Abstract
In this research study, energy, exergy and exergo-economic analysis of Montazer Ghaem gas turbine power plant which is located near Tehran, capital city of Iran is carried out. The results of this study reveal that the highest exergy destruction occurs in the combustion chamber (CC), where the large temperature difference is the major source of the irreversibility. In addition, the effects of the gas turbine load variations and ambient temperature are investigated to see how system performance changes: the gas turbine is significantly affected by the ambient temperature which leads to a decrease in net power output. The results of the load variation of the gas turbine show that a reduction in gas turbine load results in a decrease in the exergy efficiency of the cycle as well as all the components. As was expected, an increase in ambient temperature has a negative effect on the exergy efficiency of the cycle, so this factor could be countered by using gas turbine air inlet cooling methods. In addition, an exergo-economic analysis is conducted to determine the cost of exergy destruction in each component and to determine the cost of fuel. The results show that combustion chamber has the largest cost of exergy destruction, which is in line with the exergy analysis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Power generation is a fundamental pillar of infrastructure for other industries and for industrial growth and development. Rapid growth in demand for electricity in certain countries is driving heavy investment in new power plants over the short term. Gas turbine power plants present a prime option in the energy mix. Awareness of limited hydro-carbon resources, environmental and economic concerns, and ever-increasing demand for electricity necessitate the design of optimal gas turbine power plants in terms of technical and cost aspects. Exergy analysis is based on the first and second laws of thermodynamics and makes it possible to characterize the optimal analysis technique on energy systems as well as to identify energy levels and thermodynamic adverse processes clearly in a system. This method is used to describe different energy flows and contributes to reductions in several losses that may occur in the system. Thermodynamics have been used for almost a century to model energy systems, including advanced power plants. The first law of thermodynamics is usually used to model a system; it cannot determine the source of irreversibilities in the system under consideration. In energy systems analysis, which is essentially based on the first law of thermodynamics, there is no difference between various energy states. For instance, a thermal energy unit that has been desorbed by a condenser in a steam turbine power plant is equal to one output work unit from a turbine in the same power plant.
As a result, an analysis based on energy equilibrium may be misleading due to its failure to provide information about internal losses in the system. For example, analysis of energy in adiabatic systems like adiabatic compressors, combustion chambers and or thermal converters may lead to a hasty conclusion that there is no energy loss in this equipment. Nevertheless, even without adapting second law techniques, an experienced designer knows that with respect to their capabilities in feeding various processes and capacity for conversion into other forms of energy, they have some different qualities. It is thus obvious that to conduct an efficiency analysis of energy systems performance criteria must be devised for evaluating thermodynamic efficiency. One may refer to the thermal efficiency of power cycles and or yield coefficient of heat exchangers, as examples of performance criteria. However, like energy analysis, such criteria are mainly based on the first law of thermodynamics, where downgrade of energy quality is not considered. Similarly, results obtained by these criteria may be interpreted only within the field of limited processes, and many pieces of equipment and processes lack criteria of this kind. For this reason, it seems that a thermodynamic concept in which the second law of thermodynamics (downgrade of energy quality) is considered could be used without limitation for conducting an effective analysis of all processes of energy conversion.
The potential for conducting useful mechanical work by means of energy consumption is the criterion of the exergy method for numerical evaluation of the quality of different states of energy. A criterion that is formed according to the second law of thermodynamics may be adapted for all energy conversion systems and its result could be interpreted independently of the type of equipment. Exergetic analysis is used to address the magnitude, place, numerical value and the reasons for occurrence of thermodynamic inefficiencies; based on its results the efficiency of the consuming systems and energy converter may be improved. In addition, by adapting this analysis, one may remove the ambiguities that are created due to first law analyses and criteria. In the next section of this paper, we will explain the meaning and provide a history of exergy subjects, and detail their concept and computation technique. In the following section, exergy analysis and its relationships with the Montazer Ghaem gas turbine power plant are examined. Although exergy is a new term, the primary evaluations on the rate of energy convertibility of a system into work hark back to the time of definition and presentation of the second law of thermodynamics. By publishing a paper in 1824, Sadi Carnot showed that the conversion of thermal energy into mechanical work might be limited in thermal machines. The essay was hailed as the first accurate numerical analysis of the quality of different energy modes and the ability to convert them into each other.
“Work potential” and “Maximum usable work” from a certain amount of energy was examined after the mathematical formulation of the second law in works by Clausius, Thomson, Maxwell and especially Gibbs. For the first time, Gouy and Stodola separately and clearly defined work potential in 1889 and 1898, respectively. During the 1930s, attention was drawn toward the practical dimensions of this concept, and industrial progress ensued. In the same year, by purposing some essays, Bosnjakovic documented techniques of the second law of thermodynamics to analyze energy systems. Subsequently, in 1956, Rant defined the work potential of energy precisely and employed the term “Exergy” for the first time in denoting this quantity. The 1980s and 1990s saw increasing attention and credibility being lent to exergy analysis, and several conferences were held to support and develop this field of applied thermodynamics. The continuum of papers inspired by these conferences led to the documentation of the current forms of exergetic topics.
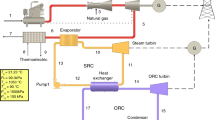
Many researchers including Kotas [1], Moran and Shapiro [2] conducted exergy analyses for combined cycle power plants and calculated losses in different parts. In an essay, Facchini et al. [3] carried out an exergy analysis of a combined cycle power plant and concluded that the maximum losses occur inside the combustion chamber, because of the great difference between the flame temperature and operating fluid, and concluded that exergy analysis was a helpful concept for comparing performance in gas turbine cycles. Looking at recent studies indicates that they tried to improve efficiency and output power in these power plants. Bassily [4] simulated and reduced losses for a triple pressure combined cycle power plant; he took a recovery boiler with seven pinch points and examined the impact on them of input temperature inside the gas turbine. His aim was to lower the temperature on the pinch points. Sung and Kim [5] carried out an exergy analysis of a gas turbine cycle at different loads and concluded that the chemical reactions that occurred in the combustion chamber as well as different high temperatures between the flame and operating fluid, would cause maximum losses in gas turbine cycles. Javadabadi et al. [6] conducted an exergy analysis of the gas turbine cycle of a 116 MW power plant and concluded that the impact of rising input temperature in gas turbine turbines may improve total exergy efficiency of the gas turbine cycle, and would reduce exergy losses. Similarly, they came to the result that maximum losses will occur in the combustion chamber in a gas-fired power plant. Ahmadi et al. [7] carried out an exergy analysis on a gas turbine power plant with input air as coolant into a compressor (Fog System). Their results showed that although application of a Fog System led to improvement in output power in the gas cycle, but it would increase exergetic losses of the cycle. Thus, the importance of exergy analysis is clear in power production cycles. The present study comprised a comparative exergy and exergo-economic analysis of the Montazer Ghaem power plant shown in Fig. 7.1 at different loads and ambient temperatures. In brief, the study consists of the following elements:
-
Exergy analysis of a typical GT power plant.
-
Analysis of system performance at different ambient temperatures and partial loads.
-
Exergo-economic analysis of the gas turbine power plant.
7.2 Exergy Analysis
Exergy is the maximum theoretical useful work that may be received from energy in a system of ideal machines. It is clear that exergy is not stored in a single process, but may be destroyed due to irreversibility. In this method, it is possible to analyse each element of the cycle separately and to obtain the share of each one in total loss of the cycle. Regarding gas turbine power plants, with respect to input fuel or any input flow into the power plant, one may obtain the maximum capacity of the power plant by exergy analysis. The exergy of matter flow may be divided into its major components including kinetic exergy, potential exergy, physical exergy and chemical exergy. In this research paper, due to their dispensable rates, kinetic and potential terms are ignored. Physical exergy is defined as the maximum theoretical useful work obtained as a system interacts with an equilibrium state [8]. Chemical exergy is associated with the departure of the chemical composition of a system from its chemical equilibrium. Chemical exergy is an important part of exergy in the combustion process [9]. Applying the first and second laws of thermodynamics, the following exergy balance is obtained:
In this formula ex is the total specific exergy and \( \dot{E}{x}_D \) is the exergy destruction rate, other terms in this equation are defined as [10]:
Where T is the absolute temperature (K) and subscripts i and 0 refer to ambient conditions. The mixture chemical exergy is obtained by following relations [11]:
The following equation is used to calculate the fuel exergy [12]:
For most of usual gaseous fuels, the ratio of chemical exergy to lower heating value is usually close to 1. Since the main fuel used in power plants is methane, one may write [1]:
In this paper, exergy analysis of Montazer Ghaem gas turbine power plant is conducted. Initially, exergy of different points of the cycle, which are characterized in Fig. 7.1, were computed and then, exergetic losses and their exergetic efficiency were calculated by writing down exergetic balance for each element in the gaseous cycle. In Table 7.1, the exergy destruction rate and exergy efficiency equations for plant components are given.
7.3 Exergo-Economic Analysis
The goal of conducting thermo-economic investigations of systems is to minimize the cost of exergy. In exergy costing, a certain cost is determined for each of the exergetic flows. The cost balance may be considered for the total system, and input and output exergies to/from the total system may be priced. A cost balance that is recorded for kth element denotes that the sum cost rates in exergies of output flows are equal to the total cost rates of exergies in input flows plus the cost rate of the capital investment, operating and maintenance. For each flow line in the system, a parameter called the flow cost rate ($/s) was defined. Thus, for a system that receives heat and produces work, the exergetic balance may be written as follows [13]:
The exergy product is the partial of the system and is defined as a target for application of that element in the system. Moreover, the exergy fuel of the system may be defined as those exergies that are consumed to produce the exergy product of the given system components, where we indicate them by \( {\dot{E}}_P,{\dot{E}}_F \) respectively. Similarly, the cost rates of fuel and product are indicated by \( {\dot{C}}_F,{\dot{C}}_P \) respectively. In the exergetic balance that is written for an element of a system, there is no term that directly denotes cost of exergy destruction. For this reason, the cost caused by exergy destruction is called the latent cost in the elements of the system. Exergy destruction cost is considered an important parameter in the exergo-economic analysis.
Where \( {\dot{E}}_{F,k} \) represents the fuel exergy rate for kth element, and \( {\dot{E}}_{P,k} \) stands for the product exergy rate of kth element and \( {\dot{E}}_{D,k} \) is the exergy destruction rate of that element due to the irreversibilities, respectively. Assuming that the product E P,k is fixed and that the unit cost of fuel c F,k of the kth component is independent of the exergy destruction, we can define the cost of exergy destruction by the equation [11]:
More details of the exergoeconomic analysis, cost balance equations and exergoeconomic factors are completely discussed in references [12, 14, 15]. Several methods have been suggested to express the purchase cost of equipment in terms of design parameters in Eq. (7.9) [9, 11, 16]. In this paper we have used the cost functions that are suggested by Ahmadi et al. [17]. To convert the capital investment into cost per unit time one may write:
Where Z k is the purchase cost of kth component in U.S dollars, N is the annual number of operating hours of the unit, φ = 1.06 [17] is the maintenance factor and the Capital Recovery Factor (CRF) depends on the interest rate as well as estimated equipment life; CRF is determined using the relation [17]:
Where i is the interest rate and n is the total operating period of the system in years. For each component of the Montazer Ghaem gas turbine power plant, the term \( {\dot{C}}_{D,k}+{\dot{Z}}_k \) is calculated to give insight into purchase cost and exergy destruction cost.
7.4 Results and Discussion
In this section, results of exergy and exergo-economic analysis are presented. Figures 7.2 and 7.3 show the exergy destruction rate and exergy efficiency of different elements in the gas turbine cycle of the Montazer Ghaem power plant, respectively. These figures signify that the combustion chamber has the maximum rate of exergy destruction and the minimum rate of exergy efficiency among other elements. This is due to the chemical reactions inside the combustion chamber as well as high temperature differences between the operating fluid and flame. At the same time, it is observed that by lowering the irreversibility load, all elements of this cycle are reduced and thus exergy efficiency is improved.
In Figs. 7.4 and 7.5, it is seen that by raising ambient temperature, exergy destruction rate is increased in the compressor since the ratio of pressure in the compressor is the same in three states and with respect to reduction of density of input air, the compressor needs more consuming work and thus the exergy destruction of the compressor is increased. However, the exergy destruction rate of the combustion chamber is reduced by raising the temperature since both discharge of the input fluid reduces and fuel discharge decreases, whereas fuel exergy is tangibly reduced so exergy destruction is lowered overall. At the same time, it is observed that the exergy destruction rate of the turbine is improved by a rise in temperature.
In Figs. 7.6 and 7.7, the exergy efficiency of total gas turbine cycle is given for different loads and ambient temperatures respectively. From these results, it is seen that the rate of total exergy destruction in the total gas turbine cycle is improved by increasing the load and raising ambient temperature. Table 7.2 shows that in exergo-economic analysis, the combustion chamber is the major component for exergy loss, since cost of exergy destruction is also higher in the combustion chamber than in other elements. These results suggest total agreement between the exergy analysis and the exergo-economic analysis.
7.5 Conclusions
In the current paper, exergetic analysis is carried out for a typical gas turbine power plant at different working conditions. For each element in the power plant, exergy efficiency and exergy destruction ratio are computed in three loads of 50, 75 and 100 MW for 4 °C as ambient temperature as well as 85 MW for 4, 15 and 34 °C ambient temperatures. The exergy efficiency of total cycle was obtained in all conditions. Results indicate that the combustion chamber may be considered as the foremost factor for exergy destruction and relatively low efficiency. This is due to higher fuel exergy and chemical reactions of fuel with air, and heat transfer inside the combustion chamber.
The other interesting result is that by reducing the load in all elements, the rate of exergy efficiency is decreased. This point may imply that the power plant achieves maximum efficiency at its nominal load. Rising temperatures have an opposite trend against load increase and may cause reductions in the exergy efficiency of all elements and, hence, the relative efficiency of the whole power plant. Thus, it can be concluded that the best working conditions considered for the power plant are: 100 MW load at 4 °C.
By considering technical conditions, exergo-economic analysis of power plants may play an effective role in informing the management of technical conditions. Similarly, this analysis may reflect the importance of paying attention to the exergy efficiency of power plants and improvements through identifying the price of exergy destruction proportional to the fuel price and the price of purchasing elements. The results of this study indicate that the combustion chamber attracts the maximum cost in terms of exergy destruction and, thus, constitutes the prime target for optimization efforts. It should be noted that the results that were obtained from exergo-economic analysis, comply with the results coming from exergy analysis, and these verify the accuracy and authenticity of both methods.
References
Kotas TJ (1985) The exergy method in thermal plant analysis. Butterworths, London
Moran MJ, Shapiro HN (2000) Fundamentals of engineering thermodynamics, 4th edn. Wiley, New York
Facchini B, Fiaschi D, Manfrida G (2000) Exergy analysis of combined cycles using latest generation gas turbine. ASME J Engrg Gas Turbine Power 122:233–238
Bassily AM (2005) Modeling, numerical optimization, and irreversibility reduction of a triple-pressure reheat combined cycle. Int J Energy 32(5):778–794
Song TW, Sohn JL, Kim JH, Kim TS, Ro ST (2002) Exergy-based performance analysis of the heavy duty gas turbine in part-load operating condition. Int J Exergy 2:105–112
Ebadi MJ, Gorji-Bandpy M (2005) Exergetic analysis of gas turbine plants. Int J Exergy 2(4):31–39
Ahmadi P, Abadi A, Ghaffarizadeh AR, Naghib I (2008) Effect of Fog inlet air cooling method on combined cycle power plant output power. 16th Annual (International) Conference on Mechanical Engineering-ISME. Shahid Bahonar University of Kerman, Iran
Dincer I, Rosen MA (1999) Energy environment and sustainable development. Appl Energy 64:427–440
Cihan A, Hacıhafızoglu O, Kahveci K (2006) Energy-exergy analysis and modernization suggestions for a combined-cycle power plant. Int J Energy Res 30:115–126
Ahmadi P, Rosen MA, Dincer I (2011) Greenhouse gas emission and exergo environmental analyses of a trigeneration energy system. Int J Greenh Gas Control 5:1540–1549
Bejan A, Tsatsaronis G, Moran M (1996) Thermal design and optimization. Wiley, New York
Ameri M, Ahmadi P, Hamidi A (2009) Energy, exergy and exergoeconomic analysis of a steam power plant (a case study). Int J Energy Res 33:499–512
Ameri M, Enadi N (2012) Thermodynamic modeling and second law based performance analysis of a gas turbine power plant (exergy and exergoeconomic analysis). J Power Technol 92(3):183–191
Sahoo PK (2008) Exergoeconomic analysis and optimization of a cogeneration system using evolutionary programming. Appl Therm Eng 28(13):1580–1588
Ahmadi P, Dincer I (2011) Thermodynamic analysis and thermoeconomic optimization of a dual pressure combined cycle power plant with a supplementary firing unit. Energy Convers Manag 52(5):2296–2308
Roosen P, Uhlenbruck S, Lucas K (2003) Pareto optimization of a combined cycle power system as a decision support tool for trading off investment vs. operating costs. Int J Therm Sci 42:553–560
Ahmadi P, Barzegar Avval H, Ghaffarizadeh A, Saidi MH (2011) Thermo economic-environmental multi-objective optimization of a gas turbine power plant with preheater using evolutionary algorithm. Int J Energy 35(5):389–403
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Nomenclature
Nomenclature
- \( \dot{C} \) :
-
Cost per unit of exergy ($/MJ)
- \( {\dot{C}}_D \) :
-
Cost of exergy destruction ($/h)
- CRF :
-
Capital recovery factor
- ex :
-
Specific exergy (kJ/kg)
- \( \dot{e}x \) :
-
Specific exergy rate (kW/kg)
- \( \dot{E}x \) :
-
Exergy flow rate (kW)
- h :
-
Specific enthalpy (kJ/kg)
- LHV :
-
Lower heating value (kJ/kg)
- \( \dot{m} \) :
-
Mass flow rate (kg/s)
- P:
-
Pressure (kPa)
- \( \dot{Q} \) :
-
Heat transfer rate (kW)
- R :
-
Gas constant (kJ/kg K)
- s:
-
Specific entropy (kJ/kg K)
- T:
-
Temperature (K)
- \( \dot{W} \) :
-
Work transfer rate (kW)
- \( \dot{Z} \) :
-
Capital cost rate ($/s)
- Z k :
-
Component purchase cost ($)
- η ex :
-
Exergy efficiency
- φ :
-
Maintenance factor
- ξ :
-
Coefficient of fuel chemical exergy
- C :
-
Compressor
- CC :
-
Combustion chamber
- ch :
-
Chemical
- D :
-
Destruction
- e :
-
Exit condition
- GT :
-
Gas turbine
- f :
-
Fuel
- i :
-
Inlet Condition
- k :
-
Component
- ph :
-
Physical
- 0 :
-
Reference ambient condition
- ⋅:
-
Rate
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mousafarash, A., Ahmadi, P. (2014). Exergy and Exergo-Economic Based Analysis of a Gas Turbine Power Generation System. In: Dincer, I., Midilli, A., Kucuk, H. (eds) Progress in Sustainable Energy Technologies Vol II. Springer, Cham. https://doi.org/10.1007/978-3-319-07977-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-07977-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07976-9
Online ISBN: 978-3-319-07977-6
eBook Packages: EnergyEnergy (R0)