Abstract
Psychiatric disorders are common and are responsible for significant human morbidity and mortality. Although current treatment approaches are effective for many patients, a substantial minority remains treatment-resistant. Advances in the understanding of how disturbances in brain networks lead to psychiatric manifestations and the observation of the effectiveness of deep brain stimulation (DBS) in disorders of motor circuitry such as Parkinson’s disease and dystonia has motivated its investigation in other circuit-based disorders. This chapter reviews the clinical experience of DBS in the major mood and anxiety disorders, namely major depression and obsessive–compulsive disorder. We review the circuitry of these conditions, as well as target selection for these and other emerging DBS indications in psychiatry. Neuromodulation is becoming an increasingly important component of the care of psychiatric patients, and DBS, in properly selected patients, and guided by an expert multi-disciplinary team, may offer promise for patients with few treatment alternatives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Major Depressive Disorder
- Anorexia Nervosa
- Deep Brain Stimulation
- Essential Tremor
- Medial Forebrain Bundle
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
13.1 Introduction
The global burden of neuropsychiatric disease is substantial, and expected to rise significantly in the next few decades. The World Health Organization (WHO) estimates that one in four patients who seek medical help do so for a mental or behavioural illness, with major depressive disorder (MDD) ranking as the third most important cause of global disease burden (Kessler et al. 2003, 2005). As the population ages, and the prevalence of these conditions increases, there will be an urgent need to develop novel therapeutic interventions.
For patients with the most common forms of mental illness, including MDD and obsessive–compulsive disorder (OCD), some effective treatments are available, including pharmacological and psychotherapeutic options whether alone or in combination. For example, current guidelines for the management of MDD emphasize a step-wise, graded approach to medical management, which achieves a satisfactory clinical response in up to two-thirds of patients (Kennedy et al. 2009; Lam et al. 2009). Despite these and other available therapies, however, a substantial proportion of patients remain resistant to treatment (Giancobbe et al. 2009; Kennedy et al. 2009). For these patients neuromodulation may be an option.
Brain circuit-based neuromodulation for psychiatric illness is not new, and its history extends to the 1930s with the early development of electroconvulsive therapy (ECT). The idea that electrical currents can be used to ‘reset’ activity in pathological brain structures took hold early, and with significant advances in anaesthesia, patient monitoring, and psychopharmacology, ECT today is an important part of the treatment algorithm for several psychiatric conditions (Lipsman et al. 2013a). Other non-invasive approaches currently under active investigation include repetitive transcranial magnetic stimulation (rTMS), deep TMS and magnetic seizure therapy (MST).
Alternatives to non-invasive neuromodulation are invasive surgical procedures which can more directly target pathological circuits and structures. Early operations, such as limbic leucotomy, have now been supplanted by far more refined, safer and image-guided procedures. Deep brain stimulation (DBS) is one such procedure. The last 15 years have seen a surge of interest in DBS for psychiatric disorders, an interest that has been driven primarily by three factors:
-
1.
Safety and efficacy: DBS is part of the standard treatment of patients with Parkinson’s disease (PD), dystonia and essential tremor, with over 100,000 patients treated globally to date (Lozano et al. 2013). In properly selected patients, and guided by expert multi-disciplinary teams, DBS is effective in controlling symptoms and improving quality of life in these disorders. The ability of DBS to reversibly modulate activity in motor circuits has led to its investigation in other circuit-based conditions, including psychiatric disorders.
-
2.
Neuroimaging: Advances in structural and functional neuroimaging are providing insights into the mechanisms of psychiatric conditions. Imaging studies have helped generate hypothesis-driven investigations of neural circuitry and suggested critical nodes within disrupted circuits that can be targeted with DBS.
-
3.
Definition of treatment-resistance: Although pharmacological treatments are available for most psychiatric illnesses, a large minority of patients remains treatment-resistant. Specific approaches for these patients, who are susceptible to the effects of both polypharmacy and chronic mental illness, are required.
DBS is currently under investigations for a number of neurological and psychiatric conditions. A survey of the National Institutes of Health (NIH) clinical trial registry reveals that at least 21 distinct indications are now under Phase I, ‘first-in-man’, investigation (Fig. 13.1). Several of these, such as Parkinson’s disease, epilepsy and depression, have progressed to Phase III, randomized controlled, multi-centre trials. It appears therefore that the fastest growing indications for investigative DBS are psychiatric, which as of today are among the strongest drivers of progress in DBS research. There are currently trials investigating DBS for major depression, bipolar disorder, OCD, Tourette’s syndrome, anorexia nervosa, schizophrenia, obesity, Alzheimer’s disease and addiction. This chapter will focus on two of the most common indications, MDD and OCD, and provide an overview of the circuitry of these conditions, the targets currently employed, and a summary of results to date. We also discuss some of the emerging indications in the last 5 years, and hypothesize about the future psychiatric DBS, and neuromodulation for these disorders.
Clinical trials in deep brain stimulation, according to the National Institute of Health (NIH) clinical trials registry (From Lozano and Lipsman (2013) with permission)
13.2 MDD
13.2.1 Epidemiology and Phenomenology
MDD is among the most common psychiatric conditions, with a lifetime prevalence of up to 16 % in the general adult population (Kessler et al. 2003). The condition is highly heterogeneous, and encompasses much more than just depressed mood. Patients often endorse varying degrees of amotivation, apathy, and anhedonia, as well as lack of energy, and sleep and appetite disturbances. The involvement of multiple ‘systems’ including mood, cognitive, perceptual and vegetative functions suggests broad circuit dysfunction involving several, primarily limbic, networks. It is not possible, therefore, to ascribe MDD to dysfunction of a specific structure, but instead MDD should be viewed as a network disorder, much like Parkinson’s disease. Similarly, it is not possible to link MDD to dysfunction in one neurotransmitter system, such as serotonin. Indeed, neurobiological models suggest that dopaminergic and noradrenergic dysfunction contribute in important ways to MDD, as evidenced, in part, by the successful use of anti-depressants that target these systems (Lam et al. 2009). An improved understanding of MDD aetiology, and the development of novel therapies, would therefore need to account for its heterogeneous clinical and neurobiological picture.
13.2.2 Neurocircuitry
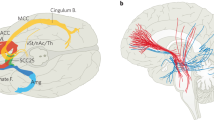
Much of the progress in elucidating MDD circuitry has been driven by advances in neuroimaging, and particularly functional imaging which permits a real-time view of the active brain. These studies have identified critical ‘nodes’ in primarily limbic circuits that are dysfunctional in the pathologically depressed state. For example, metabolic imaging with fluorodeoxyglucose (FDG)-PET has shown that the subcallosal cingulate (SCC) region is hyperactive in unmedicated depressed patients as well as in healthy subjects experiencing sadness (Kennedy et al. 2007; Mayberg 1997; Mayberg et al. 1999). This hyperactivity normalizes with remission of the depression following medical, psychotherapeutic or DBS treatment (Kennedy et al. 2001, 2007; Mayberg et al. 2005). Other regions implicated in depression include those involved in reward- and decision-making pathways, such as the nucleus accumbens and dorsolateral prefrontal cortex (DLPFC) (Price and Drevets 2010). Anhedonia, or the lack of pleasure in typically pleasurable activities, is a major component of depression, and has been linked to dysfunction in the nucleus accumbens (NAcc) in patients and pre-clinical models (Bewernick et al. 2010; Price and Drevets 2010). The DLPFC participates in decision-making and has reciprocal projections with both anterior cingulate and medial prefrontal cortical regions, which participate in affect- or reward-guided decisions, both dysfunctional in MDD. The DLPFC is also a TMS target in depression, where its modulation is linked to improvements in mood (Lipsman et al. 2013a). All of these structures, and the SCC in particular, project widely in the brain, along pathways subserving many of MDD’s cardinal symptoms. The SCC, for example, projects to amygdala and the insula, which participate in vegetative and homeostatic control, as well as to the medial prefrontal and anterior cingulate region, which participate in decision-making (Hamani et al. 2011). In this way, dysfunction in SCC can be linked to several key MDD symptoms. More broadly, such work can identify specific regions for DBS targeting, which can further be tailored according to the predominant clinical picture.
13.2.3 DBS Targets
Several brain targets for the management of treatment-refractory MDD with DBS are currently under investigation. These include SCC, NAcc, ventral caudate/ventral striatum (VC/VS), inferior thalamic peduncle (ITP), lateral habenula (Hab), and medial forebrain bundle (MFB) (Table 13.1). Below we review the targets that have accumulated the most experience to date.
13.2.3.1 SCC
SCC (aka subgenual cingulate gyrus, ‘Area 25’) is a region below the genu of the corpus callosum that sits at the confluence of at least three white matter pathways. These pathways, projecting to orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC) and cingulate connect higher order, ‘top-down’ cortical structures with subcortical modulatory regions. As described above, the SCC has been functionally linked to a depressed state, and more broadly to the regulation of negative emotions. Currently, the most experience with MDD DBS worldwide is with the SCC target. The SCC DBS experience started in 2003 and published in 2005 in six patients with chronic, resistant disease (Mayberg et al. 2005). Four of these patients experienced a greater than 50 % reduction in depression scores (HAMD: Hamilton Depression Rating Scale), with two patients achieving a clinical remission. This patient group has since been expanded and data on 20 patients and followed for between 3 and 6 years have been presented (Kennedy et al. 2011). Response and remission rates at last follow-up were 64 and 43 %, respectively. Two other groups have reported their series with SCC DBS and found similar results. In one paper, eight patients were followed to 1 year with the authors finding response and remission rates of 63 and 50 % (Puigdemont et al. 2011). Another group followed 17 patients (10 with MDD and 7 with bipolar depression), and found that at 2 years following surgery, 92 % were responders and 58 % were in remission (Holtzheimer et al. 2012). An additional multi-centre trial of SCC DBS found response rates of 48 % at 6 months and 29 % at 12 months (Lozano et al. 2012). Such results, in the context of severe, unremitting chronic depression, are promising and have led to the design of phase III, randomized controlled trials. The results of these trials will help establish whether DBS at this target should be an accepted treatment for this specific patient population.
13.2.3.2 NAcc and VC/VS
The prominence of anhedonia in MDD motivated the investigation of targets along reward pathways with DBS. NAcc is a grey matter region comprised of an anatomic core and shell and which exists at the ventral confluence of the caudate and putamen (hence, ventral striatum). Neurophysiological studies in animal and human models have directly linked neuronal firing in the NAcc to the receipt and expectation of reward, and imaging studies using both PET and fMRI have linked NAcc striatal dysfunction to MDD (Patel et al. 2012).
Several studies have investigated NAcc and VC/VS with DBS in open-label, prospective trials. Malone et al. (2009) operated on 15 patients using the VC/VS target and at between 6 and 51 months follow-up found that eight patients were treatment responders, and six were in remission. Bewernick et al. (2010, 2012) targeted the NAcc in ten patients and at 1 year had a 50 % response rate, with further significant effects on anhedonia, as well as on comorbid anxiety. The similar results in these studies, from different centres and employing slightly different targets is encouraging, suggesting that DBS of reward pathways can have relatively stereotypic and reproducible response rates. VC/VS has also been investigated in refractory OCD (see below) and the experience with depression arose from the observation of improved mood in OCD patients. Given the established role of NAcc in reward pathways, and the preclinical and imaging literature linking NAcc activity to reward, it may be that NAcc, and its afferent/efferent projections may be the preferred target for primarily anhedonic MDD.
13.2.3.3 MFB
Rather than targeting distinct nuclei, as is the case for motor-circuit conditions such as Parkinson’s disease, DBS in depression often targets axonal pathways in an effort to broadly modulate network wide activity. A recent example is MFB, an axon pathway that is part of the dopaminergic mesolimbic system connecting the ventral tegmental area (VTA) with NAcc and other key subcortical structures. Schlaepfer et al. (2013) described their experience of MFB DBS in seven patients with treatment-refractory MDD, and found that stimulation was associated with robust, and rapid, remission of depression. The rapidity of the response, which occurred within hours and days, contrast the typical time to response with SCC and NAcc DBS. In addition, the effect was seen in virtually every patient implanted, with six of seven patients classified as treatment responders at 12–33 weeks follow-up. These results, which require further investigation and validation in larger, blinded trials, are intriguing and suggest a more ‘direct’ route to mood change than previously observed. Coupling MFB stimulation with neuroimaging, and particularly dopamine or FDG PET, would provide additional insights into the mechanisms of the clinical response.
13.3 OCD
13.3.1 Epidemiology and Phenomenology
OCD is among the most common anxiety disorders, with a population prevalence of up to 2–3 % (Lipsman et al. 2007). The condition is marked by obtrusive, repetitive and anxiogenic thoughts (obsessions), as well as time-consuming, disproportionate, and anxiolytic behaviours (compulsions). Although obsessions and compulsions can occur in the same patient, some suffer only from obsessions or compulsions. For example, whereas some patients will have contamination obsessions and/or compulsions (i.e. ‘washers’) others will have compulsions to count (i.e. ‘checkers’). Heterogeneity in OCD is the rule, although it appears that activation of fear and anxiety circuitry is a common thread.
OCD exists at the interface between psychiatry and neurology given the prominence of physical behaviours that patients believe are ‘outside of their control’. Behaviours, or compulsions, that are meant to relieve anxiety become reinforcing, leading to a vicious cycle of thoughts and actions. Current treatment strategies include medications targeting primarily the serotonergic system, and psychotherapeutic treatments that attempt to break the cycle by altering pathological cognitions. As in depression, a substantial proportion of patients, often up to a third, remain significantly disabled, despite optimal guideline-concordant care. For these patients, novel treatment strategies, including neurosurgery, are being investigated.
13.3.2 Neurocircuitry
Similar to mood disorders, advances in neuroimaging have led to a better understanding of OCD circuitry. Such studies have implicated decision-making and fear circuitry, as well as pure motor pathways in the basal ganglia (Greenberg et al. 2010b). For example, among the most consistently activated structures is OFC that is known to participate heavily in judgement, executive functioning, impulse control and emotion-guided decision-making (Kent et al. 2003). Both fMRI and PET studies have further shown significant amygdalar activation in response to provocative, disease-relevant stimuli, as well as a failure of cortical structures, and OFC in particular, to downregulate activity within the amygdale (Saxena et al. 1999, 2004; Swedo et al. 1992). The argument for overlap between anxiety and motor circuitry is strengthened by the large number of patients with Tourette’s syndrome who are diagnosed with comorbid OCD (Cummings and Frankel 1985). It is further not uncommon for patients with striatal and other basal-ganglia pathology to develop tic- and OCD-like behaviours (Cummings and Frankel 1985). Indeed, the complex regionalization of neuroanatomy, particularly within motor circuitry is now being recognized. The subthalamic nucleus, for example, a traditionally ‘motor’ structure is now known to have distinct associative and limbic components, with unique efferent and afferent projections. The existence of parallel, yet overlapping, circuits within the same 5-mm structure, highlights the intimate relationship between these pathways, and how dysfunction in one ‘critical node’ can have network wide influence, and lead to a broad constellation of symptoms.
13.3.3 DBS Targets
OCD was the first psychiatric condition to be investigated with DBS, in a report published in 1999 that described the anterior limb of the internal capsule (ALIC) as the target (Nuttin et al. 1999). Since then, several structures have been investigated, including the subthalamic nucleus (STN), VC/VS and ITP. Below we review the targets that have accumulated the most experience to date (Table 13.2).
13.3.3.1 ALIC
The ALIC is a projection pathway connecting cortical structures in the frontal and medial frontal lobe with subcortical structures and thalamus. The importance of the ALIC to disorders of mood and anxiety was recognized early, and severing this cortical–subcortical connection was the objective of initial attempts at psychiatric surgery with limbic leucotomy (Dax et al. 1948). Leucotomy has long been abandoned, replaced with stereotactic capsulotomy, a more precise and safe lesioning of the ALIC which for many years was the standard surgical approach for treatment-refractory anxiety and mood disorders. The development of DBS permitted implantation of electrodes at the capsulotomy target without causing a lesion. Nuttin et al. (1999) described their experience with ALIC DBS in OCD in two publications, their initial experience in 1999 in four patients and a subsequent publication in 2003 in six patients (Nuttin et al. 1999). Of the patients who had pre- and post-operative assessments with the Yale-Brown Obsessive Compulsive Scale (YBOCS), 75 % saw a reduction in scores of greater than 35 %, indicating a clinically meaningful response. Abelson et al. (2005) also performed a double-blind, sham stimulation study in four patients who underwent ALIC DBS and found that one patient saw a greater than 35 % YBOCS reduction during the double-blind period.
13.3.3.2 STN
The STN is an ovoid grey matter structure that is a component of the ‘indirect’ motor pathway. STN receives largely inhibitory input from the globus pallidus externus (GPe) and excitatory input directly from the motor cortex, and sends excitatory output to the globus pallidus internus (GPi) and substantia nigra reticulata (SNr).
The STN is a major DBS target for patients with disabling PD, and it was in the course of PD surgery that its putative role in OCD was hypothesized. Mallet et al. (2002) reported their experience of STN DBS in two patients with comorbid PD and OCD, and found that in addition to improvements in motor scores, patients experienced significant reductions in OCD scores post-operatively. This work led to a randomized, double-blind trial of STN DBS for OCD, wherein 16 patients underwent the procedure of which 12 saw at least a 25 % reduction in YBOCS scores (Mallet et al. 2008).
13.3.3.3 VC/VS
The DBS target with the most experience to date in OCD is the VC/VS. Anatomically, the VC/VS is closely related to the NAcc which exists at the ventral interface of caudate and putamen. Sturm et al. (2003) initially reported that three out of four patients who underwent VC/VS DBS for OCD saw ‘near total recovery’ at 24–30 months follow-up, although no YBOCS data were provided (Greenberg et al. 2010a; Greenberg et al. 2006) operated on ten patients with severe OCD using the same target, and found that of eight patients who were followed to 3 years, 50 % had a greater than 35 % reduction in YBOCS scores, indicating a treatment response. Such results are similar to those reported by Denys et al. (2010), who found 9 of 16 patients were responders at 8 months follow-up, with a mean YBOCS reduction of 46 % in open-label follow-up. Most recently, Tsai et al. (2012) reported their experience with VC/VS stimulation in four severe OCD patients and found that at 15-month follow-up, there was a mean 31 % reduction in YBOCS scores as well as 32 % reduction in depression ratings. Such results provide further support for the role of VC/VS in both mood and anxiety pathways.
13.3.3.4 ITP
The ITP consists of a relatively small bundle of projection fibres connecting OFC with the thalamus. ITP stimulation has been proposed for both refractory OCD and MDD, and a small experience with this target is accumulating. Jimenez-Ponce et al. reported the initial experience with ITP stimulation in five patients with OCD finding a mean 49 % reduction in YBOCS scores at 12-month follow-up (Jimenez-Ponce et al. 2009). The patient group, however, was highly heterogeneous and included some with comorbid schizophrenia and addiction. Larger studies, in more homogeneous cohorts will provide additional data about this target, but such results do to confirm earlier case reports that described the safety and efficacy of ITP stimulation (Jimenez et al. 2007).
13.4 Other Emerging Indications
All psychiatric indications for DBS are currently investigational. Although a Food & Drug Administration (FDA) humanitarian device exemption (HDE) exists for the use of DBS in OCD in the United States, DBS is not yet a standard of care for any psychiatric condition. However, the early promise of DBS in psychiatry has motivated its investigation in other circuit-based disorders, such as Alzheimer’s disease and anorexia nervosa.
13.4.1 Alzheimer’s Disease (AD)
AD is a neurodegenerative condition marked by severe memory and cognitive impairments. Given its relationship to advancing age and associated significant disability, demographic changes in the years to come will lead to an exponential rise in cases. The societal and public health costs will be enormous, and compounded by the abject lack of any effective treatments, despite decades of intense research (Laxton et al. 2013; Laxton and Lozano 2013).
It is becoming clear that AD is a disorder of brain networks, similar to PD and other neurodegenerative disorders. Focal disturbances lead to local disruption of neuronal activity, which is then propagated to synaptically connected structures within a functional network (Smith et al. 2012). By focally targeting disrupted networks, DBS has been proposed as a means of modulating activity in these circuits thereby restoring function. To date, two targets have been proposed for DBS in AD (Table 13.3), the fornix and the nucleus basalis of meynert (NBM). As part of Papez Circuit, the fornix is the principle outflow tract from the hippocampus and projects to the mammillary bodies via post-commissural fibres. A case report in 2008 described stimulation of the hypothalamic fornix in a case of severe obesity, which instead of curbing appetite led to significant improvements in verbal memory as well as spontaneous recall of autobiographical events (Hamani et al. 2008). This motivated a pilot trial of fornix DBS in six patients with mild AD, which found a significant slowing or stabilization of disease in two patients, with concomitant increases in cerebral glucose utilization in key memory circuits, including the default mode network (Laxton et al. 2010). An additional case report of fornix DBS in a patient with AD also found stabilization of memory scores at 12 months following stimulation (Fontaine et al. 2013). A large, multi-centre phase II/III trial is now being conducted to study the potential of this approach.
The NBM is a prime source of cholinergic transmission, and projects widely to key memory-related structures. Two case reports have described NBM DBS in patients with dementia, one with Alzheimer’s type and the other with Parkinson’s (Freund et al. 2009; Turnbull et al. 1985). The AD saw little clinical improvement but significant changes in glucose utilization on PET scan. The PD patient did see significant cognitive improvements following stimulation. The entorhinal cortex (EC) has also been explored as a stimulation target for memory enhancement. To date, however, this work has been limited to patients without preexisting memory disturbance, and exclusively tested in patients undergoing epilepsy surgery (Suthana et al. 2012, 2013). In these patients, who had hippocampal depth electrodes in place for seizure localization, researchers were able to demonstrate significant improvements in spatial memory with EC stimulation; an intriguing result that requires additional investigation.
13.4.2 Anorexia Nervosa (AN)
AN is a psychiatric condition marked by severe disturbances in weight, body- and self-perception. A common condition, with a lifetime general population prevalence of between 0.3 and 0.9 % anorexia nervosa is approximately ten times more common in females than in males (Bulik et al. 2005; Smink et al. 2012). The most obvious and striking feature of AN is a severe state of emaciation and malnourishment. As a result of chronic starvation patients are at risk for serious medical and metabolic complications that can affect virtually any body system. AN has the highest mortality rate of any psychiatric disease with mortality rates ranging from 5 to 15 % (Attia 2010; Hoek 2006; Morris and Twaddle 2007).
The imaging and anatomic literatures suggest that dysfunction in emotional circuits contribute to AN, as well as subsequent related dysfunctions in reward, perception and body-homeostatic control. Evidence is also emerging that depression, anxiety and dysregulated emotional processing impede effective therapies and lead to worse clinical outcomes (Kaye and Bailer 2011; Kaye et al. 2009). Circuit models have linked AN symptoms to interconnected regions and structures, such as the parietal lobe, insula and SCC. DBS, by intervening in a critical node in the AN circuit, may influence both core pathology and the symptoms that impede effective treatment. For these reasons, DBS for treatment-refractory AN has been proposed.
There are several published case reports and series’ investigating DBS in AN. Two case reports evaluated DBS of the SCC and NAcc in patients with comorbid AN/MDD and AN/OCD, respectively (Israel et al. 2010; McLaughlin et al. 2012). In both cases, long-term follow-up was associated with improvements in weight, and in the OCD case, improvements in anxiety. One case series evaluated NAcc DBS in four adolescent females with acute AN (average illness duration 18 m), and found at mean 38 m follow-up, a mean 65 % increase in body mass index (Wu et al. 2012). A pilot trial investigating SCC DBS in six patients with chronic, refractory AN showed that at 6–9 months follow-up, four patients were at BMIs significantly higher than baseline, with five patients showing significant improvements in mood and/or anxiety (Lipsman et al. 2013c). At 6 months, DBS was also associated with significant changes in cerebral glucose metabolism on PET scans compared to baseline, most notably showing a reversal of known parietal hypometabolism. With the psychometric results preceding changes in weight, such results suggest that DBS may be influencing the most common obstacles to enduring behaviour change in these patients. Additional, larger trials, utilizing sham stimulation control, are now required to validate these results.
13.5 Future Directions
The future of DBS in psychiatry will see both technical and conceptual advances. The former will see smaller, rechargeable batteries make the procedure safer and the clinical benefits longer lasting. Improved imaging will allow more accurate targeting, as advances in tractography will permit placement of stimulating electrodes at key junctional pathways that maximize the modulatory effect. One key area of interest will be elucidating biomarkers for psychiatric conditions as well as predictors of treatment response. Recent work in MDD has shown, for example, that glucose utilization patterns in the insula, can predict response to a first-line depression treatment (McGrath et al. 2013). Similarly, work done using EEG has shown that preoperative power in specific frequency bands can predict outcomes following SCC DBS in depression (Broadway et al. 2012). Such work can help elucidate the characteristics of patients that do and do not respond to stimulation. It may also be that in the future, emerging technologies such as radiofrequency-guided nanotechnology and focused ultrasound will obviate the need for cranial access (Lipsman et al. 2013b; Stanley et al. 2012).
The number of indications for DBS in psychiatry will also continue to expand. Changes in the Diagnostic and Statistical Manual (DSM), such as a shift away from categorical labels and towards classification of broad behaviours and cognitions, will help tailor treatments to specific dysfunctional circuits. Improving our understanding of the neural circuitry maintaining these disorders will help to further define the role of DBS in their management.
References
Abelson JL, Curtis GC, Sagher O et al (2005) Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 57(5):510–516. doi:10.1016/j.biopsych.2004.11.042
Anderson D, Ahmed A (2003) Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation. Case report. J Neurosurg 98(5):1104–1108
Aouizerate B, Cuny E, Martin-Guehl C et al (2004) Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg 101(4):682–686. doi:10.3171/jns.2004.101.4.0682
Attia E (2010) Anorexia nervosa: current status and future directions. Annu Rev Med 61:425–435. doi:10.1146/annurev.med.050208.200745
Bewernick BH, Hurlemann R, Matusch A et al (2010) Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry 67(2):110–116. doi:10.1016/j.biopsych.2009.09.013
Bewernick BH, Kayser S, Sturm V et al (2012) Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology 37(9):1975–1985. doi:10.1038/npp.2012.44
Broadway JM, Holtzheimer PE, Hilimire MR et al (2012) Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology 37(7):1764–1772. doi:10.1038/npp.2012.23
Bulik CM, Reba L, Siega-Riz AM et al (2005) Anorexia nervosa: definition, epidemiology, and cycle of risk. Int J Eat Disord 37(Suppl):S2–S9. doi:10.1002/eat.20107; discussion S20–S21
Cummings JL, Frankel M (1985) Gilles de la Tourette syndrome and the neurological basis of obsessions and compulsions. Biol Psychiatry 20(10):117–126
Dax EC, Freudenberg RK, Reitman F et al (1948) Prefrontal leucotomy; a review. Postgrad Med J 24(274):415–426
Denys D, Mantione M, Figee M et al (2010) Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 67(10):1061–1068. doi:10.1001/archgenpsychiatry.2010.122
Fontaine D, Mattei V, Borg M et al (2004) Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. J Neurosurg 100(6):1084–1086. doi:10.3171/jns.2004.100.6.1084
Fontaine D, Deudon A, Lemaire JJ et al (2013) Symptomatic treatment of memory decline in Alzheimer’s disease by deep brain stimulation: a feasibility study. J Alzheimers Dis 34(1):315–323. doi:10.3233/JAD-121579
Franzini A, Messina G, Cordella R et al (2010) Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus 29(2):E13. doi:10.3171/2010.5.FOCUS1094
Freund HJ, Kuhn J, Lenartz D et al (2009) Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol 66(6):781–785. doi:10.1001/archneurol.2009.102
Giacobbe P, Mayberg HS, Lozano AM (2009) Treatment resistant depression as a failure of brain homeostatic mechanisms: implications for deep brain stimulation. Exp Neurol 219(1):44–52. doi:10.1016/j.expneurol.2009.04.028
Goodman WK, Foote KD, Greenberg BD et al (2010) Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry 67(6):535–542. doi:10.1016/j.biopsych.2009.11.028
Greenberg BD, Malone DA, Friehs GM et al (2006) Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 31(11):2384–2393. doi:10.1038/sj.npp.1301165
Greenberg BD, Gabriels LA, Malone DA Jr et al (2010a) Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 15(1):64–79. doi:10.1038/mp.2008.55
Greenberg BD, Rauch SL, Haber SN (2010b) Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology 35(1):317–336. doi:10.1038/npp.2009.128
Hamani C, McAndrews MP, Cohn M et al (2008) Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 63(1):119–123. doi:10.1002/ana.21295
Hamani C, Mayberg H, Stone S et al (2011) The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry 69(4):301–308. doi:10.1016/j.biopsych.2010.09.034
Hoek HW (2006) Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 19(4):389–394. doi:10.1097/01.yco.0000228759.95237.78
Holtzheimer PE, Kelley ME, Gross RE et al (2012) Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69(2):150–158. doi:10.1001/archgenpsychiatry.2011.1456
Israel M, Steiger H, Kolivakis T et al (2010) Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biol Psychiatry 67(9):e53–e54. doi:10.1016/j.biopsych.2009.11.016
Jimenez F, Velasco F, Salin-Pascual R et al (2007) Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl 97(Pt 2):393–398
Jimenez F, Nicolini H, Lozano AM et al (2012) Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. doi:10.1016/j.wneu.2012.07.010
Jimenez-Ponce F, Velasco-Campos F, Castro-Farfan G et al (2009) Preliminary study in patients with obsessive-compulsive disorder treated with electrical stimulation in the inferior thalamic peduncle. Neurosurgery 65(6 Suppl):203–209. doi:10.1227/01.NEU.0000345938.39199.90; discussion 209
Kaye WH, Bailer UF (2011) Understanding the neural circuitry of appetitive regulation in eating disorders. Biol Psychiatry 70(8):704–705. doi:10.1016/j.biopsych.2011.08.018
Kaye WH, Fudge JL, Paulus M (2009) New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 10(8):573–584. doi:10.1038/nrn2682
Kennedy SH, Evans KR, Kruger S et al (2001) Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 158(6):899–905
Kennedy SH, Konarski JZ, Segal ZV et al (2007) Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry 164(5):778–788. doi:10.1176/appi.ajp.164.5.778
Kennedy SH, Milev R, Giacobbe P et al (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. IV Neurostimulation therapies. J Affect Disord 117(Suppl 1):S44–S53. doi:10.1016/j.jad.2009.06.039
Kennedy SH, Giacobbe P, Rizvi SJ et al (2011) Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 168(5):502–510. doi:10.1176/appi.ajp.2010.10081187
Kent JM, Rauch SL (2003) Neurocircuitry of anxiety disorders. Curr Psychiatry Rep 5(4):266–273
Kessler RC, Berglund P, Demler O et al (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289(23):3095–3105. doi:10.1001/jama.289.23.3095
Kessler RC, Berglund P, Demler O et al (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62(6):593–602. doi:10.1001/archpsyc.62.6.593
Lam RW, Kennedy SH, Grigoriadis S et al (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III Pharmacotherapy. J Affect Disord 117(Suppl 1):S26–S43. doi:10.1016/j.jad.2009.06.041
Laxton AW, Lozano AM (2013) Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg 80(3–4):S28.e21–S28.e28. doi:10.1016/j.wneu.2012.06.028
Laxton AW, Tang-Wai DF, McAndrews MP et al (2010) A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 68(4):521–534. doi:10.1002/ana.22089
Laxton AW, Lipsman N, Lozano AM (2013) Deep brain stimulation for cognitive disorders. Handb Clin Neurol 116:307–311. doi:10.1016/B978-0-444-53497-2.00025-5
Lipsman N, Neimat JS, Lozano AM (2007) Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery 61(1):1–11. doi:10.1227/01.neu.0000279719.75403.f7; discussion 11–13
Lipsman N, Sankar T, Downar J et al (2013a) Neuromodulation for treatment-refractory major depressive disorder. CMAJ 186(1):33–39. doi:10.1503/cmaj.121317
Lipsman N, Schwartz ML, Huang Y et al (2013b) MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol 12(5):462–468. doi:10.1016/S1474-4422(13)70048-6
Lipsman N, Woodside DB, Giacobbe P et al (2013c) Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. doi:10.1016/S0140-6736(12)62188-6
Lozano AM, Lipsman N (2013) Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77(3):406–424. doi:10.1016/j.neuron.2013.01.020
Lozano AM, Giacobbe P, Hamani C et al (2012) A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg 116(2):315–322. doi:10.3171/2011.10.JNS102122
Mallet L, Mesnage V, Houeto JL et al (2002) Compulsions, Parkinson’s disease, and stimulation. Lancet 360(9342):1302–1304. doi:10.1016/S0140-6736(02)11339-0
Mallet L, Polosan M, Jaafari N et al (2008) Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. NEnglJMed 359(20):2121–2134. doi:10.1056/NEJMoa0708514
Malone DA Jr, Dougherty DD, Rezai AR et al (2009) Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65(4):267–275. doi:10.1016/j.biopsych.2008.08.029
Mayberg HS (1997) Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9(3):471–481
Mayberg HS, Liotti M, Brannan SK et al (1999) Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156(5):675–682
Mayberg HS, Lozano AM, Voon V et al (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45(5):651–660. doi:10.1016/j.neuron.2005.02.014
McGrath CL, Kelley ME, Holtzheimer PE et al (2013) Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 70:821–829. doi:10.1001/jamapsychiatry.2013.143
McLaughlin NC, Didie ER, Machado AG et al (2012) Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder. Biol Psychiatry. doi:10.1016/j.biopsych.2012.09.015
Morris J, Twaddle S (2007) Anorexia nervosa. BMJ 334(7599):894–898. doi:10.1136/bmj.39171.616840.BE
Nuttin B, Cosyns P, Demeulemeester H et al (1999) Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 354(9189):1526. doi:10.1016/S0140-6736(99)02376-4
Nuttin BJ, Gabriels L, van Kuyck K et al (2003) Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am 14(2):267–274
Patel SR, Sheth SA, Mian MK et al (2012) Single-neuron responses in the human nucleus accumbens during a financial decision-making task. J Neurosci 32(21):7311–7315. doi:10.1523/JNEUROSCI.0027-12.2012
Plewnia C, Schober F, Rilk A et al (2008) Sustained improvement of obsessive-compulsive disorder by deep brain stimulation in a woman with residual schizophrenia. Int J Neuropsychopharmacol 11(8):1181–1183. doi:10.1017/S1461145708009188
Price JL, Drevets WC (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35(1):192–216. doi:10.1038/npp.2009.104
Puigdemont D, Perez-Egea R, Portella MJ et al (2011) Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. Int J Neuropsychopharmacol 15:1–13. doi:10.1017/S1461145711001088
Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, Phelps ME, Baxter LR Jr (1999) Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology 21(6):683–693. doi:10.1016/S0893-133X(99)00082-2
Saxena S, Brody AL, Maidment KM et al (2004) Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry 161(6):1038–1048
Schlaepfer TE, Cohen MX, Frick C et al (2008) Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33(2):368–377. doi:10.1038/sj.npp.1301408
Schlaepfer TE, Bewernick BH, Kayser S et al (2013) Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry 73(12):1204–1212. doi:10.1016/j.biopsych.2013.01.034
Smink FR, van Hoeken D, Hoek HW (2012) Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep 14(4):406–414. doi:10.1007/s11920-012-0282-y
Smith GS, Laxton AW, Tang-Wai DF et al (2012) Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol 69(9):1141–1148. doi:10.1001/archneurol.2012.590
Stanley SA, Gagner JE, Damanpour S et al (2012) Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 336(6081):604–608. doi:10.1126/science.1216753
Sturm V, Lenartz D, Koulousakis A et al (2003) The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat 26(4):293–299
Suthana N, Fried I (2013) Deep brain stimulation for enhancement of learning and memory. Neuroimage 85(3):996–1002. doi:10.1016/j.neuroimage.2013.07.066
Suthana N, Haneef Z, Stern J et al (2012) Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med 366(6):502–510. doi:10.1056/NEJMoa1107212
Swedo SE, Pietrini P, Leonard HL et al (1992) Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch Gen Psychiatry 49(9):690–694
Tsai HC, Chang CH, Pan JI et al (2012) Pilot study of deep brain stimulation in refractory obsessive-compulsive disorder ethnic Chinese patients. Psychiatry Clin Neurosci 66(4):303–312. doi:10.1111/j.1440-1819.2012.02352.x
Turnbull IM, McGeer PL, Beattie L et al (1985) Stimulation of the basal nucleus of Meynert in senile dementia of Alzheimer’s type. A preliminary report. Appl Neurophysiol 48(1–6):216–221
Wu H, Van Dyck-Lippens PJ, Santegoeds R et al (2012) Deep-brain stimulation for anorexia nervosa. World Neurosurg 98(5):1104–1108. doi:10.1016/j.wneu.2012.06.039/10.3171/jns.2003.98.5.1104
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lipsman, N., Lozano, A.M. (2015). Deep Brain Stimulation for Psychiatric Disorders. In: Itakura, T. (eds) Deep Brain Stimulation for Neurological Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-08476-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-08476-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08475-6
Online ISBN: 978-3-319-08476-3
eBook Packages: MedicineMedicine (R0)