Abstract
Laboratory and field experiments indicate that the presence of Limnoperna fortunei decreases concentrations of particulate organic matter and increases ammonia, nitrate, and especially phosphate. Long-term series of field data partially confirm these results. After having been colonized by the mussel, a 47 km2 reservoir developed higher concentrations of ammonia and phosphates, a higher P:N ratio, more transparency, less seston, and less phytoplankton and primary production. Phytoplankton clearance rates by the mussel vary widely, suggesting that “normal” values for adult organisms are around 100 mL/ind./h, or ca. 2–4 mL/mg DW/h. Data on grazing selectivity are inconclusive, but seem to indicate highest impacts on small (< 1 mm) particles. Large plankton are negatively selected, but they may account for greater proportions of total biomass in the diet. Studies on consumption of toxic cyanobacteria yield conflicting results, but large golden mussel populations significantly enhance blooms of colonial Microcystis spp. through changes in nutrient availability, size-selective grazing, promotion of colony formation, and reduced grazing of toxic cells. These toxic blooms, in turn, suppress reproduction of the mussel, most probably killing the larvae. Growth of periphyton and aquatic macrophytes are enhanced significantly by the golden mussel.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Limnoperna fortunei
- Golden mussel
- Ecological impact
- Nutrient recycling
- Phytoplankton grazing
- Grazing selectivity
- Cyanobacterial blooms
- Microcystis
Introduction
The influence of filter-feeding organisms on water-column properties, in particular the concentration of bioseston and modifications in the concentration and proportions of nutrients, has been investigated for decades in both marine and freshwater environments. Interest in this topic stems from the fact that filtration is among the most widespread feeding modes in a vast array of aquatic animals (Jørgensen 1966), and because it profoundly affects many water-column traits, as well as sediment characteristics. Filtration-related changes brought about by introduced species, in particular bivalves, have received special attention because they modify historical, preintroduction conditions, and because some nonindigenous species can attain very high densities and become invasive, thus enhancing their otherwise moderate impact on waterbodies. Figure 1 offers a visual overview of some of the most important modifications observed in association with the introduction of zebra and quagga mussels in Europe and North America (MacIsaac 1996; Karatayev et al. 2002; Kelly et al. 2010; Nalepa and Schloesser 2014), and of the golden mussel in Asia and South America (Mansur et al. 2012; Boltovskoy and Correa 2015). Even though this diagram includes but a fraction of the actual relationships that come into effect when one of these species colonizes a hitherto uninvaded waterbody, the maze of interactions is remarkable. It is noteworthy that the same ecosystem trait, process or component can be influenced by several different effects associated with the presence of mussels, and that the directions of these impacts can often be opposing. Further complications in pinpointing and quantifying impacts stem from the fact that many of these effects are site-dependent, which means that they can vary widely among waterbodies, or even in different areas of the same waterbody, and also as a function of time elapsed after initial introduction. Some shifts can be very strong during the initial postintroduction years and wane thereafter; others persist through time, and still others only become evident several years after introduction (Burlakova et al. 2005; Burlakova et al. 2006).
Generalized schematic diagram of some salient effects of filter-feeding mussels on freshwater bodies (excluding relationships with fishes). Effects analyzed and demonstrated for L. fortunei are denoted in bold characters. Notice that several of the impacts shown have opposite effects on the same process or component; for example, clarification of the water and nutrient recycling can favor phytoplankton growth, but grazing and enhancement of periphyton and macrophytes can depress phytoplankton abundance (conflicting effects are denoted with the same color)
This section reviews current knowledge of the effects of the introduction of Limnoperna fortunei on nutrients and phytoplankton abundance and composition, as well as some consequences of the observed changes. As elsewhere in this volume, this chapter focuses on the golden mussel, rather than on invasive freshwater bivalves in general. As discussed below, while the mechanisms by which L. fortunei influences waterbodies are practically identical to those of the dreissenids, the final outcome of these interactions is often very dissimilar (Boltovskoy et al. 2006, 2013; Boltovskoy and Correa 2015).
Nutrient Recycling
Short-term (24 h) experiments investigating effects of L. fortunei on nutrient concentrations and proportions have been carried out in laboratory settings and in field-deployed mesocosms (Cataldo et al. 2005; Cataldo et al. 2012a).
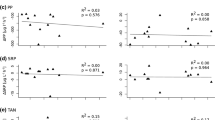
Laboratory experiments were conducted using plastic containers with 60 L. fortunei 18–27 mm in length in 15 L of water obtained in the Río de la Plata estuary (Cataldo et al. 2005). Nutrient concentrations were measured at 0, 3, 6, 12 and 24 h. In controls without mussels, none of the measurements at 24 h differed significantly from initial (0 h) conditions. In containers with mussels, only total N and total P remained constant. Particulate N and P dropped to < 25 % of their initial values (N from 1149 to 0 µg/L, and P from 122 to 31 µg/L). Ammonia, nitrate and phosphate, on the other hand, increased conspicuously (ammonia from 1190 to 1620 µg/L, nitrate from 1532 to 2333 µg/L, and phosphate from 121 to 212 µg/L) (Figs. 2a, b and 3a, b).
A similar experiment was performed in 400 l mesocosms deployed in a shallow, coastal area of the reservoir Embalse de Río Tercero, a medium-sized (47 km2), meso-eutrophic waterbody (chlorophyll a around 3–6 µg/L; Boltovskoy et al. 2009a) located in central Argentina (32.37ºS, 64.77ºW) (Cataldo et al. 2012a). Four polyethylene terephthalate cylindrical mesocosms (75 cm in diameter, 105 cm high) with their bottoms sealed off with a polyethylene liner were filled with reservoir water to about 15 cm from the rim. Each of two of the mesocosms were stocked with ~ 1700 mussels 14–35 mm in length, collected nearby, whereas the other two were used as controls (no mussels). As in the previous experiment, nutrient concentrations were measured at 0, 3, 6, 12, and 24 h. All variables remained practically constant in the controls (as in the lab experiments). In the mesocosms stocked with mussels, total N and P changed negligibly. Particulates dropped significantly (particulate N from 25 to 13 µg/L, particulate P from 122 to 31 µg/L), and ammonia, nitrate and phosphate increased strongly (ammonia from 0 to 220 µg/L, nitrate from 10 to 40 µg/L, and phosphate from 1 to 13 µg/L) (Fig. 2c and d; 3c and d).
It is noteworthy that, after 24 h, in both lab and mesocosm experiments, increases in the concentration of phosphate were much higher than those of nitrate (Figs. 2 and 3), thus modifying not only the total amount of major nutrients available for the autotrophs, but also their proportions. These results are generally similar to those obtained by Kawase (2011) in his 6-h filtration experiments with L. fortunei, where he recorded significant drops in turbidity and in the concentrations of particulate organic C and N.
Results obtained with longer incubations agreed with the above trends during the initial 24-h period, but subsequently the behavior of nutrients changed significantly. Cataldo et al. (2012b) assessed the effects of L. fortunei on water column properties of Salto Grande Reservoir (Uruguay River, Argentina-Uruguay) using three pairs of floating 400-L mesocosms (Fig. 4a and b) stocked with either 100 or 300 mussels 16–20 mm in length, and without mussels (controls). Immediately after deployment (day 0), and on days 1, 2, 3, 7, 14, 21, 28, and 35 the following parameters were assessed (Fig. 4c): water temperature, concentrations of ammonia, nitrate, phosphate, and chlorophyll a, quantification and identification of phytoplanktonic algae, and evaluation of the size and density of Microcystis spp. colonies. Accumulated sediments were retrieved from each mesocosm at the end of the experiment to assess their wet and dry weight, and organic matter contents. All mesocosms were provided with four PVC plates suspended within the enclosure; these plates were retrieved on day 35 and periphytic organic matter and chlorophyll a were measured.
In the absence of L. fortunei, ammonia dropped from ca. 10 µg/L to below detection levels by the end of the incubation. In the mesocosms with mussels, on the other hand, ammonia increased from 10 µg/L (on day 0) to around 20–30 µg/L (on day 35; Fig. 5a). For nitrate the pattern was different; in agreement with short-term studies, the presence of mussels enhanced nitrate concentrations until day 3, but from then on nitrate decreased gradually until termination of the experiment (day 35), when it was at around 40–60 % of initial values (Fig. 5b). In the controls, nitrate concentrations dropped from day 1, and by day 35 were at ~ 20 % of initial values. Phosphate concentrations increased on day 1 (in the mesocosms with 100 mussels; as in the short term surveys), or day 3 (in mesocosms with 300 mussels), and from there on showed moderate variations (with the exception of a second peak on day 28 in the mesocosms with 300 mussels). In the controls, phosphate values were very low throughout the entire experimental period (Fig. 5c).
Changes in the concentrations of ammonia (a), nitrate (b), and phosphate (c) in mesocosms with and without L. fortunei throughout a 35-day experimental period. (From Cataldo et al. 2012b)
While the usefulness of short and medium-term experiments for identifying the effects of invasive mussels on nutrients and the biota is beyond doubt, their ability to predict impacts over the long term (years to decades) is limited. Long-term impacts cannot be unequivocally assessed through laboratory tests or enclosure experiments, whereas field sampling programs very seldom cover periods long enough for such analyses. Furthermore, many of the variables that play fundamental roles in lakes, rivers and reservoirs (e.g., nearshore vs. offshore partitioning, external inputs of nutrients and organic matter, regional differences in substrate type, vertical mixing, and many others, see reviews in Kelly et al. 2010, Bootsma and Liao 2014) are not effective in experimental settings. For L. fortunei, the only survey available where the effects of this mussel were quantified on the basis of a long-term series of field data is the one carried out in Embalse de Río Tercero reservoir by Boltovskoy et al. (2009a). This reservoir, which was colonized by the golden mussel around 1998, has been monitored regularly since 1996. In 2006, average mussel densities over the entire reservoir were estimated at 960 ind./m2 (see Fig. 11 in Chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume), suggesting that these populations could potentially filter a volume equivalent to that of the entire water body every 2–8 days (Boltovskoy et al. 2009a). Comparison of data collected between 1996 and 2008 point at significant changes in several water column properties, especially at the station located in the area of highest mussel densities. Total N in the water increased 300 % (most probably largely on account of ammonia), ammonia increased ca. 400 %, and phosphate increased 200 %. Nitrate remained at pre-2000 levels. The phosphate:nitrate ratio increased from 0.061 (before 2000) to 0.112 (after 2000) (Fig. 6), probably as a result of high rates of phosphate release due to mobilization of iron-bound phosphorus in the anoxic guts of the mussels (Turner 2010). These shifts are particularly noticeable when contrasting the periods 1996–2000 versus 2002–2007, which suggests that it took the mussel around four years to build up a population large enough to start affecting the reservoir water (see Chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume).
Mean concentrations of N and P in the reservoir Embalse de Río Tercero in 1996–2000 (before the waterbody was influenced by the presence of L. fortunei, which invaded in 1998), and in 2002–2007 (with high L. fortunei population densities). (Based on data from Boltovskoy et al. 2009a)
These results generally confirm previous information based on studies with Dreissena spp. (Karatayev et al. 2002; Turner 2010; Zhang et al. 2011), yet their effects on phytoplankton are different (see below).
Enhanced mineralization associated with the presence of mussels is not restricted to organic matter, but may also affect other substances present in the water, including pesticides. Di Fiori et al. (2012), concluded that concentrations of glyphosate, a phosphonate compound widely used as an herbicide for weed control of several genetically modified crops (soybean, maize, cotton, canola) decreased by 40 % in the presence of large mussels. The pathways responsible for this decrease, however, are still poorly understood.
Phytoplankton Grazing: Rates and Impact
Clearance rates of L. fortunei have been estimated in several studies, but the range of values reported is very large: 0.2 to 725 mL/ind./h, or 0.1 to 29.5 mL/mg dry tissue (DT)/h (Table 1). This spread is largely associated with differences in methodology. With very few exceptions (e.g., Gazulha et al. 2012a, b), studies did not differentiate actually ingested particles from those discarded as pseudofeces; the latter are embedded in mucus, rejected and settled on the bottom, thus disappearing from the water column and ending up being included in the estimates of consumption. Differences in food type and toxicity, pre-experiment starvation times, mussel size, experiment duration, amount of suspended solids, temperature, resuspension mechanisms used, pH, and water flow, among other factors, also strongly affect grazing estimates (Morton 1983). Despite their spread, these data suggest that normal filtration rates for adult individuals 15–25 mm in length range around 100 mL/ind./h, or ca. 2–4 mL/mg DW/h. Younger individuals and warmer temperatures yield higher specific filtration rates (Sylvester et al. 2005).
These figures are roughly within the range of those reported for many other freshwater mussels (Karatayev et al. 1997; Sylvester et al. 2005), very few of which, however, attain densities similar to those observed in L. fortunei beds. On suitable substrates golden mussel densities normally range around 5000 ind./m2, and can occasionally exceed 200,000 ind./m2 (Sylvester et al. 2007; Spaccesi and Rodrigues Capitulo 2012; see Chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume). Thus, strong impacts on the water column are due to high mussel densities, rather than to exceptional individual filtration rates.
Results of laboratory and mesocosm experiments indicate strong drops in algal numbers over short periods. Studies carried out in a laboratory recirculating system and in 400-l mesocosms (Cataldo et al. 2005; 2012a; see above) showed dramatic drops in phytoplankton cell numbers over 24 h (Fig. 7a, d). Clearance and grazing rates were not monotonic, but changed over the course of the experiment. Clearance was found to be highest at 6 h, and decreased afterwards (Fig. 7b, e). Similar trends have been reported for L. fortunei by other authors (Pestana et al. 2009; Frau et al. 2013). However, because algal densities decrease with time, the numbers of algae eliminated from the water column (either ingested or rejected as pseudofeces) decrease very sharply (Fig. 7c, f). Decreased pumping (= clearance) rates are probably a response to satiation (Fig. 7a), low phytoplankton densities (Fig. 7d), or both. Longer-term experiments, however, indicate that after this initial decline algal numbers recover partially, most probably stimulated by higher nutrient availability and increasing light penetration (Cataldo et al. 2012b; Fig. 8). These results seem to mimic natural conditions; the few colonized waterbodies for which there are adequate historical records dropped in phytoplankton abundance and production after having been invaded by L. fortunei. The reservoir Embalse de Río Tercero lost about 30–40 % of its seston load, represented chiefly by algae (Fig. 9b), >40 % of its planktonic primary production (Fig. 9d), and became significantly clearer (Fig. 9a) (Boltovskoy et al. 2009a). Information on potential impacts in the main South American waterbodies colonized by the mussel—the large floodplain rivers (see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume) is still scant. Based on data from two tributaries of the Middle Paraná River collected before and after colonization by the mussel, Rojas Molina and José de Paggi (2008) concluded that zooplankton abundance (especially Rotifera) and chlorophyll a declined as a result of the invasion of L. fortunei (see Chapter “Impacts of Limnoperna fortunei on Zooplankton” in this volume). Similar trends have also been suggested for a marginal lagoon and a tributary of the Middle Paraná River (Devercelli and Peruchet 2008). However, these rivers and associated marginal floodplains are relatively open systems strongly influenced by variable conditions in their upper reaches, and are subject to wide seasonal and interannual variations largely depending on precipitation and runoff regimes (see below), which complicates interpretation of causal relationships. Furthermore, in recent decades their catchment basins have been strongly modified by growing human populations and increasing land use for agricultural purposes, with the enhancement of the input of fertilizer-derived nutrients, pesticides and waste products, which further hinders pinpointing the concomitant effects of the invasive mussel.
Changes in algal densities (a, d), clearance rates (b, e), and grazing rates (c, f) by L. fortunei fed natural plankton along a 24-h experimental period in laboratory conditions (Río de la Plata, a-c) and in 400-l mesocosms (Embalse de Río Tercero reservoir, d-e). (Based on data from Cataldo et al. 2005; Cataldo et al. 2012a)
Changes in the concentrations of chlorophyll a (a) and algal cells (b) in mesocosms with and without L. fortunei throughout a 35-day experimental period. (From Cataldo et al. 2012b)
Changes in several water-column properties in the reservoir Embalse de Río Tercero between 1996 and 2007. Curves (3-point running means; 5-point for d) are based on measurements at approximately 3-month intervals (ca. 70 data points). Shaded areas indicate periods with modified conditions (presumably due to colonization by the mussel). Red broken lines denote means for each period (significantly different at p = 0.05 for all parameters except chlorophyll a, where p = 0.069; Scheffé’s post hoc tests). Inset map shows the position of the sampling station in the reservoir (star). (Modified from Boltovskoy et al. 2009a)
Phytoplankton Grazing: Selectivity
Several surveys have addressed the issue of selectivity in L. fortunei grazing, using both natural, mixed plankton, and various combinations of cultured algae, with somewhat dissimilar results. In the 24-h mesocosm experiment performed in Embalse de Río Tercero described above, Cataldo et al. (2012a) found no association between prey cell size (across a range of 5–280,596 µm3) and consumption rate. Laboratory experiments with Microcystis viridis, Pseudoanabaena sp. and Selenastrum capricornutum also yielded similar filtration rates for the three species (Rückert et al. 2004). On the other hand, strong selectivity was found when the mussel was fed a wider range of planktonic organisms, including zooplankton. Fachini et al (2012) reported negative selection for large (1–20 mm) filamentous algae and copepods, and positive selection for Rotifera, small (< 1 mm) filamentous algae, and several solitary algal cells, concluding that small to moderately sized particles and organisms with limited escape responses are favored by the mussel (see Chapter “Impacts of Limnoperna fortunei on Zooplankton” in this volume). Filtration experiments (72 h) performed in 200-L containers with natural plankton suggested that small flagellates are avoided by the mussel, whereas diatoms are positively selected (Frau et al. 2013).
Contrasting results may be partly explained by the fact that most studies did not differentiate particles actually ingested from those that are collected, embedded in mucus, and rejected as mucus-bound clumps that do not return to the water-column, but settle on the bottom. Gazulha et al. (2012b) differentiated between ingested particles and particles expelled as pseudofeces, and concluded that filtration rates of single-celled, colonial and filamentous cyanobacteria are similar, but while single cells are ingested, filamentous, and colonial forms are massively rejected as pseudofeces. A similar result was obtained when feeding L. fortunei with a mixture of cyanobacteria and diatoms. The diatom Nitzschia palea was found to disappear from the water faster than the cyanobacterium Microcystis aeruginosa, but ingestion rates were significantly higher for the latter, whereas N. palea was rejected (Gazulha et al. 2012a).
In summary, results available to date are still scarce and contradictory. Aside from the fact that the proportions of large organisms, especially those with well-developed avoidance abilities, are lower in the diet than in the water (although positive selection for several planktonic animals was also reported; see Chapter “Impacts of Limnoperna fortunei on Zooplankton” in this volume), data on grazing selectivity of the golden mussel are inconclusive. Furthermore, while impacts on larger plankton are probably lower than those on smaller particles, selectivity studies based on filtration experiments can be misleading with regard to the relative importance of the different items in the diet of L. fortunei, because the proportion of total biomass ingested may be dominated by large prey items (Rojas Molina et al. 2010; see Chapter “Impacts of Limnoperna fortunei on Zooplankton” in this volume).
Enhancement of Cyanobacterial Blooms
Blooms of toxic cyanobacteria, especially Microcystis spp., are usually associated with eutrophication and, in particular, with elevated P:N ratios (Smith 1983; Smith and Bennett 1999). Extensive river damming worldwide has created thousands of new waterbodies where stagnancy, enhanced vertical stratification, and growing nutrient input from agricultural land use boosts growth of cyanobacteria (Pizzolón et al. 1999; Jeong et al. 2003; Ruibal Conti et al. 2005; Relyea 2006). In recent decades, an additional bloom-enhancing effect has been described: some waterbodies have been observed to develop more frequent and stronger toxic cyanobacterial blooms after having been colonized by the zebra mussel (Bykova et al. 2006). Observational and experimental data on the effects of L. fortunei show that it also has a very significant impact on the abundance of Cyanobacteria.
In the experiment described above using 400-L mesocosms (Fig. 4), Cataldo et al. (2012a) found that Microcystis spp. densities increased from undetectable levels to ca. 1500–2000 cells/mL after 35 days in enclosures without mussels (Fig. 10a); however, in mesocosms with mussels, Microcystis spp. numbers soared to > 200,000 cells/ml (Fig. 10b). Most significantly, colonial forms almost exclusively accounted for this increase, whereas solitary cells of Microcystis spp. remained at very low levels throughout the experiment (Fig. 10b). During the first week, only solitary cells of Microcystis spp. were found in all mesocosms. After 2 weeks, in the mesocosms without mussels the proportion of solitary Microcystis cells dropped to 34 %, and varied around 20–50 % until the end of the experiment. In contrast, in the mesocosms with mussels, solitary cells were dominant until week 3, being completely replaced thereafter by colonial individuals (Fig. 10b). Most significantly, this growth in the presence of mussels was accompanied by a strong increase in the size of Microcystis spp. colonies (Fig. 10c). In controls without mussels, the mean size of Microcystis spp. colonies remained around 50 µm (maximum dimension) throughout the entire experimental period. In the mesocosms with mussels, on the other hand, on week 4 all Microcystis cells were in colonies ~ 135 µm in size, and by week 5, they attained a mean size of ~ 179 µm (Fig. 10c). Other cyanobacterial species present in the enclosures (Anabaena circularis, Aphanocapsa delicatissima, Chroococcus minutus, Pseudoanabaena mucicola) also grew more in the presence of L. fortunei than in the controls, but their densities remained low.
Changes in the abundance of Microcystis spp. solitary cells and cells belonging to a colony in 400-L mesocosms without L. fortunei (a) and stocked with 300 mussels (b). Changes in mean size of Microcystis spp. colonies through time in mesocosms without mussels and in the presence of 300 mussels (c). (From Cataldo et al. 2012b)
Interpretation of these results suggests that there are several mechanisms converging to enhance Microcystis spp. densities in the presence of the mussels: (1) Changes in nutrient availability, (2) changes in the P:N ratio, (3) size-selective grazing, whereby small, solitary cells are eliminated more effectively than colonies, (4) promotion of colony-formation by chemical signals that trigger aggregation of solitary cells in order to avoid grazing, and (5) Microcystin toxicity, deterring grazing as Microcystis spp. biomass builds up (Cataldo et al. 2012b).
Nutrient availability and higher P:N ratios have long been identified as cyanobacterial bloom-enhancing triggers (Smith 1983; Smith and Bennett 1999). Promotion of colony-formation in various autotrophs, including Microcystis spp., by predator-produced chemical signals has been described repeatedly (Yang et al. 2005). Selective grazing of solitary cells is supported by some previous results with L. fortunei (see above). Grazing of toxic Microcystis spp. by the golden mussel, on the other hand, is still a debatable issue. Some surveys have concluded that both toxic and nontoxic strains of Microcystis spp. are actively consumed by adult mussels (Rückert et al. 2004; Gazulha et al. 2012a). Fachini (2011) found that toxic and nontoxic strains of Microcystis aeruginosa are consumed alike (at microcystin LR concentrations in water around 6–9 ppb), but at lower ingestion rates than other algae (Monorhaphidium sp.). In contrast, preliminary tests performed in Salto Grande Reservoir, where very dense blooms of Microcystis spp. are a recurrent summer through autumn phenomenon (Chalar 2009; O’ Farrell et al. 2012; Boltovskoy et al. 2013), indicate that at dissolved microcystin LR concentrations above 2 ppb the mussel ceases to filter, whereas at levels above 8 ppb significant mortality is observed (at 8 ppb 5 % of the mussels die after 2 h, whereas at 30 ppb mortality reaches 90 %) (Boltovskoy et al. 2009b). Interestingly, a similar controversy is also found in studies on the effects of Microcystis spp. on Dreissena polymorpha. Some surveys concluded that zebra mussels fed Microcystis spp. show significantly reduced grazing and acute irritant responses (Juhel et al. 2006a; Juhel et al. 2006b), or selectively reject them, especially large colonial aggregates of the unpalatable toxic strains (Vanderploeg et al. 2001), whereas others concluded that toxic Microcystis spp. are consumed as effectively as other algae, suggesting that the mussel could control cyanobacterial blooms (Dionisio Pires et al. 2010). This suggests that consumption of toxin-producing strains of these cyanobacteria varies depending on conditions that we still do not fully understand.
In any case, regardless of L. fortunei's tolerance to microcystin, the results of Cataldo et al. (2012b) strongly suggest that the golden mussel boosts the growth of cyanobacteria. A major difference with the zebra mussel, however, is that while D. polymorpha enhances cyanobacterial numbers only in lakes with low to moderate P concentrations (< 25 µg total P/L; Nicholls et al. 2002; Raikow et al. 2004; Sarnelle et al. 2005; Knoll et al. 2008), L. fortunei in South America does so at very high total P levels (between 50 and > 100 µg total P/L in the reservoir where these experiments were carried out, Chalar 2006; Cataldo et al. 2012b; O’ Farrell et al. 2012).
A remarkable consequence of mussel-induced toxic cyanobacterial growth is that these blooms suppress the bivalve’s reproduction. This effect has been suggested by several laboratory and field studies (Boltovskoy et al. 2009b; Gazulha 2010; Gazulha et al. 2012b), and confirmed by the analysis of 9 years of observational data in Salto Grande reservoir. Recurrent blooms of Microcystis spp. in Salto Grande Reservoir interrupt production of larvae at a time when in all other waterbodies investigated (without cyanobacterial blooms) mussel reproduction is maximum (late spring–early autumn; Boltovskoy et al. 2013).
Periphyton and Aquatic Macrophytes
An indirect consequence of fast nutrient regeneration rates and clarification of the water column by elimination of suspended organic and inorganic matter (including nutrient-consuming phytoplankton) through filter feeding is enhanced growth of periphyton and aquatic macrophytes (Fig. 1; Pillsbury and Lowe 1994; Karatayev et al. 1997; Zhu et al. 2006; Karatayev et al. 2007; Kelly et al. 2010). Data for the golden mussel indicate that it has also had these effects in some of the reservoirs surveyed.
In the mesocosm survey in Salto Grande Reservoir (Fig. 4; Cataldo et al. 2012a), periphytic chlorophyll a in the enclosures with mussels was ca. 16 times higher than in those without L. fortunei 5 weeks after deployment. Periphyton biomass increased in both control and experimental enclosures, but the proportion of algal biomass was significantly higher in the latter, indicating a shift from heterotrophic to autotrophic dominance.
Since it was first filled in 1934, the reservoir Embalse de Río Tercero had no significant macrophyte populations. It was colonized by L. fortunei in 1998, and since around 2000 the macrophyte Elodea callitrichoides has been a dominant feature of this waterbody, forming large beds along several coastal stretches (Boltovskoy et al. 2009a). At approximately the same time, coot and grebe (Fulica leucoptera, Fulica armillata, Podilymbus podiceps) populations in the reservoir increased noticeably, most probably in response to the expansive growth of the beds of aquatic plants on which the birds feed. Coots and grebes have also been observed to retrieve clusters of L. fortunei from the bottom (M. Hechem, pers. comm.), suggesting that they also feed on the mussel too (as other coot species feed on D. polymorpha in North America; Molloy et al. 1997).
Concluding Remarks
Functional similarities between freshwater invasive byssate mussels, including L. fortunei and Dreissena spp., are responsible for similar forcing mechanisms, particularly in their effects on nutrient recycling and pelagic-benthic coupling (Karatayev et al. 1997; Boltovskoy et al. 2006; Ward and Ricciardi 2007; Kelly et al. 2010; Burlakova et al. 2012). However, intrinsic differences between L. fortunei and dreissenids, as well as environmental differences between the waterbodies invaded, are responsible for significant contrasts in ecosystem responses. For example, considerably higher calcium concentrations in European and North American waters than in South American waters (Karatayev et al. 2007) are presumably responsible for the fact that in the former empty mollusc shells represent an important source of substrate for D. polymorpha (Strayer et al. 1996; Burlakova et al. 2006; Strayer and Malcom 2006), whereas in South America dead mussels shells dissolve before they are colonized (Boltovskoy et al. 2006; Karatayev et al. 2007) (see Chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume). For reasons we still do not fully understand, in North American waterbodies Dreissena spp. enhance cyanobacterial blooms only when total P concentrations are below 20–25 µg/l (Nicholls et al. 2002; Raikow et al. 2004; Sarnelle et al. 2005; Knoll et al. 2008), but in South America L. fortunei boosts growth of Microcystis spp. even at levels as high as 50–100 µg P/L (Cataldo et al. 2012b).
Impacts of bivalve grazing on phytoplankton obviously depend on mussel densities and on the abundance of particulate organic matter (POM) in the water column. While mussel densities are generally comparable for these invaders (Karatayev et al. 2010), the attributes of the water bodies invaded are not. The Río de la Plata floodplain river system invaded by L. fortunei has very marked differences with the colder, clearer and more oligotrophic North American waterbodies colonized by Dreissena. One salient difference are the concentrations of POC, typically around 0.15–1 mg/L in the Great Lakes (Fanslow et al. 1995; Barbiero and Tuchman 2004; Johengen et al. 2008), but several times higher in the Río de la Plata watershed—about 3.5 mg/L (Depetris 1976; Depetris and Paolini 1991; Depetris and Pasquini 2007). Sylvester et al. (2005) estimated the energy that can be obtained by a filtering mussel from the phytoplankton and from the seston in general in the lower delta of the Paraná River. Their assessment indicates that phytoplankton alone cannot meet the energetic demands of L. fortunei, but when POM is considered the requirements of juvenile and adult mussels are exceeded 15–22 fold. Furthermore, although freshwater suspension feeding organisms are generally thought to be inefficient at using dissolved organic carbon (DOC) as a source of food (Lopez 1988), both veligers and adults of D. polymorpha have been shown to use DOC intensively, obtaining up to 50 % of their metabolic needs for carbon from this source (Roditi et al. 2000; Banard et al. 2006; Baines et al. 2007). No data are available for the golden mussel, but functional similarities with zebra mussels indicate that L. fortunei may also be able to use DOC. This suggests that filter-feeding organisms in these riverine systems are not food-limited (but they may be food-limited in some of the lentic bodies of water colonized: Boltovskoy et al. 2009b). This assumption contrasts sharply with some of the described impacts of Dreissena spp. in the northern hemisphere, where competition for food with the invader has been found to have strong effects on zooplankton and fish communities (Lozano et al. 2001; Bartsch et al. 2003; Strayer et al. 2004).
Indigenous filter-feeding benthic animals and fishes in the Paraná watershed are scarce, and the dominant feeding modes are associated with detrital and sedimentary organic matter (José de Paggi and Paggi 2007; Rossi et al. 2007). Thus, much of the organic matter carried downstream (around 1 Tg/y, Depetris and Pasquini 2007), is flushed out into the ocean through the Río de la Plata estuary. Since the 1990s, L. fortunei, the first abundant macrobenthic filter-feeder in this system, has been intercepting an important proportion of this POM and retaining it in the system for use by a wide array of animals. This trophic shift involves not only L. fortunei larvae and adults, but also many other invertebrates whose abundances are enhanced by L. fortunei beds, and the organic matter-enriched sediments derived from the “shunt” of suspended POM to the bottom as feces and pseudofeces (Sardiña et al. 2008; Kelly et al. 2010) (see Chapter “Relationships of Limnoperna fortunei with Benthic Animals” in this volume). These organic matter-rich sediments are the main source of food for many organisms, including some of the most abundant fish species, like Prochilodus lineatus, whose biomass represents > 60 % of the overall fish biomass in the Paraná-Uruguay system (Bonetto 1998). On local scales some of the consequences of this invasion have been explored, but on the ecosystem scale we still know very little. Boltovskoy et al. (2006) suggested that enhanced feeding conditions for larval and adult fishes may be responsible for the three-fold increase in Argentine freshwater fish landings after introduction of L. fortunei. However, interpretation of these cause-effect relationships should be made with caution because several factors, including changes in fishing regulations, fishing pressure, fish export trends, etc. changed during the same period.
References
Baines SB, Fisher NS, Cole JJ (2007) Dissolved organic matter and persistence of the invasive zebra mussel (Dreissena polymorpha) under low food conditions. Limnol Oceanogr 52:70–78
Banard C, Martineau C, Frenette JJ, Dodson JJ, Vincent WF (2006) Trophic position of zebra mussel veligers and their use of dissolved organic carbon. Limnol Oceanogr 51:1473–1484
Barbiero RP, Tuchman ML (2004) The deep chlorophyll maximum in Lake Superior. J Great Lakes Res 30(Suppl 1):256–268
Bartsch LA, Richardson WB, Sandheinrich MB (2003) Zebra mussels (Dreissena polymorpha) limit food for larval fish (Pimephales promelas) in turbulent systems: a bioenergetics analysis. Hydrobiologia 495:59–72
Boltovskoy D, Correa N (2015) Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia 746:81–95
Boltovskoy D, Correa N, Cataldo D, Sylvester F (2006) Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata watershed and beyond. Biol Invasions 8:947–963
Boltovskoy D, Karatayev A, Burlakova L, Cataldo D, Karatayev V, Sylvester F, Mariñelarena A (2009a) Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei . Hydrobiologia 636:271–284
Boltovskoy D, Sylvester F, Otaegui A, Leytes V, Cataldo D (2009b) Environmental modulation of the reproductive activity of the invasive mussel Limnoperna fortunei in South America. Austral Ecol 34:719–730
Boltovskoy D, Correa N, Bordet F, Leites V, Cataldo D (2013) Toxic Microcystis (cyanobacteria) inhibit recruitment of the bloom-enhancing invasive bivalve Limnoperna fortunei . Freshw Biol 58:1968–1981
Bonetto AA (1998) Panorama sinóptico sobre la ictiofauna, la pesca y la piscicultura en los ríos de la cuenca del Plata con especial referencia al Paraná. Revista de Ictiología 6:3–15
Bootsma HA, Liao Q (2014) Nutrient cycling by dreissenid mussels. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 555–574
Burlakova LE, Karatayev AY, Padilla DK (2005) Functional changes in benthic freshwater communities after Dreissena polymorpha (Pallas) invasion and consequences for filtration. In: Dame RF, Olenin S (eds) The comparative roles of suspension-feeders in ecosystems. Springer, Amsterdam, pp 263–275
Burlakova LE, Karatayev AY, Padilla DK (2006) Changes in the distribution and abundance of Dreissena polymorpha within lakes through time. Hydrobiologia 571:133–146
Burlakova LE, Karatayev AY, Karatayev VA (2012) Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 685:121–134
Bykova O, Laursen A, Bostan V, Bautista J, Mc Carthy L (2006) Do zebra mussels (Dreissena polymorpha) alter lake water chemistry in a way that favours Microcystis growth? Sci Total Environ 371:362–372
Cataldo D, O’Farrell I, Paolucci EM, Sylvester F, Boltovskoy D (2005) Efectos de Limnoperna fortunei sobre el fitoplancton y los nutrientes. In: Tercer Congreso Argentino de Limnología, CAL III, Chascomús (Argentina)
Cataldo D, O’Farrell I, Paolucci E, Sylvester F, Boltovskoy D (2012a) Impact of the invasive golden mussel (Limnoperna fortunei) on phytoplankton and nutrient cycling. Aquat Invasions 7:91–100
Cataldo D, Vinocur A, O’Farrell I, Paolucci E, Leites V, Boltovskoy D (2012b) The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande Reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680:25–38
Chalar G (2006) Dinámica de la eutrofización a diferentes escalas temporales: Embalse Salto Grande (Argentina-Uruguay). In: Tundisi JG, Matsumura Tundisi T, Sidagis Galli C (eds) Eutrofização na América do Sul: causas, conseqüências e tecnologias de gerenciamento e controle. Instituto Internacional de Ecologia e Gerenciamento Ambiental, Academia Brasileira de Ciencias, Conselho Nacional de Desenvolvimento Científico e Tecnológico, InterAcademy Panel on International Issues, InterAmerican Network of Academies of Sciences, Sao Carlos, pp 87–101
Chalar G (2009) The use of phytoplankton patterns of diversity for algal bloom management. Limnol—Ecol Manage Inland Waters 39:200–208
Depetris PJ (1976) Hydrochemistry of the Paraná River. Limnol Oceanogr 21:736–739
Depetris PJ, Paolini JE (1991) Biogeochemical aspects of South American rivers: the Paraná and the Orinoco. In: Degens ET, Kempe S, Richey JE (eds) Biogeochemistry of major world rivers. Wiley, Chichester, pp 105–125
Depetris PJ, Pasquini AI (2007) The geochemistry of the Paraná River: an overview. In: Iriondo MH, Paggi JC, Parma MJ (eds) The Middle Paraná River. Limnology of a subtropical wetland. Springer Verlag, Berlin, pp 143–174
Devercelli M, Peruchet E (2008) Trends in chlorophyll-a concentration in urban water bodies within different man-used basins. Annales de Limnologie—Int J Limnol 44:75–84
Di Fiori E, Pizarro H, dos Santos Afonso M, Cataldo D (2012) Impact of the invasive mussel Limnoperna fortunei on glyphosate concentration in water. Ecotoxicol Environ Safety 81:106–113
Dionisio Pires LM, Ibelings BW, van Donk E (2010) Zebra mussels as a potential tool in the restoration of eutrophic shallow lakes, dominated by toxic cyanobacteria. In: van der Velde G, Rajagopal S, Bij de Vaate A (eds) The Zebra Mussel in Europe. Backhuys Publishers, Leiden, pp 361–372
Fachini A (2011) Filtração do bivalve invasor Limnoperna fortunei (Dunker, 1857), o mexilhão dourado, sobre a comunidade planctônica natural e na presença de cianobactéria tóxica. MSc Thesis, Universidade Federal do Rio Grande do Sul (Brazil), pp 1–68
Fachini A, Gazulha V, Pedrozo CS (2012) Os impactos do mexilhão-dourado sobre a comunidade planctônica. In: Mansur MCD, Santos CP, Pereira D, Padula Paz IC, Leite Zurita ML, Raya Rodriguez MT, Vilar Nehrke M, Aydos Bergonci PE (eds) Moluscos límnicos invasores no Brasil. Biologia, prevenção, controle. Redes Editora, Porto Alegre, pp 255–261
Fanslow DL, Nalepa TF, Lang GA (1995) Filtration rates of the zebra mussel (Dreissena polymorpha) on natural seston from Saginaw Bay, Lake Huron. J Great Lakes Res 21:489–500
Frau D, Rojas Molina F, Devercelli M, José de Paggi S (2013) The effect of an invading filter-feeding bivalve on a phytoplankton assemblage from the Paraná system: a mesocosm experiment. Mar Freshw Behav Physiol 45:303–316
Gazulha V (2010) O mexilhão dourado Limnoperna fortunei (Dunker, 1857) na presença de cianobactérias: taxas de filtração, comportamento alimentar e sobrevivência. PhD Thesis, Universidade Federal do Rio Grande do Sul (Brazil), pp 1–104
Gazulha V (2012) O impacto de Limnoperna fortunei sobre as cianobacterias. In: Mansur MCD, Santos CP, Pereira D, Padula Paz IC, Leite Zurita ML, Raya Rodriguez MT, Vilar Nehrke M, Aydos Bergonci PE (eds) Moluscos límnicos invasores no Brasil. Biologia, prevenção, controle. Redes Editora, Porto Alegre, pp 249–254
Gazulha V, Mansur MCD, Cybis LF, Azevedo SMFO (2012a) Feeding behavior of the invasive bivalve Limnoperna fortunei (Dunker, 1857) under exposure to toxic cyanobacteria Microcystis aeruginosa . Braz J Biol 72:41–49
Gazulha V, Mansur MCD, Cybis LF, Azevedo SMFO (2012b) Grazing impacts of the invasive bivalve Limnoperna fortunei (Dunker, 1857) on single-celled, colonial and filamentous cyanobacteria. Braz J Biol 72:33–39
Jeong KS, Kim DK, Wigham P, Joo GJ (2003) Modelling Microcystis aeruginosa bloom dynamics in the Nakdong River by means of evolutionary computation and statistic approach. Ecol Model 161:67–68
Johengen TH, Biddanda BA, Cotner JB (2008) Stimulation of Lake Michigan plankton metabolism by sediment resuspension and river runoff. J Great Lakes Res 34:213–227
Jørgensen CB (1966) Biology of suspension feeding. Pergamon Press, Oxford, pp 1–357
José de Paggi S, Paggi JC (2007) Zooplankton. In: Iriondo MH, Paggi JC, Parma MJ (eds) The middle Parana River. Limnology of a subtropical wetland. Springer, Berlin, pp 229–249
Juhel G, Davenport J, O' Halloran J, Culloty S, Ramsay R, James K, Furey A, Allis O (2006a) Pseudodiarrhoea in zebra mussels Dreissena polymorpha (Pallas) exposed to microcystins. J Exp Biol 209:810–816
Juhel G, Davenport J, O'Halloran J, Culloty SC, O’Riordan RM, James KF, Furey A, Allis O (2006b) Impacts of microcystins on the feeding behaviour and energy balance of zebra mussels, Dreissena polymorpha: a bioenergetics approach. Aquat Toxicol 79:391–400
Karatayev AY, Burlakova LE, Padilla DK (1997) The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in Eastern Europe. J Shellfish Res 16:187–203
Karatayev AY, Burlakova LE, Padilla DK (2002) Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe: distribution, impacts and management. Kluwer Academic Publishers, Dodrecht, pp 433–446
Karatayev AY, Boltovskoy D, Padilla DK, Burlakova LE (2007) The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. J Shellfish Res 26:205–213
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010) Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves. J Shellfish Res 29:975–984
Kawase M (2011) Feeding and filtration in Limnoperna lacustris . http://www.mizuho-c.ac.jp/images/library/kiyo_05/amckiyo-no05-05.pdf. Accessed 11 Feb 2014 [In Japanese]
Kelly DW, Herborg L-M, MacIsaac HJ (2010) Ecosystem changes associated with Dreissena invasions: recent developments and emerging issues. In: van der Velde G, Rajagopal S, Bij de Vaate A (eds) The Zebra Mussel in Europe. Backhuys Publishers, Leiden, pp 199–210
Knoll LB, Sarnelle O, Hamilton SK, Kissman CEH, Wilson AE, Rose JB, Morgan MR (2008) Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Can J Fish Aquat Sci 65:448–455
Lopez GR (1988) Comparative ecology of the macrofauna of freshwater and marine muds. Limnol Oceanogr 33:946–962
Lozano SJ, Scharold JV, Nalepa TF (2001) Recent declines in benthic macroinvertebrate densities in Lake Ontario. Can J Fish Aquat Sci 58:518–529
MacIsaac HJ (1996) Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. Am Zool 36:287–299
Mansur MCD, Santos CP, Pereira D, Padula Paz IC, Leite Zurita ML, Raya Rodriguez MT, Vilar Nehrke M, Aydos Bergonci PE (eds) (2012) Moluscos límnicos invasores no Brasil. Biologia, prevenção, controle. Redes Editora, Porto Alegre, pp 1–411
Molloy DP, Karatayev AY, Burlakova EB, Kurandina DP, Laruelle F (1997) Natural enemies of zebra mussels: predators, parasites, and ecological competitors. Rev Fish Sci 5:27–97
Morton B (1983) Feeding and digestion in Bivalvia. In: Wilbur KM, Saleuddin ASM (eds) The Mollusca, vol 5: Physiology, Part 2. Academic Press, New York, pp 65–147
Nalepa TF, Schloesser DW (eds) (2014) Quagga and zebra mussels: biology, impacts, and control. 2nd Edition. CRC Press, Boca Raton, pp 1–815
Nicholls KH, Heintsch L, Carney E (2002) Univariate step-trend and multivariate assessments of the apparent effects of P loading reductions and zebra mussels on the phytoplankton of the Bay of Quinte, Lake Ontario. J Great Lakes Res 28:15–31
O’Farrell I, Bordet F, Chaparro G (2012) Bloom forming cyanobacterial complexes co-occurring in a subtropical large reservoir: validation of dominant eco-strategies. Hydrobiologia 698:175–190
Pestana D, Ostrensky A, Pereira Boeger WA, Pie MR (2009) The effect of temperature and body size on filtration rates of Limnoperna fortunei (Bivalvia, Mytilidae) under laboratory conditions. Braz Arch Biol Technol 52:135–144
Pillsbury RW, Lowe RL (1994) The impact of zebra mussels on benthic algal communities in Saginaw Bay, Lake Huron. In: 4th International Zebra Mussel Conference, Madison (USA)
Pizzolón L, Tracanna B, Prosperi C, Guerrero JM (1999) Cyanobacterial blooms in Argentinean inland waters. Lakes & Reserv: Res Manage 4:101–105
Raikow DF, Sarnelle O, Wilson AE, Hamilton SK (2004) Dominance of the noxious cyanobacterium Microcystis aeruginosa in low-nutrient lakes is associated with exotic zebra mussels. Limnol Oceanogr 49:482–487
Relyea RA (2006) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 16:2027–2034
Roditi HA, Fisher NS, Sañudo-Wilhelmy SA (2000) Uptake of dissolved organic carbon and trace elements by zebra mussels. Nature 407:78–80
Rojas Molina F, Jose de Paggi SB (2008) Zooplankton in the Paraná River floodplain (South America) before and after the invasion of Limnoperna fortunei (Bivalvia). Wetlands 28:695–702
Rojas Molina F, Paggi JC, Devercelli M (2010) Zooplanktophagy in the natural diet and selectivity of the invasive mollusk Limnoperna fortunei . Biol Invasions 12:1647–1659
Rossi L, Cordiviola E, Parma MJ (2007) Fishes. In: Iriondo MH, Paggi JC, Parma MJ (eds) The middle Parana River. Limnology of a subtropical wetland. Springer, Berlin, pp 305–325
Rückert G, Campos MCS, Rolla ME (2004) Alimentação de Limnoperna fortunei (Dunker 1857): taxas de filtração com ênfase ao uso de Cyanobacteria. Acta Scientiarum 26:421–429
Ruibal Conti AL, Guerrero JM, Regueira JM (2005) Levels of microcystins in two Argentinean reservoirs used for water supply and recreation: differences in the implementation of safe levels. Environ Toxicol 20:263–269
Sardiña P, Cataldo D, Boltovskoy D (2008) The effects of the invasive mussel, Limnoperna fortunei, on associated fauna in South American freshwaters: importance of physical structure and food supply. Fund Appl Limnol/Archiv für Hydrobiologie 173:135–144
Sarnelle O, Wilson AE, Hamilton SK, Knoll LB, Raikow DF (2005) Complex interactions between the zebra mussel, Dreissena polymorpha, and the harmful phytoplankter, Microcystis aeruginosa . Limnol Oceanogr 50:896–904
Smith VH (1983) Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221:669–671
Smith VH, Bennett SJ (1999) Nitrogen:phosphorus supply ratios and phytoplankton community structure in lakes. Archiv für Hydrobiologie 146:37–53
Spaccesi FG, Rodrigues Capitulo A (2012) Benthic communities on hard substrates covered by Limnoperna fortunei Dunker (Bivalvia, Mytilidae) at an estuarine beach (Río de la Plata, Argentina). J Limnol 71:144–153
Strayer DL, Malcom HM (2006) Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshw Biol 51:117–130
Strayer DL, Powell J, Ambrose P, Smith LC, Pace ML, Fischer DT (1996) Arrival, spread, and early dynamics of a zebra mussel (Dreissena polymorpha) population in the Hudson River estuary. Can J Fish Aquat Sci 53:1143–1149
Strayer DL, Hattala KA, Kahnle AW (2004) Effects of an invasive bivalve (Dreissena polymorpha ) on fish in the Hudson River estuary. Can J Fish Aquat Sci 61:924–941
Sylvester F, Dorado J, Boltovskoy D, Juárez A, Cataldo D (2005) Filtration rates of the invasive pest bivalve Limnoperna fortunei as a function of size and temperature. Hydrobiologia 534:71–80
Sylvester F, Boltovskoy D, Cataldo D (2007) Fast response of freshwater consumers to a new trophic resource: Predation on the recently introduced Asian bivalve Limnoperna fortunei in the lower Parana River, South America. Austral Ecol 32:403–415
Turner CB (2010) Influence of zebra (Dreissena polymorpha) and quagga (Dreissena rostriformis) mussel invasions on benthic nutrient and oxygen dynamics. Can J Fish Aquat Sci 67:1899–1908
Vanderploeg HA, Liebig JR, Carmichael WW, Agy MA, Johengen TH, Fahnenstiel GL, Nalepa TF (2001) Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can J Fish Aquat Sci 58:1208–1221
Ward JM, Ricciardi A (2007) Impacts of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Divers Distrib 13:155–165
Yang Z, Kong FX, H.S. C, Shi XL (2005) Observation on colony formation of Microcystis aeruginosa induced by filtered lake water under laboratory conditions. Annales de Limnologie 41:169–173
Zhang H, Culver D, Boegman L (2011) Dreissenids in Lake Erie: an algal filter or a fertilizer? Aquat Invasions 6:175–194
Zhu B, Fitzgerald DG, Mayer CM, Rudstam LG, Mills EL (2006) Alteration of ecosystem function by zebra mussels in Oneida Lake: impacts on submerged macrophytes. Ecosystems 9:1017–1028
Acknowledgments
This work was partially financed by grants from the University of Buenos Aires, Argentina (UBA X-020 and 20020100100035) and from the Argentine Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 2007 1968) to DB.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Boltovskoy, D., Correa, N., Sylvester, F., Cataldo, D. (2015). Nutrient Recycling, Phytoplankton Grazing, and Associated Impacts of Limnoperna fortunei . In: Boltovskoy, D. (eds) Limnoperna Fortunei. Invading Nature - Springer Series in Invasion Ecology, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-319-13494-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-13494-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13493-2
Online ISBN: 978-3-319-13494-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)