Abstract
The nutritional conditions supporting growth and maximum dye removal by Aspergillus lentulus have been investigated. Initially a composite media containing yeast extract, glucose and mineral components was used and the effect of various components on dye removal was studied. For maximum dye removal (≈100%), ≥0.5% (w/v) glucose and ≥0.25% (w/v) yeast extract were essential. While glucose played an important role in pellet formation, which in turn was important for dye removal, yeast extract contributed towards higher biomass production. Mineral components (except NH4NO3) did not affect dye removal significantly. Next the alternate sources of carbon (molasses, jaggery, starch and sodium acetate) and nitrogen (peptone, urea, ammonium nitrate, sodium nitrate and ammonium chloride) were tested. Among carbon sources, all the sources produced almost complete dye removal in 48 h (more than 97% in 24 h), except sodium acetate (64% in 48 h). All the tested nitrogen sources resulted in >90% dye removal in 48 h. Yeast extract and peptone gave best results with high dye removal rate (9.8 and 8.1 mg/l/h, respectively). However, among the low cost alternates, urea and NH4Cl came out to be suitable sources due to the high uptake capacity of the biomass produced coupled with high dye removal rate in case of NH4Cl. Therefore, a combination of urea and NH4Cl was tested, which produced complete dye removal with a high dye removal rate (10 mg/l/h). Finally the modified composite media containing urea and NH4Cl as nitrogen sources and glucose as carbon source was utilized for effluent treatment. Results indicated that performance of modified composite media was at par with composite media for supporting growth of A. lentulus and dye removal from the textile effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Removal of dyes from industrial effluents has assumed great significance due to their health hazards (Joshi et al. 2004; Mathur et al. 2005). Biological techniques being eco friendly have been extensively researched in the last decade and can be carried out using microbial biomass in either growing or non-growing form (Robinson et al. 2001). The process of dye removal through growing cells has certain advantages over non-growing cells (biosorption) which is often more sensitive to process parameters like pH, temperature and the class of dyes that are to be removed. Also, in the former case, dye removal can take place simultaneously along with microbial cell growth (Kaushik and Malik 2009a). This eliminates the requirement for separate biomass production, harvesting and storage. However, growing microbial cells have complex nutritional requirements. So far glucose, yeast extract, peptone etc. have been extensively studied as the nutrient sources to support microbial growth and dye removal (Martins et al. 1999; Liu et al. 2006; Moosvi et al. 2007; Mohana et al. 2008). Nevertheless, in order to reduce the process cost for industrial effluent treatment, it is very important to know that which components are absolutely essential to generate maximum dye removal. Further, the process should be versatile and have the flexibility to achieve optimum dye removal on diverse nutrient sources. In this regard, earlier researchers have investigated the effect of various alternate carbon (Kapdan et al. 2000; Sumathi and Manju 2000) and nitrogen (Hatvani and Mecs 2002; Rigas and Dritsa 2006) sources. These results indicate that critical carbon and nitrogen requirement for optimum dye removal may be diverse and strain specific.
In the previous studies, a fungal isolate Aspergillus lentulus FJ172995 was investigated for the removal of Acid Navy Blue dye. Results indicated that the isolate was able to bring about 100% (Acid Navy Blue, 100 mg/l) removal within 20 h of incubation when provided with complete set of nutrients (Kaushik and Malik 2009b). However, requirement of expensive nutritional resources shall imply high cost burdens for dye removal. Hence, the current study aims at simplifying the nutritional supplementation by identifying the most critical nutrients required to support fungal growth and dye removal. The conventional composite media used in the present study contained three major components i.e. glucose, yeast extract and mineral components. In the first phase experiments were conducted to establish which component out of the above three is crucial for dye removal. In the next phase, attempts were made to replace the selected crucial media components with cheaper and locally available materials.
Materials and methods
Dyes and chemicals
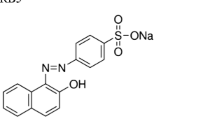
The dye used in the present investigation, Acid Navy Blue (C.I. Acid Blue 120), was provided by Department of Textile Technology, IIT Delhi which is an anionic azo dye and find extensive use in dyeing of woollen and silk fibres. The chemical structure of Acid Navy Blue is presented in Fig. 1. Absorption maximum (λmax) of the dye was obtained by scanning the dye solution over visible range. Standard curve was plotted by measuring absorbance at absorption maxima for each dye. The stock solution of 10,000 mg/l dye was prepared in distilled water. All the other chemicals used were of analytical grade and were obtained from Merck and Qualigens.
Microorganism and growth conditions
The experiments on dye removal were performed with a strain of Aspergillus lentulus FJ172995 previously isolated from the textile effluent from Baddi (Himachal Pradesh, India) where large scale textile units are located. The fungal isolate was maintained on slants of Potato Dextrose Agar. Freshly revived cultures were used for all the experiments.
The growth media having the composition: (NH4NO3, 0.5 g/l; MgSO4.7H2O, 0.1 g/l; K2HPO4, 0.5 g/l; NaCl, 1 g/l; Glucose, 10 g/l; Yeast extract, 2.5 g/l, pH: 6.5) was used for all the experiments unless otherwise stated. The flasks were agitated in a shaker (Scigenics Biotech, Orbitek) at 180 rpm and 30°C.
Effect of initial glucose and yeast extract concentration on dye removal by A. lentulus
All the experiments concerning dye removal were conducted at 200 mg/l initial dye concentration. Effect of varying glucose concentration was estimated by studying the dye removal by A.lentulus at different initial glucose concentration (0–1%). Rest of the media components were kept same as mentioned earlier. Flasks containing 100 ml sterilized media were aseptically inoculated with 5% spore suspension (having spore concentration of 6.25 × 106/ml). To test the effect of yeast extract concentration (0–1%), flasks having 100 ml sterilized media with 1% glucose concentration and other media components were inoculated as stated earlier. Suitable biotic controls without dye for both cases were run to evaluate the effect of dye on growth of A. lentulus. Abiotic control (uninoculated) was also included to see any interaction between media components and the dye. All the flasks were incubated in a shaker at 180 rpm and 30°C. Samples were withdrawn from the flask at regular intervals and analyzed for dye removal (%).
Effect of various mineral components of the media on dye removal by A. lentulus
Effect of each of the mineral component of the media (NH4NO3, MgSO4, K2HPO4 and NaCl) as described in “Microorganism and growth conditions” was evaluated to study the dye removal and biomass production by A. lentulus. For this, series of flasks containing media was prepared in which one of the mineral component except the others was missing. The flasks were designated as NH4NO3 − (without NH4NO3), MgSO4 − (without MgSO4), K2HPO4 − (without K2HPO4) and NaCl− (without NaCl).
Effect of alternate carbon and nitrogen sources on dye removal by A. lentulus
Different carbon sources at 1% concentration were included in the media to test their efficacy in supporting the growth of A. lentulus and dye removal. Various carbon sources tested were glucose, starch, molasses, jaggery and sodium acetate.
Similarly different nitrogen sources (yeast extract, peptone, urea, ammonium chloride, ammonium nitrate and sodium nitrate) were also tested. The amount of nitrogen source was so adjusted to provide 10 mM nitrogen for the growth of the fungal isolate. The composition of rest of the media was as follow: glucose, 10 g/l; MgSO4.7H2O, 0.1 g/l; K2HPO4, 0.5 g/l, pH: 6.5. Control flasks were also run along with the experimental flasks. Initial dye concentration in each of the experimental flasks was kept at 200 mg/l and the flasks were inoculated and incubated in a shaker as described previously.
Dye removal from textile effluent by A. lentulus
Textile effluent was procured from a small scale textile unit located at Sanganer, Jaipur, Rajasthan (India). The effluent was characterized using standard methods of wastewater analysis (APHA). The pH of the effluent was 8.11 and no adjustments in the initial pH were made prior to the effluent treatment with A. lentulus. Dye removal studies were conducted to test the effect of supplementation on dye removing efficiency of A. lentulus. The series of flasks containing the neat effluent (NE) and effluent supplemented with nutrients [modified composite media (SEMM) and composite media (SECM)] were inoculated with spore suspension and incubated at 180 rpm and 30°C. Since the objective was to test the minimum possible and low cost supplementation, hence based on the results, a modified composite media containing half the glucose and a combination of NH4Cl and urea as nitrogen source was tested. The composition of the modified composite media (MM) was K2HPO4, 0.5 g/l; MgSO4·7H2O, 0.1 g/l; NH4Cl, 0.2 g/l; Urea, 0.3 g/l; Glucose, 5 g/l. For comparison, a series of flasks containing effluent supplemented with composite media (SECM) was also tested. To assess the role of native micro flora, in each series flasks containing unsterilized effluent were also included.
Analytical techniques
The concentration of Acid Navy Blue dye was determined by measuring the absorbance of test samples through spectrophotometer (Systronics Visiscan 167) at 561 nm. A calibration plot between concentration and absorbance of Acid Navy Blue was used for determination of dye concentration. Dye removal (%) was calculated using the equation:
where A 0 is the initial absorbance, A t is the absorbance at incubation time, t. Test samples were analyzed for absorbance after centrifuging (Sigma 4K5) the samples at 10,000 rpm for 10 min.
The dry cell weight of the biomass formed at the end of the incubation period was measured by filtering out the contents of the flasks through pre-dried and pre-weighed Whatman No. 1 filter paper and drying it overnight at 60°C to a constant weight. Glucose content in the samples was assayed through dinitrosalicylic acid method (DNS) for reducing sugar (Miller 1959) at 540 nm. Microscopic photographs were taken through bright field microscopy (Leica DM2500) at a magnification of 10×.
Statistical analysis
All the studies were conducted in triplicates and the results are presented as means of the replicates along with standard deviation (represented as error bars). One way and two-way ANOVA were used, as required, to statistically analyze the results with the limit of significance set at P < 0.05 (SPSS Package, Version 16).
Results
Effect of initial glucose and yeast extract concentration on dye removal by A. lentulus
Effect of initial glucose concentration on dye removal by A. lentulus was tested at different glucose concentrations ranging from 0 to 1%. Results show (Fig. 2) that dye removal significantly increases with time (till 48 h) and also with an increase in glucose concentration (P < 0.05). Marked increase in dye removal at higher glucose concentration could be attributed to better growth and higher biomass production (Fig. 3). After 72 h of incubation 78.2 and 92.4% dye was removed at 0.1 and 0.2% glucose concentration, respectively. Almost complete dye removal was obtained at 0.5 and 1% glucose concentration within 24 h, with no significant difference. Low percentage of dye removal (47.8%) as well as biomass (0.6 g/l) was obtained in 72 h in the absence of glucose thus establishing the importance of glucose as a carbon source required for the growth of the fungus. Similarly, Sumathi and Manju (2000) reported enhancement of Drimarene Blue uptake by A. foetidus with increasing concentrations of glucose which was related to increased growth rates and biomass production of the fungus. However, Assadi et al. (2001) reported an initial increase in removal of colour from textile waste water by Phanerochaete chrysosporium as the glucose concentration was increased from 0.1 to 0.3% (dye removal increased from 90 to 95%) but further increase in glucose concentration resulted in decrease in dye removal (85% decolourization at 0.7% glucose concentration). Thus, it is felt that glucose concentration giving best dye removal results needs to be optimized for a particular strain.
Like carbon, nitrogen is also an essential nutrient and is a constituent of proteins. Hence, studies have been conducted to establish the effect of nitrogen concentration on the dye removal by the fungi. Figure 4 depicts the effect of yeast extract (widely studied nitrogen source) concentration on dye removal by A. lentulus. Results showed a significant increase in biomass production (P < 0.05) as the yeast extract concentration in the medium was increased from 0 to 1% (Fig. 3). This also resulted in significantly quicker dye removal as complete dye removal was obtained within 20 h with yeast extract concentration ranging from 0.25 to 1%. Complete dye removal was obtained in 48 h with 0.1% yeast extract concentration whereas 84.56% dye was removed in the absence of yeast extract in 48 h. This suggests the crucial role of yeast extract in promoting the growth of the fungus. When two-way ANOVA was applied to the results, it was found that the difference between dye removal values beyond 20 h of incubation for yeast extract concentrations ranging from 0.25 to 1% was insignificant. Therefore, 0.25% yeast extract concentration was regarded as the optimum concentration required to bring about complete dye removal.
In the present study when the results for dye removal at 24 h are compared in the absence of glucose (49%) and yeast extract (84.4%), glucose seems to play more important role than yeast extract. Similarly, Parshetti et al. (2007) reported the superiority of glucose over peptone (nitrogen source) for decolourization of Reactive Blue-25 by A. ochraceus. Addition of glucose in the growth media brought about complete dye decolourization in 7 days, addition of peptone lead to complete dye decolourization in 15 days, whereas when both glucose and peptone were added, 11 days were needed to achieve complete dye decolourization.
Glucose is required for the pellet formation of the fungus (Liao et al. 2007). A pellet is formed due to aggregation of the fungal mycelia under the shaking condition during its growth period. In the presence of dye, the growing pellet absorbs and internalizes the dye which can be microscopically seen being concentrated in the centre of the pellet. Therefore, proper pellet formation is crucial in achieving efficient dye removal. In the optimal conditions (1% glucose and 0.25% yeast extract), the pellet size of 1.5–2 mm is achieved. Microscopic pictures of the fungal pellets obtained at different concentrations of glucose are presented in Fig. 5a–e. In the absence of glucose, fungal pellets were malformed and very small in size. It is seen that as the concentration of glucose in growth media is increased from 0 to 1%, the size of the fungal pellet is also increased from 0.5 to 1.5 mm. Liao et al. (2007) also observed that carbon sources (PDB and glucose) played a crucial role in determining pellet size in Rhizopus oryzae. PDB produced more biomass as compared to glucose but with a smaller pellet size. There was no substantial change in the pellet size of the fungus as yeast extract was added to the media in varying proportion. But as the yeast extract concentration was increased, higher amount of fungal biomass was produced. Also, pellets grown in the presence of other inorganic nitrogen sources show larger mycelial extensions as compared to those grown in the presence of yeast extract.
Effect of various mineral components of the media on dye removal by A. lentulus
After establishing the importance of carbon and nitrogen sources in the growth media, effect of various mineral components on dye removal efficiency of A. lentulus was evaluated by omitting one of the components from the media at a time. From the results it is evident that when compared with the control flask (in which all the mineral components were present) absence of NaCl did not affect the dye removal efficiency as well as biomass production significantly (Fig. 6). Dye removal efficiency was significantly (P < 0.05) reduced in the absence of K2HPO4 (96.91%) and MgSO4 (97.46%). Lowest removal was obtained in the absence of NH4NO3 (90.29%) which is a source of nitrogen.
This indicated that NH4NO3 is an important source of nitrogen which is a constituent of all the proteins (including enzymes) and is required for the growth and metabolism of the fungus. K2HPO4 acts as a buffer and is also a source of phosphorus to the fungus, and MgSO4 is an important source of sulphur. It should also be noted that although these components were omitted from the media at a time, yet substantial growth and dye removal was observed. This could be explained by the fact that in all the cases glucose, which is an important source of carbon and yeast extract, a source of nitrogen and various growth factors and minerals were present in the media.
Effect of alternate carbon and nitrogen sources on dye removal by A. lentulus
Glucose and yeast extract are widely used in investigations involving fungal growth for treatment of dye waste water. However, non-availability of glucose in remote areas and high cost of yeast extract may hinder their large scale utility for industrial applications. Therefore, various carbon and nitrogen sources were tested to evaluate their ability to produce fungal biomass and support dye removal. Various carbon sources tested were starch, molasses, jaggery and sodium acetate; glucose was taken as standard. From Fig. 7 it is evident that although biomass produced with molasses (5.1 g/l), jaggery (5.4 g/l) and starch (5.5 g/l) was slightly lower than that of glucose (5.8 g/l), more than 97% removal was obtained within 24 h with these sources. This, reduction in biomass and dye removal was insignificant when compared to glucose at P < 0.05 level of significance. However, very low biomass (3.3 g/l) was produced with sodium acetate as the carbon source and so does the lower dye removal (64%).
For alternate nitrogen sources, yeast extract (taken as standard), peptone, urea, ammonium chloride, ammonium nitrate and sodium nitrate were tested for supporting growth and dye removal. More than 90% removal was achieved with all the tested nitrogen sources in 48 h but it remained incomplete in case of NH4Cl (96.4%) and NaNO3 (91.5%) till the end of the experiment (Fig. 8). For rest of the nitrogen sources, time required for complete dye removal varied due to difference in dye removal rates. Further, the sugar consumption rates and hence the amount of sugar consumed for maximum dye removal in each case also varied. Performance of these sources is tabulated in Table 1 in terms of maximum dye removal, mg dye removed/g sugar consumed and dye removal rate at equilibrium. Peptone proved to be most efficient source with 97.6 mg dye removal/g of glucose consumed followed by urea (45.8 mg/g) and yeast extract (39 mg/g). Maximum dye removal rate of 9.8 mg/l/h was obtained with yeast extract followed by peptone (8.1 mg/l/h) and ammonium chloride (7.7 mg/l/h). Comparing these results, it was found that peptone and yeast extract were the two most efficient nitrogen sources for dye removal by A. lentulus. However, looking at the cost of these sources, their application in waste water treatment is limited. To devise suitable alternatives, urea and ammonium chloride can be tried in combination as NH4Cl displayed a high dye removal rate while urea showed high dye removal yield. Both these nitrogen sources also resulted in high uptake capacity (98.9 mg/g for urea and 85.1 mg/g for NH4Cl) of the fungal biomass. Promising results have been obtained when these two sources were used in combination for dye removal by the fungus (dye removal rate of 10 mg/l/h was achieved with 2:1 ratio of urea: NH4Cl).
Thus, composite media was modified by replacing yeast extract and NH4NO3 with urea and NH4Cl so that final nitrogen content remains as 10 mM. Owing to its low cost, glucose was retained in the modified composite media as the carbon source but its amount was reduced to 5 g/l. The efficiency of this modified composite media was then tested on the real textile effluent.
Dye removal from textile effluent by A. lentulus
The characterization of the textile effluent generated from dyeing operation in a small scale textile unit is shown in Table 2. It was an orange coloured effluent with alkaline pH. Efficiency of A. lentulus in removing dye from this textile effluent was evaluated in various conditions. Experiments were set up with neat (unsupplemented) and supplemented effluent. Two types of media were used for supplementation: composite media and modified composite media (where yeast extract was replaced by a combination of urea and ammonium chloride). Negligible dye removal was observed in the flasks containing neat effluent even after 72 h of incubation (Fig. 9). Neither the native microbes nor the fungal isolate was able to grow in the absence of nutrients. This implies that the effluent in itself was incapable of supporting the microbial growth. When supplementation was provided with modified composite media, (the flask containing sterilized effluent with fungal inoculum), 56.4% dye was removed in 72 h. In the flasks containing unsterilized effluent and no fungal inoculum, native microbes (present in the effluent) could bring about only 0.8% dye removal in 72 h. This shows that native microbes are not able to bring about dye removal even after nutrient supplementation. Nevertheless when unsterilized effluent was inoculated with fungus (A. lentulus + native microbes) 65.9% dye removal was obtained. Pellets of A. lentulus were prominently visible and biomass measurements showed ample growth of the fungus. This suggests that A. lentulus which is much more efficient than native microbes can very well compete and bring about even higher removal synergistically. The results were at par when composite media containing yeast extract was used for supplementation. Thus, these results indicate the suitability of urea and ammonium chloride as nitrogen source (in place of expensive yeast extract) along with glucose as the carbon source for supporting the growth and dye removal by A. lentulus from textile effluent.
Efficiency of A. lentulus for removing dye from neat textile effluent (NE) and supplemented textile effluent (SE) in various conditions; NE (U-I): Unsterilized, without inoculum; NE (U+I) Unsterilized, with inoculum; NE (S+I): Sterilized, with inoculum; SEMM (U-I): Unsterilized, without inoculum; SEMM (U+I): Unsterilized, with inoculum; SEMM (S+I): Sterilized, with inoculum and SECM (U+I): Unsterilized, with inoculum. MM denotes supplementation with modified composite media and CM denotes supplementation with composite media (temperature: 30°C; pH: 8.1)
Thus, applicability of A. lentulus in treating dye effluent can be clearly established. Also, A. lentulus is capable of removing various azo dyes like Acid Navy Blue, Acid Sulphone Blue, Fast Red A (Kaushik and Malik 2009b). Azo dyes are widely used dyes in the textile industries and account for over 60% of the total number of dyes being manufactured (Fu and Viraraghavan 2001). Thus, an isolate capable of removing these dyes efficiently, holds a potential to be exploited for treating dye effluent from textile industries.
Conclusions
From the studies conducted so far, it can be concluded that glucose (carbon source) and yeast extract (nitrogen source) can be replaced by certain locally available materials for supporting fungal growth and dye removal. Starch, molasses and jaggery can efficiently replace glucose without negatively impacting the dye removal. Among the nitrogen sources, yeast extract and peptone performed best in terms of dye removal rate. While replacing these sources with low-cost alternates, a significant reduction in dye removal tae was observed. Nevertheless, a combination of urea and ammonium chloride proved to be suitable nitrogen sources with high dye removal rate. Thus a modified composite media was formulated comprising half the glucose as the carbon source and combination of urea and ammonium chloride as the nitrogen source. The efficiency of modified composite media was tested for dye removal from real textile effluents. It was found that performance of modified composite media was at par with composite media, thus eliminating the use of expensive yeast extract for treatment of dye effluents. Further, investigations on the optimization of this modified composite media through response surface methodology can be taken up to enhance the performance and dye removal by A. lentulus.
References
Assadi MM, Rostami K, Shahvali M, Azin M (2001) Decolorization of textile wastewater by Phanerochaete chrysosporium. Desalination 141:331–336
Fu Y, Viraraghavan T (2001) Fungal decolourization of dye wastewaters: a review. Bioresour Technol 79:251–262
Hatvani N, Mecs I (2002) Effect of the nutrient composition on dye decolourisation and extracellular enzyme production by Lentinus edodes on solid medium. Enzyme Microb Technol 30:381–386
Joshi M, Bansal R, Purwar R (2004) Colour removal from textile effluents. Indian J Fiber Textile Res 29:239–259
Kapdan IK, Kargia F, McMullanb G, Marchant R (2000) Effect of environmental conditions on biological decolourization of textile dyestuff by C. versicolor. Enzyme Microb Technol 26:381–387
Kaushik P, Malik A (2009a) Fungal dye decolourization: recent advances and future potential. Environ Int 35:127–141
Kaushik P, Malik A (2009b) Microbial decolourization of textile dyes through isolates obtained from contaminated sites. J Sci Ind Res 68:325–331
Liao W, Liu Y, Frear C, Chen S (2007) A new approach of pellet formation of a filamentous fungus—Rhizopus oryzae. Bioresour Technol 98:3415–3423
Liu GF, Zhou JT, Wang J, Song ZY, Qv YY (2006) Bacterial decolorization of azo dyes by Rhodopseudomonas palustris. World J Microbiol Biotechnol 22:1069–1074
Martins MAM, Cardoso MH, Queiroz MJ, Ramalho MT, Campos AMO (1999) Biodegradation of azo dyes by the yeast Candida zeylanoides in batch aerated cultures. Chemosphere 38:2455–2460
Mathur N, Bhatnagar P, Bakre P (2005) Assessing mutagenicity of textile dyes from Pali (Rajasthan) using AMES bioassay. Appl Ecol Environ Res 4:111–118
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mohana S, Shrivastava S, Divecha J, Madamwar D (2008) Response surface methodology for optimization of medium for decolorization of textile dye Direct Black 22 by a novel bacterial consortium. Bioresour Technol 99:562–569
Moosvi S, Kher X, Madamwar D (2007) Isolation, characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dyes Pigments 74:723–729
Parshetti GK, Kalme SD, Gomare SS, Govindwar SP (2007) Biodegradation of Reactive Blue-25 by Aspergillus ochraceus NCIM-1146. Bioresour Technol 98:3638–3642
Rigas F, Dritsa V (2006) Decolourisation of a polymeric dye by selected fungal strains in liquid cultures. Enzyme Microb Technol 39:120–124
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Sumathi S, Manju BS (2000) Uptake of reactive textile dyes by Aspergillus foetidus. Enzyme Microb Technol 27:347–355
Acknowledgments
Department of Science & Technology, Government of India is gratefully acknowledged for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaushik, P., Malik, A. Effect of nutritional conditions on dye removal from textile effluent by Aspergillus lentulus . World J Microbiol Biotechnol 26, 1957–1964 (2010). https://doi.org/10.1007/s11274-010-0376-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0376-9