Abstract
Clinical human and experimental animal studies have facilitated our understanding of the essential structures in the initiation, transmission, modulation, and maintenance of headache. Pain-sensitive cranial structures, such as the dura mater encephali and large intracerebral blood vessels, and the cranial and cervical muscles and ligaments, which are innervated by primary afferent neurons originating from the trigeminal ganglia, take pivotal role in headache generation. Nociceptive impulses originated in the peripheral structures are then transmitted to central structures via second-order trigeminal neurons in the trigeminocervical complex in the brainstem. In addition to bottom organization of headache, top-down modulation of nociceptive stimuli from cranial structures through descending pathways is also discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Trigeminovascular system
- Meningeal afferents
- Trigeminocervical complex
- Central trigeminal pain pathways
- Content
1.1 Introduction
Clinical and experimental observations provide evidence for an essential contribution of peripheral, intracranial, as well as extracranial nociceptive processes in the generation of headaches [1]. A large body of evidence supports the hypothesis that most types of headaches, including migraine, are of trigeminovascular origin, caused or influenced by nociceptive afferents innervating the cranial meninges, particularly the dura mater encephali and large intracerebral blood vessels [2]. The primary role of the meningeal sensory innervation in generating headaches fits well to the intraoperative studies of Ray and Wolff and other investigators [3, 4], who demonstrated that headache-like pain, but not other sensations, can be evoked by electrical, mechanical, thermal, or chemical stimulation of dural blood vessels and sinuses or large intracerebral arteries. Importantly, the painful sensations were referred to the trigeminal dermatomes where typically headaches are localized [5]. These early studies formed the basis of many anatomical and physiological examinations in animals regarding the pathophysiology of headaches. Recordings of action potentials from trigeminal nerves [6] and the trigeminal ganglion [7] as well as higher neurons in the spinal trigeminal nucleus [8, 9] and in the thalamus [10, 11] provided further evidence for an important role of the trigeminovascular system in meningeal nociception.
1.2 Peripheral Structures Involved in Headache States
1.2.1 Trigeminal and Other Cranial Nerves Associated with Headache
1.2.1.1 Organization of the Trigeminal Ganglion and Meningeal Representation
Due to the limited experimental access to intracerebral arteries, most of the morphological and nearly all functional studies have focused on the innervation of the cranial dura mater and the dural venous sinuses. Using neuronal tracing, afferents around the middle meningeal artery have been found predominantly originating in the ophthalmic division (V1) of the ipsilateral trigeminal ganglion but to a minor extent also in the maxillary (V2) and mandibular (V3) divisions [12, 13]. The basal dura mater in the middle cranial fossa was represented mainly in V3. New retrograde tracings in the rat confirmed that meningeal nerves innervating the territorium of the middle cranial fossa, which is mainly supplied by the middle meningeal artery, origin predominantly in V3 and to a lesser extent in V2 [14]. The finding that all three divisions of the trigeminal nerve, though not equally, contribute to the innervation of the meninges is in accordance with old anatomic observations in primates [15]. Moreover, retrograde labeling of nerve fibers around basal intracranial arteries and the superior sagittal sinus, from which in humans headache can be provoked [3], appeared not only in the rat trigeminal ganglia but also in the first and second spinal ganglia [16] projecting to the cervical dorsal horn.
1.2.1.2 Afferent Innervation of the Meninges and Intracerebral Arteries
The innervation of the human cranial dura mater, which has firstly been described centuries ago by the anatomists Arnold [17] and Luschka [18], is regarded as pivotal for the generation or aggravation of headaches. Neuroanatomical studies demonstrated the close relationship between meningeal blood vessels and nerve fibers of different origin (Fig. 1.1a). Besides the trigeminal fibers originating in the ipsilateral trigeminal ganglion [19, 20], a network of sympathetic fibers mainly from the superior cervical ganglion [21, 22] and a comparatively sparse innervation by parasympathetic fibers originating in the sphenopalatine and otic ganglia has been described [23, 24]. The innervation of intracerebral (pial) blood vessels is similarly organized [25] but with a higher proportion of parasympathetic fibers coming mainly from the internal carotid and sphenopalatine ganglia [26].
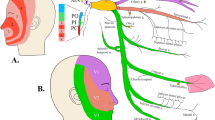
Histochemical demonstration of afferent and efferent innervation of the rat dura mater and markers of trigeminal ganglion neurons. (a) Confocal image of putative sympathetic and afferent nerve fibers in the rat dura mater labeled by tyrosine hydroxylase (TH, green) and calcitonin gene-related peptide (CGRP, red) immunofluorescence. Both TH- and CGRP-immunoreactive fibers form a dense network around the middle meningeal artery (MMA). Scale bar 200 μm. (b) Confocal image of trigeminal ganglion neurons labeled by retrograde tracing with Texas red from the temporal muscle (see red nerve fiber, arrowhead) and with Rhodamine green from the parietal dura mater. The yellow neuron with mixed red and green innervates both temporalis muscle and dura mater by afferent collaterals. Scale bar 50 μm. (c) Confocal image of a trigeminal ganglion section immunohistochemically stained for purinergic (P2X 3 ) receptor channels and nuclei (DAPI). The majority of neuronal cell bodies, mostly small ones, are P2X3 immunopositive. Scale bar 50 μm (Courtesy of S. Vilotti, SISSA Trieste)
Several immunohistochemical studies described neuropeptide-immunoreactive nerve fibers in the dura mater [27–29] and around cerebral (pial) blood vessels in different species including humans [30, 31]. Meningeal nerve fibers immunoreactive for substance P (SP), neurokinin A (NKA), and calcitonin gene-related peptide (CGRP) are thought to belong to the afferent (trigeminal and spinal sensory) system, while nerve fibers immunopositive for neuropeptide Y (NPY) are most likely of sympathetic and those immunoreactive for vasoactive intestinal polypeptide (VIP) of parasympathetic origin [21, 19]. The peptidergic nerve fibers form a dense network around blood vessels but can also be found in nonvascular regions [29, 20]. On the light microscopic level, sympathetic nerve fibers can be labeled by tyrosine hydroxylase (TH) immunoreactivity and thereby discriminated from peptidergic afferents (Fig. 1.1a). It is important to note, however, that a major proportion of trigeminal afferents does not express neuropeptides [32]. For differentiation of sensory fibers, other markers have been used such as neurofilament 200, which is present in myelinated fibers, and isolectin B4, which characterizes mostly unmyelinated, non-peptidergic fibers [33].
Electron microscopic examinations revealed myelinated (Aδ possibly Aβ) and unmyelinated (C) nerve fibers in the cranial dura mater [29, 34, 35]. An attempt was made to classify the C fibers according to their three-dimensional structure and their content of different kinds of vesicles into afferent and autonomic fibers [36]. The majority of meningeal C and Aδ fibers terminate as free nerve endings, but encapsulated Ruffini-like receptors and lamellated nerve terminals have additionally been described in higher vertebrates including man, particularly at sites where cerebral veins enter the sagittal sinus [34]. Myelinated and unmyelinated axons terminate also within the arachnoid granulations at different tissue structures suggesting that they have different mechano- and chemoreceptive functions [37].
1.2.1.3 Extracranial Collaterals of Meningeal Afferent Innervation
Long ago anatomical studies by Luschka [18] on the primate and human meningeal innervation reported on nerves that penetrate the skull, believed to innervate extracranial tissues. Recently a role for pericranial afferents in headache generation is again a matter of discussion [1]. Histological examinations in the mouse have revealed peripherin- and CGRP-immunopositive nerve fibers traversing the bones of the calvaria between the galea aponeurotica and the meninges [38]. The historical intraoperative data from Wolff’s group, who observed that noxious stimulation not only of dural but also extracranial structures like pericranial muscles and arteries can cause headache, support this concept [3]. Likewise, further experimental and clinical observations indicated that noxious activation of afferents in pericranial tissues, particularly in the temporal and occipital–cervical regions, can contribute to headache generation [39, 40] and peripheral sensitization in migraine pain [41].
A couple of new studies have been made using in vitro and in vivo neuronal tracing and electron microscopy in rodents and human skulls to investigate extracranial projections from meningeal nerves and their origin in the trigeminal ganglion [14]. In particular, anterograde and retrograde neuronal in vitro tracing with DiI revealed nerve fiber bundles leaving the skull through emissary canals and fissures to innervate the pericranial temporal, parietal, and occipital periosteum as well as deep layers of the temporal and upper neck muscles. A variety of functional measurements in rats confirmed the afferent nature of extracranial afferent collaterals and the impact of their activation on the intracranial secretion of neuropeptides and their vasodilatory function [42]. Following in vivo tracing with different dextran amines applied to the dura mater and the pericranial muscles, some neurons were detected in the trigeminal ganglion containing tracer from both structures (Fig. 1.1b). These data affirmed functional afferent connections between intra- and pericranial tissues and provide a new view on the influence of extracranial meningeal afferent projections on meningeal nociception and headache generation.
1.2.2 Molecular Signature of Trigeminal Afferents and Neurogenic Inflammation of the Dura Mater
Meningeal afferents convey the nociceptive information to the central nervous system, but through the antidromic release of vasoactive peptides from their perivascular peripheral terminals, they also promote a sterile “neurogenic inflammation” in the meningeal tissue characterized by vasodilatation and increased permeability of blood vessels [43, 44]. Neurogenic inflammation is considered to contribute to the peripheral mechanisms in the pathophysiology of headaches.
1.2.2.1 Receptors, Transduction, and Conduction Channels
Although headache-like pain is the only sensation induced by activation of intracranial afferents, regardless of the mode of their stimulation [5, 4], the nerve fibers innervating meningeal tissues consist of a heterogenous population based on their morphological and immunohistochemical properties (Fig. 1.2).
Schematic representation of the trigeminovascular system of the cranial dura mater with arterial vessel (AV), venous vessel (VV), central canal (circle) and mast cell (MC) and the afferent projection to the trigeminocervical complex with a second-order neuron in the subnucleus caudalis (Vc) and an inhibitory interneuron. One and the same single (Aδ or C) fiber may innervate the dura mater and, with collaterals projecting through the skull, the periosteum. Afferent fibers may contain and release CGRP in the periphery and the CNS. The left inset shows some important transduction channels (TRPV1, TRPA1, P2X3, ASIC3), receptors (5-HT1, PAR-2), and voltage-gated conduction channels (Cav, Nav, Kv) and the proposed signaling between the afferent ending, AV and MC. The right inset shows the proposed nociceptive transmission in superficial laminae of the trigeminocervical complex to Vc neurons expressing glutamate (Glu) receptor channels (NMDA, AMPA) and metabotropic glutamate receptors (mGluR). CGRP signaling is probably between terminals of primary afferents facilitating neurotransmitter release. Inhibitory neurons act pre- and postsynaptically on GABA and glycine (Gly) receptors, targeted by descending serotonergic and noradrenergic inhibitory pathways and segmental connections
1.2.2.1.1 TRP Channels
Chemosensitive meningeal afferents likely contribute to sensitization of the nociceptive pathway [45]. Successful prevention of cluster headache and migraine attacks with the topical, desensitizing application of capsaicin to the patients’ nasal mucosa has focused attention to the significant role of capsaicin-/chemosensitive population of trigeminal afferents [46, 47]. Chemosensitive meningeal afferents express different members of the transient receptor potential (TRP) channel family. Sixteen percent of the neurons in the trigeminal ganglion of humans and 21–31 % of dural afferent neurons in rodents have been found to express the transient receptor potential vanilloid 1 (TRPV1) channel [48, 32]. The TRPV1 receptor is a nonspecific cation channel, which can be activated by noxious heat, acidic pH (pH < 5.3), and different compounds like some endogenous membrane lipid metabolites (anandamide, N-arachidonoyl dopamine) and exogenous capsaicin or resiniferatoxin.
The transient receptor potential ankyrin 1 (TRPA1) ion channel, another member of the TRP receptor superfamily, has recently emerged as another important receptor activated by noxious chemical agents [49]. TRPA1 receptors can be activated by noxious cold, different environmental irritants like acrolein, and also by pungent ingredients of plant origin, like cinnamaldehyde and umbellulone that is the major volatile constituent of the “headache tree” Umbellularia californica [50, 51]. Endogenous activators of the receptor recently defined are some prostaglandin metabolites, hydrogen peroxide and nitroxyl (HNO), the one-electron-reduced sibling of nitric oxide (NO) [52]. Both endogenous and exogenous activators of the TRPA1 modify cysteine residues of the receptor (e.g., by forming disulfide bonds). Similar to TRPV1, activation of trigeminal afferents through TRPA1 receptors induces nociceptive responses and release of CGRP (Fig. 1.2). Histological and functional observations have revealed colocalization of TRPV1 and TRPA1 in trigeminal ganglion neurons [50, 53].
In humans, inhaled irritants may stimulate TRPA1 receptors of extracranial trigeminal afferents that innervate the nasal mucosa and may project collaterals to meningeal blood vessels. Nociceptive stimulation of extracranial tissues may activate intracranial collaterals by an axon reflex mechanism, releases vasoactive neuropeptides in meningeal tissue, and increases intracranial blood flow [42].
1.2.2.1.2 ASIC Channels
Some dural afferents express acid-sensing ion channels (ASICs), predominantly the ASIC3 subtype responding to low meningeal pH [54]. ASICs belong to the ENaC/DEG (epithelial amiloride-sensitive Na+ channel and degenerin) family of ion channels [55]. Relative small changes in the meningeal proton concentration activate the ASIC3 channel initiating an afferent signal in the trigeminal nociceptive pathway. The reason for an acidification of the local meningeal pH can be ischemia of the dura mater possibly developing as a consequence of cortical spreading depression that has been linked to the aura phase of migraine attacks [56, 57]. Degranulation of dural mast cells as a result of neurogenic inflammation of the dura mater can be an additional source of acidic metabolites leading to the activation of sensory nerve endings.
1.2.2.1.3 Purinergic Receptors
Purinergic (P2X2 and P2X3) receptor channels that may contribute to the transduction of nociceptive signals have been localized to trigeminal ganglion neurons innervating the dura mater (Fig. 1.1c). Purine receptor immunoreactivity is present predominantly in medium- and small-sized neurons that are mainly non-peptidergic and unmyelinated [33]. An important pathophysiological function of purinergic receptors may be peripheral sensitization of the trigeminal nociceptive pathway through the communication between different clusters of neurons within the trigeminal ganglion. In vitro studies have provided evidence that CGRP release from neurons stimulates the ERK1/2 MAP kinase signaling pathway in surrounding satellite glial cells and increases P2Y1,2 receptor-mediated intracellular calcium responses, which leads to the release of inflammatory cytokines from the activated satellite cells. Increased levels of cytokines have been shown to result in local inflammatory reactions and modulation of the neuronal function [58, 59]. By this way, CGRP may function as a paracrine factor to stimulate adjacent glial cells within a cluster and to cause excitation of more distant neurons and glial cells located in other clusters, thereby propagating an inflammatory signal across the entire ganglion [33, 60].
1.2.2.1.4 5-HT Receptors
One of the most effective classes of drugs for the treatment of migraine pain is the triptans, serotonin 1B/1D/1F (5-HT1B/1D/1F) receptor agonists. While the 5-HT1B receptors appear to be located primarily on vascular smooth muscle mediating vasoconstriction, the 5-HT1D/1F receptors are located on the peripheral and central terminals of meningeal afferents [61, 62] (Fig. 1.2). Activation of these G protein-coupled receptors inhibits the release of transmitters from the trigeminal afferents leading to the attenuation of the central transmission of nociceptive signals.
The presence of 5-HT7 receptors on trigeminal nerve endings and middle meningeal arteries has been demonstrated recently. Vasodilatation induced by the activation of trigeminal 5-HT7 receptors seems to be the result of CGRP release from the nerve terminals [63].
1.2.2.1.5 Calcium Channels
Activation of voltage-gated P/Q-type and N-type calcium channels has a key role in the regulation of synaptic function. Clinical and experimental data provide evidence that changes in the channel structure influence the release of neurotransmitters in the nociceptive transmission (Fig. 1.2). A rare hereditary form of migraine with aura and hemiparesis is the familial hemiplegic migraine type 1 (FHM-1). The FHM-1 gene encodes the pore-forming CaV2.1 subunit of P/Q-type Ca2+ channels. The mutation of the channel structure results in a gain of P/Q-type channel activity in trigeminal neurons and a selective increase in low-voltage-activated T-type currents in the small (IB4−) neuron population. This condition may lead to hyperexcitability of small, probably peptidergic, trigeminal neurons [64, 65].
Clinical and experimental observations provide evidence for N-type voltage-gated calcium channels (CaV2.2) as therapeutic targets for chronic pain conditions. N-type calcium channels are present in the presynaptic terminals of primary afferent sensory neurons, especially in Aδ and C fibers [66]. Blocking the channel function has a significant antinociceptive effect. In an experimental migraine model, inhibition of the channel function reduced neurotransmitter release from the primary sensory neurons and decreased the excitability of second-order neurons in the trigeminal brainstem [67].
1.2.2.1.6 Sodium Channels
The voltage-gated sodium channels with the pore-forming alpha-subunits NaV1.7 and NaV1.8 have emerged as molecules involved in peripheral pain processing and in the development of an increased pain sensitivity associated with inflammation [68]. In experimental models of meningeal nociception, amitriptyline, a tricyclic antidepressant, which is used to prevent migraine attacks, blocked the NaV1.8 currents in trigeminal ganglion neurons and alleviated nociceptive behavior induced by electrical stimulation of the superior sagittal sinus. These results strongly support the contribution of NaV1.8 channels to the pathophysiology of migraine and provide a novel guideline to migraine prophylaxis [69].
1.2.2.1.7 Potassium Channels
A subtype of voltage-gated K+ channels, KV7 expressed in nociceptors, is recognized to be one of the most important regulators of resting membrane potential and action potential firing threshold. Expression of this K+ channel contributes strongly to the excitability of the nociceptors. The analgesic drug flupirtine opens KV7 channels and by this way exerts an analgesic effect in migraine, chronic musculoskeletal pain, and neuralgia [70, 71]. Some of the nonsteroidal anti-inflammatory drugs such as diclofenac used in migraine therapy have also strong KV7 channel opener activity, which may at least partly be responsible for their analgesic effect [72]. Recent observations indicate an NO-mediated KV7 channel inhibition in trigeminal ganglion neurons that correlate with increased excitability and release of CGRP in nociceptors. It was suggested to contribute to excitatory effects of NO in headaches [73].
1.2.2.2 Mast Cells and Immunocytes in the Dura Mater
Activated meningeal nociceptors releasing the neuropeptides CGRP and SP, which induce direct vascular effects, can also activate and degranulate dural mast cells [74]. The local release of inflammatory mediators such as histamine from activated mast cells is believed to further stimulate meningeal nociceptors possibly promoting headache (Fig. 1.2). Clinical observations have shown that infusion of histamine induces headaches preferentially in migraineurs [75, 76].
The cranial dura mater is rich in connective tissue-type mast cells in both humans [77] and rodents [78] that are in close apposition to meningeal nociceptive nerve fibers and blood vessels [78]. This anatomical situation allows a multidirectional communication between blood vessels, nociceptors, and mast cells leading to the sensitization or activation of the trigeminal nociceptive pathway and changes in meningeal blood flow [79]. Direct vascular effects of histamine released by activated mast cells are mediated by multiple receptors localized on different histological components of the arterial vessel wall (Fig. 1.2). Relaxation of dural arteries is mediated by H2 receptors of vascular smooth muscle cells and by endothelial H1 receptors. In addition, H1 receptors on smooth muscle cells may mediate vasoconstriction [80].
Additional molecules known to be released from activated mast cells such as prostaglandins, leukotrienes, cytokines, and tryptase may also take part in the sensitization or activation of meningeal nociceptors [81]. The serine protease tryptase released from mast cells upon stimulation cleaves and activates the proteinase-activated receptor 2 (PAR-2) of meningeal nociceptors amplifying the initial vasodilation caused by sensory neuropeptides and possibly also the central transmission of nociceptive signals [82] (Fig. 1.2).
Resident macrophages of the dura mater expressing the inducible nitric oxide synthase (iNOS) are considered to play a significant role in delayed headache induced by infusion of the so-called NO-donor nitroglycerin. Following administration of nitroglycerin, a strong activation of iNOS was observed in the macrophages along the branches of the middle meningeal artery together with an upregulation of pro-inflammatory cytokines. Cytokines and NO synthesized by macrophages can sensitize small unmyelinated trigeminal afferents and generate headache. Activation of trigeminal afferents, in turn, promotes neuropeptide release and local blood flow changes in the meninges [83]. Nuclear factor kappa B (NF-κB) seems to mediate the transcriptional signal to iNOS and inflammatory cytokines. Since NF-κB can be activated by diverse pathological and inflammatory stimuli such as oxidative stress and bacterial and viral metabolites, its activation may provide the substrate within meningeal macrophages that contributes to headaches in response to different exogenous agents in susceptible individuals [84].
1.3 Central Structures Involved in Headache States
1.3.1 Subcortical Structures Implicated in Headaches
1.3.1.1 Morphofunctional Organization of the Trigeminocervical Complex and Meningeal Representation
The central processes of trigeminal ganglion neurons forming the trigeminal nerve enter the brainstem at the pontine level and terminate in the trigeminocervical complex, which consists of the pontine principal sensory nucleus (Vp) and the spinal trigeminal nucleus (Vsp) (Fig. 1.2). Basically, the thick myelinated mechanoreceptive trigeminal afferents terminate in the Vp, whereas both large-diameter and small-diameter fibers descend in the spinal trigeminal tract (SVT) projecting to the Vsp, which is subdivided into three subnuclei [85]: a rostral subnucleus oralis (Vo), a middle subnucleus interpolaris (Vi), and a caudal subnucleus caudalis (Vc). The Vc is often referred to as the medullary dorsal horn (MDH) because of the smooth transition to the anatomically and functionally similar spinal dorsal horn. Olszewski [85] identified three histologically different regions in the MDH: an outer marginal region, the substantia gelatinosa, and a deep magnocellular region. Later Gobel et al. [86] proposed a laminar subdivision of the MDH similar to Rexed’s nomenclature of the spinal dorsal horn [87] in which lamina I corresponds to the marginal layer, lamina II to the substantia gelatinosa, and laminae III and IV to the magnocellular region. The most ventral lamina V merges with the medullary reticular formation [88] without clear boundary. Groups of neurons intermingled in the spinal trigeminal tract down to the transition of Vi and Vc are referred to as the interstitial islands of Cajal or as the paratrigeminal or interstitial nucleus [89]. The neurons of these islands are regarded as nociceptive, similar to the neurons in laminae I and II of the Vc [10, 90].
Anatomical and electrophysiological studies [91, 92] revealed that the TBNC is topographically organized in ventrodorsal and in rostrocaudal direction. Mandibular afferents terminate preferentially in the dorsal region of each trigeminal subnucleus (dorsomedial in the MDH), ophthalmic afferents terminate ventrally (ventrolateral in MDH), and maxillary terminals are interposed. The rostrocaudal organization of the trigeminocervical complex is less clear, but within the Vc the rostrocaudal axis of the face is represented from rostral to caudal [93]. Early anatomical [94] and neurophysiological [95] studies suggest that each subnucleus receives information from all parts of the head. Jacquin et al. found mandibular nerves in the rat projecting to all trigeminal subnuclei, although the anterior oral afferents tended to terminate most heavily in the rostral trigeminocervical complex, whereas the posterior perioral–auricular afferents terminated preferentially in the caudal aspect of the complex [96]. Tracing from the superficial temporal artery revealed afferent terminals mainly in the rostral cervical spinal dorsal horn and sparsely in the Vi and Vc of the spinal trigeminal nucleus [97]. Because of a lack of tracing studies, it is not clear if a similar somatotopic distribution in ventrodorsal and rostrocaudal directions exists for intracranial trigeminal structures.
Also based on clinical observations and animal studies, it has been recognized that the Vc is primarily responsible for processing nociceptive and thermoreceptive information from the face and head, whereas the Vp is involved in processing tactile information (Fig. 1.2). Isolated lesions of the Vc caused ipsilaterally complete or partial loss of pain and temperature sensation, whereas tactile sensations remained nearly intact [98]. This clinical experience led Sjoqvist [99] to develop the method of trigeminal tractotomy for the relief of facial pain, in which the spinal trigeminal tract at the level of the obex was transected. The clinical data were supplemented with a large body of neurophysiologic evidence based on trigeminal tractotomy or experimental lesions of different subnuclei demonstrating that the Vc in the perception of pain in trigeminal tissues is essential, whereas for the processing of nociceptive information from intraoral and orofacial tissues, more rostral regions of the trigeminocervical complex are important as well [100, 101].
Transganglionic cholera toxin and HRP tracing of afferents innervating the rat superior sagittal sinus labeled central terminals in the ipsilateral Vc and Vi but also in the ventrolateral area of the C1–C3 spinal dorsal horn on both sides [16]. Labeling was seen in laminae I and II with HRP but in laminae III and IV with cholera toxin [16]. These findings are largely confirmed by electrophysiological recordings from second-order neurons with afferent input from the dura mater in rat [9, 102].
Apart from the abovementioned study [97], the projection of nociceptive afferents to specific laminae of the trigeminocervical complex has again mainly been studied for facial inputs using axonal tracing in cat and rat. Hayashi and Jacquin et al. found high-threshold mechanoreceptive (nociceptive) Aδ afferents forming extensive terminal arbors in the superficial Vi and, most pronounced, in lamina I and, to a lesser extent, outer lamina II of Vc [96, 103]. In the rat, a second termination area was localized in laminae III to V of Vc [96]. Corneal afferents, which are thought to be mainly nociceptive, terminate mainly in the outer laminae of Vc [104]. Corresponding to the distribution of nociceptive afferent terminals in the Vsp, SP- and CGRP-immunoreactive nerve fibers have been demonstrated in different species preferentially around the substantia gelatinosa of Vc and the transition zone between Vi and Vc (Vi/Vc) [105, 106]. Colocalization of immunoreactivity for TRPV1 receptors with SP and CGRP was found in axon collaterals in the dorsal parts of Vp, Vo, and Vi and in terminals and fibers throughout lamina I and the outer zone of lamina II (IIo) of the Vc [107]. Trigeminal rhizotomy in the cat caused disappearance of most of the CGRP-immunoreactive fibers throughout the trigeminocervical complex, whereas a considerable number of SP-immunoreactive fibers remained intact [108, 109] suggesting that these are of central origin.
Electron microscopic immunohistochemistry in the cat Vsp revealed CGRP immunoreactivity within the substantia gelatinosa in axon terminals which were presynaptic to dendritic profiles and postsynaptic to other fibers [110]. Likewise, the presence of CGRP receptors in rat and human Vc was found by immunohistochemistry restricted to trigeminal afferent endings in superficial layers (laminae I and II) of the Vc [111, 112]. This implies that CGRP as a central neuromodulator may exert its effects presynaptically to spinothalamic and other second-order neurons (Fig. 1.2).
1.3.1.2 Projections to Thalamic and Other Subcortical Nuclei
Nociceptive information from cranial and upper cervical structures is transmitted via the trigeminal and spinal afferent system to the higher diencephalic and cortical pain-associated areas. According to the well-delineated conventional pathway, the cell bodies of second-order neurons in the trigeminocervical complex mainly project to third-order neurons in the thalamus and then carry information to somatosensory cortical areas. The majority of the caudal part of the trigeminal nucleus (Vc) and the cervical dorsal horn neurons send axons to two contralateral thalamic nuclei that relay ascending somatosensory information to the primary somatic sensory cortex: the ventroposteromedial thalamic nucleus (VPM) and the posterior thalamic nuclear complex (Po) [113–115]. Trigeminal Vc neurons also project to the posterior part of the ventral medial nucleus (VMpo), the ventral caudal part of the medial dorsal nucleus (MDvc), and the nucleus submedius (Sm) of the thalamus [116] (Fig. 1.3). Projections to the thalamus have distinct molecular characteristics as glutamatergic trigeminothalamic projection neurons in the Vc; Vi and Vo mainly express the glutamate transporter VGLUT2. The sensitization of second-order and/or third-order neurons within the system is an important factor in the development of allodynia during the migraine attack. A recent study revealed that the thalamic reticular nucleus (TRN) was activated following cortical spreading depression (CSD) ipsilaterally in freely moving conscious rodents [117, 118]. Unilateral TRN activation during CSD implicates its potential role in lateralized headache perception and attention, regarding the fact that the parabrachial nuclei (PBN) and amygdala have direct projections on the TRN involved in drawing attention to emotional stimuli [119].
Schematic representation of ascending nociceptive pathways in perception of headache. Bipolar neurons in the trigeminal ganglion (TG) conduct noxious signals from peripheral nociceptors of perivascular trigeminal afferents to nociceptive laminae in the spinal trigeminal nucleus (Vc) through Aδ and C fibers. Transmission of nociceptive impulses to second-order neurons in the dorsal Vc is a critical step in pain transmission. Trigeminal projections from the Vc to third-order neurons in the thalamic ventroposteromedial (VPM) nuclei are relayed to the primary and secondary somatosensory cortex. The latter pathway is also known as a lateral pain system and related to the sensory discriminative aspects of headache perception. The Vc also projects to a large number of the brainstem and diencephalic structures including the rostral ventrolateral medulla (RVLM), parabrachial nucleus (PBN), periaqueductal gray matter (PAG), amygdala, and hypothalamus. The pathway from Vc-PBN-amygdala-medial thalamus to the anterior cingulate cortex (ACC) and insula is known as medial pain pathway and is involved in affective aspects of headache. The prefrontal cortex plays a role in cognitive evaluation of headache perception. The thalamic reticular nucleus (TRN) seems to be involved in attention and emotional components of pain perception. Reciprocal connections between subcortical structures and cerebral cortex are not shown for clarity. ACC anterior cingulate cortex, Amyg amygdala, Ins insular cortex, Noc nociceptor, MDvc thalamic mediodorsal ventrocaudal nucleus, PAG periaquaductal gray matter, PBN parabrachial nucleus, PFC prefrontal cortex, Po posterior group, RVLM rostroventrolateral medulla, SI and SII primary and secondary somatosensory cortices, Vc caudal trigeminal brainstem nucleus, VMpo thalamic ventromedial posterior nucleus, VPM ventroposteromedial thalamic nucleus, TG trigeminal ganglia, TRN thalamic reticular nucleus
Tracing studies revealed that the Vc also projects to other brainstem and diencephalic structures such as the brainstem reticular formation, nucleus of the solitary tract, superior salivatory nucleus, A5 cell group region, lateral periaqueductal gray matter, inferior colliculus, parabrachial nuclei, hypothalamus, and cerebellum [114, 120]. Trigeminal neurons send axons to the ipsilateral cerebellum, and trigeminocerebellar projection neurons predominantly express the glutamate transporter VGLUT1 [121]. Trigeminal projections also target the lateral reticular formation, mainly the rostral ventrolateral medullary reticular formation (RVLM), which participates in viscero-sympathetic reflexes. It was shown that trigemino-RVLM axons can be CGRP immunopositive [122].
The parabrachial nuclei (PBN), particularly their lateral parts, are an important projection site for trigeminal nociceptive information. The majority of nociceptive neurons in lamina I mainly in the Vc and Vi send axons to the PBN where many neurons respond preferably to noxious stimuli [90, 123, 124]. When retrograde tracer was injected into the PBN, projection neurons were detected ipsilaterally in lamina I of the Vc [125] though bilateral projection from the Vc to parabrachial nuclei has been identified [124]. Many of the neurons in the lateral parabrachial area project to the ventromedial hypothalamus and central nuclei of amygdala. Parabrachial-projecting neurons in the Vc have a topographic distribution [126]. Corneal afferents rather synapse with parabrachial-projecting neurons in the trigeminal nucleus and barely target neurons projecting to the thalamus. The trigeminal projections to the PBN are related with autonomic emotional responses to pain [127–129]. It is noteworthy that the PBN has reciprocal connections with forebrain areas and the insular cortex [130] and receives input from the amygdala [131]. The PBN was activated during CSD in rodents [132].
The amygdala, as a part of the trigemino–parabrachio–amygdaloid pathway, is another important subcortical nociceptive relay station. The central nucleus of the amygdala receives noxious information indirectly from the superficial lamina of the Vc through the lateral parabrachial area [133]. The amygdala, particularly the basolateral amygdaloid (BLA) nucleus, projects to the perirhinal area, the agranular insular area, and the mediodorsal thalamic nucleus. A direct amygdalofugal pathway to the trigeminal nuclear complex also exists in rodents. Particularly, the central amygdaloid nucleus sends extensive unilateral projections to all the trigeminal sensory nuclei, in addition to relatively light projections to the contralateral Vc [134]. Activation of amygdaloid nuclei along with the ipsilateral Vc was identified following CSD in awake freely moving rodents [135].
The perception of trigeminal pain is significantly modulated by the hypothalamus in the diencephalon. A considerable number of Vc neurons directly send their axons to hypothalamic regions [102]. Vc neurons projecting to the hypothalamus respond exclusively to noxious stimulation of the dura mater. In turn, the paraventricular nucleus, the lateral hypothalamic area, the perifornical hypothalamic area, the A11 nucleus, and the retrochiasmatic area send projections to the Vc. Hypothalamic projections are preferentially involved in the processing of meningeal and cutaneous inputs from the ophthalmic branch of the trigeminal nerve [136]. The latter finding indicates a somatotopic modulation of the Vc neurons by the hypothalamus. Descending hypothalamic projections to the Vc are bilateral, except those from the paraventricular nucleus that exhibit an ipsilateral predominance.
1.3.1.3 Descending Antinociceptive Systems
The transmission of nociceptive information to second-order trigeminal neuron is controlled by the inhibitory pathway descending from the periaqueductal gray matter (PAG) and from the rostral ventromedial medulla (RVM) (Figs. 1.3 and 1.4). The PAG located in the mesencephalon around the Sylvius aqueduct is the key structure in descending pain modulation with its powerful inhibitory properties on pain perception. Higher cerebral cortical structures, such as the hypothalamus and amygdala, have been also implicated in the descending modulation of nociceptive activity [137–139]. Cortical inputs from pain-processing cortical areas such as the somatosensory areas, the insular cortex, the prefrontal cortex, and the anterior cingulate cortex to the PAG were demonstrated in several species [140, 141]. It is suggested that the RVM is the final relay station for descending antinociceptive information from the forebrain [142], as inputs from higher brain centers converge on the PAG and the RVM to exert pain-suppressive effects. Stimulation of the RVM as well as PAG stimulation has been shown to suppress nociceptive responses [143].
Schematic representation of top-down, descending modulation of headache. The transmission of nociceptive information to second-order trigeminal neurons is controlled by inhibitory pathways descending from the periaqueductal gray (PAG) and from the rostral ventromedial medulla (RVM). Cortical inputs from pain-processing cortical areas such as the somatosensory areas, the insular cortex, the prefrontal cortex, and the anterior cingulate cortex project to the PAG. The hypothalamus, amygdala, PBN, locus coeruleus, and Raphé nuclei send projections to the descending pain inhibitory system. The dorsolateral prefrontal cortex (DLPFC) is related to attentional modulation of pain, the rostral anterior cingulate cortex (rACC) is implicated in placebo response, and morphine, noradrenaline, and serotonin are essential players in that system. rACC rostral anterior cingulate cortex, Amy amygdala, Ins insular cortex, LC locus coeruleus, PAG periaquaductal gray matter, PBN parabrachial nucleus, DLPFC dorsolateral prefrontal cortex, RN Raphé nucleus, RVM rostroventromedial medulla, SI primary somatosensory cortex, Vc caudal trigeminal brainstem nucleus
There are distinct neuronal subpopulations within the RVM that project caudally. Depending on the features of nociceptive stimuli (such as strength and duration), RVM neurons could yield either excitatory (on cells) or inhibitory (off cells) response to a noxious stimulus. Both neuronal subtypes are activated by electrical stimulation of the PAG. Morphine applied into the PAG or given systemically suppresses on-cell activity and increases off-cell activity [144]. Supraspinal opioid receptors play a key role in descending inhibitory controls relaying through the PAG and RVM.
In addition, there are serotonergic RVM cells projecting to the spinal cord [145] which contribute to descending antinociceptive inhibition by stimulation of the RVM or PAG [138, 146]. Descending projections from the noradrenergic neuronal cell groups in the locus coeruleus, subcoeruleus, A5, and A7 have a significant antinociceptive influence through spinal α2-adrenoceptors [146, 147]. It is notable that the locus coeruleus has direct inputs from the central nucleus of the amygdala, preoptic area, and paraventricular nucleus of the hypothalamus. GABAergic and glycinergic interneurons within the nociceptive laminae of the Vc mediate inhibitory effect on the transduction of nociceptive impulses to second-order neurons [148].
The amygdala plays a key role in emotional behavior, as inputs from the trigemino–parabrachio–amygdaloid pathway contribute to pain-induced changes in affective behavior and direct amygdalofugal projections to the PAG–RVM system provide feedback modulation of emotions on pain [137]. Through the latter pathway, application of opioids into the amygdala has been shown to induce antinociceptive effects [137] (Fig. 1.4). The parabrachial nuclei have direct projections to the trigeminocervical complex [149], and their electrical stimulation also exerted inhibitory effects on the activity of nociceptive neurons in the Vc.
Neurons of the paraventricular nucleus of the hypothalamus (PVN) send descending projections to laminae I and II of the Vc as well as the superior salivatory nucleus (Fig. 1.4). The latter nucleus gives rise to parasympathetic outflow to the cephalic vasculature, particularly in response to trigeminal activation, and could modulate neurogenic inflammation in the meninges [56, 150, 151]. Stimulation of the hypothalamic A11 nucleus has been shown to decrease the dural stimulation-evoked responses of Vc neurons. As a whole, those findings support the top-down modulation of the hypothalamus on Vc activities particularly driven by meningeal nociceptors.
The hypothalamic nuclei, particularly the paraventricular nucleus and/or arcuate nucleus, are involved in stress-induced analgesia [152]. Electrical stimulation of the hypothalamus results in antinociception. The hypothalamic PVN, PAG, and central nuclei of the amygdala take part in the stress-induced analgesic system [153]. It is notable that the rostral ACC, which is rich in opiate receptors, also participates in the endogenous analgesia [154]. During the analgesia induced by opioids or placebo, functional connectivity between the rACC and PAG has been found [155].
1.3.2 Cortical Areas Associated with Discriminative and Affective Aspects of Nociception
Perception of headache is a complex function of the cerebral cortex and involves distinct parts of the brain, which processes sensory discriminative, affective–emotional, and metacognitive aspects of nociception. Pain studies demonstrated that the activation of a cortical network of brain structures involving the somatosensory cortices SI and SII, the insular cortex, the anterior cingulate cortex (ACC), and the frontal cortex (DLPFC, orbitofrontal) is associated with nociceptive experience.
1.3.2.1 Cortical Structures of Discriminative Head Pain
The sensory discriminative and affective–motivational aspects of pain are transmitted through different systems and encoded in distinct cortical regions. The bottom-up organization of pain perception occurs through at least two major ascending pathways, the lateral and medial nociceptive systems [156]. The lateral nociceptive system comprises lamina I neurons projecting to the primary and secondary somatosensory cortex via the lateral thalamus and is involved in sensory discrimination of nociception [157, 158] (Fig. 1.3). Accordingly, it was demonstrated that manipulation of pain intensity was associated with changes mainly in the SI cortex, while the subjective ratings of pain unpleasantness were correlated with activity in the ACC [159].
Nociceptive stimulation of the ophthalmic branch of the trigeminal nerve, which preferably provides nociceptive information mediating headache, also activates similar cerebral cortical regions such as the somatosensory cortex, the insula, and the anterior cingulate cortex [160]. Descending cortical projections from the cerebral cortex to the trigeminal nucleus caudalis (Vc) have been demonstrated. Cortical projections originate contralaterally from insular (Ins) and primary somatosensory (SI) cortices. Projections from the primary sensory cortex terminate in deeper lamina, while projections from the insular cortex target solely superficial nociceptive laminae (laminae I and II) [161, 162] and inhibit trigeminal nociception, and meningeal-driven nociceptive inputs onto Vc were shown to be facilitated and inhibited by projections from the insula and SI, respectively [162].
1.3.2.2 Cortical Structures of Affective Pain Modulation
The medial nociceptive system that is directed toward the anterior cingulate cortex (ACC) through the parabrachial nucleus (PBN), parafascicular nucleus, and amygdala is involved in the affective–emotional aspects of nociception [157, 163, 164] (Fig. 1.3). Furthermore nociceptive information may be transmitted to the forebrain from the PBN and amygdala. The prefrontal cortex and the orbitofrontal cortex are implicated in the evaluation of affective experiences [165]. These pathways may contribute to the emotional aspects of pain and to the interactions between hedonic and cognitive processes of pain.
The majority of trigeminal ganglion neurons project multisynaptically to the anterior cingulate cortex (ACC). Iwata et al. [164] demonstrated by employing extracellular unit recordings that the ascending somatosensory pathways to the ACC from the trigeminal primary afferents arise mainly from Aδ and Aβ fibers but not from C fibers. The ACC predominantly receives projections from the lateral parabrachial nucleus. The parabrachial nuclei convey incoming nociceptive information from the Vc to the basolateral amygdaloid BLA nucleus [133]. The anatomical connections from Vc-parabrachial-BLA-ACC are thought to be involved in emotional and autonomic functions during trigeminal nociception.
The insula and amygdala, as components of the medial nociceptive pathway, have been implicated in evaluative and affective processes. The insular cortex sends projections to trigeminal nucleus caudalis. Tracing studies identified that many neurons in the granular and dysgranular insular cortex project to the laminae I/II of Vc bilaterally with a contralateral predominance. It is important to note that the direct projections from the insula only target to laminae I/II while sparing the lamina V of Vc [166]. Strong projections from the insular cortex to the bilateral rostral ventromedial medulla (RVM) and the nucleus of the solitary tract were detected. Bilateral insular projections with an ipsilateral predominance to the parabrachial nucleus were shown [161]. The insula is one of the important cortical pain-associated centers, and nociceptive processing of Vc neurons may be directly modulated through the insula or indirectly through brainstem nuclei such as PAG, PBN, and RVM. The insular cortex is pivotal in interoception and homeostatic functions [167]. Lesions of the insula are often associated with increased tolerance of pain.
Pain perception was also modulated by expectations and attention, and studies implicated the role of the dorsolateral prefrontal cortex (DLPFC) and the orbitofrontal cortex (OFC) during distraction and anticipation. The DLPFC may have a “top-down” mode of inhibition on the ascending nociceptive systems and is related with attentional modulation of pain (Figs. 1.3 and 1.4). In support of the pain modulatory function of the DLPFC, the anatomical connections between prefrontal cortices and the midbrain structure periaqueductal gray (PAG) were demonstrated by using diffuse tensor imaging [168]. Increased DLPFC activity was correlated with reduction in the affective component of nociceptive pain [169] and increased activity in the anterior cingulate cortex (ACC) along with increased activity in the PAG. Since the DLPFC activation was associated with decrease of nociception, repetitive transcranial magnetic stimulation (TMS) application to DLPFC was used for chronic migraine and fibromyalgia management [169, 170].
1.3.2.3 Functional Connectivity and Cortical Networks
Acute pain is a complex experience that is associated with activation of many structures in the brain that is often called pain matrix.The main components of the pain network are cortical structures of SI, SII, IC, ACC and PFC and the thalamus, which is the gate to sensorial input to the cerebral cortex and pacemaker for thalamocortical oscillations. The functional connectivity of such a network is important, and recent imaging studies have been focused on the alterations of functional connectivity during resting state (default mode network) or task performance. Chronic headache disorders have often been reported to be associated with changes in the brain networks during resting state and/or in response to stimuli.
Resting-state abnormalities were found in brain regions associated with pain processing and cognition in migraine patients [171–173]. Functional connectivity studies in patients suffering from temporomandibular disorders (TMD) revealed an increased connectivity of the anterior insula and anterior cingulate cortex. The latter finding was suggested to indicate an adaptation of the pain modulatory system early in the chronification process [174].
The resting-state functional connectivity of the hypothalamus was increased with parts of the frontal, parietal, and temporal cortex interictally in cluster headache patients [175]. However, the increased resting-state functional connectivity of the hypothalamus with the ACC and the posterior cingulate cortex (PCC) was detected during acute spontaneous cluster headache attacks. Functional MR studies revealed a diffuse abnormality of brain functional connectivity in cluster headache patients, which extends beyond the pain matrix primarily to cerebellar, frontal, and occipital areas [176, 177]. A recent case study demonstrated that cerebral activation of the ipsilateral trigeminal root entry zone, ventral pons, red nucleus, basal ganglia, cerebellum, prefrontal cortex, insula, and cingulate cortex was associated with ipsilateral hypothalamic activation during the cluster headache attack [178].
Structural MR studies in medication overuse headache (MOH) patients demonstrated increased gray matter thickness in the midbrain including periaqueductal gray matter and nucleus cuneiformis, which was partially reversed by the treatment in parallel to clinical improvement. It was proposed that the decreased gray matter in the orbitofrontal cortex could be predictive of poor response to treatment in MOH [179].
1.4 Gender Differences in Headache Anatomy
Gender differences in the epidemiology of headache disorders are well known; however, experimental headache studies conducted on females are significantly scarce [180, 181]. After reviewing the literature on headache and gender differences, it can be concluded that the medial pain pathway related to the affective–motivational aspects of headache seems to be more involved in women, which is briefly presented in the following section: (a) Capsaicin-induced trigeminal sensitization as detected by the visual flair and allodynic areas was greater in women particularly during menstruation phase compared to men [182]. (b) In women the resting cerebral metabolic glucose utilization in the orbitofrontal area was greater than in men [183]. (c) During negative affects such as anxiety and anger, the cerebellum, midbrain, thalamus, and ACC were more activated in women [184]. (d) In default mode network, there is a difference in insular processing between men and women’s brain [185]. (e) In migraineurs, the posterior insular and precuneus cortices are thicker in women [186]. (f) Heat pain induced greater activation in the PFC, ACC, insula, and thalamus in women [187–189].
Experimental animal studies are in line with human data: (a) In female mice, the functional connectivity between nodes of descending antinociception and affective system was stronger compared to male mice [63]. (b) Female rats exhibited significant sex differences in activation pattern of temporomandibular joint-responding neurons in the trigeminal brainstem and their projections on subcortical structures [190]. (c) In female rats, the density of dural mast cells is higher than in males, and estradiol promotes an increase in mast cell numbers along with a change in the phenotype [191]. (d) There are estrogen receptors and terminals in PBN subregions that are related to pain modulation [192]. (e) Estrogens modify nitroglycerin-induced c-fos expression in PVH, SON, SPVC, and CGRP expression in female rat brain [193]. (f) Cortical spreading depression susceptibility of FHM-1 knock-in mice was increased in female sex which was influenced by gonadal hormones [194, 180].
References
Olesen J, Burstein R, Ashina M, Tfelt-Hansen P (2009) Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 8:679–690. doi:10.1016/S1474-4422(09)70090-0
Pietrobon D, Striessnig J (2003) Neurobiology of migraine. Nat Rev Neurosci 4:386–398. doi:10.1038/nrn1102
Ray BS, Wolff HG (1940) Experimental studies on headache: pain sensitive structures of the head and their significance in headache. Arch Surg 1:813–856
Feindel W, Penfield W, Mcnaughton F (1960) The tentorial nerves and localization of intracranial pain in man. Neurology 10:555–563
Penfield W, McNaughton M (1940) Dural headache and innervation of the dura mater. Arch Neurol Psychiatry 44:43–75
Bove GM, Moskowitz MA (1997) Primary afferent neurons innervating guinea pig dura. J Neurophysiol 77:299–308
Strassman AM, Raymond SA, Burstein R (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384:560–564. doi:10.1038/384560a0
Strassman A, Mason P, Moskowitz M, Maciewicz R (1986) Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res 379:242–250
Schepelmann K, Ebersberger A, Pawlak M et al (1999) Response properties of trigeminal brain stem neurons with input from dura mater encephali in the rat. Neuroscience 90:543–554
Davis KD, Dostrovsky JO (1988) Properties of feline thalamic neurons activated by stimulation of the middle meningeal artery and sagittal sinus. Brain Res 454:89–100
Burstein R, Jakubowski M, Garcia-Nicas E et al (2010) Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68:81–91. doi:10.1002/ana.21994
Mayberg MR, Zervas NT, Moskowitz MA (1984) Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol 223:46–56. doi:10.1002/cne.902230105
Steiger HJ, Meakin CJ (1984) The meningeal representation in the trigeminal ganglion–an experimental study in the cat. Headache 24:305–309
Schueler M, Neuhuber WL, De Col R, Messlinger K (2014) Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache 54:996–1009. doi:10.1111/head.12371
McNaughton M (1938) The innervation of the intracranial blood vessels and dural sinuses. Assoc Res Nerv Ment Dis 18:178–200
Liu Y, Broman J, Edvinsson L (2004) Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience 129:431–437. doi:10.1016/j.neuroscience.2004.07.045
Arnold F (1831) Der Kopfteil des vegetativen Nervensystems beim Menschen. K. Groos, Heidelberg
Luschka H (1856) Die Nerven der harten Hirnhaut. H. Laupp, Tübingen
O’Connor TP, van der Kooy D (1986) Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci Off J Soc Neurosci 6:2200–2207
Strassman AM, Weissner W, Williams M et al (2004) Axon diameters and intradural trajectories of the dural innervation in the rat. J Comp Neurol 473:364–376. doi:10.1002/cne.20106
Edvinsson L, Uddman R (1981) Adrenergic, cholinergic and peptidergic nerve fibres in dura mater–involvement in headache? Cephalalgia Int J Headache 1:175–179
Keller JT, Marfurt CF, Dimlich RV, Tierney BE (1989) Sympathetic innervation of the supratentorial dura mater of the rat. J Comp Neurol 290:310–321. doi:10.1002/cne.902900210
Amenta F, Sancesario G, Ferrante F, Cavallotti C (1980) Acetylcholinesterase-containing nerve fibers in the dura mater of guinea pig, mouse, and rat. J Neural Transm 47:237–242
Edvinsson L, Hara H, Uddman R (1989) Retrograde tracing of nerve fibers to the rat middle cerebral artery with true blue: colocalization with different peptides. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 9:212–218. doi:10.1038/jcbfm.1989.31
Hardebo JE, Arbab M, Suzuki N, Svendgaard NA (1991) Pathways of parasympathetic and sensory cerebrovascular nerves in monkeys. Stroke J Cereb Circ 22:331–342
Suzuki N, Hardebo JE (1991) The pathway of parasympathetic nerve fibers to cerebral vessels from the otic ganglion in the rat. J Auton Nerv Syst 36:39–46
Von Düring M, Bauersachs M, Böhmer B et al (1990) Neuropeptide Y- and substance P-like immunoreactive nerve fibers in the rat dura mater encephali. Anat Embryol (Berl) 182:363–373
Keller JT, Marfurt CF (1991) Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol 309:515–534. doi:10.1002/cne.903090408
Messlinger K, Hanesch U, Baumgärtel M et al (1993) Innervation of the dura mater encephali of cat and rat: ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol (Berl) 188:219–237
Edvinsson L, Brodin E, Jansen I, Uddman R (1988) Neurokinin A in cerebral vessels: characterization, localization and effects in vitro. Regul Pept 20:181–197
You J, Gulbenkian S, Jansen Olesen I et al (1995) Peptidergic innervation of guinea-pig brain vessels: comparison with immunohistochemistry and in vitro pharmacology in rostrally and caudally located arteries. J Auton Nerv Syst 55:179–188
Huang D, Li S, Dhaka A et al (2012) Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain 8:66. doi:10.1186/1744-8069-8-66
Staikopoulos V, Sessle BJ, Furness JB, Jennings EA (2007) Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 144:208–216. doi:10.1016/j.neuroscience.2006.09.035
Andres KH, von Düring M, Muszynski K, Schmidt RF (1987) Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 175:289–301
Fricke B, Andres KH, Von Düring M (2001) Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech 53:96–105. doi:10.1002/jemt.1074
Messlinger K (1996) Functional morphology of nociceptive and other fine sensory endings (free nerve endings) in different tissues. In: The polymodal receptor: a gateway to pathological pain. Elsevier, Amsterdam, pp 273–298
Von Düring M, Andres KH (1991) Sensory nerve fiber terminals in the arachnoid granulations of non-human primates. Neurosci Lett 127:121–124
Kosaras B, Jakubowski M, Kainz V, Burstein R (2009) Sensory innervation of the calvarial bones of the mouse. J Comp Neurol 515:331–348. doi:10.1002/cne.22049
Calhoun AH, Ford S, Millen C et al (2010) The prevalence of neck pain in migraine. Headache 50:1273–1277. doi:10.1111/j.1526-4610.2009.01608.x
Svensson P, Ashina M (2006) Human studies of experimental pain from muscle. In: The headaches. Lippincott Williams & Wilkins, Philadelphia, pp 627–635
Malick A, Burstein R (2000) Peripheral and central sensitization during migraine. Funct Neurol 15(Suppl 3):28–35
Schueler M, Messlinger K, Dux M et al (2013) Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain 154:1622–1631. doi:10.1016/j.pain.2013.04.040
Moskowitz MA, Buzzi MG (1991) Neuroeffector functions of sensory fibres: implications for headache mechanisms and drug actions. J Neurol 238(Suppl 1):S18–S22
Moskowitz MA (1993) Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 43:S16–S20
Goadsby PJ (2007) Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med 13:39–44. doi:10.1016/j.molmed.2006.11.005
Sicuteri F, Fusco BM, Marabini S et al (1989) Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin J Pain 5:49–53
Marks DR, Rapoport A, Padla D et al (1993) A double-blind placebo-controlled trial of intranasal capsaicin for cluster headache. Cephalalgia Int J Headache 13:114–116
Hou M, Uddman R, Tajti J et al (2002) Capsaicin receptor immunoreactivity in the human trigeminal ganglion. Neurosci Lett 330:223–226
Belvisi MG, Dubuis E, Birrell MA (2011) Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest 140:1040–1047. doi:10.1378/chest.10-3327
Jordt S-E, Bautista DM, Chuang H-H et al (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265. doi:10.1038/nature02282
Nassini R, Materazzi S, Vriens J et al (2012) The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain J Neurol 135:376–390. doi:10.1093/brain/awr272
Eberhardt M, Dux M, Namer B et al (2014) H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun 5:4381. doi:10.1038/ncomms5381
Salas MM, Hargreaves KM, Akopian AN (2009) TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29:1568–1578. doi:10.1111/j.1460-9568.2009.06702.x
Yan J, Edelmayer RM, Wei X et al (2011) Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain 152:106–113. doi:10.1016/j.pain.2010.09.036
Wemmie JA, Price MP, Welsh MJ (2006) Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29:578–586. doi:10.1016/j.tins.2006.06.014
Bolay H, Reuter U, Dunn AK et al (2002) Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8:136–142. doi:10.1038/nm0202-136
Lambert GA, Michalicek J (1994) Cortical spreading depression reduces dural blood flow–a possible mechanism for migraine pain? Cephalalgia Int J Headache 14:430–436; discussion 393–394
Rothwell NJ, Hopkins SJ (1995) Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci 18:130–136
Vitkovic L, Bockaert J, Jacque C (2000) “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem 74:457–471
Magni G, Ceruti S (2013) P2Y purinergic receptors: new targets for analgesic and antimigraine drugs. Biochem Pharmacol 85:466–477. doi:10.1016/j.bcp.2012.10.027
Amrutkar DV, Ploug KB, Hay-Schmidt A et al (2012) mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain 153:830–838. doi:10.1016/j.pain.2012.01.005
Buzzi MG, Moskowitz MA (1991) Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine. Cephalalgia Int J Headache 11:165–168
Wang X, Fang Y, Liang J et al (2014) 5-HT7 receptors are involved in neurogenic dural vasodilatation in an experimental model of migraine. J Mol Neurosci MN. doi:10.1007/s12031-014-0268-9
Tao J, Liu P, Xiao Z et al (2012) Effects of familial hemiplegic migraine type 1 mutation T666M on voltage-gated calcium channel activities in trigeminal ganglion neurons. J Neurophysiol 107:1666–1680. doi:10.1152/jn.00551.2011
Cao Y-Q, Tsien RW (2005) Effects of familial hemiplegic migraine type 1 mutations on neuronal P/Q-type Ca2+ channel activity and inhibitory synaptic transmission. Proc Natl Acad Sci U S A 102:2590–2595. doi:10.1073/pnas.0409896102
Westenbroek RE, Hoskins L, Catterall WA (1998) Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci Off J Soc Neurosci 18:6319–6330
Ripsch MS, Ballard CJ, Khanna M et al (2012) A peptide uncoupling CRMP-2 from the presynaptic Ca(2+) channel complex demonstrates efficacy in animal models of migraine and aids therapy-induced neuropathy. Transl Neurosci 3:1–8. doi:10.2478/s13380-012-0002-4
Lampert A, O’Reilly AO, Reeh P, Leffler A (2010) Sodium channelopathies and pain. Pflugers Arch Eur J Physiol 460:249–263. doi:10.1007/s00424-009-0779-3
Liang J, Liu X, Pan M et al (2014) Blockade of Nav1.8 currents in nociceptive trigeminal neurons contributes to anti-trigeminovascular nociceptive effect of amitriptyline. Neuromolecular Med 16:308–321. doi:10.1007/s12017-013-8282-6
Devulder J (2010) Flupirtine in pain management: pharmacological properties and clinical use. CNS Drugs 24:867–881. doi:10.2165/11536230-000000000-00000
Mastronardi P, D’Onofrio M, Scanni E et al (1988) Analgesic activity of flupirtine maleate: a controlled double-blind study with diclofenac sodium in orthopaedics. J Int Med Res 16:338–348
Peretz A, Degani N, Nachman R et al (2005) Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 67:1053–1066. doi:10.1124/mol.104.007112
Ooi L, Gigout S, Pettinger L, Gamper N (2013) Triple cysteine module within M-type K+ channels mediates reciprocal channel modulation by nitric oxide and reactive oxygen species. J Neurosci Off J Soc Neurosci 33:6041–6046. doi:10.1523/JNEUROSCI.4275-12.2013
Ottosson A, Edvinsson L (1997) Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia Int J Headache 17:166–174
Krabbe AA, Olesen J (1980) Headache provocation by continuous intravenous infusion of histamine. Clinical results and receptor mechanisms. Pain 8:253–259
Lassen LH, Thomsen LL, Olesen J (1995) Histamine induces migraine via the H1-receptor. Support for the NO hypothesis of migraine. Neuroreport 6:1475–1479
Varatharaj A, Mack J, Davidson JR et al (2012) Mast cells in the human dura: effects of age and dural bleeding. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 28:541–545. doi:10.1007/s00381-012-1699-7
Dimlich RV, Keller JT, Strauss TA, Fritts MJ (1991) Linear arrays of homogeneous mast cells in the dura mater of the rat. J Neurocytol 20:485–503
Dux M, Sántha P, Jancsó G (2012) The role of chemosensitive afferent nerves and TRP ion channels in the pathomechanism of headaches. Pflugers Arch Eur J Physiol 464:239–248. doi:10.1007/s00424-012-1142-7
Dux M, Schwenger N, Messlinger K (2002) Possible role of histamine (H1- and H2-) receptors in the regulation of meningeal blood flow. Br J Pharmacol 137:874–880. doi:10.1038/sj.bjp.0704946
Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77:1033–1079
Dux M, Rosta J, Sántha P, Jancsó G (2009) Involvement of capsaicin-sensitive afferent nerves in the proteinase-activated receptor 2-mediated vasodilatation in the rat dura mater. Neuroscience 161:887–894. doi:10.1016/j.neuroscience.2009.04.010
Reuter U, Bolay H, Jansen-Olesen I et al (2001) Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain J Neurol 124:2490–2502
Reuter U, Chiarugi A, Bolay H, Moskowitz MA (2002) Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol 51:507–516
Olszewski J (1950) On the anatomical and functional organization of the spinal trigeminal nucleus. J Comp Neurol 92:401–413
Gobel S, Falls WM, Hockfield S (1977) The division of the dorsal and ventral horns of the mammalian caudal medulla into eight layers using anatomical criteria. In: Pain in the trigeminal region. Elsevier/North-Holland Biomedical Press, Amsterdam/New York, pp 443–453
Rexed B (1952) The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol 96:414–495
Nord SG, Kyler HJ (1968) A single unit analysis of trigeminal projections to bulbar reticular nuclei of the rat. J Comp Neurol 134:485–494. doi:10.1002/cne.901340407
Phelan KD, Falls WM (1989) The interstitial system of the spinal trigeminal tract in the rat: anatomical evidence for morphological and functional heterogeneity. Somatosens Mot Res 6:367–399
Hayashi H, Tabata T (1989) Physiological properties of sensory trigeminal neurons projecting to mesencephalic parabrachial area in the cat. J Neurophysiol 61:1153–1160
Hayashi H, Sumino R, Sessle BJ (1984) Functional organization of trigeminal subnucleus interpolaris: nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol 51:890–905
Strassman AM, Vos BP (1993) Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol 331:495–516. doi:10.1002/cne.903310406
Yokota T, Nishikawa N (1980) Reappraisal of somatotopic tactile representation within trigeminal subnucleus caudalis. J Neurophysiol 43:700–712
Torvik A (1956) Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol 106:51–141
Kruger L, Siminoff R, Witkovsky P (1961) Single neuron analysis of dorsal column nuclei and spinal nucleus of trigeminal in cat. J Neurophysiol 24:333–349
Jacquin MF, Barcia M, Rhoades RW (1989) Structure-function relationships in rat brainstem subnucleus interpolaris: IV. Projection neurons. J Comp Neurol 282:45–62. doi:10.1002/cne.902820105
Liu Y, Zhang M, Broman J, Edvinsson L (2003) Central projections of sensory innervation of the rat superficial temporal artery. Brain Res 966:126–133
Lisney SJ (1983) Some current topics of interest in the physiology of trigeminal pain: a review. J R Soc Med 76:292–296
Sjoqvist O (1938) Studies on pain conduction in the trigeminal nerve. A contribution to the surgical treatment of facial pain. Acta Psychiatry Scand 17(Suppl):1–139
Young RF (1982) Effect of trigeminal tractotomy on dental sensation in humans. J Neurosurg 56:812–818. doi:10.3171/jns.1982.56.6.0812
Broton JG, Rosenfeld JP (1986) Cutting rostral trigeminal nuclear complex projections preferentially affects perioral nociception in the rat. Brain Res 397:1–8
Malick A, Strassman RM, Burstein R (2000) Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol 84:2078–2112
Hayashi H (1985) Morphology of terminations of small and large myelinated trigeminal primary afferent fibers in the cat. J Comp Neurol 240:71–89. doi:10.1002/cne.902400106
Panneton WM, Burton H (1981) Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol 199:327–344. doi:10.1002/cne.901990303
Pearson JC, Jennes L (1988) Localization of serotonin- and substance P-like immunofluorescence in the caudal spinal trigeminal nucleus of the rat. Neurosci Lett 88:151–156
Boissonade FM, Sharkey KA, Lucier GE (1993) Trigeminal nuclear complex of the ferret: anatomical and immunohistochemical studies. J Comp Neurol 329:291–312. doi:10.1002/cne.903290302
Bae YC, Oh JM, Hwang SJ et al (2004) Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol 478:62–71. doi:10.1002/cne.20272
Henry MA, Johnson LR, Nousek-Goebl N, Westrum LE (1996) Light microscopic localization of calcitonin gene-related peptide in the normal feline trigeminal system and following retrogasserian rhizotomy. J Comp Neurol 365:526–540. doi:10.1002/(SICI)1096-9861(19960219)365:4<526::AID-CNE2>3.0.CO;2-6
Tashiro T, Takahashi O, Satoda T et al (1991) Distribution of axons showing calcitonin gene-related peptide- and/or substance P-like immunoreactivity in the sensory trigeminal nuclei of the cat. Neurosci Res 11:119–133
Henry MA, Nousek-Goebl NA, Westrum LE (1993) Light and electron microscopic localization of calcitonin gene-related peptide immunoreactivity in lamina II of the feline trigeminal pars caudalis/medullary dorsal horn: a qualitative study. Synap N Y N 13:99–107. doi:10.1002/syn.890130202
Lennerz JK, Rühle V, Ceppa EP et al (2008) Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507:1277–1299. doi:10.1002/cne.21607
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L (2013) Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain Off J Am Pain Soc 14:1289–1303. doi:10.1016/j.jpain.2013.03.010
Guy N, Chalus M, Dallel R, Voisin DL (2005) Both oral and caudal parts of the spinal trigeminal nucleus project to the somatosensory thalamus in the rat. Eur J Neurosci 21:741–754. doi:10.1111/j.1460-9568.2005.03918.x
Mantle-St John LA, Tracey DJ (1987) Somatosensory nuclei in the brainstem of the rat: independent projections to the thalamus and cerebellum. J Comp Neurol 255:259–271. doi:10.1002/cne.902550209
Ring G, Ganchrow D (1983) Projections of nucleus caudalis and spinal cord to brainstem and diencephalon in the hedgehog (Erinaceus europaeus and Paraechinus aethiopicus): a degeneration study. J Comp Neurol 216:132–151. doi:10.1002/cne.902160203
Craig AD (2004) Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol 477:119–148. doi:10.1002/cne.20240
Bolay H, Tepe N, Filiz A et al (2013) The thalamic reticular nucleus is activated by cortical spreading depression in freely moving rats: prevention by acute valproate. Cephalalgia 33:5–6
Tepe N, Filiz A, Akcali D et al (2015) The thalamic reticular nucleus is activated by cortical spreading depression in freely moving rats: prevention by acute valproate administration. Eur J Neurosci. 41(1):120–8.
Zikopoulos B, Barbas H (2012) Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci Off J Soc Neurosci 32:5338–5350. doi:10.1523/JNEUROSCI.4793-11.2012
Bernard JF, Peschanski M, Besson JM (1989) A possible spino (trigemino)-ponto-amygdaloid pathway for pain. Neurosci Lett 100:83–88
Ge S-N, Li Z-H, Tang J et al (2014) Differential expression of VGLUT1 or VGLUT2 in the trigeminothalamic or trigeminocerebellar projection neurons in the rat. Brain Struct Funct 219:211–229. doi:10.1007/s00429-012-0495-1
Panneton WM, Gan Q (2014) Direct reticular projections of trigeminal sensory fibers immunoreactive to CGRP: potential monosynaptic somatoautonomic projections. Front Neurosci 8:136. doi:10.3389/fnins.2014.00136
Barnett EM, Evans GD, Sun N et al (1995) Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci Off J Soc Neurosci 15:2972–2984
Feil K, Herbert H (1995) Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J Comp Neurol 353:506–528. doi:10.1002/cne.903530404
Mitchell JL, Silverman MB, Aicher SA (2004) Rat trigeminal lamina I neurons that project to thalamic or parabrachial nuclei contain the mu-opioid receptor. Neuroscience 128:571–582. doi:10.1016/j.neuroscience.2004.07.026
Aicher SA, Hermes SM, Hegarty DM (2012) Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience. doi:10.1016/j.neuroscience.2012.11.033
Saper CB (1995) The spinoparabrachial pathway: shedding new light on an old path. J Comp Neurol 353:477–479. doi:10.1002/cne.903530402
Sessle BJ (1999) Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci J Can Sci Neurol 26(Suppl 3):S7–S11
Gauriau C, Bernard J-F (2002) Pain pathways and parabrachial circuits in the rat. Exp Physiol 87:251–258
Yasui Y, Takada M, Mitani A et al (1985) Direct cortical projections to the parabrachial nucleus in the cat. J Comp Neurol 234:77–86
Tokita K, Inoue T, Boughter JD (2009) Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 161:475–488. doi:10.1016/j.neuroscience.2009.03.046
Moskowitz MA, Nozaki K, Kraig RP (1993) Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci Off J Soc Neurosci 13:1167–1177
Jasmin L, Burkey AR, Card JP, Basbaum AI (1997) Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. J Neurosci Off J Soc Neurosci 17:3751–3765
Lazarov NE, Usunoff KG, Schmitt O et al (2011) Amygdalotrigeminal projection in the rat: an anterograde tracing study. Ann Anat Anat Anz Off Organ Anat Ges 193:118–126. doi:10.1016/j.aanat.2010.12.004
Akcali D, Sayin A, Sara Y, Bolay H (2010) Does single cortical spreading depression elicit pain behaviour in freely moving rats? Cephalalgia Int J Headache 30:1195–1206. doi:10.1177/0333102409360828
Abdallah K, Artola A, Monconduit L et al (2013) Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS One 8:e73022. doi:10.1371/journal.pone.0073022
Helmstetter FJ, Tershner SA, Poore LH, Bellgowan PS (1998) Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Res 779:104–118
Aimone LD, Gebhart GF (1988) Serotonin and/or an excitatory amino acid in the medial medulla mediates stimulation-produced antinociception from the lateral hypothalamus in the rat. Brain Res 450:170–180
Messlinger K, Dostrovsky JO, Strassman A (2006) Anatomy and physiology of head pain. In: The headaches, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 95–109
An X, Bandler R, Ongür D, Price JL (1998) Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol 401:455–479
Mantyh PW (1982) Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol 206:146–158. doi:10.1002/cne.902060205
Gebhart GF (2004) Descending modulation of pain. Neurosci Biobehav Rev 27:729–737. doi:10.1016/j.neubiorev.2003.11.008
Morgan MM, Heinricher MM, Fields HL (1992) Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience 47:863–871
Pertovaara A, Almeide A (2006) Endogenous pain modulation, chapter 13, descending inhibitory systems. Handb Clin Neurol 81(3rd series vol. 3), 179–192
Lakos S, Basbaum AI (1988) An ultrastructural study of the projections from the midbrain periaqueductal gray to spinally projecting, serotonin-immunoreactive neurons of the medullary nucleus raphe magnus in the rat. Brain Res 443:383–388
Kwiat GC, Basbaum AI (1992) The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens Mot Res 9:157–173
Yaksh TL (1985) Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav 22:845–858
Iliakis B, Anderson NL, Irish PS et al (1996) Electron microscopy of immunoreactivity patterns for glutamate and gamma-aminobutyric acid in synaptic glomeruli of the feline spinal trigeminal nucleus (Subnucleus Caudalis). J Comp Neurol 366:465–477. doi:10.1002/(SICI)1096-9861(19960311)366:3<465::AID-CNE7>3.0.CO;2-2
Yoshida A, Chen K, Moritani M et al (1997) Organization of the descending projections from the parabrachial nucleus to the trigeminal sensory nuclear complex and spinal dorsal horn in the rat. J Comp Neurol 383:94–111
Delépine L, Aubineau P (1997) Plasma protein extravasation induced in the rat dura mater by stimulation of the parasympathetic sphenopalatine ganglion. Exp Neurol 147:389–400. doi:10.1006/exnr.1997.6614
Johnson K, Bolay H (2006) Neurogenic inflammatory mechanisms in migraine. In: The headaches, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 309–319
Millan MJ, Gramsch C, Przewłocki R et al (1980) Lesions of the hypothalamic arcuate nucleus produce a temporary hyperalgesia and attenuate stress-evoked analgesia. Life Sci 27:1513–1523
Butler RK, Finn DP (2009) Stress-induced analgesia. Prog Neurobiol 88:184–202. doi:10.1016/j.pneurobio.2009.04.003
Frost JJ, Mayberg HS, Sadzot B et al (1990) Comparison of [11C]diprenorphine and [11C]carfentanil binding to opiate receptors in humans by positron emission tomography. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 10:484–492. doi:10.1038/jcbfm.1990.90
Valet M, Sprenger T, Boecker H et al (2004) Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain–an fMRI analysis. Pain 109:399–408. doi:10.1016/j.pain.2004.02.033
Treede RD, Kenshalo DR, Gracely RH, Jones AK (1999) The cortical representation of pain. Pain 79:105–111
Gauriau C, Bernard J-F (2004) A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468:24–56. doi:10.1002/cne.10873
Gauriau C, Bernard J-F (2004) Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci Off J Soc Neurosci 24:752–761. doi:10.1523/JNEUROSCI.3272-03.2004
Rainville P, Duncan GH, Price DD et al (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971
May A, Kaube H, Büchel C et al (1998) Experimental cranial pain elicited by capsaicin: a PET study. Pain 74:61–66
Sato F, Akhter F, Haque T et al (2013) Projections from the insular cortex to pain-receptive trigeminal caudal subnucleus (medullary dorsal horn) and other lower brainstem areas in rats. Neuroscience 233:9–27. doi:10.1016/j.neuroscience.2012.12.024
Noseda R, Constandil L, Bourgeais L et al (2010) Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci Off J Soc Neurosci 30:14420–14429. doi:10.1523/JNEUROSCI.3025-10.2010
Price DD, Dubner R (1977) Neurons that subserve the sensory-discriminative aspects of pain. Pain 3:307–338
Iwata K, Miyachi S, Imanishi M et al (2011) Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Exp Neurol 227:69–78. doi:10.1016/j.expneurol.2010.09.013
Lorenz J, Cross DJ, Minoshima S et al (2002) A unique representation of heat allodynia in the human brain. Neuron 35:383–393
Dubner R, Bennett GJ (1983) Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci 6:381–418. doi:10.1146/annurev.ne.06.030183.002121
Craig AD (2003) A new view of pain as a homeostatic emotion. Trends Neurosci 26:303–307
Hadjipavlou G, Dunckley P, Behrens TE, Tracey I (2006) Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain 123:169–178. doi:10.1016/j.pain.2006.02.027
Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain J Neurol 126:1079–1091
Brighina F, Piazza A, Vitello G et al (2004) rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci 227:67–71. doi:10.1016/j.jns.2004.08.008
Zhao L, Liu J, Dong X et al (2013) Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain 14:85. doi:10.1186/1129-2377-14-85
Tessitore A, Russo A, Giordano A et al (2013) Disrupted default mode network connectivity in migraine without aura. J Headache Pain 14:89. doi:10.1186/1129-2377-14-89
Schwedt TJ, Schlaggar BL, Mar S et al (2013) Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 53:737–751. doi:10.1111/head.12081
Ichesco E, Quintero A, Clauw DJ et al (2012) Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache 52:441–454. doi:10.1111/j.1526-4610.2011.01998.x
Qiu E, Wang Y, Ma L et al (2013) Abnormal brain functional connectivity of the hypothalamus in cluster headaches. PLoS One 8:e57896. doi:10.1371/journal.pone.0057896
Rocca MA, Valsasina P, Absinta M et al (2010) Central nervous system dysregulation extends beyond the pain-matrix network in cluster headache. Cephalalgia Int J Headache 30:1383–1391. doi:10.1177/0333102410365164
Yang P-F, Qi H-X, Kaas JH, Chen LM (2014) Parallel functional reorganizations of somatosensory areas 3b and 1, and S2 following spinal cord injury in squirrel monkeys. J Neurosci Off J Soc Neurosci 34:9351–9363. doi:10.1523/JNEUROSCI.0537-14.2014
Morelli N, Rota E, Gori S et al (2013) Brainstem activation in cluster headache: an adaptive behavioural response? Cephalalgia Int J Headache 33:416–420. doi:10.1177/0333102412474505
Riederer F, Gantenbein AR, Marti M et al (2013) Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci Off J Soc Neurosci 33:15343–15349. doi:10.1523/JNEUROSCI.3804-12.2013
Bolay H, Berman NEJ, Akcali D (2011) Sex-related differences in animal models of migraine headache. Headache 51:891–904. doi:10.1111/j.1526-4610.2011.01903.x
Peterlin BL, Gupta S, Ward TN, Macgregor A (2011) Sex matters: evaluating sex and gender in migraine and headache research. Headache 51:839–842. doi:10.1111/j.1526-4610.2011.01900.x
Gazerani P, Andersen OK, Arendt-Nielsen L (2005) A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain 118:155–163. doi:10.1016/j.pain.2005.08.009
Andreason PJ, Zametkin AJ, Guo AC et al (1994) Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res 51:175–183
Wager TD, Phan KL, Liberzon I, Taylor SF (2003) Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19:513–531
Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D (2011) Local brain connectivity and associations with gender and age. Dev Cogn Neurosci 1:187–197. doi:10.1016/j.dcn.2010.10.001
Maleki N, Becerra L, Brawn J et al (2012) Concurrent functional and structural cortical alterations in migraine. Cephalalgia Int J Headache 32:607–620. doi:10.1177/0333102412445622
Paulson PE, Minoshima S, Morrow TJ, Casey KL (1998) Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76:223–229
Derbyshire SWG, Nichols TE, Firestone L et al (2002) Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain Off J Am Pain Soc 3:401–411
De Leeuw R, Albuquerque RJC, Andersen AH, Carlson CR (2006) Influence of estrogen on brain activation during stimulation with painful heat. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg 64:158–166. doi:10.1016/j.joms.2005.10.006
Chang Z, Okamoto K, Bereiter DA (2012) Differential ascending projections of temporomandibular joint-responsive brainstem neurons to periaqueductal gray and posterior thalamus of male and female rats. Neuroscience 203:230–243. doi:10.1016/j.neuroscience.2011.11.042
Boes T, Levy D (2012) Influence of sex, estrous cycle, and estrogen on intracranial dural mast cells. Cephalalgia Int J Headache 32:924–931. doi:10.1177/0333102412454947
Saleh TM, Connell BJ, McQuaid T, Cribb AE (2003) Estrogen-induced neurochemical and electrophysiological changes in the parabrachial nucleus of the male rat. Brain Res 990:58–65
Greco R, Mangione A, Siani F et al (2013) Effects of CGRP receptor antagonism in nitroglycerin-induced hyperalgesia. Cephalalgia Int J Headache 34:594–604. doi:10.1177/0333102413517776
Eikermann-Haerter K, Baum MJ, Ferrari MD et al (2009) Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann Neurol 66:564–568. doi:10.1002/ana.21779
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bolay, H., Messlinger, K., Dux, M., Akcali, D. (2015). Anatomy of Headache. In: Ashina, M., Geppetti, P. (eds) Pathophysiology of Headaches. Headache. Springer, Cham. https://doi.org/10.1007/978-3-319-15621-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-15621-7_1
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15620-0
Online ISBN: 978-3-319-15621-7
eBook Packages: MedicineMedicine (R0)