Abstract
This chapter details the neural circuitry that controls the mechanisms that regulate urinary and intestinal functions, describing the afferent and efferent neuronal pathways between the brain and the pelvic organs.
After a functional anatomical approach, the neurophysiological investigations valuable in the assessment of patients with urogenital and anorectal dysfunctions are reported. On assessing a patient with neurogenic bladder and bowel dysfunction, different neurophysiological methods are described, in particular electromyography (EMG), sacral reflexes, somatosensory-evoked potential (SEP), motor-evoked potential (MEP), sympathetic skin response (SSR), and pudendal nerve terminal motor latency (PNTML). Moreover, technical advice is given, reporting normative values and discussing neurophysiological tests clinical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pelvic Floor
- Transcranial Magnetic Stimulation
- Pelvic Floor Muscle
- External Anal Sphincter
- Pudendal Nerve
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Urinary and fecal continence, micturition and defecation, and sexual arousal and orgasm are dependent on the integrity of the central and peripheral nervous pathways to the sacral region. The coordination between bladder, urethra, anorectum, and sphincters is mediated by a complex neural control system in the brain, spinal cord, peripheral ganglia, and peripheral nerves.
3.1 Neural Control of Urinary Tract
Bladder and urethra have two primary functions: the storage and the periodic elimination of urine; these activities need the neural coordination in the central, somatic, and autonomic peripheral nervous systems. The voluntary control of micturition requires complex connections between sympathetic and parasympathetic autonomic nerves, pudendal somatic nerves, and many areas in the brain. Parasympathetic preganglionic cholinergic outflow, giving excitatory input to the bladder, arises in the sacral parasympathetic nucleus (SPN), localized in the intermediolateral column from the S2–S4 spinal segments. The parasympathetic fibers then travel through pelvic nerves to intramural bladder ganglia and pelvic plexus, where the postganglionic fibers induce detrusor contraction and urinary flow. The parasympathetic activation of M3 muscarinic and P2X purinergic receptors is involved in voiding reflex, while nitric oxide transmission mediates inhibition of urethral smooth muscle. The sympathetic system, which plays a primary role in the continence mechanism inhibiting the parasympathetic action, originates from the intermediolateral columns in the T11–L2 spinal cord segments. Preganglionic may synapse on postganglionics in the paravertebral sympathetic chain or pelvic plexus; hypogastric nerve, conveying sympathetic afferents and efferents, releases noradrenaline (NA) on bladder and urethra. The sympathetic efferents therefore activate β3-adrenergic inhibitory receptors in the detrusor muscle relaxing the bladder and α1-adrenergic excitatory receptors in the bladder neck and urethra allowing the continence and urine storage and preventing retrograde ejaculation. The somatic cholinergic pathway originating from S2–S4 motor neurons in Onuf’s nucleus (ON) innervates, through pudendal nerves, the external urethral sphincter (EUS) and pelvic floor muscles (Fig. 3.1). The somatic afferents from the bladder neck and the urethra are conveyed from pudendal nerves, the dorsal horn of the spinal cord, while the sensation of bladder fullness travels by pelvic and hypogastric nerves to the spinal cord.
The afferent Aδ fibers, lightly myelinated, and the unmyelinated C fibers travel through pelvic and hypogastric nerves. While Aδ fibers respond to active contraction and passive distension, conveying information about bladder filling, C fibers are insensitive to bladder filling under physiological conditions and activated only in pathological conditions.
These fibers convey nociceptive, volume, and tension information in the somatosensory pathways from the sacral dermatomes through the spinal cord to the CNS. The activity of volume and tension mechanoreceptors during bladder filling is conveyed to the dorsal horns by the pelvic nerves. A rostral intersegmental pathway projects to the thoracolumbar cord, stimulating sympathetic preganglionics, thus promoting continence via the hypogastric nerves. The persistence of low detrusor pressure, the absence of involuntary contractions, and the increased pressures at urethral level are the result of storage reflexes. Pelvic organ afferents can inhibit the sacral preganglionics to the bladder and induce increased urethral pressure. This guarding reflex is known as visceral-visceral reflex. This fact explains the possible therapeutic utility of intravaginal electrostimulation in the treatment of urgency-frequency syndrome. Another guarding reflex exists, in which afferents from pelvic organs and bladder filling stimulate ON to increase the outlet urethral resistance.
Urinary bladder and the other functional unit consisting of bladder neck, urethra, and EUS are controlled and regulated by various central neural circuits, involving midbrain periaqueductal gray (PAG), cell groups in the preoptic and caudal hypothalamus, pontine micturition center (PMC), also known as Barrington nucleus, and medial frontal cortex. PMC is activated during voiding (M-region) and bladder filling (L-region or pontine storage center) and appears to initiate and coordinate lower urinary tract function. This notion is supported by neurophysiological data; moreover, PET scanning of the human brain during micturition documents increased metabolic activity in the pons as well as in cortical and subcortical areas, giving further evidence for pontine involvement in urinary storage and release (Fig. 3.2).
The cortical (prefrontal cortex, insula, anterior cingulate cortex, cerebellum), subcortical (basal ganglia, thalamus, hypothalamus), and pontine circuitry accomplishes three major functions: amplification of bladder contraction to allow complete micturition, control of micturition frequency, and coordination of the activity of lower urinary tract muscles (Fig. 3.3). Overlapping between voluntary control and a reflex mechanism is allowed by sympathetic, parasympathetic, and somatic peripheral innervation of bladder and urethra. Higher centers in the CNS induce a modulatory effect over PMC, primarily mediated by an inhibitory input. The PMC appears to initiate and coordinate lower urinary tract function, pairing detrusor contraction with inhibition of urethral outlet, while sacral micturition center triggers an involuntary reflex detrusor contraction in response to bladder filling. In fact, two distinct voiding reflex pathways exist: a suprasacral reflex physiologically active in normal subjects and a sacral reflex which allows voiding in pathological conditions.
Such an anatomo-functional complexity allows to define urinary continence, as suggested by C.J. Fowler, “a severe test of neurological integrity” [1].
In fact, neurogenic lower urinary tract (LUT) disorders are neuroanatomically divided into suprapontine, spinal (infrapontine-suprasacral), sacral, and peripheral, showing different patterns of voiding dysfunctions. Based on knowledge of voiding centers, these different clinical features can be explained. The suprapontine, supraspinal neurological lesion induces a detrusor hyperreflexia with normal sphincter synergy (DHSS), caused by the loss of inhibition of sacral micturition center. The patient with DHSS has a volume trigger point, at which the bladder contracts, which is considerably lower than the normal bladder capacity, complaining urinary frequency, urgency, and incontinence. Spinal (infrapontine-suprasacral) lesions induce a disruption of connections between the PMC and the sacral center, causing loss of PMC activity and subsequent loss of coordinated relaxation of the EUS during bladder contraction. This loss results in detrusor sphincter dyssynergia (DSD), paired with detrusor hyperreflexia (as with suprapontine, supraspinal lesions) due to uninhibited bladder contractions. DHSS and DSD are dangerous pathological conditions, commonly leading to upper urinary tract damages, reflecting high intravesical pressures needed to obtain urinary flow, progressively impairing kidney function to the point of kidney failure.
Those with suprasacral injuries and intact bladder sensation usually complain of urgency-frequency syndrome; incontinence without awareness may be shown.
Patients with sacral lesions usually complain of suprapubic fullness, inability to void, and incontinence. Traumas are the most common cause responsible for conus and cauda equina lesions. Clinical findings reflect urinary retention with overflow incontinence and elevated post-void residual (PVR).
Peripheral nerve lesions may involve parasympathetic, sympathetic, and/or somatic nerves.
Usually, parasympathetic involvement results in detrusor areflexia; large bladder capacities and chronic bladder overdistension with increased PVR may be seen in case of motor and sensory nerve impairment. Sympathetic lesions alone may cause incontinence due to impaired internal sphincter closure. Patients with peripheral nerve diseases usually complain of suprapubic fullness and inability to void, showing urodynamic findings of detrusor areflexia.
Urinary symptoms and signs may differ from expectations because of incomplete suprapontine, spinal, sacral, and peripheral lesions, coexisting involvement of central and peripheral neurological pathways or other factors, such as drugs, prostate obstruction, or cognitive impairment.
3.2 Neural Control of Intestinal Tract
Bowel activity and secretion in the gastrointestinal (GI) tract are connected and modulated by the cortical activity and controlled by intrinsic and extrinsic GI innervation of smooth muscle layers and glands. Intrinsic innervation relays on the enteric nervous system (ENS), which is the largest nerve cells accumulation outside the brain, having about 100 million neurons and extending throughout the length of GI tract. The neurons of the ENS are organized into two plexuses, myenteric or Auerbach plexus, between the longitudinal and circular smooth muscle layers, and submucosal or Meissner plexus, that influences the absorptive and secretory functions of the enteric mucosa. Extrinsic innervation depends on parasympathetic and sympathetic preganglionics. Sympathetic output originates in the prevertebral ganglia, while parasympathetic innervation is allowed by dorsal motor vagal nucleus (DMV) of the medulla oblongata and sacral parasympathetic nucleus of the spinal cord.
Despite the close anatomical relationship between the rectum and anal canal, there are clear differences in their innervation. Afferent innervation of the rectum derives from the pelvic nerve (Aδ and C fibers), sensitive to rectal distension. Aδ fibers rapidly adapt to changes in rectal distension, while C fibers are slowly adapting and respond to the intensity of rectal distension [2]. Sensations from the rectum can be poorly localized, while the high density of afferent pathways and receptors in the anal canal allows localization of the sensations and sensory definition of the quality of content.
The motor control of anorectum and pelvic floor results from parasympathetic, sympathetic, and somatic nerves. Parasympathetic pathways originate from the parasympathetic nucleus located at S2–S4 segments, having both excitatory and inhibitory components. The excitatory part induces colonic propulsive activity during defecation, while the inhibitory part permits adaption of colonic volume to the content and relaxation of the colon ahead of fecal material.
Rectoanal inhibitory reflex, consisting of anal relaxation, induced by rectal distension, is mediated by a nitric oxide pathway involving intrinsic nerves. Tonically active sympathetic excitatory neurons that innervate internal anal sphincter allow closure of the anal canal at rest. Anal sphincter and pelvic floor somatic innervation originates from Onuf’s nucleus motor neurons at S2–S4 levels. The external anal sphincter (EAS) contributes 30–50 % of resting anal tone, while internal anal sphincter (IAS), regulated by sympathetic nerves, provides most of the resting anal pressure. The puborectalis muscle (PRM) is, moreover, tonically active and permits maintenance of the resting anorectal angle. PRM contraction in fact is fundamental to preserve fecal continence, and its relaxation is necessary for normal bowel emptying. The rectum is functionally different from colon because of its function as a reservoir opposed to a transit function. The rectal compliance is the adaptive capacity of this reservoir to increase its distension to luminal content.
Small volumes of feces propulsed slowly to an almost empty rectum result in an increased rectal compliance, while rapid and large masses distending the rectal wall induce activation of rectoanal inhibitory reflex and the desire to void.
Defecation is mediated by a coordinated relaxation of pelvic floor, IAS and EAS, and an increase in rectal pressure. However, evidence is emerging of an existing association between symptoms of impaired defecation and psychological state.
Gastrointestinal symptoms are also the most important non-motor manifestation of Parkinson disease (PD) and parkinsonism, with constipation as the most prominent manifestation resulting from poor colonic peristalsis and defecatory dysfunction [3]. A wide pattern of cortical areas is involved in anorectal stimulation, including areas that process cognitive and affective aspects of sensation (prefrontal cortex, anterior cingulate cortex and insula) and areas activated during spatial discrimination (primary and secondary somatosensory cortex). Anal canal stimulation results in activation of similar cortical areas than those involved during rectal stimulation, but the former results in activation at a more superior level of primary somatosensory cortex without anterior cingulate cortex activation. It seems that viscera have a greater limbic cortex representation than somatic structures, thus explaining the greater autonomic responses evoked by visceral sensation in comparison with somatic sensation [4].
3.3 Neurophysiological Evaluation of Pelvic Floor
Neurophysiological evaluation of patients affected by urinary, fecal, and sexual disorders usually follows surgical and clinical evaluation and, almost always, other investigations. Although neurophysiological investigations are performed worldwide, their application to pelvic floor disorders is limited to a few centers. In patients with pelvic floor disorders, EAS EMG is the single most useful diagnostic test, particularly for focal sacral lesions. EAS muscle is, in fact, readily accessible and evaluated without discomfort. However, no consensus statement for a standardized approach to LUT and anorectal neurogenic disorders has been reached, and the role of different tests has not been clearly defined yet.
Clinical history and neurological examination should always be performed to propose a diagnosis of neurogenic pelvic dysfunction and to plan further electrophysiological tests [5, 6]. Examination usually includes anal sphincter tone, strength in the S1–S2 innervated muscles (gastrocnemius, gluteal muscles), sensation extending from the soles of the feet to the perianal area, and presence of anal and bulbocavernous reflexes. Anal reflex is induced by pricking or scratching the perianal skin area, whereas bulbocavernosus reflex is evoked by a nonpainful clitoral or gland squeeze [7, 8]. Clinically elicited reflexes may be extinguished by mild or severe nerve lesions, whereas the same reflexes can be recorded neurophysiologically, though with a prolonged latency and reduced amplitude, also in almost complete nerve lesions.
Extensive neurophysiological investigations should be performed in any patient with LUT and anorectal disorders of suspected central or peripheral neurogenic etiology. These tests include concentric needle EMG of different pelvic floor muscles, measurement of sacral reflex latency (pudendo-anal or bulbocavernosus reflex) [9], pudendal and anal somatosensory-evoked potentials (SEPs), and motor-evoked potentials (MEPs) from pelvic floor and EAS muscles by transcranial and lumbosacral magnetic stimulation. Pudendal nerve terminal motor latency (PNTML) has been used in different clinical conditions, but its clinical value has been questioned because the reproducibility, sensitivity, and specificity are uncertain. The recording of a sympathetic skin response (SSR) from the saddle region is useful for testing the lumbosacral autonomic sympathetic system. Unfortunately, a clinically useful test for evaluating the sacral parasympathetic system, which is crucial for LUT and anorectal functioning, has not been found yet.
Tests are usually capable of demonstrating neuropathic lesions and helping to define the specific affected sensory, motor, or autonomic pathway. Severity of lesions can be also assessed, and the underlying mechanisms can be revealed. Even when all other functional tests do not show altered findings, the electrophysiological tests can be positive, therefore leading to a surgical or conservative approach and assessing the prognosis.
3.3.1 Electromyography (EMG)
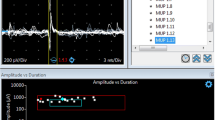
Needle EMG is the most important neurophysiological technique for evaluating patients with suspected neurogenic etiology of pelvic floor dysfunction [10]. EMG assessment of the pelvic floor, EAS, and EUS muscles is mainly indicated to evaluate: (1) the presence of pathological spontaneous activity, fibrillation potentials and positive sharp waves, and denervation of muscle fibers, (2) the presence of muscle fiber reinnervation [11], (3) normal mild continuous tonic contraction in the EAS, PRM [12], and EUS and adequate contraction or relaxation during squeeze or straining, and (4) recruitment pattern and motor-unit potential (MUP) waveform [13]. It is sometimes difficult to discriminate MUPs from fibrillation potentials and positive sharp waves in partial denervation of sphincter muscles during relaxation; in this case, the needle evaluation of bulbocavernosus muscle is useful as no ongoing activity of motor units is recorded [14]. The most important parameters in the analysis of MUP are amplitude, duration, area, number of phases and turns, and firing rate that can be automatically evaluated by advanced EMG systems provided with special software of analysis.
However, in the EAS muscle the best diagnostic parameters seem to be MUP duration, area, and number of turns [15]. Completely or partially denervated pelvic muscles may be reinnervated by axonal regrowth from the proximal nerves; thus a recording of bi and triphasic motor units, soon becoming polyphasic with prolonged duration, can be shown. The EAS muscle needle EMG examination is the test most commonly used to assess the functional state of pelvic floor and sacral myotomes; in fact EAS is easy to access, its needle evaluation is not very painful and very useful information can be acquired. EAS examination holds the central position in Podnar and Vodusek’s algorithm for electrodiagnostic evaluation of the sacral nervous system [6]. With the patient in a comfortable Sims position with knees and hips flexed, after grounded electrically at the thigh, a standard concentric needle EMG electrode is inserted into the subcutaneous portion of the EAS muscle to a depth of 3–5 mm under the mucosa, 1 cm from the anal orifice [6, 16]. Both left and right halves of the subcutaneous EAS muscle must be examined separately, starting on the side with the clinical evidence of sphincter dysfunction (episiotomy scar tissue, patulous anus). If partial or complete atrophy of the subcutaneous EAS muscle is appreciated, a concentric needle electrode can be introduced 1–3 cm deeper through the skin to evaluate spontaneous activity, recruitment pattern, and functional contractile capacity of the deeper EAS and 4–5 cm deeper for examination of the PRM. In the presence of fibrosis, there is a loss of pelvic floor muscle contractile capacity, and consequently, no spontaneous activity or MUP is recognized. When the needle advances in the EAS muscle, continuous firing of low-threshold MUPs is normally appreciated, and during a brief period of relaxation, the presence of spontaneous activity, fibrillation, or jasper potentials can be recorded.
EMG recordings from the EAS are performed at rest and during squeezing, coughing, and straining that simulates rectal evacuation. In healthy subjects, squeeze and cough increase the MUP recruitment pattern, whereas strain decreases or inhibits MUP firing.
Needle examination of the bulbocavernosus muscle is indicated when no EMG signals are recorded in the subcutaneous or deeper EAS muscles [17].
Kinesiological EMG (KEMG) is used to assess and record patterns of individual anterior or posterior muscles during functional maneuvers. An abnormal pattern during bladder filling or emptying, anal closure, squeezing, coughing, and straining can be recorded by surface or needle electrodes. The utility of this technique is to reveal possible dyssynergic contraction pattern of EUS concomitant with detrusor contraction (during urodynamic test) and analogous inappropriate PRM activation on attempt of evacuation. In patients with anal incontinence, during manometric balloon retaining test, KEMG can show absent or insufficient EAS activation.
3.3.2 Sacral Reflexes
Sacral reflexes are motor responses, derived from pelvic striated floor and sphincter muscles, to electrical stimulation of the dorsal penile or clitoral nerve, perianal skin, bladder neck, or proximal urethra. Sacral reflexes evaluate the functional status of the afferent neural fibers of the clitoris or penis, the S2–S4 spinal segments, and the efferent pathways to EAS and bulbocavernosus muscles [7, 9, 18]. The central circuit at the spinal level is complex and probably involves many sacral interneurons.
The motor response in EAS and BC muscle is recorded either with a concentric needle or wire electrodes and can be analyzed separately for each side of both muscles. These sacral reflexes, named pudendo-anal and bulbocavernosus reflex, reveal two components with different thresholds at the electrical stimulation: a first component with a shorter latency of 28–45 ms, probably oligosynaptic, and a second component with a longer latency at about 50–75 ms, typical for a polysynaptic response (Fig. 3.4). The first component is morphologically constant, is stable, and does not habituate, while the second component or long latency response is not always demonstrable and rapidly habituates [19]. The cutaneoanal reflex, described in 1891 by Rossolimo, like the other two reflexes consists of two or three motor contractions (early response at 5 ms, intermediate at 15 ms, and late at about 50 ms) of EAS muscle in response to scratching or pricking the perianal skin. This reflex, which is abolished by transection of the posterior S4 roots, shows marked habituation, is quite variable (35–80 ms), and therefore cannot be used as a diagnostic tool [20].
Vesicourethral and vesico-anal reflexes are described following stimulation of the bladder neck and mucosa, but their usefulness as a diagnostic tool is considered to be limited.
Recently, a technique for transcutaneous electrical stimulation of the S3 motor root, recording from EAS muscle, has also been described [21].
Method: A common scheme of sacral-evoked responses consists in the anterior electrical stimulation (penile/clitoral, bladder neck site) and recording by needle electrode from different pelvic muscles (BC, EAS, levator ani). Sacral reflexes are useful in different pelvic floor disorders and have been recommended for the assessment of cauda equina, conus, and medullaris lesions. In the presence of unilateral/asymmetrical lesions of pudendal nerves, sacral roots, or lumbosacral plexus, these reflexes may show a reduction of responses amplitude and/or increased latencies.
Only the largest myelinated, fastest fibers convey the neurophysiological signals traveling in the afferent limb of these reflexes. Many disorders of bladder, bowel, and sexual function are unfortunately the result of unmyelinated fiber dysfunction; therefore, conduction in these fibers is not tested by these procedures, and autonomic and small-fiber neuropathies may not be revealed by these tests.
3.3.3 Somatosensory-Evoked Potential (SEP)
Pudendal SEP is a method for evaluating the afferent sensory pathway to the parietal cortex, and it is used in investigating central and peripheral neurological diseases that affect pelvic floor functional integrity. SEPs findings may help in showing lesions in somatosensory pathways, localizing them and defining a prognostic value.
In a similar way to the other neurophysiological tests, pudendal SEPs may be normal in latency and amplitude also in case of an underlying organic disease. The peripheral electrical stimulation used to obtain an SEP activates predominantly, if not entirely, the large diameter fast-conducting group Ia muscle and group II cutaneous afferent fibers. Loss of posterior, dorsal column or lemniscal sensory pathways is invariably associated with abnormal SEPs, indicating that within the spinal cord the SEPs are mediated predominantly via these tracts. Generally SEPs are best recorded over the somatosensory cortex, and several of their components are widely distributed over the scalp [22]. The pudendal SEPs technique, first described by Haldeman in 1983 [23], depends on the recording by a disk electrode affixed to the scalp of a typical “W-shaped” waveform, as a response that appears with a given latency depending on site stimulation. Although several studies have shown that SEPs can effectively be recorded after dorsal penile and clitoral stimulation [23–27], only few investigations have been published concerning anal somatosensory-evoked responses [28, 29]. It is necessary to remind that pudendal SEPs after anal and dorsal penile/clitoral nerve stimulation cannot be considered to produce equivalent results due to separate branches of the pudendal nerve innervating the pelvic region. Therefore, obtaining separate reference values in both sexes for anal and penile/clitoral latencies when evaluating pelvic floor neurophysiology is considered to be relevant [30]. The analogous morphology of pudendal and tibial SEPs might suggest a common neurophysiological mechanism to produce both responses.
Method: The responses are bipolarly recorded using surface electrodes from the scalp, 2 cm behind Cz, referred to Fz or Fpz (10–20 EEG International System), roughly overlying the sensorimotor cortex for the genital and anal area. Electrical stimulation is performed by means of a bipolar surface electrode positioned at the anal orifice, at the base of the penis or cranial to the clitoris. The typical recording consists of a series of waves that reflects sequential activation of neural structures along the somatosensory pathways. A first positive peak can be recorded in normal subjects at about 42 ms using a stimulus intensity of two to four times the sensory threshold. Later negative and positive peaks show a large variability in amplitude between individuals (Fig. 3.5). SEPs amplitudes have, however, not been found to differentiate between normal and pathologic responses. SEPs can be used in perineology to confirm and localize sensory abnormalities affecting anal or genitourinary neural pathways [6, 10]. Some authors [25] have already discussed the limitations of pudendal SEPs, showing that sometimes in pathological conditions penile/clitoral SEPs are normal. Pudendal SEPs are considered to be useful in diagnosing impotence associated with spinal cord injury [31] and diabetic neuropathy [32], while in case of primary erectile dysfunction, their utility is debated [33].
3.3.4 Motor-Evoked Potential (MEP)
Conventional electrophysiological methods that activate the descending cortico-motoneuronal pathways use the electrical and magnetic stimulation technique. However, transcranial magnetic stimulation (TMS) has the advantages of being painless and capable of stimulating also the more deeply situated nervous structures; electrical stimulation is therefore mainly reserved for intraoperative monitoring. TMS has been commonly used to assess the central and peripheral conduction time to skeletal muscles of the upper and lower limbs and to evaluate the integrity and function of the corticospinal pathways [34, 35]. TMS is also applied to study the corticospinal pathway to the pelvic floor muscles, including EAS, which is the most common target muscle from which MEPs are recorded [36, 37], and EUS and PRM, whose recordings are poorly reproducible [38]. The intensity of TMS necessary to obtain an EAS MEP is much higher than the intensity to elicit a MEP in the limbs. This fact can be explained by the cortical representation of the anogenital area that is localized deep within the motor strip in the interhemispheric fissure.
This method investigates the motor efferent pathway from the brain and lumbosacral roots to the EAS, allowing to determine the total conduction time and the lumbosacral latency. Cortical magnetic stimulation is usually performed in two conditions: at rest, with EAS relaxed (MEPs mean latency of about 27 ms), and during facilitation (MEPs mean latency of about 23 ms) due to a voluntary mild contraction of pelvic floor and EAS muscles. The magnetic stimulation applied over the lower lumbar spine is known to activate the lumbosacral ventral roots at their exit from the vertebral canal. MEPs from lumbosacral magnetic stimulation are obtained only during rest condition at about 6 ms [37], since facilitation does not modify latencies during peripheral nerve stimulation (Fig. 3.6).
Motor-evoked potentials from EAS after transcranial magnetic stimulation of the motor cortex and after lumbar magnetic stimulation. Upper two traces represent cortical motor superimposed responses at rest and during facilitation, bottom traces show MEPs from EAS muscle after magnetic stimulation applied over the lower lumbar spine
Method: Magnetic shocks are delivered by a magnetic simulator; different shapes of coils exist, each of which produces different magnetic field patterns. The coil produces, normally, a peak magnetic field strength of 1.5 T, being placed flat on the scalp, centered on Cz (10–20 I.E.) to stimulate the motor cortex and on the lumbosacral region (L3–L4 interspace) to stimulate the lumbosacral roots. EMG recordings are taken from EAS using a needle electrode placed approximately 1 cm lateral to the anal orifice. The ground electrode is located around the upper portion of the leg. The different types of MEP abnormalities, i.e., responses with decreased amplitude or delayed latency, may imply axonal or demyelinative impairment underlying the different clinical pathological conditions. Corticospinal abnormalities detected by this method in patients with neurogenic bladder and bowel disorders have been reported [39–41].
3.3.5 Sympathetic Skin Response (SSR)
SSR is a technique that records changes in skin conductance after activation of sweat glands in skin areas rich in eccrine glands (commonly palmar, plantar, saddle sites) under the neural control of sympathetic cholinergic (sudomotor) fibers. SSR is the only neurophysiological technique directly testing sympathetic fibers. Potentials generated by SSR can be recorded in response to various stimuli; these include electrical peripheral nerve stimulation, acoustic stimuli, and magnetic stimulation of the nerves or brain, although magnetic stimulation lacks specificity in terms of sensory pathways involved [42, 43]. SSR is dependent on integrity of peripheral sympathetic cholinergic pathways, as it is preserved in selective sympathetic adrenergic failure, and it is absent in pure autonomic failure (PAF) (with sympathetic adrenergic and cholinergic failure) and in pure cholinergic dysautonomia. Different areas in cerebral cortex and in the brainstem have been proposed as generator sites for the sensory signals of the SSR [44].
Method: SSRs are recorded from palmar, plantar, and saddle surfaces, both left and right, using surface electrodes. Electrodes are placed on the volar site and on the corresponding area of the dorsal aspect of the hand or foot. For perineum recordings, the active electrode is attached to the perineum (below the scrotum) and the reference electrode to the iliac crest with the ground on the leg. This kind of recording from the perineal region increases the diagnostic sensitivity when evaluating sympathetic function within the thoracolumbar spinal cord [45]. Only few studies exist regarding the relationship between bladder dysfunction and SSR anomalies. In particular a lack of SSR in bladder neck dyssynergia and in foot following spinal cord injury has been shown [46].
3.3.6 Pudendal Nerve Terminal Motor Latency (PNTML)
Pudendal nerve inferior rectal branches can be evaluated measuring PNTML, which is the technique most commonly used for assessment in patients with idiopathic neurogenic fecal incontinence [16]. PNTML technique, first described in 1984 by Kiff and Swash, is determined by recording the anal sphincter motor potential evoked by stimulation of the pudendal nerve into the rectum with a special bipolar surface electrode known as St. Mark’s electrode. The stimulating electrode is fixed on the tip of a gloved index finger, while the two recording electrodes, which pick up the contraction response of EAS, are placed at the base of the finger. On insertion of the finger into the rectum, an electrical stimulation is given near the ischial spine.
Pudendal nerve is therefore stimulated as it leaves the pelvis, before branching into perineal nerve and inferior rectal nerve, which innervate periurethral striated muscle and anal sphincter respectively.
Mean latencies of the responses from the anal sphincter are 2.1 ± 0.2 ms; however, PNTML using St. Mark’s electrode permits to stimulate the terminal pudendal nerve branches only near their motor points, preventing complete evaluation of pudendal nerve (Fig. 3.7). Moreover the recorded response is frequently of low amplitude and impaired by stimulus artifact. The test owes its popularity to different studies showing abnormal latencies in various clinical situations [47–49]. In fact, pudendal neuropathy is seen in up to 70 % of patients with fecal incontinence and in more than 50 % of patients with sphincter injury [50].
However, PNTML clinical value has been questioned, and two consensus statements, uroneurological and gastroenterological, did not propose this test for evaluating patients with bladder and bowel dysfunction [17, 51]. American Gastroenterological Association medical position statement in 1999 concluded that PNTML cannot be recommended for evaluating patients with fecal incontinence because: (a) PNTML has a poor correlation with clinical symptoms and histologic findings, (b) the technique does not discriminate between muscle weakness caused by pudendal nerve injury and muscle injury in patients with fecal incontinence, (c) there is a lack of sensitivity and specificity for detecting EAS weakness, (d) it is considered to be an operator-dependent technique, and (e) the test does not predict surgical outcome [51, 52].
References
Fowler CJ (1999) Neurological disorders of micturition and their treatment. Brain 122:1213–1231
Mayer EA, Gebhart GF (1994) Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 107:271–293
Abbott RD, Petrovitch H, White LR et al (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57:456–462
Hobday DI, Aziz Q, Thacker N et al (2001) A study of the cortical processing of ano-rectal sensation using functional MRI. Brain 124:361–368
Podnar S (2003) Electrodiagnosis of the anorectum: a review of techniques and clinical applications. Tech Coloproctol 7:71–76
Podnar S, Vodusek DB (2001) Protocol for clinical neurophysiologic examination of the pelvic floor. Neurourol Urodyn 20:669–682
Pedersen E, Klemar B, Schrøder HD et al (1982) Anal sphincter responses after perianal electrical stimulation. J Neurol Neurosurg Psychiatry 45:770–773
Swash M (1982) Early and late components in the human anal reflex. J Neurol Neurosurg Psychiatry 45:767–769
Vodusek DB, Janko M, Lokar J (1983) Direct and reflex responses in perineal muscles on electrical stimulation. J Neurol Neurosurg Psychiatry 46:67–71
Fowler CJ (2001) A neurologist’s clinical and investigative approach to patients with bladder, bowel and sexual dysfunction. In: Fowler CJ, Brady CM, Frohman EM, Sakakibara R, Stewart JD (eds) Neurologic bladder, bowel, and sexual dysfunction. Elsevier, Amsterdam, pp 1–6
Podnar S (2004) Criteria for neuropathic abnormality in quantitative anal sphincter electromyography. Muscle Nerve 30:596–601
Floyd WF, Walls EW (1953) Electromyography of the sphincter ani externus in man. J Physiol 122:599–609
Swash M (1992) Electromyography in pelvic floor disorders. In: Henry MM, Swash M (eds) Coloproctology and the pelvic floor. Butterworth Heinemann, Oxford, pp 184–195
Podnar S (2007) Neurophysiology of the neurogenic lower urinary tract disorders. Clin Neurophysiol 118:1423–1437
Podnar S, Mrkaić M (2002) Predictive power of motor unit potential parameters in anal sphincter electromyography. Muscle Nerve 26:389–394
Cheong DM, Vaccaro CA, Salanga VD et al (1995) Electrodiagnostic evaluation of fecal incontinence. Muscle Nerve 18:612–619
Fowler CJ, Benson JT, Craggs MD et al (2002) Clinical neurophysiology. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence. 2nd international consultation on incontinence. Plymbridge Distributors, Plymouth, pp 389–424
Ertekin C, Reel F (1976) Bulbocavernosus reflex in normal men and in patients with neurogenic bladder and/or impotence. J Neurol Sci 28:1–15
Vodusek DB, Janko M (1990) The bulbocavernosus reflex. A single motor neuron study. Brain 113:813–820
Vodusek DB, Amarenco G, Podnar S (2009) Clinical neurophysiological tests. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence. Health Publications, Plymouth, pp 523–540
Pelliccioni G, Scarpino O (2006) External anal sphincter responses after S3 spinal root surface electrical stimulation. Neurourol Urodyn 25:788–791
Aminoff MJ, Eisen AA (1998) AAEM minimonograph 19: somatosensory evoked potentials. Muscle Nerve 21:277–290
Haldeman S, Bradley WE, Bhatia NN et al (1983) Cortical evoked potentials on stimulation of pudendal nerve in women. Urology 21:590–593
Guérit JM, Opsomer RJ (1991) Bit-mapped imaging of somatosensory evoked potentials after stimulation of the posterior tibial nerves and dorsal nerve of the penis/clitoris. Electroencephalogr Clin Neurophysiol 80:228–237
Delodovici ML, Fowler CJ (1995) Clinical value of the pudendal somatosensory evoked potential. Electroencephalogr Clin Neurophysiol 96:509–515
Loening-Baucke V, Read NW, Yamada T et al (1994) Evaluation of the motor and sensory components of the pudendal nerve. Electroencephalogr Clin Neurophysiol 93:35–41
Yang CC, Kromm BG (2004) New techniques in female pudendal somatosensory evoked potential testing. Somatosens Mot Res 21:9–14
Haldeman S, Bradley WE, Bhatia N (1982) Evoked responses from the pudendal nerve. J Urol 128:974–980
Remes-Troche JM, Tantiphlachiva K, Attaluri A et al (2011) A bi-directional assessment of the human brain-anorectal axis. Neurogastroenterol Motil 23:240–248
Pelliccioni G, Piloni V, Sabbatini D et al (2014) Sex differences in pudendal somatosensory evoked potentials. Tech Coloproctol 18:565–569
Ashraf VV, Taly AB, Nair KP et al (2005) Role of clinical neurophysiological tests in evaluation of erectile dysfunction in people with spinal cord disorders. Neurol India 53:32–35
Sartucci F, Piaggesi A, Logi F et al (1999) Impaired ascendant central pathways conduction in impotent diabetic subjects. Acta Neurol Scand 99:381–386
Kaiser T, Jost WH, Osterhage J et al (2001) Penile and perianal pudendal nerve somatosensory evoked potentials in the diagnosis of erectile dysfunction. Int J Impot Res 13:89–92
Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 1:1106–1107
Rothwell JC, Hallett M, Berardelli A et al (1999) Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52:97–103
Opsomer RJ, Caramia MD, Zarola F et al (1989) Neurophysiological evaluation of central-peripheral sensory and motor pudendal fibres. Electroencephalogr Clin Neurophysiol 74:260–270
Pelliccioni G, Scarpino O, Piloni V (1997) Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral magnetic stimulation: normative data. J Neurol Sci 149:69–72
Brostrom S, Jennum P, Lose G (2003) Motor evoked potentials from the striated urethral sphincter and puborectal muscle: normative values. Neurourol Urodyn 22:306–313
Gunnarsson M, Ahlmann S, Lindstrom S et al (1999) Cortical magnetic stimulation in patients with genuine stress incontinence: correlation with results of pelvic floor exercises. Neurourol Urodyn 18:437–444
Brostrom S, Frederiksen JL, Jennum P et al (2003) Motor evoked potentials from the pelvic floor in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 74:498–500
Jennum P, Jensen L, Fenger K et al (2001) Motor evoked potentials from the external anal sphincter in patients with autosomal dominant pure spastic paraplegia linked to chromosome 2p. J Neurol Neurosurg Psychiatry 71:561–562
Shahani BT, Halperin JJ, Boulu P et al (1984) Sympathetic skin response: a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry 47:536–542
Uncini A, Pullman SL, Lovelace RE et al (1988) The sympathetic skin response: normal values, elucidation of afferent components and application limits. J Neurol Sci 87:299–306
Vetrugno R, Liguori R, Cortelli P et al (2003) Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res 13:256–270
Rodic B, Curt A, Dietz V et al (2000) Bladder neck incompetence in patients with spinal cord injury: significance of sympathetic skin response. J Urol 163:1223–1227
Schurch B, Curt A, Rossier AB (1997) The value of sympathetic skin response recordings in the assessment of the vesicourethral autonomic nervous dysfunction in spinal cord injured patients. J Urol 157:2230–2233
Snooks SJ, Badenoch DF, Tiptaft RC et al (1985) Perineal nerve damage in genuine stress urinary incontinence. An electrophysiological study. Br J Urol 57:422–426
Gilliland R, Altomare DF, Moreira H Jr et al (1998) Pudendal neuropathy is predictive of failure following anterior overlapping sphincteroplasty. Dis Colon Rectum 41:1516–1522
Bakas P, Liapis A, Karandreas A et al (2001) Pudendal nerve terminal motor latency in women with genuine stress incontinence and prolapse. Gynecol Obstet Invest 51:187–190
Roig JV, Villoslada C, Lledo S et al (1995) Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum 38:952–958
Barnett JL, Hasler WL, Camilleri M (1999) American Gastroenterological Association medical position statement on anorectal testing techniques. American Gastroenterological Association. Gastroenterology 116:732–760
Madoff RD, Parker SC, Varma MG et al (2004) Faecal incontinence in adults. Lancet 364:621–632
Acknowledgments
The authors would like to thank Mrs. Rosa Luana and Mr. Marzio Marcellini for their skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pelliccioni, G., Pelliccioni, P. (2015). Neurophysiology and Neurophysiological Evaluation of the Pelvic Floor. In: Martellucci, J. (eds) Electrical Stimulation for Pelvic Floor Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-06947-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-06947-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06946-3
Online ISBN: 978-3-319-06947-0
eBook Packages: MedicineMedicine (R0)