, M. R., & Kesner, R. P. (2008). Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus, 18(9), 955–964.
Hunsaker, M. R., & Kesner, R. P. (2013). The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and Biobehavioral Reviews, 37(1), 36–58. doi:10.1016/j.neubiorev.2012.09.014.
Ishizuka, N., Weber, J., & Amaral, D. G. (1990). Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. Journal of Comparative Neurology, 295, 580–623.
Jackson, M. B. (2013). Recall of Spatial Patterns Stored in a Hippocampal Slice By Long-Term Potentiation. Journal of Neurophysiology. doi:10.1152/jn.00533.2013.
Jezek, K., Henriksen, E. J., Treves, A., Moser, E. I., & Moser, M.-B. (2011). Theta-paced flickering between place-cell maps in the hippocampus. Nature, 278, 246–249.
Jung, M. W., & McNaughton, B. L. (1993). Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus, 3, 165–182.
Kesner, R. P. (2007). Behavioral functions of the CA3 subregion of the hippocampus. Learning and Memory, 14(11), 771–781. doi:10.1101/lm.688207.
Kesner, R. P. (2013). An analysis of the dentate gyrus function. Behavioural Brain Research, 254, 1–7. doi:10.1016/j.bbr.2013.01.012.
Kesner, R. P., & Rolls, E. T. (2001). Role of long term synaptic modification in short term memory. Hippocampus, 11, 240–250.
Kesner, R. P., & Rolls, E. T. (2015). A computational theory of hippocampal function, and tests of the theory: new developments. Neuroscience and Biobehavioral Reviews 48, 92–147.
Kesner, R. P., Gilbert, P. E., & Barua, L. A. (2002). The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience, 116, 286–290.
Kesner, R. P., Hunsaker, M. R., & Warthen, M. W. (2008). The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behavioral Neuroscience, 122(6), 1217–1225.
Kesner, R. P., Morris, A. M., & Weeden, C. S. S. (2012). Spatial, temporal, and associative behavioral functions associated with different subregions of the hippocampus. In T. R. Zentall & E. A. Wasserman (Eds.), Oxford handbook of comparative cognition (pp. 322–346). Oxford: Oxford University Press.
Killian, N. J., Jutras, M. J., & Buffalo, E. A. (2012). A map of visual space in the primate entorhinal cortex. Nature, 491(7426), 761–764. doi:10.1038/nature11587.
Kohonen, T. (1977). Associative memory: A system theoretical approach. New York: Springer.
Kohonen, T. (1984). Self-organization and associative memory. Berlin: Springer-Verlag.
Kohonen, T., Oja, E., & Lehtio, P. (1981). Storage and processing of information in distributed memory systems. In G. E. Hinton & J. A. Anderson (Eds.), Parallel models of associative memory (pp. 129–167). Hillsdale: Lawrence Erlbaum.
Kondo, H., Lavenex, P., & Amaral, D. G. (2009). Intrinsic connections of the macaque monkey hippocampal formation: II. CA3 connections. Journal of Comparative Neurology, 515(3), 349–377.
Kropff, E., & Treves, A. (2008). The emergence of grid cells: Intelligent design or just adaptation? Hippocampus, 18(12), 1256–1269.
Lassalle, J. M., Bataille, T., & Halley, H. (2000). Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiology of Learning and Memory, 73, 243–257.
Lavenex, P., & Amaral, D. G. (2000). Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus, 10, 420–430.
Lavenex, P., Suzuki, W. A., & Amaral, D. G. (2004). Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. Journal of Comparative Neurology, 472, 371–394.
Lee, I., & Kesner, R. P. (2004). Encoding versus retrieval of spatial memory: Double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus, 14, 66–76.
Leutgeb, S., & Leutgeb, J. K. (2007). Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning and Memory, 14(11), 745–757.
Leutgeb, J. K., Leutgeb, S., Moser, M. B., & Moser, E. I. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961–966.
Levy, W. B. (1989). A computational approach to hippocampal function. In R. D. Hawkins & G. H. Bower (Eds.), Computational models of learning in simple neural systems (pp. 243–305). San Diego: Academic.
Lynch, M. A. (2004). Long-term potentiation and memory. Psychological Review, 84, 87–136.
Marr, D. (1971). Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 262, 23–81.
McClelland, J. L., McNaughton, B. L., & O’Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102, 419–457.
McHugh, T. J., Jones, M. W., Quinn, J. J., Balthasar, N., Coppari, R., Elmquist, J. K., et al. (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science, 317(5834), 94–99.
McNaughton, B. L. (1991). Associative pattern completion in hippocampal circuits: New evidence and new questions. Brain Research Review, 16, 193–220.
McNaughton, B. L., & Morris, R. G. M. (1987). Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences, 10(10), 408–415.
McNaughton, B. L., & Nadel, L. (1990). Hebb-Marr networks and the neurobiological representation of action in space. In M. A. Gluck & D. E. Rumelhart (Eds.), Neuroscience and connectionist theory (pp. 1–64). Hillsdale: Erlbaum.
McNaughton, B. L., Barnes, C. A., & O’Keefe, J. (1983). The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Experimental Brain Research, 52, 41–49.
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I., & Moser, M.-B. (2006). Path integration and the neural basis of the ‘cognitive map.’ Nature Reviews Neuroscience, 7, 663–678.
Morris, R. G. M. (1989). Does synaptic plasticity play a role in information storage in the vertebrate brain? In R. G. M. Morris (Ed.), Parallel distributed processing: Implications for psychology and neurobiology (pp. 248–285). Oxford: Oxford University Press.
Morris, R. G. (2003). Long-term potentiation and memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 643–647.
Morris, R. G., Moser, E. I., Riedel, G., Martin, S. J., Sandin, J., Day, M., & O’Carroll, C. (2003). Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 773–786.
Moscovitch, M., Rosenbaum, R. S., Gilboa, A., Addis, D. R., Westmacott, R., Grady, C., et al. (2005). Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. Journal of Anatomy, 207, 35–66.
Naber, P. A., Lopes da Silva, F. H., & Witter, M. P. (2001). Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus, 11, 99–104.
Nakashiba, T., Cushman, J. D., Pelkey, K. A., Renaudineau, S., Buhl, D. L., McHugh, T. J., et al. (2012). Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell, 149(1), 188–201. doi:S0092-8674(12)00157-2[pii]10.1016/j.cell.2012.01.046.
Nakazawa, K., Quirk, M. C., Chitwood, R. A., Watanabe, M., Yeckel, M. F., Sun, L. D., et al. (2002). Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science, 297, 211–218.
Nakazawa, K., Sun, L. D., Quirk, M. C., Rondi-Reig, L., Wilson, M. A., & Tonegawa, S. (2003). Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron, 38, 305–315.
Nakazawa, K., McHugh, T. J., Wilson, M. A., & Tonegawa, S. (2004). NMDA receptors, place cells and hippocampal spatial memory. Nature Reviews Neuroscience, 5, 361–372.
O’Keefe, J. (1979). A review of the hippocampal place cells. Progress in Neurobiology, 13, 419–439.
O’Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon Press.
O’ Keefe, J., & Speakman, A. (1987). Single unit activity in the rat hippocampus during a spatial memory task. Experimental Brain Research, 68, 1–27.
Pitkanen, A., Kelly, J. L., & Amaral, D. G. (2002). Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus, 12, 186–205.
Rajji, T., Chapman, D., Eichenbaum, H., & Greene, R. (2006). The role of CA3 hippocampal NMDA receptors in paired associate learning. The Journal of Neurosciences, 26(3), 908–915.
Robertson, R. G., Rolls, E. T., & Georges-François, P. (1998). Spatial view cells in the primate hippocampus: Effects of removal of view details. Journal of Neurophysiology, 79, 1145–1156.
Rolls, E. T. (1987). Information representation, processing and storage in the brain: Analysis at the single neuron level. In J.-P. Changeux & M. Konishi (Eds.), The neural and molecular bases of learning (pp. 503–540). Chichester: Wiley.
Rolls, E. T. (1989a). Functions of neuronal networks in the hippocampus and cerebral cortex in memory. In R. M. J. Cotterill (Ed.), Models of brain function (pp. 15–33). Cambridge: Cambridge University Press.
Rolls, E. T. (1989b). Functions of neuronal networks in the hippocampus and neocortex in memory. In J. H. Byrne & W. O. Berry (Eds.), Neural models of plasticity: Experimental and theoretical approaches (pp. 240–265). San Diego: Academic.
Rolls, E. T. (1989c). The representation and storage of information in neuronal networks in the primate cerebral cortex and hippocampus. In R. Durbin, C. Miall, & G. Mitchison (Eds.), The computing neuron (pp. 125–159). Wokingham: Addison-Wesley.
Rolls, E. T. (1990a). Functions of the primate hippocampus in spatial processing and memory. In D. S. Olton & R. P. Kesner (Eds.), Neurobiology of comparative cognition (pp. 339–362). Hillsdale: L. Erlbaum.
Rolls, E. T. (1990b). Theoretical and neurophysiological analysis of the functions of the primate hippocampus in memory. Cold Spring Harbor Symposia in Quantitative Biology, 55, 995–1006.
Rolls, E. T. (1991). Functions of the primate hippocampus in spatial and non-spatial memory. Hippocampus, 1, 258–261.
Rolls, E. T. (1995). A model of the operation of the hippocampus and entorhinal cortex in memory. International Journal of Neural Systems, 6, 51–70.
Rolls, E. T. (1996a). Roles of long term potentiation and long term depression in neuronal network operations in the brain. In M. S. Fazeli & G. L. Collingridge (Eds.), Cortical plasticity (pp. 223–250). Oxford: Bios.
Rolls, E. T. (1996b). A theory of hippocampal function in memory. Hippocampus, 6, 601–620.
Rolls, E. T. (1999). Spatial view cells and the representation of place in the primate hippocampus. Hippocampus, 9, 467–480.
Rolls, E. T. (2008). Memory, attention, and decision-making: A unifying computational neuroscience approach. Oxford: Oxford University Press.
Rolls, E. T. (2010a). Attractor networks. WIREs Cognitive Science, 1i, 119–134.
Rolls, E. T. (2010b). A computational theory of episodic memory formation in the hippocampus. Behavioural Brain Research, 205, 180–196.
Rolls, E. T. (2012a). Advantages of dilution in the connectivity of attractor networks in the brain. Biologically Inspired Cognitive Architectures, 1, 44–54. doi: 10.1016/j.bica.2012.03.003.
Rolls, E. T. (2012b). Invariant visual object and face recognition: Neural and computational bases, and a model, VisNet. Frontiers in Computational Neuroscience, 6, 35, 1–70.
Rolls, E. T. (2013). A quantitative theory of the functions of the hippocampal CA3 network in memory. Frontiers in Cellular Neuroscience, 7, 98. doi:10.3389/fncel.2013.00098.
Rolls, E. T. (2014). Emotion and decision-making explained. Oxford: Oxford University Press.
Rolls, E. T. (2016). Cerebral cortex: Principles of operation. Oxford: Oxford University Press.
Rolls, E. T., & Deco, G. (2002). Computational neuroscience of vision. Oxford: Oxford University Press.
Rolls, E. T., & Deco, G. (2010). The noisy brain: Stochastic dynamics as a principle of brain function. Oxford: Oxford University Press.
Rolls, E. T., & Kesner, R. P. (2006). A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology, 79, 1–48.
Rolls, E. T., & Stringer, S. M. (2000). On the design of neural networks in the brain by genetic evolution. Progress in Neurobiology, 61, 557–579.
Rolls, E. T., & Stringer, S. M. (2005). Spatial view cells in the hippocampus, and their idiothetic update based on place and head direction. Neural Networks, 18, 1229–1241.
Rolls, E. T., & Treves, A. (1990). The relative advantages of sparse versus distributed encoding for associative neuronal networks in the brain. Network, 1, 407–421.
Rolls, E. T., & Treves, A. (1998). Neural networks and brain function. Oxford: Oxford University Press.
Rolls, E. T., & Treves, A. (2011). The neuronal encoding of information in the brain. Progress in Neurobiology, 95, 448–490.
Rolls, E. T., & Webb, T. J. (2012). Cortical attractor network dynamics with diluted connectivity. Brain Research, 1434, 212–225.
Rolls, E. T., & Xiang, J.-Z. (2005). Reward-spatial view representations and learning in the hippocampus. Journal of Neuroscience, 25, 6167–6174.
Rolls, E. T., & Xiang, J.-Z. (2006). Spatial view cells in the primate hippocampus, and memory recall. Reviews in the Neurosciences, 17, 175–200.
Rolls, E. T., Robertson, R. G., & Georges-François, P. (1997a). Spatial view cells in the primate hippocampus. European Journal of Neuroscience, 9, 1789–1794.
Rolls, E. T., Treves, A., Foster, D., & Perez-Vicente, C. (1997b). Simulation studies of the CA3 hippocampal subfield modelled as an attractor neural network. Neural Networks, 10, 1559–1569.
Rolls, E. T., Treves, A., Robertson, R. G., Georges-François, P., & Panzeri, S. (1998). Information about spatial view in an ensemble of primate hippocampal cells. Journal of Neurophysiology, 79, 1797–1813.
Rolls, E. T., Stringer, S. M., & Trappenberg, T. P. (2002). A unified model of spatial and episodic memory. Proceedings of the Royal Society of London B, 269, 1087–1093.
Rolls, E. T., Xiang, J.-Z., & Franco, L. (2005). Object, space and object-space representations in the primate hippocampus. Journal of Neurophysiology, 94, 833–844.
Rolls, E. T., Stringer, S. M., & Elliot, T. (2006). Entorhinal cortex grid cells can map to hippocampal place cells by competitive learning. Network: Computation in Neural Systems, 17, 447–465.
Rolls, E. T., Dempere-Marco, L., & Deco, G. (2013). Holding multiple items in short term memory: A neural mechanism. PLoS One, 8, e61078.
Rondi-Reig, L., Libbey, M., Eichenbaum, H., & Tonegawa, S. (2001). CA1-specific N-methyl-D-aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task. Proceedings of the National Academy of Sciences of the United States of America, 98, 3543–3548.
Samsonovich, A., & McNaughton, B. L. (1997). Path integration and cognitive mapping in a continuous attractor neural network model. Journal of Neuroscience, 17, 5900–5920.
Schultz, S., & Rolls, E. T. (1999). Analysis of information transmission in the Schaffer collaterals. Hippocampus, 9, 582–598.
Schultz, S., Panzeri, S., Rolls, E. T., & Treves, A. (2000). Quantitive model analysis of a Schaffer collateral model. In P. Hancock, P. Foldiak, & R. Baddeley (Eds.), Information theory and the brain (pp. 257–272,Chap. 14). Cambridge: Cambridge University Press.
Schwindel, C. D., & McNaughton, B. L. (2011). Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Progress in Brain Research, 193, 163–177. doi:B978-0-444-53839-0.00011-9[pii]10.1016/B978-0-444-53839-0.00011-9.
Sidhu, M. K., Stretton, J., Winston, G. P., Bonelli, S., Centeno, M., Vollmar, C., et al. (2013). A functional magnetic resonance imaging study mapping the episodic memory encoding network in temporal lobe epilepsy. Brain, 136, 1868–1888. doi:10.1093/brain/awt099.
Simmen, M. W., Treves, A., & Rolls, E. T. (1996). Pattern retrieval in threshold-linear associative nets. Network, 7, 109–122.
Stefanacci, L., Suzuki, W. A., & Amaral, D. G. (1996). Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology, 375, 552–582.
Stella, F., Cerasti, E., & Treves, A. (2013). Unveiling the metric structure of internal representations of space. Frontiers in Neural Circuits, 7, 81. doi:10.3389/fncir.2013.00081.
Storm-Mathiesen, J., Zimmer, J., & Ottersen, O. P. (Eds.). (1990). Understanding the brain through the hippocampus (Vol. 83). Oxford: Elsevier.
Stringer, S. M., & Rolls, E. T. (2002). Invariant object recognition in the visual system with novel views of 3D objects. Neural Computation, 14, 2585–2596.
Stringer, S. M., & Rolls, E. T. (2006). Self-organizing path integration using a linked continuous attractor and competitive network: Path integration of head direction. Network: Computation in Neural Systems, 17, 419–445.
Stringer, S. M., Rolls, E. T., Trappenberg, T. P., & Araujo, I. E. T. (2002a). Self-organizing continuous attractor networks and path integration. Two-dimensional models of place cells. Network: Computation in Neural Systems, 13, 429–446.
Stringer, S. M., Trappenberg, T. P., Rolls, E. T., & Araujo, I. E. T. (2002b). Self-organizing continuous attractor networks and path integration: One-dimensional models of head direction cells. Network: Computation in Neural Systems, 13, 217–242.
Stringer, S. M., Rolls, E. T., & Trappenberg, T. P. (2004). Self-organising continuous attractor networks with multiple activity packets, and the representation of space. Neural Networks, 17, 5–27.
Stringer, S. M., Rolls, E. T., & Trappenberg, T. P. (2005). Self-organizing continuous attractor network models of hippocampal spatial view cells. Neurobiology of Learning and Memory, 83, 79–92.
Suzuki, W. A., & Amaral, D. G. (1994a). Perirhinal and parahippocampal cortices of the macaque monkey—cortical afferents. Journal of Comparative Neurology, 350(4), 497–533.
Suzuki, W. A., & Amaral, D. G. (1994b). Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. Journal of Neuroscience, 14, 1856–1877.
Taylor, J. G. (1999). Neural “bubble” dynamics in two dimensions: Foundations. Biological Cybernetics, 80, 393–409.
Tonegawa, S., Nakazawa, K., & Wilson, M. A. (2003). Genetic neuroscience of mammalian learning and memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 787–795.
Treves, A. (1990). Graded-response neurons and information encodings in autoassociative memories. Physical Review A, 42, 2418–2430.
Treves, A. (1991). Dilution and sparse coding in threshold-linear nets. Journal of Physics A, 24, 327–335.
Treves, A. (1995). Quantitative estimate of the information relayed by Schaffer collaterals. Journal of Computational Neuroscience, 2, 259–272.
Treves, A., & Rolls, E. T. (1991). What determines the capacity of autoassociative memories in the brain? Network, 2, 371–397.
Treves, A., & Rolls, E. T. (1992). Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus, 2, 189–199.
Treves, A., & Rolls, E. T. (1994). A computational analysis of the role of the hippocampus in memory. Hippocampus, 4, 374–391.
van Haeften, T., Baks-te-Bulte, L., Goede, P. H., Wouterlood, F. G., & Witter, M. P. (2003). Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus, 13, 943–952.
Van Hoesen, G. W. (1982). The parahippocampal gyrus. New observations regarding its cortical connections in the monkey. Trends in Neuroscience, 5, 345–350.
Walters, D. M., Stringer, S. M., & Rolls, E. T. (2013). Path integration of head direction: Updating a packet of neural activity at the correct speed using axonal conduction delays. PLoS One, 8, e58330.
Wang, S. H., & Morris, R. G. (2010). Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annual Review of Psychology, 61, 49–79, C41–44.
Webb, T., Rolls, E. T., Deco, G., & Feng, J. (2011). Noise in attractor networks in the brain produced by graded firing rate representations. PLoS One, 6, e23630.
Wills, T. J., Lever, C., Cacucci, F., Burgess, N., & O’Keefe, J. (2005). Attractor dynamics in the hippocampal representation of the local environment. Science, 308, 873–876.
Witter, M. P. (1993). Organization of the entorhinal-hippocampal system: A review of current anatomical data. Hippocampus, 3, 33–44.
Witter, M. P. (2007). Intrinsic and extrinsic wiring of CA3: Indications for connectional heterogeneity. Learning and Memory, 14(11), 705–713.
Witter, M. P., Naber, P. A., van Haeften, T., Machielsen, W. C., Rombouts, S. A., Barkhof, F., et al. (2000a). Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus, 10, 398–410.
Witter, M. P., Wouterlood, F. G., Naber, P. A., & Van Haeften, T. (2000b). Anatomical organization of the parahippocampal-hippocampal network. Annals of the New York Academy of Sciences, 911, 1–24.
Zhang, K. (1996). Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: A theory. Journal of Neuroscience, 16, 2112–2126.
Zilli, E. A. (2012). Models of grid cell spatial firing published 2005–2011. Front Neural Circuits, 6, 16. doi:10.3389/fncir.2012.00016.
Acknowledgments
Different parts of the research described here were supported by Program Grants from the Medical Research Council, by a Human Frontier Science Program Grant, by an EEC BRAIN grant, by the MRC Oxford Interdisciplinary Research Centre in Cognitive Neuroscience, by the Oxford McDonnell-Pew Centre in Cognitive Neuroscience, and by the Oxford Centre for Computational Neuroscience. The author has performed the experimental and theoretical work which is incorporated in some of the ideas presented here on the hippocampus with many colleagues, including Alessandro Treves, Simon Stringer, Ray Kesner, Robert Robertson, Pierre Georges-François, Shane O’Mara, and Alain Berthoz, and their contributions are sincerely acknowledged. Pdfs of some of the papers cited are available at www.oxcns.org.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rolls, E. (2016). Pattern Completion and Pattern Separation Mechanisms in the Hippocampus. In: Jackson, P., Chiba, A., Berman, R., Ragozzino, M. (eds) The Neurobiological Basis of Memory. Springer, Cham. https://doi.org/10.1007/978-3-319-15759-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-15759-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15758-0
Online ISBN: 978-3-319-15759-7
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)