Abstract
Synthesis of nanoparticles using biomaterials such as plants is regarded as a cost-effective and reliable approach. This article reviews published papers related to the use of live plants in extracellular and intracellular gold and silver nanoparticles synthesis. Using live plants for the generation of extracellular nanoparticles provides a reliable and simple approach, through the elimination of various production steps. This review showed that root exudates of living plant species contain biomolecules such as enzymes, proteins, phenolics, polysaccharides, and amino acids, which contribute to the rapid and environmentally friendly production of gold and silver nanoparticles. Living plants have been successful in synthesizing intra- and extracellular gold and silver nanoparticles of different shapes like cubic, spherical, rod, triangle, and also in different sizes. Further, the factors and mechanisms that contribute to both the intracellular and extracellular synthesis of these nanoparticles by living plants have been briefly discussed. This study summarizes the important contribution of living plants to the phytosynthesis of gold and silver nanoparticles and their possible applications in diverse fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology research involves the manipulation of atoms and molecules in different ways in order to provide an understanding on how materials can be formed [1]. The design of a safe and environmentally friendly method to synthesize metal nanoparticles (NPs) is a key requirement in the field of nanotechnology [2, 3]. To achieve this, it is necessary to use biomaterials to produce low-cost, energy-efficient, and non-toxic metal NPs [4, 5]. NPs with different chemical compositions are typically synthesized under brutal conditions including temperature extremities, pH, and pressure [6, 7]. In addition, these processes are environmentally undesirable and burdensome, generate larger molecules, and cluster as capping agents do not restrict them [8, 9].
In contrast, biologically synthesized NPs are carried out under environmentally benign conditions such as atmospheric pressure, physiological pH, and room temperatures. These are efficient, environmentally friendly, and cost-effective methods [10]. Various species including fungi [11] and microorganisms [12] have been used to synthesize NPs for various purposes. Applications of biologically synthesized NPs include wastewater treatment [13], drug delivery [14, 15], antimicrobial activities [16, 17], and biosensors [18, 19].
Furthermore, in the last three decades, the synthesis of NPs based on plant materials has been studied, whereas in the last ten and half years, the production of NPs using living plants has only been investigated [20]. Plant extracts have recently been properly evaluated in NPs-mediated synthesis [21,22,23,24]. Although a range of studies have used plant biomass [25, 26] and extracts in various ways to synthesize NPs, few studies have used living plants. The utilization of extracts from plant parts of varying plant species have demonstrated the use of potassium chloroaurate (KAuCl4)- and silver nitrate (AgNO3)-mediated synthesis for gold nanoparticles (AuNPs) and silver (AgNPs) [27,28,29,30].
NPs, particularly biosynthesized by live plants as a result of absorption of soluble salts and reduction of metallic ions, have been used extensively in numerous applications such as plant imaging [31], pathogen sensing, proton conductive plants, improved CO2 capture, bacteria-free nitrogen fixation, drought and fungi resistance, and enhanced photosynthesis and photocatalysis [32,33,34,35] as well as a range of environmental and industrial reactions [36].
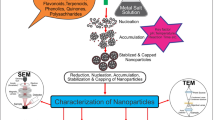
This article reviews both the extracellular and intracellular Au and AgNPs synthesis by living plants along with their applications. Further discussed is the mechanisms and factors contributing to the synthesis of these NPs by living plants. Finally, challenges and future prospects have been highlighted.
Extracellular Au and AgNPs synthesis
As a promising approach to metal NPs synthesis, live plants have proven to be successful. Plant cells and exudates are viewed as possible biofactories for the synthesis of NPs such as Au and Ag (Table 1). However, only a few research studies have shown that root exudates of live plant species can contribute to extracellular synthesis of NPs over the past ten years. This biosynthesis approach to environmentally sustainable NPs has many benefits, including the capability to expand the process and its economic viability. It removes the need for NPs extraction from the plant and reduces the manufacturing procedure in contrast with conventional NPs synthesis processes.
Plants are known to produce NPs extracellularly. For example, Raju et al. [10] showed for the first time the extracellular AuNPs synthesis employing live peanut seedlings (Fig. 1). Within the cellular membranes and in solution, the synthesized AuNPs were extremely stable. Microscopic studies with the transmission electron (TEM) showed that extracellular AuNPs were monodispersed with size between 4 and 6 nm. Also, AuNPs were developed extracellularly after interactions between the root exudates (of B. juncea and M. sativa) and aqueous solution of KAuCl4 that reduced Au3+ to AuNPs in another research [37]. Most of the particles were 5–10 nm and 10–20 nm for B. juncea and M. sativa, respectively.

Reproduced from Ref. [10])
(a) Plant that grew on paper, (b) 24-h, (c) 48-h, (d) 72-h exposed seedlings, and (e) 10−4 M HAuCl4 solution (
Also, it has been documented that cowpea seedlings grown in HAuCl4 did not exhibit any plant growth suppression, even at levels up to 1 mM [38]. Surprisingly, during germination and seedling development, cowpea turned transparent pale-yellow HAuCl4 solution colloidal purple. These purple colloid suspensions have shown a unique surface plasmon resonance (SPR) spectra absorption which corresponds to AuNPs. The presence of crystalline AuNPs in these purple colloids was verified by XRD and TEM analysis. Every cowpea germination released ̴ 35 GAE nmoles of phenolics, and since phenolics help in the production of AuNPs, it was considered that the reduction of Au3+ to AuNPs was related to phenolics. The results showed that seed coat of cowpeas is Au3+ resistant as germination released phenolics which have the capability to minimize toxic Au3+ to less noxious AuNPs.
Pardha-Saradhi et al. [39] assessed the extracellular production of root surface AuNPs using 16 species of living plants from 11 different families. All the intact plants that had their root system turn the transparent pale-yellow colloid salty solution of Au purple or golden. The generation of crystalline AuNPs with size between 5 and 100 nm was confirmed by TEM, energy-dispersive X-ray (EDX) spectroscopy, and powder XRD. The decrease in 2,6-dichlorophenolindophenol through the root system of intact plants also confirmed that surfaces of the roots have strong decreasing strength needed for reducing Au3+ to AuNPs. These findings clearly show that the intact plant roots can be exploited under ambient conditions for the bulk exogenous synthesis of AuNPs in an aqueous environment.
In the case of Ag, Raju et al. [40] reported on the extracellular reduction of Ag ions to AgNPs using living peanut plants as shown in Fig. 2. TEM analysis revealed that the NPs formed were polydispersed and were of different shapes and dimensions. The NPs had spherical, triangular, square, hexagonal, and rod shapes. Most of the synthesized NPs had spherical form with a size of 56 nm. EDS evaluation verified that the NPs were of Ag and XRD also confirmed the crystalline structure of the AgNPs.

Reproduced from Ref. [40])
Peanut seedling exposed to deionized water (control), peanut seedling exposed to 1 mM AgNO3 solution for 24, 48, and 72 h. Inset image shows change in color of the root of peanut seedling exposed to 1 mM AgNO3 solution. C control, E exposed (
In a recent study by Ali et al. [41], AgNPs were synthesized exogenously under combined stresses of AgNO3 and several levels of drought stress simulated by polyethylene glycol (PEG) with living plant, T. apollinea. In order to assess the toxicological impact of the plant treatments, biomass, cell death, and H2O2 content were measured. Day 6 plants were more adversely affected than day 3 plants and at higher drought levels (Fig. 3). EDX, UV, SEM, XRD, and FTIR were used to identify and characterize the T. apollinea-synthesized AgNPs. The NPs were spherical and cubic forms, along with various phytochemicals as potential capping agents.

Adapted from Ref. [41])
The effect of AgNO3 and PEG treatments on plant phenotypes (
According to another research by Pardha-Saradhi et al. [42], studies were carried out to determine whether the reduction strength of root system surface can be exploited to reduce Ag+ to extracellular AgNPs. The intact root system of 16 species plants from 11 separate families of angiosperms converted transparent colorless AgNO3 solutions into pale brown solutions, as clearly shown in Fig. 4. UV spectra of these turbid brown solutions showed AgNPs-specific SPR peak at 380–450 nm. The presence of distinct AgNPs between 5 and 50 nm was reported in TEM combined with EDX. Selected area electron diffraction (SAED) and powder XRD patterns of the AgNPs revealed Bragg reflections, characteristic of crystalline Ag0 FCC structure, and Ag2O cubic structure. Ag0/Ag2O NPs were as well produced under strict sterile conditions with intact plant system in a manner similar to the ones reported under non-sterile conditions. The findings revealed the capacity of root system utilization in producing Ag0/Ag2O-NPs regardless of microorganisms.

Reproduced from Ref. [42])
Potential of intact plants root system for AgNPs generation. Root system of intact plants of (a) Phyllanthus fraternus, (b) Portulaca grandiflora, (c) Triticum aestivum, (d) Amaranthus gracilis, and (e) Vernonia cinerea showing the ability to modify transparent colorless AgNO3 with different concentrations (mM) turbid brown. No color change was noted in tubes containing various amounts of AgNO3 incubated without plants under similar conditions (
Furthermore, sodium citrates method was compared to the root system capacity of the intact plants of V. mungo and T. aestivum for the generation of AuNPs at room temperature and under strict sterile conditions. 0.02% sodium citrate (i.e., used in the Lee–Meisel process for producing AuNPs) failed even after incubation for 12 h at room temperature, to display any color changes in AgNO3 solution or the unique SPR of the AgNPs in the absorption spectra.
As can be seen in Fig. 5, the authors reported that the root system of intact V. mungo and T. aestivum plants incubated under environmental and sterile conditions changed the color of transparent AgNO3 to colloidal brown solution within 6 h and the AgNPs unique SPR peak was clearly revealed. When used at a concentration 50 times that of Lee–Meisel approach, sodium citrate therefore changed transparent colorless AgNO3 solutions grayish colloidal; nevertheless, the intensity of color and the SPR peak specific to AgNPs was substantially less than when a root system of intact plants was used in ambient conditions in the absorption spectrum of these colloidal solutions (Fig. 5). Dynamic light dispersion studies showed that with the root systems of V. mungo and T. aestivum, the mean particle size of AgNPs was smaller (20 nm) than with 1% sodium citrate-synthesized AgNPs (33 nm) under ambient conditions. These findings show clearly that the intact plant root system can be used for fast AgNPs synthesis under safer and clean conditions.

Reproduced from Ref. [42])
Potential of sodium citrate and root system of intact plants of 4-day-old V. mungo and T. aestivum to generate AgNPs. 1% sodium citrate (a) and root system of intact plants of V. mungo (b) and T. aestivum (c) incubated in AgNO3 of different concentrations (mM) for 6 h, showing alteration in color and turning clear solution colloidal under sterile conditions at room temperature. UV–Vis spectra of resultant colloidal solutions formed by 1% sodium citrate (d), V. mungo (e) and T. aestivum (f) (
Metal accumulation and intracellular synthesis of Au and AgNPs
Haverkamp et al. [43] recorded the first synthesis of mixed metal NPs in live B. Juncea plants that indicated that plants could be used for the development of catalysts with unique compositions, perhaps even the ones that are difficult to synthesize using conventional methods. The NPs were an alloy of Cu, Ag, and Au. STEM and energy-dispersive X-ray analytics (EDX) validated the structure and composition of the nanoalloys. Their study showed that NP alloys can be produced by changing the components of metal in a growth medium by the use of live plants.
A similar report by Anderson et al. [36] demonstrated assisted accumulation of gold up to a concentration greater than 3500 mg kg−1 (0.35%) dry weight, and discrete biogenic AuNPs were observed in shoot and leaf biomass of B. juncea growing on a mine waste. Moreover, Au and Ag nanoalloys were observed when equal Ag+ concentrations were used. The presence of Cu and Ag, however, reduced the size of the AuNPs. Also, the presence of Cu and/or Ag limited the extent of Au reduction to AuNPs in the plant tissues. As a viable technology to build high-concentration Au structures in a carbon matrix, this biogenic synthesis approach was therefore proposed for novel applications. According to another study, the known metallophytes, B. juncea and M. sativa, were evaluated for their capability to sequester and isolate Au from aqueous solutions of KAuCl4 [37]. Once isolated, some of the metal particles were stored as NPs throughout the cortex, epidermis, and vascular tissue for both species, but particularly found in the xylem parenchyma cells. Particle sizes, in general, ranged between 2 nm and 1 µm in M. sativa and 2 nm and 2 µm in B. juncea and were dependent on location; NPs located at the roots had an identical size distribution in both species, whereas the distribution within aboveground tissues differed between B. juncea and M. sativa, with B. juncea showing a much broader particle size ranges. Report from a different study emphasized the ability of desert plant Chilopsis linearis Sweet (desert willow) to absorb Au from gold-enriched media at varying plant developmental stages. The plants were exposed to 20, 40, 80, 160, and 320 mg Au L−1 in agar-based growth media for 13, 18, 23, and 35 d. The amount of Au and its oxidation state within plants were measured using an inductively coupled plasma/optical emission spectrometer (ICP/OES) and X-ray absorption spectroscopy (XAS), respectively. The XAS data indicate that desert willow produced AuNPs in plant tissue. The AuNPs were developed by exposing the plants to 160 mg Au L−1, which gave average sizes of 8, 18, and 35 Å, respectively, in the root, the leaves, and the stem, respectively. The AuNPs generated by plants were of average sizes and were related to the total tissue Au concentration and location in the plant [44].
Studies on in planta synthesis of AuNPs were also carried out in roots of Arabidopsis thaliana seedlings treated with 10 mg L−1 KAuCl4 for 7 d. TEM of KAuCl4-treated seedlings showed the presence of monodispersed AuNPs of different shapes (spherical, triangular, and exotic) and sizes (20–50 nm) in the root biomatrix. There was a significant induction of FRO2 in KAuCl4-treated roots, and therefore its likely involvement in the bioreduction of Au3+ was assumed [45].
Similar research by Taylor et al. [46] demonstrated the genetic and physiological response of Arabidopsis thaliana L. in Au solution sorption with an estimated 10 to 15% of Au translocated to the shoot tissues, and this demonstrated that Au3+ entered the shoot tissues directly, via passive uptake, and accumulated as AuNPs in the root tissues (Fig. 6). M. sativa L. was also utilized in NPs uptake from hydroponic culture and demonstrated that the NPs (in the range of 5 and 100 nm diameter) were not accumulated directly by the plants.

Reproduced from Ref. [46])
Arabidopsis absorption of gold. Appearance of 6-week-old, hydroponically grown plants (a) before and (b) 24 h after treatment with gold, as K(AuCl4). (c) Levels of gold in plant tissues after 24 h, a and b are significantly different from each other (p, 0.005) within each treatment. Results are the mean from three biological replicates 6 SD. Electron micrographs of plant tissues dosed with 500 mg L−1 gold, as K(AuCl4). (D) Leaf mesophyll, (e) leaf vascular tissue, (f) root cortex, and (g) root vascular tissue. NP, gold nanoparticle; Cy, cytoplasm; Cw, cell wall; Vw, vascular wall (
The growth of Sesbania seedlings in KAuCl4 solution led to gold uptake, eventually producing stable AuNPs in tissues of the plant [47]. TEM revealed that monodispersed nanospheres were spread intracellularly, perhaps by reduction of the metal ions by the secondary metabolites present in cells of the plant. The highly efficient biotransformation of Au3+ into AuNPs by the plant tissues was characterized by X-ray absorption near edge spectroscopy (XANES) and extended X-ray absorption fine structure.
In reducing aqueous 4-nitrophenol (4-NP), the catalytic role of biosynthesized AuNPs by U. armoricana was elucidated. Light-assisted in vivo AuNPs synthesis was first reported in aqueous solutions of dilute Au3+ salts by the living marine green algae (U. armoricana). After illumination of the living plant, the production of AuNPs was extremely rapid (15 min) compared to very slow rates (over 2 weeks) synthesis AuNPs without illumination. U. armoricana was found to be highly effective in AuNPs uptake, suggesting that the algae, along with its fast generation of AuNPs, remained alive as TEM demonstrated that the cell structure and thylakoid membranes were intact. The AuNPs were formed along the cell walls and in the chloroplasts. The dried U. armoricana-supported AuNPs demonstrated efficient catalytic reduction of 4-NP [48]. In a study by Starnes et al. [49], Au accumulation in different plant species (cucumber, oregano, red clover, sunflower, ryegrass, and alfalfa) was evaluated. Intake of gold at the roots varied from 500 ppm (ryegrass) to 2500 ppm (alfalfa). Alfalfa was therefore chosen for further studies because of its potential to accumulate relatively large amounts of gold through the root. Initial analysis showed that most of the AuNPs were produced within 6 h of treatment with most of the size range within 10–30 nm. A variety of treatments also showed spherical AuNPs (1–50 nm).
An investigation by Kumari et al. [50] focused at studying the intracellular synthesis of AgNPs by the legume, Vigna radiata. TEM revealed spatial distribution of AgNPs in the cytoplasmic spaces, chloroplast, vacuolar and nucleolar plant regions. The phytotoxic parameters such as percent seed germination and shoot elongation were left almost unchanged at low AgNO3 doses (20–50 mg L−1). But at greater degrees of exposure (100 mg L−1), the percent seed germination and shoot and root elongation declined, showing that the phytotoxicity depended on concentration. This study revealed that the intracellular synthesis of AgNPs by V. radiata, particularly at lower doses of AgNO3, could be used for large-scale NPs production as a sustainable and environmentally safe technology. Marchiol et al. [51] studied the intracellular production of AgNPs utilizing B. juncea, F. rubra, and M. sativa. The plants were grown in Hoagland’s solution for 30 d and then exposed for 24 h to a solution of 1000 mg L−1 AgNO3. Even though the exposure time was short, Ag absorption and translocation to the plant leaves were extremely high, attaining 6156 and 2459 mg kg−1 in B. juncea and F. rubra, respectively. TEM images of plant fractions indicated the intracellular formation of AgNPs in the roots, stems, and leaves of the plants. In the roots, AgNPs were present in the cortical parenchymal cells, on the cellular walls of the xylem vessels and in regions linked to the pits. In leaf tissues, AgNPs of varying shapes and sizes were found close to the cellular walls, in the cytoplasm as well as within chloroplasts. AgNPs were not seen in the phloem of the three species of the plant. This is the first findings of AgNPs synthesis in the living plants of F. rubra.
A research aimed at investigating the intracellular generation of AgNPs and their movement from the root to the shoots in maize was successful. TEM revealed spatially distributed AgNPs along with some important plant nutrient elements in various locations of the maize plant [52]. The possible relationship between the synthesized NPs and several nutrient elements of plant tissue was explained by 2D proton-induced X-ray emission of silver, chlorine as well as various nutrient elements. As the first study on the synthesis of AgNPs in live maize plants, the research provided direct evidence of NPs synthesis related to the distribution of the nutrient elements in plant, which is important for study in the synthetic application of NPs in crop plants and a clue for prospective implications of plant crops to synthesize eco-friendly NPs in Ag-contaminated sites. Another recent study focused on synthesizing AgNPs in living Arabidopsis and 2D distribution of Ag and other minerals (Ca, P, S, Mg, and CI) in the Arabidopsis tissues [53]. The Ag concentrations in the plant tissues were determined by ICP/OES, which showed that the greater proportion of Ag was in the roots. TEM showed spherical AgNPs located in the plant cells wall, plasma membrane, and cytoplasmic vacuoles. The distributions of Ag and Cl were consistent in plant tissues by 2D proton-induced X-ray emission, which showed that AgCl NPs may have been synthesized.
The intracellular synthesis of NPs was investigated by the use of three sprouts plants (bean, radish, and alfalfa). AgNPs and AuNPs (20 to 25 nm) were mainly synthesized intracellularly and located in the vascular cylinders and cortex. Amino acids and peptides were believed to reduce Ag ions to AgNPs. Alfalfa was the most resistant to Ag ions during inhibition tests for root growth and as such were more suitable than radish and bean plants for use in green synthesis of NPs [54]. Intracellular NPs that ranged between 5 and 50 nm in oval form (Fig. 7) were also reported by Raju et al. [10].

Reproduced from Ref. [10])
(a) and (b) HRTEM images at various scales of intracellular AuNPs synthesized by seedling of peanut (
Aside AgNPs and AuNPs synthesis, live plants have also been successful in synthesizing NPs such as PdNPs and metal–organic frameworks. Parker et al. [55] showed live Arabidopsis potential in generating PdNPs without the need for toxic chemicals or energy-intensive technologies in a relatively simple method. The PdNPs show exceptional catalytic activity by producing much greater yields than the commercial Pd catalyst in Suzuki–Miyaura reactions.
Additionally, Richardson and Liang [32] showed the synthesis of two types of metal–organic frameworks (MOFs), zinc(2‐methylimidazole)2 and lanthanide2(terephthalate)3, within a variety of living plants, using both the plant clippings and full intact plants. Synchrotron studies led to elucidating the kinetics and crystal phases of the nano-biohybrid plant. Metal salts were small enough to withstand adhesive and cohesive forces in plants, and precursors formed around biomolecules in the plants, which allowed for MOFs to expand, as shown in Fig. 8. Luminescent MOF-enhanced plants have been used for small molecular sensing and can result in more complex nano-biohybrid sensors and species benefited by higher efficiencies of completely intact plants [31, 32].

Adapted from Ref. [32])
Illustration of MOF formation inside of plants. (a) Plants are augmented with two different classes of MOFs (
Factors and mechanisms that contribute to NPs synthesis by living plants
NPs are formed in living plants by reduction of the metal ions absorbed as soluble salts. However, there is no clear explanation of the process underlying NPs formation in planta.
Plant metabolism is most likely to play a significant role in NPs biosynthesis. The contents of reducing sugars and antioxidant compounds were suggested as part of the AgNPs biosynthesis [51]. However, as the reducing sugars between species were very different, it was proposed that a single substance is unlikely to be responsible for the reduction process. Kumari et al. [50] demonstrated that the level of plant metabolites such as total phenolics, lipids, alkaloids, terpenoids, and amino acid increased by 65%, 133%, 19%, 67%, and 35%, respectively, in AgNO3 (100 mg L−1)-treated plants in comparison with the control. In the treated plants, the protein and sugar content also decreased by 38% and 27%, respectively. Amino acids and peptides were also believed to reduce Ag ions to AgNPs [54].
In order to appreciate the mechanism of the reduction processes, Beattie and Haverkamp [56] verified that the locations of the most abundant reduction of Au and Ag metal salts to NPs were the chloroplasts, regions of high reducing sugar (glucose and fructose) content. They suggested that these sugars were responsible for the reduction of Au and Ag with reduction potentials of over + 0.16 V and that the amount of reducing sugar present or produced determines the quantity of NPs that may be formed. In addition, AuNPs developed using the plants most critical organelle, chloroplasts, was shown to be successful [38]. Light-driven donation of electrons by chloroplasts to metal ions was exploited. According to the authors, in the presence of light of 600 µmol m−2 s−1 photon flux density (PFD), the chloroplasts isolated from Potamogeton nodosus (an aquatic plant) and Spinacia oleracea (a terrestrial plant) turned Au3+ solutions purple, with intensification of the color with time. However, the color of Au3+ solutions did not change in the dark. These findings clearly show that photosynthetic electron transport can reduce Au3+ to AuNPs when exposed to light. The chloroplasts considerably improved their capability of generating AuNPs as the PFD increases, further showing strong prospects of light-driven photosynthetic electron transport in synthesizing NPs [38].
Furthermore, micro- and macronutrients in plants can impact NPs synthesis as Tong et al. [52] demonstrated that AgNPs synthesized by maize were substantially linked to other elements in the plant, such as K, Ca, P, and S. It was speculated that the gold was absorbed by the plant mainly in ionic form and that the plants reacted to the exposure of gold by up-regulating genes for plant stress and down-regulating specific metal transporters to reduce gold absorption [46]. There was up-regulation of genes involved in the plant (Arabidopsis) stress response, such as glutathione transferases, cytochromes P450, glucosyl transferases, and peroxidases. The results showed that considerable down-regulation of discreet numbers of genes encoding proteins were involved in transportation of cadmium, copper, iron, and nickel ions, as well as aquaporins, attached to Au [46].
The findings of Starnes et al. [49] also showed how unique properties of NPs can be achieved by manipulating their geometries in planta. Interestingly, growth manipulations led to a notable change in the relative number of spherical, triangular, hexagonal, and rectangular AuNPs, which provided empirical evidence for their feasibility in planta engineering. The molecular mechanism for the absorption and reduction of KAuCl4 to AuNPs with various geometries by the roots was, however, not studied. Jain et al. [45] published their findings regarding molecular evidence toward the role of genes taking part in Fe homeostasis during in planta synthesis of AuNPs in roots of A. thaliana. First, they assessed the dosage-dependent impacts of KAuCl4 treatment on primary root length (PRL) and meristematic root activity in transgenic CycB1;1::uidA. Compared to control seedling (0 mg L−1 KAuCl4), PRL and meristematic activity of primary and lateral roots demonstrated incremental attenuation in seedlings treated with greater concentrations of KAuCl4 (25 mg L−1 or above). Heightened expression levels of Fe transporters IRT1 and IRT2 additionally suggested their prospective role in transport of bioreduced Au3+ across the membranes of the root. Expression levels of the other genes taking part in Fe homeostasis and also different members of phosphate, zinc, and potassium transporter families were left unaffected by the KAuCl4 treatment. An increased Au content in Fe-deprived roots moreover provided evidence that shows the particular role of a subset of Fe-responsive genes during in planta synthesis of AuNPs. Greater concentrations of KAuCl4 (≥ 25 mg L−1) showed inhibition of the growth of the roots. Monodispersed AuNPs of varying sizes and shape were detected in root of seedlings grown in medium supplemented with 10 mg L−1 KAuCl4. KAuCl4 treatment under Fe-deprived conditions increased Au content in shoot and roots.
Moreover, root enzymes can reduce metals to form NPs. A suggested participation of plasma membrane-bound dehydrogenases (a root enzyme) in reducing Ag+ and production of Ag0/Ag2O-NPs by intact plants roots also reduced triphenyltetrazolium to triphenylformazan and impermeable ferricyanide to ferrocyanide. Root enzyme extract caused the reduction of triphenyltetrazolium to triphenylformazan and Ag+ to AgNPs in the presence of NADH, clearly demonstrating the prospects of dehydrogenases to reduce Ag+ to AgNPs, which led to the production of Ag0/Ag2O-NPs as schematically demonstrated in Fig. 9 [42].

Reproduced from Ref. [42])
Schematic representation of the process involved in the reduction of Ag+ and formation of silver NPs at the root surface of live plants (
Concentrating synthesized NPs from plants
A method for concentrating intracellular NPs from plants was proposed using enzymatic digestion [57]. Moderate digestive conditions were used to prevent an increase in the size of AuNPs, as a suitable material for catalysis. XANES showed that the plant contained roughly equivalent amounts of Au0 and oxidized Au+1. 55–60 wt% of the plants was enzymatically dissolved, and no noticeable increase in the overall gold content of the samples was observed as a result of the loss of soluble gold fraction. However, the AuNPs concentration increased twofold. Approximately 95 wt% of the starting dry biomass was required to be solubilized in order to achieve a concentration that is appropriate for catalytic reactions, but this was not accomplished [57].
Challenges and future prospects
This review has summarized and illustrated the safer and faster synthesis of NPs, in particular AuNPs and AgNPs, by different living plant species. Biomolecules such as root enzymes, proteins/amino acids, and polysaccharides found in root exudates aid in reducing and capping of ions from solution to form extracellular NPs. Vital organelles and chloroplasts also contribute to the synthesis of intracellular NPs.
Moreover, this review has shown that over the past decade, the use of living plants to synthesize NPs has only concentrated much on Au and AgNPs, and this indicates an opportunity for researchers to explore the potential of living plants to synthesize other NPs such as Fe, Cu, Ni, and Pt. Also, the association of intracellular NPs with major plant nutrients [46] shows how NPs can be beneficial in the agricultural field, especially in the development of sensors and plant growth enhancers for improved crop productivity [58, 59]. In this way, a synthesis technique on plants opens up a fascinating possibility in which NPs can be captured in biomass, in a film or generated in solution, all of which have interesting applications such as production of nano-pesticides [60, 61] and antibacterial activities against plant pathogens [62, 63]. Though in planta engineering of NPs can facilitate the generation of NPs as well as complex metal alloys, the challenge lies in the efficient methods to concentrate the intracellular NPs from the plants for use in novel processes.
Another concern lies in the deleterious effect of the exposed metal ions such as AgNO3 on the physiological performance of living plants. For instance, AgNO3 interaction slowed down the growth of mustard seedlings by imparting toxicity; however, biologically synthesized AgNPs interactions imposed less stress conditions on the growth and metabolism of mustard seedlings [64]. Similar findings have also been reported [65, 66]. Additionally, Girilal et al. [67] showed that biologically synthesized metal NPs have less stress effects on plants as compared to chemically synthesized NPs.
Overall, living plants have been very efficient in synthesizing NPs in a faster and safer approach, as Pardha-Saradhi et al. [42] demonstrated how roots of intact plants were faster in generating AgNPs compared to the chemical method where sodium citrate was used. Future studies can test more suitable living plant species in infield application, soil-based systems, and the use of waste streams containing metal ions (bio-nanomining) [68] to act as precursor to synthesize both extracellular and intracellular NPs.
References
Farokhzad OC, Langer R (2009) Impact of nanotechnology on drug delivery. ACS Nano 3:16–20
Joshi N, Jain N, Pathak A et al (2018) Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J Sol-Gel Sci Technol 86:682–689. https://doi.org/10.1007/s10971-018-4666-2
Husen A, Siddiqi KS (2014) Phytosynthesis of nanoparticles: concept, controversy and application. Nanoscale Res Lett 9:1–24
Chauhan R, Kumar A, Abraham J et al (2013) A biological approach to the synthesis of silver nanoparticles with Streptomyces sp JAR1 and its antimicrobial activity. mdpi.com 81:607–621. https://doi.org/10.3797/scipharm.1302-02
Kasthuri J, Veerapandian S, Rajendiran N (2009) Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces
Khatoon UT, Nageswara Rao GVS, Mohan KM et al (2017) Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 146:259–265
Zhu H, Wang X, Li Y, et al (2009) Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem Commun. 5118–5120. https://pubs.rsc.org/en/content/articlehtml/2009/cc/b907612c
Masala O, Seshadri R (2004) Synthesis routes for large volumes of nanoparticles. Annu Rev Mater Res 41–81
Hao E, Bailey RC, Schatz GC et al (2004) Synthesis and optical properties of “branched” gold nanocrystals. Nano Lett 4:327–330
Raju D, Mehta UJ, Ahmad A (2012) Phytosynthesis of intracellular and extracellular gold nanoparticles by living peanut plant (Arachis hypogaea L.). Biotechnol Appl Biochem 59:471–478
Guilger-Casagrande M, Lima R de (2019) Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol Frontiers Media S.A.; [cited 2020 Sep 21]. p 287. http://www.pmc/articles/PMC6818604/?report=abstract
Patil MP, Kangjae M, Niyonizigiye I et al (2019) Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf B Biointerfaces 183:110455
Mehrotra T, Nagabooshanam S, Singh R (2019) Electrochemical evaluation of bacillus species for rapid biosynthesis of silver nanoparticles: application in domestic wastewater treatment. In: 2019 6th International Conference on Signal Processing Integral Networks, SPIN 2019. Institute of Electrical and Electronics Engineers Inc., pp 456–460
Yew YP, Shameli K, Miyake M et al (2020) Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: a review. Arab J Chem Elsevier B.V., 2287–2308
Santra TS, Tseng (Kevin) F-G, Barik TK (2015) Biosynthesis of silver and gold nanoparticles for potential biomedical applications—a brief review. J Nanopharmaceut Drug Deliv 2:249–265
Bhuyan T, Mishra K, Khanuja M et al (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Vijayakumar S, Krishnakumar C, Arulmozhi P et al (2018) Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmis pentaphylla (Retz.) DC. Microb Pathog 116:44–48
Wang T, Yang L, Zhang B et al (2010) Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf B Biointerfaces 80:94–102
Ismail M, Khan MI, Akhtar K et al (2018) Biosynthesis of silver nanoparticles: a colorimetric optical sensor for detection of hexavalent chromium and ammonia in aqueous solution. Phys E Low-Dimens Syst Nanostruct 103:367–376
Marchiol L (2012) Synthesis of metal nanoparticles in living plants. Ital J Agron Page Press Publications; 2012 [cited 2020 Sep 21]. pp 274–282. https://www.agronomy.it/index.php/agro/article/view/ija.2012.e37
Pagar T, Ghotekar S, Pagar K et al (2019) A review on bio-synthesized Co3O4 nanoparticles using plant extracts and their diverse applications. J Chem Rev 1:260–270
Akintelu SA, Folorunso AS (2020) A review on green synthesis of zinc oxide nanoparticles using plant extracts and its biomedical applications. Bionanoscience. Springer; pp 1–16. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s12668-020-00774-6
Bouafia A, Laouini SE, Ouahrani MR (2020) A review on green synthesis of CuO nanoparticles using plant extract and evaluation of antimicrobial activity. Asian J Res Chem 13:65
Burlacu E, Tanase C, Coman N-A et al (2019) A review of bark-extract-mediated green synthesis of metallic nanoparticles and their applications. Molecules 24:4354
Armendariz V, Herrera I, Peralta-Videa JR et al (2004) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 6:377–382. https://doi.org/10.1007/s11051-004-0741-4
Raut RW, Kolekar NS, Lakkakula JR et al (2010) Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L.) pierre. Nano-Micro Lett 2:106–113
Tarannum N, Divya GYK (2019) Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv 9:34926–34948
Song JY, Jang HK, Kim BS (2009) Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem 44:1133–1138
Aljabali A, Akkam Y, Al Zoubi M et al (2018) Synthesis of gold nanoparticles using leaf extract of Ziziphus zizyphus and their antimicrobial activity. Nanomaterials 8:174
Santhoshkumar J, Rajeshkumar S, Venkat Kumar S (2017) Phyto-assisted synthesis, characterization and applications of gold nanoparticles—a review. Biochem Biophys Rep 11:46–57. https://doi.org/10.1016/j.bbrep.2017.06.004
Dong R, Li Y, Li W, et al (2019) Recent developments in luminescent nanoparticles for plant imaging and photosynthesis. J Rare Earths Chin Soc Rare Earths 903–915
Richardson JJ, Liang K (2018) Nano-biohybrids: in vivo synthesis of metal–organic frameworks inside living plants. Small 14:1–7
Tang B, Liu J, Fan L et al (2018) Green preparation of gold nanoparticles with Tremella fuciformis for surface enhanced Raman scattering sensing. Appl Surf Sci 427:210–218. https://doi.org/10.1016/j.apsusc.2017.08.008
Li X, Sun H, Mao X et al (2020) Enhanced photosynthesis of carotenoids in microalgae driven by light-harvesting gold nanoparticles. ACS Sustain Chem Eng 8:7600–7608. https://doi.org/10.1021/acssuschemeng.0c00315
Giraldo JP, Landry MP, Faltermeier SM et al (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13:400–408
Anderson CWN, Bhatti SM, Gardea-Torresdey J et al (2013) In vivo effect of copper and silver on synthesis of gold nanoparticles inside living plants. ACS Sustain Chem Eng 1:640–648. https://doi.org/10.1021/sc400011s
Bali R, Harris AT (2010) Biogenic synthesis of Au nanoparticles using vascular plants. Ind Eng Chem Res 49:12762–12772. https://doi.org/10.1021/ie101600m
Shabnam N, Pardha-Saradhi P, Sharmila P (2014) Phenolics impart Au3+-stress tolerance to cowpea by generating nanoparticles. PLoS One 9
Pardha-Saradhi P, Yamal G, Peddisetty T et al (2014a) Root system of live plants is a powerful resource for the green synthesis of Au-nanoparticles. RSC Adv 4:7361–7367
Raju D, Paneliya N, Mehta UJ (2014) Extracellular synthesis of silver nanoparticles using living peanut seedling. Appl Nanosci 4:875–879. https://doi.org/10.1007/s13204-013-0269-y
Ali M, Mosa K, El-Keblawy A et al (2019) Exogenous production of silver nanoparticles by Tephrosia apollinea living plants under drought stress and their antimicrobial activities. Nanomaterials 9:1716
Pardha-Saradhi P, Yamal G, Peddisetty T et al (2014b) Reducing strength prevailing at root surface of plants promotes reduction of Ag+ and generation of Ag0/Ag2O nanoparticles exogenously in aqueous phase. Bansal V, editor. PLoS One 9:e106715. https://doi.org/10.1371/journal.pone.0106715
Haverkamp RG, Marshall AT, Van Agterveld D (2007) Pick your carats: nanoparticles of gold-silver-copper alloy produced in vivo. J Nanopart Res 9:697–700
Rodriguez E, Parsons JG, Peralta-Videa JR et al (2007) Potential of Chilopsis Linearis for gold phytomining: using xas to determine gold reduction and nanoparticle formation within plant tissues. Int J Phytoremed 9:133–147. https://doi.org/10.1080/15226510701232807
Jain A, Sinilal B, Starnes DL et al (2014) Role of Fe-responsive genes in bioreduction and transport of ionic gold to roots of Arabidopsisthaliana during synthesis of gold nanoparticles. Plant Physiol Biochem 84:189–196. https://doi.org/10.1016/j.plaphy.2014.09.013
Taylor AF, Rylott EL, Anderson CWN et al (2014) Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS One 9
Sharma NC, Sahi SV, Nath S et al (2007) Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol 41:5137–5142
Mukhoro OC, Roos WD, Jaffer M et al (2018) Very green photosynthesis of gold nanoparticles by a living aquatic plant: photoreduction of AuIII by the seaweed Ulva armoricana. Chem A Eur J 24:1657–1666. https://doi.org/10.1002/chem.201704448
Starnes DL, Jain A, Sahi SV (2010) In planta engineering of gold nanoparticles of desirable geometries by modulating growth conditions: an environment-friendly approach. Environ Sci Technol 44:7110–7115. https://doi.org/10.1021/es101136q
Kumari R, Singh JS, Singh DP (2017) Biogenic synthesis and spatial distribution of silver nanoparticles in the legume mungbean plant (Vigna radiata L.). Plant Physiol Biochem 110:158–166
Marchiol L, Mattiello A, Pošćić F et al (2014) In vivo synthesis of nanomaterials in plants: location of silver nanoparticles and plant metabolism. Nanoscale Res Lett 9:1–11
Tong X, Guo N, Dang Z et al (2018) In vivo biosynthesis and spatial distribution of Ag nanoparticles in maize (Zea mays L.). IET Nanobiotechnol 12:987–993
Xu H, Yu T, Fu Y et al (2020) Biosynthesis of Ag nanoparticles and twodimensional element distribution in Arabidopsis. IET Nanobiotechnol 14:325–330
Park S, Sung HK, Kim Y (2016) Green synthesis of metal nanoparticles using sprout plants: pros and cons. J Nanosci Nanotechnol 16:4444–4449
Parker HL, Rylott EL, Hunt AJ et al (2014) Supported palladium nanoparticles synthesized by living plants as a catalyst for suzuki-miyaura reactions. Marr A, editor. PLoS One 9:e87192. https://doi.org/10.1371/journal.pone.0087192
Beattie IR, Haverkamp RG (2011) Silver and gold nanoparticles in plants: sites for the reduction to metal. Metallomics 3:628–632
Marshall AT, Haverkamp RG, Davies CE et al (2007) Accumulation of gold nanoparticles in Brassic juncea. Int J Phytoremed 9:197–206
Shojaei TR, Salleh MAM, Tabatabaei M, et al (2018) Applications of nanotechnology and carbon nanoparticles in agriculture. Synth Technol Appl Carbon Nanomater 247–277
Rastogi A, Tripathi DK, Yadav S, et al (2019) Application of silicon nanoparticles in agriculture [Internet]. 3 Biotech. Springer Verlag, p 90. org/https://doi.org/10.1007/s13205-019-1626-7
Fang IJ, Trewyn BG (2012) Application of mesoporous silica nanoparticles in intracellular delivery of molecules and proteins. Methods Enzymol 41–59
Gahukar RT, Das RK (2020) Plant-derived nanopesticides for agricultural pest control: challenges and prospects. Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-020-0066-2
Vanti GL, Kurjogi M, Basavesha KN et al (2020) Synthesis and antibacterial activity of solanum torvum mediated silver nanoparticle against Xxanthomonas axonopodis pv.punicae and Ralstonia solanacearum. J Biotechnol 309:20–28
Paulkumar K, Gnanajobitha G, Vanaja M, et al (2014) Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci World J
Vishwakarma K, Shweta UN et al (2017) Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci 8:1501. https://doi.org/10.3389/fpls.2017.01501/full
Wang J, Koo Y, Alexander A et al (2013) Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ Sci Technol 47:5442–5449. https://doi.org/10.1021/es4004334
Feizi H, Moghaddam PR (2011) Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth article in biological trace element research. Springer 146:101–106
Girilal M, Mohammed Fayaz A, Elumalai LK et al (2018) Comparative stress physiology analysis of biologically and chemically synthesized silver nanoparticles on Solanum Lycopersicum L. Colloids Interface Sci Commun 24:1–6. https://doi.org/10.1016/j.colcom.2018.02.005
Wong-Pinto L, Menzies A, Ordóñez JI (2020) Bionanomining: biotechnological synthesis of metal nanoparticles from mining waste—opportunity for sustainable management of mining environmental liabilities. Appl Microbiol Biotechnol 104:1859–1869. https://doi.org/10.1007/s00253-020-10353-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saim, A.K., Kumah, F.N. & Oppong, M.N. Extracellular and intracellular synthesis of gold and silver nanoparticles by living plants: a review. Nanotechnol. Environ. Eng. 6, 1 (2021). https://doi.org/10.1007/s41204-020-00095-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-020-00095-9