Abstract
The field of nanotechnology has created great interest among researchers due to its remarkable outcomes in different fields of optoelectronics, medical, pharmaceuticals, chemical, and agricultural importance. It is an emerging cutting-edge technology involving different methodologies for the synthesis of nanoparticles of particular size and shapes. Development of experimental protocols for synthesis of metal nanoparticles of specific size and shape is a necessary advancement of nanotechnology. Although physical and chemical methods have been successfully used to synthesize metal nanoparticles, there is a persistent necessity to develop eco-friendly and sustainable techniques for the synthesis of nanoparticles. Biosynthesis of nanoparticles using a number of fungi, bacteria, actinomycetes, lichen, and viruses have been reported till date but the plant system has emerged as an efficient system due to its distinctive characters like easy availability, low cost, green approach, simpler downstream processing, etc. In the plant system, biosynthesis process is more useful if nanoparticles are produced extracellularly using plants or their extracts and in a controlled approach related to their size, dispersity, and shape. Plant system can also be suitably scaled up for large-scale synthesis of nanoparticles. However, some aspects like role of different biomolecules in synthesis of nanoparticles, understanding the biological mechanism of synthesis process needs to be considered elaborately. In this chapter, we have discussed briefly about plants as a prominent tool for the synthesis of metal nanoparticles. Moreover, different methods of synthesis of nanoparticles, different mechanisms involved in the synthesis process, and also the potential applications of metal nanoparticles have also been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Nanotechnology is an interdisciplinary field of science including physics, chemistry, biology, and material science. The nanoparticles are an indispensable part of nanotechnology (Parashar et al. 2009a; Bankar et al. 2010; Zhang et al. 2011; Mahdavi et al. 2013). Engineered metal nanoparticles are produced by a number of physical and chemical methods. However, these methods are harmful as the chemicals used are generally toxic, flammable, and not easily disposable due to environmental issues, expensive, and have low production rate (Kasthuri et al. 2008; Bankar et al. 2010; Nagajyothi and Lee 2011). Thus, instead of using toxic chemicals for the synthesis of metal nanoparticles, the use of biological entities has received substantial consideration in the field of nanobiotechnology (Logeshwari et al. 2013). The biological methods for the synthesis of metal nanoparticles are regarded as safe, cost-efficient, sustainable, and toward greener approach (Marchiol 2012). Hence, extensive contribution have been made to employ biological systems for the synthesis of metal nanoparticles at ambient temperature and pressure conditions without the use of any toxic chemicals and also without production of any poisonous byproducts (Kumar and Yadav 2009; Satyavathi et al. 2010; Gopalkrishnan et al. 2012). A variety of microorganisms including bacteria, fungi, and yeasts have been harnessed as potential nanofactories for intra and extracellular synthesis of metal nanoparticles (Sharma et al. 2009; Mallikarjuna et al. 2011; Renugadevi and Aswini 2012; Iravani and Zolfaghari 2013). However, the use of plant system for the production of metal nanoparticles is an upcoming research field (Iravani 2011). The use of plants for the synthesis of metal nanoparticles offers an environment friendly, cost-effective, and legitimate alternative for large-scale production of metal nanoparticles (Marchiol 2012; Logeshwari et al. 2013).
The present chapter deals with the use of plants for the synthesis of metal nanoparticles and the several aspects related to the process, the mechanism of synthesis in plants, and the applications of the system.
12.2 Plants as the System of Choice
Among the different living systems harnessed for the synthesis of metal nanoparticles, plants have found predominant application in the synthesis process as the use of plants for the biosynthesis of metal nanoparticles could be beneficial compared to other biological agents (Rai et al. 2008; Mude et al. 2009; Jha and Prasad 2010; Duran et al. 2011; Renugadevi and Aswini 2012; Dinesh et al. 2012). In the case of plant systems, the elaborate process of maintaining cell cultures is eliminated (Marchiol 2012). Also, biological synthesis of metal nanoparticles involves synthesis in a controlled manner according to their size, dispersity, and shape (Shankar et al. 2004; Ankamwar et al. 2005; Parashar et al. 2009a, b).
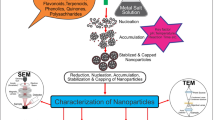
The plant system can also be duly scaled up for large-scale synthesis of nanoparticles (Rai et al. 2008). Different metals especially silver and gold have been extensively studied for the phytosynthesis of metal nanoparticles employing plant extracts and plant biomass (Marchiol 2012; Mahdavi et al. 2013) (Fig. 12.1 ). It has been depicted that many plant species can actively uptake and bioreduce metal ions from soil and solutions during detoxification process and thereby form insoluble complexes with the metal ion in the form of nanoparticles (Goldsbrough 2000). This natural phenomenon of heavy metal tolerance of plants attracted researchers to explore the related biological mechanisms as well as physiology and genetics of metal tolerance in hyperaccumulator plants (Baker and Brooks 1989; Memon and Schröder 2008). Thus, the researchers concentrated on the use of plants with potential in phytomining and phytoremediation of heavy metals in order to phytosynthesize metallic nanoparticles. Gardea-Torresdey et al. (2002) presented the first report of synthesis of nanoparticles using alfalfa seedlings which is considered as a hyperaccumulator plant. It was revealed that gold nanoparticles, ranging in size from 2 to 20 nm, could be synthesized inside live plants.
Also, trending research in biosynthesis of nanometals using plant extracts, fruit extract, and bark and root extracts has opened a new era in easy, fast, and eco-friendly methods for the synthesis of metal nanoparticles (Sharma et al. 2009; Thakkar et al. 2010; Iravani et al. 2011; Iravani and Zolfaghari 2013). Many researchers have explored the phytosynthesis of metal nanoparticles using different plant extracts and their potential applications (Gardea-Torresdey et al. 2002; Shankar et al. 2004; Chandran et al. 2006; Harris and Bali 2007; Haverkamp and Marshall 2009; Mude et al. 2009; Jha and Prasad 2010; Duran et al. 2011; Renugadevi and Aswini 2012; Dinesh et al. 2012; Mahdavi et al. 2013).
Thus, green nanotechnology has involved a lot of interest of researchers compared to other biological systems and includes a wide range of procedures that reduce or eliminate toxic substances to restore the environment. Also, the phytosynthesis of metal nanoparticles using plant extracts and other parts of living plants has become a current substitute for the production of metal nanoparticles. As phytosynthesis of metal nanoparticles involves use of environmental friendly, nontoxic, and safe reagents.
12.2.1 Mechanism of Synthesis
The precise mechanism for the formation of metal nanoparticles using plants is not yet known, nor investigated in depth (Rai et al. 2008; Haverkamp and Marshall 2009). Biosynthesis of metal nanoparticles is a bottom-up approach of synthesis where reduction/oxidation is the main reaction by which synthesis takes place (Marchiol 2012). Various microorganisms such as bacteria, fungi, and yeasts are considered as nanofactories for intra- and extracellular synthesis of metal nanoparticles (Lovley et al. 1987; Ahmad et al. 2003; Husseiny et al. 2007; Singaravelua et al. 2007). Whereas, use of plant system for biosynthesis of metal nanoparticle is a comparatively new and under advancement research technique (Marchiol 2012).
The bioreduction of metal nanoparticles in plants occurs by a combination of bioactive compounds present in plant extracts like enzymes, proteins, amino acids, vitamins, polysaccharides, etc. (Iravani 2011). Several researchers have reported efficient and rapid extracellular synthesis of silver, gold, copper, and gold nanoparticles using extracts of several plants; for example, Aloe vera (Chandran et al. 2006), Medicago sativa (Gardea-Torresdey et al. 2002), Azadirachta indica (Shankar et al. 2004), Avena sativa (Armendariz et al. 2004), Emblica officinalis (Ankamwar et al. 2005), Humulus lupulus (Rai et al. 2006), Spinacia oleracea and Lactuca sativa (Kanchana et al. 2011), Capsicum annum (Jha and Prasad 2011), Tridax procumbens (Gopalkrishnan et al. 2012), and Sargassum muticum (Mahdavi et al. 2013).
Shankar et al. (2004) employed neem (A. indica) leaf extract for the synthesis of silver nanoparticles. The FTIR spectra showed the presence of reducing sugars and flavones or terpenoids in the sample. Hence, it was supposed that the reducing sugars are responsible for the reduction of silver ion to silver nanoparticles while the flavones or terpenoids act as the capping agent. The TEM images of the reaction mixture gave a picture of synthesis of polydisperse spherical nanoparticles. While, the XRD spectra confirmed the crystalline nature of nanoparticles.
Capsicum annum extract was used by Li et al. (2007) for the synthesis of silver nanoparticles. The fruit extract depicted rapid change in coloration from green to dark-brown marking synthesis of silver nanoparticles. The UV-visible spectra demonstrated peak at 440 nm and the TEM images confirmed the synthesis of spherical nanoparticles. In this study, mechanism of recognition-reduction-limited nucleation and growth for the synthesis of nanoparticles was proposed by the authors. It was projected that the silver ions undergo electrostatic interaction with the proteins present in the extract which leads to the formation of silver complex. Further, the flexible linkages of proteins and other biomolecules lead to the synthesis of stable spherical nanoparticles (Fig. 12.2).
Schematic representation of mechanism of synthesis of silver nanoparticles (Li et al. 2007, Reproduced with permission from Royal Society of Chemistry)
Bioactive compound phyllanthin isolated from Phyllanthus amarus was harnessed by Kasthuri et al. (2008) for the biosynthesis of silver nanoparticles. In the study, UV-visible absorbance peak at 439 nm with a shift at 446 nm was observed and the TEM analysis of the nanoparticles depicted synthesis of quasispherical nanoparticles with average size about 30 nm. The cyclic voltammetry measurements showed that upon addition of phyllanthin extract to the reaction medium, the cathodic peak gets shifted toward the negative direction suggesting that the silver nanoparticles gets stabilized by the phyllanthin extract. The FTIR spectra also revealed that the –OCH3 group of the phyllanthin extract plays a leading role in the formation and stabilization of nanoparticles (Fig. 12.3).
Schematic representation of formation of phyllanthin stabilized gold and silver nanoparticles (Kasthuri et al. 2008, Reproduced with permission from Springer.com)
In a similar way, latex extract of Jatropha curcas was also harnessed for the synthesis of silver nanoparticles by Bar et al. (2009a). The HRTEM images of the study illustrated two broad range distributions of nanoparticles, with diameter 20–30 nm and some larger diameter an uneven shapes. The XRD spectra of the biosynthesized silver nanoparticles revealed the crystalline nature of silver nanoparticles, the EDX spectra also showed strong signal of silver. The latex of J. curcas was observed to curcacycline A, curcacycline B, and curcain. Thus, it was hypothesized that the silver ions get entrapped into the core structure of curcacycline A or curcacycline B and get reduced and stabilized in situ by the amide group which results in the formation of silver nanoparticles and the enzyme curcain functions as a stabilizing agent of the nanoparticles.
Bar et al. (2009b) used the seed extract of J. curcas for the synthesis of silver nanoparticles. The UV-visible spectra of the silver nanoparticles depicted an absorbance peak at 425 nm and the HRTEM and XRD studies also showed predominant synthesis of spherical nanoparticles with polycrystalline nature. The FTIR study of the biosynthesized silver nanoparticles demonstrated that the amide groups were responsible for the reduction of silver ions while, the proteins acted as stabilizing agent for the nanoparticles.
Singh et al. (2011) also harnessed latex of Calotropis procera for biosynthesis of zinc oxide (ZnO) nanoparticles. The TEM and SEM studies of the biosynthesized nanoparticles depicted formation of spherical-shaped nanoparticles, granular in nature, and average size 5–40 nm. The XRD study also revealed the presence of crystalline-natured zinc oxide nanoparticles. In the above study, latex of C. procera plant was supposed to be the reducing as well as the stabilizing agent for the synthesis of zinc oxide nanoparticles.
Yadav and Rai (2011) demonstrated the synthesis of silver nanoparticles using Holarrhena antidysenterica and studied the mechanistic aspects related to it. In the study, the authors proposed the possible role of terpenoids for the bioreduction of silver ions. The proteins were observed to act as an encapsulating and stabilizing agent to protect agglomeration of silver nanoparticles. The ESI (Elemental Spectroscopy Imaging) analysis of the silver nanoparticles also confirmed the stabilization of nanoparticles by proteins. The FTIR spectra of the silver nanoparticles depicted well-known signatures of amide linkages in proteins.
Green synthesis of palladium (Pd) nanoparticles was depicted by Petla et al. (2012) using Glycine max (soyabean) leaf extract. The change in coloration of the soyabean leaf extract after treatment with palladium ions from orange to dark-brown was marked as the synthesis of Pd nanoparticles. The UV-vis spectra at 420 nm also confirmed the formation of nanoparticles. The authors believed that the proteins and some of the amino acids present in the leaf extract were responsible for the synthesis of Pd nanoparticles. The FTIR analysis also corroborated that the amino acids were not only involved in the synthesis process but also acted as surfactants inhibiting rapid agglomeration of nanoparticles.
Tridax procumbens was exploited for the synthesis of copper oxide nanoparticles by Gopalkrishnan et al. (2012). In the study, the authors observed that the water soluble carbohydrates present in plants were responsible for the reduction of copper ions and formation of copper oxide nanoparticles. The antibacterial activity of copper oxide nanoparticles was also checked against E. coli. It was found that nanoparticles at concentration of 20 µg cm−3 inhibited 65 % bacterial growth while, nanoparticles at a concentration of 30 µg cm−3 inhibited 100 % bacterial growth.
Mahdavi et al. (2013) exploited green biosynthesis method for reduction of ferric chloride solution with brown seaweed (BS, S. muticum). The water extract of brown seaweed containing sulfated polysaccharides was considered as the main factor which acted as the reducing agent and efficient stabilizer for iron oxide nanoparticles. The structure and properties of the iron oxide nanoparticles were investigated using X-ray Diffraction, Fourier Transform Infrared Spectroscopy, Field Emission Scanning Electron Microscopy (FESEM), Energy Dispersive X-ray Fluorescence Spectrometry (EDXRF), Vibrating Sample Magnetometry (VSM), and Transmission Electron Microscopy. The average particle diameter of iron oxide nanoparticles was found to be 18 ± 4 nm. The X-ray diffraction study showed that the nanoparticles are crystalline in nature, with a cubic shape.
Iravani and Zolfaghari (2013) synthesized silver nanoparticles using Pinus eldarica bark extract. The effects of quantity of the extract, substrate concentration, temperature, and pH on the formation of silver nanoparticles were also studied. The TEM images depicted that biosynthesized silver nanoparticles were predominantly spherical in shape with approximately size range of 10–40 nm.
Logeswari et al. (2013) reported biosynthesis of silver nanoparticles by commercially available plant powders, such as Solanum tricobatum, Syzygium cumini, Centella asiatica, and Citrus sinensis. The characterization of silver nanoparticles was done by UV–Vis Spectrophotometer, X-Ray Diffractometer (XRD), Atomic Force Microscopy (AFM), and Fourier Transform Infrared (FTIR) Spectroscopy. The AFM study showed irregular shapes of silver nanoparticles, and the size was found to be 53, 41, 52, and 42 nm, corresponding to S. cumini, C. sinensis, S. tricobatum and C. asiatica, respectively. The FTIR Spectroscopy confirmed the presence of protein as the stabilizing agent surrounding the silver nanoparticles.
12.3 Applications
-
Metal nanoparticles offer great interest in different disciplines including biotechnology/biomedicine, bioremediation, agriculture, catalyst, biosensors, etc. Functionalized nanoparticles present immense potential in catalysis, bio-labeling, and bioseparation (Gupta et al. 2012).
-
Nanotube membranes are harnessed as channels for separation of molecules and ions between solutions hence, used as biomembranes. These nanotube biomembranes separate nanoparticles based on their size while, membrane with dimension 20–60 nm can be used to separate proteins (Gupta et al. 2012).
-
Magnetic nanoparticles are used as effective molecular carrier for gene separation. Magnetic nanoparticles also show potential application in drug delivery. In this process, magnetic nanoparticles are injected to drug molecule to be attached, these particles are then guided toward the chosen site under localized magnetic field and can carry large doses of drugs (Lu et al. 2007; Perez-Martinez et al. 2012).
-
Application of metal nanoparticles as catalyst is an immensely growing field. Nanoparticles due to their distinctive properties form an ideal component for catalyst. Platinum and gold bimetallic nanoparticles are used as electrocatalyst for polyelectrolyte fuel cells for the conversion of exhaust heat to energy (Toshima 2013). Titanium, gold, and silver heterostructures have also depicted electrochemical properties and are thus used as a photocatalyst (Zhang et al. 2013; Kawamura et al. 2013).
-
Metal nanoparticles have also depicted biological applications like silica-coated nanoparticles are biocompatible structures used in artificial implants and drug delivery due to their high stability, surface properties, and compatibility (Dikpati et al. 2012; Perez-Martinez et al. 2012). Polyethylenimine-derived (PEI) nanoparticles and dendrimers have also shown applications like gene delivery, catalysis, and electronics (Perez-Martinez et al. 2012; Dikpati et al. 2012).
-
Magnetite nanoparticles demonstrate application in wastewater treatment and removal of heavy metals from water through single-step removal of some model organophosphorus pesticide from water along with some microorganisms (Das et al. 2009). Magnetite nanoparticles are also harnessed as adsorbents for separating and removing the contaminants in water by applying external magnetic fields (Carlos et al. 2013).
-
Metal nanoparticles with unique properties also offer use in the detection and destroying of pesticides (Argay et al. 2012). The optical properties of nanoparticles related to their size and surface helps in the detection of pesticides. However, for destruction of pesticides photocatalytic oxidation method employing titanium nanoparticles is used (Argay et al. 2012).
12.4 Future Prospects and Conclusion
Varying number of chemical, physical, and biological methods are used for the production of metal nanoparticles. But, most of these methods are still in the development stage and thus problems are faced regarding the stability and aggregation of metal nanoparticles and morphology and size distribution. It is observed that the metal nanoparticles synthesized by plants are more stable in comparison with those produced by other organisms. Plants reduce metal ions faster than fungi or bacteria. In addition, use of plants offers an easy and safe green method to scale-up production of well-dispersed metal nanoparticles. Hence, researchers have focused their attention on understanding the biological mechanisms and enzymatic processes for synthesis of metal nanoparticle using plants and detection of biomolecules involved in the synthesis of metallic nanoparticles. Many biomolecules present in plant extracts like proteins/enzymes, amino acids, polysaccharides, alkaloids, alcoholic compounds, and vitamins are found to be involved in bioreduction, formation, and stabilization of metal nanoparticles. The future investigations related to the use of plant system would focus toward the optimization of reaction conditions and engineering the recombinant organisms for production of high amounts of proteins, enzymes, and biomolecules involved in biosynthesis and stabilization of nanoparticles. Understanding the biochemical processes/pathways involved in plant heavy metal detoxification, accumulation, and resistance will also be studied to improve nanoparticle production. Genetic modification of plants with improved metal tolerance and accumulation capacities is also a future approach to increase the production of metal nanoparticle synthesis.
References
Ahmad A, Senapati S, Khan MI et al (2003) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilicactinomycete Thermomonospora sp. Langmuir 19:3550–3553
Ankamwar B, Chaudhary M, Sastry M (2005) Gold nanotriangles biologically synthesized using tamarind leaf extract and potential applications in vapour sensing. Syn React Inorg Metal-Org Nano-Met Chem 35(1):19–26
Aragay G, Pino F, Merkoci A (2012) Nanomaterials for sensing and destroying pesticides. Chem Rev 112:5317–5338
Armendariz V, Gardea-Torresdey JL, Herrera I et al (2004) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 6(4):377–382
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyper accumulate chemical elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Bankar A, Joshi B, Kumar AR et al (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A 368:58–63
Bar H, Bhui DK, Sahoo GP et al (2009a) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A 339:134–139
Bar H, Bhui DK, Sahoo GP et al (2009b) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A 348:212–216
Carlos L, Einschlag FSG, Gonzalez MC et al (2013) Applications of magnetite nanoparticles for heavy metal removal from wastewater. doi:10.5772/54608
Chandran SP, Ahmad A, Chaudhary M et al (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22(2):577–583
Das SK, Das AR, Guha AK (2009) Gold nanoparticles: microbial synthesis and application in water hygiene management. Langmuir 25(14):8192–8199
Dikpati A, Madgulkar AR, Kshirsagar SJ et al (2012) Targeted drug delivery to CNS using nanoparticles. JAPS J 2(1):179–191
Dinesh S, Karthikeyan S, Arumugam P (2012) Biosynthesis of silver nanoparticles from Glycyrrhiza glabra root extract. Arch Appl Sci Res 4(1):178–187
Duran N, Marcato PD, Duran M et al (2011) Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi and plants. Appl Microbiol Biotechnol 90:1609–1624
Gardea-Torresdey JL, Gombez E, Parsons JG et al (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2(4):397–401
Goldsbrough P (2000) Metal tolerance in plants: the role of phyto-chelatins and metallothioneins. In: Terry N, Banelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishing, Boca Raton, pp 221–234
Gopalkrishnan K, Ramesh C, Raghunathan V et al (2012) Antibacterial activity of Cu2O nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline. Digest J Nanomat Biostruct 7(2):833-839
Gupta S, Sharma K, Sharma R (2012) Myconanotechnology and applications of nanoparticles in biology. Recent Res Sci Technol 4(8):36–38
Harris AT, Bali R (2007) On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopar Res 11051:9288–9293
Haverkamp RG, Marshall AT (2009) The mechanism of nanoparticle formation in plants: limits on accumulation. J Nanopar Res 11:1453–1463
Husseiny MI, AbdEl-Aziz M, Badr Y et al (2007) Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A 67:1003–1006
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650
Iravani S and Zolfaghari B (2013) Green synthesis of silver nanoparticles using Pinuseldarica bark extract. Biomed Res Int Article ID:639725
Jha AK, Prasad K (2010) Green synthesis of silver nanoparticles using Cycas leaf. Int J Green Nanotechnol Phys Chem 1:110–117
Jha AJ, Prasad K (2011) Green fruit of chili (Capsicum annum L.) synthesizes nano silver. Digest J Nanomater Biostruct 6:1717–1723
Kanchana A, Agarwal I, Sunkar S et al (2011) Biogenic silver nanoparticles from Spinaciaoleracea and Lactuca sativa and their potential antimicrobial activity. Digest J Nanomater Biostruct 6:741–1750
Kasthuri J, Kanthiravan K, Rajendiran N (2008) Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopar Res 15:1075–1085
Kawamura G, Nogami M, Matsuda A (2013) Shape-controlled metal nanoparticles and their assemblies with optical functionalities. J Nanomater Article ID:631350
Kumar V, Yadav SK (2009) Plant-mediaed synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84:151–157
Li S, Qui L, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annum L. extract. Green Chem 9:852–858
Logeshwari P, Silambarasan S, Abraham J (2013) Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica F 20(3):1049–1054
Lovley DR, Stolz JF Jr, Nord GL et al (1987) Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 330:252–254
Lu AH, Salabas EL, Schuth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization and application. Angew Chem Int Ed 46:1222–1244
Mahdavi M, Namvar F, Ahmad MB et al (2013) Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 18:5954–5964
Mallikarjuna K, Narasimha G, Dilip GR et al (2011) Green synthesis of silver nanoparticles using Ocimumleaf extract and their characterization. Digest J Nanomater Biostruct 6(1):181–186
Marchiol L (2012) Synthesis of metal nanoparticles in living plants. Ital J Agron 7:274–282
Memon AR, Schröder P (2008) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res 16:162–175
Mude N, Ingle A, Gade A et al (2009) Synthesis of silver nanoparticles by the callus extract of Carica papaya: a first report. Plant Biochem Biotechnol 18:83–86
Nagajyothi PC, Lee KD (2011) Synthesis of plant mediated silver nanoparticles using Dioscorea batatas rhizome extract and evaluation of their antimicrobial activities. J Nanomater Article ID:573429
Parashar V, Parashar R, Sharma B et al (2009a) Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Digest J Nanomater Biostruct 4(1):45–50
Parashar UK, Saxena PS, Shrivastava A (2009b) Bioinspired synthesis of silver nanoparticles. Digest J Nanomater Biostruct 4(1):159–166
Perez-Martinez FC, Carrion B, Cena V (2012) The use of nanoparticles for gene therapy in the nervous system. J Alzheimer’s Dis 31:697–710
Petla RK, Vivekanandhan S, Misra M et al (2012) Soyabean (Gylcine max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol 3:14–19
Rai A, Singh A, Ahmad A et al (2006) Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 22:736–741
Rai M, Yadav A, Gade A (2008) Current trends in phytosynthesis of metal nanoparticles. Crit Rev Biotechnol 28(4):277–284
Renugadevi K, Aswini RV (2012) Microwave irradiation assisted synthesis of silver nanoparticles using Azadirachta indica leaf extract as a reducing agent and in vitro evaluation of its antibacterial and anticancer activity. Int J Nanomater Biostruct 2(2):5–10
Satyavathi R, Krishna MB, Rao SV et al (2010) Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in non-linear optics. Adv Sci Lett 3:1–6
Shankar SS, Ahmad A, Rai A et al (2004) Rapid synthesis of Au, Ag and bimetallic Au core-Ag shell nanoparticles by using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275(5):496–502
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83–96
Singaravelua G, Arockiamaryc JS, Ganesh Kumar V et al (2007) Novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B 57:97–101
Singh RP, Shukla VK, Yadav RS et al (2011) Biological approach of zinc oxide nanoparticles formation and its characterization. Adv Mat Lett 2(4):313–317
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomedicine 6(2):257–262
Toshima N (2013) Metal nanoparticles for energy conversion. Pure Appl Chem 85(2):437–451
Yadav A, Rai M (2011) Bioreduction and mechanistic aspects involved in the synthesis of silver nanoparticles using Holarrhena antidysenterica. J Bionanosci 5:70–73
Zhang X, Yan S, Tyagi RD et al (2011) Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82(11):489–494
Zhang G, Duan H, Lu B et al (2013) Electrospinning directly synthesized metal nanoparticles decorated on both sidewalls of TiO2 nanotubes and their applications. Nanoscale 5:5801–5808
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yadav, A., Rai, M. (2015). Phytosynthesis of Metal Nanoparticles. In: Siddiqui, M., Al-Whaibi, M., Mohammad, F. (eds) Nanotechnology and Plant Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-14502-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-14502-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14501-3
Online ISBN: 978-3-319-14502-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)