Abstract
Mycoremediation of dye-bearing effluents has gained considerable importance in the last decade. However, a deep understanding of the dye removal mechanism as well as the optimization of the dye removal process is essential while designing the operational strategy for mycoremediation. This chapter describes the recent advancements based on the use of various analytical as well as statistical tools in elucidating the mechanism of dye removal process and optimizing the conditions for efficient removal. In order to connote mycoremediation at industrial level, various reactor designs and management of dye-laden fungal biomass have been discussed. The chapter concludes with the potential of various innovations such as the microbial formulations that shall prove handy in translating mycoremediation at industrial scale.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- High Performance Liquid Chromatography
- Response Surface Methodology
- Taguchi Method
- Fungal Biomass
- Lignin Peroxidase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Dye bearing industrial effluent can cause environmental problems unless it is properly treated before disposal. The complex aromatic structure of the dyes is resistant to light, biological activity, ozone and other degradative environmental conditions. Thus, conventional wastewater treatment is less effective. There are various methods for the treatment of wastewater (Forgacs et al. 2004), which broadly fall into three categories: physical (adsorption, coagulation /flocculation , membrane filtration etc.), chemical (chemical oxidation, photo-catalytic oxidation , electrolysis, Fenton reagent etc.) and biological (biosorption, enzymatic degradation etc.). Present treatment processes are largely based on the principles of flocculation with lime and ferrous sulphate, adsorption on activated carbon , nano-filtration, reverse osmosis and solar evaporation (Ranganathan et al. 2007). But owing to their high maintenance cost, prerequisite for preliminary treatment steps and land requirement, these are not economically viable for small enterprises and hence, there is a need to look for suitable decentralized technologies. Being eco-friendly, microbial decolorization (through bacteria, fungi and algae) is receiving much attention for the treatment of textile dye waste water (Sarayu and Sandhya 2012).
Considering the choice of microbes and the characteristics of textile effluent, fungi can be considered as the most suitable organism for the remediation purpose. Owing to their high cell-to-surface ratio, these organisms have a greater contact with the environment. Also, the extra-cellular nature of fungal enzymes allows them to thrive and tolerate high concentrations of toxicants. The fungi have a strong potential for mycoremediation in non-sterile open environment (Fig. 1). The mycelial growth gives a competitive advantage over single cells, such as bacteria and yeasts, especially with respect to the colonization of insoluble substrates. They have high surface-to-cell ratio characteristics of filaments that maximize both mechanical and enzymatic contact with the substrate. The extracellular nature of the degradative enzymes enables fungi to tolerate higher concentrations of toxic chemicals than what would be possible, if these compounds had to be brought into the cell. In this case, insoluble compounds, that cannot cross a cell membrane, are also susceptible to attack.
Simple model of fungal action on naturally-occurring and/or anthropogenically—derived organic and inorganic substrates (modified after Gadd 2007)
A lot of research has focused on mycoremediation, which could offer an attractive decentralized system. However, there has not been much success in translating these results into actual applications. A critical insight into the mechanism or pathways of dye transformation as well as process optimization is important while designing the operational strategy for mycoremediation. Several analytical and statistical tools have been described in the recent studies to achieve this. Moreover, some studies focus on the appropriate bioreactor design as per the underlying mechanism of fungal dye decolorization , while a very few investigations are available on the management of the dye laden fungal biomass. This chapter describes the recent innovations and vital advancements in mycoremediation targeting the ease of application.
2 Mechanism of Dye Removal by Fungi
Researchers have been employing various fungi as listed in Table 1, for dye removal studies either in living or dead form (Kaushik and Malik 2009). Three principal mechanisms are involved during the dye removal process mediated by fungi; biosorption, bioaccumulation and biodegradation. Biosorption is a metabolically independent process which involves the binding of solutes to the fungal biomass and thus can occur in either living or dead biomass (Srinivasan and Viraraghavan 2010). Biodegradation is an energy intensive and metabolically dependent process, where the complex dye molecules are broken down into simpler molecules through the action of certain enzymes. Bioaccumulation is also energy and metabolically dependent process, where actively growing cells accumulate the pollutants inside their cytoplasm (Chojnacka 2010).
2.1 Dye Biosorption
Left over spent fungal biomass, which is a by-product of industrial fermentations, is a very good and cheap source to be used in extensive use for dye biosorption (Fomina and Gadd 2014). Various functional groups , that are present on the fungal cell wall i.e. amino, carboxyl, thiol and phosphate groups, can bind dye molecules (Tan et al. 2011; Fan et al. 2012). Biosorption of dye molecules onto the surface of fungal cells is a quick process and often gets completed in a few hours. Dye biosorption process is also affected by various process parameters, such as pH, temperature, initial dye concentration and type of dye present in the solution (Srinivasan and Viraraghavan 2010). Therefore, to obtain efficient dye removal, it is necessary to optimize various process parameters. Moreover, selection of a fungal strain for dye biosorption should be made in a way that it is capable of removing wide variety of dyes belonging to different classes.
The biosorption capacity of biomass can be further increased by certain physical (drying, autoclaving) or chemical (organic, inorganic) pre-treatments (Viraraghavan and Srinivasan 2011). Immobilization in alginate beads (Prigione et al. 2008) or loofa-sponge (Iqbal and Saeed 2007) has been reported to enhance biosorption capacity. Biosorbent can be regenerated by treatment with certain chemicals, such as alkalis, chelating agents etc. Recovery of the adsorbent and dye makes the treatment process more economical. It is observed that most of the studies are performed with the help of dye solutions. However, mixed effluent from textile industries containing mixtures of dyes and certain other chemicals may interfere with the process of dye removal through biosorption. Therefore, more studies should be performed utilizing the mixtures of dyes and industrial effluent.
2.2 Dye Bioaccumulation
Majority of the reports, that report bioaccumulation as the principle mechanism for dye removal, are focused on the use of single cell fungi (Dias et al. 2010; Das et al. 2011) and cyanobacteria (Silva-Stenico et al. 2012). However, a few studies report bioaccumulation by mycelial fungi, such as A. niger (Taskin and Erdal 2010) and P. oxalicum (Xin et al. 2010). Bioaccumulation of dyes by fungi is mediated by initial biosorption to the fungal cell wall which is metabolism-independent and then accumulation into the cytoplasm which is metabolism-dependent (Wang and Hu 2008).
2.3 Dye Biodegradation
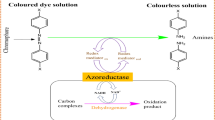
Biodegradation is described as the breakdown of chemical compounds which is mediated by the action of biological enzymes. Complete biodegradation is the total breakdown of organic molecules into water, carbon dioxide and/or any other inorganic end products which is known as mineralization . White-rot fungi secrete certain ligninolytic enzymes that bind non-specifically to the substrate. Therefore, they are capable of degrading a wide variety of recalcitrant compounds and complex mixtures of pollutants, such as dyes. Since these ligninolytic enzymes secreted by fungi are extracellular, therefore, problem related to substrate diffusion limitation into the cell, which is commonly encountered in bacteria, is not observed in fungi. Also, enzyme secretion by white-rot fungi depends on nutrient limitation (nitrogen or carbon) and is not altered by the presence of pollutants. This implies that for dye removal through biodegradation as the principle mechanism, acclimatization of the fungi with the pollutants may be skipped (Ge et al. 2004). Laccase, Manganese Peroxidase (MnP), Manganese Independent Peroxidase (MIP) , Lignin Peroxidase (LiP) , Tyrosinase etc. are the various enzymes that are involved in the degradation of the dye.
For biodegradation, fungal cells have to be in their growing form. This limits their application in treatment of toxic dye effluents . Nevertheless, the enzyme activity is often not altered by the presence of other pollutants, but biosorption as a process is influenced by the factors, like ionic strength, ionic state of dye and biosorbent. Thus, biodegradation, as a means for dye removal, has its own advantages. The growth of the fungus, enzyme production and subsequent dye decolorization are effected by the culture conditions, like initial dye concentration , pH, agitation , media components, presence of heavy metals etc. (Martorell et al. 2012; Daâssi et al. 2013; Jin and Ning 2013; Moreira-Neto et al. 2013). Growth of the fungus is also affected by the nutritional conditions of the environment. Table 2 summarizes the key differences among three principle mechanisms for dye removal process. It is important to note that during the process of dye removal through fungi, multiple mechanisms may be operative either simultaneously or sequentially. For example, removal of Acid Navy Blue by A. lentulus pre dominantly followed bioaccumulation mechanism , while biosorption played a minor role (Kaushik and Malik 2010, 2013). Also, each mechanism i.e. biosorption, bioaccumulation or biodegradation, has its own advantages and limitations. Therefore, it is always beneficial to use a consortium of microbes for such treatment processes over pure cultures, as it promotes the dye removal process through multiple mechanisms, making it more efficient in terms of percentage removal, time required and supplementation needs.
3 Analytical Tools to Study Dye Removal Process
Monitoring of dye removal process is based on the study of dye concentration at a given time or by estimating the production of metabolites/intermediates or dye by products. Dye concentration can be estimated through various measurements, such as Total Organic Carbon (TOC), Chemical Oxygen Demand (COD) or by measuring absorbance of the solution at the absorption maxima of the dye with a UV-Vis spectrometer. However, these techniques are not selective in nature and affected by other contaminants that may be present in the solution. More specific analytical techniques have been used by the researchers to monitor the dye removal process as well as to determine the dye intermediates that are released as a result of degradation process. The choice of analytical technique depends on the type of dye removal process involved and type of the dye. These analytical techniques can be categorized into two types: separation techniques and spectroscopic techniques . Separation techniques include various chromatographic techniques , such as High Performance Liquid Chromatography (HPLC) , Gas Chromatography Mass Spectrometry (GCMS) , Liquid Chromatography Mass Spectrometry (LCMS) , High Performance Thin Layer Chromatography (HPTLC) etc. which help in the identification of the intermediate compounds released during the dye removal process as well as the final degradation products formed as a result of biodegradation. Spectroscopic techniques , such as UV-Vis spectroscopy, have been widely used by the researchers to quantify the dye removal process in terms of reduction brought about in the absorbance value (at absorption maxima) of the dye. In addition to this, Fourier Transform Infra-Red Spectroscopy (FTIR) and Nuclear magnetic Resonance (NMR) have also been utilized as an essential tool for estimating the dye degradation pathway. A recent review by Fernández et al. (2010) has listed the advantages and limitations of these two types of analytical techniques in Table 3.
Apart from these techniques, enzymatic assays, that involve the detection of various dye degrading enzymes, have been used by the researchers to confirm biodegradation process during dye removal. Various white-rot fungi , such as Trametes versicolor , Phanerochaete chrysosporium , Pleurotus ostreatus etc., have been reported to produce extracellular ligninolytic enzymes , such as lignin peroxidase (LiP) , laccase, manganese peroxidase (MnP) etc. which cause dye degradation. The assay to detect the presence of an enzyme is based on either of the following two principles: disappearance of the substrate or the appearance of the new product, essentially a colored compound which can be quantified by spectroscopic techniques .
Table 4 shows various studies which adopted described analytical techniques in examining the dye removal process. Recently, use of high performance capillary electrophoresis has gained a lot of importance (Zhao et al. 2007; Lu et al. 2008) in determining the dye and their intermediates or by-products (which have ionic character). During dye degradation process, the dye peak undergoes a continuous change. Initially, it decreases and then certain new peaks appear which later on disappear due to the formation of small undetectable compounds. Use of a MS detector helps in a more accurate identification of such compounds. For the study of intermediate compounds from sulfonated azo dyes , electro spray ionization (ESI) source can be employed which causes minimal fragmentation of such dyes (Zhao et al. 2007; Lu et al. 2008; Gomi et al. 2011).
Apart from monitoring the dye removal process, the morphological changes in fungal biomass can also be a useful aid in establishing the correlation between dye removal process and the microbial agent present in the solution. Various microscopic techniques , such as light microscopy, scanning and transmission electron microscopy , may provide useful insights into the mechanism involved in the dye removal process. Biosorption or bioaccumulation process by fungal biomass can conclusively be explained through light microscopy techniques. Figure 2 shows the phase contrast micrographs of Acid Navy Blue laden pellets of Aspergillus lentulus . The size variation in the pellet structure, if any, resulting from various cultivation conditions, such as nutrient sources, can also be visualized through microscopy (Kaushik and Malik 2010b).
Microscopic pictures of A. lentulus grown in different initial glucose concentrations: a 0 %, b 0.1 %, c 0.2 %, d 0.5 % and e 1 % on the pellet (Magnification: 10X) (Kaushik and Malik 2010b)
Chakraborty et al. (2013) used light microscopy to show the biosorption of Congo red dye on the biomass of Alternaria alternate. Also, SEM micrographs were used by Chakraborty et al. (2013) to describe the amorphous nature of the fungal biomass after dye removal process. Similarly, Transmission electron microscopy can also be utilized to examine the difference obtained in the morphological structure of the fungi prior to and after the biosorption of dye. Das et al. (2006) demonstrated with the help of TEM micrographs that the cells of Rhizopus oryzae in presence of Rhodamine B dye exhibit electron dense molecules mainly in the region of cell surfaces, whereas these are absent in control cells. In living cells, the dye molecules accumulate in the cytoplasm as granules, whereas, in starved cells, dye molecules mainly bind on the cell surface and a very small amount is transported to the cytoplasm.
Moreover, any toxicity response , exhibited by the fungi towards the test dye, also becomes evident through these techniques. Figure 3 shows the difference in the mycelial structure of the fungus Aspergillus lentulus which was grown in the presence of various dyes (Kaushik and Malik 2013). The broad and flattened hyphae exhibited by A. lentulus in presence of dye Methylene Blue as compared to that shown in the presence of Acid Navy Blue and in absence of any dye, shows the toxicity of Methylene Blue dye to the fungi. SEM or TEM technique coupled with Energy Dispersive X-Ray (SEM-EDX/TEM-EDX) can be a useful tool for estimating and quantifying the presence of dye molecules on/inside the fungal biomass after dye biosorption (Kaushik 2011).
SEM micrographs showing fungal pellets grown in different conditions a presence of Methylene Blue (10 mg l−1), b presence of Methylene Blue (50 mg l−1), c presence of Acid Navy Blue (100 mg l−1) and d absence of dye (Magnification: X 5,000) (Kaushik and Malik 2013)
Thus, it can be concluded that the analytical techniques are an important tool to study the dye removal process and utilization of these techniques in combination can provide a detailed insight into the process of dye removal, its mechanism, dye degradation pathway and study of degradation metabolites. This can further aid in the development of suitable reactor design /technology for the treatment dye effluents.
4 Statistical Tools Required for Process Optimization
Design of experiment (DOE) is an important tool to study any process involving multiple variables that affect it. Through this approach, a process under study is described through a mathematical model and an experimental design is created to obtain a set of experiments to collect the data to be analyzed using the model equations. DOE approach enables the researcher to optimize the conditions for maximizing the process and aids in selecting the principle factor affecting it. DOE methodology is superior to the conventional approach of one variable at a time (OVAT) analysis, being less labour intensive and time consuming. It has an added advantage of providing the interaction studies of various variables affecting the process which is not possible through OVAT analysis . The two most widely applied tools of DOE utilized in dye removal process are Taguchi method and response surface methodology (Fig. 4).
4.1 Response Surface Methodology (RSM)
RSM is a method that utilizes statistical and mathematical techniques to optimize a process in which the output or response is influenced by different factors or variables. RSM analyzes the effect of independent variables alone and in combination and generates a mathematical model (Bas and Boyaci 2007). The term independent variables refer to the experimental variables that can be changed independently of each other. In a typical dye removal process, these variables can be pH, temperature, initial dye concentration , nutrient concentration, contact time etc. A response is defined as a dependent variable which is measured as an output of the experiment. Percentage dye removal, residual dye concentration and biomass generated can be regarded as the response of a dye removal process. To obtain the best response value of dye removal process, the independent variables need to be optimized (Kaushik and Malik 2009). The optimization study using RSM can be divided into three stages. In the first stage, the independent variables and their levels are determined. Screening experiments are performed to determine the important parameters that influence the process of dye removal. Further, depending upon the direction in which these parameters affect the process, their levels (ranging from -1 to +1) are determined. In the second stage, the experimental design is selected and the model equation is predicted and verified. Generally a full quadratic equation (second order) is used in RSM.
In the third stage, responses of surface plot and contour plot are obtained and their optimum points are determined (Bas and Boyaci 2007). Many researchers have employed RSM to optimize the dye removal process by fungi in growing mode in terms of initial dye concentration (Alam et al. 2009; Gönen and Aksu 2009a; Sharma et al. 2009; Srinivasan and Murthy 2009; Das et al. 2010), pH (Sharma et al. 2009; Qu et al. 2010), temperature (Sharma et al. 2009; Qu et al. 2010) and nutritional conditions (Gönen and Aksu 2009b; Kaushik and Malik 2011; Aghaie-Khouzani et al. 2012; Papadopoulou et al. 2013). However, certain factors must be considered while selecting RSM for biological processes. For example, it is not necessary that all the systems, that show curved graph, fit to second order polynomial and thus require to be converted to other forms, such as the log values or by changing the range of parameters (Bas and Boyaci 2007). This limits its use in those biological processes which cannot be described by a second order polynomial equation .
4.2 Taguchi Method
Taguchi method was initially developed as a tool for improving the quality in engineering methodology and obtaining a robust design (Wang et al. 2002). However, it has been also employed to optimize the condition of dye removal process (Engin et al. 2008; Yildiz 2008). Daneshvar et al. (2007) applied Taguchi method in optimizing the process of biological degradation of Malachite Green with respect to temperature, initial pH, type of algae, dye concentration and time of reaction. Revankar and Lele (2007) optimized the fermentation medium for Ganoderma sp. to obtain the maximum removal of amaranth dye using Taguchi methodology.
Taguchi method of statistical optimization involves fractional factorial experimental design which is a part of total possible combinations which are required to estimate the important factors affecting the process and their interaction (Kim et al. 2004). Taguchi method utilizes orthogonal array method , the matrices of which vary with the number of factors and interactions. For instance, 8-trial orthogonal array (L-8 matrix) is used when the number of factors is less than 7 and 16-trial orthogonal array (L-16 matrix) is used when the number of factors is less than 15. Taguchi method takes into account the “signal (S)” and “noise (N)” ratio to measure the quality characteristic of the process or system which deviates from the desired value. The “signal” represents the desirable and “noise” represents the undesirable values for the output characteristics. This S/N ratio varies according to the type of characteristics and can be calculated as follow:
if nominal is the best characteristic;
if smaller is the best characteristic;
and if larger is the best characteristic;
where, ‘Σ’ is the average of observed data, ‘\( S^{2}_{y} \) ’ is the variation of ‘y’, ‘n’ is the number of observations and ‘y’ is the observed data.
Although Taguchi method provides better graphic visualization in determining the optimal conditions, the extent of influence each factor exerts in the output of any process needs further analysis through ANOVA. Nevertheless, this method requires less data to find optimum conditions as compared to RSM . Also, since Taguchi method minimizes the experimental runs, it is recommended to use this method when the number of variables under study is large and also when the experimental time run is lengthy and costly.
Thus, it can be concluded that application of various optimization tools is desirable in a process like dye removal which is effected by multiple factors and their interaction. Thus, optimizing the conditions to obtain the best possible combination of the process parameters and nutrients, makes the process viable and economic in terms of cost, time and waste production. For example, a process optimization study as described by Kaushik and Malik (2011), resulted in 85 % cost reduction, wherein the yeast extract from the unoptimized media was replaced by low cost nitrogen supplements (urea and ammonium chloride). Further, this resulted in higher uptake capacity of the fungal biomass (A. lentulus), decreasing the production of excess biomass and reducing the production of dye laden waste sludge.
5 Reactor Designs Based on Different Mechanisms
Reactor scale studies are often necessary to evaluate the efficiency of the developed technology at industrial scale. Different reactor designs have been proposed by various researchers, depending upon the principle mechanism involved in the process of dye removal. The design of the bioreactor for dye removal process basically depends on two major factors, first being the type of organism and its growth properties i.e. whether it is a unicellular or multicellular microbe; whether it exhibits mycelial or pelleted growth etc. and second being the mechanism i.e. biosorption (the microbe need not be provided with nutrient support but contact between the biosorbent and dye should be maximized), bioaccumulation (nutrient support is required along with aeration) and biodegradation (nutrient support along with optimum pH and temperature for maximal enzyme activity needs special attention). Figure 5 provides a schematic representation of packed bed reactor (often suitable for dye biosorption) developed for the removal of Violet 14R dye by the dried and powdered biomass of A. lentulus (Singh 2010). The height of biomass bed can be varied depending upon the contact time required for accomplishing efficient dye removal. Second most common reactor design for dye biosorption is based on the phenomenon of immobilization where the biosorbent is immobilized on an inert carrier. Gupta (2010) utilized the blocks of ceramic monoliths for the immobilization of A. lentulus which were then utilized for the biosorption of Acid Navy Blue dye (Fig. 6).
Schematic representation of a packed bed bioreactor developed for the biosorption of Violet 14R dye by A. lentulus biomass (Singh 2010)
Use of ceramic monoliths for the immobilization of fungi; a schematic representation of a monolith b monolith before biosorption c monolith with immobilized biomass of A. lentulus after biosorption of Acid Navy Blue d dye samples obtained from flask containing fungi immobilized monoliths e dye samples obtained from flask containing control monoliths; without immobilized biomass (Gupta 2010)
For dye removal process requiring the use of living cells, Mishra and Malik (2013) have proposed the use of three most commonly used reactor designs; continuous stirred-tank reactor, expanded and packed bed reactors and airlift bioreactors (Fig. 7). All these three designs require a constant supply of nutrient and air so as to keep the cells in their live state. One such design has been demonstrated by Xin et al. (2012b) where they have utilized air lift column bioreactor to obtain the pelleted growth of the fungi for removing dye through bioaccumulation mechanism. The air lift bioreactors, promoting pelleted growth of the fungi, have an added advantage where problems related to biomass clogging are minimized resulting in higher mass transfer and dye removal. Moreover, pelleted biomass allows quick separation of dye laden biomass from the treated wastewater.
Bioreactor designs: continuous stirred-tank reactor a; Expanded Bed and Packed Bed b and Airlift bioreactors c (Mishra and Malik 2013)
Reactor designs based on biodegradation process may utilize either the microbial biomass capable of producing the enzyme responsible for dye removal or the purified enzyme, such as LiP, laccase etc. The microbial cells may be immobilized in a suitable bioreactor design for the production of enzyme which then brings about the decolorization. For example, Dominguez et al. (2001) studied degradation of Poly R-478 dye by three enzymes i.e. MnP, LiP and laccase produced by Phanerochaete chrysosporium immobilized on cubes of nylon sponge in a bioreactor based on a standard rotating drum configuration. Similar attempt was made by Kasinath et al. (2003), in which they immobilized the white rot fungus Irpex lacteus on polyurethane foam and pine wood to study the degradation of Remazol Brilliant Blue R dye in a packed bed bioreactor with the help of MnP and laccase enzyme. Recently, researchers have also attempted the immobilization of purified enzymes to carry out the degradation process, such as the immobilization of laccase enzyme obtained from Myceliophthora thermophila on Eupergit support in a packed bed bioreactor (Lloret et al. 2012).
Other than basic designs, sequential reactor configurations , employing anaerobic and aerobic conditions, have also been proposed by many researchers. Zee and Villaverde (2005), Khehra et al. (2006) have proposed the sequential bioreactors based on initial anaerobic treatment, followed by aerobic treatment, where the final degradation of the products of anaerobic cleavage occurs. However, carcinogenic aromatic amines , produced during the anaerobic degradation of azo dyes, are not effectively removed in the subsequent aerobic step, thus limiting the use of this type of sequential treatment (Mohanty et al. 2006). Reverse of this treatment process i.e. the aerobic degradation, followed by anaerobic treatment, has been proposed by Novotný et al. (2011) for wastewater containing high concentration of dye and organics. Aerobic step based on the enzymatic degradation by fungi Irpex lacteus in a fungal trickling filter (FTF) bioreactor , followed by anaerobic degradation in bacterial reactors , resulted in efficient decolorization in first step and marked TOC reduction in second step. Thus, sequential bioreactors , based on combined aerobic-anaerobic treatment of dye wastewater, have a large potential. Another approach, which may be utilized for enhancing removal of dye, is the coupling of two mechanisms, such as chemical and biological method. Sudarjanto et al. (2006) integrated the chemical and biological degradation methods to degrade Reactive Azo Red 195A . For this, two reactors i.e. photoreactor and bioreactor were used in a series. Advanced oxidation of dye by UV/H2O2 was carried out in photoreactor, followed by aerobic biodegradation in bioreactor containing microbial biofilms . Shoabargh et al. (2013) have coupled photodegradation and enzymatic process of Acid Orange 7 dye degradation, using a rectangular recycling photo-bioreactor containing glucose oxidase (GOx) immobilized on TiO2/polyurethane (PU).
Thus, it seems that recent technical advancements, employing combination of techniques, should be further explored so as to attain a design more suitable for dye containing effluents which can be acceptable in terms of inputs required, cost and time taken for the treatment.
6 Management of Dye Laden Fungal Biomass
The major hindrance faced after dye removal process is the disposal of dye laden microbial biomass. Only a few studies address to the problem posed by the generation of dye laden microbial slurry. Nigam et al. (2000) utilized different agricultural residues, such as wheat straw, wood chips and corn-cob shreds for the biosorption of mixture of dyes containing Cibacron Red, Remazol Navy Blue, Remazol Red , Cibacron Orange , Remazol Golden Yellow , Remazol Blue, Remazol Turquoise Blue and Remazol Black B dyes . After biosorption, the waste slurry is utilized as a substrate for solid state fermentation by two white-rot fungi ; Phanerochaete chrysosporium and Coriolus versicolor. After the enzymatic degradation, the spent fermentation slurry was used as a soil conditioner. A variant approach was adopted by Kaushik et al. (2013), wherein the spent fermentation slurry, containing fungal biomass of A. lentulus and corn cob, was utilized for the biosorption of Acid Navy Blue dye and the disposal of dye laden slurry was accomplished through vermicomposting , resulting in the production of compost ideal for disposal in the soil. Although these studies provide encouraging results to overcome the waste management problem, still more studies are required in this regard to provide viable and sustainable options.
7 Future Perspectives
Currently biological removal of synthetic dyes is a widely researched topic. Yet, certain research gaps can still be identified which are important to be addressed for field application of the technology.
Majority of the reports available on this aspect indicate the optimum pH for fungal growth to be in acidic range as well as in mesophilic range. On the contrary, the pH of the dye effluent usually lies in the alkaline range and is released from the industry at high temperatures. Therefore, for a biological process to be effective at the industrial scale, it is important that the selected microbe should be able to grow in alkaline pH and to withstand high temperatures as well. Apart from containing a mixture of dyes, the effluent contains many other compounds, i.e. salts which can interfere with the process of dye removal. Thus, in addition to the studies with single dye solution, removal of dye mixtures and the effect of dye auxiliaries and validating the efficiency of the isolates in real effluent should be taken up.
Also, in order to ensure commercial application in remote industries, it is important to develop the seed culture in a form which can be easily transported and stored without requiring much energy inputs. Recently, attempts have been made to develop a carrier based microbial formulation for remediation of heavy metals and dyes from wastewater (Sharma 2009; Kaushik 2011; Mishra 2013).
8 Conclusion
Synthetic dyes are widely used in textile, dyeing, tanning, food and paper industries and are released into the environment through waste waters coming out from these industries. A lot of research has focused on mycoremediation of synthetic dyes which could offer an attractive decentralized system. A critical assessment of the recent studies indicates that a strong foundation of an efficient mycoremediation process can be laid by meticulously choosing fungal strains tolerant to high pH, temperature and salt concentration, and designing consortiums of such strains with variable mechanisms of dye sequestration . This could be the first step to ensure a reliable process performance. Further, the process cost, inputs, rates and dye uptake capacities can be optimized through statistical process optimization tools that help ascertain interactive effect of several process variables. Significant advancements have been made in the area of appropriate bioreactor designs which need to be testified at pilot scales. Further, integration of physico-chemical techniques with mycoremediation offers a novel approach for handling synthetic dyes . Nevertheless, more efforts must go in to address the need for user-friendly formulated products (of robust fungal strains/consortium) as well as fate of dye laden fungal sludge to establish the mycoremediation tool as eco-friendly process.
References
Aghaie-Khouzani M, Forootanfar H, Moshfegh M, Khoshayand MR, Faramarzi MA (2012) Decolorization of some synthetic dyes using optimized culture broth of laccase producing ascomycete Paraconiothyrium variabile. Biochem Eng J 60:9–15
Akar T, Divriklioglu M (2010) Biosorption applications of modified fungal biomass for decolorization of Reactive Red 2 contaminated solutions: batch and dynamic flow mode studies. Bioresour Technol 101:7271–7277
Aksu Z, Balibek E (2010) Effect of salinity on metal-complex dye biosorption by Rhizopus arrhizus. J Environ Manage 91:1546–1555
Alam MZ, Mansor MF, Jalal KCA (2009) Optimization of decolorization of methylene blue by lignin peroxidase enzyme produced from sewage sludge with Phanerocheate chrysosporium. J Hazard Mater 162:708–715
Anastasi A, Spina F, Prigione V, Tigini V, Giansanti P, Varese GC (2010) Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Bioresour Technol 101:3067–3075
Bas D, Boyacı IH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78:836–845
Casas N, Blánquez P, Gabarrell X, Vicent T, Caminal G, Sarrà M (2007) Degradation of orange G by laccase: fungal versus enzymatic process. Environ Technol 28:1103–1110
Chakraborty S, Basak B, Dutta S, Bhunia B, Dey A (2013) Decolorization and biodegradation of congo red dye by a novel white rot fungus Alternaria alternata CMERI F6. Bioresour Technol 147:662–666
Chojnacka K (2010) Biosorption and bioaccumulation—the prospects for practical applications. Environ Int 36:299–307
Daâssi D, Mechichi T, Nasri M, Couto SR (2013) Decolorization of the metal textile dye Lanaset Grey G by immobilized white-rot fungi. J Environ Manage 129:324–332
Daneshvar N, Khataee AR, Rasoulifard MH, Pourhassan M (2007) Biodegradation of dye solution containing Malachite Green: Optimization of effective parameters using Taguchi method. J Hazard Mater 143:214–219
Das D, Charumathi D, Das N (2011) Bioaccumulation of the synthetic dye Basic Violet 3 and heavy metals in single and binary systems by Candida tropical is grown in a sugarcane bagasse extract medium: modelling optimal conditions using response surface methodology (RSM) and inhibition kinetics. J Hazard Mater 186:1541–1552
Das D, Charumathi D, Das N (2010) Combined effects of sugarcane bagasse extract and synthetic dyes on the growth and bioaccumulation properties of Pichia fermentans MTCC 189. J Hazard Mater 183:497–505
Das SK, Bhowal J, Das AR, Guha AK (2006) Adsorption behavior of Rhodamine B on Rhizopus oryzae biomass. Langmuir 22:7265–7272
Dias AA, Lucas MS, Sampaio A, Peres JA, Bezerra RMF (2010) Decolorization of azo dyes by yeasts. In: Erkurt HA (Ed) Biodegradation of azo dyes. The Handbook of Environmental Chemistry, vol 9. Springer, Berlin, pp 183–193
Dominguez A, Rivela I, Couto SR, Sanroman MA (2001) Design of a new rotating drum bioreactor for ligninolytic enzyme production by Phanerochaete chrysosporium grown on an inert support. Process Biochem 37:549–554
Engin AB, Özdemir O, Turan M, Turan AZ (2008) Color removal from textile dye bath effluents in a zeolite fixed bed reactor: determination of optimum process conditions using Taguchi method. J Hazard Mater 159:348–353
Fan H, Yang JS, Gao TG, Yuan HK (2012) Removal of a low-molecular basic dye (Azure Blue) from aqueous solutions by a native biomass of a newly isolated Cladosporium sp.: kinetics, equilibrium and biosorption simulation. J Taiwan Inst Chem Eng 43:386–392
Fernández C, Larrechi MS, Callao MP (2010) An analytical overview of processes for removing organic dyes from wastewater effluents. Trend Anal Chem 29:1202–1211
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol. doi:10.1016/j.biortech.2013.12.102
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res 111:3–49
Ge Y, Yan L, Qinge K (2004) Effect of environment factors on dye decolorization by P. sordida ATCC90872 in an aerated reactor. Process Biochem 39:1401–1405
Gomi N, Yoshida S, Matsumoto K, Okudomi M, Konno H, Hisabori T, Sugano Y (2011) Degradation of the synthetic dye amaranth by the fungus Bjerkandera adusta Dec 1: inference of the degradation pathway from an analysis of decolorized products. Biodegradation 22:1239–1245
Gönen F, Aksu Z (2009a) Single and binary dye and heavy metal bioaccumulation properties of Candida tropicalis: Use of response surface methodology (RSM) for the estimation of removal yields. J Hazard Mater 172:1512–1519
Gönen F, Aksu Z (2009b) Predictive expressions of growth and Remazol Turquoise Blue-G reactive dye bioaccumulation properties of Candida utilis. Enzym Microb Technol 45:15–21
Gupta A (2010) Design of a flow column for dye removal from acid navy blue solution using fungal biomass. Minor Project Report, Indian Institute of Technology Delhi
Iqbal M, Saeed A (2007) Biosorption of reactive dye by loofa sponge-immobilized fungal biomass of Phanerochaete chrysosporium. Process Biochem 42:1160–1164
Jin X, Ning Y (2013) Laccase production optimization by response surface methodology with Aspergillus fumigatus AF1 in unique inexpensive medium and decolorization of different dyes with the crude enzyme or fungal pellets. J Hazard Mater 262:870–877
Kasinath A, Novotný C, Svobodová K, Patel KC, Šašek V (2003) Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed-bed bioreactor. Enzyme Microb Technol 32:167–173
Kaushik P (2011) Decolorization of industrial waste water and xylanase production by Aspergillus lentulus. PhD Thesis. Indian Institute of Technology Delhi
Kaushik P, Malik A (2009) Fungal dye decolorization: recent advances and future potential. Environ Int 35:127–141
Kaushik P, Malik A (2010a) Alkali, thermo and halo tolerant fungal isolate for the removal of textile dyes. Colloids Surf, B 81:321–328
Kaushik P, Malik A (2010b) Effect of nutritional conditions on dye removal from textile effluent by Aspergillus lentulus. World J Microbiol Biotechnol 26:196–1957
Kaushik P, Malik A (2011) Process optimization for efficient dye removal by Aspergillus lentulus FJ172995. J Hazard Mater 185:837–843
Kaushik P, Malik A (2013) Comparative performance evaluation of Aspergillus lentulus for dye removal through bioaccumulation and biosorption. Environ Sci Pollut Res 20:2882–2892
Kaushik P, Malik A, Satyawati S (2013) Vermicomposting: an eco-friendly option for disposal of fermentation and dye decolorization waste. Clean: Soil, Air, Water 41:616–621
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2006) Biodegradation of azo dye C.I. Acid Red 88 by an anoxic-aerobic sequential bioreactor. Dyes Pigm 70:1–7
Kim ST, Park MS, Kim HM (2004) Systematic approach for the evaluation of the optimal fabrication conditions of a H2S gas sensor with Taguchi method. Sens Actuators B 102:253–260
Lade HS, Waghmode TR, Kadam AA, Govindwar SP (2012) Enhanced biodegradation and detoxification of disperse azo dye Rubine GFL and textile industry effluent by defined fungal-bacterial consortium. Int Biodeterior Biodegrad 72:94–107
Levin L, Melignani E, Ramos AM (2010) Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour Technol 101:4554–4563
Lloret L, Hollmann F, Eibes G, Feijoo G, Moreira MT, Lema JM (2012) Immobilisation of laccase on Eupergit supports and its application for the removal of endocrine disrupting chemicals in a packed-bed reactor. Biodegradation 23:373–386
Lu Y, Phillips DR, Lu L, Hardin IR (2008) Determination of the degradation products of selected sulfonated phenylazonaphthol dyes treated by white rot fungus Pleurotus ostreatus by capillary electrophoresis coupled with electrospray ionization ion trap mass spectrometry. J Chromatogr A 1208:223–231
Martorell MM, Pajot HF, Rovati JI, Figueroa LIC (2012) Optimization of culture medium composition for manganese peroxidase and tyrosinase production during Reactive Black 5 decolorization by the yeast Trichosporon akiyoshidainum. Yeast 29:137–144
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43:1162–1222
Mishra A (2013) Development of biological system employing microbial consortium for pollutant removal from mixed waste stream. PhD Thesis. Indian Institute of Technology Delhi
Mohanty S, Dafale N, Rao NN (2006) Microbial decolorization of reactive black-5 in a two-stage anaerobic-aerobic reactor using acclimatized activated textile sludge. Biodegradation 17:403–413
Moreira-Neto SL, Mussatto SI, Machado KMG, Milagres AMF (2013) Decolorization of salt-alkaline effluent with industrial reactive dyes by laccase-producing basidiomycetes strains. Lett App Microbiol 56:283–290
Nigam P, Armour G, Banat IM, Singh D, Marchant R (2000) Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresour Technol 72:219–226
Novotný C, Svobodová K, Benada O, Kofroňová O, Heissenberger A, Fuchs W (2011) Potential of combined fungal and bacterial treatment for color removal in textile wastewater. Bioresour Technol 102:879–888
Papadopoulou K, Kalagona IM, Philippoussis A, Rigas F (2013) Optimization of fungal decolorization of azo and anthraquinone dyes via Box-Behnken design. Int Biodeterior Biodegrad 77:31–38
Parshetti GK, Kalme SD, Gomare SS (2007) Biodegradation of reactive blue-25 by Aspergillus ochraceus NCIM-1146. Bioresour Technol 98:3638–3642
Prigione V, Varese GC, Casieri L, Marchisio VF (2008) Biosorption of simulated dyed effluents by inactivated fungal biomasses. Bioresour Technol 99:3559–3567
Qu Y, Shi S, Mab F, Yan B (2010) Decolorization of reactive dark blue K-R by the synergism of fungus and bacterium using response surface methodology. Bioresour Technol 101:8016–8023
Ranganathan K, Karunagaran K, Sharma DC (2007) Recycling of wastewaters of textile dyeing industries using advanced treatment technology and cost analysis—case studies. Resour Conserv Recycl 50:306–318
Revankar MS, Lele SS (2007) Synthetic dye decolorization by white rot fungus, Ganoderma sp. WR-1. Bioresour Technol 98:775–780
Sarayu K, Sandhya S (2012) Current technologies for biological treatment of textile wastewater—a review. Appl Biochem Biotechnol 167:645–661
Sharma P, Singh L, Dilbaghi N (2009) Response surface methodological approach for the decolorization of simulated dye effluent using Aspergillus fumigatus Fresenius. J Hazard Mater 161:1081–1086
Sharma S (2009) Chromium removal from industrial effluents using fungal isolate. PhD Thesis, Indian Institute of Technology Delhi
Shoabargh S, Karimi A, Dehghan G, Khataee A (2013) A hybrid photocatalytic and enzymatic process using glucose oxidase immobilized on TiO2/polyurethane for removal of a dye. J Ind Eng Chem. doi:10.1016/j.jiec.2013.11.058
Silva-Stenico ME, Vieira FDP, Genuário DB, Silva CSP, Moraes LAB, Fiore MF (2012) Decolorization of textile dyes by cyanobacteria. J Braz Chem Soc 23:1863–1870
Singh R (2010) Design of a Flow Column for removal of reactive dye. Minor Project Report, Indian Institute of Technology Delhi
Sivasamy A, Sundarabal N (2011) Biosorption of an Azo Dye by Aspergillus niger and Trichoderma sp. fungal biomasses. Curr Microbiol 62:351–357
Srinivasan A, Viraraghavan T (2010) Decolorization of dye wastewaters by biosorbents: a review. J Environ Manage 91:1915–1929
Srinivasan SV, Murthy DVS (2009) Statistical optimization for decolorization of textile dyes using Trametes versicolor. J Hazard Mater 165:909–914
Sudarjanto G, Keller-Lehmann B, Keller J (2006) Optimization of integrated chemical-biological degradation of a reactive azo dye using response surface methodology. J Hazard Mater B138:160–168
Tan C, Li M, Lin YM, Lu XQ, Chen ZL (2011) Biosorption of basic orange from aqueous solution onto dried A. filiculoides biomass: Equilibrium, kinetic and FTIR studies. Desalination 266:56–62
Tan CY, Li G, Lu XQ, Chen ZL (2010) Biosorption of basic orange using dried A. filiculoides. Ecol Eng 36:1333–1340
Tan L, Li H, Ning S, Xu B (2014) Aerobic decolorization and degradation of azo dyes by suspended growing cells and immobilized cells of a newly isolated yeast Magnusiomyces ingens LH-F1. Bioresour Technol. doi:10.1016/j.biortech.2014.02.063
Taskin M, Erdal S (2010) Reactive dye bioaccumulation by fungus Aspergillus niger isolated from the effluent of sugar fabric-contaminated soil. Toxicol Ind Health 26:239–247
Telke AA, Kadam AA, Jagtap SS, Jadhav JP, Govindwar SP (2010) Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol Bioprocess Eng 15:696–703
Viraraghavan T, Srinivasan A (2011) Fungal biosorption and biosorbents. In: Kotrba P, Mackova M, Macek T (Eds) Microbial biosorption of metals, Springer, Berlin, pp 143–158
Vitor V, Corso CR (2008) Decolorization of textile dye by Candida albicans isolated from industrial effluents. J Ind Microbiol Biotechnol 35:1353–1357
Wang BE, Hu YY (2008) Bioaccumulation versus adsorption of reactive dye by immobilized growing Aspergillus fumigatus beads. J Hazard Mater 157:1–7
Wang SM, Giang YS, Ling YC (2002) Taguchi’s method in optimizing the experimental conditions of simultaneous supercritical fluid extraction and chemical derivatization for the gas chromatographic-mass spectrometric determination of amphetamine and methamphetamine in aqueous matrix. Forensic Sci J 1:47–53
Xin B, Chen G, Zheng W (2010) Bioaccumulation of Cu-complex reactive dye by growing pellets of Penicillium oxalicum and its mechanism. Water Res 44:3565–3572
Xin B, Xia Y, Zhang Y, Aslam H, Liu C, Chen S (2012a) A feasible method for growing fungal pellets in a column reactor inoculated with mycelium fragments and their application for dye bioaccumulation from aqueous solution. Bioresour Technol 105:100–105
Xin B, Zhang Y, Liu C, Chen S, Wu B (2012b) Comparison of specific adsorption capacity of different forms of fungal pellets for removal of Acid Brilliant Red B from aqueous solution and mechanisms exploration. Process Biochem 47:1197–1201
Xiong XJ, Meng XJ, Zheng TL (2010) Biosorption of C.I. Direct Blue 199 from aqueous solution by nonviable Aspergillus niger. J Hazard Mater 175:241–246
Yildiz YS (2008) Optimization of Bomaplex Red CR-L dye removal from aqueous solution by electrocoagulation using aluminum electrodes. J Hazard Mater 153:194–200
Zee FP, Villaverde S (2005) Combined anaerobic-aerobic treatment of azo dyes—a short review of bioreactor studies. Water Res 39:1425–1440
Zhao X, Hardin IR, Hwang HM (2006) Biodegradation of a model azo disperse dye by the white rot fungus Pleurotus ostreatus. Int Biodeterior Biodegrad 57:1–6
Zhao X, Lu Y, Phillips DR, Hwang HM, Hardin IR (2007) Study of biodegradation products from azo dyes in fungal degradation by capillary electrophoresis/electrospray mass spectrometry. J Chromatogr A 1159:217–224
Acknowledgments
Financial assistance from Department of Science and Technology, Indian Council of Agricultural Research, Government of India and CSIR Senior Research Fellowship and Research Associateship to one of the authors (PK), are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kaushik, P., Malik, A. (2015). Mycoremediation of Synthetic Dyes: An Insight into the Mechanism, Process Optimization and Reactor Design. In: Singh, S. (eds) Microbial Degradation of Synthetic Dyes in Wastewaters. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-10942-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-10942-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10941-1
Online ISBN: 978-3-319-10942-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)