Abstract
Limnoperna fortunei (the golden mussel), Dreissena polymorpha (the zebra mussel), and Dreissena rostriformis bugensis (the quagga mussel) are considered among the most aggressive freshwater invaders. All three species share several biological traits, such as their sessile mode of life attached to hard substrata by a byssus (although quagga mussels can also dwell on muddy bottoms), similar sizes, similar longevity, and similar time to sexual maturity. The spawning period, however, is usually longer for L. fortunei. Ecologically, they also share similarities (e.g., suspension feeding mode), but the dreissenids thrive and reproduce in colder waters (especially D. r. bugensis), and are significantly less tolerant to low pH and calcium concentrations, hypoxic conditions, and pollution. Rates of intrabasin spread of L. fortunei in South America are roughly similar to those of D. polymorpha in North America, but interbasin spread is generally faster for the zebra mussel, probably partly due to cultural and economic differences between their respective invasive ranges. Geographic spread of quagga mussels has been much slower than that of zebra mussels, but once the former colonize waterbodies already populated by zebra mussels, they usually become dominant, both spatially and numerically. Judging from their respective environmental tolerance limits, in particular calcium concentrations, it is expected that both species of Dreissena may eventually colonize much of Europe, Asia, and North America, but colonization of South America, Africa, and Australia is less likely. In contrast, L. fortunei, which tolerates much lower calcium concentrations, could spread to areas presently occupied by the dreissenids as well as Africa and Australia. Should the three species overlap, it seems likely that L. fortunei will outcompete the dreissenids in warmer, more polluted, less oxygenated, and more acidic waters as well as in waters with lower calcium concentrations. However, the outcome of their competitive interactions when conditions are suitable for all three species is unclear. L. fortunei and both species of Dreissena are functionally similar, and as a consequence, many of their impacts on the systems they invade are also similar, yet the magnitude of these effects, and in some cases even their sign, can differ widely depending on the invasive species and environmental constraints. Future research on the golden mussel should focus on shedding light on the many unknown aspects of its biology and ecology, which are particularly critical for a comprehensive assessment of its interactions with local biota.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Limnoperna fortunei
- Dreissena polymorpha
- Dreissena rostriformis bugensis
- Ecological impact
- Distribution
- Environmental tolerance
- Geographic spread
Introduction
Although Limnoperna fortunei (the golden mussel) is taxonomically unrelated to Dreissena polymorpha (the zebra mussel) and Dreissena rostriformis bugensis (the quagga mussel), they have similar life histories, share many ecological traits, and are functionally similar. Therefore, their ecological and economic impacts on waterbodies they invade are often similar as well. Due to their high rates of spread, large numbers of colonized waterbodies, and the extent of their ecological and economic impacts, both species of Dreissena and L. fortunei are considered among the most aggressive freshwater invaders (Karatayev et al. 2007a, 2010a). All three are spreading at virtually all spatial scales and are expected to continue doing so (Karatayev et al. 2007a, 2007b, 2011, 2015; Pollux et al. 2010; Benson 2014; Boltovskoy and Correa 2015).
The overall impact of an invader depends on many factors, including, among others, the number of waterbodies colonized, its total population density in a given waterbody, its population dynamics, and distribution within a waterbody (Karatayev et al. 2010b, 2011). The number of waterbodies colonized will depend on the invader’s ability to use different transport vectors, propagule pressure, environmental limits, and its life history and biological parameters (e.g., fecundity, growth, and survival), which ultimately determine total population size, population dynamics, and distribution within an invaded waterbody. Therefore, to accurately predict the potential spread and ecological impacts of invaders, it is essential to know their environmental limits and their biology. Although D. polymorpha is among the best studied freshwater invertebrates, less data are available for D. r. bugensis and L. fortunei (Karatayev et al. 2007a, 2007b, 2015, 2014a; Nalepa 2010; Boltovskoy and Correa 2015).
The aims of this chapter are to review similarities and differences among L. fortunei and both species of Dreissena in their biological traits, environmental limits, rates of spread, population dynamics, and ecological impacts, and to identify the essential information needed to better understand their geographic spread and their effects on ecosystems.
Life History
L. fortunei belongs to the largely marine bivalve family Mytilidae, while zebra and quagga mussels belong to the Dreissenidae, which is of brackish water origin. L. fortunei and Dreissena spp. represent an unusual ecological type in freshwaters and have traits typical of marine mussels, including free-swimming larvae and a sessile, attached adult stage.
Extensive research has been conducted on the biology, reproduction, growth and other life history traits of D. polymorpha, but relatively fewer studies have focused on D. r. bugensis and L. fortunei (Karatayev et al. 2007a, 2007b, 2014a, 2015; Nalepa 2010). We know that all three species mature at approximately the same age, and have a similar body size (Table 1).
In L. fortunei and D. polymorpha gonads are fully developed by the spring, and spawning typically occurs in spring–summer (Lvova and Makarova 1994; Boltovskoy et al. 2009b; see Chapter “Reproductive Output and Seasonality of Limnoperna fortunei” in this volume). In cold deep waters, gonads of D. polymorpha and D. r. bugensis may be ripe for a longer period of time during the year and spawning extends over more months, producing smaller recruitment events over a longer period (Bacchetta et al. 2010; Nalepa 2010). The duration of the reproductive period depends on the temperature regime and is longer in warmer regions. In the northern part of its range, spawning of Dreissena spp. lasts for about 3–5 months (Lvova and Makarova 1994). In Lake Mead (Arizona-Nevada, USA), however, quagga mussel veligers are present in the plankton year round, suggesting a much longer spawning season (Wong et al. 2012), similar to the spawning season of golden mussels in the tropics and subtropics (up to 10 months, Boltovskoy et al. 2009b). In contrast, in temperate and cold-temperate areas (Japan, Korea) L. fortunei produces larvae for only 1 month or less each year (Choi and Shin 1985; Nakano et al. 2010a) (see Figs. 2 and 3 in Chapter “Reproductive Output and Seasonality of Limnoperna fortunei”, this volume).
For L. fortunei, most studies from Asia and South America concur that reproduction starts when water temperatures reach around 15–18 °C (Morton 1977; Choi and Shin 1985; Cataldo and Boltovskoy 2000; Nakano et al. 2010a; Brugnoli et al. 2011). So far, the golden mussel has not been reported from waterbodies where year round temperatures are below 15–18 °C, although there are many records from areas where water temperature is always above 15–18 °C (Mata 2011; Oliveira et al. 2011). Interestingly, in these tropical waterbodies the reproductive cycle is less regular, and slows noticeably in the winter (July–August in the southern hemisphere). Even when water temperatures are well above the threshold for reproduction (see chapter “Reproductive Output and Seasonality of Limnoperna fortunei” in this volume), larval production typically decreases or even ceases in the winter. Zebra mussels usually initiate spawning when water temperatures reach 12–15 °C, typically in the late spring (May to June in the northern hemisphere), and continue to spawn until the end of summer (August or September) (Table 1; Sprung 1987; Borcherding 1991; Lvova et al. 1994a; Karatayev et al. 1998, 2010b; Pollux et al. 2010). Quagga mussels, which usually live deeper, can spawn at water temperatures as low as 4.5–6.0 °C (Nalepa 2010). However, in areas where they co-occur with zebra mussels, both dreissenid species may initiate spawning at the same time (e.g., 18–20°C; Claxton and Mackie 1998).
Thus, the golden mussel requires higher temperatures for reproduction (15–18 °C), followed by the zebra mussel (12–15 °C), and the quagga mussel can reproduce in much colder waters (variable, but occasionally as low as 5–6 °C).
Fecundity data are only available for D. polymorpha (Table 1). Female zebra mussels can spawn up to 106 eggs, and males up to nearly 1010 sperm, comprising more than 30 % of their body weight prior to spawning (Sprung 1991). In the absence of similar data for quagga mussels, their fecundity is often assumed to be the same as for zebra mussels (e.g., Keller et al. 2007). However, this may be not the case. Zebra and quagga mussels have very different population dynamics in the waterbodies they invade. The time lag between when a species is first detected in a waterbody and when it reaches its maximum population size being much shorter for zebra mussels (2.5 ± 0.2 years) than for quagga mussels (12.2 ± 1.5 years) (Karatayev et al. 2011). The shorter lag time for zebra mussels may reflect their higher reproductive potential. Information for L. fortunei is still too scant and fragmentary for comparison, but the few data at hand seem to indicate that the lag time is closer to that of the quagga than the zebra mussel (see chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume). This, however, does not necessarily imply comparatively lower fecundity because carrying capacity depends on many intrinsic (e.g., fecundity), and extrinsic traits (e.g., predation pressure, competition, etc.).
Longevity of zebra mussels (up to 4–5 years, reviewed in Lvova et al. 1994b; Mills et al. 1996; Orlova et al. 2004; Karatayev et al. 2007b), seems somewhat greater than that of L. fortunei (around 2–3 years; Morton 1977; Boltovskoy and Cataldo 1999) (Table 1).
Environmental Limits
Temperature
The lower temperature limit for both species of Dreissena is close to 0 °C. The upper temperature limit for D. polymorpha, determined from field observations in both Europe and North America, is around 32–33 °C (Aldridge et al. 1995; Karatayev et al. 1998; Allen et al. 1999; Table 2). Field observations indicate that quagga mussels are likely somewhat less tolerant of high temperatures than zebra mussels (reviewed in Mills et al. 1996; Karatayev et al. 1998; Garton et al. 2014). Data from the Zaporozhskoe Reservoir (Ukraine) show that quagga mussels survive in waters ≤ 30.5 °C, while zebra mussels tolerate waters ≤ 33 °C (Dyga and Zolotareva 1976).
For L. fortunei, the upper thermal limit is around 35 °C, which is somewhat higher than that of both dreissenids (Table 2). In South America, minimum winter temperatures of the waterbodies colonized by L. fortunei are around 10 °C, but in Japan golden mussels survive at water temperatures of 5–6 °C (Magara et al. 2001), and in Korea L. fortunei populations have been reported from the Paldang Reservoir, which freezes for 1–2 months every winter (Choi and Kim 1985; Choi and Shin 1985; Park et al. 2013; Hae-Kyung Park, pers. comm.).
While on the basis of these data, it is tempting to speculate that low winter temperatures are unlikely to be a deterrent for the spread of L. fortunei into cooler waterbodies, minimum survival temperature may not be a good indicator of the mussel’s ability to maintain self-sustaining populations. Reproductive cycles (as evidenced by the presence of larvae in the water column) clearly show that temperature is the dominant factor for spawning (see Chapter “Reproductive Output and Seasonality of Limnoperna fortunei” in this volume). The shorter the periods of high temperature, the shorter is the spawning season. Thus, while in the Upper Paraná River, where water temperatures range around 18 to > 30 °C, larvae are produced for 9–10 months each year, in Japan, at ~ 7–25 °C, larval output is restricted to 1–2 months, and in Korea, at 0–30 °C, reproduction is restricted to around 20 days (Choi and Shin 1985; Nakano et al. 2010a; Hamada 2011; Mata 2011; see Fig. 2, 3 and 10 in Chapter “Reproductive output and Seasonality of Limnoperna fortunei” in this volume). Interestingly, in all of these waterbodies, summer water temperatures are high. Even Paldang Reservoir, which freezes in the winter, reaches ~ 30 °C in the summer (Choi and Shin 1985). This suggests that the magnitude and duration of warm summer temperatures determine whether self-sustaining populations are possible, rather than minimum winter values. Data at hand indicate that the lowest temperatures at which L. fortunei spawns are around 15–18 °C (see Chapter “Reproductive Output and Seasonality of Limnoperna fortunei” in this volume), which suggests that waterbodies whose temperature is always below these values are unlikely to be colonized by this mussel. Therefore, Andean Patagonian lakes located south of ~ 38 °S, most of which do not freeze but never reach temperatures above 13–15 °C (Baigun and Marinone 1995; Díaz et al. 2000) are most probably not at risk of colonization by L. fortunei. In contrast, the North American Great Lakes, which may freeze in the winter, but usually have 3–4 month periods when water temperatures are above 16 °C (except Lake Superior; National Oceanic and Atmospheric Administration, NOAA 2014), are probably suitable for colonization by L. fortunei.
Salinity
In Europe and North America, D. polymorpha can form stable populations at salinities below 6 ‰, which is only slightly higher than the limit for quagga mussels (Table 2). For L. fortunei, constant salinities around 2 ‰ are the upper limit for extended survival (Huang et al. 1981; Angonesi et al. 2008; Barbosa and Melo 2009; Sylvester et al. 2013). However, at intermittent saltwater-freshwater conditions, such as those normally present in tidal estuaries, golden mussels can tolerate short periods (hours) of salinities up to 23 ‰ without significant mortality (Sylvester et al. 2013; see Chapter “Chemical Strategies for the Control of the Golden Mussel (Limnoperna fortunei) in Industrial Facilities” in this volume). This suggests that tests at constant salinity underestimate the tolerance of this species, and probably other freshwater molluscs, to saltwater exposure. Because estuarine ports represent ~ 70 % of nonmarine ports globally, they constitute major donor and recipient hotspots for the spread of nonnative species into continental aquatic ecosystems via shipping. It is probable that the tolerance of L. fortunei to estuarine conditions contributes to this species’ success as an invader (Sylvester et al. 2013).
pH and Calcium
Zebra mussels are restricted to waters with neutral or alkaline pH (> 7.3–7.5; Table 2). To our knowledge, there are no published data on pH limits for quagga mussels. Both in Europe and in North America, zebra mussels have colonized many more waterbodies than have quagga mussels. With few exceptions, almost all lakes had already been colonized by zebra mussels when quagga mussels invaded, suggesting that the pH limits for both species of Dreissena largely overlap. The threshold for calcium needed to support sustainable populations of zebra mussels is > 23 mg/L (Fig. 1, Table 2), although values as low as 8–15 mg/L have been reported (Mellina and Rasmussen 1994; Jones and Ricciardi 2005). However, these lower values may reflect limits for the survival of adult mussels, rather than the establishment of locally sustainable populations (Sprung 1987).The calcium limits for both species of Dreissena are substantially higher than those for L. fortunei (Fig. 1). Calcium is generally scarce in South American floodplain rivers colonized by the golden mussel (3-9 mg/L; Maglianesi 1973, Bonetto et al. 1998), and values as low as 1 mg/L of Ca and pH&<6 have been reported from some areas successfully colonized by L. fortunei, such as the Upper Paraguay River (Oliveira et al. 2011).
Mean calcium concentrations in rivers on different continents (Wetzel 1975) and minimum calcium requirements for Dreissena polymorpha and Limnoperna fortunei
Dissolved Oxygen
D. polymorpha is intolerant of even moderate hypoxia. Although it may colonize the deep oxygenated areas of some lakes, it usually is restricted to littoral and sublittoral zones (reviewed in Karatayev et al. 1998, 2015) (Table 2). In contrast, D. r. bugensis survives at lower oxygen concentrations than the zebra mussel (Shkorbatov et al. 1994), which may be related to its lower respiration rate (Stoeckmann 2003), and at least partially explains the ability of quagga mussels to colonize the profundal zone of deep lakes. However, both species of Dreissena are absent from hypoxic areas (e.g., central basin of Lake Erie; Karatayev et al. 2014c).
In contrast, L. fortunei survives in areas with very low oxygen concentrations, high organic loads, and industrial pollution (Villar et al. 1999; Belaich et al. 2006; Boltovskoy et al. 2006; Perepelizin and Boltovskoy 2011; Bonel et al. 2013; Young et al. 2014). In the delta of the Lower Paraná River, dense L. fortunei beds are present in the vicinity of urbanized and industrialized areas which discharge untreated domestic and industrial wastes. These waters and sediments contain pollutants at levels several times above those considered hazardous for aquatic life (e.g., Zn, Cr, Cu, Benzo[a]pyrene, polychlorinated biphenyls (PCBs), etc.), where other organisms (e.g., the Asian clam, Corbicula fluminea) do not survive (Cataldo et al. 2001a, 2001b).
Substrata
Within a waterbody, one of the main factors that affect the distribution of both L. fortunei and Dreissena spp. is the availability of suitable substrata. These mussels usually require hard substrate for attachment, and therefore their distribution is extremely patchy, with harder and coarser substrata yielding the highest densities and biomass of mussels. The most favorable substrata for these species are rocks, gravel, shells, and consolidated sediments (Karatayev et al. 1998, 2010a; Boltovskoy et al. 2006, 2009a; Burlakova et al. 2006).
In South American rivers, where hard substrata are scarce, plants may constitute important sites for attachment. Roots, rhizomes, and stolons of the water hyacinth (Eichhornia crassipes, Eichhornia azurea) seem to be particularly important substrata. Although densities of L. fortunei on these plants are comparatively low (see Chapter “Limnoperna fortunei Colonies: Structure, Distribution and Dynamics” in this volume), the abundance and widespread distribution of species of Eichhornia make them key elements of seeding sites (Callil et al. 2006; Marçal and Callil 2008, 2012; Rojas Molina 2010; Rojas Molina et al. 2010; Ohtaka et al. 2011; see Fig. 3e in Chapter “L imnoperna fortunei colonies: structure, distribution and dynamics” in this volume).
D. polymorpha and L. fortunei usually avoid pure mud, where they only occur on isolated hard objects, such as wood fragments, shells, stones, or artificial substrata (e.g., discarded debris). Mussels can use the hard fragments for initial attachment and subsequently attach to each other forming druses (Karatayev et al. 1998, 2010a, 2015).
Although the pattern of distribution of L. fortunei across substrate types is similar to D. polymorpha, the golden mussel appears to reach higher densities and especially higher biomass per unit area (Karatayev et al. 2010a). Both zebra and golden mussels are largely limited to the littoral zone and usually avoid soft sediments of the cold profundal zone (Karatayev et al. 1998, 2010a, 2015; Burlakova et al. 2006; Boltovskoy et al. 2009a). It is not clear, however, if golden mussels favor the shallow, coastal fringe because that is where hard substrata are most often found (Boltovskoy et al. 2009a), or because they prefer shallower sites, regardless of substrate type. Colonization of artificial substrata suggests that recruits prefer settling at depth (6–18 m), rather than closer to the surface (Morton 1977; Nakano et al. 2010b; Brugnoli et al. 2011), but this pattern may also reflect differences in predation pressure. Comparison of samples scraped from the concrete wall of a penstock of the hydroelectric Yacyretá power plant (Upper Paraná River) yielded higher densities at 10 m (248,200 ind./m2), than at the surface (170,400 ind./m2), and at 40 m (54,400 ind./m2) (Darrigran et al. 2007). While differences in densities at these three depths may reflect differences in hydrodynamics specific to this particular water intake structure, they still show that when offered adequate substrate, within these limits depth does not curtail the survival of L. fortunei.
In addition to the littoral zone, quagga mussels can colonize silty sediments, especially those found in the profundal zones of deep large lakes (Patterson et al. 2005; Watkins et al. 2007; Nalepa et al. 2009a; Nalepa 2010; Karatayev et al. 2015, 2014c). In these soft sediments, D. r. bugensis usually has a more even distribution across the bottom, and rarely forms large druses. Instead, single mussels or small aggregations almost float on the surface of the silty bottom (Nalepa 2010; Karatayev et al. 2014c). Therefore, in deep lakes with large profundal zones, quagga mussels may be found at higher overall numbers across the whole lake than either zebra or golden mussels.
Rate of Spread
Of the three species considered in this chapter, D. polymorpha has by far the longest and the best-documented history of invasion. This species began to spread from its native range in Europe in the early 1800s (Karatayev et al. 2007b, 2011, 2015; Pollux et al. 2010; van der Velde et al. 2010; bij de Vaate et al. 2014).
At the global scale, three major phases in the spread of the zebra mussel can be recognized: (1) An initial exponential phase in the nineteenth century in Europe, where it spread at a rate of ~ 3.9 geographic regions (countries, or geographic provinces within large countries) per decade; (2) A period of extremely slow spread for almost a century during the industrial revolution and increased water pollution; and (3) A second period of exponential spread that started in the 1960s, and included expansion in both Europe and North America (where zebra mussels were introduced in the 1980s, Carlton 2008), when it spread at an average rate ~ 6.6 regions/decade (Karatayev et al. 2011).
Although there was extensive ship traffic between areas inhabited by D. r. bugensis (the Dnieper-Bug Liman and the lower reaches of the Southern Bug River in Ukraine) and other regions of eastern and western Europe through the middle of the twentieth century, quagga mussels remained restricted to their native range until the 1940s (Zhulidov et al. 2004; Karatayev et al. 2007b, 2011; Son 2007; van der Velde et al. 2010; Zhulidov et al. 2010). Starting in the mid-1980s, quagga mussels spread in Europe and North America (where this species was first discovered in 1989, Mills et al. 1993) at a rate of 7.4 regions/decade, which is significantly faster than the initial spread of zebra mussels in Europe, but similar to the current rate of spread of zebra mussels at a global scale (Karatayev et al. 2011, 2014a). The delay in the spread of D. r. bugensis was likely due to its inability to use mechanisms and vectors responsible for the spread as efficiently as D. polymorpha. Quagga mussels appear to be less resistant to dislodgment than zebra mussels (Mackie 1991; Dermott and Munawar 1993; Peyer et al. 2009, 2010). As a result, zebra mussels may be more likely to remain attached to boat hulls than quagga mussels, facilitating their transport to new habitats.
In Europe, most waterbodies had already been colonized by zebra mussels long before quagga mussels began to spread, making it difficult to compare their rates of spread. However, North America was colonized by both species at approximately the same time (1980s), in the same area (Lake Erie), making their rates of spread in North America directly comparable. By 2008, zebra mussels had colonized twice as many US states as quagga mussels, almost eight times more counties, and over 15 times more waterbodies (Karatayev et al. 2011). By 2010, 25 years after their introduction into North America, D. polymorpha had colonized 17 times more waterbodies than D. r. bugensis (Benson 2014). These differences clearly show that zebra mussels are far more efficient at colonizing new waterbodies than quagga mussels.
It has been shown that estimates of the rates of spread of exotic bivalves depend upon the spatial resolution of the scale of spread, and may be accelerated or slowed by various human activities (reviewed in Karatayev et al. 2007b; see Chapter “Distribution and Colonization of Limnoperna fortunei: Special Traits of an Odd Mussel” in this volume). In general, the rate of spread is slower at finer spatial scales. For example, aquatic exotic species may quickly spread along connected waterways within a recently invaded continent, and soon reach their maximum range across the continental scale. However, it takes much longer to colonize all regions within an invaded continent, and much longer again to spread to every isolated lake and river (waterbody scale) within a region. This difference in the rate of colonization across different spatial scales may be several orders of magnitude. For example, in the nineteenth century it took less than 40 years for D. polymorpha to spread across Europe, chiefly through canal systems, to present day Belarus, Poland, the Baltic states, Great Britain, the Netherlands, Germany, Belgium, and France (reviewed in Karatayev et al. 2007b). On the other hand, at the regional scale it took over 150 years for D. polymorpha to spread across geographical barriers to Alpine regions (Kinzelbach 1992), and almost 200 years to colonize Ireland (Minchin 2000) and Spain (bij de Vaate et al. 2002).
The spread of L. fortunei outside of its purported native range in China, south of the Yangtze River, into tropical Indochina (Cambodia, Laos, Thailand, Vietnam), likely occurred centuries ago (Morton and Dinesen 2010), but the first documented record of expansion was in 1965, when this species colonized Hong Kong (Morton 1975). In the late 1980s, it was recorded in Japan (Matsuda and Uenishi 1992). In the early 1990s, it spread to South America (Pastorino et al. 1993), and is presently found in Argentina, Uruguay, Paraguay, Bolivia, and Brazil (see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume). A rough comparison of the rates of spread shows that D. polymorpha spread ~ 2800 km (Minneapolis to New Orleans in 7 years (1986–1993), whereas L. fortunei spread 3400 km (Río de la Plata estuary to the Pantanal wetland) in 8–9 years (1990–1998). Thus, the rates of expansion in these areas have been generally similar, but the major pathways used for expansion likely differed. Once D. polymorpha colonized the uppermost reaches of the Mississippi River system (in 1991; Benson 2014), it swiftly expanded southwards by means of its downstream drifting larvae (Stoeckel et al. 2004). In contrast, L. fortunei first invaded the outlet of the Río de la Plata watershed (the Río de la Plata estuary), and spread northwards and upstream. Upstream expansion was obviously facilitated by attachment of adult individuals to the hulls of commercial boats that operate along the Paraná-Paraguay waterway, thus fitting the “jump dispersal” mode (MacIsaac et al. 2001). For L. fortunei, the importance of boat traffic as a dispersal vector is reinforced by the fact that in the Uruguay River, much of which is not navigable, the upstream expansion has been much slower than in the Paraná-Paraguay system (Boltovskoy et al. 2006).
Rates of spread across river basins, on the other hand, have apparently been faster for D. polymorpha, especially in the USA, than for L. fortunei. In Japan, the golden mussel is still restricted to a rather limited part of the country (see Chapter “Colonization and Spread of Limnoperna fortunei in Japan” in this volume), whereas in South America in more than 20 years only one major basin has been colonized (the Río de la Plata basin), and a few minor ones (Mar Chiquita, Patos-Mirim, Guaíba, Tramandaí; see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume).
The main mechanisms for interbasin dispersal of freshwater mussels are man-made canals and aqueducts, and overland transport. Canals and aqueducts are partly responsible for the spread of L. fortunei in China (see Chapter “Distribution and Spread of Limnoperna fortunei in China” in this volume), and in Japan (see Chapter “Colonization and Spread of Limnoperna fortunei in Japan” in this volume), but, for different reasons, their impact has been limited. In China, many of the major hydraulic projects are very recent, suggesting that the effects of invasion are still underway. In Japan, there are 400,000 km of man-made canals, many of which connect watersheds (Ministry of Agriculture, Forestry and Fisheries 2003); however, because of the country’s topography, watersheds are numerous and very small (Japan Commission on Large Dams 2009). Despite a millennium of efforts by man to reshape the drainage network (according to the International Commission on Large Dams, of the 20 oldest dams in the world, 15 are located in Japan), many are still isolated. This may explain why the overall spread of L. fortunei in Japan has been comparatively slow (see Chapter “Colonization and Spread of Limnoperna fortunei in Japan” in this volume). In contrast, there are many large, navigable rivers in the USA, and almost 20,000 man-made canals, including several major interbasin transfer aqueducts, some of which are known to have been instrumental for the rapid dispersal of dreissenids (Benson 2014). In comparison, natural basins in South America have suffered little modification (with the exception of dams, especially in the Río de la Plata watershed, see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume), and there are no man-made interbasin connections (although plans to interconnect all major navigable waterways have been under consideration for years).
For freshwater byssate mussels, overland interbasin transfer is chiefly accomplished through fouling of recreational boats (Balcom 1994; Padilla et al. 1996; Buch and McMahon 2001; Johnson et al. 2001). Thus, invasion pressure on unconnected waterbodies is highly dependent on the number of boats (chiefly trailered), which in turn is associated with income and living standard levels. By all indices, the USA has higher economic development than China and the five South American countries where L. fortunei is invasive, and most probably has a significantly higher number of recreational, trailerable watercraft.
Another potentially important factor is the number and density of waterbodies. Areas where lakes and rivers are more numerous would be more susceptible to the dispersal of aquatic species than those where such features are scarcer. In the USA, the surface of lakes and rivers accounts for 6.8 % of the total land area, which is 3–10 times higher than in any of the South American countries invaded by L. fortunei, and 2 and 24 times higher than in Japan and China, respectively (Fig. 2).
Potential for Future Spread
Dispersal of exotic species can be considered at different spatial scales, including the global, regional, local, and waterbody scales, each characterized by particular environmental constraints (Karatayev et al. 2007b). Because different exotic bivalves have different environmental limits (Table 2), their current and potential ranges are also different. Based on thermal tolerance alone, all three species have the potential to invade all continents except Antarctica, but none has fully reached this potential (Karatayev et al. 2007b). Both species of Dreissena are expanding their range in eastern and western Europe and have invaded North America. Neither has yet invaded Asia, Africa, South America, or Australia (Fig. 3). L. fortunei is spreading in Asia and has invaded South America (Fig. 3). Colonization of Asia and Africa by D. polymorpha and D. r. bugensis has been anticipated (e.g., Starobogatov and Andreeva 1994), but high calcium requirements (Ramcharan et al. 1992; Burlakova 1998; Karatayev et al. 2007b) may curtail their spread into these continents. Most Australian and South American fresh waters have low concentrations of calcium, averaging 4 and 7 mg/L, respectively, while many North American and most European freshwaters have calcium concentrations that normally exceed 20–30 mg/L (Wetzel 1975; Payne 1986; Fig. 1). In South American Patagonia (Argentina and Chile), the temperature of many lakes located along the Andes cordillera south of ~ 38°S seems adequate for colonization by zebra and quagga mussels (2–3 to ~ 16 °C), but, again, the levels of dissolved calcium are most likely too low for these mussels. Of 21 lakes analyzed by Díaz et al. (2000), only two have Ca levels above 7 mg/L.
Current and potential worldwide distribution of Limnoperna fortunei, Dreissena polymorpha, and Dreissena rostriformis bugensis. For L. fortunei, records in Indochina (denoted as native) are probably areas invaded before the twentieth century. (Distribution of D. polymorpha and D. r. bugensis in North America, courtesy of the United States Geological Service, Nonindigenous Aquatic Species)
In contrast, it seems likely that L. fortunei may colonize many of the areas presently occupied by species of Dreissena, as well as those where Dreissena cannot live, including North America, Europe, Africa, and Australia (Karatayev et al. 2007b). Temperature may represent a deterrent in some regions, but the fact that the golden mussel thrives in Paldang Reservoir (Korea) suggests that as long as peak summer temperatures are high (> 18 °C) it might establish viable populations and survive winter temperatures as low as 0 °C. Colonization of other major South American watersheds, especially those that drain into the Atlantic Ocean (Tocantins, São Francisco, Amazonas, Orinoco, Magdalena), is probably inevitable (Boltovskoy et al. 2006; Oliveira et al. 2010), but so far no records of invasion by L. fortunei have been reported from these basins.
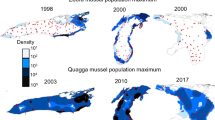
The spread of these three species is still far from complete. For example, in 2008, after more than 200 years of invasion in Belarus, only 33 % of all colonizable lakes were invaded by the zebra mussel (Karatayev et al. 2010a). Similarly, less than 10 years after the initial invasion of North America, zebra mussels had spread throughout most of the major connected river systems east of the continental divide; however, this spread has been much slower at the regional scale, and even slower at the waterbody scale (Padilla 2005). After more than 20 years of invasion, only 120 of more than 15,000 inland lakes in Wisconsin (< 1 %) were invaded by 2013 (reviewed in Karatayev et al. 2014a). To date, quagga mussels have not invaded any of the inland lakes in Belarus or in Wisconsin. Similarly, in Argentina, the golden mussel is present in a small fraction of the potentially colonizable waterbodies (Fig. 4). In Buenos Aires Province alone, < 10 % of the ~ 530 permanent lentic waterbodies (Toresani et al. 1994) are currently invaded by L. fortunei. This mussel has not yet expanded its range beyond the large Río de la Plata watershed, a few minor basins in Uruguay and southern Brazil, and a small endorheic basin (Mar Chiquita) located in central Argentina (see Chapter “Colonization and Spread of Limnoperna fortunei in South America” in this volume).
It should be noted that monitoring of South American waterbodies for the presence of golden mussels is nowhere as systematic and thorough as that for dreissenids in the northern hemisphere. None of the countries invaded has a comprehensive program aimed at the early detection of L. fortunei, and efforts at tracking its expansion are isolated and uncoordinated. Furthermore, while so far the golden mussel has been spreading in populated and industrialized areas of the Río de la Plata watershed, where the presence of this invader seldom went unnoticed, the next major watershed, the Amazon, is largely a very sparsely populated dense rainforest where most of the population lives in a few larger cities. Thus, the presence of the golden mussel is less likely to be noticed swiftly, and it is even less likely to be reported in the literature. On the other hand, because rivers are the main paths of transportation for people and produce, once colonization of the Amazon basin starts, the spread of golden mussels will likely be very fast.
Competition
The distributional ranges of zebra and quagga mussels overlap in Europe and in North America, and both species have the potential to overlap with L. fortunei in the future (Fig. 3). When co-occurring, species with similar habitat use will be expected to compete. Zebra and quagga mussels coexist in their native range in the Dnieper River delta and Dnieper-Bug Liman, Ukraine (reviewed in Zhulidov et al. 2010; Karatayev et al. 2011, 2014a). However, where the invasive ranges of both species overlap (Fig. 3), quagga mussels seem to outcompete zebra mussels over time (Nalepa 2010; Zhulidov et al. 2010; Karatayev et al. 2011, 2014a). This is especially typical for deep waterbodies and is likely due to the greater energetic efficiency of D. r. bugensis (Mills et al. 1999; Diggins 2001; Baldwin et al. 2002; Stoeckmann 2003; Karatayev et al. 2010c; Nalepa 2010). In addition, D. r. bugensis can colonize silty habitats, is more tolerant of low oxygen levels, has higher growth rates and lower mortality, and reproduces at lower temperatures than D. polymorpha (Mills et al. 1996; Claxton and Mackie 1998; Karatayev et al. 1998, 2010c, 2014c; Nalepa 2010). These demographic and physiological traits of quagga mussels allow them to colonize the large, cold profundal zone of deep lakes, which is unsuitable for zebra mussels. Thus, they can colonize the entire lake achieving much higher total population sizes, and outcompete zebra mussels by depleting food resources to levels that are too low for zebra mussels, but sufficient to support quagga mussels (Nalepa 2010; Karatayev et al. 2011, 2014a, 2015). In contrast, due to their tolerance of some abiotic factors (Table 2), greater rate of byssal thread production, and higher attachment strength (Peyer et al. 2009, 2010), zebra mussels are likely to be better adapted to the unstable, high-energy environment of the upper littoral zone, where fluctuations in temperature, currents, and wave action are prominent (reviewed in Karatayev et al. 2011, 2014a, 2015). Zebra mussels can still have an advantage in shallow lakes and rivers and coexist with quagga mussels (Zhulidov et al. 2004, 2010; Grigorovich et al. 2008; Peyer et al. 2009; Karatayev et al. 2011, 2015). Even in Lake Erie, where ≥ 95 % of the mussels in the central and eastern basins are quagga mussels, D. polymorpha still composes 30 % of the mussels in the shallow western basin after more than 20 years of coexistence (Karatayev et al. 2014c).
Because at present L. fortunei does not co-occur with either species of Dreissena, it is difficult to predict if they will compete, and if they do, under what conditions which species will prevail. Data at hand suggest that such co-occurrence is most likely to happen in Europe, Asia, and/or North America (Fig. 3). D. polymorpha has recently been found on barges imported to Argentina from the USA for grain transport along the Paraná-Paraguay rivers (Pablo Almada, personal observation), indicating that ballast water is not the only pathway for intercontinental transport of invaders. However, no live mussels were found in the few samples examined, and even if they had zebra mussels would probably not survive in the Río de la Plata watershed (see above). From their corresponding environmental tolerance ranges (Table 2), it seems likely that L. fortunei will outcompete the dreissenids in warmer, more polluted, less oxygenated and more acidic waters, as well as in waters with lower Ca concentrations. However, the outcome of their competitive interaction when conditions are suitable for all three species is unclear. The niche of L. fortunei within a waterbody appears to be more similar to that of zebra mussels than quagga mussels, suggesting that the competition may be stronger between the former two species (Table 2). However, similarities between zebra mussels and golden mussels also suggest that the impact of D. r. bugensis on L. fortunei may be similar to the one observed on D. polymorpha.
Impacts of Invasion
L. fortunei and both species of Dreissena are functionally similar, and as a consequence, many of their impacts on the systems they invade are also similar (Table 3). All three species are ecosystem engineers, sessile suspension feeders that attach to substrate with byssal threads. All three form druses, increasing habitat complexity for other benthic invertebrates, and affect planktonic communities, trophic relationships, and nutrient cycling via their feeding and filtering activities (Karatayev et al. 1997, 2002, 2007a, 2007b, 2015; Darrigran 2002; Beekey et al. 2004; Boltovskoy et al. 2006, 2009a; Burlakova et al. 2012; Boltovskoy and Correa 2015). However, the magnitude of these effects, and in some cases even their sign, depends on the invasive species, the other species present in the native community, and waterbody type.
Effects on Benthic Invertebrates
By creating reef-like three-dimensional structure, both species of Dreissena and L. fortunei change the physical habitat and provide refuge from predation and from physical stressors (waves, currents, desiccation) for benthic organisms that would otherwise be scarce or absent. In addition to increased habitat complexity, the impact of these byssate bivalves is compounded by their role as suspension feeders. All three species increase the rates of deposition of both inorganic and especially organic material on the bottom, providing an enhanced food subsidy for benthic deposit feeders. Many studies have shown that both D. polymorpha and L. fortunei have positive effects on most native invertebrates, which take advantage of both the structural complexity and food resources provided by zebra and golden mussels (Botts et al. 1996, Karatayev et al. 1997, 2002, 2007a, 2007b, 2010a; Darrigran et al. 1998; Stewart et al. 1998; Gutierrez et al. 2003; Beekey et al. 2004; Sardiña et al. 2008, 2011; Burlakova et al. 2012; Boltovskoy and Correa 2015; see Chapter “Relationships of Limnoperna fortunei with Benthic Animals” in this volume). At the same time, a few species of invertebrates have occasionally been found to be less abundant in mussel beds than in nearby bare sediments. Sardiña et al. (2011) reported that some snails, ostracods, nematodes, and chironomids may be less abundant in L. fortunei beds than in nearby bare sediments. However, the overall diversity, density, and biomass of native invertebrates is always higher in druses and mussel beds compared to nearby bare sediments (Table 3).
While in the littoral zone the effects of D. r. bugensis are probably similar to those of zebra and golden mussels (Bially and MacIsaac 2000; Yakovleva and Yakovlev 2011), in the cold profundal zone of deep lakes (where, unlike zebra and golden mussels, quagga mussels can be very abundant; Patterson et al. 2005; Watkins et al. 2007; Nalepa 2010; Karatayev et al. 2015, 2014c), their effects are quite different. Quagga mussels usually do not create large druses, but rather live individually or form small aggregates that float on the surface of soft silt (rather than sink), separated by the length of their siphons (Dermott and Kerec 1997, Karatayev and Burlakova, personal observations). Thus, they provide fewer refugia for benthic taxa and can compete with native invertebrates for space and food decreasing their overall diversity, density, and biomass (Dermott and Kerec 1997; Lozano et al. 2001; Nalepa et al. 2007, 2009a, 2009b; Watkins et al. 2007; Soster et al. 2011; Burlakova et al. 2014; Karatayev et al. 2015, 2014c).
Effects on the Water Column
The effects of L. fortunei and dreissenids on the water column are associated with their roles as suspension feeders, and effects can be system-wide, as opposed to effects on benthic invertebrates, which are mostly local. Suspension feeding not only affects nutrients and planktonic communities, it also transfers materials from the water column to the benthos, enhancing the coupling between planktonic and benthic components of the ecosystem, which can trigger a suite of changes that increase the relative importance of the benthic community—a process sometimes referred to as “benthification” (Mayer et al. 2014). The intensity and extent of these effects depend on many factors, including mussel population density and distribution in a waterbody, food resources available for the bivalves, water mixing rates, lake morphology, and plankton turnover rates (Karatayev et al. 1997, 2002; Kelly et al. 2010; Boltovskoy and Correa 2015). Because D. polymorpha is usually restricted to the littoral zone, its impacts may be significantly greater in small, shallow lakes than in large, deep ones (Karatayev et al. 2015). The impacts of L. fortunei may be similar, but this has not been confirmed by ad hoc studies. In contrast, quagga mussels are found throughout the entire waterbody, and, in deep lakes, they have larger total population sizes. As a consequence, they may have greater system-wide effects than golden or zebra mussels (reviewed in Karatayev et al. 2015).
Although there are more data on the system-wide impacts of zebra mussels than those of quagga and golden mussels, because of their functional similarity, their impacts on waterbodies are likely to be similar (Table 3), although the final outcome may differ depending on waterbody characteristics. The feeding activity of these invasive bivalves boosts nutrient concentrations and alters their proportions, in particular increasing the phosphorus to nitrogen (P:N) ratio (Conroy and Culver 2005; Cataldo et al. 2012b). Consumption of organic particles, including phyto- and zooplankton, and the rejection of organic and inorganic suspended matter as feces and pseudofeces decreases plankton densities and turbidity, which in turn favors light penetration and growth of macrophytes and periphyton. These effects have been described repeatedly in European, Asian, and North and South American waterbodies colonized by dreissenids or L. fortunei (see references in Table 3; see Chapter “Nutrient Recycling, Phytoplankton Grazing, and Associated Impacts of Limnoperna fortunei” in this volume). However, their net impacts on the systems investigated are not necessarily identical, especially when comparing cold-temperate North American lakes with tropical and subtropical South American freshwater habitats. The Paraguay-Paraná-Uruguay floodplain river system invaded by L. fortunei is quite different than the colder, clearer, and more oligotrophic North American waterbodies colonized by Dreissena. A particularly important contrast are the mean concentrations of particulate organic carbon (POC), which are much higher in South America (about 3.5 mg/L in the Paraná River, 20–40 % of it labile and available for biologic consumption; Depetris 1976; Depetris and Pasquini 2007) than in many of the waterbodies invaded by Dreissena (typically around 0.15–1 mg/L in the Great Lakes; Fanslow et al. 1995; Barbiero and Tuchman 2004; Johengen et al. 2008). Filtering organisms are generally scarce and probably not food limited in South America (Sylvester et al. 2005), which suggests that competitive impacts with suspension-feeding native animals, such as those described in North America (Bartsch et al. 2003; Thorp and Casper 2003; Raikow 2004), are less likely in South America. Furthermore, indigenous suspension-feeding organisms in the Río de la Plata watershed are scarce, and the main source of energy for animals is of detrital origin. Most of the suspended organic matter is flushed out into the ocean through the Río de la Plata estuary (~ 1,000,000–2,000,000 t of POC per year; Depetris and Kempe 1993; Guerrero et al. 1997). L. fortunei, the only abundant macrobenthic suspension-feeder, intercepts part of this organic matter and retains it in the system for use by a wide array of animals. The ecosystem-wide effects of this new energetic subsidy to the benthos have not been investigated, but are likely significant (Boltovskoy et al. 2006).
One of the most contentious questions is the impact of exotic bivalves on toxic cyanobacteria, in particular Microcystis spp. Several authors have suggested that Dreissena spp. promote toxic blooms via selective grazing and rejection of toxic strains of blue-green algae and excretion of soluble waste products at low nitrogen to phosphorus ratios (Conroy and Culver 2005; Bykova et al. 2006; Fishman et al. 2009). Other studies (in both North America and Europe) have found that zebra mussels may actively consume and reduce the density of Microcystis spp. (Baker et al. 1998; Strayer et al. 1999; Dionisio Pires et al. 2005, 2010). It was suggested that the positive effect of dreissenids on Microcystis spp. is restricted to lakes with low to moderate total phosphorus concentrations (< 25 µg total P/L), whereas those with high nutrient loadings are not affected (Vanderploeg et al. 2001; Nicholls et al. 2002; Sarnelle et al. 2005; Knoll et al. 2008). In contrast, L. fortunei boosts Microcystis spp. growth at very high P concentrations (50–100 µg/L; Cataldo et al. 2012b).
Trophic Interactions with Fishes
In Europe and North and South America, dreissenids and L. fortunei provide an abundant food resource for fishes. At least 38 species of fish in Europe and in North America feed on Dreissena spp. (Molloy et al. 1997), and almost 50 species of fish consume L. fortunei in South America (see Chapter “Trophic Relationships of Limnoperna fortunei with Adult Fishes” in this volume). The importance of mussels in fish diets varies depending on the feeding mode and fish age, season of the year, and the morphology of the waterbody (Karatayev et al. 2002, 2007a; Strayer et al. 2004; Boltovskoy and Correa 2015).
In dreissenid-invaded areas, shortly after invasion there has been an increase in benthivorous fishes, especially in the littoral zone. This is true even for those that do not feed on dreissenids because of the increase in biomass of native benthic invertebrates that occurs with invasion (Karatayev et al. 2002, 2007a; Higgins and Vander Zanden 2010; Kelly et al. 2010; Burlakova et al. 2012). In Europe, a shift to dreissenid-based diets has resulted in increased growth, average and maximum sizes, and condition for some species of fish (Lyagina and Spanowskaya 1963; Poddubnyi 1966). In contrast, in the profundal zone of the Great Lakes, the introduction of zebra, and especially quagga mussels has been linked to the decline in the abundance, condition, and growth of several fish species. This effect has been associated with a decrease in their main food, the amphipod Diporeia spp., and to the lower energy content of the new food resource (mussels), which replaced the original forage base (Lozano et al. 2001; Hoyle et al. 2008; Nalepa et al. 2009a, 2009b; Rennie et al. 2009). Limited data suggest that dreissenids can have both negative and positive effects on planktivorous fishes. Suspension feeding by mussels can reduce planktonic food resources. Increased water transparency can result in increased predation on larval fish, but may also facilitate prey capture by visual fish predators (Francis et al. 1996; Mayer et al. 2001, 2014; Mills et al. 2003). In the long term, however, the effects of Dreissena spp. on fish were found to decrease with time (Strayer et al. 2014).
Following the introduction of the golden mussel in South America, several fish species shifted their diet from plants and detritus to the energetically more profitable L. fortunei (Boltovskoy et al. 2006; see Chapter “Trophic Relationships of Limnoperna fortunei with Adult Fishes” in this volume). L. fortunei is consumed not only by fishes that can detach mussels from a clump and grind their valves, but also by species that swallow whole individuals, and even others that nibble on extended siphons and mantle edges. Many of these midsized fishes are in turn consumed by larger, piscivorous species with high commercial value, suggesting that improved feeding conditions for their prey are likely to have a positive impact on these large species as well.
Consumption of L. fortunei veligers by fish larvae is probably even more significant than the consumption of adult mussels. Of 25 larval fish taxa surveyed in the Paraná, Paraguay, and Uruguay rivers, 18 feed on veligers, especially their earliest life stage (protolarvae) (Paolucci et al. 2007; Paolucci 2010; see Chapter “Trophic Relationships of Limnoperna fortunei with Larval Fishes” in this volume). Veligers are not only more abundant and easier to capture than crustacean zooplankton, but they also represent an energetically more profitable food resource yielding significantly higher growth rates than crustaceans (Paolucci et al. 2010b).
Concluding Remarks
Limnoperna fortunei was originally described in 1856 (as Volsella fortunei; Dunker 1856), and subsequently referred to under various different names including Modiola lacustris, Limnoperna lacustris, Modiola siamensis, Limnoperna siamensis, Modiola cambodgensis, Modiola (Limnoperna) siamensis (Morton and Dinesen 2010), in chiefly taxonomic and distributional studies. It was a species of little interest until it invaded Japan and South America around 1990. After that time, the number of publications dedicated to the golden mussel soared from < 0.3/year as of 1992, to > 20/year after 1993 (see “Preface” in this volume). The striking similarity between L. fortunei and species of Dreissena has been noticed since the very first detailed studies of the biology of the golden mussel, when it invaded Hong Kong ~ 1965 (Morton 1975). By then, D. polymorpha had been expanding across Europe for centuries, and there was abundant information on its biology, ecology, and impacts. Thus, using Dreissena as a model and a recurrent reference in subsequent literature on the golden mussel was an obvious outcome.
The growing body of information on L. fortunei clearly shows that, indeed, parallels with the dreissenids, in particular with D. polymorpha, are numerous and warranted. However, proven similarities also encouraged ascribing to L. fortunei processes, and particularly impacts, reported for zebra mussels in the northern hemisphere. Although many researchers were cautious in their conclusions, stating that such effects were merely a possibility, others were not. These assumptions had a snowball effect whereby subsequent publications indiscriminately extrapolated results on the impacts of zebra mussels in the northern hemisphere to those of L. fortunei in South America.
Boltovskoy and Correa (2015) noted that “Complications for interpreting the effects of L. fortunei on the ecosystem are even more critical when attempting to label the impacts as negative or positive. A basic precautionary principle and the long list of examples where introduced species have been shown to have devastating effects on the biota (Simberloff 2003) clearly support the need to make all efforts possible to keep biological invasions at bay, or to eradicate them if feasible. However, once a nonnative species has been introduced and its eradication is out of the question (as is the case of L. fortunei), analyses of its interactions with the local biota should be based on evidence, rather than on extrapolations from other invasives and geographic areas. Much of the literature on the golden mussel has been oriented at forcibly demonstrating the environmental harm caused by this invader, thus biasing if not the results, the interpretation of the evidence obtained (Bujes et al. 2007; Defeo et al. 2013)”.
As shown in this review, impacts of these invasive mussels vary widely among geographic areas and waterbodies, and even in different sectors within the same waterbody. Furthermore, interactions with the local biota change as a function of mussel species, their densities, and with time after initial colonization. Using data on the much more thoroughly researched dreissenids furnished useful guidelines for defining potential interactions and fruitful research topics, but it has also tended to hinder assessment of differences between the golden mussel and Dreissena spp., many of which have been shown to be responsible for quite dissimilar environmental impacts (Boltovskoy et al. 2006; Boltovskoy and Correa 2015). We contend that in order to effectively widen our current knowledge, research on L. fortunei should center on identifying contrasts and dissimilarities with dreissenids, rather than on confirming parallels.
Future research should aim at shedding light on the many unknown aspects of the biology and ecology of the golden mussel, which are particularly critical for a comprehensive assessment of its interactions with the local biota. So far, only a few effects at local scales have been explored, whereas at the ecosystem scale our understanding of interactions of L. fortunei with the environment is still very limited. For example, although mussel densities are a key element for gauging the impacts of the invader on ecosystems, so far only one attempt has been made at assessing this parameter over an entire waterbody (Boltovskoy et al. 2009a). Several potential traits (e.g., fecundity, metabolism) and interactions of utmost importance (e.g., biomagnification and transfer of contaminants, thermal shifts due to changes in water transparency, the homogenization of faunal composition across environments, facilitation of other invasive species, changes in macrophyte growth, modifications in benthic oxygenation, overgrowth of other organisms, trophic relationships with waterfowl and aquatic vertebrates other than fishes, etc.) have practically not been addressed so far.
References
Aldridge DW, Payne BS, Miller AC (1995) Oxygen consumption, nitrogenous excretion, and filtration rates of Dreissena polymorpha at acclimation temperature between 20 and 32 °C. Can J Fish Aquat Sci 52:1761–1767
Allen YC, Thompson BA, Ramcharan CW (1999) Growth and mortality rates of the zebra mussel, Dreissena polymorpha, in the Lower Mississippi River. Can J Fish Aquat Sci 56:748–759
Angonesi LG, da Rosa NG, Bemvenuti CE (2008) Tolerance to salinities shocks of the invasive mussel Limnoperna fortunei under experimental conditions. Iheringia (Série Zoologia) 98:66–69
Bacchetta R, Mantecca P, Vailati G (2010) Reproductive behaviour of zebra mussels living in shallow and deep water in the South Alps lakes. In: van der Velde G, Rajagopal S, Bij de Vaate A (eds) The Zebra Mussel in Europe. Backhuys Publishers, Leiden, pp 161–168
Baigun C, Marinone MC (1995) Cold-temperate lakes of South America: do they fit northern hemisphere models? Archiv Hydrobiologie-Beiheft Ergebnisse der Limnologie 135:23–51
Baker SM, Levinton JS, Kurdziel JP, Shumway SE (1998) Selective feeding and biodeposition by zebra mussels and their relation to changes in phytoplankton composition and seston load. J Shellfish Res 17:1207–1213
Balcom N (1994) Zebra mussel awareness and boat use patterns among boaters using three “high risk” Connecticut lakes. In: 4th International Zebra Mussel Conference, Madison (Wisconsin)
Baldwin BS, Mayer MS, Dayton J, Pau N, Mendilla J, Sullivan M, Moore A, Ma A, Mills EL (2002) Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Can J Fish Aquat Sci 59:680–694
Barbiero RP, Tuchman ML (2004) The deep chlorophyll maximum in Lake Superior. J Great Lakes Res 30(Supplement 1):256–268
Barbosa FG, Melo AS (2009) Modelo preditivo de sobrevivência do Mexilhão Dourado (Limnoperna fortunei) em relação a variações de salinidade na Laguna dos Patos, RS, Brasil. Biota Neotropica 9:407–412
Bartsch LA, Richardson WB, Sandheinrich MB (2003) Zebra mussels (Dreissena polymorpha) limit food for larval fish (Pimephales promelas) in turbulent systems: a bioenergetics analysis. Hydrobiologia 495:59–72
Beekey MA, McCabe DJ, Marsden JE (2004) Zebra mussels affect benthic predator foraging success and habitat choice on soft sediments. Oecologia 141:164–170
Belaich M, Oliver C, Pilloff M, Porta A (2006) Evaluation of a biomarker of Cd(II) exposure on Limnoperna fortunei. Environ Pollut 144:280–288
Benson AJ (2014) Chronological history of zebra and quagga mussels (Dreissenidae) in North America, 1988–2010. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control. 2nd edition CRC Press, Boca Raton, pp 9–32
Bially A, MacIsaac HJ (2000) Fouling mussels (Dreissena spp.) colonize soft sediments in Lake Erie and facilitate benthic invertebrates. Freshw Biol 43:85–97
bij de Vaate A, Greijdanus-Klaas M, Smit H (1992) Densities and biomass of zebra mussels in the Dutch part of the lower Rhine. In: Neumann D, Jenner HA (eds) The zebra mussel Dreissena polymorpha. Ecology, biology monitoring and first applications in the water quality management. Gustav Fisher, Stuttgart, pp 67–77
bij de Vaate A, Jazdzewski K, Ketelaars HAM, Gollasch S, van der Velde G (2002) Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59:1159–1174
bij de Vaate A, van der Velde G, Leuven RSEW, Heiler KCM (2014) Spread of the quagga mussel (Dreissena rostriformis bugensis) in western Europe. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control. Second edition. CRC Press, Boca Raton, pp 83–92
Boltovskoy D, Cataldo D (1999) Population dynamics of Limnoperna fortunei, an invasive fouling mollusc, in the lower Paraná River (Argentina). Biofouling 14:255–263
Boltovskoy D, Correa N (2015) Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia. 746:81–95
Boltovskoy D, Correa N, Cataldo D, Sylvester F (2006) Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata watershed and beyond. Biol Invasions 8:947–963
Boltovskoy D, Karatayev A, Burlakova L, Cataldo D, Karatayev V, Sylvester F, Mariñelarena A (2009a) Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636:271–284
Boltovskoy D, Sylvester F, Otaegui A, Leytes V, Cataldo D (2009b) Environmental modulation of the reproductive activity of the invasive mussel Limnoperna fortunei in South America. Austral Ecol 34:719–730
Bonel N, Solari LC, Lorda J (2013) Differences in density, shell allometry and growth between two populations of Limnoperna fortunei (Mytilidae) from the Río de la Plata basin, Argentina. Malacologia 56:43–58
Bonetto C, Villar C, De Cabo L, Vaithiyanathan P (1998) Hydrochemistry of a large floodplain river. Verhandlungen der Internationalen Vereinigung Theoretische und Angewandte Limnologie 26:899–902
Borcherding J (1991) The annual reproductive cycle of the freshwater mussel Dreissena polymorpha Pallas in lakes. Oecologia 87:208–218
Botts PS, Patterson BA, Schloesser DW (1996) Zebra Mussel effects on benthic invertebrates: physical or biotic? J N Am Benthol Soc 15:179–184
Brugnoli E, Dabezies MJ, Clemente JM, Muniz P (2011) Limnoperna fortunei (Dunker 1857) en el sistema de embalses del Rio Negro, Uruguay. Oecologia Australis 15:576–592
Buch KL, McMahon RF (2001) Assessment of potential for dispersal of aquatic nuisance species by recreational boaters into the western United States. The University of Texas at Arlington, Arlington, pp 1–70
Bujes CS, Ely I, Verrastro L (2007) Trachemys dorbigni (Brazilian Slider) diet. Herpetol Rev 38:335
Burlakova LE (1998) Ecology of Dreissena polymorpha (Pallas) and its role in the structure and function of aquatic ecosystems. PhD Thesis, Academy of Sciences of Belarus (Belarus), pp 1–167
Burlakova LE, Karatayev AY, Padilla DK (2000) The impact of Dreissena polymorpha (Pallas) invasion on unionid bivalves. Int Rev Hydrobiol 85:529–541
Burlakova LE, Karatayev AY, Karatayev VA (2012) Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 685:121–134
Burlakova LE, Karatayev AY, Pennuto C, Mayer C (2014) Changes in Lake Erie benthos over the last 50 years: historical perspectives, current status, and main drivers. J Great Lakes Res. 40:560–573
Bykova O, Laursen A, Bostan V, Bautista J, Mc Carthy L (2006) Do zebra mussels (Dreissena polymorpha) alter lake water chemistry in a way that favours Microcystis growth? Sci Total Environ 371:362–372
Callil CT, Mansur MCD, Marcelo SM (2006) Bivalves invasores no Pantanal. In: XVII Encontro Brasileiro de Malacologia, Rio De Janeiro (Brazil)
Carlton JT (2008) The zebra mussel Dreissena polymorpha found in North America in 1986 and 1987. J Great Lakes Res 34:770–773
Cataldo D, Boltovskoy D (2000) Yearly reproductive activity of Limnoperna fortunei (Bivalvia) as inferred from the occurrence of its larvae in the plankton of the lower Paraná River and the Río de la Plata estuary (Argentina). Aquatic Ecol 34:307–317
Cataldo D, Boltovskoy D, Stripeikis J, Pose M (2001a) Condition index and growth rates of field caged Corbicula fluminea (Bivalvia) as biomarkers of pollution gradients in the Paraná River delta (Argentina). Aquatic Ecosyst Health Manag 4:187–201
Cataldo D, Colombo JC, Boltovskoy D, Bilos C, Landoni P (2001b) Environmental toxicity assessment in the Paraná River delta (Argentina): simultaneous evaluation of select pollutants and mortality rates of Corbicula fluminea (Bivalvia) early juveniles. Environ Pollut 112:379–389
Cataldo D, O´Farrell I, Paolucci E, Sylvester F, Boltovskoy D (2012a) Impact of the invasive golden mussel (Limnoperna fortunei) on phytoplankton and nutrient cycling. Aquat Invasions 7:91–100
Cataldo D, Vinocur A, O´Farrell I, Paolucci E, Leites V, Boltovskoy D (2012b) The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande Reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680:25–38
Claxton WT, Mackie GL (1998) Seasonal and depth variations in gametogenesis and spawning of Dreissena polymorpha and Dreissena bugensis in eastern Lake Erie. Can J Zool 76:2010–2019
Conroy J, Culver DA (2005) Do dreissenid mussels affect Lake Erie ecosystem stability processes? Am Midland Nat 153:20–32
Choi SS, Shin CN (1985) Study on the early development and larvae of Limnoperna fortunei. Korean J Malacol 1:5–12 [In Korean]
Darrigran GA (2002) Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol Invasions 4:145–156
Darrigran GA, Martin SM, Gullo B, Armendariz L (1998) Macroinvertebrates associated with Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) in Río de la Plata, Argentina. Hydrobiologia 367:223–230
Darrigran GA, Penchaszadeh PE, Damborenea C (1999) The reproductive cycle of Limnoperna fortunei (Dunker, 1857) (Mytilidae) from a neotropical temperate locality. J Shellfish Res 18:361–365
Darrigran GA, Damborenea C, Greco N (2007) An evaluation pattern for antimacrofouling procedures: Limnoperna fortunei larvae study in a hydroelectric power plant in South America. Ambio 36:575–579
Defeo O, Castrejón M, Ortega L, Kuhn AM, Gutiérrez NL, Castilla JC (2013) Impacts of climate variability on Latin American small-scale fisheries. Ecol Soc 18:30
Depetris PJ (1976) Hydrochemistry of the Paraná River. Limnol Oceanogr 21:736–739
Depetris PJ, Kempe S (1993) Carbon dynamics and sources in the Paraná River. Limnol Oceanogr 38:382–395
Depetris PJ, Pasquini AI (2007) The geochemistry of the Paraná River: an overview. In: Iriondo MH, Paggi JC, Parma MJ (eds) The Middle Paraná River. Limnology of a subtropical wetland. Springer Verlag, Berlin, pp 143–174
Dermott R, Kerec D (1997) Changes to the deep water benthos of eastern Lake Erie since the invasion of Dreissena: 1979–1993. Can J Fish Aquat Sci 54:922–930
Dermott R, Munawar M (1993) Invasion of Lake Erie offshore sediments by Dreissena, and its ecological implications. Can J Fish Aquat Sci 50:2298–2304
Díaz M, Pedrozo F, Baccala N (2000) Summer classification of Southern Hemisphere temperate lakes (Patagonia, Argentina). Lakes & Reservoirs: Res Manag 5:213–229
Di Fiori E, Pizarro H, dos Santos Afonso M, Cataldo D (2012) Impact of the invasive mussel Limnoperna fortunei on glyphosate concentration in water. Ecotoxicol Environ Safety 81:106–113
Diggins TP (2001) A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. J Great Lakes Res 27:457–466
Dionisio Pires LM, Bontes BM, Van Donk E, Ibelings BW (2005) Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. J Plankton Res 27:331–339
Dionisio Pires LM, Ibelings BW, van Donk E (2010) Zebra mussels as a potential tool in the restoration of eutrophic shallow lakes, dominated by toxic cyanobacteria. In: van der Velde G, Rajagopal S, Bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 361–372
Dunker W (1856) Mytilacea nova collectionis Cumingianæ. Proc Zool Soc Lond 24:358–366
Dyga AK, Zolotareva VI (1976) Biology of Dreissena bugensis Andrusov of Zaporozhskoe Reservoir and their role in water purification. In: Andrushaitis GP et al. (eds) Abstracts of the Third Congress of the All-Union Hydrobiological Society. Zinatne Press, Riga, pp 237–239 [In Russian]
Fachini A (2011) Filtração do bivalve invasor Limnoperna fortunei (Dunker, 1857), o mexilhão dourado, sobre a comunidade planctônica natural e na presença de cianobactéria tóxica. MSc Thesis, Universidade Federal do Rio Grande do Sul (Brazil), pp 1–68
Fahnenstiel GL, Pothoven SA, Nalepa TF, Vanderploeg HA, Klarer DM, Scavia D (2010) Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. J Great Lakes Res 36:20–29
Fanslow DL, Nalepa TF, Lang GA (1995) Filtration rates of the zebra mussel (Dreissena polymorpha) on natural seston from Saginaw Bay, Lake Huron. J Great Lakes Res 21:489–500
Fishman DB, Adlerstein SA, Vanderploeg HA, Fahnenstiel GL, Scavia D (2009) Causes of phytoplankton changes in Saginaw Bay, Lake Huron, during the zebra mussel invasion. J Great Lakes Res 35:482–495
Francis JT, Robillard SR, Marsden JE (1996) Yellow perch management in Lake Michigan: a multi-jurisdictional challenge. Fisheries 21:18–20
Garton DW, McMahon R, Stoeckmann AM (2014) Limiting environmental factors and competitive interactions between zebra and quagga mussels in North America. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edition. CRC Press, Boca Raton, pp 383–402
Grigorovich IA, Angradi TR, Stepien CA (2008) Occurrence of the quagga mussel (Dreissena bugensis) and the zebra mussel (Dreissena polymorpha) in the upper Mississippi River system. J Freshw Ecol 23:429–435
Guerrero R, Acha EM, Framiñan MB, Lasta CA (1997) Physical oceanography of the Río de la Plata Estuary, Argentina. Cont Shelf Res 17:727–742
Gutierrez JL, Jones CG, Strayer DL, Iribarne OO (2003) Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101:79–90
Hallstan S, Grandin U, Goedkoop W (2010) Current and potential distribution of the zebra mussel (Dreissena polymorpha) in Sweden. Biol Invasions 12:285–296
Hamada M (2011) Relationship between water temperature and period of occurrence and attachment in planktonic larvae of golden mussel Limnoperna fortunei, in the Yahagi River. Report of Yahagi River Institute 15:45–54 [In Japanese]
Higgins SN, Vander Zanden MJ (2010) What a difference a species makes: a meta–analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol Monogr 80:179–196
Hoyle JA, Bowlby JN, Morrison BJ (2008) Lake whitefish and walleye population responses to dreissenid mussel invasion in eastern Lake Ontario. Aquat Ecosyst Health Manag 11:403–411
Huang Z, Li C, Zhang L, Li F, Zheng C (1981) The distribution of fouling organisms in Changjiang River estuary. Oceanologia et Limnologia Sinica 12:531–537
Hunter RG, Simons KA (2004) Dreissenids in Lake St. Clair in 2001: evidence for population regulation. J Great Lakes Res 30:528–537
Jantz B, Neumann D (1992) Shell growth and aspects of the population dynamics of Dreissena polymorpha in the River Rhine. In: Neumann D, Jenner HA (eds) The zebra mussel Dreissena polymorpha. Ecology, biology monitoring and first applications in the water quality management. Gustav Fisher, Stuttgart, pp 49–66
Japan Commission on Large Dams (2009) Dams in Japan. Past, present and future. CRC Press, Boca Raton, pp 1–232
Johengen TH, Biddanda BA, Cotner JB (2008) Stimulation of Lake Michigan plankton metabolism by sediment resuspension and river runoff. J Great Lakes Res 34:213–227
Johnson LE, Ricciardi A, Carlton JT (2001) Overland dispersal of aquatic invasive species: a risk assessment of transient recreational boating. Ecol Appl 11:1789–1799
Jones LA, Ricciardi A (2005) Influence of physicochemical factors on the distribution and biomass of invasive mussels (Dreissena polymorpha and Dreissena bugensis) in the St. Lawrence River. Can J Fish Aquat Sci 62:1953–1962
Karatayev AY, Burlakova LE, Padilla DK (1997) The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in Eastern Europe. J Shellfish Res 16:187–203
Karatayev AY, Burlakova LE, Padilla DK (1998) Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). J Shellfish Res 17:1219–1235
Karatayev AY, Burlakova LE, Padilla DK (2002) Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe: Distribution, impacts and management. Kluwer Academic Publishers, Dodrecht, pp 433–446
Karatayev AY, Burlakova LE, Padilla DK (2006) Growth rate and longevity of Dreissena polymorpha (Pallas): a review and recommendations for future study. J Shellfish Res 25:1–23
Karatayev AY, Boltovskoy D, Padilla DK, Burlakova LE (2007a) The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. J Shellfish Res 26:205–213
Karatayev AY, Padilla DK, Minchin D, Boltovskoy D, Burlakova LE (2007b) Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biol Invasions 9:161–180
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010a) Limnoperna fortunei versus Dreissena polymorpha: Population densities and benthic community impacts of two invasive freshwater bivalves. J Shellfish Res 29:975–984
Karatayev AY, Burlakova LE, Padilla DK (2010b) Dreissena polymorpha in Belarus: history of spread, population biology, and ecosystem impacts. In: van der Velde G, Rajagopal S, bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 45–58
Karatayev AY, Mastitsky SE, Padilla DK, Burlakova LE, Hajduk MH (2010c) Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia 668:183–194
Karatayev AY, Burlakova LE, Mastitsky SE, Padilla DK, Mills EL (2011) Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. J Shellfish Res 30:923–931
Karatayev AY, Burlakova LE, Padilla DK (2014a) General overview of zebra and quagga mussels. What we do and do not know. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 695–703
Karatayev AY, Burlakova LE, Padilla DK (2015) Zebra versus quagga mussels: Spread, population dynamics and ecosystem impacts. Hydrobiologia 746:97–112
Karatayev AY, Burlakova LE, Pennuto C, Ciborowski J, Karatayev VA, Juette P, Clapsadl M (2014c) Twenty five years of changes in Dreissena spp. populations in Lake Erie. J Great Lakes Res 40:550–559
Kawase M (2011) Feeding and filtration in Limnoperna lacustris. Available via http://www.mizuho-c.ac.jp/images/library/kiyo_05/amckiyo-no05-05.pdf. Accessed 11 Feb 2014 [In Japanese]
Keller RP, Drake JM, Lodge DM (2007) Fecundity as a basis for risk assessment of nonindigenous freshwater molluscs. Conserv Biol 21:191–200
Kelly DW, Herborg L-M, MacIsaac HJ (2010) Ecosystem changes associated with Dreissena invasions: recent developments and emerging issues. In: van der Velde G, Rajagopal S, bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 199–210
Kinzelbach R (1992) The main features of the phylogeny and dispersal of the zebra mussel Dreissena polymorpha. In: Neumann D, Jenner HA (eds) The zebra mussel Dreissena polymorpha. Ecology, biology monitoring and first applications in the water quality management. Gustav Fisher, Stuttgart, pp 5–17
Knoll LB, Sarnelle O, Hamilton SK, Kissman CEH, Wilson AE, Rose JB, Morgan MR (2008) Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Can J Fish Aquat Sci 65:448–455
Lozano SJ, Scharold JV, Nalepa TF (2001) Recent declines in benthic macroinvertebrate densities in Lake Ontario. Can J Fish Aquat Sci 58:518–529
Lucy FE, Burlakova LE, Karatayev AY, Mastitsky SE, Zanatta DT (2014) Zebra mussel impacts on unionids. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 623–646
Luferov VP (1965) Organisms living anabiotically frozen in ice of the littoral zone of Rybinskoe Reservoir. Trudy Instituta Biologii Vnutrennikh Vod Akademii Nauk SSSR 8:151–154 [In Russian]
Lvova AA (1977) The ecology of Dreissena polymorpha (Pall.) in Uchinskoe Reservoir. PhD Thesis, Moscow State University (Russia), pp 1-116 [In Russian]
Lvova AA, Makarova GE (1994) Reproduction. In: Starobogatov JI (ed) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, pp 138–148 [In Russian]
Lvova AA, Karatayev AY, Makarova GE (1994a) Planktonic larva. In: Starobogatov JI (ed) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, pp 138–148 [In Russian]
Lvova AA, Makarova GE, Miroshnichenko MP (1994b) Growth of the Dreissena in different parts of the distribution area. In: Starobogatov JI (ed) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, pp 156–179 [In Russian]
Lyagina TN, Spanowskaya VD (1963) Morphological peculiarities of some fish in the Uchinskoe water storage. In: Uchinskoe and Mozhaiskoe Reservoirs: hydrobiological and ichthyological studies. Moscow State University, Moscow, pp 269–310 [In Russian]
Lyakhnovich VP, Karatayev AY, Andreev NI, Andreeva SI, Afanasiev SA, Dyga AK, Zakutskiy VP, Zolotareva VI, Lvova AA, Nekrasova MY, Osadchikh VF, Pligin YV, Protasov AA, Tischikov GM (1994) Living conditions. In: Starobogatov YI (ed) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, pp 109–119 [In Russian]
MacIsaac HJ, Grigorovich IA, Ricciardi A (2001) Reassessment of species invasions concepts: the Great Lakes basin as a model. Biol Invasions 3:405–416
Mackie GL (1991) Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219:251–268
Magara Y, Matsui Y, Goto Y, Yuasa A (2001) Invasion of the non-indigenous nuisance mussel, Limnoperna fortunei, into water supply facilities in Japan. J Water Supply: Res Technol—Aqua 50:113–124
Maglianesi RE (1973) Principales características químicas y físicas del las aguas del Alto Paraná y Paraguay Inferior. Physis 32:185–197
Mansur MCD, Santos CP, Darrigran G, Heydrich I, Callil CT, Cardoso FR (2003) Primeiros dados quali-quantitativos do mexilhão-dourado, Limnoperna fortunei (Dunker), no Delta do Jacuí, no Lago Guaíba e na Laguna dos Patos, Rio Grande do Sul, Brasil e alguns aspectos de sua invasão no novo ambiente. Revista Brasileira de Zoologia 20:75–84
Marçal SF, Callil CT (2008) Structure of invertebrates community associated with Eichhornia crassipes Mart. (Solms-Laubach) after the introduction of Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) in the Upper Paraguay River, MT, Brazil. Acta Limnologica Brasiliensia 20:359–371
Marçal SF, Callil CT (2012) Limnoperna fortunei associada a macrófitas aquáticas na bacia do Rio Paraguai, Mato Grosso. In: Mansur MCD, Santos CP, Pereira D, Padula Paz IC, Leite Zurita ML, Raya Rodriguez MT, Vilar Nehrke M, Aydos Bergonci PE (eds) Moluscos límnicos invasores no Brasil. Biologia, prevenção, controle. Redes Editora, Porto Alegre, pp 201–206
Mata FAR (2011) Abundância e distribuição temporal de Limnoperna fortunei Dunker, 1857 (Mollusca, Bivalvia) e os impactos da incrustação em usinas geradoras de energia elétrica. MSc Thesis, Universidade Federal de Ouro Preto (Brazil), pp 1–91
Matsuda M, Uenishi M (1992) A newly introduced mussel, Limnoperna lacustris (V. Martens) (Mollusca: Mytilidae) in Lake Biwa, Shiga Prefecture, Japan. Annual Rep Biwako Bunkakan 10:45 [In Japanese]
Matthews MA, McMahon RF (1994) The survival of zebra mussels (Dreissena polymorpha) and asian clams (Corbicula fluminea) under extreme hypoxia. In: 4th International Zebra Mussel Conference, Madison (USA)
Mayer CM, Rudstam LG, Mills EL, Cardiff SG, Bloom CA (2001) Zebra mussels (Dreissena polymorpha), habitat alteration and yellow perch (Perca flavescens) foraging: system-wide effects and behavioural mechanisms. Can J Fish Aquat Sci 58:2459–2467
Mayer CM, Burlakova LE, Eklöv P, Fitzgerald D, Karatayev AY, Ludsin SA, Millard S, Mills EL, Ostapenya AP, Rudstam LG, Zhu B, Zhukova TV (2014) Benthification of freshwater lakes. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 575–585
McMahon RF, Bogan AE (2001) Mollusca: Bivalvia. In: Thorn JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, pp 331–429
Mellina E, Rasmussen JB (1994) Patterns in the distribution and abundance of zebra mussel (Dreissena polymorpha) in rivers and lakes in relation to substrate and other physicochemical factors. Can J Fish Aquat Sci 51:1024–1036
Mills EL, Dermott RM, Mellina E, Conn DB, Spidle AP (1993) Colonization, ecology, and population structure of the ‘‘Quagga’’ mussel (Bivalvia: Dreissenidae). Can J Fish Aquat Sci 50:2305–2314
Mills EL, Rosenberg G, Spidle AP, Ludyanskiy M, Pligin Y (1996) A review of the biology of the quagga mussel (Dreissena bugensis), a second species of freshwater dreissenid introduced to North America. Am Zool 36:271–286
Mills EL, Chrisman JR, Baldwin B, Owens RW, O’Gorman R, Howell T, Roseman EF, Raths MK (1999) Changes in the dreissenid community in the Lower Great Lakes with emphasis on Southern Lake Ontario. J Great Lakes Res 25:187–197
Mills EL, Casselman JM, Dermott R, Fitzsimons JD, Gal G, Holeck KT, Hoyle JA, Johannsson OE, Lantry BF, Makarewicz JC, Millard ES, Munawar IF, Munawar M, O’Gorman R, Owens RW, Rudstam LG, Schaner T, Stewart TJ (2003) Lake Ontario: food web dynamics in a changing ecosystem (1970–2000). Can J Fish Aquat Sci 60:471–490
Minchin D (2000) Dispersal of zebra mussel in Ireland. Verhandlungen der Internationalen Vereinigung Theoretische und Angewandte Limnologie 27:1576–1579
Ministry of Agriculture, Forestry and Fisheries (2003) The global diversity of irrigation. Available via http://www.maff.go.jp/j/nousin/keityo/mizu_sigen/index.html. Accessed 5 April 2014
Molloy DP, Karatayev AY, Burlakova EB, Kurandina DP, Laruelle F (1997) Natural enemies of zebra mussels: predators, parasites, and ecological competitors. Rev Fish Sci 5:27–97
Montalto L, Oliveros OB, Ezcurra De Drago I, Demonte LD (1999) Peces del Río Parana medio predadores de una especie invasora: Limnoperna fortunei (Bivalvia, Mytilidae). Revista de la Facultad de Bioquímica y Ciencias Biológicas de la Universidad Nacional del Litoral 3:85–101
Morton B (1975) The colonization of Hong Kong’s raw water supply system by Limnoperna fortunei (Dunker 1857) (Bivalvia: Mytilacea) from China. Malacol Rev 8:91–105
Morton B (1977) The population dynamics of Limnoperna fortunei (Dunker 1857) (Bivalvia: Mytilacea) in Plover Cove Reservoir, Hong Kong. Malacologia 16:165–182
Morton B, Dinesen G (2010) Colonization of Asian freshwaters by the Mytilidae (Bivalvia): A comparison of Sinomytilus harmandi from the Tonle-Sap River, Phnom Penh, Cambodia, with Limnoperna fortunei. Molluscan Res 30:57–72
Nakano D, Kobayashi T, Sakaguchi I (2010a) Differences in larval dynamics of golden mussel Limnoperna fortunei between dam reservoirs with and without an aeration system. Landsc Ecol Eng 6:53–60
Nakano D, Kobayashi T, Sakaguchi I (2010b) Predation and depth effects on abundance and size distribution of an invasive bivalve, the golden mussel Limnoperna fortunei, in a dam reservoir. Limnology 11:259–266
Nalepa TF (2010) An overview of the spread, distribution, and ecological impacts of the quagga mussel, Dreissena rostriformis bugensis, with possible implications to the Colorado River system. In: Colorado River basin science and resource management symposium. Coming together, coordination of science and restoration activities for the Colorado River ecosystem, Scottsdale (USA)
Nalepa TF, Hartson DJ, Fanslow DL, Lang GA, Lozano SJ (1998) Decline in benthic macroinvertebrate populations in southern Lake Michigan, 1980–1993. Can J Fish Aquat Sci 55:2402–2413
Nalepa TF, Fahnenstiel GL, Johengen TH (1999) Impacts of the zebra mussel (Dreissena polymorpha) on water quality: A case study in Saginaw Bay, Lake Huron. In: Claudi R, Leach JH (eds) Non-indigenous freshwater organisms in North America: their biology and impact. CRC Press, Boca Raton, pp 255–271
Nalepa TF, Fanslow DL, Pothoven SA, Foiley III AJ, Lang GA (2007) Long-term trends in benthic macroinvertebrate populations in Lake Huron over the past four decades. J Great Lakes Res 33:421–436
Nalepa TF, Fanslow DL, Lang GA (2009a) Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to invasive mussel Dreissena rostriformis bugensis. Freshw Biol 54:466–479
Nalepa TF, Pothoven SA, Fanslow DL (2009b) Recent changes in benthic macroinvertebrate populations in Lake Huron and impact on the diet of lake whitefish (Coregonus clupeaformus). Aquat Ecosyst Health Manag 12:2–10
Nalepa TF, Fanslow DL, Pothoven SA (2010) Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. J Great Lakes Res 36:5–19
Nicholls KH, Heintsch L, Carney E (2002) Univariate step-trend and multivariate assessments of the apparent effects of P loading reductions and zebra mussels on the phytoplankton of the Bay of Quinte, Lake Ontario. J Great Lakes Res 28:15–31
NOAA (2014) NOAA CoastWatch Great Lakes Node. Available via http://coastwatch.glerl.noaa.gov/. Accessed 6 June 2014
Ohtaka A, Tetsuya N, Takahiro K, Haruo K, Yuji A, Sokrithy IM, Rachna C, Shinji T (2011) Composition of aquatic invertebrates associated with macrophytes in Lake Tonle Sap, Cambodia. Limnology 12:137–144
Oliveira MD, Hamilton SK, Jacobi CM (2010) Forecasting the expansion of the invasive golden mussel Limnoperna fortunei in Brazilian and North American rivers based on its occurrence in the Paraguay River and Pantanal wetland of Brazil. Aquat Invasions 5:59–73
Oliveira MD, Calheiros DF, Jacobi CM, Hamilton SK (2011) Abiotic factors controlling the establishment and abundance of the invasive golden mussel Limnoperna fortunei. Biol Invasions 13:717–729
Orlova MI (1987) Differences in salinity tolerance of Dreissena in the lower Dnieper River and the Dnieper-Bug Liman. In: Likharev IM (ed) Abstracts of the 8th meeting on the investigation of molluscs. Nauka Press, Leningrad, pp 261–263 [in Russian]
Orlova MI, Muirhead J, Antonov PI, Shcherbina GK, Starobogatov I, Biochino GI, Therriault TW, MacIsaac HJ (2004) Range expansion of quagga mussels (Dreissena rostriformis bugensis) in the Volga River and Caspian Sea basin. Aquat Ecol 38:561–573
Padilla DK (2005) The potential for zebra mussels as a model for invasion biology. American Malacological Bulletin, 20:123–131
Padilla DK, Chotkowski MA, Buchan LAJ (1996) Predicting the spread of zebra mussels (Dreissena polymorpha) to inland waters using boater movement patterns. Glob Ecol Biogeogr Lett 5:353–359
Paolucci EM (2010) Impacto del molusco invasor Limnoperna fortunei sobre el ecosistema: interacción trófica y efectos sobre las larvas de peces nativos. PhD Thesis, Universidad de Buenos Aires (Argentina), pp 1–162
Paolucci EM, Cataldo DH, Fuentes CM, Boltovskoy D (2007) Larvae of the invasive species Limnoperna fortunei (Bivalvia) in the diet of fish larvae in the Paraná River, Argentina. Hydrobiologia 589:219–233
Paolucci EM, Cataldo DH, Boltovskoy D (2010a) Prey selection by larvae of Prochilodus lineatus (Pisces): indigenous zooplankton versus larvae of the introduced bivalve Limnoperna fortunei. Aquat Ecol 44:255–267
Paolucci EM, Thuesen EV, Cataldo DH, Boltovskoy D (2010b) Veligers of an introduced bivalve (Limnoperna fortunei) are a new food resource that enhances growth of larval fish in the Paraná River (South America). Freshw Biol 55:1831–1844
Park H-K, Cho K-H, Won DH, Lee J, Kong D-S, Jung D-I (2013) Ecosystem responses to climate change in a large on-river reservoir, Lake Paldang, Korea. Climatic Change 120:477–489
Pastorino G, Darrigran GA, Martín SM, Lunaschi L (1993) Limnoperna fortunei (Dunker, 1857) (Mytilidae), nuevo bivalvo invasor en aguas del Río de la Plata. Neotropica 39:101–102
Patterson MWR, Ciborowski JJH, Barton DR (2005) The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie, 2002. J Great Lakes Res 31(Supplement 2):223–237
Payne AI (1986) The ecology of tropical lakes and rivers. Wiley, New York, pp 1–310
Penchaszadeh PE, Darrigran GA, Angulo C, Averbuj A, Brögger M, Dogliotti A, Pírez N (2000) Predation of the invasive freshwater mussel Limnoperna fortunei (Dunker, 1857) (Mytilidae) by the fish Leporinus obtusidens Valenciennes, 1846 (Anostomidae) in the Rio de la Plata, Argentina. J Shellfish Res 19:229–231
Perepelizin PV, Boltovskoy D (2011) Resistance of the invasive pest mussel Limnoperna fortunei to anoxia. J Am Water Works Assoc 103:79–85
Peyer SM, McCarthy AJ, Lee CE (2009) Zebra mussels anchor byssal threads faster and tighter than quagga mussels in flow. J Exp Biol 212:2026–2035
Peyer SM, Hermanson JC, Lee CE (2010) Developmental plasticity of shell morphology of quagga mussels from shallow and deep-water habitats of the Great Lakes. J Exp Biol 2013:2602–2609
Poddubnyi AG (1966) Adaptive response of Rutilus rutilus to variable environmental conditions. Proc Institute of Biology of Inland Waters of the Academy of Science of the USSR 10:131–138 [In Russian]
Pollux BJ, van der Velde G, bij de Vaate A (2010) A perspective on global spread of Dreissena polymorpha: a review on possibilities and limitations. In: van der Velde G, Rajagopal S, bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 45–58
Pothoven SA, Fahnenstiel GL (2013) Recent change in summer chlorophyll a dynamics of southeastern Lake Michigan. J Great Lakes Res 39:287–294
Pothoven SA, Fahnenstiel GL (2014) Lake Michigan after dreissenid mussel invasion. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control, 2nd edn. CRC Press, Boca Raton, pp 545–553
Raikow DF (2004) Food web interactions between larval bluegill (Lepomis macrochirus) and exotic zebra mussels (Dreissena polymorpha). Can J Fish Aquat Sci 61:497–504
Ramcharan CW, Padilla DK, Dodson SI (1992) Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Can J Fish Aquat Sci 49:2611–2620
Rennie MD, Sprules WG, Johnson TB (2009) Resource switching in fish following a major food web disruption. Oecologia 159:789–802
Roe SL, MacIsaac HJ (1997) Deepwater population structure and reproductive state of quagga mussels (Dreissena bugensis) in Lake Erie. Can J Fish Aquat Sci 54:2428–2433
Rojas Molina F (2010) Efectos del molusco invasor Limnoperna fortunei (Dunker) sobre el zooplancton del paraná medio. PhD Thesis, Universidad de Buenos Aires (Argentina), pp 1–205
Rojas Molina F, Paggi JC, Devercelli M (2010) Zooplanktophagy in the natural diet and selectivity of the invasive mollusk Limnoperna fortunei. Biol Invasions 12:1647–1659
Rojas Molina F, Paggi JC, Devercelli MD (2011) Vulnerability of microcrustaceans to predation by the invasive filter-feeding mussel Limnoperna fortunei (Dunker). Marine and Freshwater Behaviour and Physiology 44:329–338
Rojas Molina F, Jose de Paggi SB, Frau D (2012) Impacts of the invading golden mussel Limnoperna fortunei on zooplankton: A mesocosm experiment. Zool Stud 51:733–744
Sardiña P, Cataldo D, Boltovskoy D (2008) The effects of the invasive mussel, Limnoperna fortunei, on associated fauna in South American freshwaters: importance of physical structure and food supply. Fund Appl Limnol/Archiv Hydrobiologie 173:135–144
Sardiña P, Chaves E, Marchese M (2011) Benthic community responses to invasion by the golden mussel, Limnoperna fortunei Dunker: biotic homogenization vs environmental driving forces. J N Am Benthol Soc 30:1009–1023
Sarnelle O, Wilson AE, Hamilton SK, Knoll LB, Raikow DF (2005) Complex interactions between the zebra mussel, Dreissena polymorpha, and the harmful phytoplankter, Microcystis aeruginosa. Limnol Oceanogr 50:896–904
Scarabino F (2004) Conservación de la malacofauna uruguaya. Comunicaciones de la Sociedad Malacológica del Uruguay 8:267–273
Schloesser DW, Masteller EC (1999) Mortality of unionid bivalves (Mollusca) associated with dreissenid mussels (Dreissena polymorpha and D. bugensis) in Presque Isle Bay, Lake Erie. Northeast Nat 6:341–352
Sherman JJ, Murray BA, Woolnough DA, Zanatta DT, Uzarski DG (2013) Assessment of remnant unionid assemblages in a selection of Great Lakes coastal wetlands. J Great Lakes Res 39:201–210
Shevtsova LV (1989) Importance of the Bug River Dreissena for decreasing organic matter and for the sedimentation of suspended substances. Hydrobiol J 25:47–51
Shkorbatov GL, Karpevich AF, Antonov PI (1994) Ecological physiology. In: Starobogatov JI (ed) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, pp 67–108 [In Russian]
Simberloff D (2003) Confronting introduced species: a form of xenophobia? Biol Invasions 5:179–192
Son MO (2007) Native range of the zebra mussel and quagga mussel and new data on their invasions within the Ponto-Caspian region. Aquat Invasions 2:169–179
Soster FM, McCall PL, Herrmann KA (2011) Decadal changes in the benthic invertebrate community in western Lake Erie between 1981 and 2004. J Great Lakes Res 37:226–237
Spiridonov YI (1972) Polarographic investigation of the respiration of Dreissena. In: Topics in physiological and population ecology, Saratov University Press, Saratov (Russia), pp 15–21 [In Russian]
Sprung M (1987) Ecological requirements of developing Dreissena polymorpha eggs. Archiv Hydrobiologie 79:69–86
Sprung M (1991) Costs of reproduction: A study on metabolic requirements of the gonads and fecundity of the bivalve Dreissena polymorpha. Malacologia 33:63–70
Starobogatov YI, Andreeva SI (1994) Range. In: Starobogatov YI (ed) Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Nauka Press, Moscow, pp 47–53 [In Russian]
Stewart TW, Miner JG, Lowe RL (1998) Quantifying mechanisms for zebra mussel effects on benthic macroinvertebrates: organic matter production and shell-generated habitat. J N Am Benthol Soc 17:81–94
Stoeckel JA, Rehmann CR, Schneider DW, Padilla DK (2004) Retention and supply of zebra mussel larvae in a large river system: importance of an upstream lake. Freshw Biol 49:919–930
Stoeckmann A (2003) Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Can J Fish Aquat Sci 60:126–134
Strayer DL, Caraco NF, Cole JJ, Findlay S, Pace ML (1999) Transformation of freshwater ecosystems by bivalves. A case study of zebra mussels in the Hudson River. Bioscience 49:19–27
Strayer DL, Hattala KA, Kahnle AW (2004) Effects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Can J Fish Aquat Sci 61:924–941
Strayer DL, Hattala KA, Kahnle AW, Adams RD (2014) Has the Hudson River fish community recovered from the zebra mussel invasion along with its forage base? Can J Fish Aquat Sci. 71:1146–1157
Sylvester F (2006) Biología alimentaria y ecología del molusco invasor Limnoperna fortunei (Mytilidae) en el Paraná inferior y Río de la Plata. PhD Thesis, Universidad de Buenos Aires (Argentina), pp 1–160
Sylvester F, Dorado J, Boltovskoy D, Juárez A, Cataldo D (2005) Filtration rates of the invasive pest bivalve Limnoperna fortunei as a function of size and temperature. Hydrobiologia 534:71–80
Sylvester F, Boltovskoy D, Cataldo D (2007a) Fast response of freshwater consumers to a new trophic resource: Predation on the recently introduced Asian bivalve Limnoperna fortunei in the lower Parana River, South America. Austral Ecol 32:403–415
Sylvester F, Boltovskoy D, Cataldo D (2007b) The invasive bivalve Limnoperna fortunei enhances benthic invertebrate densities in South American floodplain rivers. Hydrobiologia 589:15–27
Sylvester F, Cataldo D, Notaro C, Boltovskoy D (2013) Fluctuating salinity improves survival of the invasive freshwater golden mussel at high salinity: implications for the introduction of aquatic species through estuarine ports. Biol Invasions 15:1355–1366
Thorp JH, Casper AF (2003) Importance of biotic interactions in large rivers: an experiment with planktivorous fish, dreissenid mussels and zooplankton in the St Lawrence River. River Res Appl 19:265–279
Toresani NI, López HL, Gómez SE (1994) Lagunas de la Provincia de Buenos Aires. Dirección de Intereses Marítimos, Dirección Provincial de Pesca e Intereses Maritimos, Subcretaría de Pesca, Intereses Maritimos y Producciones Intensivas, Ministerio de la Producción de la Provincia de Buenos Aires, La Plata, pp 1–109
Vanderploeg HA, Liebig JR, Carmichael WW, Agy MA, Johengen TH, Fahnenstiel GL, Nalepa TF (2001) Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can J Fish Aquat Sci 58:1208–1221
Vanderploeg HA, Nalepa TF, Jude DJ, Mills EL, Holeck KT, Liebig JR, Grigorovich IA, Ojaveer H (2002) Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can J Fish Aquat Sci 59:1209–1228
van der Velde G, Rajagopal S, bij de Vaate A (2010) From zebra mussels to quagga musels: an introduction to the Dreissenidae. In: van der Velde G, Rajagopal S, bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 1–10
Villar C, Stripeikis J, D’Huicque L, Tudino M, Troccoli O, Bonetto C (1999) Cd, Cu and Zn concentrations in sediments and the invasive bivalves Limnoperna fortunei and Corbicula fluminea at the Rio de la Plata basin, Argentina. Hydrobiologia 416:41–49
Watkins JM, Dermott R, Lozano SJ, Mills EL, Rudstam LG, Scharold JV (2007) Evidence for remote effects of dreissenids mussels on the amphipod Diporea: analysis of Lake Ontario benthic surveys, 1997–2003. J Great Lakes Res 33:642–657
Wetzel RG (1975) Limnology. Saunders, Philadelphia, pp 1–860
Wong WH, Gerstenberger S, Baldwin W, Moore B (2012) Settlement and growth of quagga mussels (Dreissena rostriformis bugensis Andrusov, 1897) in Lake Mead, Nevada-Arizona, USA. Aquat Invasions 7:7–19
Yakovleva AV, Yakovlev VA (2011) Impact of Dreissena polymorpha and Dreissena bugensis on zoobenthos structure in the upper reaches of the Kuybyshev Water Reservoir, Russia. Rus J Biol Invasions 2:312–319
Young SS, Yang HN, Huang DJ, Liu SM, Huang YH, Chiang CT, Liu JW (2014) Using benthic macroinvertebrate and fish communities as bioindicators of the Tanshui River Basin around the greater Taipei area—multivariate analysis of spatial variation related to levels of water pollution. Int J Environ Res Public Health 11:7116–7143
Zhulidov AV, Pavlov DF, Nalepa TF, Scherbina GH, Zhulidov DA, Gurtovaya TY (2004) Relative distributions of Dreissena bugensis and Dreissena polymorpha in the Lower Don River System, Russia. Int Rev Hydrobiol 89:326–333
Zhulidov AV, Kozhara AV, Scherbina GH, Nalepa TF, Protasov A, Afanasiev SA, Pryanichnikova EG, Zhulidov DA, Gurtovaya TY, Pavlov DF (2010) Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biol Invasions 12:1923–1940
Acknowledgments
This work was partially financed by grants from the University of Buenos Aires, Argentina (UBA X-020 and 20020100100035) and from the Argentine Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 2007 1968) to D. Boltovskoy.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Karatayev, A., Boltovskoy, D., Burlakova, L., Padilla, D. (2015). Parallels and Contrasts Between Limnoperna fortunei and Species of Dreissena . In: Boltovskoy, D. (eds) Limnoperna Fortunei. Invading Nature - Springer Series in Invasion Ecology, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-319-13494-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-13494-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13493-2
Online ISBN: 978-3-319-13494-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)