Abstract
Chronic immune activation is encountered in different pathologies including granulomatous and functional bowel diseases, cancer, aging, atherosclerosis, and obesity. Persistence of chronic inflammatory stimuli over time creates a biologic background for immunosenescence and favors neopterin formation with the enhanced tryptophan (Trp) degradation in diseases concomitant with cellular immune activation. Trp degradation leads to the generation of several neuroactive compounds by three distinct pathways.
Indoleamine 2,3-dioxygenase (IDO) induction leads to many complex changes within the affected cells resulting in immunosuppression through breakdown of Trp. Thus, neopterin concentrations as well as IDO expression significantly increase in inflammatory bowel diseases (IBD) such as ulcerative colitis and Crohn’s disease.
However, irritable bowel syndrome (IBS) is linked with abnormal serotonin functioning and immune activation. In this case, enteric serotonin (5-HT) signaling may be defective and inactivated by the serotonin-selective reuptake transporter (SERT) in the enterocytes. A positive correlation is evident between IBS severity and kynurenine (Kyn) to Trp ratio which is significantly correlated with the rise of interferon (IFN)-gamma.
The dual host-protective and tumor-promoting actions of immunity are referred to as cancer immunoediting. IDO-reactive T cells are able to recognize and kill tumor cells as well as IDO-expressing dendritic cells (DCs). IDO activation leads to immunosuppression through breakdown of Trp in the tumor microenvironment and tumor-draining lymph nodes. C-C chemokine receptor type 4 (CCR4)+ forkhead boxp3(Foxp3)+ regulatory T (Treg) cells create a favorable environment for tumor escape from host immune responses. Thus, Foxp3+/IDO+ tumors are associated with more advanced disease.
Age-related changes in the immune system are known as immunosenescence. A causal relationship is evident between the Trp metabolism and immune deficiency in elderly. Eventually, the reduced serum Trp concentrations and increased Kyn levels indicate increased chronic low-grade inflammation in elderly. In this case, IDO-induced Trp degradation is associated with increase in neopterin and nitrite levels. The amounts of neopterin produced by activated macrophages correlate with their capacity to release reactive oxygen species (ROS). Melatonin not only improves the antioxidant potential of the cell by stimulating the synthesis of antioxidant enzymes but also reduces free radical generation. The decline in melatonin production in aged individuals is a primary contributing factor for the development of age-associated neuronal damage.
IDO activity also has a significant positive correlation in both sexes with carotid artery intima/media thickness as an early marker of atherosclerosis. Enhanced Trp degradation in patients with coronary heart disease correlates with enhanced neopterin formation. In addition to elevated Kyn to Trp ratio, neopterin concentrations correlate with the abdominal obesity and metabolic syndrome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tryptophan
- Kynurenine
- Neopterin
- Ulcerative colitis
- Crohn’s disease
- Irritable bowel syndrome
- Immune escape mechanisms
- Obesity
- Aging
6.1 Introduction
Tryptophan (Trp) is an indispensable amino acid that should be supplied by dietary protein. L-Tryptophan metabolism is associated with numerous physiological functions and leads to the generation of several neuroactive compounds by three distinct pathways (Ruddick et al. 2006). First of all through the kynurenine (Kyn) pathway, while a large amount of Trp is oxidatively metabolized in the liver, simultaneously a small amount of Trp degradation can occur extrahepatically (Wirleitner et al. 2003). In this respect the conversion of Trp to Kyn is catalyzed by either the ubiquitous indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO) which is localized in the liver (Ruddick et al. 2006). In the central compound of this pathway, Kyn can turn into free radical generator 3-hydroxykynurenine, kynurenic acid (KA), and quinolinic acid (QA). KA is an N-methyl-D-aspartate (NMDA) receptor and alpha7 nicotinic acetylcholine receptor (alpha7nAChR) antagonist at physiological concentrations through its competitive blockade of the glycine co-agonist site (Schwarcz and Pellicciari 2002). QA has excitotoxic properties due to potent activation of NR2A and NR2B; NMDA receptor subtypes and its ability to generate free radicals are independent of receptor activation (Schwarcz and Pellicciari 2002). The activity of TDO can be increased by L-Trp and its analogs via an allosteric binding site and is competitively inhibited by some common indoleamines including tryptamine (Ruddick et al. 2006). IDO is stimulated during cellular immune responses preferentially by T-helper (Th)1-type cytokine interferon-gamma (IFN-gamma). IDO induction has been correlated with the conversion of Trp to Kyn and simultaneously induction of guanosine triphosphate cyclohydrolase (GTPCH), which is the key enzyme in pteridine biosynthesis. Therefore, IDO is recognized as one of the prominent mediators of immune regulation by metabolic pathways. IDO activity is best characterized by the Kyn to Trp ratio which correlates with concentrations of immune activation markers such as neopterin (Taylor and Feng 1991; Schröcksnadel et al. 2006). Thus, increased neopterin formation with the enhanced Trp degradation is only observed in diseases concomitant with cellular immune activation (Widner et al. 2002). In this context a significant correlation between Kyn-Trp ratio and neopterin concentrations indicates the involvement of IDO during the degradation of Trp (García-Lestón et al. 2012). Therefore, immunosuppressant substances are effective by inhibiting IDO activity and neopterin production simultaneously in a similar dose-dependent manner (Schroecksnadel et al. 2011).
In another pathway, a small portion of Trp is used for the synthesis of serotonin. Serotonin (5-hydroxytryptamine, 5-HT) is a key neurotransmitter that modulates a wide variety of functions in both peripheral organs and the central nervous system (CNS). The predominant site of 5-HT synthesis throughout the gastrointestinal tract is the enterochromaffin (EC) cells of the intestinal mucosa (Martel 2006). 5-HT is synthesized through the actions of two different tryptophan hydroxylases, tryptophan hydroxylase (TpH)-1 and TpH-2, which are found in EC cells and neurons, respectively (Gershon and Tack 2007). Tetrahydrobiopterin (BH4) is essential for the biosynthesis of serotonin, which serves as cofactor for tryptophan hydroxylase. GTPCH 1 is the first and rate-limiting enzyme for BH4 biosynthesis (Nagatsu and Ichinose 1999; Ichinose et al. 2013). The effects of 5-HT occur via seven distinct families of 5-HT receptors (5-HTRs). Six of them are G-protein coupled, whereas the remaining 5-HT3R is ionotropic (Hoyer et al. 2002; Hannon and Hoyer 2008). The synaptic concentration of released serotonin is regulated by the serotonin transporter (SERT) that removes serotonin from the synapse. Intestinal 5-HT is inactivated by metabolic degradation after SERT-mediated uptake into enterocytes or neurons. Furthermore, inhibition of SERT causes an increase in transmural transport of 5-HT in intestinal segments and augments the extracellular concentration of 5-HT (Martel 2006). Since Trp is known as the primary amino acid precursor of serotonin, systemic Trp depletion results in decreased serotonin synthesis (Bell et al. 2001; van Donkelaar et al. 2011). Consequently, the two metabolic pathways, Kyn and 5-HT, compete for their reciprocal precursor, Trp. Eventually, KA concentrations reduce and 5-HT synthesis increases. Although serotonergic metabolism in the intestinal mucosa is not affected by acute Trp depletion, profound effects on systemic concentrations of serotonergic metabolites are evident (Keszthelyi et al. 2012).
Another pathway that is activated in response to signals from the circadian clock and arylalkylamine N-acetyltransferase (AANAT; serotonin N-acetyltransferase) is the first rate-limiting enzyme in melatonin production and converts serotonin to N-acetyl serotonin. AANAT also constitutes a key interface between melatonin production and regulatory mechanisms (Coon et al. 2002). Actually, synthesis of melatonin starts with hydroxylation of L-Trp to 5-hydroxytryptophan. 5-Hydroxytryptophan is converted to serotonin. Serotonin is subsequently converted to N-acetylserotonin by the enzyme AANAT (Slominski et al. 2012). AANAT mRNA is uniformly distributed in the pineal gland but is limited primarily to the photoreceptor outer segments in the retina. Furthermore, the conversion of N-acetylserotonin to melatonin is achieved by the enzyme of hydroxyindole-O-methyltransferase (HIOMT). This enzyme is present in high amount in the pineal gland, but it is nearly undetectable in the retina (Coon et al. 2002). Circadian clocks in the vertebrate retina optimize retinal function by driving rhythms in gene expression, photoreceptor outer segment membrane turnover, and visual sensitivity (Iuvone et al. 2005). Most of the regulatory functions of melatonin are mediated by two high-affinity G-protein-coupled receptors, named MT1and MT2 (Dubocovich et al. 2010), which are mainly expressed in the CNS but are also present in different peripheral organs (Slominski et al. 2012). The third melatonin-binding site MT3 is an enzyme named quinone reductase 2 (QR2). Protective effect of melatonin against oxidative stress is provided by the activation of MT3/QR2. All three melatonin receptors can be found in the gut (Chen et al. 2011). G-protein-coupled membrane receptors of melatonin modulate several intracellular messengers such as cyclic adenosine monophosphate (cAMP) and [Ca2+] which are highly effective in the production of melatonin (Klein 2007).
Chronic immune activation is encountered in different pathologies including granulomatous and functional bowel diseases (Prior et al. 1986; Clarke et al. 2009), atherosclerosis (Blasi 2008), cancer (Dalgleish and O’Byrne 2002), and obesity (Brandacher et al. 2007; Duncan and Schmidt 2001). Persistence of chronic inflammatory stimuli over time creates a biologic background for immunosenescence and favors the susceptibility to inflammatory age-related diseases (Franceschi et al. 2000; Candore et al. 2006). In this chapter, inflammatory bowel disease, irritable bowel syndrome, cancer, aging, atherosclerosis, and obesity are taken into consideration in terms of chronic immune activation and Trp metabolism.
6.2 Chronic Immune Activation in Inflammatory and Functional Bowel Diseases
Inflammatory bowel disease (IBD) results from an inappropriate immune response that occurs in genetically susceptible individuals. It represents a complex interaction between the intestinal immune system, environmental circumstances, and microbial factors (Danese and Fiocchi 2006). Actually, IBDs consist of two distinct pathologies: ulcerative colitis and Crohn’s disease. The incidence and prevalence of IBD are increasing with time and in different regions around the world. In time-trend analyses, 75 % of Crohn’s disease studies and 60 % of ulcerative colitis studies have an increased incidence of statistical significance. The highest reported prevalence values for IBD were in Europe, 505 per 100,000 persons and 322 per 100,000 persons, and in North America, 249 per 100,000 persons and 319 per 100,000 persons, for ulcerative colitis and for Crohn’s disease, respectively (Molodecky et al. 2012).
The mechanisms of cell entry into the intestinal mucosa, bacterial and foreign antigen invasion, angiogenesis, and the control of gut inflammation through intestinal microvasculature are the most important issues considering the pathogenesis of IBD (Danese 2011).
Regardless of pathogenetic mechanisms, evaluation of urinary neopterin excretion in untreated ulcerative colitis patients shows a striking correlation between neopterin levels and the severity of disease. When the chronic cellular immune activation underlying ulcerative colitis is subsided, neopterin levels decrease and clinical remission is achieved (Niederwieser et al. 1985). Furthermore, fecal neopterin concentration is also increased in patients with clinically active or inactive Crohn’s disease and in patients with clinically active ulcerative colitis when compared with controls. Therefore, neopterin represents a remarkable biomarker for the activity of IBD (Husain et al. 2013). On the other hand, expression of IDO mRNA is markedly induced in perifollicular regions of lymphoid follicles in colonic tissues of IBD patients. IDO is primarily expressed in CD123+ mononuclear cells. Upregulation of IDO is detected by the increase of Kyn and Kyn/Trp in supernatants from colonic tissues (Wolf et al. 2004). In a similar manner with neopterin, increase in IDO expression in the lesions of ulcerative colitis or Crohn’s disease is positively related to the severity of inflammation. IDO-positive mononuclear cells also express CD11c, CD68, and toll-like receptor (TLR)4 (Zhou et al. 2012). Deficient TLR and nucleotide-binding-oligomerization domain function due to genetic variability is associated with an increased susceptibility to the development of inflammatory bowel disease (Mueller and Podolsky 2005). Actually, nucleotide-binding-oligomerization domain-containing-2 (NOD2) acts as a bacterial sensor in dendritic cells (DCs), and NOD2 variants are associated with Crohn’s disease. DCs from individuals with Crohn’s disease expressing Crohn’s disease-associated NOD2 are defective in autophagy induction, bacterial trafficking, and antigen presentation (Cooney et al. 2010).
On the other hand, an induction of mRNA for TLR2, TLR4, and TLR5 expression in inflammation-associated human intestinal macrophages also contributes to the inflammatory process (Hausmann et al. 2002). TLRs play essential roles in innate immune responses by recognizing various pathogen-derived components. In this respect, they activate various transcription factors such as nuclear factor-kappa B (NF-kappaB), activating protein-1, and interferon regulatory factors, which are responsible for inflammatory responses. In addition, TLRs also mediate alternative pathways by utilizing TLR3, TLR4, TLR7/8, and TLR9. Specific combination of these adapter molecules induces type I interferon responses (Kawai and Akira 2006). Consequently, the classical proinflammatory TLR signaling pathway leads to the synthesis of inflammatory cytokines and chemokines, such as interleukin (IL)-1beta, IL-6, IL-8, IL-12, and tumor necrosis factor (TNF)-alpha, which are causally involved in the pathogenesis of IBD. Thus, treatment with the TNF-blocking antibody, “infliximab,” indicates good clinical response to anti-TNF-alpha agents. This is accompanied with reduced IDO expression (Wolf et al. 2004; Frazão et al. 2013).
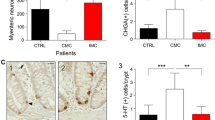
Chronic or recurrent abdominal pain or discomfort along with altered bowel function characterizes the irritable bowel syndrome (IBS) (Fukudo 2013). Gastrointestinal comorbidities, such as functional dyspepsia, gastroesophageal reflux disease, functional constipation, and anal incontinence, occur in almost 50 % of the patients. A broad variety of extraintestinal comorbidities, such as fibromyalgia, chronic fatigue syndrome, and chronic pelvic pain, are best documented and appear in up to 65 % (Riedl et al. 2008). A web-based survey that was carried out shown that the subtypes of IBS were mixed IBS 36 %, IBS with diarrhea 33 %, IBS with constipation 18 %, and unsubtyped IBS 11 % (Krogsgaard et al. 2013). It is also thought that the disorder of the autonomic nervous system function, the neuro-immune axis, and the brain-gut-microbiota axis profiles are unique in IBS patients. Since 5-HT neurotransmission in IBS patients is regulated with the 5-HT3 antagonists, 5-HT4 agonists, and antidepressants, 5-HT appears to be strongly associated with brain-gut function (Fukudo 2013). Successive potentiation of 5-HT and desensitization of its receptor could account for the symptoms seen in diarrhea-predominant and constipation-predominant IBS, respectively (Gershon 2004). Hence, IBS is a complex disorder that is associated with altered gastrointestinal motility, secretion, and sensation. Actually, 5-HT modulates sensation and perception of visceral stimulation at peripheral and central sites. However, enteric 5-HT signaling may be defective and inactivated by the SERT in the enterocytes or neurons. Tegaserod, a 5-HT4 partial agonist, is used in constipation-predominant IBS, while alosetron, a 5-HT3 antagonist, is used in IBS with diarrhea (Sikander et al. 2009; Crowell and Wessinger 2007). Furthermore, mucosal 5-HT, TpH-1 mRNA, SERT mRNA, and SERT immunoreactivity are all significantly reduced in both IBS with constipation and IBS with diarrhea (Coates et al. 2004). These data suggested that reduced SERT in the IBS patients can be one of the factors contributing to the development of both diarrhea and constipation. Thus, SERT immunoreactivity intensity of all IBS, IBS with diarrhea and IBS with constipation, patients significantly differs from that of healthy controls (El-Salhy et al. 2013). There are conflicting data on the efficacy of selective 5-HT reuptake inhibitors in IBS, the association of the SERT gene promoter polymorphism serotonin transporter-linked polymorphic region (5-HTTLPR) with IBS, and the expression pattern of SERT in the intestinal mucosa of IBS patients (Colucci et al. 2008).
According to these evidences, IBS has been linked with abnormal serotonin functioning and immune activation. On the one hand, Trp is used as a substrate for serotonin biosynthesis, but it can alternatively be catabolized to Kyn by the enzyme IDO. While a positive correlation between IBS severity and Kyn to Trp ratio is evident in these patients, increase in IFN-gamma activity is significantly correlated with the rise of Kyn-Trp ratio (Fitzgerald et al. 2008). In this case two alternatives may be valid. Firstly, the increased Kyn-Trp originates from the increased activity of hepatic TDO; the alternative scenario of increased IDO activity is equally valid. However, the elevated neopterin levels in the IBS cohort strongly suggest that IDO is the main enzymatic player. Although, the majority of the neopterin measurements are below the cutoff value, 10 nM level, which are considered to be reliably indicative of a disease state (Schroecksnadel et al. 2005a). In some cases although both plasma Kyn levels and the Kyn-Trp ratio are significantly increased in the IBS cohort, no difference is found in plasma L-Trp levels between IBS patients and healthy subjects. These patients show significant increases in neopterin levels but below the cutoff value (Clarke et al. 2009). These evidences confirm that low-level chronic immune activation may be valid in IBS. Additionally, significant imbalances in Trp concentrations and its metabolites may be frequently observed. This phenomenon might be associated either with a disturbance in albumin binding of Trp and an overcompensatory response to decreased Trp concentrations or a dysfunctional serotonergic system in IBS (Chen and Guillemin 2009; Shufflebotham et al. 2006).
As stated above, IBS patients exhibit a distinct Trp degradation profile through downstream of overall TLR activation that is different from that of healthy controls. However, TLR4 activation for Trp metabolism appears equivalent in both healthy controls and some subgroups of IBS patients (Clarke et al. 2012). Indeed, colonic gene and protein expression of TLR2 and TLR4 differs significantly between the subgroups of IBS patients, providing further support for the hypothesis of altered intestinal immune activation. A significant increase of TLR2 and TLR4 was shown only in diarrhea mixed bowel pattern (IBS-M) subgroup compared with healthy subjects. These results support the hypothesis, at least in constipation and IBS-M patients, that the innate immune system plays a key role in the pathophysiology of the disease. Thus the increased colonic expression of TLR2 and TLR4 in IBS-M patients are accompanied by the impaired expression of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and enhanced production of mucosal proinflammatory cytokines, IL-8 and IL1-beta (Belmonte et al. 2012).
In fact IBS patients showed a significant amount, 72 % increase in number of mucosal immune cells, CD3+, CD4+, and CD8+ T cells, and mast cells compared to controls (Cremon et al. 2009). The increased level of T-cell activation is consistent with the hypothesis of low-grade immune activation in IBS (Ohman et al. 2009). Mild inflammation is involved in diarrhea-predominant IBS patients as proinflammatory cytokine TNF-alpha is significantly higher, although no difference in anti-inflammatory cytokine is observed (Rana et al. 2012). Although IBS is characterized by the increase of proinflammatory cytokines, IL-6 and IL-8, IBS patients with certain extraintestinal comorbid conditions are distinguished by additional elevations in IL-1beta and TNF-alpha (Scully et al. 2010).
6.3 Immune Escape Mechanism in Cancer
The dual host-protective and tumor-promoting actions of immunity are referred to as cancer immune editing which is comprised of elimination, equilibrium, and escape phases (Vesely and Schreiber 2013). IDO-reactive T cells are peptide-specific, cytotoxic effector cells. Hence, IDO-specific T cells effectively disrupt IDO+ cancer cell lines of different origin. IDO-specific cytotoxic T lymphocytes (CTLs) recognize and kill IDO+-matured CD19+ plasmacytoid DC, which mediates immune suppression. Indeed, IDO is upregulated in DC in tumor-draining lymph nodes and creates a tolerogenic microenvironment (Sørensen et al. 2009).
DNA molecules containing unmethylated CpG oligodeoxynucleotides (ODN) have potent immunostimulatory effects on plasmacytoid DCs through TLR9 recognition and signaling. Human plasmacytoid DCs are activated by CpG-ODN-mediated TLR9 ligation. Later, they can induce the generation of CD4+ CD25+ forkhead boxp3(Foxp3)+ regulatory T cells (Tregs) from CD4+CD25 T cells (Moseman et al. 2004). In this process, human plasmacytoid DCs express high levels of IDO mRNA and protein in response to TLR9 ligation and use the IDO pathway to induce the differentiation of CD4+CD25+Foxp3+ Tregs from CD4+CD25- T cells. IDO inhibitor, 1-methyl-D-tryptophan, significantly impedes plasmacytoid DC-driven inducible Treg generation and suppressor cell function. However, Kyn supplementation suppresses the effect of 1-methyl-D-tryptophan and restores the differentiation of Treg cells (Chen et al. 2008).
IDO is spontaneously recognized by CTLs in patients with cancer (Sørensen et al. 2009). Thus, IDO-specific T cells are present in peripheral blood as well as in the tumor microenvironment. These IDO-reactive T cells are able to recognize and kill tumor cells as well as IDO-expressing DCs, that is, one of the main immune-suppressive cell populations (Sørensen et al. 2011). Inhibition of the expression and activity of IFN-gamma-induced IDO in bone marrow-derived dendritic cells (BMDCs) through the suppression of the activity of Janus kinase/signal transducers and activators of transcription (JAK/STAT) and protein kinase C causes antitumor activity by regulating CD8+ T-cell polarization and CTLs activity (Noh et al. 2013).
Spontaneous CTL reactivity against IDO exists not only in patients with cancer but also in healthy persons. IDO+ DCs inhibit T-cell proliferation because of Trp depletion and accumulation of toxic Trp metabolites (Platten et al. 2005; Munn and Mellor 2007). Actually, Trp metabolites of the Kyn pathway, such as 3-hydroxyanthranilic and QA, can induce the selective apoptosis of Th1 cells and can also effectively suppress T-cell proliferation (Fallarino et al. 2003). Furthermore, CTLs starved of Trp are unable to proliferate and go into G1 cell-cycle arrest (Munn et al. 2005).
Moreover, IDO-expressing plasmacytoid DCs activate the general control non-derepressible-2 (GCN2) kinase pathway in responding T cells. GCN2 kinase acts as a molecular sensor for T cells during IDO-induced Trp depletion and related immunosuppression (Munn et al. 2005). Endoplasmic reticulum (ER) transmembrane signaling protein, unfolded protein response (UPR)-mediated downregulation of protein synthesis, is accompanied by increased phosphorylation of eukaryotic translation initiation factor 2alpha (eIF2alpha). UPR initiates a rapid block in translation of cyclin D1 mRNA, and the cyclin D-dependent kinase activity is lost. During ER stress, one of the mammalian eIF2alpha kinases, protein kinase RNA-activated (PKR)-like ER kinase (PERK), contributes to cyclin D1 translation attenuation and provokes G1 arrest (Brewer et al. 1999). When considering all, both PERK and GCN2 contribute to the ER stress-mediated regulation of eIF2alpha phosphorylation and translation of cyclin D1 (Hamanaka et al. 2005). Consequently, the activation of GCN2 triggers a stress response program that can result in cell-cycle arrest, differentiation, adaptation, or apoptosis via eIF2alpha phosphorylation (De Haro et al. 1996).
Functionally, Trp-deprived DCs show a reduced capacity to stimulate T cells, which can be restored by blockade of specific immunoglobulin (Ig)-like transcripts (ILTs), ILT3. Trp deprivation generates human monocyte-derived DCs with a marked upregulation of the inhibitory receptors ILT3 and ILT4 and increases the capacity to induce CD4+CD25+Foxp3+ Tregs in an ILT3-dependent manner. Moreover, ILT3high ILT4high DCs lead to the induction of CD4+CD25+Foxp3+ Tregs with suppressive activity from CD4+CD25- T cells. The generation of ILT3high ILT4high DCs with tolerogenic properties by Trp deprivation is linked to a stress response pathway mediated by the GCN2 kinase (Brenk et al. 2009).
IDO activation leads to many complex changes within the affected cells resulting in immunosuppression through breakdown of Trp in the tumor microenvironment and tumor-draining lymph nodes (Soliman et al. 2010). In human malignancies, overexpression of IDO can facilitate immune escape which is under control of tumor suppressor gene bridging integrator 1 (Bin1). Thus, Bin1 loss contributes to immune escape in cancer by increasing the STAT1 and NF-kappaB-dependent expression of IDO (Muller et al. 2005). Since IDO represents an antitumoral immune effector mechanism, IDO also can cause immune system failure by inhibiting T-cell responses. Therefore, tumor cells can escape from immune system through IDO activity. Kyn-Trp ratio correlates strongly with the concentrations of cytokine IL-6, soluble IL-2 receptor-alpha, TNF-alpha receptor, and the macrophage marker neopterin. In this respect accelerated Trp degradation represents an immune escape mechanism (Sperner-Unterweger et al. 2011). Within the tumor microenvironment, not only tumor cells but also other infiltrating cells such as DCs, monocytes, and others can be sources of IDO. In addition to the Trp depletion, accumulation of its metabolites into the tumor environment also propagates the suppression of antitumor immune responses (Zamanakou et al. 2007). On the other hand, Engin et al. have found that certain colon cancer subsets are different in their ability to express IDO, while significant correlation between IDO activity and immunostaining scores indicates an immunosuppressive activity in patients with high IDO expression in colorectal cancer. Thus, high total IDO immunostaining score is a strong predictor for immune tolerance, lymphatic invasion, and subsequent lymph node metastasis (Engin et al. 2010).
Treg cells have been defined as a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self-antigens (Sakaguchi 2005, 2006). CD4+ Treg cells are abundant in tumor tissues and prevent the induction of effective antitumor immunity. They express C-C chemokine receptor 4 (CCR4) in tumor tissues. CCR4+ Treg cells are predominant among tumor-infiltrating Foxp3+ T cells (Sugiyama et al. 2013). The chemokines which are specific ligands for CCR4 that are produced by tumor cells attract CCR4+ Treg cells to the tumor. These cells create a favorable environment for tumor escape from host immune responses. Thus, anti-CCR4 monoclonal antibodies eliminate the suppressive effect of CCR4+ Treg cells on the host immune response to tumor cells (Ishida and Ueda 2006). Actually, Foxp3+ Treg cells are associated with more advanced disease in cancers. As IDO promotes differentiation of Treg cells, it may become a suitable target to abolish the development of T-cell tolerance against the cancer development. Node-positive disease almost exclusively occurs in patients with Foxp3+/IDO+ tumors. Actually, the combined expression and immunosuppressive effects of IDO and Foxp3 on metastatic lymph nodes support this assumption (Mansfield et al. 2009). Most Treg cells are defined based on expression of CD4, CD25, and the transcription factor, Foxp3. The combination of expression of CD4, CD25, and CD127 represents highly purified population of Treg cells and has an efficient suppressor function (Liu et al. 2006). Indeed, natural Treg cells have been observed to predominantly infiltrate tumor masses especially in the early phase of tumor progression (Yamaguchi and Sakaguchi 2006).
Activation of IDO in either tumor cells or nodal regulatory DCs appears to be sufficient to facilitate tumoral immune escape (Munn and Mellor 2007). Additionally, most human tumors can overexpress IDO (Uyttenhove et al. 2003). For instance, IDO is also expressed in human breast cancer cells. Estrogen receptor-negative breast cancer cells may evade the attention of the immune system through the expression of IDO together with its main substrate, L-Trp transport, into these cells (Travers et al. 2004).
In the tumor-draining lymph nodes (TDLNs), there are three strong regulatory mechanisms. IDO, functional activation of Tregs, and the inhibitory programmed cell death 1/programmed cell death 1 ligand (PD-1/PD-L) pathway are tightly linked and constitute an immunosuppressive milieu. When IDO+ plasmacytoid DCs present antigen to effector T cells in the presence of mature, resting Tregs, this initiates a GCN2-dependent activation of the Tregs by IDO. While GCN2 signaling is critical for allowing IDO-induced functional activation, Trp metabolites complete the full activation of the Tregs. Seventy-five to 90 % of this constitutive Treg activity in TDLNs is mediated via IDO-induced, PD-1/PD-L-dependent pathway. IDO-induced Treg activation is prevented by blockade of CTLs antigen 4, and IDO-Treg-PD-1/PD-L pathway is interrupted (Sharma et al. 2007). Eventually, the combination of these IDO-expressing plasmacytoid DCs and IDO-activated Treg cells renders the local milieu in the TDLNs profoundly inhibitory for T-cell activation (Munn and Mellor 2006).
On the other hand, Tregs exposed to certain inflammatory signals from activated DCs or TLR ligands can lose their suppressor activity (Pasare and Medzhitov 2003) and may alter their phenotype (be “reprogrammed”) to resemble proinflammatory effector cells. The reprogrammed Treg cells downregulate Foxp3 expression and express proinflammatory cytokines, IFN-gamma, IL-17, and TNF-alpha. This phenotype conversion requires DC-Treg cell contact, which causes IL-6 secretion by the DC, and occurs in an antigen-specific manner (Radhakrishnan et al. 2008). That means IDO plus effector T cells activate Foxp3+ Tregs for suppression. In the absence of IDO, Tregs can lose their suppressor phenotype and undergo conversion to a Th17-like phenotype. Most of the reprogrammed Tregs coexpress IL-2 and TNF-alpha, in addition to IL-17 and IL-22. Only a small number of reprogrammed cells express interferon-gamma or IL-10. Thus, reprogrammed Treg is a source of multiple proinflammatory cytokines. Upregulation of IL-17 in Tregs is driven by IL-6. However, IL-6 expression occurs only when IDO is blocked (Sharma et al. 2009).
Trp degradation is also detectable in patients with gynecological cancer. The relationship between Kyn-Trp and neopterin concentrations indicates that cellular immune activation rather than tumor-mediated IDO activity is responsible for the degradation of Trp (Schroecksnadel et al. 2005b). However, immunosuppressants are effective to inhibit IDO activity and neopterin production in a similar and dose-dependent manner (Schroecksnadel et al. 2011).
6.4 Aging and Chronic Immune Activation
Aging is associated with increased levels of circulating cytokines and proinflammatory markers. Age-related changes in the immune system, known as immunosenescence, and increased secretion of cytokines by adipose tissue represent the major causes of chronic inflammation (Michaud et al. 2013). Actually, impairment of immune defense with aging is a part of the age-associated neuroendocrine disorders which consist of hypertension, obesity, dyslipidemia, type 2 diabetes, menopause, late-onset depression, vascular cognitive impairment, and some forms of cancer (Oxenkrug 2010). On the other hand, progressive increase in Trp catabolism is also a part of the normal aging process (Frick et al. 2004). In this regard, a causal relationship is evident between the Trp metabolism and immune deficiency in elderly. Thus, neopterin, Kyn-Trp ratio, and all Kyn metabolites are 20–30 % higher in the older group, whereas Trp is 7 % lower (Theofylaktopoulou et al. 2013). Eventually, the reduced serum Trp concentrations and increased Kyn levels indicate increased chronic low-grade inflammation in elderly. In this case IDO-induced Trp degradation is associated with increase in neopterin and nitrite levels (Capuron et al. 2011). In addition to rising neopterin and Kyn levels, KA and homocysteine concentrations as well as the Kyn-Trp ratio also increase with older age. In this respect increasing neopterin concentrations and Kyn-Trp ratio in older age are associated with immune activation especially of the T-cell/macrophage system (Frick et al. 2004; Urbańska et al. 2006). As mentioned above, neopterin and Trp metabolites are strong predictive markers of the normal aging process and comorbidities of aging such as cardiovascular and neurodegenerative diseases or malignant tumors. Actually, aging and related pathological conditions critically involve an overwhelming production of reactive oxygen species (ROS) (Becker et al. 2014). During the exposure to oxidative stress, neopterin derivatives exhibit distinct biochemical effects, most likely via interactions with reactive oxygen or nitrogen intermediates (Hoffmann et al. 2003). The amounts of neopterin produced by activated monocytes/macrophages correlate with their capacity to release ROS. With this background, neopterin concentrations in body fluids can be taken into consideration as a degree of oxidative stress emerging during cell-mediated immune response (Murr et al. 1999). In this case the increased synthesis of BH4 in pteridine pathway is an adaptive response to inflammation; however, inflammation-induced oxidative stress could oxidize BH4 (Huang et al. 2005). In fact the enhanced production of neopterin occurs at the expense of BH4 formation (Fuchs et al. 2009). BH4 is the essential cofactor in the enzymatic hydroxylation of phenylalanine, tyrosine, and Trp. It is synthesized from GTP, and synthesis steps are catalyzed by GTPCH I, 6-pyruvoyl-tetrahydropterin synthase, and sepiapterin reductase (Shintaku 2002).
IFN-gamma-induced IDO promoter activity is enhanced synergistically by TNF-alpha. IFN-gamma-responsive elements, IFN regulatory factor-1, and two IFN-gamma-stimulated response elements (ISRE-1 and ISRE-2) are critical for this synergistic activation (Robinson et al. 2005). The transcriptional regulation of GTPCH I is important in the control of BH4 metabolism during the coordinated induction of GTPCH I and inducible nitric oxide synthase (iNOS) gene expression. However, the combination of TNF-alpha and IFN-gamma induces a strong activation of GTPCH I mRNA, protein, and BH4 production (Peterson and Katusic 2005). Eventually, TNF-alpha acts synergistically with the Th1 type cytokine, IFN-gamma in age-related changes in both pteridine and Kyn pathways. Meanwhile, BH4 serves as an essential NOS cofactor, and Kyn catabolites, quinolinic acids, and picolinic acids transcriptionally activate iNOS. These evidences indicate that there is a connection between arginine and Trp metabolic pathways in the generation of reactive nitrogen intermediates in aging (Melillo et al. 1994). Consequently, demand for BH4 might be increased under the condition of Kyn-induced activation of iNOS triggered by IFN-gamma-induced upregulation of Kyn pathway (Oxenkrug 2007). The deficiency of BH4 results in uncoupling of NOS and shifting of arginine metabolism to the production of superoxide anion rather than nitric oxide (NO) (Pou et al. 1992).
Actually, IFN-gamma does not play a role in redox modulation of IDO activity in DCs. The cystine/glutamate antiporter controls intracellular and extracellular redox. Mattox et al. showed that the antiporter control of redox regulates IDO enzymatic activity and IDO protein levels in DCs. IDO-competent DCs arise under pathophysiologic conditions, which are characterized by imbalances in systemic redox as occurs in obesity and aging (Mattox et al. 2012). Blocking the antiporter activity exhausts intracellular glutathione and interferes with DC differentiation from monocyte precursors, thereafter significantly reducing DC presentation of exogenous antigen to T cells (D’Angelo et al. 2010).
Several intermediate products of the Kyn pathway are known to be neurotoxic. Among them, the NMDA receptor agonist and neurotoxin, QA, is likely to be most important in terms of biological activity (Stone 2001). Anthranilic acid, 3-hydroxyanthranilic acid (3-HAA), and 3-hydroxykynurenine (3-HK) have been shown to generate free radicals leading to neuronal damage similar to QA (Stone 2001). During the Trp supplementation, Trp can be used for the synthesis of serotonin, melatonin, and nicotinamide adenine dinucleotide (NAD+) besides the Kyn production (Ruddick et al. 2006; Penberthy 2007). Moreover, under conditions of Trp depletion, supplementation with Trp downregulates enzymes directing Trp to non-NAD+-dependent pathways. This suggests a shift of all available Trp catabolism to NAD+ synthesis (Penberthy 2007). Kyn causes intracellular NAD+ depletion and reduces cell viability at greater than physiological concentrations (Braidy et al. 2009). The third metabolic pathway of L-Trp degradation leads to synthesis of its major metabolite melatonin. Melatonin not only improves the antioxidant potential of the cell by stimulating the synthesis of antioxidant enzymes but also reduces free radical generation and keeps the adequate mitochondrial adenosine triphosphate (ATP) synthesis. The decline in melatonin production in aged individuals is one of the primary contributing factors for the development of age-associated neuronal damage (Pandi-Perumal et al. 2013).
6.5 Atherosclerosis
Atherosclerosis is a chronic inflammatory disease initiated by the retention and accumulation of low-density lipoprotein (LDL) in the artery wall, leading to maladaptive responses of macrophages and T cells (Tabas et al. 2007). It could be caused by an immune reaction against autoantigens at the endothelial level, the most relevant of which are oxidized LDL and heat shock proteins (Blasi 2008). IDO suppresses T-cell activity and is upregulated by various inflammatory stimuli. IDO activity has a significant positive correlation in both sexes with carotid artery intima/media thickness as an early marker of atherosclerosis (Niinisalo et al. 2008; Pertovaara et al. 2007). Enhanced Trp degradation was reported in patients with coronary heart disease and was found to correlate with enhanced neopterin formation. In cardiovascular disease, IFN-gamma is the most important trigger for the formation and release of ROS. Chronic ROS production leads to the depletion of antioxidants. Furthermore, oxidative stress plays a major role in the atherogenesis and progression of cardiovascular disease (Schroecksnadel et al. 2006). In these patients, as traditional cardiovascular disease risk factors, IFN-gamma activity, plasma neopterin, and plasma Kyn-Trp ratio provide similar risk estimates for major coronary events and mortality (Pedersen et al. 2011). Neopterin and Kyn do not necessarily only serve as passive markers of IFN-gamma activity. Neopterin is released in parallel with its partially reduced derivative 7,8-dihydroneopterin (Fuchs et al. 2009). IFN-gamma-stimulated human macrophages generate ROS as well as neopterin and 7,8-dihydroneopterin. These pteridines may also have antioxidant effects depending on the circumstances (Herpfer et al. 2002).
The Kyn metabolite, 3-HAA, has immune regulatory properties and can inhibit Th1 and Th2 cells while increasing the amount of Tregs (Platten et al. 2005; Hayashi et al. 2007). Thus, 3-HAA modulates systemic adaptive immune responses and inhibits oxidized LDL (oxLDL) uptake in macrophages. Consequently, 3-HAA reduces local inflammation and atherosclerosis by impairing local antigen presentation and vascular infiltration of T cells (Zhang et al. 2012). However, 3-HAA, but not L-Kyn, markedly inhibits antigen-independent proliferation of CD8+ T cells induced by IL-2, IL-7, and IL-15 (Weber et al. 2006). A marked immunosuppressive effect of IDO expression is evident on human CD4+ and CD8+ T cells. Nevertheless, there is a significant difference in the suppressive effect of IDO on proliferation of CD8+ compared to that of CD4+ T cells (Forouzandeh et al. 2008). Actually, IFN-gamma is synthesized by CD4+ Th1 cells. This cytokine, a key regulator of immune function, is highly expressed in atherosclerotic lesions and has emerged as a significant factor in atherogenesis (McLaren and Ramji 2009). Neopterin is produced by human macrophages upon activation by proinflammatory stimuli like Th1-type cytokine IFN-gamma. Neopterin has prooxidative properties. Elevated neopterin concentrations are an independent marker for cardiovascular disease (Fuchs et al. 2009). Additionally, high neopterin levels also predict independently adverse prognosis in coronary artery disease patients (Grammer et al. 2009; Ray et al. 2007; Avanzas et al. 2005). Patients with hypertension and chest pain, but without obstructive coronary artery disease and developed adverse events during follow-up period, have significantly higher neopterin levels compared with patients without events (Avanzas et al. 2004). Aging vasculature generates an excessive ROS and NO. Consequently, it facilitates the formation of the deleterious radical, peroxynitrite. Main sources of ROS are mitochondrial respiratory chain and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, although NOS uncoupling could also account for ROS generation. The redox-sensitive transcription factor, NF-kappaB, is upregulated in vascular cells and drives a proinflammatory shift (El Assar et al. 2013).
Actually, IFN-gamma regulates a number of steps during atherogenesis. Its cellular actions in human macrophages are mediated through the regulation of STAT1. IFN-gamma-induced expression of key genes implicated in atherosclerosis is extracellular signal-regulated kinase (ERK) 1/2 dependent. The ERK pathway is required for the IFN-gamma-induced activity of STAT1 and monocyte chemoattractant protein-1 promoter (Li et al. 2010). At the same time IFN-gamma is also a principal inducer of neopterin and Kyn formation. Positive correlation between circulating neopterin and Kyn-Trp ratio levels reflects IFN-gamma activity.
When macrophages are exposed to oxLDLs, increased nuclear factor erythroid 2-related factor 2 (Nrf2) expression protects the macrophages from oxLDL-mediated injury via expression of antioxidant enzymes, including catalase, glutathione peroxidase (GPx), glutathione reductase, glutathione S-transferase, and NADPH/quinone oxidoreductase 1 (Zhu et al. 2008). Circulating adipocyte fatty acid-binding protein (FABP4) levels are associated with long-term prognosis in patients with coronary heart disease and may represent an important pathophysiological mediator of atherosclerosis (Von Eynatten et al. 2012). In macrophages, FABP4 coordinates cholesterol trafficking and inflammatory responses. Nrf2 is a redox-sensitive transcription factor and provides a primary cellular defense against the oxidative stress. Akt and ERK/Nrf2-dependent FABP4 upregulation pathway in human macrophages responds to the oxidative effect of polyunsaturated fatty acids (Lázaro et al. 2013). The kelch-like ECH-associated protein (Keap1)-Nrf2-ARE (antioxidant response element) signaling pathway elicits an adaptive response for cell survival. During cell stress, Keap1 disrupts Nrf2, and Nrf2 translocates to the nucleus and upregulates genes containing an antioxidant response element in their promoter regions (Wakabayashi et al. 2010). The activation of Nrf2 suppresses IFN-gamma production while inducing the production of the Th2 cytokines IL-4, IL-5, and IL-13 (Rockwell et al. 2012). In fact the dual neuroprotective treatment with nicotinamide and an Nrf2 inducer indicates that redox environment is more important than ROS for neuron survival in aging (Ghosh et al. 2014).
Raising the bioavailability of NO in primary human endothelial cells by activating Nrf2 impairs the presence of superoxide and the subsequent formation of peroxynitrite. Eventually, active Nrf2 elicits an antioxidant response in endothelial cells and reduces endothelial NOS (eNOS) expression. BH4 levels are important to keep eNOS in the coupled and NO-producing state. Reduced BH4 levels lead to downregulated eNOS expression in an Nrf2-dependent manner. Activation of Nrf2 downregulates eNOS levels via elevation of heme oxygenase (HO-1) activity (Heiss et al. 2009). HO-1 is important to prevent the endothelium from atherosclerosis. 3-HAA induces HO-1 expression and stimulates nuclear translocation of Nrf2 in human endothelial cells. Nrf2-dependent HO-1 expression induced by 3-HAA inhibits the monocyte chemoattractant protein (MCP)-1 secretion, vascular cell adhesion molecule (VCAM)-1 expression, and the activation of transcriptional NF-kappaB in endothelial cells. Subsequently, TNF-alpha stimulated vascular injury and inflammation is suppressed in atherosclerosis (Pae et al. 2006).
Oxidant compounds such as hydrogen peroxide (H2O2) have been shown to stimulate the release of arachidonic acid (AA) in a number of cell systems (Xu et al. 2003). Involvement of AA and its metabolites in the stimulation of both ERK and c-Jun-N-terminal kinase (JNK) following the oxidative stress evoked by H2O2 induces a cell-cycle arrest (Tournier et al. 1997). Fatty acid, AA, interferes with the transcriptional function of the IFN-gamma signaling pathway by reducing phosphorylation of STAT1. AA modulates the immunosuppressive activity of IDO by inhibiting the IFN-gamma/STAT1/IDO pathway (Bassal et al. 2012).
IDO expression is impaired in early prediabetic nonobese diabetic mouse strain. Virtually, IFN-gamma fails to induce IDO expression in cells with defective STAT1 phosphorylation in IFN-gamma-induced IDO signaling pathway of these animals (Hosseini-Tabatabaei et al. 2012).
The NF-kappaB subunits p65 and STAT1 cooperate to control iNOS gene transcription in response to proinflammatory cytokines (Burke et al. 2013). iNOS generates high concentrations of NO which is easily converted to peroxynitrite and superoxide in the prooxidant environment, a characteristic in essential hypertension. iNOS upregulates arginase activity, which limits NO production through eNOS and causes hypertension-associated endothelial dysfunction (Santhanam et al. 2007). Acute iNOS inhibition increases NO-dependent vasodilation likely through eNOS-mediated mechanisms (Smith et al. 2011).
The metabolism of arginine to NO is functionally in contrast with the metabolism of Trp to Kyn. Similar to iNOS, IDO is expressed in inflammatory conditions via IFN-gamma induction. IFN-gamma-induced endothelial IDO converts Trp to N-formylkynurenine, which decomposes spontaneously to Kyn. Kyn could directly modulate vascular tone and significantly attenuate the contractile response via activation of soluble guanylate cyclase (sGC). Eventual activation of adenylate cyclase by Kyn contributes to vessel relaxation via a cAMP-dependent pathway (Wang et al. 2010). Hence, Kyn formation within atherosclerotic arteries possibly represents a counter-regulatory protective mechanism (Niinisalo et al. 2010).
6.6 Human Hypertryptophanemia
Aging is characterized by a proinflammatory status which could contribute to the onset of major age-related diseases such as cardiovascular diseases, neurodegeneration, osteoarthritis and osteoporosis, and diabetes. In human hypertryptophanemia or in other neurodegenerative diseases, Trp accumulates in the body. Subsequent events leading to the brain injury are involved in oxidative stress damage (Feksa et al. 2008). Actually, Trp significantly decreases the brain antioxidant defenses by reducing the values of total radical-trapping antioxidant potential, total antioxidant reactivity, and glutathione. Consequently, the overall content of antioxidant capacity of the brain is reduced by Trp. Furthermore, the Trp-induced increase of thiobarbituric acid-reactive substances is fully prevented by glutathione and by combination of catalase plus superoxide dismutase (Feksa et al. 2006). More recent studies of Trp loading have indicated that high doses of Trp cause an abnormal white blood cell accumulation in tissues (Gross et al. 1996, 1999; Ronen et al. 1999), suggesting that Trp or its metabolites are active in modulating immune system activity.
6.7 Obesity-Related Chronic Immune Activation
Obesity-related immune-mediated systemic inflammation is associated with the induction of the Trp-Kyn pathway which reflects the IDO activation. Although a markedly increased Kyn-Trp ratio is evident in adult obese subjects with metabolic syndrome, obese juveniles show contrary decrease in Kyn-Trp ratio (Mangge et al. 2014). In any case plasma Trp concentration of obese individual is reduced independent of the weight reduction and dietary intake. Because of the changes in Trp metabolism, serotonin production may decrease. Impaired satiety due to subsequent insufficient serotonin synthesis causes overfeeding and obesity (Brandacher et al. 2007). Kyn-Trp ratio and all kynurenines, except anthranilic acid, are 2–8 % higher in overweight and 3–17 % higher in obese, than in normal-weight individuals (Theofylaktopoulou et al. 2013). Bariatric surgery significantly diminishes immune mediators by substantial weight reduction. In addition to elevated levels of neopterin, Trp depletion still persists (Brandacher et al. 2006). Neopterin concentrations correlate with abdominal obesity and metabolic syndrome (MetS), which is the cause of increased mortality risk. Accordingly, neopterin concentrations also correlate with high-density lipoprotein (HDL) cholesterol, insulin resistance, and plasma pyridoxal-5′-phosphate (Oxenkrug et al. 2011). Dysregulation of Trp-Kyn and Kyn-NAD metabolic pathways plays an important role in the occurrence of insulin resistance. Thus, the key enzymes of Kyn-NAD pathway require pyridoxal-5-phosphate as a cofactor. Obesity, cardiovascular diseases, or aging associated by excessive Kyn and xanthurenic acid formation in combination with pyridoxal-5-phosphate deficiency impair the biological activity of insulin (Oxenkrug 2013). Inflammation is associated with a T-cell infiltration in obese adipose tissue, with predominance of Th17 in the omental compartment and of Treg in the subcutaneous depot. The Th17/Treg balance is decreased in subcutaneous fat and correlates with IDO1 activation. In contrast, in the omental compartment, despite IDO1 activation, the Th17/Treg balance control is impaired (Wolowczuk et al. 2012).
6.8 Conclusion
During the chronic immune activation, Trp-consuming pathways display extremely simple response to highly complex immune mechanisms. Trp depletion and Trp metabolites influence the immune response modulation and immune tolerance. The activation of IDO through IFN-gamma leads to many complex changes within the affected cells resulting in immunosuppression through breakdown of Trp. Despite the evidences, IDO is not a sole factor in chronic immune activation encountered diseases. In particular suppression of tumor-specific host immune response suggests that IDO might support the tumor progression by providing immune escape. Persistence of chronic inflammatory stimuli over time creates a biologic background for immunosenescence and favors the susceptibility to inflammatory age-related diseases. Thus, for better understanding of the mechanisms underlying the interaction between IDO and chronic immune activation-related disorders, further studies should be planned in more details.
References
Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, Quiles J, Zouridakis E, Kaski JC (2004) Prognostic value of neopterin levels in treated patients with hypertension and chest pain but without obstructive coronary artery disease. Am J Cardiol 93:627–629
Avanzas P, Arroyo-Espliguero R, Quiles J, Roy D, Kaski JC (2005) Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur Heart J 26:457–463
Bassal NK, Hughes BP, Costabile M (2012) Arachidonic acid and its COX1/2 metabolites inhibit interferon-γ mediated induction of indoleamine-2,3 dioxygenase in THP-1 cells and human monocytes. Prostaglandins Leukot Essent Fat Acids 87:119–126
Becker K, Schroecksnadel S, Gostner J, Zaknun C, Schennach H, Uberall F, Fuchs D (2014) Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 21:164–171
Bell C, Abrams J, Nutt D (2001) Tryptophan depletion and its implications for psychiatry. Br J Psychiatry 178:399–405
Belmonte L, Beutheu Youmba S, Bertiaux-Vandaële N, Antonietti M, Lecleire S, Zalar A, Gourcerol G, Leroi AM, Déchelotte P, Coëffier M, Ducrotté P (2012) Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS One 7:e42777
Blasi C (2008) The autoimmune origin of atherosclerosis. Atherosclerosis 201:17–32
Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ (2009) Effects of kynurenine pathway metabolites on intracellular NAD synthesis and cell death in human primary astrocytes and neurons. Int J Tryptophan Res 2:61–69
Brandacher G, Winkler C, Aigner F, Schwelberger H, Schroecksnadel K, Margreiter R, Fuchs D, Weiss HG (2006) Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg 16:541–548
Brandacher G, Hoeller E, Fuchs D, Weiss HG (2007) Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab 8:289–295
Brenk M, Scheler M, Koch S, Neumann J, Takikawa O, Häcker G, Bieber T, von Bubnoff D (2009) Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J Immunol 183:145–154
Brewer JW, Hendershot LM, Sherr CJ, Diehl JA (1999) Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci U S A 96:8505–8510
Burke SJ, Updegraff BL, Bellich RM, Goff MR, Lu D, Minkin SC Jr, Karlstad MD, Collier JJ (2013) Regulation of iNOS gene transcription by IL-1β and IFN-γ requires a coactivator exchange mechanism. Mol Endocrinol 27:1724–1742
Candore G, Colonna-Romano G, Balistreri CR, Di Carlo D, Grimaldi MP, Listì F, Nuzzo D, Vasto S, Lio D, Caruso C (2006) Biology of longevity: role of the innate immune system. Rejuvenation Res 9:143–148
Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D (2011) Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry 70:175–182
Chen Y, Guillemin GJ (2009) Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res 2:1–19
Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR (2008) The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 181:5396–5404
Chen CQ, Fichna J, Bashashati M, Li YY, Storr M (2011) Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 17:3888–3898
Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG (2009) Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol 9:6
Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG (2012) A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol 3:90
Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL (2004) Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126:1657–1664
Colucci R, Blandizzi C, Bellini M, Ghisu N, Tonini M, Del Tacca M (2008) The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med 14:295–304
Coon SL, Del Olmo E, Young WS 3rd, Klein DC (2002) Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87). J Clin Endocrinol Metab 87:4699–4706
Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A (2010) NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 16:90–97
Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G (2009) Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol 104:392–400
Crowell MD, Wessinger SB (2007) 5-HT and the brain-gut axis: opportunities for pharmacologic intervention. Expert Opin Investig Drugs 16:761–765
D’Angelo JA, Dehlink E, Platzer B, Dwyer P, Circu ML, Garay J, Aw TY, Fiebiger E, Dickinson BL (2010) The cystine/glutamate antiporter regulates dendritic cell differentiation and antigen presentation. J Immunol 185:3217–3226
Dalgleish AG, O’Byrne KJ (2002) Chronic immune activation and inflammation in the pathogenesis of aids and cancer. Adv Cancer Res 84:231–276
Danese S (2011) Role of the vascular and lymphatic endothelium in the pathogenesis of inflammatory bowel disease: ‘brothers in arms’. Gut 60:998–1008
Danese S, Fiocchi C (2006) Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol 12:4807–4812
De Haro C, Méndez R, Santoyo J (1996) The eIF-2alpha kinases and the control of protein synthesis. FASEB J 10:1378–1387
Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010) International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 62:343–380
Duncan BB, Schmidt MI (2001) Chronic activation of the innate immune system may underlie the metabolic syndrome. Sao Paulo Med J 119:122–127
El Assar M, Angulo J, Rodríguez-Mañas L (2013) Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65C:380–401
El-Salhy M, Wendelbo I, Gundersen D (2013) Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep 8:451–455
Engin AB, Engin A, Gonul II, Karamercan A, Sepici-Dincel A, Dursun A (2010) Tumor invasion pattern and related serum tryptophan and neopterin concentrations in colorectal carcinomas. Pteridines 16:35
Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, Puccetti P (2003) T cell apoptosis by kynurenines. Adv Exp Med Biol 527:183–190
Feksa LR, Latini A, Rech VC, Wajner M, Dutra-Filho CS, de Souza Wyse AT, Wannmacher CM (2006) Promotion of oxidative stress by L-tryptophan in cerebral cortex of rats. Neurochem Int 49:87–93
Feksa LR, Latini A, Rech VC, Feksa PB, Koch GD, Amaral MF, Leipnitz G, Dutra-Filho CS, Wajner M, Wannmacher CM (2008) Tryptophan administration induces oxidative stress in brain cortex of rats. Metab Brain Dis 23:221–233
Fitzgerald P, Cassidy Eugene M, Clarke G, Scully P, Barry S, Quigley Eamonn MM, Shanahan F, Cryan J, Dinan TG (2008) Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil 20:1291–1297
Forouzandeh F, Jalili RB, Germain M, Duronio V, Ghahary A (2008) Differential immunosuppressive effect of indoleamine 2,3-dioxygenase (IDO) on primary human CD4+ and CD8+ T cells. Mol Cell Biochem 309:1–7
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Frazão JB, Errante PR, Condino-Neto A (2013) Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch Immunol Ther Exp (Warsz) 61:427–443
Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D (2004) Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem 37:684–687
Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra-Sanchez L, Kaski JC (2009) The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem 16:4644–4653
Fukudo S (2013) Stress and visceral pain: focusing on irritable bowel syndrome. Pain 154(Suppl 1):S63–S70
García-Lestón J, Roma-Torres J, Mayan O, Schroecksnadel S, Fuchs D, Moreira AO, Pásaro E, Méndez J, Teixeira JP, Laffon B (2012) Assessment of immunotoxicity parameters in individuals occupationally exposed to lead. J Toxicol Environ Health A 75:807–818
Gershon MD (2004) Review article: serotonin receptors and transporters: roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 20(Suppl 7):3–14
Gershon MD, Tack J (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132:397–414
Ghosh D, Levault KR, Brewer GJ (2014) Dual-energy precursor and nuclear erythroid-related factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species. Neurobiol Aging 35:179–190
Grammer TB, Fuchs D, Boehm BO, Winkelmann BR, Maerz W (2009) Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen risk and cardiovascular health study. Clin Chem 55:1135–1146
Gross B, Ronen N, Reznick A, Mokady S, Honigman S, Livne E (1996) Biochemical and morphological changes observed in rat muscles following consumption of excessive 1-tryptophan and atherogenic diets. Adv Exp Med Biol 398:575–578
Gross B, Ronen N, Honigman S, Livne E (1999) Tryptophan toxicity – time and dose response in rats. Adv Exp Med Biol 467:507–516
Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA (2005) PERK and GCN2 contribute to eIF2alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell 16:5493–5501
Hannon J, Hoyer D (2008) Molecular biology of 5-HT receptors. Behav Brain Res 195:198–213
Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W, Rogler G (2002) Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 122:1987–2000
Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, Lee J, Raz E (2007) 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A 104:18619–18624
Heiss EH, Schachner D, Werner ER, Dirsch VM (2009) Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem 284:31579–31586
Herpfer I, Greilberger J, Ledinski G, Widner B, Fuchs D, Jurgens G (2002) Neopterin and 7,8-dihydroneopterin interfere with low density lipoprotein oxidation mediated by peroxynitrite and/or copper. Free Radic Res 36:509–520
Hoffmann G, Wirleitner B, Fuchs D (2003) Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res 52:313–321
Hosseini-Tabatabaei A, Jalili RB, Li Y, Kilani RT, Moeen Rezakhanlou A, Ghahary A (2012) Mechanism underlying defective interferon gamma-induced IDO expression in non-obese diabetic mouse fibroblasts. PLoS One 7(5):e37747
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Huang A, Zhang Y, Chen K, Hatakeyama K, Keaney JF Jr (2005) Cytokine-stimulated GTP cyclohydrolase I expression in endothelial cells requires coordinated activation of nuclear factor-kappaB and Stat1/Stat3. Circ Res 96:164–171
Husain N, Tokoro K, Popov JM, Naides SJ, Kwasny MJ, Buchman AL (2013) Neopterin concentration as an index of disease activity in Crohn’s disease and ulcerative colitis. J Clin Gastroenterol 47:246–251
Ichinose H, Homma D, Sumi-Ichinose C, Nomura T, Kondo K (2013) GTP cyclohydrolase regulation: implications for brain development and function. Adv Pharmacol 68:23–35
Ishida T, Ueda R (2006) CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci 97:1139–1146
Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS (2005) Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res 24:433–456
Kawai T, Akira S (2006) TLR signaling. Cell Death Differ 13:816–825
Keszthelyi D, Troost FJ, Jonkers DM, van Donkelaar EL, Dekker J, Buurman WA, Masclee AA (2012) Does acute tryptophan depletion affect peripheral serotonin metabolism in the intestine? Am J Clin Nutr 95:603–608
Klein DC (2007) Arylalkylamine N-acetyltransferase: “the timezyme”. J Biol Chem 282:4233–4237
Krogsgaard LR, Engsbro AL, Bytzer P (2013) The epidemiology of irritable bowel syndrome in Denmark. A population-based survey in adults ≤50 years of age. Scand J Gastroenterol 48:523–529
Lázaro I, Ferré R, Masana L, Cabré A (2013) Akt and ERK/Nrf2 activation by PUFA oxidation-derived aldehydes upregulates FABP4 expression in human macrophages. Atherosclerosis 230:216–222
Li N, McLaren JE, Michael DR, Clement M, Fielding CA, Ramji DP (2010) ERK is integral to the IFN-γ-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol 185:3041–3048
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711
Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R, Schnedl WJ, Reininghaus E, Paulmichl K, Weghuber D, Fuchs D (2014) Obesity-related dysregulation of the Tryptophan-Kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 22:195–201
Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH (2009) Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast. BMC Cancer 9:231
Martel F (2006) Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res 54:73–76
Mattox ML, D’Angelo JA, Grimes ZM, Fiebiger E, Dickinson BL (2012) The cystine/glutamate antiporter regulates indoleamine 2,3-dioxygenase protein levels and enzymatic activity in human dendritic cells. Am J Clin Exp Immunol 1:113–123
McLaren JE, Ramji DP (2009) Interferon: a master regulator of atherosclerosis. Cytokine Growth Factor Rev 20:125–135
Melillo G, Cox GW, Biragyn A, Sheffler LA, Varesio L (1994) Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J Biol Chem 269:8128–8133
Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F (2013) Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14:877–882
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142:46–54
Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W (2004) Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol 173:4433–4442
Mueller T, Podolsky DK (2005) Nucleotide-binding-oligomerization domain proteins and toll-like receptors: sensors of the inflammatory bowel diseases’ microbial environment. Curr Opin Gastroenterol 21:419–425
Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC (2005) Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 11:312–319
Munn DH, Mellor AL (2006) The tumor-draining lymph node as an immune-privileged site. Immunol Rev 213:146–158
Munn DH, Mellor AL (2007) Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 117:1147–1154
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL (2005) GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22:633–642
Murr C, Fuith LC, Widner B, Wirleitner B, Baier-Bitterlich G, Fuchs D (1999) Increased neopterin concentrations in patients with cancer: indicator of oxidative stress? Anticancer Res 19:1721–1728
Nagatsu T, Ichinose H (1999) Regulation of pteridine-requiring enzymes by the cofactor tetrahydrobiopterin. Mol Neurobiol 19:79–96
Niederwieser D, Fuchs D, Hausen A, Judmaier G, Reibnegger G, Wachter H, Huber C (1985) Neopterin as a new biochemical marker in the clinical assessment of ulcerative colitis. Immunobiology 170:320–326
Niinisalo P, Raitala A, Pertovaara M, Oja SS, Lehtimäki T, Kähönen M, Reunanen A, Jula A, Moilanen L, Kesäniemi YA, Nieminen MS, Hurme M (2008) Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest 68:767–770
Niinisalo P, Oksala N, Levula M, Pelto-Huikko M, Jarvinen O, Salenius JP, Kytomaki L, Soini JT, Kahonen M, Laaksonen R, Hurme M, Lehtimaki T (2010) Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann Med 42:55–63
Noh KT, Chae SH, Chun SH, Jung ID, Kang HK, Park YM (2013) Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 431:348–353
Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M (2009) T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol 104:1205–1212
Oxenkrug GF (2007) Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Ann N Y Acad Sci 1122:35–49
Oxenkrug GF (2010) Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci 1199:1–14
Oxenkrug G (2013) Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol 48:294–301
Oxenkrug G, Tucker KL, Requintina P, Summergrad P (2011) Neopterin, a marker of interferon-gamma-inducible inflammation, correlates with pyridoxal-5′-phosphate, waist circumference, HDL-cholesterol, insulin resistance and mortality risk in adult Boston community dwellers of Puerto Rican origin. Am J Neuroprot Neuroregen 3:48–52
Pae HO, Oh GS, Lee BS, Rim JS, Kim YM, Chung HT (2006) 3-Hydroxyanthranilic acid, one of L-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis 187:274–284
Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, Hardeland R, Cardinali DP (2013) Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res 23:267–300
Pasare C, Medzhitov R (2003) Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033–1036
Pedersen ER, Midttun Ø, Ueland PM, Schartum-Hansen H, Seifert R, Igland J, Nordrehaug JE, Ebbing M, Svingen G, Bleie Ø, Berge R, Nygård O (2011) Systemic markers of interferon-γ-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 31:698–704
Penberthy TW (2007) Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Curr Drug Metab 8:245–266
Pertovaara M, Raitala A, Juonala M, Lehtimäki T, Huhtala H, Oja SS, Jokinen E, Viikari JS, Raitakari OT, Hurme M (2007) Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 148:106–111
Peterson TE, Katusic ZS (2005) Transcribing the cross-talk of cytokine-induced tetrahydrobiopterin synthesis in endothelial cells. Circ Res 96:141–143
Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L (2005) Treatment of auto-immune neuroinflammation with a synthetic tryptophan metabolite. Science 310:850–855
Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM (1992) Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 267:24173–24176
Prior C, Bollbach R, Fuchs D, Hausen A, Judmaier G, Niederwieser D, Reibnegger G, Rotthauwe HW, Werner ER, Wachter H (1986) Urinary neopterin, a marker of clinical activity in patients with Crohn’s disease. Clin Chim Acta 155:11–21
Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR (2008) Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol 181:3137–3147
Rana SV, Sharma S, Sinha SK, Parsad KK, Malik A, Singh K (2012) Pro-inflammatory and anti-inflammatory cytokine response in diarrhoea-predominant irritable bowel syndrome patients. Trop Gastroenterol 33:251–256
Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, Braunwald E (2007) Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 115:3071–3078
Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser AS, Klapp BF, Mönnikes H (2008) Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res 64:573–582
Robinson CM, Hale PT, Carlin JM (2005) The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res 25:20–30
Rockwell CE, Zhang M, Fields PE, Klaassen CD (2012) Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol 188:1630–1637
Ronen N, Livne E, Gross B (1999) Oxidative damage in rat tissue following excessive 1-tryptophan and atherogenic diets. Adv Exp Med Biol 467:497–505
Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA (2006) Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 8:1–27
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6:345–352
Sakaguchi S (2006) Regulatory T cells. Springer Semin Immunopathol 28:1–2
Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE (2007) Inducible no synthase dependent s-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res 101:692–702
Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D (2006) Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 364:82–90
Schroecksnadel K, Winkler C, Wirleitner B, Schennach H, Fuchs D (2005a) Aspirin down-regulates tryptophan degradation in stimulated human peripheral blood mononuclear cells in vitro. Clin Exp Immunol 140:41–45
Schroecksnadel K, Winkler C, Fuith LC, Fuchs D (2005b) Tryptophan degradation in patients with gynecological cancer correlates with immune activation. Cancer Lett 223:323–329
Schroecksnadel K, Frick B, Winkler C, Fuchs D (2006) Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr Vasc Pharmacol 4:205–213
Schroecksnadel S, Sucher R, Kurz K, Fuchs D, Brandacher G (2011) Influence of immunosuppressive agents on tryptophan degradation and neopterin production in human peripheral blood mononuclear cells. Transpl Immunol 25:119–123
Schwarcz R, Pellicciari R (2002) Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303:1–10
Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, Quigley EM (2010) Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol 105:2235–2243
Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 117:2570–2582
Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH (2009) Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 113:6102–6111
Shintaku H (2002) Disorders of tetrahydrobiopterin metabolism and their treatment. Curr Drug Metab 3:123–131
Shufflebotham J, Hood S, Hendry J, Hince DA, Morris K, Nutt D, Probert C, Potokar J (2006) Acute tryptophan depletion alters gastrointestinal and anxiety symptoms in irritable bowel syndrome. Am J Gastroenterol 101:2582–2587
Sikander A, Rana SV, Prasad KK (2009) Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta 403:47–55
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351:152–166
Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA (2011) Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58:935–942
Soliman H, Mediavilla-Varela M, Antonia S (2010) Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J 16:354–359
Sørensen RB, Berge-Hansen L, Junker N, Hansen CA, Hadrup SR, Schumacher TN, Svane IM, Becker JC, Thor Straten P, Andersen MH (2009) The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS One 4:e6910
Sørensen RB, Hadrup SR, Svane IM, Hjortsø MC, Thor Straten P, Andersen MH (2011) Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 117:2200–2210
Sperner-Unterweger B, Neurauter G, Klieber M, Kurz K, Meraner V, Zeimet A, Fuchs D (2011) Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology 216:296–301
Stone TW (2001) Endogenous neurotoxins from tryptophan. Toxicon 39:61–73
Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jäger E, Sakaguchi S (2013) Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 110:17945–17950
Tabas I, Williams KJ, Boren J (2007) Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116:1832–1844
Taylor MW, Feng GS (1991) Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 5:2516–2522
Theofylaktopoulou D, Midttun Ø, Ulvik A, Ueland PM, Tell GS, Vollset SE, Nygård O, Eussen SJ (2013) A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol 173:121–130
Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, Saunier B (1997) Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase). Eur J Biochem 244:587–595
Travers MT, Gow IF, Barber MC, Thomson J, Shennan DB (2004) Indoleamine 2,3-dioxygenase activity and L-tryptophan transport in human breast cancer cells. Biochim Biophys Acta 1661:106–112
Urbańska EM, Luchowski P, Luchowska E et al (2006) Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol Rep 58:507–511
Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274
van Donkelaar EL, Blokland A, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J (2011) Mechanism of acute tryptophan depletion: is it only serotonin? Mol Psychiatry 16:695–713
Vesely MD, Schreiber RD (2013) Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci 1284:1–5
Von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H (2012) Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol 32:2327–2335
Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak MK, Misra V, Biswal S, Yamamoto M, Kensler TW (2010) Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci Signal 3:ra52
Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF Jr, Hunt NH, Stocker R (2010) Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 16:279–285
Weber WP, Feder-Mengus C, Chiarugi A, Rosenthal R, Reschner A, Schumacher R, Zajac P, Misteli H, Frey DM, Oertli D, Heberer M, Spagnoli GC (2006) Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur J Immunol 36:296–304
Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D (2002) Neopterin production, tryptophan degradation, and mental depression–what is the link? Brain Behav Immun 16:590–595
Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D (2003) Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem 10:1581–1591
Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, Fuchs D, Brandacher G, Winkler C, Geboes K, Rutgeerts P, Tilg H (2004) Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol 113:47–55
Wolowczuk I, Hennart B, Leloire A, Bessede A, Soichot M, Taront S, Caiazzo R, Raverdy V, Pigeyre M, ABOS Consortium, Guillemin GJ, Allorge D, Pattou F, Froguel P, Poulain-Godefroy O (2012) Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol 303:R135–43
Xu J, Yu S, Sun AY, Sun GY (2003) Oxidant-mediated AA release from astrocytes involves cPLA(2) and iPLA(2). Free Radic Biol Med 34:1531–1543
Yamaguchi T, Sakaguchi S (2006) Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol 16:115–123
Zamanakou M, Germenis AE, Karanikas V (2007) Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett 111:69–75
Zhang L, Ovchinnikova O, Jönsson A, Lundberg AM, Berg M, Hansson GK, Ketelhuth DF (2012) The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J 33:2025–2034
Zhou L, Chen H, Wen Q, Zhang Y (2012) Indoleamine 2,3-dioxygenase expression in human inflammatory bowel disease. Eur J Gastroenterol Hepatol 24:695–701
Zhu H, Jia Z, Zhang L, Yamamoto M, Misra HP, Trush MA, Li Y (2008) Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med (Maywood) 233:463–474
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Engin, A.B. (2015). Evaluation of Tryptophan Metabolism in Chronic Immune Activation. In: Engin, A., Engin, A. (eds) Tryptophan Metabolism: Implications for Biological Processes, Health and Disease. Molecular and Integrative Toxicology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-15630-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-15630-9_6
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-15629-3
Online ISBN: 978-3-319-15630-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)