Abstract
The enrichment of the biosphere with reactive nitrogen from anthropogenic origin, in combination with increased consumption of vegetables and (preserved) animal products, has led to increased intake by humans of nitrite and nitrate. Nitrate and nitrate-forming salts are among the key components of fertilizers and the increased dependency of farming practices on such fertilizers over several decades has led to increasing levels of human exposure. Elemental nitrogen is fixed by several mechanisms including lightning fixation and by symbiotic organisms in the soil. Plants and vegetables use these nitrogen intermediates as an energy source necessary for growth. Understanding nitrogen fixation in the environment by micro-organisms will help in our understanding of the role of nitrite, nitrite, and nitric oxide in humans. The scope of this chapter is to review the mechanisms of nitrogen fixation and how this relates to human nitrogen cycle and human health and disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Nitrogen is the most abundant element in the atmosphere.
-
N2 is chemically inert.
-
Conversion to biologically active, “fixed,” nitrogen requires considerable energy input.
-
Nitrogen , inorganic nitrogen oxides, and organic nitrogen participate in complex biological cycle.
-
The individual steps in the cycle may be used for respiration, anabolic processes, detoxification, or host defense.
-
Plants and animals often have symbiotic relationships with specific micro-organisms which catalyze the parts of the cycle which they are unable to do.
-
Mammals, including humans, have a complex nitrogen cycle which is, as yet, only partly understood.
-
An understanding of the handling of nitrogen by micro-organisms may broaden our understanding of the role of nitrate, nitrite, and nitric oxide in health and disease.
Introduction
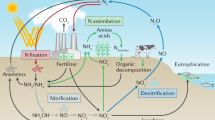
Nitrogen atoms are a constituent part of a vast array of biologically important chemicals from the complex, such as proteins and nucleic acids, to the apparently more simple diatomic nitric oxide. Nitrogen (N2) gas makes up approximately 78 % of the atmosphere. It is highly inert, being bound together by a triple bond that requires considerable energy to break (bond energy 940 kJ mol–1); higher organisms lack the apparatus to do so. The conversion of nitrogen from inert N2 to fixed , and, thus, biologically available nitrogen is essential to life on earth and requires dedicated multistep enzymatic pathways; vast amounts of energy in the case of lightning; or a combination of heat, pressure, and metallic catalysts in the Haber–Bosch process . The overview of the biological nitrogen cycle is illustrated in Fig. 2.1.
Theories exist that life on earth in its earliest and most primitive stages, when the gaseous constitution of the atmosphere was very different from that found today, synthesized NO to detoxify ozone or oxygen [1] or, indeed, developed NO reductases to remove NO from the cell. Whatever the reason, the chemical properties of NO , NO2 - , and NO3 − played a key role in the lives of the most primitive organisms. It is striking that as evolution progressed, higher organisms became utterly dependent on the nitrogen fixing and cycling abilities of their more primitive antecedents. Indeed, the ability to process nitrogen is no less important to familiar plants such as clover and their symbionts to extremophile bacteria .
The surge in knowledge and interest in the microbial/environmental nitrogen cycle is mirrored by the burgeoning interest in the mammalian nitrate cycle and the role of its multifarious reactive nitrogen oxide intermediates in health and disease. The two are, however, rarely considered together.
Nitrate, Agriculture, and Economics
The importance of nitrogen for plant growth was realized in the nineteenth century, first by Boussingault and later by Gilbert and Lawes’ Rothamsted experiments [2]. Thus, readily available sources of fixed nitrogen were of considerable economic value. Guano , the nitrogen-rich desiccated droppings of sea birds , proved to be a very valuable export commodity for Peru in the early and mid-1800s. Indeed, such was the economic value of fixed nitrogen that Chile contested a war with Bolivia and Peru from 1879 to 1883 [3]. Bolivia increased taxation on the export of nitrate mined by Chilean companies. In retaliation, Chile seized the Bolivian port of Antofagasta. Bolivia declared war on Chile with Peru joining the Bolivian side in line with a secret treaty. Chile won the conflict that ensued; consequently, the nitrate-rich zones of Bolivia and Peru were ceded to Chile. The redrawn national boundaries also left Bolivia landlocked.
While these sources were economically important at the time, they did not represent a sustainable source of fixed nitrogen. Sustained plant growth requires the continued availability of fixed nitrogen, which can be derived from any of the following sources.
Nitrogen Fixation
Atmospheric nitrogen can be fixed via the following processes
-
1.
Microbial dinitrogen fixation
-
2.
Industrially by the Haber–Bosch process
-
3.
Fossil fuel combustion
-
4.
Lightning
Microbial Dinitrogen Fixations
The conversion of atmospheric nitrogen to a fixed, biologically available nitrogen species is the exclusive preserve of prokaryotes, eubacteria, and archea. Eukaryotes are not capable of this action. Collectively these organisms are known as diazotrophs . They fall into three distinct groups: free living, symbiotic (within plant roots), and those with a loose association with another organism, typically plant roots.
Nitrogen fixation can only occur in anaerobic conditions. Nitrogenase , the enzyme responsible for fixing atmospheric nitrogen , is exquisitely sensitive to oxygen . To maintain anaerobic conditions, a variety of approaches may be adopted: a very high respiration rate to maintain a low internal oxygen tension and/or binding of O2 to leghemoglobin, a hemoglobin homolog, in the roots of leguminous plants [4].
The symbiotic relationship between rhizobia and leguminous plants is worthy of special consideration. The plant provides rhizobia , which are obligate anaerobes , with an anaerobic environment and sucrose in return for fixed nitrogen. The rhizobia infect plant roots of compatible species. A signaling cascade takes place between the plant and rhizobia resulting in changes in gene transcription in the plant driving root nodule formation and progressive infection of the nodule. Fixed nitrogen in the form of NH3 is transferred from an infected root cell to an adjacent uninfected cell for incorporation into amides, typically glutamate or asparagine, or ureides, such as allantoin for transport to the upper part of the plant.
Haber–Bosch
Such was the economic importance of fixed nitrogen for agriculture that the development of an industrial process became a prime concern for the chemical industry. In 1910 Fritz Haber began to develop what would ultimately become known as the Haber–Bosch process. This process, still widely used today, involves reacting N2 and H2 gases at approximately 500 °C and a pressure of 300 atm over an iron catalyst to yield ammonia.
Some estimates suggest that 40 % of the plant-derived protein consumed by humans globally is derived from fertilizers utilizing nitrogen fixed by the Haber–Bosch process [5].
Fossil Fuel Combustion
The burning of fossil fuel for energy is responsible for less than 10 % of the fixed biologically available nitrogen deposited on the terrestrial surface [6]. The majority of the nitrogen made biologically available via this route is already fixed. Burning fossil fuels simply releases it from long-term sequestration in geological stores [7]. At sufficiently high temperatures, some de novo nitrogen fixation occurs.
Atmospheric Nitrogen Fixation by Lightning
While nitrogen fixation by lightning is a relatively minor player in the overall turnover of nitrogen within the global cycle, it is worth mentioning as it serves as a potent reminder of the magnitude of the energy investment required to fix nitrogen. The conversion of N2 to NO and rapid oxidation to NO2 (nitrogen dioxide) in the upper atmosphere by lightning is notoriously difficult to study and quantify. The primary interest in this process pertains to its importance to stratospheric and tropospheric chemistry and its impact on climate [8], as comparatively little of the nitrogen fixed in this way finds its way into biological systems.
Regardless of the specific mechanism, once fixed, the nitrogen will enter anyone of a variety of biological pathways.
Nitrate Assimilation in Plants and Bacteria
Most plants do not have a symbiont partner to provide them with fixed nitrogen. For those plants and bacteria unable to fix nitrogen independently, nitrate, found in soil, is the preferred source of fixed nitrogen. Nitrate is initially reduced to nitrite and ultimately ammonium before being acted upon by glutamate synthase and incorporated into carbon skeletons as described earlier for transport elsewhere in the plant. Certain plant species reduce nitrate rapidly on entry into the root system. Others transport unmetabolized nitrate to the upper part of the plant.
Nitrification
Decomposition of organic nitrogen-containing compounds such as proteins by a wide variety of bacteria and fungi produces ammonium. This ammonium is oxidized to nitrite and ultimately nitrate in reactions catalyzed by the chemolithoautotrophs nitrosomonas and nitrobacter which use the energy released to assimilate carbon from CO2.
It is worth noting that under certain circumstances, nitrifiers are capable of denitrification.
Denitrification
Fixed nitrogen is converted back to inert nitrogen gas by micro-organisms, which utilize this multistep reductive process as an alternative respiratory pathway to oxygen-dependent respiration in anaerobic or near anaerobic conditions. Oxygen respiration is more energetically favorable and is, thus, preferred. It is only when oxygen tension falls to levels that limit the microbe’s ability to respire that the enzymes of denitrification are expressed. Conversely, in conditions of rising oxygen tension, denitrification is inhibited.
Some organisms are only capable of catalyzing part of the denitrification pathway. This may represent a defense mechanism in some important human pathogens , such as Neisseria gonorrhoeae [9], which may use it as a device for surviving NO attack from the host.
Denitrification is a four-stage process with each step catalyzed by complex multisite metalloenzymes.
Typically, nitrate reduction by membrane-bound nitrate reductase enzyme (NAR) occurs on the cytoplasmic side. Some organisms express a periplasmic nitrate reductase , NAP. Subsequent steps occur in the periplasm or on the periplasmic side of membrane-bound proteins. Thus, denitrifying bacteria have developed specialized nitrate and nitrite transport mechanisms [10]. The nitrite produced by this process is further reduced to nitric oxide by either a heme or copper containing nitrite reductase , NIR [11]. The NO generated must be rapidly reduced to N2O as accumulation of NO would be fatal to the organism. Bacterial nitric oxide reductases (NOR) contain the typical components of the main catalytic subunit of heme/copper cytochrome oxidases [12]. The final step in the denitrification pathway is the reduction of N2O to N2 by another copper containing enzyme, N2O reductase; while it represents the terminal step in a complete denitrification pathway, it also represents a separate and independent respiratory process [13].
It is worth noting that NO, N2O, or N2 may be released in the denitrification process. The stoichiometry of the gas mix will be affected by the relative activities of the pertinent reductases.
The denitrification process is usually thought to occur in aerobic soil or sediment environments. Recently, it has emerged that a complete denitrification pathway exists in human dental plaque [14], a finding that may have important implications in the mammalian nitrate cycle.
Dissimilatory Reduction of Nitrate to Ammonia
A third route for nitrate reduction exists that provides neither energy nor components for anabolic processes within the cell. Dissimilatory nitrate reduction appears to exist as a method for detoxifying excess nitrite following respiratory reduction of nitrate or balancing an excessive quantity of reductants [15]. It is exclusively an anaerobic process. Under certain conditions such as may be found during photoheterotrophic growth [16], in anaerobic marine sediments, and for some organisms in the human gastrointestinal tract [17] this may be the principal nitrate reduction pathway. A reductant-rich environment has a paucity of electron acceptors. Reduction of NO2 − to NH4 + consumes six electrons compared with the two to three utilized in denitrification [18]. In these circumstances, dissimilatory nitrate reduction to ammonia is a key to maintaining the overall redox balance of the cell.
Anammox
The biological nitrogen cycle was widely held to be complete until the discovery of a pathway for anaerobic ammonium oxidation in 1990 [19]. When a wastewater plant reported higher than expected generation of dinitrogen gas, investigations revealed that certain bacteria were able to oxidize ammonium using nitrite as the electron acceptor to generate energy for growth in anoxic conditions. This process was thought to provide sufficient energy for slow growth only, with a bacterial doubling time of 11 days [20]. It has since emerged that anammox may provide for more rapid growth with a doubling time of 1.8 days, approaching that of ammonium oxidizers [21]:
As biologically available nitrogen is converted back to dinitrogen gas , anammox can be thought of as a form of denitrification. This entire complex pathway is illustrated in Fig. 2.1.
Nitrogen Balance in Mammals
In humans, nitrogen is principally ingested as protein. The average US young adult consumes approximately 90 g of protein daily [22]. This equates to about 14.5 g of nitrogen, nearly all of which is excreted in the urine as urea, creatinine, uric acid, and ammonia. Only a tiny proportion of this nitrogen is converted to nitric oxide via the nitric oxide synthase pathway. In healthy humans, about 1 mmol of nitrate is generated from l-arginine and then nitric oxide oxidation, which represents about one-thousandth of the amount of nitrogen ingested; during illness, such as gastroenteritis, this can increase by as much as eightfold [23].
Ruminants , and other mammals which rely on symbiotic bacteria to metabolize cellulose, can also make use of nonprotein nitrogen sources for protein synthesis. Rumen bacteria can convert urea to ammonia which is used to produce amino acids that can be incorporated into mammalian proteins [24].
l-Arginine Nitric Oxide Synthase Pathway
Three distinct NOS isoforms exist in mammals : inducible, neuronal, and endothelial. The loci of each gives an indication as to the pluripotent effects of nitric oxide in mammals, with roles in host defense, neuronal and other cellular signaling pathways, and vascular control. l-arginine provides organic nitrogen as a substrate which, with 2O2 and NADPH as a cofactor, is converted to nitric oxide and l-citrulline. The nitric oxide produced has a very short half-life, being rapidly oxidized in the presence of superoxide or oxyhemoglobin to nitrate, which enters the mammalian nitrate cycle.
Dietary Sources of Nitrate and Nitrite
Certain foods such as green leafy vegetables and beetroot are particularly rich in nitrate. Consumption of a typical Western diet results in the ingestion of approximately 1–2 mmol nitrate per day. Nitrite, because of its antibotulism effect , has been used as preservative and colorant for centuries. In addition, humans are also exposed to biologically active nitrogen oxides from the combustion of fossil fuels or inhalation of tobacco smoke.
Enterosalivary Circulation of Nitrate/Nitrite/NO
The remarkable symbiosis between legumes and nitrogen-fixing rhizobia finds its counterpoint in the relationship between nitrate-reducing bacteria hidden in crypts in mammalian tongues. Nitrate from the diet is rapidly and completely absorbed from the upper gastrointestinal tract . This, along with nitrate derived from the oxidation of NO synthesized by the l-arginine NOS pathway , is actively taken up the salivary glands. The resulting salivary nitrate concentration may be ten times greater than the plasma nitrate concentration. In crypts on the dorsum of the tongue, facultative anaerobes, e.g., Veillonella species , utilize nitrate as an alternative electron acceptor [25]. The nitrite released elevates salivary nitrite to levels 1000 times that of plasma in the resting state. In the presence of acid-generating plaque bacteria , some nitrite is chemically reduced to nitric oxide [26]. The remaining salivary nitrite is then swallowed. In the acidic environment of the stomach, some of this nitrite is further reduced to nitric oxide [27] which has an important role in both protection against enteric pathogens and regulation of gastric blood flow and mucous production [28, 29]. Some of the nitrite is absorbed from the stomach with important consequences for mammalian vascular physiology. This pathway is illustrated in Fig. 2.2.
Simplified representation of the enterosalivary circulation of nitrate . Nitrate (represented by the black arrows) derived from the diet is swallowed. It is rapidly and completely absorbed in the upper gastrointestinal tract. Approximately 25 % is concentrated in the salivary glands and secreted into the mouth. Here it is reduced to nitrite (represented by the gray arrows) by facultative anaerobes on the dorsum of the tongue and swallowed. Some of the nitrite undergoes acidic reduction to nitric oxide in the stomach, with the remainder being absorbed. The fate of nitrite is discussed in depth later. Sixty percent of ingested nitrate is lost in the urine within 48 h
In 1996, it was discovered that nitric oxide is continually released from the surface of normal human skin [30]. Although it was initially thought that nitric oxide synthase would be responsible, inhibition of this enzyme by infusing monomethyl l-arginine into the brachial artery showed that this was not the case. Further studies showed that nitrate is excreted in human sweat and reduced to nitrite by skin bacteria. As normal skin is slightly acidic (pH around 5.5) this nitrite is reduced to nitric oxide. The function of this NO is thought to be to inhibit skin pathogens—particularly fungi [31]—and, intriguingly, when normal saliva is applied to healthy skin, the high concentrations of nitrite considerably increase nitric oxide synthesis , perhaps to protect against infection and encourage wound healing [32].
Breast milk has recently been shown to contain variable amounts of nitrite and nitrate, and it has been suggested that conversion of these anions to more reactive nitrogen oxides may be a factor underlying the protective effect of breastfeeding against infant gastroenteritis [33].
Urinary Nitrate Excretion
Approximately 60 % of the nitrate ingested or endogenously synthesized will be lost in the urine within 48 h [34]. The discovery of complete denitrification pathways in human dental plaque flora [14] may offer a clue as to the fate of at least part of the remainder. Nitrate is freely filtered at the glomerulus. Studies in dogs suggest that as much as 90 % may be reabsorbed by the renal tubules [35]. Nitrite is not found in human urine under normal physiological conditions. Its presence indicates infection with nitrate-reducing organisms. Detection of urinary nitrite is in widespread use as a simple bedside test for diagnosing urinary tract infection.
Conclusion
When considering the biological nitrogen cycle as part of the global nitrogen cycle, mammalian nitrogen cycling is often considered separately from the processes occurring in plants and micro-organisms. Scientific interest in both these spheres has undergone a resurgence over the last 20–30 years. The complexity of the processes and relationships between animals, plants, and micro-organisms is still being unraveled.
References
Feelisch M, Martin JF. The early role of nitric oxide in evolution. Trends Ecol Evol. 1995;10(12):496–9.
Addiscott T. Nitrate, agriculture and the environment. Oxford: CABI; 2005.
Kiernan VG. Foreign interests in the War of the Pacific. Hisp Am Hist Rev. 1955;35(1):14–36.
Harutyunyan EH et al. The structure of deoxy- and oxy-leghaemoglobin from Lupin. J Mol Biol. 1995;251(1):104–15.
Smil V. Enriching the earth: Fritz Haber, Carl Bosch, and the transformation of world food production. Cambridge: MIT; 2004.
Schlesinger WH. On the fate of anthropogenic nitrogen. Proc Natl Acad Sci. 2009;106(1):203–8.
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl. 1997;7(3):737–50.
Schumann U, Huntrieser H. The global lightning-induced nitrogen oxides source. Atmos Chem Phys. 2007;7(14):3823–907.
Barth KR, Isabella VM, Clark VL. Biochemical and genomic analysis of the denitrification pathway within the genus Neisseria. Microbiology. 2009;155(12):4093–103.
Moir JW, Wood NJ. Nitrate and nitrite transport in bacteria. Cell Mol Life Sci. 2001;58(2):215–24.
Cutruzzolà F. Bacterial nitric oxide synthesis. Biochim Biophys Acta. 1999;1411(2–3):231–49.
Hendriks J, Oubrie A, Castresana J, Urbani A, Gemeinhardt S, Saraste M. Nitric oxide reductases in bacteria. Biochim Biophys Acta. 2000;1459(23):266–73.
Zumft W. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61(4):533–616.
Schreiber F, Stief P, Gieseke A, Heisterkamp I, Verstraete W, de Beer D, et al. Denitrification in human dental plaque. BMC Biol. 2010;8(1):24.
Moreno-Vivián C, Ferguson SJ. Definition and distinction between assimilatory, dissimilatory and respiratory pathways. Mol Microbiol. 1998;29(2):664–6.
Berks BC, Ferguson SJ, Moir JWB, Richardson DJ. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim Biophys Acta. 1995;1232(3):97–173.
Parham NJ, Gibson GR. Microbes involved in dissimilatory nitrate reduction in the human large intestine. FEMS Microbiol Ecol. 2000;31(1):21–8.
Bothe H, Ferguson SJ, Newton WE. Biology of the nitrogen cycle. 1st ed. Amsterdam: Elsevier; 2007.
Jetten MSM, Strous M, Pas-Schoonen KT, Schalk J, Dongen UGJM, Graaf AA, et al. The anaerobic oxidation of ammonium. FEMS Microbiol Rev. 1998;22(5):421–37.
Strous M, Heijnen JJ, Kuenen JG, Jetten MSM. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl Microbiol Biotechnol. 1998;50(5):589–96.
Isaka K, Date Y, Sumino T, Yoshie S, Tsuneda S. Growth characteristic of anaerobic ammonium-oxidizing bacteria in an anaerobic biological filtrated reactor. Appl Microbiol Biotechnol. 2006;70(1):47–52.
Fulgoni III VL. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87(5):1554S–7.
Forte P, Dykhuizen RS, Milne E, McKenzie A, Smith CC, Benjamin N. Nitric oxide synthesis in patients with infective gastroenteritis. Gut. 1999;45(3):355–61.
Huntington GB, Archibeque SL. Practical aspects of urea and ammonia metabolism in ruminants. J Anim Sci. 2000;77(E-Suppl):1–11.
Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113(1):14–9.
Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate [see comment]. Nat Med. 1995;1(6):546–51.
Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, et al. Stomach NO synthesis [see comment]. Nature. 1994;368(6471):502.
Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119(2):512–20.
Dykhuizen RS, Frazer R, Duncan C, Smith CC, Golden M, Benjamin N, et al. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob Agents Chemother. 1996;40(6):1422–5.
Weller R, Pattullo S, Smith L, Golden M, Ormerod A, Benjamin N. Nitric oxide is generated on the skin surface by reduction of sweat nitrate. J Invest Dermatol. 1996;107(3):327–31.
Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J Appl Microbiol. 2001;90(4):648–52.
Benjamin N, Pattullo S, Weller R, Smith L, Ormerod A. Wound licking and nitric oxide [see comment]. Lancet. 1997;349(9067):1776.
Hord NG, Ghannam JS, Garg HK, Berens PD, Bryan NS. Nitrate and nitrite content of human, formula, bovine and soy milks: implications for dietary nitrite and nitrate recommendations. Breastfeed Med. 2011;6(6):393–9.
Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res. 1983;43(4):1921–5.
Godfrey M, Majid DS. Renal handling of circulating nitrates in anesthetized dogs. Am J Physiol. 1998;275(1 Pt 2):F68–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Gilchrist, M., Benjamin, N. (2017). From Atmospheric Nitrogen to Bioactive Nitrogen Oxides. In: Bryan, N., Loscalzo, J. (eds) Nitrite and Nitrate in Human Health and Disease. Nutrition and Health. Humana Press, Cham. https://doi.org/10.1007/978-3-319-46189-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-46189-2_2
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-46187-8
Online ISBN: 978-3-319-46189-2
eBook Packages: MedicineMedicine (R0)