Abstract
This chapter focuses on the nitrogen (N) cycle, a complex network of mainly microbial transformations in which various nitrogen compounds are interconverted. Both microorganisms and plants absorb N from and excrete N into the environment. First, N assimilation is addressed (22.1), after which N transformations by microorganisms are described (22.2). In paragraph 22.3 both plant and microbial N cycling are discussed at the ecosystem level, followed by paragraph 22.4, where the use of N by humans and the consequences for the N cycle are reviewed. Finally, in 22.5 the conclusions and outlook are presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anammox Bacterium
- Nitrification Inhibitor
- Increase Crop Productivity
- Nitric Oxide Reductase
- Intensive Animal
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 N Metabolism of Plants
After the discovery in the early 1900s that N compounds could increase crop productivity, this topic was intensively studied. Generally N is a limiting nutrient for plant production and mineralization, hence N availability is an important controlling factor for ecosystem processes. The N cycle is also tightly coupled to the carbon (C) cycle. Access to N dictates both the photosynthetic activity, which is the main C input in plants, and the production of protein (Larcher 2001) . N compounds are incorporated into plant material when (1) the N compound is available, when (2) the plant has adequate uptake systems and (3) when all assimilatory complexes are present and active .
N Sources
In terrestrial ecosystems, the soil acts as a nutrient reserve for plants, where 98 % of the mineral nutrient supply is bound in humus, organic matter and insoluble compounds and only less than 0.2 % is dissolved in water. The soil’s N content depends mostly on microbial mineralization of organic matter into inorganic N such as ammonium (NH4 +) or nitrate (NO3 −). N availability for plants is affected by the root structure and density, Radial Oxygen Loss (ROL), root exudates and symbioses with microorganisms (Jackson et al. 2008) . In anoxic environments such as wetlands, ROL is of special interest as it supplies oxygen (O2) to microorganisms (Lamers et al. 2012) .
Uptake of N
Terrestrial plants control mineral uptake via their roots. Furthermore N uptake and assimilation are always dependent on the availability of other nutrients, especially phosphorus. Although both inorganic and organic N (i.e. amino acids) can be taken up by plants, most studies have focused on inorganic N sources. Energetic costs of N uptake and assimilation are highest for NO3 −, followed by NH4 + and lowest for amino acids (Lambers et al. 1998) . NO3 − first has to be reduced to NH4 + before it can be assimilated. Terrestrial plants adapted to low pH and redox potential preferentially take up NH4 +. Subsequently, the release of H+ acidifies the rhizosphere, affecting the rhizosphere microbiome and causing reduced nitrification rates . Plants adapted to soils with a higher pH use preferentially NO3 − as it causes less soil acidification compared to NH4 + (Lamers et al. 2012; Larcher 2001; Britto and Kronzucker 2002) .
Terrestrial plants have both high and low affinity transport systems (HATS and LATS) for the active uptake of NO3 − and NH4 +. HATS take up inorganic N between 1 μM and 1 mM. LATS are only expressed when inorganic N is above 0.5 mM. Only for NO3 − uptake, inducible transporters are known. Uptake of NO3 − induces HATS, followed by positive feedback on the putative transporters, causing an increased uptake of NO3 −. Subsequently, in the cytoplasm NO3 − is first reduced to nitrite (NO2 −) by nitrate reductase (Nas), followed by nitrite reductase (Nir) into NH4 +. Uptake of NH4 + directly from the environment is mediated by ammonium transporters (AmtB) that are located in the cell membrane. Regulation of NO3 − absorption via HATS depends on the N status of the whole plant, whereas NH4 + uptake via HATS is locally regulated in the roots (Jackson et al 2008) . Passive uptake of inorganic N is mediated by LATS, which are energetically costly due to poor regulation.
The uptake and assimilation of N is not very different for (heterotrophic) microorganisms. AmtB’s are responsible for the uptake of ammonium, NO3 − uptake is mediated by ABC transport proteins or exchanged by NarK antiporters. NO2 −uptake is catalyzed by FocA.
Assimilation and Incorporation of N
The different steps in N assimilation in plant cells are comparable to assimilation in microorganisms. When NO3 − is transported to the cytoplasm, it is reduced to NO2 − by an assimilatory Nas. Subsequently Nir at the expense of ferrodoxin or NAD(P)H reduces NO2 − to NH4 +. NH4 + is used for the production of glutamate for which several systems exist. Glutamine synthetase forms glutamine from glutamate and NH4 +. Next, glutamine 2-oxoglutarate aminotransferase (GOGAT; glutamate synthase), catalyzes the synthesis of 2 glutamate from glutamine and 2-oxoglutarate. Furthermore, glutamate dehydrogenase, can convert glutamate back into 2-oxoglutarate and NH4 + during N remobilization. Subsequently, glutamate and glutamine are used to produce various amino acids (mostly aspartate and aspargine) via transamination. Via negative feedback glutamine concentrations regulate the N uptake by HATS. Internal transport of N occurs primarily as amino acids via xylem and phloem, and can be stored in young shoots, leaves, buds, seeds and storage organs of plants, from where it can be remobilized if needed (Jackson et al. 2008) .
N Requirements
N requirements differ greatly between plant species. Severe insufficient N supply often results in dwarf growth. Ultimately this leads to sclerosis, where plants have small cells and thickened cell walls. Excessive amounts of N, especially of NH4 +, can become toxic for plants. Most plausible processes involved in NH4 + toxicity are energetically costly membrane effluxes, reduced photosynthesis, displacement of cations and interference of hormone metabolism. Long term excessive N uptake will eventually lead to lowered resistance against abiotic and biotic stressors and delayed reproduction of the plant (Britto and Kronzucker 2002) .
2 Microbial N Cycle
After reviewing the N processes of plants, the N transformations of microorganisms will be discussed in the next paragraphs.
N2 Fixation
Nitrogen fixation is the only known biological process where dinitrogen gas (N2) is transformed into NH4 +. This energetically costly process (16 ATP per molecule of N2 fixed) is exclusively performed by N fixing microorganisms (diazotrophs) . The enzyme nitrogenase (nif) catalyzes this reaction and becomes inactive when exposed to O2. Diazotrophs occur free living and in symbiosis. The best studied diazotrophic symbiosis is that between legumes and Rhizobia sp., but it is also known for other plants, mosses and between microorganisms (Chap. 21). Even though direct evidence is missing, it seems likely that bryophytes were the first land plants that started to live in symbiosis with diazotrophs.
Nitrification
The subsequent oxidation of ammonium via NO2 − to NO3 − is being studied since the nineteenth century, as this process causes significant losses of fixed N that are no longer available for crops. Early studies focused on the metabolism of Nitrosomonas spp. that thrive at high NH4 + concentrations. The key enzyme ammonium monooxygenase (AmoA) produces hydroxylamine (NH2OH) and is present in large amounts in their membrane systems. NH2OH is converted into NO2 − by an hydroxylamine oxidoreductase (Hao). Further studies indicated that Nitrosospira spp. might be more relevant at lower NH4 + concentrations. NH4 + oxidizers mostly live in close proximity to NO2 − oxidizing bacteria (NOB) that convert the toxic NO2 − rapidly into NO3 − by a nitrite oxidoreductase (NxrAB) system . Nitrospira and Nitrospina spp. thrive at low NO2 − environments like freshwater and marine systems, respectively. In contrast to the expectation, only low numbers of bacterial nitrifiers were observed in marine surveys. About 20 % of the cells were of Archaeal origin, but their metabolism remained unknown until metagenomic inventories showed the presence of amoA genes on fosmids that also contained 16S rRNA gene copies of these Archaea. The isolation of the first archaeal NH4 + oxidizer Nitrosopumilis maritimus from a marine aquarium showed that Archaeal Ammonium-Oxidizers (AOA) have a very high affinity for NH4 + probably reflecting their low NH4 + habitats.
Denitrification
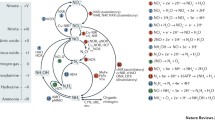
Denitrification is the oldest known process of the N cycle. In this process NO and N2O are produced from NO3 − and NO2 − and N2 is the final product. Most denitrifiers are facultative anaerobes, but some species may continue to denitrify in the presence of O2, a process known as aerobic denitrification. The denitrification trait is widespread among Bacteria, Archaea and can even be found in some Eukaryotes. The reduction of oxidized N species (NOx) is catalyzed by metalloenzymes that contain molybdenum, iron or copper. The electrons needed for the reduction are derived from oxidation of inorganic or organic sources Fig. 22.1).
Schematic overview of N transformation from ammonium (NH 4 +) and/or nitrate (NO 3 −) perspective. Corresponding genes are printed in italic (grey), where nif nitrogenase, amo ammonium monooxygenase, hao hydroxylamine oxidoreductase, nxr nitrite oxidoreductase, nar respiratory nitrate reductase, nap periplasmic nitrate reductase, nir nitrite reductase, nor nitric oxide reductase, nos nitrous oxide reductase, nrf multiheme nitrite reductase, nod putative NO dismutase and hzs hydrazine synthase. Enzymes nar and nap are involved in all conversions of NO3 − to NO2 −.Glutamine synthetase (GS) and glutamine 2-oxoglutarate aminotransferase (GOGAT) genes, both involved in assimilation of NH4 +. Modified and extended with permission after Burgin and Hamilton (2007)
Denitrification is probably the most intensively studied process of the N cycle, because it may be responsible for more than 20 % of fixed N losses in agriculture. Initial studies focused on the identification of intermediates and later emphasis was on the regulation and enzymology of the process. Many organisms possess a nitrate reductase either located at the cytoplasmic membrane (NarGH) or in the periplasm (NapAB), that produces the important intermediate NO2 −, which is subsequently converted into NO by either a nirK or a nirS nitrite reductase. Various membrane-bound nitric oxide reductases exist. Finally N2O is converted by a nitrous oxide reductase (NosZ) .
In laboratory cultures, one single species may convert NO3 − all the way to N2, in nature the reactions are probably divided between different species, and co-cultures are rather rule than exception. In natural habitats denitrifiers will have to compete with dissimilatory nitrate reduction to ammonia (DNRA) and anammox for NO3 − and NO2 −, and the outcome of the competition may be dependent on the C/N ratio (Fig. 22.2). For a longtime it was believed that methane (CH4) could not be used by denitrifiers, because its activation would require O2. However, in 2006 a co-culture of Bacteria and Archaea was enriched that could perform CH4 dependent denitrification (Raghoebarsing et al. 2006) . By increasing the NO2 − concentration, the Archaea disappeared from the community and Bacteria named Methylomirabilis oxyfera became dominant. M. oxyfera was shown to have a peculiar denitrification pathway involving the dismutation of NO into O2 and N2 by a putative NO dismutase (Ettwig et al. 2010) . Recently it has been established that Archaea can convert NO3 − to NO2 − and maybe even further to NH4 + at the expense of CH4 (Haroon et al. 2013) .
Branching diagram with a simplified overview of NO3 − transformations under different conditions, indicated by the different colors. Depicted in green is CH4 availability, blue is the carbon input, red represents iron (Fe) concentrations, in yellow the free sulfide concentrations (H 2 S, S 0, FeS), finally in different brown shades are the C/N ratio under the different Fe or free sulfide concentrations. (Conc. the concentration; DNRA Dissimilatory Nitrate Reduction to Ammonium; AOM Anaerobic Oxidation of Methane). Adapted and extended with permission from Burgin and Hamilton (2007)
DNRA
DNRA is one of the least studies aspects of the N cycle. Many microorganisms are able to perform the DNRA reaction especially at low NO3 − and high C concentrations. First NO3 − is converted to NO2 − by a nitrate reductase. In the second step a multiheme nitrite reductase (nrfA) converts the NO2 − directly to NH4 +. Electrons needed for reduction are derived by fermentation of organic compounds or by sulfide oxidation. DNRA is a difficult pathway to detect and needs sophisticated stable isotope experiments. An elegant example is the study of Lam et al. (2009) that investigated the N cycle pathways in the Chilean OMZ (Oxygen Minimum Zone) . By applying a complementary array of methods, they were able to show that DNRA may contribute up to 40 % of the N flux in this OMZ.
Anaerobic Ammonium Oxidation
Only in 1995 the first publication on the disappearance of NH4 + from an anoxic denitrifying pilot plant was reported. After complaints by the citizens of Delft that the Gist Brocades pilot plants produced too much hydrogen sulfide, the waste water engineers added copious amounts of calcium nitrate to prevent sulfate reduction. Inadvertently they created favourable conditions for anaerobic ammonium oxidizing (anammox) bacteria to proliferate. Biomass of the pilot plant was subsequently used to start new more defined enrichment cultures, first as fluidized bed reactors, later as sequencing batch reactors, yielding enough anammox biomass to perform the necessary experiments. Inhibitors studies with antibiotics showed that the process was bacterial, while 15N stable isotopes studies indicated the production of the rocket fuel hydrazine (N2H4). As the enrichments yielded 70–90 % anammox dominance, physical purification methods based on gradient centrifugation had to be applied. This gave sufficient purified cells to do crucial 15N and 14C experiments showing the autotrophic nature of the anammox bacteria. From the purified cells, the 16S rRNA gene could be amplified, and the anammox bacteria were shown to belong to the phylum of the Planctomycetes. Electron microscopic analysis showed that anammox bacteria have a unique cell plan with a specialized compartment harboring the enzymes responsible for the anammox reactions (Van Niftrik and Jetten 2012) . Analysis of the fatty acids of the cells and organelle indicated that anammox bacteria have both ether and ester lipids of concatenated cyclobutane rings that from a kind of staircase structure, hence their name ladderane lipids. These are unique and can be used as specific anammox biomarkers.
After the availability of suitable diagnostic tools, several expeditions to OMZs were organized. Indeed it could be documented that in those OMZs, anammox bacteria were present and active. Taken together they could account for half of the loss of fixed N from the systems, making them important players in the global N cycle. Recently it was also shown that anammox can contribute significantly to the N-loss in terrestrial ecosystems such as wetlands and river sediments.
After the genome of the first anammox bacterium was resolved, the molecular mechanisms were elucidated (Kartal et al. 2011) . The crucial intermediates were NO and N2H4 and a unique enzyme complex hydrazine synthase was identified. Application of anammox bacteria together with partial nitrification may result in more sustainable waste water treatment systems saving on O2 and electricity usage, methanol consumption, and ecological footprint (Kartal et al. 2010) . Based on these advantages, already more than 20 full scale anammox plants have been build worldwide, and many more are commissioned.
3 N Cycle in Ecosystems
After the introduction of the N cycle processes, the following section will focus on the cooperation and competition for N compounds between plants and microorganisms. The competition is controlled by metabolic limitations and environmental conditions.
Plant vs Plant N Competition
Plants have evolved diverse adaptations to cope with nutrient limitations . Resource depletion has been hypothesized as the strategy in plant-plant competition for N. By taking up more N compounds than directly necessary, N can become rapidly depleted in the environment and thus limiting for competitors. Competition for N resources between plants also occurs indirectly. Microorganisms flourish in the rhizosphere due to high litter production by roots. Plants modulate their rhizospheric microbiome (see Chap. 43) by attracting certain species, which have the potential to enhance N uptake for the plant.
Plant vs Microorganisms
The trade-off between plants and microorganisms in competition for N, is a much debated topic. In terrestrial ecosystems, the classical paradigm stated that microorganisms are stronger competitors for N than plants, hence plants would only use the microbial N left-overs. In the late 1990s, a new hypothesis was developed that put less emphasis on mineralization and underlined depolymerization of the complex N compounds present in soil by microorganisms, as the key process and bottleneck in N cycling (Jackson et al. 2008) .
Short term experiments with 15N additions showed that microorganisms take up organic and inorganic N faster than roots. Microorganisms have high substrate affinities, low volume to surface ratio’s and fast turnover rates compared to plants and therefore are stronger competitors. In the long run plants assimilated most of the 15N due to the gradual release of 15N that was first mineralized by the microorganisms. It is this temporal difference that determines the competition for N between plants and microbes in the end (Jackson et al. 2008; Kuzyakov and Xu 2013) . The most direct competition between plants and microbes occurs at the level of the available inorganic N. Nitrifiers have to compete for available NH4 + with all NH4 + assimilating plants and microorganisms, whereas the processes of denitrification and DNRA compete for NO3 − with plant and microbial NO3 − assimilation.
Competition for NH4 +
Fertilization experiments with inorganic N have either shown about equal N uptake rates for both plants and microorganisms, hence both were simultaneously limited in N. In a NH4 + fertilization experiment plant removal resulted in increased nitrification rates. This indicated that plants and autotrophic nitrifiers compete for NH4 + , with plants being the stronger competitors (Kaye and Hart 1997) .
Competition for NO3 −
In rice soils, a study of NO3 − assimilation by plant and microorganisms showed that fertilization with labeled NO3 − always resulted in more 15NO3 − ending up in the microbial than in plant N pools. As rice grows in flooded soils under anaerobic conditions, ROL will release oxygen into the rhizosphere. The supplied O2 stimulates nitrification, and subsequently NO3 − can enter the anoxic zone where denitrification and anammox cause fixed N to be lost to the atmosphere . By blocking transport of O2 and N2 via the aerenchym through clipping of rice plants below the water surface, nitrification rates rapidly decreased and in the longer term also denitrification rates decreased (Arth et al. 1998; Matheson et al. 2002) .
O2 is an important regulator of NO3 − partitioning between denitrification and DNRA. It was shown that microcosms planted with Glyceria declinata experienced more O2 intrusion and higher redox potentials compared to unplanted microcosms, leading to higher denitrification than DNRA rates (Matheson et al. 2002) . In a study with young-barley, short term rewetting increased nitrification rates, whereas long term rewetting also stimulated denitrification (Højberg et al. 1996) . Since the role of denitrification in N loss might be overestimated (Burgin and Hamilton 2007) , future studies in anoxic systems should also analyze the role of anammox and DNRA.
Microorganism vs Microorganisms
Also microorganisms among each other have to interact and/or compete for NH4 +and NOx. Nitrifiers (AOA, AOB, NOB) live in close proximity to ensure rapid conversion of NH4 + to NO3 − without intermediate NO2 − accumulation. Availability of O2 is an important factor determining whether anammox or nitrifiers can convert NH4 + first, and whether NOB or anammox can convert NO2 − subsequently. In systems with fluctuating O2 and NH4 + concentrations like wastewater systems, sediments or OMZs, consortia of AOA, AOB, NOB and anammox have been found to be active (Lam et al. 2009) . Low ammonium and O2 concentrations seem to favor AOA. Laboratory studies under O2 limitation showed that AOA can thrive very well in co-cultures with anammox bacteria. Several guilds may have to compete for NO3 − and the outcome of the competition is mostly determined by the quality and quantity of the electron donor. Burgin and Hamilton (2007) made an elegant scheme to predict the occurrence of the various NO3 − reducing processes (Fig. 22.2). At low organic carbon, denitrification and anammox will compete, and above a C/N ratio of 2, denitrification may be favored. Under high energy or carbon input DNRA may be the most important process, while denitrification is dominant at low C/N ratios in the absence of NH4 +. At high CH4 concentration AOM Archaea may play a significant role in nitrate reduction and DNRA, and M. oxyfera in NO2 − conversion to N2.
4 Anthropogenic N Use and Changes in N Cycle
The global cycling of N has doubled over the last century, starting with the application of the Haber-Bosch process (N2 + 3 H2 → NH3) in 1913. At the expense of fossil fuel, artificial fertilizer could be produced, and thus increased crop productivity and harvest. Though, what was not realized at the time is that the use of (excess) N fertilizer has severe ecological impacts.
Studies on N fertilizer use have primarily focused on loss of N fertilizers into other (pristine) ecosystems. Only part of the N that is applied as fertilizer is taken up by microorganisms and plants and later on removed via harvest of the crops. The remainder will enter the N cycle of the ecosystem. According to Burgin and Hamilton (2007) the most desirable way to reduce high N levels in ecosystems is via permanent removal by denitrification (or anammox), because other N transformations may result in even more harmful N-compounds.
The main processes studied with respect to N loss from ecosystems are NO3 − leaching , ammonia volatilization and loss as NOx or N2 gas (Cameron et al. 2013) . NO3 − leaching depends on nitrate loading of the soil and the levels of drainage that occur. NO3 − is a large problem for water quality and can affect human health . Via groundwater the leached NO3 − enters rivers and lakes, where it might stimulate algal blooms and cause biodiversity loss. NO3 − losses from fertilizer-use can be reduced by using adequate and efficient fertilizer levels to prevent N excess, by optimizing plant N uptake to avoid N losses and if all else fails nitrification inhibitors can be applied. Fertilizer-use is nowadays highly restricted and managed so that fertilizers are used in an efficient manner, with amounts that are matched to the rate of plant growth.
Ammonia (NH3) volatilization is especially a problem in areas surrounding intensive animal farms. Most important sources are animal urine and feces, but also N fertilizers contribute to NH3 volatilization. Once volatilized, NH3 deposits cause acidification and eutrophication. NH3 volatilization can be best reduced by applying fertilizers beneath the soil surface or just before rain, and by reducing intensive animal farming.
Ultimately, N can be lost to the atmosphere via nitrification or denitrification in the form of NO, N2O or N2. In particular, NO and N2O (NOx) form a serious problem since they deplete the ozone layer and contribute substantially to climate change . The global warming potential of N2O is 298 times that of CO2. To reduce NOxformation, nitrification inhibitors combined with optimized fertilizer application may diminish nitrification. Methods to reduce denitrification include changing the soil physiochemical parameters (i.e. increasing pH by applying lime, or increase aeration of the soil, as described in Cameron et al. 2013) .
5 Conclusions and Outlook
N cycling has been studied intensively for over decades. Although the knowledge on the N cycle has increased, the role of anammox, the contribution of AOM dependent conversions of nitrogen and the interactions in the N cycle deserve more attention. Ultimately, improving our understanding of the N cycle will help to retain and restore balances in ecosystem N cycles which have been affected by anthropogenic activities.
References
Arth I, Frenzel P, Conrad R (1998) Denitrification coupleD to nitrification in the rhizosphere of rice. Soil Biol Biochem 30:509–515
Britto DT, Kronzucker HJ (2002) Ammonium toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162:145–173
Ettwig KF, Butler MK, Le Paslier D et al (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548
Haroon MF, Hu S, Shi Y et al (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570
Højberg O, Binnerup S, Sørensen J (1996) Potential rates of ammonium oxidation, nitrite oxidation, nitrate reduction and denitrification in the young barley rhizosphere. Soil Biol Biochem 28:47–54
Jackson LE, Burger M, Cavagnaro TR (2008) Roots, nitrogen transformations, and ecosystem services. Annu Rev Plant Biol 59:341–363
Kartal B, Kuenen JG, van Loosdrecht MCM (2010) Engineering. Sewage treatment with anammox. Science 328:702–703
Kartal B, Maalcke WJ, De Almeida NM et al (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–130
Kaye J, Hart S (1997) Competition for nitrogen between plants and soil microorganisms. Trends Ecol Evol 5347:139–141
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Lam P, Lavik G, Jensen MM et al (2009) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci U S A 106:4752–4757
Lambers H, Chapin III FS, Pons TL (1998) Plant physiological ecology, 1st edn. Larcher publisher Springer Verlag, Berlin
Lamers LPM, Van Diggelen JMH, Op den Camp HJM et al (2012) Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: a review. Front Microbiol 3:156
Larcher W (2001) Physiological plant ecology, 4th edn. Lambers publisher Springer Science + Business media, New York
Matheson F, Nguyen M, Cooper A (2002) Fate of 15 N-nitrate in unplanted, planted and harvested riparian wetland soil microcosms. Elsevier Ecol Eng 19:249–264
Van Niftrik L, Jetten MSM (2012) Anaerobic ammonium-oxidizing bacteria: unique microorganisms with exceptional properties. Microbiol Mol Biol Rev 76:585–596
Raghoebarsing AA, Pol A, Van de Pas-Schoonen KT et al (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921
Acknowledgements
We would like to thank our co-workers and collaborators and granting agencies for their continuous support (ERC 232937, ERC 339880, Spinozapremie 2012 and OCW-NWO Gravitation Grant SIAM 024.002.002).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kox, M., Jetten, M. (2015). The Nitrogen Cycle. In: Lugtenberg, B. (eds) Principles of Plant-Microbe Interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-08575-3_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-08575-3_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08574-6
Online ISBN: 978-3-319-08575-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)