Abstract
Polyhydroxyalkanoates (PHAs), a class of naturally occurring, optically active, aliphatic biopolyesters are gaining tremendous attention as they are the potential substitute to the nonbiodegradable petrochemical-based synthetic plastics. Microorganisms synthesize PHA in the form of intracellular, spherical, nano-sized inclusions, the size and number of which vary depending upon the bacteria. They are potentially useful in industries, with the major application of PHAs being in the medical field, especially in tissue engineering. In recent years, Gram-positive organisms such as Bacillus and Streptomyces species have been recognized as potential strains for commercial scale PHA production as lipopolysaccharide is one of the major contaminants known to copurify when extracted from Gram-negative bacteria.
Isolation of bacteria from diverse coastal and marine arenas carried out to obtain PHA producers revealed the highest heterotrophic bacterial load in the mangrove area. However, the distribution of PHA producers was the highest in the nutrient-limited marine sediment samples. Based on the intensity as well as the duration of maximum PHA production on glucose, 11 Gram-positive, rod-shaped sporulating isolates were selected for further studies. These isolates belonged to the genus Bacillus, with the majority being Bacillus megaterium. Nile blue A staining of the isolates revealed the presence of intracellular PHA granules which exhibited bright orange-red fluorescence when viewed under fluorescent light. Further, characterization of the polymer extracted from these bacteria using Fourier transform infrared (FTIR) spectroscopy confirmed the aliphatic nature of the PHA polymer. Different strains of Bacillus species obtained in the present study can be exploited for the production of PHAs for biomedical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Conventional synthetic plastics derived from petroleum have become an inevitable part of our day to day life. With the exponential growth in human population, the unconditional use of these plastics has led to the accumulation of large amounts of nonbiodegradable waste in the environment. The disposal of these harmful wastes is a serious global problem. Many countries are now trying to overcome this problem by conducting programmes such as solid waste management (Rohini et al. 2006). These include the development of biodegradable plastics to reduce the plastic wastes in the environment. Secondly, the enticement to use biodegradable plastics is also related to the rapid depletion of nonrenewable crude oil resources (Leong et al. 2014; Naik et al. 2008; Philip et al. 2007; Reddy et al. 2008; Suriyamongkol et al. 2007).
Biodegradable plastics are biological polymers that are enzymatically degraded to carbon dioxide and water under aerobic conditions, and to methane and inorganic compounds anaerobically (Naik et al. 2008). They are largely divided into three categories: chemically synthesized polymers, starch-based biodegradable plastics and polyhydroxyalkanoates (PHAs) .
Among the several biodegradable polymers , PHAs, a class of naturally occurring, optically active, aliphatic biopolyesters are currently receiving tremendous attention from both academic and industrial point of view. These microbial bioplastics possess material properties similar to petro-based synthetic plastics such as polypropylene but unlike petroplastics they are completely biodegradable, biocompatible, nontoxic and can be produced using renewable carbon sources (Madison and Huisman 1999; Sheu et al. 2009; Valappil et al. 2007).
PHAs are therefore considered suitable for commercial exploitation and have gained applications in several fields such as medicine, pharmacy, agriculture, food and packaging industry, as raw materials for synthesizing enantiomerically pure chemicals and in the production of paints (Anderson and Dawes 1990; Rawte and Mavinkurve 2001; Rehm 2007; Sudesh et al. 2000).
8.2 Bacterial Polyhydroxyalkanoates and Their Chemical Structure
PHAs are structurally simple macromolecules synthesized by a wide variety of Gram-positive and Gram-negative bacteria including members of the family halobacteriaceae of the archaea (Anderson and Dawes 1990; Brandl et al. 1990; Hezayen et al. 2002; Madison and Huisman 1999; Philip et al. 2007). Marine prokaryotes accumulate PHAs up to 80 % of their dry cell weight (DCW) especially when present in “high-nutrient” econiche (Philip et al. 2007; Valappil et al. 2007; Weiner 1997). Synthesis of PHA occurs when a carbon source is present in excess and one of the essential growth nutrients is limiting (Anderson and Dawes 1990; Madison and Huisman 1999; Rehm 2007).
Polymer production is initiated when acetyl coenzyme A (CoA) is restricted from entering the tricarboxylic acid (TCA) cycle due to nutrient limitation. This results in shunting the acetyl units from the TCA cycle into PHA production (Lenz and Marchessault 2005). The polymer acts as a sink for carbon and reducing equivalents which is mobilized by intracellular depolymerases as soon as the supply of limiting nutrient is restored (Anderson and Dawes 1990; Byrom 1994; Gao et al. 2001; Madison and Huisman 1999).
PHA is deposited in the cell cytoplasm as discrete, insoluble “inclusions or granules” (Fig. 8.1) (Anderson and Dawes 1990; Rehm 2007). Being highly refractile, the granules can be easily visualized using phase contrast light microscope (Dawes and Senior 1973). These granules are lipidic in nature and therefore stained with Sudan black B (Burdon 1946). A more specific dye that binds to PHA is the oxazine as well as the fluorescent oxazone form (Nile red) of Nile blue A . Both Nile blue A and Nile red can also be used to detect PHAs inside the growing bacterial cells (Ostle and Holt 1982; Spiekermann et al. 1999; Wu et al. 2003).
Electron microscopy image of Pseudomonas aeruginosa accumulating polyester granules. (Rehm 2007)
In vivo, PHA is present in an amorphous state (Revol 1989). Extraction of the polyester using organic solvents makes it highly crystalline. The extracted polymer exhibits material properties similar to synthetic plastics such as high molecular weight. The molecular weight of PHA synthesized usually ranges from 2 × 105 to 3 × 106 Daltons (Da) and depends upon the microorganism, carbon source used as well as the growth conditions (Byrom 1994; Madison and Huisman 1999; Ojumu et al. 2004; Sudesh et al. 2000).
8.2.1 Chemical Structure
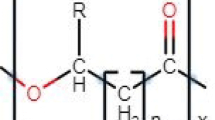
PHAs are made up of 3-hydroxyfatty acid (3HA) monomers that are arranged in a linear, head-to-tail fashion, i.e. the ester bond is formed between the carboxyl group of one monomeric unit with the hydroxyl group of the adjacent monomeric unit. The HA monomers that are incorporated into the polyester through the native cell metabolism are strictly in the (R)-configuration due to the stereospecificity of PHA synthase (Sudesh et al. 2000). This stereoregularity makes the polymer optically active.
The general structure of PHA is shown in Fig. 8.2 (Rehm 2007). The hydroxyl substituted carbon in the (R)-configuration contains an alkyl group that can vary from methyl to tridecyl. The basic unit and most abundant PHA in nature is poly-3-hydroxybutyrate, (PHB). It is the simplest PHA with respect to the chemical structure having a methyl (–CH3) group in the alkyl side chain. The PHB homopolymer is made up of repeating units of (R)-3-hydroxybutyrate (3HB). It is hard and brittle. Incorporation of 3-hydroxyvalerate (3HV) monomers along with (3HB) yields a copolymer P(3HB-co-3HV), which is an elastomer similar to polypropylene. Due to the flexibility of the copolymer, it can be melt processed into a variety of consumer products including plastics, films and fibres (Anderson and Dawes 1990; Madison and Huisman 1999; Rawte and Mavinkurve 2001; Rehm 2007).
Reaction catalyzed by the polyester synthases. (Rehm 2007)
The saturated alkyl side chain of PHA can also be substituted with aromatic, unsaturated, halogenated, epoxidized and branched monomers. Similarly, the position of the hydroxyl group can also be varied to obtain 4-, 5- or 6-hydroxyacid. Some eubacteria are also capable of synthesizing polythioesters using mercaptoacids as a carbon source (Lutke-Eversloh et al. 2001). Overall, the size of the alkyl side chain as well as the monomeric composition determines the material properties of PHA. Therefore, by manipulating these features, new polymers with desired material properties can be synthesized (Anderson and Dawes 1990; Madison and Huisman 1999).
8.2.2 Classification of PHA
PHAs are divided into two broad groups based on the substrate specificity of PHA synthase to accept 3HAs of a certain carbon chain length (Naik et al. 2008; Philip et al. 2007):
-
Short-chain-length (SCL) PHAs
-
Medium-chain-length (MCL) PHAs
The SCL PHAs consist of HA monomers which are composed of 3–5 carbon atoms. These polymers are stiff and brittle. They possess a high degree of crystallinity, lack toughness and show thermoplastic material properties similar to polypropylene. MCL PHAs consist of 6–14 carbon-containing HA monomers and are generally flexible, have low crystallinity, tensile strength and melting point. They are elastomeric in nature, hence opening new opportunities for their application as rubbers and elastomers (Anderson and Dawes 1990; Gagnon et al. 1992; Ojumu et al. 2004; Suriyamongkol et al. 2007).
Recently, bacteria able to synthesize PHAs containing both SCL and MCL monomeric units consisting of 3–14 carbon atoms have been reported. These copolymers have properties in between that of SCL and MCL PHAs depending on the mole ratio of SCL to MCL monomers, further improving their physical and thermal properties. For example, the incorporation of small amounts of MCL monomer, 3-hydroxyhexanoate (3-HHx) into PHB backbone alters the material properties of PHA. The resulting PHA formed is similar to that of low-density polyethylene and therefore suitable for commercial applications (Madison and Huisman 1999; Philip et al. 2007; Yu 2007).
8.3 PHA Biosynthesis
Biosynthesis of PHA in bacteria is divided into three phases (Fig. 8.3) (Steinbuchel and Valentin 1995):
Phases of PHA biosynthesis in bacteria. (Steinbuchel and Valentin 1995)
-
Phase I: Entry of the carbon source from the surrounding environment into the cell either by simple diffusion or a specific membrane transport system.
-
Phase II: Conversion of the carbon source into hydroxyacyl Coenzyme A (CoA) thioesters.
-
Phase III: Polymerization of hydroxyacyl CoA thioester precursors by the key enzyme, PHA synthase to produce PHA.
8.4 Distribution, Isolation and Identification of Bacteria Producing PHA
The biodegradable and biocompatible biopolymer, PHA, has been long since recognized as the potential substitute for the petro-based synthetic plastics (Anderson and Dawes 1990). However, synthesis of this biopolymer at an industrial scale has been limited owing to its high production cost, depending mainly on the bacterial strain used. In addition, the chemical composition of the polymer which greatly influences its material properties is also determined by the type of bacteria synthesizing PHA (Chien et al. 2007; Rawte et al. 2002). Therefore, attempts are now being made to isolate efficient and high yielding bacterial strains which synthesize PHAs with novel monomeric composition within a short incubation period thus cutting down on the overall production cost (Chien et al. 2007).
In the recent years, studies are directed towards exhaustive screening of samples from diverse environments such as soil (Anil Kumar et al. 2007; Halami 2008), activated sludge (Borah et al. 2002; Omar et al. 2001; Reddy et al. 2009), but till date, only a few reports on the isolation of bacteria from marine and mangrove ecosystems are available (Arun et al. 2009; Chien et al. 2007; Rawte et al. 2002; Sathiyanarayanan et al. 2013; Wei et al. 2011). The sediments obtained from these econiches are rich in bacterial flora that can utilize diverse carbon compounds (formed as a result of breakdown of the decomposing detrital matter) for PHA production (Bhosle and Mavinkurve 1980; Matondkar et al. 1980). Interestingly, the PHAs thus synthesized possess novel chemical composition that can be exploited for commercial applications (Weiner 1997). However, the marine environment which provides such a virtually untapped resource for the isolation of novel PHA-producing bacteria has not been explored adequately as yet.
Hence, the present study was undertaken in the quest of isolating potential PHA producers specifically from marine and coastal ecosystems. The sites included coastal beaches, mangrove area and plant leaf litter area in Goa. The sediments collected from these econiches were processed for the determination of total viable count (TVC) of the heterotrophic bacteria. The results of TVC obtained for sediments of coastal beaches were hundred-fold lower than that of mangrove as well as plant leaf litter area. The highest heterotrophic bacterial count of 13.8 × 106 colony forming unit per gram (cfu g−1) dry weight was obtained for the sediment collected from the mangrove area while the lowest count of 16.6 × 104 cfu g−1 sediment dry weight was obtained for one of the coastal beach sediment sample (Caranzalem). Such low heterotrophic bacterial counts in the beach sediment samples as compared to that of marine (Palaniappan and Krishnamurthy 1985) and mangrove sediments (Matondkar et al. 1981) have also been reported by Prabhu et al. (1990).
So far, there is only one report on the isolation of PHA-producing bacteria isolated from marine and mangrove ecosystems in Goa (Rawte et al. 2002). In this report, higher heterotrophic bacterial load has been demonstrated in the mangrove sediments and lower bacterial counts in the coastal beach sediment samples. The fluctuation of the bacterial population in these ecological niches could be attributed to various environmental factors in such econiches, widely differing from each other (Nair and LokaBharathi 1980). For example, in the mangrove area, there is a continuous leaf fall, which is being degraded and mineralized resulting in the source of nutrients for microorganisms. This is reflected as high load of bacteria possessing diverse hydrolytic enzymes which are involved in the degradation process (Bhosle and Mavinkurve 1980; Matondkar et al. 1981; Rawte et al. 2002). Hence, such an ecosystem can be expected to be rich in PHA-accumulating bacteria. The heterotrophic bacterial population present in the marine sediments plays a significant role also in nutrient and energy cycle but the bacterial load is reported to decline with depth, lowest counts being obtained at a depth below 1000 m (Nair et al. 1989; ZoBell 1963). The lower TVC obtained in the case of sediments collected from various coastal beaches could be attributed to the sandy nature of these sediments. Higher bacterial counts have been reported by Nair et al. (1978) in clay and clayey-sand sediments rather than fine sand, suggesting that the bacterial population possess a negative relationship with the particle size and a significant direct relation to the organic matter.
Further, random selection of the culturally dissimilar colonies obtained from the respective sediments was carried out. The percentage variation of the total isolates selected from different econiches was found to be the highest in samples obtained from marine sediments and minimum in coastal beach sediments (Fig. 8.4a). Similar observation on the percentage distribution of Gram-positive isolates indicated maximum number of these organisms obtained from marine sediments and minimum number from coastal beach sediments (Fig. 8.4b). The predominance of Gram-positive bacteria in the marine sediments has also been reported by Palaniappan and Krishnamurthy (1985) and Rawte et al. (2002). The percentage distribution of the bacterial population in the beach sediment samples has also been reported to be greatly affected by factors such as the moistening of beaches by rain water, river influence and human activities (Nair and LokaBharathi 1980).
Finally, screening of all the isolates obtained from these diverse econiches for PHA production was carried out. Interestingly, the percentage distribution of the total number of PHA producers showed a slightly different trend. In this case, the highest percentage of PHA producers was obtained from the marine sediments and that of the mangrove sediment was found to be the lowest (Fig. 8.4c) perhaps because mangroves are a nutrient-rich ecosystem (Matondkar et al. 1980; Rawte et al. 2002). PHA serves as a carbon and energy reserve, the accumulation of PHA offers a selective advantage for the survival of bacteria in samples with low nutrients (Lopez et al. 1995). The higher percentage of PHA producers in the marine sediments suggests the scarcity of nutrients in this ecosystem creating unbalanced nutritional conditions ideal for the growth of PHA-accumulating bacteria.
The bacterial isolates that exhibited maximum PHA accumulation using glucose as a sole source of carbon and for a long duration of time were selected for further studies. These included five Gram-positive, rod-shaped, sporulating bacterial strains each from coastal beaches and plant leaf litter sediments and only one Gram-positive, rod-shaped, sporulating bacterial isolate from the mangrove sediment.
Identification of all these isolates was carried out as per the cultural, morphological and biochemical tests described in Priest et al. (1988) and Bergey’s Manual of Systematic Bacteriology (Sneath et al. 1986). The data were analyzed numerically, using the simple matching coefficient (SSM). Clustering was achieved by unweighted pair group average linkage (UPGMA). The computations were performed by using Probiosys program.
From the results obtained, all the selected isolates were tentatively identified to species level, with eight of the isolates closely related to Bacillus megaterium and one isolate to Bacillus licheniformis. However, the remaining two isolates were identified only up to the genus level, i.e. Bacillus sp. Microscopic staining of the isolates using Nile blue A was carried out to detect the presence of intracellular PHA granules. These granules exhibited bright orange–red fluorescence when viewed under fluorescent light (Fig. 8.5). This microscopic staining method has also been used for the preliminary screening of bacterial isolates accumulating PHA by Rawte et al. (2002) as well as for visualization of intracellular PHA inclusions during the kinetic studies of growth (McCool et al. 1996). McCool et al. (1996) have also determined the PHA quantity inside the cells of Bacillus megaterium during exponential and stationary phases of growth and indicated this staining method to be a reliable technique for PHA estimation.
Fourier transform-infrared (FTIR) spectra of PHA purified from these Bacillus isolates exhibited a strong band at 1743 cm−1 corresponding to ester carbonyl (C = O) stretching frequency (Fig. 8.6). The bands at 2980–3027 and 1219–1392 cm−1 represented the typical C–H stretching and bending vibrations of the aliphatic portion of the compound, respectively. A distinct broadband at 3440 cm−1 indicated the free O–H stretching of the polymer end groups. Moreover, the infrared (IR) spectra of these polymers were found to be super-imposable with that reported previously (Pal and Paul 2001). Hence, the FTIR analysis revealed that the polymer produced in these Bacillus isolates was aliphatic in nature. Characterization of PHA using FTIR analysis has also been reported by Arun et al. (2009); Prabhu et al. (2009) and Rohini et al. (2006). Further, purification of the PHA polymer along with the intact bilayer membrane, i.e. the “native” PHA granules was achieved using sucrose density gradient. Figure 8.7 reveals the scanning electron micrograph (SEM) of purified native PHA granules from isolate NQ-11/A2 (Prabhu et al. 2010).
8.5 Conclusions and Future Prospects
The present study was initiated to isolate bacteria from different marine and coastal arenas with the hope of obtaining PHA producers, potentially useful for industrial applications. Out of the several isolates obtained, five Gram-positive, rod-shaped sporulating isolates each from coastal beaches and plant leaf litter area and one Gram-positive, rod-shaped sporulating isolate from mangrove area. Biochemical identification of these isolates revealed that all of them belonged to the genus Bacillus, with the majority being Bacillus megaterium . Nile blue A staining of all the 11 isolates revealed the presence of intracellular PHA granules. FTIR analysis confirmed the aliphatic nature of the polymer produced by these bacteria.
The major application of PHA being in the medical field, especially in tissue engineering needs the polymer to be free from any contaminating substances like the endotoxins. Lipopolysaccharide (LPS) is one of the major contaminants known to copurify along with PHA when extracted from Gram-negative bacteria. Hence, in the recent years, Gram-positive organisms such as Bacillus and Streptomyces species have been recognized as potential strains for commercial scale PHA production. Different strains of Bacillus species obtained in the present study could be exploited for the production of PHA in biomedical applications.
References
Anderson, A. J., & Dawes, E. A. (1990). Occurrence, metabolism, metabolic role and Industrial uses of bacterial polyhydroxyalkanoates. Microbiology Reviews, 54, 450–472.
Anil Kumar, P. K., Shamala, T. R., Kshama, L., Prakash, M. H., Joshi, G. J., Chandrashekar, A., Lathakumari, K. S., & Divyashree, M. S. (2007). Bacterial synthesis of poly (hydroxybutyrate-co-hydroxyvalerate) using carbohydrate rich mahua (Madhuca sp) flowers. Journal of Applied Microbiology, 103, 204–209.
Arun, A., Arthi, R, Shanmugabalaji, V., & Eyini, M. (2009). Microbial production of poly-β-hydroxybutyrate by marine microbes isolates from various marine environments. Bioresource Technology, 100, 2320–2323.
Bhosle, N. B., & Mavinkurve, S. (1980). Hydrocarbon utilizing microorganisms from Dona Paula Bay, Goa. Marine Environmental Research, 4, 53–58.
Borah, B., Thakur, P. S., & Nigam, J. N. (2002). The influence of nutritional and environmental conditions on the accumulation of poly-β-hydroxybutyrate in Bacillus mycoides RLJ B-017. Journal of Applied Microbiology, 92, 776–783.
Brandl, H., Gross, R. A., Lenz, R. W., & Fuller, R. C. (1990). Plastics from bacteria and for bacteria: Poly(beta-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. Advances in Biochemical Engineering/Biotechnology, 41, 77–93.
Burdon, K. L. (1946). Fatty material in bacteria and fungi revealed by staining dried, fixed slide preparations. Journal of Bacteriology, 52, 665–678.
Byrom, D. (1994). Polyhydroxyalkanoates. In: D. P. Mobley (Ed.), Plastic from microbes: Microbial synthesis of polymers and polymer precursors (pp. 5–33). Munich: Hanser.
Chien, C-C., Chen, C-C., Choi, M-H., Kung, S-S., & Wei, Y-H. (2007). Production of poly-β-hydroxybutyrate (PHB) by Vibrio spp. isolated from marine environment. Journal of Biotechnology, 132, 259–263.
Dawes, E. A., & Senior, P. J. (1973). The role and regulation of energy reserve polymers in micro-organisms. Advances in Microbial Physiology, 10, 135–266.
Gagnon, K. D., Lenz, R. W., Farris, R. J., & Fuller, R. C. (1992). Crystallization behaviour and its influence on the mechanical properties of a thermoplastic elastomer produced by Pseudomonas oleovorans. Macromolecules, 25, 3723–3728.
Gao, D., Maehara, A., Yamane, T., & Ueda, S. (2001). Identification of the intracellular polyhydroxyalkanoate depolymerase gene of Paracoccus denitrificans and some properties of the gene product. FEMS Microbiology Letters, 196, 159–164.
Halami, P. M. (2008). Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World Journal of Microbiology and Biotechnology, 24, 805–812.
Hezayen, F. F., Steinbüchel, A., & Rehm, B. H. A. (2002). Biochemical and enzymological properties of the polyhydroxybutyrate synthase from the extremely halophilic archaeon strain 56. Archives Biochemistry and Biophysis, 403, 284–291.
Lenz, R. W., & Marchessault, R. H. (2005). Bacterial polyesters: Biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules, 6, 1–8.
Leong, Y. K., Show, P. L., Ooi, C. W., Ling, T. C., & Lan, J. C. (2014). Current trends in polyhydroxyalkanoates (PHAs) biosynthesis: Insights from the recombinant Escherichia coli. Journal of Biotechnology , 180, 52–65.
Lopez, N. I., Floccari, M. E., Steinbuchel, A., Garcia, A. F., & Mendez, B. S. (1995). Effect of poly(3-hydroxybutyrate) (PHB) content on the starvation of bacteria in natural waters. FEMS Microbiology Ecology, 16, 95–102.
Lutke-Eversloh, T., Bergander, K., Luftmann, H., & Steinbuchel, A. (2001). Identification of a new class of biopolymer: bacterial synthesis of a sulfur analogue to poly(3-hydroxybutyrate) (PHB). Microbiology, 147, 11–19.
Madison, L. L., & Huisman, G. W. (1999). Metabolic engineering of poly(3-Hydroxyalkanoates): From DNA to plastic. Microbiology and Molecular Biology Reviews, 63, 21–53.
Matondkar, S. G. P., Mahtani, S., & Mavinkurve, S. (1980). The fungal flora of the mangrove swamps of Goa. Mahasagar-Bulletin of National Institute of Oceanography, 13, 281–283.
Matondkar, S. G. P., Mahtani, S., & Mavinkurve, S. (1981). Studies on mangrove swamps of Goa: Heterotrophic bacterial flora from mangroves swamps. Mahasagar-Bulletin of National Institute of Oceanography, 14, 325–327.
McCool, G. J., Fernandez, T., Li, N., & Cannon, M. C. (1996). Polyhydroxyalkanoate inclusion-body growth and proliferation in Bacillus megaterium. FEMS Microbiology Letters, 138, 41–48.
Naik, S., Venu Gopal, S. K., & Somal, P. (2008). Bioproduction of polyhydroxyalkanoates from bacteria: A metabolic approach. World Journal of Microbiology and Biotechnology, 24, 2307–2314.
Nair, S., & LokaBharathi, P. A. (1980). Heterotrophic bacterial population in tropical sandy beaches. Mahasagar-Bulletin of the National Institute of Oceanography, 13, 261–267.
Nair, S., LokaBharathi, P. A., & Achuthankutty, C. T. (1978). Distribution of heterotrophic bacteria in marine sediments. Indian Journal of Marine Science, 7, 18–22.
Nair, S., LokaBharathi, P. A., & Achuthankutty, C. T. (1989). Heterotrophic bacteria and biochemical activities off the west coast of India. Mahasagar-Bulletin of the National Institute of Oceanography, 12, 75–81.
Ojumu, T. V., Yu, J., & Solomon, B. O. (2004). Production of polyhydroxyalkanoates, a bacterial biodegradable polymer. African Journal of Biotechnology, 3, 18–24.
Omar, S., Rayes, A., Eqaab, A., Vob, I., & Steinbuchel, A. (2001). Optimization of cell growth and poly (3-hydroxybutyrate) accumulation on date syrup by a Bacillus megaterium strain. Biotechnology Letters, 23, 1119–1123.
Ostle, A. G., & Holt, J. G. (1982). Nile blue A as a Fluorescent stain for poly-b-hydroxybutyrate. Applied Environmental Microbiology, 44, 238–241.
Pal, S., & Paul, A. K. (2001). Studies of poly(3-hydroxybutyric acid) inclusions in whole cells of Azotobacterchroococcum MAL-201. Current Science, 81, 210–212.
Palaniappan, R., & Krishnamurthy, K. (1985). Heterotrophic bacteria in near shore waters of Bay of Bengal and the Arabian Sea. Indian Journal of Marine Science, 14, 113–114.
Philip, S., Keshavarz, T., & Roy, I. (2007). Polyhydroxyalkanoates: biodegradable polymers with a range of applications. Journal of Chemical Technology and Biotechnology, 82, 233–247.
Prabhu, S. K., Subramanian, B., & Mahadevan, A. (1990). Occurrence and distribution of heterotrophic bacteria off Madras coast. Mahasagar-Bulletin of the National Institute of Oceanography, 23, 43–47.
Prabhu, N. N., Santimano, M. C., & Garg, S. (2009). Studies on polyhydroxyalkanote production by a marine Bacillus sp. NQ-11/A2 isolated from continental shelf sediment. Journal of Current Sciences, 14, 265–273.
Prabhu, N. N., Santimano, M. C., Bhosle, S. N., Mavinkurve, S., & Garg, S. (2010). Native granule associated SCL-PHA synthase from a marine derived Bacillus sp. NQ-11/A2. Antonie van Leeuwenhoek, 97, 41–50.
Priest, F.G., Goodfellow, M., Todd, C. (1988). A numerical classification of the genus Bacillus. Journal of General Microbiology, 134, 1847–1882.
Rawte, T., & Mavinkurve, S. (2001). Biodegradable plastics—Bacterial polyhydroxyalkanoates. Indian Journal of Microbiology, 41, 233–245.
Rawte, T., Padte, M., & Mavinkurve, S. (2002). Incidence of marine and mangrove bacteria accumulating polyhydroxyalkanoates on the mid-west coast of India. World Journal of Microbiology and Biotechnology, 18, 655–659.
Reddy, S. V., Thirumala, M., Reddy, T. V. K., & Mahmood, S. K. (2008). Isolation of bacteria producing polyhydroxyalkanoates (PHA) from municipal sewage sludge. World Journal of Microbiology and Biotechnology, 24, 2949–2955.
Reddy, S. V., Thirumala, R. M., & Mahmood, S. K. (2009). A novel Bacillus sp. accumulating poly (3-hydroxbutyrate-CO-3-hydroxyvalerate) from a single carbon substrate. Journal of Indian Microbiology and Biotechnology, 36, 837–843.
Rehm, B. H. A. (2007). Biogenesis of microbial Polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bioparticles. Current Issues in Molecular Biology, 9, 41–62.
Revol, J. F., Chanzy, H. D., Deslandes, Y., & Marchessault, R. H. (1989). High resolution electron microscopy of poly(β-hydroxybutyrate). Polymer, 30, 1973–1976.
Rohini, D., Phadnis, S., & Rawal, S. K. (2006). Synthesis and characterization of poly-β-hydroxybutyrate from Bacillus thuringiensis R1. Indian Journal of Biotechnology, 5, 276–283.
Sathiyanarayanan, G., Kiran, G. S., Selvin, J., & Saibaba, G. (2013). Optimiation of polyhydroxybutyrate production by marine Bacillus megateriumMSBN04 under solid state culture. International Journal of Biological Macromolecules, 60, 253–261.
Sheu, D-S., Lai, Y-W., Chang, R-C., & Chen, W-M. (2009). Detection of polyhydroxyalkanoate synthase activity on a polyacrylamide gel. Analytical Biochemistry, 393, 62–65.
Sneath, P. H. A., Mair, N. S., Sharpe, M. E., & Holt, J. G. (1986). Bergey’s manual of systematic bacteriology (Vol. 2, 2nd ed., pp. 1104–1129). Baltimore: Williams and Wilkins.
Spiekermann, P., Rehm, B. H., Kalscheuer, R., Baumeister, D., & Steinbuchel, A. (1999). A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Archives of Microbiology, 171, 73–80.
Steinbuchel, A., & Valentin, H. E. (1995). Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiology Letters, 128, 219–228.
Sudesh, K., Abe, H., Doi, Y. (2000). Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Progess in Polymer Science, 25, 1503–1555.
Suriyamongkol, P., Weselake, R., Narine, S., Moloney, M., & Shah, S. (2007). Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants—a review. Biotechnology Advances, 25, 148–175.
Valappil, S. P., Boccaccini, A. R., Bucke, C., & Roy, I. (2007). Polyhydroxyalkanoates in Gram-positive bacteria: Insights from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek, 91, 1–17.
Wei, Y. H., Chen, W. C., Wu, H. S., & Janarthanan, O. M. (2011). Biodegradable and biocompatible biomaterial, polyhydroxybutyraate, produced by and indigenous Vibrio sp. BM-1 isolated from marine environment. Marine Drugs, 9, 615–624.
Weiner, R. M. (1997). Biopolymers from marine prokaryotes. Tibtech, 15, 390–427.
Wu, H. A., Sheu, D. S., & Lee, C. Y. (2003). Rapid differentiation between short-chain-length and medium-chain-length polyhydroxyalkanoate-accumulating bacteria with spectrofluorometry. Journal of Microbiological Methods, 53, 131–135.
Yu, J. (2007). Microbial production of bioplastics from renewable resources. In S.-T. Yang (Ed.), Bioprocessing for value-added products from renewable resources (pp. 585–610). Amsterdam: Elservier B.V.
ZoBell, C. E. (1963). Domain of the marine microbiologist. In C. H. Oppenheimer (Ed.), Symposium on marine microbiology (pp. 3–24). Thomas: Springfield.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Prabhu, N. (2015). Heterotrophic Bacteria Producing Polyhydroxyalkanoates: A Biodegradable Polymer. In: Borkar, S. (eds) Bioprospects of Coastal Eubacteria. Springer, Cham. https://doi.org/10.1007/978-3-319-12910-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-12910-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12909-9
Online ISBN: 978-3-319-12910-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)