Abstract
Although the incidence of intraventricular hemorrhage (IVH) and posthemorrhagic hydrocephalus (PHH) in preterm infants is decreasing, these conditions are still associated with poor neurodevelopmental and functional outcomes. Additionally, with the growing viability of infants born at younger estimated gestational ages (EGAs), these conditions remain significant burdens as IVH severity increases with prematurity. Despite improvements in neonatal care, there still lacks a uniform paradigm for the treatment and management of PHH. Patients with PHH typically are initially treated with a temporizing device that allows for these infants to develop more favorable immunologic and nutritional statuses; a permanent ventriculoperitoneal (VP) shunt is later inserted in cases of persistent ventricular dilation and symptomatic hydrocephalus. This patient population is at high risk for temporizing device and shunt complications, especially shunt obstruction and infection, slit-ventricle syndrome, and the development of loculated hydrocephalus. The impact of these complications on long-term neurodevelopmental outcomes is unclear; however, there are few alternative methods to avoid prolonged shunt dependence in these patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endoscopic Third Ventriculostomy
- External Ventricular Drainage
- Shunt Infection
- Device Infection
- Shunt Complication
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Posthemorrhagic Hydrocephalus
1.1 Introduction
Although the incidence of intraventricular hemorrhage (IVH) and posthemorrhagic hydrocephalus (PHH) in preterm infants is decreasing, these conditions are still associated with poor neurodevelopmental and functional outcomes [1]. Additionally, with the growing viability of infants born at younger estimated gestational ages (EGAs), these conditions remain significant burdens as IVH severity increases with prematurity. Despite improvements in neonatal care, there still lacks a uniform paradigm for the treatment and management of PHH. Patients with PHH typically are initially treated with a temporizing device that allows for these infants to develop more favorable immunologic and nutritional statuses; a permanent ventriculoperitoneal (VP) shunt is later inserted in cases of persistent ventricular dilation and symptomatic hydrocephalus. This patient population is at high risk for temporizing device and shunt complications, especially shunt obstruction and infection, slit-ventricle syndrome, and the development of loculated hydrocephalus. The impact of these complications on long-term neurodevelopmental outcomes is unclear; however, there are few alternative methods to avoid prolonged shunt dependence in these patients.
1.2 Pathophysiology of PHH
Preterm infants are at risk for an extensive array of neurologic complications, the most commonly observed of which is IVH. The susceptibility of these patients to cerebrovascular injury is not fully understood, but is thought to be in part due to a combination of several factors, including dysregulation of cerebral vasculature with systemic hemodynamic instability, the immaturity of several structures including the highly vascular germinal matrix, and underdeveloped cardiac and respiratory systems [2–4]. IVH typically results from rupture of vessels in the germinal matrix, a critical area of cellular proliferation in the developing brain. This area is extremely vulnerable to hemorrhage due to the fragility of its vascular network, but typically disappears around 33–35 weeks gestation.
Reports of the rate of PHH following IVH vary from 25 to 74 % [5]. The likelihood of IVH progressing to PHH depends in part on the severity of the initial hemorrhagic event, which can be assessed by the Papile grading scale [6, 7]. A grade I IVH involves less than 10 % of the ventricle, while a grade II IVH extends to less than 50 % of the ventricle. Grade III indicates involvement of more than 50 % of the ventricle with ventricular dilation, and a grade IV hemorrhage indicates ventricular dilation with periventricular white matter involvement. It has been theorized that PHH develops from IVH due to insufficient fibrinolysis of microthrombi which cause obstruction of arachnoid villi, impairing CSF resorption. Larger blood clots may also obstruct flow within regions of the ventricular system. Additionally, the breakdown of blood products is thought to result in the recruitment of pro-inflammatory factors and the deposition of extracellular matrix, leading to meningeal fibrosis and subependymal gliosis. These processes can obstruct CSF outflow from the aqueduct of Sylvius and the foramina of the fourth ventricle [5, 8].
1.3 Shunting in PHH and Complications
Clinical findings consistent with hydrocephalus, including vomiting, poor feeding, lethargy, and apnea, with increasing head circumference and fontanelle fullness along with corresponding findings of ventriculomegaly on imaging are indications for treatment. Though temporizing, nonsurgical methods such as serial lumbar punctures or ventricular taps may be attempted in some patients, many patients display persistently dilated ventricles or elevated intracranial pressures (ICP) and require surgical intervention. Previous studies of patients managed by serial tapping did not find an effect of treatment on neurodevelopmental outcomes or shunt dependence, and recurrent tapping was associated with high rates of central nervous system (CNS) infection [9]. For patients with rapidly progressive hydrocephalus, temporary external ventricular drainage (EVD) has been employed and offers the advantages of ICP monitoring and control. A catheter is inserted into the lateral ventricle and is tunneled subcutaneously under the scalp to connect to an external drainage system. However, these devices are associated with several complications, including device infection, dislocation, and occlusion, as well as overdrainage and the development of subdural hygromas. Infection rates vary from 5.4 to 7.1 % in prior reports, and infection of EVD systems have been associated with poor long-term outcomes [9–12]. Moreover, the rates of these patients who eventually need permanent shunting are high, ranging from 64 to 68 % [11, 13].
Typically, one of two temporizing devices, the ventricular reservoir or the ventriculosubgaleal shunt (VSGS), is initially placed upon diagnosis of PHH to delay placement of a permanent VP shunt. A retrospective study found that early VP shunt insertion is associated with higher rates of shunt infection and mechanical obstruction [14]. In comparing shunt outcomes between patients who initially received VP shunts and those who initially had ventricular reservoirs inserted, it was found that despite the fact that directly shunted patients were at higher weights and EGAs, they experienced higher rates of shunt revision [15]. Therefore, initial treatment with a reservoir prior to shunt insertion seems to be beneficial. The risks associated with early VP shunt insertion have been theorized to be related in part to the presence of blood breakdown products in the CSF; however, a study which examined the relationship between shunt outcomes and CSF parameters including cell count, protein level, and glucose levels did not find an association between alterations in CSF content and shunt complications [16]. Other proposed benefits of initial insertion of a temporary device include allowing time for optimization of patient factors, including nutritional and immunologic statuses, and the potential for avoiding permanent shunting; however, timing and choice of the initial intervention relies heavily on clinical judgment.
The ventricular reservoir was first introduced in the 1980s and requires CSF to be manually removed by serial reservoir taps. One of the primary complications of reservoir insertion is device infection, thought to be related to the requirement for repeated reservoir access. Early reports found infection rates ranging from 8 to 10 %, but subsequent series have reported that infection rates have decreased over time to approximately 5 % [17–21]. A proposed advantage of ventricular tapping is the removal of CSF and clearance of blood breakdown products and cellular debris; however, this theorized benefit has not been borne out in studies [22]. An important practical consideration with reservoirs is the frequency of taps and the volume of CSF removed with each tap. Depending on the rate of fluid reaccumulation and patient symptomatology, patients may require daily CSF removal. However, there is significant variability in tapping practices with respect to regularity of tapping, the amount of fluid removed, and the use of clinical features and imaging to guide tapping. A study of patients treated with reservoirs found that only taps that achieved ICPs of less than 7 cm H2O were able to achieve appreciable differences in cerebral blood flow velocity [23]. Removal of a consistent volume of CSF at regular intervals is also not ideal and results in rapid fluctuations in ICP [24]. Ultimately, most patients initially managed with reservoirs ultimately require permanent shunting, with studies reporting rates of VP shunting at 75–88 % [2].
An alternative temporizing device, the VSGS, consists of a ventricular reservoir, from which CSF is redirected to a subgaleal scalp pocket for reabsorption (Fig. 10.1). Because the device does not necessitate repeated manual CSF removal, the risk for device infection is theoretically lower. However, studies have not demonstrated a significant difference in device infection rates between patients treated with the reservoir and VSGS, with rates in VSGS patients ranging from 0 to 14 % (Table 10.1) [25, 26]. It has been proposed that the comparable risk of infection may be a result of CSF stasis within the subgaleal pocket. The rates of permanent shunt insertion in this population have also been comparable to those treated with the reservoir, at approximately 60–100 % [2, 26]. A multicenter retrospective study found that reservoir patients experienced lower rates of VP shunt insertion compared to VSGS patients [27]. However, other studies which have directly compared the two temporizing devices have found comparable rates of shunt infection and revision, permanent shunt placement, and mortality (Table 10.1) [28, 29]. In previous studies, reported VSGS revision rates ranged from 25 to 28 %; in most of these cases, revisions occurred due to the development of adhesions within the subgaleal pocket rather than shunt dysfunction [30]. Reports of mortality rates varied from 9 to 20 %; however, the causes of death were not reported, and the severity of the preceding IVH was not accounted for [30, 31]. Other less common complications seen with the VSGS include CSF leakage from the incision site, catheter migration, and intraparenchymal hemorrhage. The rates of CSF leakage vary from 4.7 to 32 % in studies; much of this variation appears to be due to surgical technique [25, 26, 32]. Intraparenchymal hemorrhage appears rare and has been reported in two studies, with three cases total in the literature [25, 26, 30]. The impact of treatment with the VSGS on long-term neurodevelopmental outcomes has yet to be fully investigated.
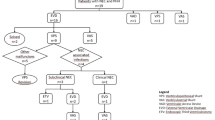
Although efforts are made to avoid permanent shunting in infants with PHH to prevent long-term shunt dependence, the overall rate of permanent shunt insertion ranges from 0 to 20 % in patients with IVH [15, 28, 33, 34]. The majority of patients initially treated with temporizing devices ultimately undergo permanent shunt placement. The most commonly inserted device is the VP shunt, although selected individuals require CSF diversion to other locations. Even after a temporizing device has been initially inserted, timing of permanent shunting is controversial and relies on clinical judgment of a patient’s surgical candidacy and evaluation of factors including infant weight, medical stability, and CSF profile [9]. Several studies have found that compared to infants with hydrocephalus of other etiologies, those with PHH are at increased risk of complications including shunt infection and occlusion requiring revision, slit-ventricle syndrome, and loculated hydrocephalus (Fig. 10.2) [35]. Rates of VP shunt infection in PHH patients have been reported at approximately 13 %, compared to 4–8.5 % overall in shunted populations [2, 5, 36–39]. The high rates of shunt infection seen in this population have been hypothesized to be in part due to an immature and dysregulated immune system. The use of antibiotic-impregnated shunt catheters has been proposed, and a prospective study of these devices reported an infection rate of 6.8 % [40]. IVH and PHH are also associated with a need for multiple shunt revisions, for which the most common indication is shunt failure secondary to obstruction [41]. A recent retrospective study reported a revision rate of 71.6 % and a multiple revision rate of 55 % for complications including obstruction and overdrainage; lower birth weight and EGA were found to be risk factors for multiple revisions [42].
1.4 Alternatives to Shunting and Adjunctive Therapies
VP shunting is currently the mainstay of treatment in PHH, but several alternative medical and surgical treatment methods have been proposed. Trials of diuretic therapy with acetazolamide or furosemide have found that drug treatment with CSF tapping is associated with higher rates of eventual permanent shunt insertion and increased risk for motor impairment and nephrocalcinosis compared to management with CSF removal alone [43, 44]. Currently, there is no evidence to support the use of diuretic therapy in PHH. The use of fibrinolytics in preventing the development of hydrocephalus in IVH has also been suggested, but two randomized trials investigating the effects of intraventricular streptokinase in PHVD have not shown any benefit with respect to rates of permanent shunting or neurodevelopmental outcomes [45]. A multicenter randomized trial on PHH prevention with intraventricular tissue plasminogen activator (tPA) with a procedure involving drainage, irrigation, and fibrinolytic therapy (DRIFT) also did not find an effect of this management protocol on the rates of permanent shunting. However, although DRIFT was found to be associated with an increased risk for secondary hemorrhage, patients in the treatment group experienced better neurodevelopmental outcomes compared to the control group, with lower rates of mortality and cognitive disability [46, 47].
A promising surgical approach for PHH is endoscopic third ventriculostomy (ETV). Though outcomes of cases managed with ETV alone are inconsistent, recent studies of ETV with choroid plexus coagulation (CPC) are encouraging. In selected PHH patients, ETV with CPC may offer a means of continued CSF diversion without long-term shunt dependence [2, 48].
1.5 Long-Term Outcomes of PHH and Shunting
Improvement in neurodevelopmental outcomes after IVH and PHH has been largely attributed to improvements in neonatal care. Early studies from 1970 to 1980 reported significant cognitive disability and mortality associated with grade IV IVH [49]. Later reports detailed high rates of neurodevelopmental problems including sensory and motor deficits, visual impairment, hearing loss, seizures, and cognitive and behavioral disturbances in patients with severe IVH [50–52]. As these patients typically also suffer from an extensive array of medical comorbidities, poor outcomes are unlikely to be due to IVH and PHH alone. However, the effect of permanent shunting on long-term outcomes is incompletely understood. Although some studies have found that shunt insertion and complications are risk factors for poor outcomes, a study of functional outcomes in PHH patients did not find differences in the rates of functional independence in shunted and non-shunted patients [53–55]. The most critical determinant of neurologic outcome appears to be IVH severity, with patients who develop periventricular hemorrhagic infarction are at highest risk for the development of complications including cerebral palsy [33].
2 Postinflammatory Hydrocephalus
2.1 Introduction
Hydrocephalus which results from CNS infection poses a unique set of challenges with respect to treatment. The pathophysiologic mechanisms of hydrocephalus development are incompletely understood, but obstruction of CSF flow is thought to result from the inflammatory debris within the subarachnoid space and meningeal scarring. Patients with persistent hydrocephalus despite infection resolution are typically treated with permanent shunt insertion. Studies have demonstrated that patients with postinflammatory hydrocephalus (PIH) are at increased risk for shunt complications, including shunt infection and obstruction requiring surgical revision, compared to patients with hydrocephalus of other etiologies. Although there have been few reports on long-term outcomes of shunting in this population, avoidance of shunt dependence remains an important goal of management. Alternative interventions including ETV have demonstrated promise in selected patients with favorable ventricular anatomy.
2.2 Pathophysiology of PIH
The mechanism of PIH is believed to be secondary to obstruction of the basal cisterns and blockage of CSF outflow secondary to inflammation and meningeal fibrosis and scarring [56]. Neonatal meningitis is also considered a risk factor for the development of multiloculated hydrocephalus [56–59]. However, the mechanism of hydrocephalus often varies by pathogen. With Toxoplasmosis gondii, the parasites are thought to cause obstruction of the ventricular system via damage to the ependymal linings of the lateral ventricles. Other studies have suggested that hydrocephalus develops as a result of leptomeningeal inflammation in reaction to the parasite. Acquisition of CMV during the in utero period is associated with the development of hydrocephalus ex vacuo secondary to cortical atrophy as well as obstructive hydrocephalus from periventricular inflammation [5].
In the postnatal period, the most common cause of PIH is bacterial infection. Studies performed in the 1980s of children afflicted with bacterial meningitis have reported PIH rates at approximately 30 % [5, 57, 60]. However, the incidence of post-meningitic hydrocephalus in the pediatric population has not been well established since advancements in neonatal care. Brain abscesses can also result in obstructive hydrocephalus from mass effect and ventricular compression. Additionally, abscesses can lead to the development of loculated hydrocephalus, thought to be due to subependymal inflammation and infarction resulting in cyst formation. The pathogenesis of hydrocephalus from viral infections varies by virus; viruses often have specific tropisms for ependymal or meningeal cells.
Special consideration should be given to hydrocephalus in patients with tuberculosis with CNS involvement. Tuberculous meningitis typically favors the base of the brain, where infection results in exudate that obstructs the basal cisterns, preventing CSF outflow from the foramina of the fourth ventricle. Less commonly, intracerebral tuberculomas can cause hydrocephalus by compression of the ventricular system. Fungal pathogens including Cryptococcus neoformans and Coccidioides immitis can cause post-meningitic hydrocephalus; however, the pathogenesis of hydrocephalus in these cases is less well understood [5].
2.3 Management of PIH and Complications
An active infection and progressive hydrocephalus presents a therapeutic challenge, as insertion of a foreign device to divert CSF may exacerbate the infection. In the acute setting, repeated lumbar or ventricular taps may be required for management. Studies from the 1960s and 1970s of post-meningitic hydrocephalus reported high mortality rates with placement of a ventricular reservoir or with EVD [61]. Persistent hydrocephalus is typically managed by VP shunt insertion, though there is considerable variability in practice depending on patient and pathogen. Depending on the pathogen, CNS infection can result in a wide range of other sequelae and complications not causally related to the development of hydrocephalus. Regardless, studies have shown that patients with PIH are at increased risk for shunt complications including shunt infection, occlusion, and multiple shunt failures and are also at risk for development of loculated hydrocephalus (Fig. 10.3) [41, 62]. Preexisting multiloculated hydrocephalus appears to increase the risk for shunt complications, especially device infection, and for poor outcomes overall. Management options include placement of multiple shunts and fenestration of intraventricular septations, but there are no evidence-based guidelines regarding treatment choice [57].
There is a dearth of literature on methods for reducing shunt complications and alternatives to shunting for PIH. A study of the use of VSGS in PIH found that compared to patients with PHH, VSGS complication rates are comparable between the two groups except with device infection, for which PIH patients experienced higher rates [63]. ETV has had encouraging results in patients with PIH, though patients need to be carefully selected based on ventricular system anatomy. In a study of non-shunted and shunt-dependent patients with PHH or PIH, the success rate of ETV in causing durable resolution of hydrocephalus was 60 % in PIH patients [64].
Recently, ETV has received attention for its promise as a cost-effective therapy for PIH in the developing world. A study of long-term outcomes in 149 Ugandan infants with PIH compared treatment with ETV with or without CPC and those treated with permanent shunt insertion and found no significant differences in survival between the two groups. Though ETV patients experienced a lower incidence of functional dependence and disability, this was attributed mostly to treatment selection and shunting of infants with the most severe cases of PIH. The authors cited that ETV success was dependent on the absence of scarring within the prepontine cistern [65].
2.4 Long-Term Outcomes of PIH
The neurodevelopmental outcomes of patients with PIH vary widely, but studies have found that these patients experience higher rates of epilepsy and cognitive and functional impairment and experience lower quality of life compared to pediatric patients with hydrocephalus of other etiologies [38, 66–71]. Most of these studies only include a small number of PIH patients; in a 2007 questionnaire-based study with four PIH patients, one patient attended normal primary school, two patients were without motor disability, and one patient eventually developed epilepsy. Outcomes were assessed with the Hydrocephalus Outcomes Questionnaire; overall, there were no significant differences between PIH patients and other hydrocephalus patients in the cognitive, physical, and social-emotional health domains [66, 72]. A study of hydrocephalus outcomes in adulthood found that patients with childhood post-meningitic hydrocephalus were at higher risk for developing cognitive impairment compared to those patients who developed hydrocephalus at older ages or as a result of a focal brain lesion. Though many studies support the idea that PIH itself is associated with poor functional outcomes, few studies have examined the impact of PIH on long-term shunt outcomes and the effects of shunting and shunt complications on neurodevelopment.
References
Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B (2011) Annual summary of vital statistics: 2008. Pediatrics 127(1):146–157. doi:10.1542/peds.2010-3175
Robinson S (2012) Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr 9(3):242–258. doi:10.3171/2011.12.PEDS11136
du Plessis AJ (2008) Cerebrovascular injury in premature infants: current understanding and challenges for future prevention. Clin Perinatol 35(4):609–641. doi:10.1016/j.clp.2008.07.010
du Plessis AJ (2009) The role of systemic hemodynamic disturbances in prematurity-related brain injury. J Child Neurol 24(9):1127–1140. doi:10.1177/0883073809339361
Cinalli G, Maixner WJ, Sainte-Rose C (2004) Pediatric hydrocephalus. Springer, Milan
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92(4):529–534
Volpe JJ (2008) Neurology of the newborn. Elsevier Health Sciences, Philadelphia
Cherian S, Whitelaw A, Thoresen M, Love S (2004) The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14(3):305–311
Shooman D, Portess H, Sparrow O (2009) A review of the current treatment methods for posthaemorrhagic hydrocephalus of infants. Cerebrospinal Fluid Res 6:1. doi:10.1186/1743-8454-6-1
Weninger M, Salzer HR, Pollak A, Rosenkranz M, Vorkapic P, Korn A, Lesigang C (1992) External ventricular drainage for treatment of rapidly progressive posthemorrhagic hydrocephalus. Neurosurgery 31(1):52–57; discussion 57–58
Rhodes TT, Edwards WH, Saunders RL, Harbaugh RE, Little CL, Morgan LJ, Sargent SK (1987) External ventricular drainage for initial treatment of neonatal posthemorrhagic hydrocephalus: surgical and neurodevelopmental outcome. Pediatr Neurosci 13(5):255–262
Berger A, Weninger M, Reinprecht A, Haschke N, Kohlhauser C, Pollak A (2000) Long-term experience with subcutaneously tunneled external ventricular drainage in preterm infants. Childs Nerv Syst 16(2):103–109; discussion 110
Cornips E, Van Calenbergh F, Plets C, Devlieger H, Casaer P (1997) Use of external drainage for posthemorrhagic hydrocephalus in very low birth weight premature infants. Childs Nerv Syst 13(7):369–374
Taylor AG, Peter JC (2001) Advantages of delayed VP shunting in post-haemorrhagic hydrocephalus seen in low-birth-weight infants. Childs Nerv Syst 17(6):328–333
Willis B, Javalkar V, Vannemreddy P, Caldito G, Matsuyama J, Guthikonda B, Bollam P, Nanda A (2009) Ventricular reservoirs and ventriculoperitoneal shunts for premature infants with posthemorrhagic hydrocephalus: an institutional experience. J Neurosurg Pediatr 3(2):94–100. doi:10.3171/2008.11.PEDS0827
Fulkerson DH, Vachhrajani S, Bohnstedt BN, Patel NB, Patel AJ, Fox BD, Jea A, Boaz JC (2011) Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in low-birth-weight premature infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr 7(2):147–151. doi:10.3171/2010.11.PEDS10244
Brouwer AJ, Groenendaal F, van den Hoogen A, Verboon-Maciolek M, Hanlo P, Rademaker KJ, de Vries LS (2007) Incidence of infections of ventricular reservoirs in the treatment of post-haemorrhagic ventricular dilatation: a retrospective study (1992-2003). Arch Dis Child Fetal Neonatal Ed 92(1):F41–F43. doi:10.1136/adc.2006.096339
Hudgins RJ, Boydston WR, Gilreath CL (1998) Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatr Neurosurg 29(6):309–313, doi:28744
Brockmeyer DL, Wright LC, Walker ML, Ward RM (1989) Management of posthemorrhagic hydrocephalus in the low-birth-weight preterm neonate. Pediatr Neurosci 15(6):302–307; discussion 308
Benzel EC, Reeves JP, Nguyen PK, Hadden TA (1993) The treatment of hydrocephalus in preterm infants with intraventricular haemorrhage. Acta Neurochir 122(3–4):200–203
Gurtner P, Bass T, Gudeman SK, Penix JO, Philput CB, Schinco FP (1992) Surgical management of posthemorrhagic hydrocephalus in 22 low-birth-weight infants. Childs Nerv Syst 8(4):198–202
Whitelaw A (2001) Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev (1):CD000216. doi: 10.1002/14651858.CD000216
Maertzdorf WJ, Vles JS, Beuls E, Mulder AL, Blanco CE (2002) Intracranial pressure and cerebral blood flow velocity in preterm infants with posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed 87(3):F185–F188
Bass JK, Bass WT, Green GA, Gurtner P, White LE (2003) Intracranial pressure changes during intermittent CSF drainage. Pediatr Neurol 28(3):173–177
Tubbs RS, Banks JT, Soleau S, Smyth MD, Wellons JC 3rd, Blount JP, Grabb PA, Oakes WJ (2005) Complications of ventriculosubgaleal shunts in infants and children. Childs Nerv Syst 21(1):48–51. doi:10.1007/s00381-004-0967-6
Fulmer BB, Grabb PA, Oakes WJ, Mapstone TB (2000) Neonatal ventriculosubgaleal shunts. Neurosurgery 47(1):80–83; discussion 83–84
Wellons JC, Shannon CN, Kulkarni AV, Simon TD, Riva-Cambrin J, Whitehead WE, Oakes WJ, Drake JM, Luerssen TG, Walker ML, Kestle JR (2009) A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr 4(1):50–55. doi:10.3171/2009.2.PEDS08400
Limbrick DD Jr, Mathur A, Johnston JM, Munro R, Sagar J, Inder T, Park TS, Leonard JL, Smyth MD (2010) Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr 6(3):224–230. doi:10.3171/2010.5.PEDS1010
Lam HP, Heilman CB (2009) Ventricular access device versus ventriculosubgaleal shunt in post hemorrhagic hydrocephalus associated with prematurity. J Matern Fetal Neonatal Med 22(11):1097–1101. doi:10.3109/14767050903029576
Koksal V, Oktem S (2010) Ventriculosubgaleal shunt procedure and its long-term outcomes in premature infants with post-hemorrhagic hydrocephalus. Childs Nerv Syst 26(11):1505–1515. doi:10.1007/s00381-010-1118-x
Acakpo-Satchivi L, Shannon CN, Tubbs RS, Wellons JC 3rd, Blount JP, Iskandar BJ, Oakes WJ (2008) Death in shunted hydrocephalic children: a follow-up study. Childs Nerv Syst 24(2):197–201. doi:10.1007/s00381-007-0408-4
Sklar F, Adegbite A, Shapiro K, Miller K (1992) Ventriculosubgaleal shunts: management of posthemorrhagic hydrocephalus in premature infants. Pediatr Neurosurg 18(5–6):263–265
Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L (2008) Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr 152(5):648–654. doi:10.1016/j.jpeds.2007.10.005
Lee IC, Lee HS, Su PH, Liao WJ, Hu JM, Chen JY (2009) Posthemorrhagic hydrocephalus in newborns: clinical characteristics and role of ventriculoperitoneal shunts. Pediatr Neonatol 50(1):26–32. doi:10.1016/S1875-9572(09)60026-7
Vinchon M, Rekate H, Kulkarni AV (2012) Pediatric hydrocephalus outcomes: a review. Fluids Barriers CNS 9(1):18. doi:10.1186/2045-8118-9-18
Kulkarni AV, Drake JM, Lamberti-Pasculli M (2001) Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94(2):195–201. doi:10.3171/jns.2001.94.2.0195
Odio C, McCracken GH Jr, Nelson JD (1984) CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child 138(12):1103–1108
Bourgeois M, Sainte-Rose C, Cinalli G, Maixner W, Malucci C, Zerah M, Pierre-Kahn A, Renier D, Hoppe-Hirsch E, Aicardi J (1999) Epilepsy in children with shunted hydrocephalus. J Neurosurg 90(2):274–281. doi:10.3171/jns.1999.90.2.0274
Renier D, Lacombe J, Pierre-Kahn A, Sainte-Rose C, Hirsch JF (1984) Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg 61(6):1072–1078. doi:10.3171/jns.1984.61.6.1072
Sciubba DM, Noggle JC, Carson BS, Jallo GI (2008) Antibiotic-impregnated shunt catheters for the treatment of infantile hydrocephalus. Pediatr Neurosurg 44(2):91–96. doi:10.1159/000113109
Lazareff JA, Peacock W, Holly L, Ver Halen J, Wong A, Olmstead C (1998) Multiple shunt failures: an analysis of relevant factors. Childs Nerv Syst 14(6):271–275
Chittiboina P, Pasieka H, Sonig A, Bollam P, Notarianni C, Willis BK, Nanda A (2013) Posthemorrhagic hydrocephalus and shunts: what are the predictors of multiple revision surgeries? J Neurosurg Pediatr 11(1):37–42. doi:10.3171/2012.8.PEDS11296
Whitelaw A, Kennedy CR, Brion LP (2001) Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev (2):CD002270. doi: 10.1002/14651858.CD002270
International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group (1998). Lancet 352 (9126):433–440
Yapicioglu H, Narli N, Satar M, Soyupak S, Altunbasak S (2003) Intraventricular streptokinase for the treatment of posthaemorrhagic hydrocephalus of preterm. J Clin Neurosci 10(3):297–299
Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, Hunt L, Carter M, Pople I (2010) Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125(4):e852–e858. doi:10.1542/peds.2009-1960
Whitelaw A, Aquilina K (2012) Management of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed 97(3):F229-3. doi:10.1136/adc.2010.190173
Warf BC, Campbell JW, Riddle E (2011) Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst 27(7):1063–1071. doi:10.1007/s00381-011-1475-0
Pikus HJ, Levy ML, Gans W, Mendel E, McComb JG (1997) Outcome, cost analysis, and long-term follow-up in preterm infants with massive grade IV germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery 40(5):983–988; discussion 988–989
Boynton BR, Boynton CA, Merritt TA, Vaucher YE, James HE, Bejar RF (1986) Ventriculoperitoneal shunts in low birth weight infants with intracranial hemorrhage: neurodevelopmental outcome. Neurosurgery 18(2):141–145
Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R (2008) Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121(5):e1167–e1177. doi:10.1542/peds.2007-0423
Gupta N, Park J, Solomon C, Kranz DA, Wrensch M, Wu YW (2007) Long-term outcomes in patients with treated childhood hydrocephalus. J Neurosurg 106(5 Suppl):334–339. doi:10.3171/ped.2007.106.5.334
Vassilyadi M, Tataryn Z, Shamji MF, Ventureyra EC (2009) Functional outcomes among premature infants with intraventricular hemorrhage. Pediatr Neurosurg 45(4):247–255. doi:10.1159/000228982
Maitre NL, Marshall DD, Price WA, Slaughter JC, O'Shea TM, Maxfield C, Goldstein RF (2009) Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics 124(6):e1153–e1160. doi:10.1542/peds.2009-0953
Resch B, Gedermann A, Maurer U, Ritschl E, Muller W (1996) Neurodevelopmental outcome of hydrocephalus following intra-/periventricular hemorrhage in preterm infants: short- and long-term results. Childs Nerv Syst 12(1):27–33
Handler L, Wright M (1978) Postmeningitic hydrocephalus in infancy. Neuroradiology 16(1):31–35
Nida TY, Haines SJ (1993) Multiloculated hydrocephalus: craniotomy and fenestration of intraventricular septations. J Neurosurg 78(1):70–76. doi:10.3171/jns.1993.78.1.0070
Albanese V, Tomasello F, Sampaolo S (1981) Multiloculated hydrocephalus in infants. Neurosurgery 8(6):641–646
Kalsbeck JE, DeSousa AL, Kleiman MB, Goodman JM, Franken EA (1980) Compartmentalization of the cerebral ventricles as a sequela of neonatal meningitis. J Neurosurg 52(4):547–552. doi:10.3171/jns.1980.52.4.0547
Lorber J, Pickering D (1966) Incidence and treatment of post-meningitic hydrocephalus in the newborn. Arch Dis Child 41(215):44–50
De Villiers J, Clüver PV, Handler L (1978) Complications following shunt operations for post-meningitic hydrocephalus. In: Treatment of hydrocephalus computer tomography. Springer, Berlin, pp 23–27
Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M (2000) Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg 92(1):31–38. doi:10.3171/jns.2000.92.1.0031
Nagy A, Bognar L, Pataki I, Barta Z, Novak L (2013) Ventriculosubgaleal shunt in the treatment of posthemorrhagic and postinfectious hydrocephalus of premature infants. Childs Nerv Syst 29(3):413–418. doi:10.1007/s00381-012-1968-5
Smyth MD, Tubbs RS, Wellons JC 3rd, Oakes WJ, Blount JP, Grabb PA (2003) Endoscopic third ventriculostomy for hydrocephalus secondary to central nervous system infection or intraventricular hemorrhage in children. Pediatr Neurosurg 39(5):258–263, doi:72871
Warf BC, Dagi AR, Kaaya BN, Schiff SJ (2011) Five-year survival and outcome of treatment for postinfectious hydrocephalus in Ugandan infants. J Neurosurg Pediatr 8(5):502–508. doi:10.3171/2011.8.PEDS11221
Platenkamp M, Hanlo PW, Fischer K, Gooskens RH (2007) Outcome in pediatric hydrocephalus: a comparison between previously used outcome measures and the hydrocephalus outcome questionnaire. J Neurosurg 107(1 Suppl):26–31. doi:10.3171/PED-07/07/026
Kao CL, Yang TF, Wong TT, Cheng LY, Huang SY, Chen HS, Chan RC (2001) The outcome of shunted hydrocephalic children. Zhonghua Yi Xue Za Zhi (Taipei) 64(1):47–53
Vinchon M, Baroncini M, Delestret I (2012) Adult outcome of pediatric hydrocephalus. Childs Nerv Syst 28(6):847–854. doi:10.1007/s00381-012-1723-y
Sgouros S, Malluci C, Walsh AR, Hockley AD (1995) Long-term complications of hydrocephalus. Pediatr Neurosurg 23(3):127–132
Vinchon M, Dhellemmes P (2006) Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst 22(7):692–697. doi:10.1007/s00381-005-0037-8
Casey AT, Kimmings EJ, Kleinlugtebeld AD, Taylor WA, Harkness WF, Hayward RD (1997) The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg 27(2):63–70
Kulkarni AV, Rabin D, Drake JM (2004) An instrument to measure the health status in children with hydrocephalus: the hydrocephalus outcome questionnaire. J Neurosurg 101(2 Suppl):134–140. doi:10.3171/ped.2004.101.2.0134
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Wang, J.Y., Ahn, E.S. (2015). Posthemorrhagic and Postinflammatory Complications. In: Di Rocco, C., Turgut, M., Jallo, G., Martínez-Lage, J. (eds) Complications of CSF Shunting in Hydrocephalus. Springer, Cham. https://doi.org/10.1007/978-3-319-09961-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-09961-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09960-6
Online ISBN: 978-3-319-09961-3
eBook Packages: MedicineMedicine (R0)