Abstract
In this study, we analyse the spatial distribution of cyanobacterial summer blooms in a large subtropical reservoir located in the Uruguay River, from 2007 to 2011; these extraordinary algal growth events are mainly represented by scum-forming and nitrogen-fixing eco-strategists of the Dolichospermum and Microcystis genera. The use of the eco-strategists approach, based on ecophysiological work and field observations, allowed us to explain the differences in the distribution pattern and temporal dynamics of both cyanobacterial complexes. Spatial differences were produced due to much higher and fluctuating cyanobacterial abundances at the right margin of the reservoir and at the littoral areas closer to the dam. Satellite imagery (LANDSAT 5 TM) clearly depicted the stronger algal development in the reservoir arms and in the section closer to the dam. The Microcystis spp. complex achieved higher density than the Dolichospermum spp. complex. We hypothesise that the hydrological cycle explains the inter-annual fluctuations of the intensity and frequency of cyanobacterial blooms, and that spatial differences in cyanobacterial presence between the reservoir arms, its margins and the main channel is mainly a response to morphometrical and hydrological characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is growing evidence that the spatial and temporal incidences of toxic algal blooms has increased steadily, thereby entailing potential risks to both human health and sustainability of ecosystems. Blooms of toxic cyanobacteria represent one of the most serious stressors in lakes, rivers, estuaries and marine environments (Ibelings & Havens, 2008). A wide range of impacts on the ecosystem generally occur when blooms with high cyanobacteria biomass persist or occur with great frequency: shading and growth inhibition of other primary producers (phytoplankton, benthic algae and vascular plants), pH raise and great diurnal fluctuations, interference of food collection for zooplankton filter feeders, increased organic loading and anoxic sediments, accumulation of ammonia in water by senescence and subsequent bacterial decomposition of the blooms and mass mortality of birds and fish. The recurrence of this scenario produces a decline in biodiversity at all levels, from phytoplankton and zooplankton to birds, as well as changes in nutrient recycling and disruptions of carbon and energy flow in pelagic and benthic food webs (Ibelings & Havens, 2008). Although the relative importance of the effect of cyanotoxins over the above-mentioned stressors is not well defined in scenarios of massive fish kills, it is certain that toxins can cause death, cancer or birth defects in mammals (Kuiper-Goodman et al., 1999), increasing the costs of management and processing required for drinking water.
Eutrophication has been traditionally indicated as the main factor responsible for cyanobacterial blooms (Reynolds, 2006; Schindler et al., 2008), but there is recent evidence suggesting that global warming may also promote these events (Antoniades et al., 2007; Paerl & Huisman, 2008; Kosten et al., 2011). Nutrient enrichment stimulates cyanobacterial growth (Smith, 1983; Moisander et al., 2009) and its threshold value depends on the combination of several factors. For example, de Tezanos Pinto & Litchman (2010) described the interactive effects of nitrogen, phosphorus and light on the development of N-fixing cyanobacteria in a laboratory experiment, and Jiang et al. (2008) screened the interactions among significant factors (nutrients, iron, temperature and light) on the growth of Microcystis aeruginosa. This introduces the paramount influence of algal entrainment in nutrient-rich mixed layers and otherwise disentrainment from well-illuminated layers, which are reflected in natural ecosystems in terms of the ratio of euphotic depth to mixing depth of the water column (Reynolds, 2006).
The recognition of the environmental conditions that facilitate and promote the establishment of different species of cyanobacteria is mandatory to predict and manage the problems caused by these noxious blooms. Mur et al. (1999) developed a scheme that groups species according to their physiological traits in six eco-strategies, thus reducing the high diversity of this group in order to facilitate the predictability of blooms. The same species can be classified in more than one category: scum-forming, stratifying, homogeneously dispersed, nitrogen-fixing, benthic and small colony-forming eco-strategists. Oliver & Ganf (2000) proposed a synthetic way of classifying species forming blooms, and described three categories according to the mixing and the light in the water column, but did not consider the dynamics of nutrients. The approaches using ecological features allow the understanding and prediction of species distributions along environmental gradients (Litchman et al., 2010). Nevertheless, the monitoring of the spatial distribution is costly and therefore spatio-temporal patterns of bloom formation in most large inland waters are poorly known (Gons et al., 2005). The assessment of the spatial distribution of phytoplankton biomass may be greatly improved by remote sensing as satellite imagery complements in situ measurements and facilitates mapping large and heterogeneous systems; this technique provides synoptic and timely information on the abundance and distribution of cyanobacterial populations that, in turn, can facilitate public health risk assessment (Wheeler et al., 2011).
In subtropical South America, most studies related to problems caused by cyanobacterial blooms are performed to assess the toxicity risk of these events. There is little ecological research designed to fully understand the causality of blooms composed by different eco-strategists. Salto Grande Reservoir, located on the Uruguay River is one of the aquatic systems more affected in the region by recurrent cyanobacterial blooms composed of species with multiple morpho-physiological adaptations. The first studies performed just after the reservoir was filled in 1979, described a central zone of high flushing rate where phytoplankton was dominated by centric diatoms, and several arms where cyanobacteria rarely thrived, always providing that water level was low (Quirós & Cuch, 1982; Quirós & Luchini, 1982). Later on, De León & Chalar (2003) and Chalar et al. (2002 provided further information regarding the differences between the tributary arms located closer to the dam. Chalar (2009) suggested that decreases in either water discharge, wind-induced resuspension or water level caused Microcystis aeruginosa bloom formation. The toxicity of these blooms has been assessed at irregular intervals since 1999 yielding positive results (Chalar et al., 2002).
In this study, we perform an overall analysis of the spatial distribution of cyanobacteria in the Salto Grande Reservoir over the last five summer seasons (2007–2011). We aim to characterise the eco-strategies of the blooms by means of comparing the species composition, their physiological features and the prevailing environmental conditions at the different sites of the reservoir. We hypothesise that the hydrological regime regulates the development and intensity of summer algal blooms and that the dominant eco-strategies depend on the environmental conditions prevailing at each location of the reservoir.
Methods
Study area
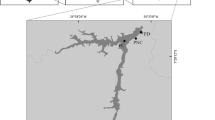
Salto Grande is a large river-like reservoir (750 km2) with multiple arms located along 100 km of the main channel of the Uruguay River (29°43′ to 31°12′S and 57°06′ to 57°55′W) (Fig. 1). It is characterised by a high water period from April to November and a low water phase during summer time (December to March). Mean flow ranges from 2,800 to 5,563 m3 s−1 with minimum and maximum records of 216 and 22,000 m3 s−1 in dry and rainy periods, respectively. The reservoir is polymictic with short-lasting stratification under low flow conditions; it has a mean depth of 6.4 m, a maximum of depth of 35 m and a mean retention time of 11.3 days. This system has a single major entrance and five lateral arms; the Arapey and Mocoretá tributaries provide water inputs of minor importance (the mean flow of the Arapey represents <4% of the Uruguay River flow), whereas the supplies of the Mandisoví, Gualeguaycito and Itapebí are so small that they are not relevant (Quirós & Cuch, 1982). The reservoir is utilised for drinking water and recreational activities, including sports and fishing. Its waters are treated for drinking purposes using conventional water treatment technology that include activated carbon filters.
Location of the Salto Grande Reservoir on the Uruguay River, indicating sampling sites:1 Dam, 2 Playa Sol, 3 Las Palmeras, 4 Los Médicos, 5 Las Perdices, 6 La Toma, 7 Los Pinos, 8 Int. Munic. Salto (IMS), 9 Gualeguaycito, 10 Itapebi, 11 Playa Sur, 12 Playa Baly, 13 Playa Grande, 14 Santa Ana, 15 Belén, 16 Monte Caseros, 17 Bella Unión
In the drainage area of the reservoir, the mean annual temperature is 19°C and the mean annual rainfall is 1,260 mm. Local winds have a NE direction during the entire year, with mean monthly velocities ranging between 10 and 12 km h−1; in summer and spring the prevailing winds are N, NE, E and SE and in autumn and winter, without being dominant, the frequency of S and SW winds increases (Rojas & Saluso, 1987).
Sampling design
We here analyse the most recent cyanobacterial records produced in routine samplings by the Joint Technical Commission of Salto Grande (CTM) and the Uruguay River Management Commission (CARU). In Fig. 1, we indicate the 17 sampling points analysed over five warm periods from 2007 to 2011; for sites 15 to 17, data are only available since 2009. CTM provided the data of water level, measured as the depth at the deepest point closest to the dam, and the inflowing discharge estimated with a mass balance calculated as a function of water level.
Samples were collected each 7–10 days from January to April at 20 cm depth below the water surface. Subsurface temperature, pH and conductivity and dissolved oxygen were measured in situ using Orion Sa 720 and YSI 58 portable electronic metres. Temperature profiles were performed at sites 1, 9 and 10 during the 2010 and 2011 summer seasons at 1 m depth intervals between the surface and the bottom. Transparency was estimated with a Secchi disc; the euphotic depth (Z eu) was estimated as 2.7 times the Secchi depth. Samples for soluble nutrients and chlorophyll a analyses were collected at the subsurface in plastic bottles pre-rinsed in lake water and preserved in dark and cold conditions until their filtration through fibreglass filters (Whatman GF/F). Nutrient determinations were only performed at sites 1, 9, 10, 14, 15, 16 and 17 from 2009 to 2011. Phosphate and nitrate were analysed following the stannous chloride method and cadmium reduction method, respectively, and ammonia was estimated by nesslerization. Total phosphorus (TP) and nitrogen (TN) were determined from unfiltered samples after digestion with persulfate (APHA, 2005). Inorganic suspended solids were evaluated by drying the non-filtrable residue at 550°C until constant weight (APHA, 2005). Concentrations of chlorophyll a, corrected for phaeopigments, were determined by spectrophotometry before and after acidification (HCl 0.1 N), using acetone at 90% solvent (Nusch, 1980). The equations published by Lorenzen (1967) were used for the calculations.
Water samples for quantitative phytoplankton analysis were preserved in PVC flasks with 1% Lugol’s iodine solution. Counts were performed according to Utermöhl (1958). Replicate chambers were left to sediment for at least 24 h. Counting errors were estimated according to Venrick (1978), accepting a maximum of 20% for the most frequent species. When dense scums occurred, countings were performed with a Neubauer hemocytometer (0.1 mm deep) under light microscope, after hot digestion with sodium hydroxide (Reynolds & Jaworski, 1978). In this study, we follow Wacklin et al. (2009) who transferred all planktic Anabaena morphotypes into the new genus Dolichospermum.
Statistical analyses
In order to study the independence between the abundance of the Microcystis and Dolichospermum complexes at the different sites and locations (beaches, tributaries, main channel, margins), we performed non parametrical analysis by Chi-Square tests. We then related the algal data of the resulting homogeneous sites to environmental variables by using the Spearman correlation index. The Canoco program was used to perform a redundant detrended analysis (RDA) using the matrix of mean annual physico-chemical and hydrological variables and the abundance of the two cyanobacterial complexes. This ‘biological matrix’ was tested separately against an environmental matrix obtained for the 17 sites without nutrient data and against a smaller matrix corresponding to the 7 sites with available nutrient information. The significance of canonical axes was analysed by a Monte Carlo permutation test (Ter Braak & Verdonschot, 1995).
Satellite imagery
We used two adjoining pairs of Landsat-5 TM images that represented contrasting scenarios in the reservoir: February 4, 2009 (extremely low waters), and March 14, 2011 (mid-waters). Both scenes were from the Landsat Path 225 and Rows 81–82. A water-only image was created using an unsupervised classification that was recoded and used to mask terrestrial areas. In order to locate the field sampling stations on satellite images and to extract the corresponding radiometric data, the images were geometrically corrected using well-distributed ground control points (GCP'S), with a positional accuracy of ±1 pixel. A radiometric correction was conducted with the Rayleigh dispersion model in order to reduce the atmospheric contribution (Stumpf, 1992). We extracted the radiometric data (reflectance) and analysed which bands or bands ratios were most suitable for creating a regression model for each image by performing Pearson correlations between satellite and in situ data. The spatial distribution of chlorophyll a was determined by applying the formulated regression model. Image processing was conducted using ERDAS Imagine 9.1 software.
Results
The fluctuation of the hydrological regime of the Salto Grande Reservoir from December 2006 to March 2011 is depicted in Fig. 2; the inflowing discharge registered a marked variation over the study period, from 434 in May 2009 to 29,730 m3 s−1 in November 2009. Major inflows occurred periodically during late winter and early spring, except for summer 2010 when high river discharge persisted till late summer; contrarily, a prolonged drought period spanned from early summer to fall 2009. Water level of the dam only fluctuated between 29.9 and 36.8 m in July 2007 and December 2009, respectively. Inflowing discharge and water level were significantly correlated (r = 0.44, P < 0.01, N = 903). A rough estimation of mean water retention time showed strong differences among summer seasons, ranging from approximately 15 days in 2010 to 66 days in 2009.
The physico-chemical characteristics of the Salto Grande Reservoir during the five warm seasons here analysed are summarised in Table 1. Water temperature ranged from 13.8°C at site 1 (dam) to 35.9°C at the beach located at site 3; higher mean annual temperatures were always registered along the shoreline sites (2007, 2008, 2010 at site 3; 2009 at site 4; 2011 at site 15). The summer of 2007 was warmest (mean 28.4°C), whereas the lowest mean temperature (26.4°C) corresponded to 2009. Waters were generally well oxygenated, though at sites 9 and 10, concentrations as low as 3 mg l−1 were measured. Highest oxygen concentrations were mostly registered at these same sites in the main channels of the inflowing tributaries, and at site 3 with a seasonal mean value of 15.7 mg l−1. Waters were slightly alkaline with mean pH higher than 7.3 achieving a maximum value of 10.1 at site 9. Mean conductivity in the reservoir was low, 55.8 μS cm−1, with higher values at the upstream sites and especially at those located at the left margin. Transparency (mean 0.66 m) was usually higher at the main channel of the river and its tributaries than in the beaches; it was inversely related to water discharge (r = −0.3, P < 0.05, N = 831). The mean concentration of inorganic suspended solids revealed a clear pattern with decreasing values from the upriver sites (20.3 mg l−1) to the dam (5.3 mg l−1).
The information regarding nutrients is scarce and available only for the sites located in the main channel of the river (1, 14) and tributaries (9, 10) and at the northern sites (15, 16, 17). Phosphorus and nitrogen inorganic dissolved fractions remained fairly constant along the reservoir, with mean concentrations of 20 and 680 μg l−1, respectively. Contrarily, TP decreased downriver from 60 to 40 μg l−1 and TN increased from 4,220 to 5,290 μg l−1; interestingly, discharge was directly correlated with TN concentration (r = 0.62, P < 0.05, N = 155) and inversely with SRP (r = −0.35, P < 0.05, N = 155). As for N:P ratios, TN:TP values were highly variable between years due to the extraordinary drought of 2009 when the enhancement of TN determined ratios as high as 331, whereas during 2010 and 2011 values ranged between 11 and 38.5. The DIN:SRP ratio ranged between 22 and 68.8, with a similar pattern at all sites decreasing from 2009 to 2011. TP (40 μg l−1) and chlorophyll a (14.8 μg l−1) mean concentrations correspond to eutrophic systems.
Phytoplanktonic chlorophyll a concentration in the Salto Grande Reservoir during the summer periods was significantly correlated with total cyanobacteria cell count (r = 0.56, P = 0.01, N = 842), which in turn was highly correlated with the abundance of species constituting the ‘Microcystis complex’ (r = 0.9, P = 0.01, N = 870) and the ‘Dolichospermum complex’ (r = 0.67, P = 0.01, N = 862). The most representative species were Microcystis aeruginosa, M. wesenbergii, Dolichospermum circinale, D. spiroides and D. planctonicum; only occasionally, Raphidiopsis mediterranea achieved relatively high abundances.
The abundance of both dominant complexes differed significantly among reservoir locations (Fig. 3), namely the beaches (2–8, 11–13 and 15–17), tributaries (9 and 10) and the middle of the main channel (1 and 14) (P < 0.05). There were significant differences between both tributaries (9 vs. 10), between the beaches located at opposite margins (2, 3, 4, 5, 11, 12, 13 vs. 6, 7, 8), and even among the beaches of the right margin located in the dam area (2, 3, 4, 5). Only two homogeneous sets of samples could be identified: the beaches at the left margin of the dam area (6, 7, 8) and at Federación (11, 12, 13). Site 3 presented the highest Microcystis mean cell density (113835.4 cells ml−1) and interestingly, despite site 9 is located in a lotic environment, its abundances were also very high (105840.4 cell ml−1). The lowest mean densities correspond to the northern site 17 at the tail of the reservoir where minimum abundances of Microcystis, Dolichospermum and total cyanobacteria were recorded (617.6, 2553.1 and 3170.7 cells ml−1, respectively), even with quite high chlorophyll concentrations (Fig. 3) that corresponded to eukaryotic algae, mostly diatoms.
There were also annual differences at each site (Fig. 3). Higher cyanobacterial development was observed during 2011 at some sites, especially at the beaches located at the right margin of the dam area (sites 3, 4 and 5), and lower densities during 2010; the Microcystis complex was responsible for this general pattern. The distribution pattern of the Dolichospermum complex differed slightly, as it was fairly abundant in 2009 and at other sites, namely those located at the beaches in Federación (sites 11, 12 and 13).
The significant correlations with the environmental variables at different sites for both cyanobacterial complexes reveal different responses (Table 2). In general terms, Microcystis appears mainly negatively correlated to discharge, while Dolichospermum negatively to water level and positively to temperature. The increase of both discharge and water level had a negative effect on cyanobacterial development whereas temperature favoured their growth. Secchi depth showed controversial responses and no correlation was observed with nitrogen and phosphorus ratios. Few weak correlations were found with nutrient concentrations: between Microcystis and SRP at site 10 (r = −0.43, P < 0.1, N = 23) and Dolichospermum and N–NH4 at sites 14 and 17 (r = 0.49, P < 0.1, N = 17 and r = 0.47, P < 0.1, N = 22, respectively).
The triplot resulting from the RDA using the abundances of the two cyanobacterial complexes and environmental variables from the 17 sites is shown in Fig. 4a. The environmental variables are significantly correlated with the first axis (P = 0.002), and the test of significance of all canonical axes is also significant (P = 0.002). The first axis explains a large proportion of variance in the cyanobacterial complex–environment relationship (75.6%) and is mainly defined by water level, oxygen, discharge and pH (intraset correlation coefficients: 0.45, −0.30, 0.29 and −0.25, respectively). The triplot represents 35% of the total variance of cyanobacterial data; temperature and oxygen were the variables most strongly correlated with the second axis (intraset correlation coefficients: 0.35 each). This analysis clearly shows that the hydrological variables were major factors controlling both complexes; Dolichospermum was benefited by the demise in water level (2009) and Microcystis was also positively influenced in warmer years (especially 2007) as depicted in the grouping of the samples corresponding to the different summer seasons. Oxygen and pH mostly respond to the metabolic activity of strong Microcystis blooms.
The influence of nutrients was analysed in a complementary RDA performed with the reduced dataset (7 sites, 3 years) (Fig. 4b). The environmental variables are significantly correlated with the first axis (P = 0.034), and the test of significance of all canonical axes is also significant (P = 0.014). The first axis explains 73.4% of variance in the cyanobacterial complex–environment relationship and is correlated with pH, TP, SRP, oxygen and water level (intraset correlation coefficients: 0.57, −0.48, −0.35, 0.34 and −0.26, respectively). The second axis is mainly associated with TP, DIN and pH level (intraset correlation coefficients: −0.36, 0.35 and −0.236, respectively). Thus, low values of water level and discharge once again evidenced the enhancement of cyanobacterial growth, but this additional analysis suggested that SRP and TP could have limited cyanobacterial growth.
The influence of temperature variation and stability of the water column on the abundance pattern of both eco-types in each summer season at deep sites (main channel and tributaries) is represented in Fig. 5 with data from site 9. Dolichospermum achieved highest densities (Fig. 5a) at elevated surface temperatures (>28.9°C), which coincided with stratification of the water column that was registered at the onset of summer (Fig. 5b). On the other hand, Microcystis increased its concentrations towards the end of summer or beginning of autumn, especially during 2011 (Fig. 5b). The pattern here illustrated for site 9, was also observed at sites 1 and 10 (data not shown).
a Dolichospermum and Microcystis temporal fluctuations at site 9 (Gualeguaycito arm) during the summers of 2010 and 2011. b Temperature profiles at site 9 for the same periods. Note the bullets in the upper panel (a) correspond to the sampling dates with highest temperatures indicated with bold lines in the lower panel (b)
Table 3 summarises the occurrence of blooms with more than 105 cyanobacterial cells ml−1 (WHO alert level 2 for drinking water supply) (Bartram et al., 1999) for each summer season at the different environments. It is evident that the worse scenarios (high cyanobacterial development) always corresponded to the beaches of the right margin located in the area close to the dam (RMDam); the higher frequency of events was observed in 2008 and 2011 when low water periods coincided with high temperatures. In the beaches along the shoreline, the light climate was good as estimated by the euphotic depth (Z eu) that generally comprised the entire water column, whereas in the open channels the relation between the mixing and euphotic depth (Z mix/Z eu) ranged between 5 and 6 in the tributaries (sites 10 and 9, respectively) and 16 at the main flow near the dam.
Mapping chlorophyll a by satellite imagery
Figure 6 presents the chlorophyll a (chl a) thematic map predicted from the March 14, 2011 satellite imagery analysis. In situ chl a range was 0.9–62 μg l−1 and the higher correlation coefficient was found between ln chl a and band 2 (b2: 0.52–0.60 μm) (r = −0.83); the derived regression model was ln chl a = 23.48–0.72 b2 (R 2 = 0.7). The image shows great spatial variations between the reservoir areas, with remarkable higher chlorophyll a concentrations at the arms. Approximately 74% of the Gualeguaycito arm area shows values exceeding the threshold of alert level 2, 18% values exceeding alert level 1 and 8% values below the alert levels for drinking water supply (Bartram et al., 1999). Similarly, 69% of the Itapebi arm area presents chlorophyll a values exceeding alert level 2, 22% values exceeding alert level 1 and 10% show values below the alert levels. On the other hand, near 100% of the area covering the main channel, the dam and beaches show lower concentrations (13–27 μg l−1) that do not exceed alert levels.
Contrarily, the low waters scenario (2009, image not shown) presents a more homogeneous distribution and lower concentrations of chlorophyll a. Both Gualeguaycito and Itapebi arms present larger areas with values that did not exceed alert levels (79 and 72% of each arm area, respectively), while 22 and 28% show concentrations exceeding alert level 1. The in situ chl a range was 7–14 μg l−1 and the higher correlation with satellite data was found with ln band 3 (b3: 0.63–0.69 μm) (r = −0.67); the regression derived model was chl a = 53.2–14.43 ln b3 (R 2 = 0.45).
Discussion
Reservoirs are worldwide endangered by the proliferation of cyanobacterial blooms (Steel & Duncan, 1999; Bittencourt-Oliveira, 2003; Naselli-Flores & Barone, 2003; Bormans et al., 2005; Znachor et al., 2006; Gemelgo et al., 2009; Sotero-Santos et al., 2008; Moisander et al., 2009). The recognition of the environmental conditions that promote and facilitate the establishment of Cyanobacteria requires the identification of the prevailing eco-strategies in each system. In several tropical to subtropical reservoirs, the co-existence of the Microcystis and Dolichospermum complexes has been repeatedly registered (Sotero-Santos et al., 2008; Li et al., 2010; Te & Gin, 2011). In the Salto Grande Reservoir, Microcystis dominated over Dolichospermum exceeding one order of magnitude its cell densities for the five last summer seasons recorded in this study. The same applies for the Kranji Reservoir (Te & Gin, 2011) and for eutrophic Czech reservoirs (Znachor et al., 2006), where Microcystis spp. (mainly M. aeruginosa) dominates over Dolichospermum spp. These species complexes were characterised by Mur et al. (1999) as scum-forming and, in the case of Dolichospermum, also as nitrogen-fixing. Both eco-strategies have the common feature to regulate buoyancy and thus, have an advantage in competing with other phytoplankton for light and nutrients. Under which conditions do these traits provide competitive advantages that can lead to extraordinary growth of the populations? Is N-fixation a beneficial trait in this enriched turbid system?
Our findings indicate that hydrological conditions were the driving factors in regulating cyanobacterial blooms in Salto Grande Reservoir: declining abundances of both eco-types are related to increasing discharge and water level as a result of flushing or dilution processes and by hindering upward flotation to the illuminated layers in deep and strongly mixed water columns through the suppression of persistent thermal stratification. Multivariate analyses showed that inter-annual differences of summer cyanobacterial development were strongly correlated with discharge and water level variations; satellite imagery supports these findings. These above-mentioned mechanisms appeared to have affected the algal abundance in Salto Grande Reservoir, triggering slightly different responses in the two blue-green complexes. Dolichospermum showed stronger proliferation during periods of lower water levels and more stratified water columns. This is reflected in the plot of the RDA (Fig. 4a) where tributary samples corresponding to low waters are associated to high cell counts of Dolichospermum (2009, 2011) and display the highest separation from those of high waters with scarce cell densities (2007, 2010). Interestingly, such high concentrations were not achieved during 2008 under comparatively low waters, probably because levels were still quite high at the beginning of summer (January). Permanently quiescent waters will more likely occur in the tributaries with little inflow (sites 9 and 10) and in sheltered beaches (site 3) at low waters, as these locations are more affected by flow decreases than the main channel. Likewise, in Australian nutrient-rich systems (Mitrovic et al., 2001; Thompson et al., 2003; Bormans et al., 2005), low flow conditions and great insolation by late spring or early summer, increased irradiance to cells in the upper mixed layer improving light conditions for Dolichospermum bloom development. Microcystis success in relatively better mixed and deeper water columns (e.g. Fig. 5) is explained by its higher ability for surface migration provided by gas vesicles with higher critical pressure and buoyant density than those present in Dolichospermum species (Visser et al., 2005 and cites therein). Huisman et al. (2004) proved that Microcystis can escape from entrainment more easily in deep than in shallow waters, and coincidentally, Chalar (2009) described enhanced Microcystis growth at the deeper sites in the Gualeguaycito and Itapebi arms of the Salto Grande Reservoir. Microcystis colony enlargement over the summer season may have favoured buoyancy regulation by late summer, due to the fact that floating velocity increases with colony size (Reynolds & Walsby, 1975). The analysis of physiological traits contributes to understand the causes of the heterogeneous spatial distribution of both complexes, as we suggest that thriving Microcystis superficial populations would be more prone to the drifting action of north-eastern winds that are dominant in the region; hence, cyanobacterial accumulation along the lee shore of the right margin is related to the scum-forming eco-strategy.
The influence of temperature also differed between both blue-green complexes and most interestingly, Dolichospermum showed different responses at different temporal scales. The inter-annual analysis performed with summer mean temperature values reveals that warmer years (e.g. 2007) boosted Microcystis blooms but did not equally promote Dolichospermum growth (Fig. 4a). The lowest mean temperature during the summer of 2009 probably caused the lower bloom frequency and impaired Microcystis growth registered in the reservoir, contrary to what would be expected during a drought period. Conversely, at a seasonal scale, Dolichospermum showed a positive relationship with temperature at most studied sites as reflected by the correlations performed using weekly values (Table 2). During each summer, maximum cell densities of Dolichospermum occurred early in the season and then dropped with early autumn decreasing temperatures (exemplified in Fig. 5a for site 9), just like in Australian systems (Thompson et al., 2003; Bormans et al., 2005). When enhanced temperatures were coupled to stratification in deeper water columns by mid-January (bold lines in Fig. 5b), Dolichospermum peaked even exceeding Microcystis cell densities.
Despite nutrient controls on phytoplankton production in reservoirs are mostly subordinated to physical factors (Forbes et al., 2008), the absence of abundant Cyanobacteria is better guaranteed at levels below 50 μg P l−1 (Dokulil & Teubner, 2000) and their presence is thought to be benefited at low TN:TP ratios (Smith, 1983). In Salto Grande, blue-greens dominated with P concentrations slightly exceeding this threshold and frequently relatively high ratios (DIN:SRP >30, TN:TP mostly exceeding 16). Our results indicate a weak correlation between SRP and discharge during summer seasons. Chalar (2006) asserted that P input occurs in winter months and thus, the lag with phytoplankton growth in warmer seasons favours P exportation from the system (low P retention: 3% of total load); the high nutrient loss probably accounts for low P inter-annual variation in spite of marked hydrological fluctuations. The RDA results show a negative effect of P on cyanobacterial growth: elevated cell density at the surface must have uptaken a great proportion of the SRP pool in the upper layers of the water column, although TP concentrations in Salto Grande were still sufficient to sustain large populations of Cyanobacteria. Although N:P ratios are imperfect predictors of cyanobacteria dominance (Reynolds, 1999; Downing et al., 2001), N-fixers dominance was found in many lakes under a boundary between the Redfield ratio (16) and ~32 (Smith et al., 1995; Nõges et al., 2008; Scott et al., 2008). During 2010, when TN:TP <16, Dolichospermum cell density increased in relation to Microcystis at most surveyed sites and even though it did not dominate in terms of cell counts, its biomass may have been higher on some occasions. The mechanistic explanation that N-fixers have a competitive advantage when N is limiting would not be the main factor explaining their relative increase, because the low heterocyte frequency (~0.07%) here encountered suggests low reliance on fixation (most filaments with heterocytes corresponded to D. planctonicum and never to D. spiroides). As Dolichospermum was significantly correlated with ammonium only at two sites, the evidence is not sufficient to suggest a preference for reduced dissolved nitrogen (Ferber et al., 2004; Znachor et al., 2006), nor increased buoyancy control under ammonium availability (Spencer & King, 1989). The complete understanding of N and P influence on the growth of these two cyanobacterial complexes requires further measurements in the shallow areas (beaches) and at different depths of the water column in the deeper channels of the reservoir.
The positive relationships of both complexes with pH and dissolved oxygen are usually expected because both parameters are related to photosynthetic activities of the cyanobacterial bloom; it is difficult to establish whether high pH is the cause or the result of increased growth of cyanobacteria. Since the CO2 fraction decreases as pH increases, this might be a limiting factor for photosynthesis. Cyanobacteria generally tend to be strong competitors under conditions of high pH and low CO2, as they possess carbon concentration mechanisms which allow them to sequester carbon in a variety of forms (Badger & Price, 2003). Nevertheless, it is difficult to distinguish the effects of these parameters individually.
As indicated by Liu et al. (2011), the dominance of cyanobacteria is a stochastic result of a combination of multidimensional environmental factors. In the Salto Grande Reservoir, such interplay produced the above described temporal pattern, which was overlapped with a distinct spatial distribution that showed higher cyanobacterial development in the arms and beaches as compared to the main channel. The beaches at the right margin of the dam area, especially site 3, were characterised by the highest frequency of blooms (alert level 2 sensu Bartram et al. (1999)) on behalf of their shallowness that ensures cell entrainment in well illuminated and warmer water columns. Moreover, most beaches located in sheltered areas along the shore line (site 3 is almost surrounded by land) and the southern arms receiving little inflow from the Gualeguaycito and Itapebí rivers, act as dead zones ideal for phytoplankton growth and accumulation. During major inflow periods (2010), the small number of blooms registered corresponded to these sheltered sites with reduced flow influence from the Uruguay River. The lower Z mix/Z eu ratios in the tributaries as compared to the main channel supports that mixing of the water column was still low to enable Cyanobacteria buoyancy to overcome mixing so that cells could spend more time in well-illuminated layers. The chlorophyll a distribution depicted in the satellite images confirms strong algal biomass concentrations in the arms and shows how the right margin beaches are more exposed to receive the drift of cyanobacteria from the Gualeguaycito arm. The influence of the prevailing NE winds supports the occurrence of a higher concentration of surface scums at the right margin. Although LANDSAT imagery allowed analysis of a large area and thus detected high biomass concentrations in stretches that were not included in the original sampling programme, its use as a monitoring tool is not highly reliable during very low water periods when interference with sediments increases. The use of a satellite sensor such as MERIS, would improve the efficiency of remote sensing under all environmental scenarios due to its better spectral resolution and more suitable bands to detect chl a in turbid waters (Gons et al., 2005; Wheeler et al., 2011).
Studies that address the effects of several environmental factors on the growth and abundance of cyanobacterial populations under field conditions with a long-term monitoring record are scarce (Wiedner et al., 2007; Liu et al., 2011). Such investigations provide the necessary information for the application of adequate management measures. A long-term ecological study on the regulated Nakdong River supported the necessity of ‘smart flow control’ in order to destruct the bloom formation (Jeong et al., 2007); similar measures were proposed by Mitrovic et al. (2010) for Australian systems. Despite, the results of this 5-year study indicate that the way to reduce cyanobacterial biomass in Salto Grande would be related to increasing the mixing of the water column, the morphology of this multiple arm reservoir discourages the success of this measure as these inflow areas act as ‘dead zones’ mostly in summer time when rivers have low discharges. In order to perform adequate plan managements, additional information is required namely nutrient data for the beaches area, dynamics of the inflowing river discharges and studies on viability and abundance of akinetes and dormancy stages in sediments.
Final remarks
Two cyanobacterial complexes dominated the Salto Grande Reservoir: Microcystis spp. and Dolichospermum spp. Their distribution was highly affected by fluctuating hydrological conditions and the morphology of the reservoir, differing along its 100 km length, between margins, over the 5-year survey and within each summer. The successful adaptation to the prevailing environment mostly by means of buoyancy control validates their classification as scum-forming eco-strategists. Nevertheless, as our results show a difference in the behaviour of both complexes that could not be clearly related to N-fixing, further studies should be performed to clarify whether this capacity constitutes a crucial strategy to develop Dolichospermum blooms under specific conditions in this eutrophic reservoir.
References
Antoniades, D., C. Crawley, M. S. V. Douglas, R. Pienitz, D. Andersen, P. T. Doran, I. Hawes, W. Pollard & W. F. Vincent, 2007. Abrupt environmental change in Canada’s northernmost lake inferred from fossil diatom and pigment stratigraphy. Geophysical Research Letters 34: L18708. doi:10.1029/2007GL030947.
APHA (American Public Health Association), 2005. Standard Methods for the Examination of Water and Wastewaters, 21st ed. APHA, Washington.
Badger, M. R. & G. D. Price, 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany 54: 609–622.
Bartram, J., M. Burch, I. R. Falconer, G. Jones & T. Kuiper-Goodman, 1999. Situation assessment, planning and management. In Chorus, I. & J. Bartram (eds), Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences. Monitoring and Management. E & FN Spon, London: 179–209.
Bittencourt-Oliveira, M. C., 2003. Detection of potential microcystin-producing cyanobacteria in Brazilian reservoirs with a mcyB molecular marker. Harmful Algae 2: 51–60.
Bormans, M., P. W. Ford & L. Fabbro, 2005. Spatial and temporal variability in cyanobacterial populations controlled by physical processes. Journal of Plankton Research 27: 61–70.
Chalar, G., L. De León, E. Brugnoli, J. Clemente & M. Paradiso, 2002. Antecedentes y nuevos aportes al conocimiento de la estructura y dinámica del Embalse Salto Grande. El Agua en Sudamérica: de la Limnología a la Gestión en Sudamérica. In Fernándes Cirelli, A. & G. Chalar (eds), Aprovechamiento y Gestión de los recursos hídricos. CYTED, Buenos Aires: 123–142.
Chalar, G. L., 2006. Eutrophication dynamics on different temporal scales: Salto Grande Reservoir (Argentina-Uruguay). In Tundisi, J. G., T. Matsumura-Tundisi & T. Sidagis (eds), Eutrophication in South America: Causes, Consequences and Technologies for Management and Control. International Institute of Ecology Inc., São Carlos: 87–101.
Chalar, G., 2009. The use of phytoplankton patterns of diversity for algal bloom management. Limnologica 39: 200–208.
De León, L. & G. Chalar, 2003. Abundancia y diversidad del fitoplancton en el Embalse de Salto Grande (Argentina-Uruguay). Ciclo estacional y distribución espacial. Limnetica 22: 103–113.
De Tezanos Pinto, P. & E. Litchman, 2010. The interactive effects of N:P ratios and light on nitrogen-fixer abundance. Oikos 119: 567–575.
Dokulil, M. T. & K. Teubner, 2000. Cyanobacterial dominance in lakes. Hydrobiologia 438: 1–12.
Downing, J. A., S. B. Watson & E. McCauley, 2001. Predicting cyanobacteria dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences 58: 1905–1908.
Ferber, L. R., S. N. Levine, A. Lini & G. P. Livingston, 2004. Do Cyanobacteria dominate in eutrophic lakes because they fix atmospheric nitrogen? Freshwater Biology 49: 690–708.
Forbes, M. G., R. D. Doyle, J. T. Scott, J. K. Stanely, H. Huang & B. W. Brooks, 2008. Physical factors control phytoplankton production and nitrogen fixation in eight Texas Reservoirs. Ecosystems 11: 1181–1197.
Gemelgo, M. C. P., J. L. N. Mucci & D. Navas-Pereira, 2009. Population dynamics: seasonal variation of phytoplankton functional groups in Brazilian reservoirs (Billings and Guarapiranga, São Paulo). Brazilian Journal of Biology 69: 1001–1013.
Gons, H. J., H. Hakvoort, S. W. M. Peters & S. G. H. Simis, 2005. Optical detection of cyanobacterial blooms. Shipboard observation and remote sensing. In Huisman, J., H. C. P. Matthijs & P. M. Viser (eds), Harmful Cyanobacteria. Springer, Dordrecht: 177–199.
Huisman, J., J. Sharples, J. Stroom, P. M. Visser, W. E. A. Kardiaa, J. M. H. Verspagen & B. Sommeijer, 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85: 2960–2970.
Ibelings, B. W. & K. H. Havens, 2008. Cyanobacterial toxins: a qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. In Hudnell, K. H. (ed.), Proceedings of the Interagency, International Symposium on Cyanobacteria Harmful Algal Blooms. Advances in Experimental Medicine and Biology, Springer, New York: 675–732.
Jeong, K.-W., D.-K. Kim & G.-J. Joo, 2007. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the coger Nakdong River (South Korea). Water Research 41: 1269–1279.
Jiang, Y., B. Ji, R. N. S. Wong & M. H. Wong, 2008. Statistical study on the effects of environmental factors on the growth and microcystins production of bloom-forming cyanobacterium—Microcystis aeruginosa. Harmful algae 7: 127–136.
Kosten, S., V. M. L. Huszar, E. Becarés, L. S. Costa, E. van Donk, L.-A. Hansson, E. Jeppesen, C. Kruk, G. Lacerot, N. Mazzeo, L. de Meester, B. Moss, M. Lürling, T. Nõges, S. Romo & M. Scheffer, 2011. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biology 18: 118–126.
Kuiper-Goodman, T., I. Falconer & J. Fitzgerald, 1999. Human health aspects. In Chorus, I. & J. Bartram (eds), Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences. Monitoring and Management. E & FN Spon, London: 113–153.
Li, Z., J. Yu, M. Yang, J. Zhang, M. D. Burch & W. Han, 2010. Cyanobacterial population and harmful metabolites dynamics during a bloom in Yanghe Reservoir, North China. Harmful Algae 9: 481–488.
Litchman, E., P. De Tezanos Pinto, C. A. Klausmeier, M. K. Thomas & K. Yoshiyama, 2010. Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia 653: 15–28.
Liu, X., X. Lu & Y. Chen, 2011. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: an 11-year investigation. Harmful Algae 10: 337–343.
Lorenzen, C. J., 1967. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnology and Oceanography 12: 343–346.
Mitrovic, S. M., L. C. Bowling & R. T. Buckney, 2001. Vertical disentrainment of Anabaena circinalis in the turbid, freshwater Darling River, Australia: quantifying potential benefits from buoyancy. Journal of Plankton Research 23: 47–55.
Mitrovic, S. M., L. Hardwick & F. Dorani, 2010. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. Journal of Plankton Research 33: 229–241.
Moisander, P. H., P. W. Lehman, M. Ochiai & S. Corum, 2009. Diversity of Microcystis aeruginosa in the Klamath River and San Francisco Bay delta, California, USA. Aquatic Microbial Ecology 57: 19–31.
Mur, L. R., O. M. Skulberg & H. Utkilen, 1999. Cyanobacteria in the environment. In Chorus, I. & J. Bartram (eds), Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences. Monitoring and Management. E & FN Spon, London: 15–40.
Naselli-Flores, L. & R. Barone, 2003. Steady-state assemblages in a Mediterranean hypertrophic reservoir. The role of Microcystis ecomorphological variability in maintaining an apparent equilibrium. Hydrobiologia 502: 133–143.
Nõges, T., R. Lagauste, P. Nõges & I. Tõnno, 2008. Critical N:P ratio for cyanobacteia and N2-fixing species in the large shallow temperate lakes Peipsi and Võrtsjärv, North-East Europe. Hydrobiologia 599: 77–86.
Nusch, E. A., 1980. Comparison of different methods for chlorophyll and phaeopigments determination. Archiv für Hydrobiologie–Beiheft Ergebnisse der Limnologie 14: 14–36.
Oliver, R. & G. Ganf, 2000. Freshwater blooms. In Whitton, B. & M. Potts (eds), The Ecology of Cyanobacteria: Their Diversity in Time and Space. Kluwer Academic Publishers, Dordrecht: 149–194.
Paerl, H. & J. Huisman, 2008. Blooms like it hot. Science 320: 57–58.
Quirós, R. & S. Cuch, 1982. Características Limnológicas del Embalse de Salto Grande II: Distribución y Dinámica de nutrientes. Instituto Nacional de Investigación y Desarrollo Pesquero. Ecología Argentina 8: 111–142.
Quirós, R. & L. Luchini, 1982. Características limnológicas del embalse Salto Grande. III. Fitoplancton y su relación con parámetros ambientales. Revista Asociación Ciencias Naturales del Litoral 13: 40–66.
Reynolds, C. S., 1999. Non-determinism to probability, or N:P in the community ecology of phytoplankton. Archiv für Hydrobiologie 146: 23–35.
Reynolds, C. S., 2006. The ecology of freshwater phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S. & G. H. M. Jaworski, 1978. Enumeration of Natural Microcystis Populations. British Phycology Journal 13: 269–277.
Reynolds, C. S. & A. E. Walsby, 1975. Water-blooms. Biological Reviews 50: 437–481.
Rojas, A. & J. H. SALUSO, 1987. Informe Climático de la Provincia de Entre Ríos. INTA EEA Paraná, Publicación Técnica N° 14. Entre Ríos, Argentina.
Schindler, D. W., R. E. Hecky, D. L. Findlay, M. P. Stainton, B. R. Parker, M. J. Paterson, K. G. Beaty, M. Lyng & S. E. M. Kasian, 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences 32: 11254–11258.
Scott, J. T., R. D. Doyle, S. Prochnow & J. D. White, 2008. Are watershed and lacustrine controls on planktonic N2 fixation hierarchically structured? Ecological Applications 18: 805–819.
Smith, V. H., 1983. Low nitrogen to phosphorus ratios favor dominante by blue-green algae in lake phytoplankton. Science 221: 669–671.
Smith, V. H., V. J. Bierman, B. L. Jones & K. E. Havens, 1995. Historical trends in the Lake Okeechobee ecosystem IV. Nitrogen:phosphorus ratios, cyanobacterial dominance, and nitrogen fixation potential. Archiv für Hydrobiologie, Monographische Beitrage 107: 71–88.
Sotero-Santos, R. B., E. Garcia Carvalho, M. J. Dellamano-Oliveira & O. Rocha, 2008. Occurrence and toxicity of an Anabaena bloom in a tropical reservoir (Southeast Brazil). Harmful Algae 7: 590–598.
Spencer, C. N. & D. L. King, 1989. Role of light, carbon dioxide and nitrogen in regulation of buoyancy, growth and bloom formation of Anabaena flos-aquae. Journal of Plankton Research 11: 283–296.
Steel, J. A. & A. Duncan, 1999. Modelling the ecological aspects of bankside reservoirs and implications for management. Hydrobiologia 395–396: 133–147.
Stumpf, R. P., 1992. Remote sensing of water clarity and suspended sediments in coastal waters. Proceedings of the First Thematic Conference on Remote Sensing for Marine and coastal Environments, Louisiana, USA.
Te, S. H. & K. Y.-H. Gin, 2011. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Algae 10: 319–329.
Ter Braak, C. J. F. & P. F. M. Verdonschot, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences 57: 255–289.
Thompson, P. A., A. M. Waite & K. McMahon, 2003. Dynamics of a cyanobacterial bloom in a hypertrophic stratified weir pool. Marine and Freshwater Research 54: 27–37.
Utermöhl, H., 1958. Zur Vervollkommung der quantitativen Phytoplankton Methodik. Mitteilungen Internationale Vereingung für Theoretische und Angewandte Limnologie 9: 1–38.
Venrick, E. L., 1978. How many cells to count? In Sournia, A. (ed.), Phytoplankton Manual. UNESCO Press, Paris: 167–168.
Visser, P. M., B. W. Ibelings, L. R. Mur & A. E. Walsby, 2005. The ecophysiology of the harmful cyanobacterium microcystis. In Huisman, J., H. C. P. Matthijs & P. M. Visser (eds), Harmful Cyanobacteria. Springer, Dordrecht: 109–142.
Wacklin, P., L. Hoffmann & J. Kimárek, 2009. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Rlfs ex Bornet et Flahault) comb. Nova. Fottea 9: 59–64.
Wheeler, S. M., L. A. Morrissey, S. N. Levine, G. P. Livingstone & W. P. Vincent, 2011. Mapping cyanobacterial blooms in Lake Champlain′s Missisquoi Bay using Quickbird and MERIS satellite. Journal of Great Lakes Research 38. http://dx.doi.org/10.1016/j.jglr.2011.06.009.
Wiedner, C., J. Rücker, R. Brüggeman & B. Nixdorf, 2007. Climate change affects timing and size of populations of an invasive cyanobacterium in temperate regions. Oecologia 152: 473–484.
Znachor, P., T. Jurczak, J. Komárková, J. Jezberová, J. Mankiewicz, K. Kaštovska & E. Zapomĕlová, 2006. Summer changes in cyanobacterial bloom composition and microcystin concentration in eutrophic Czech reservoirs. Environmental Toxicology 21: 236–243.
Acknowledgments
The authors wish to thank the Joint Technical Commission of Salto Grande (CTM) and the Uruguay River Management Commission (CARU) for field assistance and making available the data here presented and Ruben Lombardo for his assistance with multivariate analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: N. Salmaso, L. Naselli-Flores, L. Cerasino, G. Flaim, M. Tolotti & J. Padisák / Phytoplankton responses to human impacts at different scales: 16th workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP)
Rights and permissions
About this article
Cite this article
O’Farrell, I., Bordet, F. & Chaparro, G. Bloom forming cyanobacterial complexes co-occurring in a subtropical large reservoir: validation of dominant eco-strategies. Hydrobiologia 698, 175–190 (2012). https://doi.org/10.1007/s10750-012-1102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1102-4