Abstract
Background
Somatosensory evoked potentials (SEPs) of the pudendal nerve are a well-established diagnostic tool for the evaluation of pelvic floor disorders. However, the possible influence of sex differences on response latencies has not been established yet. The aim of this study was to standardize the procedures and to evaluate possible effects of gender differences on anal and penile/clitoral SEPs.
Methods
The anal and dorsal penile/clitoral SEPs were recorded in 84 healthy subjects (40 males and 44 females; mean age 47.9 ± 16.6 years, range 16–81 years; mean height 168.3 ± 20.3 cm, range 155–187 cm). Pudendal SEPs were evoked with a bipolar surface electrode stimulating the clitoris or the base of the penis and the anal orifice and recorded using scalp electrodes. The latency of the first positive component (P1) was measured. The effect and possible interaction of (a) stimulation site and (b) gender on the two variables was explored by multivariate analysis of variance (MANOVA).
Results
The examination was well tolerated and a reproducible waveform of sufficient quality was obtained in all the subjects examined. In the female subjects, a mean cortical P1 latency of 37.0 ± 2.6 and 36.4 ± 3.2 ms for anal and clitoral stimulation, respectively, was found. In the male subjects, the cortical latencies were 38.0 ± 3.5 ms for the anal stimulation and 40.2 ± 3.7 ms for the penile stimulation. At MANOVA, a statistically significant main effect of stimulation site and gender as well as a significant interaction between the two variables was found.
Conclusions
Anal and dorsal penile/clitoral SEPs represent a well-tolerated and reproducible method to assess the functional integrity of the sensory pathways in male and female subjects. Obtaining sex-specific reference data, by individual electrophysiological testing, is highly recommended because of significant latency differences between males and females, at least as far as penile/clitoral responses are concerned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatosensory evoked potentials (SEPs) recording is a powerful investigative technique, which is much used in neurological practice to investigate conduction in neural pathways extending from the peripheral site of stimulation to the parietal sensory cortex. Moreover, SEPs are part of neurophysiological assessment of the anorectal and perineal region [1–3] that is useful in patients with suspected neurogenic lesions. The pudendal SEPs technique, firstly described by Haldeman in 1983 [4], depends on the recording by a disk electrode affixed to the scalp of a typical “W-shaped” waveform, as a response which appears with a given latency depending on site stimulation. Although several studies have shown that SEPs can effectively be recorded after dorsal penile and clitoral stimulation [2, 4–11], only few investigations have been published concerning anal somatosensory evoked responses in normal subjects [12–14]. In particular, to our knowledge, no previous studies were conducted to determine sex differences, if any, in the latencies of the pudendal SEPs. Also, standardization of the technique, methodology, and possible influence of age and anatomical variables have not been described yet.
The aims of this study were to standardize the procedures and to evaluate effect of sex, age, and height on pudendal SEPs.
Materials and methods
Subjects
Eighty-four normal healthy subjects (44 females, mean age 47.61 ± 15.13 years, range 16–76 years; and 40 males, mean age 48.23 ± 18.35 years, range 12–81 years), with no known systemic disease and with no history of prior pelvic surgery or evidence of neurological, gastroenterological, and urological disorders at physical examination, were enrolled in the study after they had provided written informed consent. The study was approved by the local Ethics Committee.
Protocol
For the examination, the subjects were reclined comfortably in the right lateral position with their hips and knees flexed and the left thigh grounded electrically, the mouth maintained slightly opened. The electrophysiological studies were performed using conventional electromyographic-evoked potential (EMG-EP) equipment (Keypoint; Medtronic Functional Diagnostics, Skovlunde, Denmark) and a series of three anal and penile or clitoral SEPs were recorded for each subject, in order to show the reproducibility of the findings. Anal stimulation was performed by means of a bipolar surface electrode (Medtronic Neurodiagnostics, hand-held stimulating electrode with intensity control, 9031E0152) positioned at the anal orifice. Electrical stimulation of the dorsal nerve of the penis or clitoris was obtained by means of the same bipolar surface electrode, positioned at the base of the penis or cranial to the clitoris, the cathode being placed proximally. The intensity of the electrical stimulation was set at an average of 3–4 times the threshold level of intensity at which the subject was first able to perceive the stimulus, usually ranging from 11.0 to 45.0 mA (mean 32.5 mA for penile/clitoral and mean 28.6 mA for anal stimulation). In order to assess the tolerability of the examination at the corresponding technical setting of the chosen stimulus parameters, i.e., intensity (mA), duration (ms), and frequency (Hz), each subject was asked to describe whether or not a painful sensation was experienced during the application of the electrical stimulation.
The response was bipolarly recorded using surface electrodes from the scalp, 2 cm behind Cz, referred to Fpz (10–20 International System) [15], roughly overlying the sensorimotor cortex for the genital and anal area. Before electrode placement, the skin was gently scraped and prepared to keep the impedance at less than 5.0 kOhms. A filter setting from 5 to 3,000 Hz was used.
Response analysis
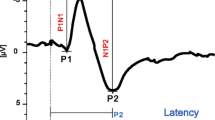
After computer-assisted averaging of 250 responses, single SEPs in the screen were analyzed by two independent experienced investigators for the identification of a sequence of four consecutive peaks that are called P1, N1, P2, and N2, respectively, using conventional nomenclature (Fig. 1). The positive peaks were labeled as P1 and P2, and negative peaks were labeled as N1 and N2. Due to its recognized smallest standard deviation [12], the latency of P1, i.e., the first positive deflection of the W-shaped averaged cortical waveform, was assumed as the most representative parameter, and its value was used for statistical analysis.
Statistical analysis
Differences in average P1 latencies between the two groups linked to gender and the influence of anthropometric factors were assessed with multivariate analysis of variance (MANOVA), using the SPSS software for Mac OS X (version 16.0, SPSS, Inc., Chicago, IL, USA). The significance level adopted was p < 0.05. The diagnostic quality of recordings was ranked by the two examiners as being (a) insufficient for proper reading, (b) sufficient (containing only limited number of artifacts), and (c) optimal (no artifact at all). The reproducibility of the examinations was also evaluated calculating the frequency with which the three series of recordings were judged to be not significantly different one another. Finally, at the end of the examination, patients were asked to express on a 0–3 point scale the discomfort, if any, experienced during stimulation of anal, penile, or clitoral sites as follows: 0 = no discomfort, 1 = minimal, 2 = mild, and 3 = severe.
Results
Measurements of anal, penile, and clitoral SEPs of sufficient quality were obtained in all the investigated subjects. Figure 1 shows an example of two superimposed anal and penile/clitoral SEPs responses in a male and a female subject. After stimulation of these two different sites, the morphological characteristics of the pudendal nerve response looked quite similar.
Average values of the P1 latencies recorded from penile/clitoral and anal SEPs are reported in Table 1.
At MANOVA, a significant interaction between the stimulation site and the sex of participants, F = 10.2, p < 0.01, was demonstrated, showing that P1 latencies of pudendal SEPs following penile/clitoral and anal stimulation site were different for males and females. In particular, males exhibited P1 latencies after penile stimulation significantly longer than females, F = 19.9, p < 0.001, demonstrating that gender is also an important factor. No interaction resulted between P1 latencies and the factors age and height. No interaction resulted between the P1 latency of the somatosensory evoked potentials obtained after anal stimulation and factors such as age, height, and sex. The results are explained in Table 2.
At penile/clitoral stimulation, 22 patients referred no discomfort, 24 patients minimal, 30 patients mild, and 8 patients severe discomfort (mean value 1.2). At anal stimulation, 25 patients referred no discomfort, 29 patients minimal, 24 patients mild, and 6 patients severe discomfort (mean value 1.1).
Discussion
SEPs are routinely used in neurology to assess the transmission of the afferent volley of the somatosensory pathways from the periphery up to the cortex. The typical recording consists of a series of waves that reflect sequential activation of neural structures along the somatosensory pathways. Together with the pudendoanal reflex, the bulbocavernosus (BC) reflex, the pudendal nerve terminal motor latency (PNTML), and the EMG of the external anal sphincter (EAS), SEPs can be used in perineology to confirm and localize sensory abnormalities affecting anal or genitourinary neural pathways [3, 16, 17]. Some authors [1, 7] have already discussed the limitations of the pudendal SEPs, showing that sometimes in pathological conditions penile/clitoral SEPs are normal. However, the same authors have stressed the importance of differentiating within pudendal SEPs the penile from the clitoral stimulation, in order to increase the sensitivity of the test. Our experience has shown that the activity of somatosensory afferents from the anal and penile/clitoral region can routinely be assessed and consistently recorded in healthy subjects with no or only minor discomfort. Moreover, when comparing pudendal SEPs in male and female subjects, a unique feature of the present study, not previously mentioned in the literature, was the significant difference which has been found between penile and clitoral SEPs. More particularly, in healthy males, in agreement with similar results reported by Kaiser in a single previous study [18], longer latencies of the P1 component after penile stimulation were observed with respect to the same parameter after anal stimulation (40.2 vs. 38.0), probably due to the longer anatomical distance covered by the depolarizing wave through the nerve pathways between the site of stimulation and that of recording. Conversely, in the female subjects, contrary to what could have been expected, not more than a slightly greater mean latency of P1 from anal SEPs was observed with respect to that from clitoral SEPs (37.0 vs. 36.4). However, Loening-Baucke et al. [2], in a study on anal and dorso-genital nerve SEPs in a group of healthy subjects of undefined sex, found shorter latencies from penile/clitoral SEPs when compared to anal SEPs. The authors accepted that this difference was difficult to explain as “the volley travels approximately an equal distance from both stimulation sites and brain.” While no reasonable explanation can be definitely put forth at present, one possible hypothesis is that a shorter distance between the stimulation site and the recording one should exist. Alternatively, a different resistance to the depolarizing wave along the course of the two neural pathways might be suggested, considering also the differences in sensory fiber diameter [19]. To add a note of complexity to the issue, it should be noted that pudendal SEPs after anal and penile/clitoral nerve stimulation cannot be considered to produce equivalent results because separate branches of the pudendal nerve innervate the pelvic region [20]. Previous studies have already confirmed this assumption, showing a functional dissociation between the two branches in patients with lower urinary tract disease [21–23]. Similarly, on assessing a patient with possible neurogenic bowel dysfunction, penile/clitoral and anal SEPs can reveal an analogous functional dissociation between latencies. Overall, these somewhat contradictory results highlight the importance of obtaining separate reference values in both sexes for anal and penile/clitoral latencies when evaluating pelvic floor neurophysiology. Unfortunately, with the exception of the paper of Blaivas [24] on the bulbocavernosus reflex and that of Podnar [25] on the penilocavernosus reflex, the issue of normative data related to sex differences has received little attention in the literature until now. To our knowledge, the present paper is the first to compare penile/clitoral and anal pudendal SEPs in healthy male and female subjects. A limitation of our study is the sample size that is not sufficient to establish normative data and further studies are needed to define normal values of pudendal SEPs in males and females. The examination should be considered part of a global neurophysiological assessment [26] that includes also bulbocavernosus reflex activity [24], EMG examination of the EAS [27, 28], and motor evoked potentials from the EAS by cortical and lumbosacral magnetic stimulation [29–31]. All these different electrodiagnostic techniques should be considered in patients with suspected neurogenic etiology of their bladder, bowel, and sexual dysfunction [3, 16, 17]. The standardization of the method we propose may provide the basis for future electrodiagnostic studies in pathological states, including double (urinary and fecal) incontinence, pelvic prolapse, obstructed defecation, and chronic pelvic pain syndromes due to pudendal nerve neuropathy.
Conclusions
Penile/clitoral SEPs and anal SEPs can easily and consistently be obtained and recorded by a skilled clinical neurophysiologist interested in the use of such techniques in the pelvic floor region. Obtaining sex-specific reference data, differentiated for stimulation site, is mandatory for each electrophysiological laboratory, as described in the present study, to allow proper application in clinical practice.
References
Vodusek DB, Amarenco G, Podnar S (2009) Clinical neurophysiological tests. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence, 4th edn. Health Publications Ltd, Plymouth, pp 523–540
Loening-Baucke V, Read NW, Yamada T, Barker AT (1994) Evaluation of the motor and sensory components of the pudendal nerve. Electroencephalogr Clin Neurophysiol 93:35–41
Podnar S (2003) Electrodiagnosis of the anorectum: a review of techniques and clinical applications. Tech Coloproctol 7:71–76
Haldeman S, Bradley WE, Bhatia NN, Johnson BK (1983) Cortical evoked potentials on stimulation of pudendal nerve in women. Urology 21:590–593
Opsomer RJ, Guerit JM, Wese FX, Van Cangh PJ (1986) Pudendal cortical somatosensory evoked potentials. J Urol 135:1216–1218
Guérit JM, Opsomer RJ (1991) Bit-mapped imaging of somatosensory evoked potentials after stimulation of the posterior tibial nerves and dorsal nerve of the penis/clitoris. Electroencephalogr Clin Neurophysiol 80:228–237
Delodovici ML, Fowler CJ (1995) Clinical value of the pudendal somatosensory evoked potential. Electroencephalogr Clin Neurophysiol 96:509–515
Vodusek DB (1990) Pudendal SEP and bulbocavernosus reflex in women. Electroencephalogr Clin Neurophysiol 77:134–136
Podnar S, Vodusek DB, Trsinar B, Rodi Z (1997) A method of uroneurophysiological investigation in children. Electroencephalogr Clin Neurophysiol 104:389–392
Yang CC, Kromm BG (2004) New techniques in female pudendal somatosensory evoked potential testing. Somatosens Mot Res 21:9–14
Cavalcanti GA, Bruschini H, Manzano GM et al (2007) Pudendal somatosensory evoked potentials in normal women. Int Braz J Urol 33:815–821
Haldeman S, Bradley WE, Bhatia NN, Johnson BK (1982) Pudendal evoked responses. Arch Neurol 39:280–283
Haldeman S, Bradley WE, Bhatia N (1982) Evoked responses from the pudendal nerve. J Urol 128:974–980
Remes-Troche JM, Tantiphlachiva K, Attaluri A et al (2011) A bi-directional assessment of the human brain-anorectal axis. Neurogastroenterol Motil 23:240–248
Jasper HH (1958) The 10–20 electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 10:371–375
Fowler CJ (2001) Neurologist’s clinical and investigative approach to patients with bladder, bowel and sexual dysfunction. In: Fowler CJ, Sakakibara R, Frohman EM, Steward JD (eds) Neurologic bladder, bowel and sexual dysfunction. Elseviers, Amsterdam, pp 1–6
Podnar S, Vodusek DB (2001) Protocol for clinical neurophysiologic examination of the pelvic floor. Neurourol Urodyn 20:669–682
Kaiser T, Jost WH, Osterhage J, Derouet H, Schimrigk K (2001) Penile and perianal pudendal nerve somatosensory evoked potentials in the diagnosis of erectile dysfunction. Int J Impot Res 13:89–92
Campbell WW, Ward LC, Swift TR (1981) Nerve conduction velocity varies inversely with height. Muscle Nerve 4:520–523
Bharucha AE (2006) Pelvic floor: anatomy and function. Neurogastroenterol Motil 18:507–519
Blaivas JG, Labib KL, Bauer SB, Retik AB (1977) A new approach to electromyography of the external urethral sphincter. J Urol 117:773–777
Blaivas JG, Scott RM, Labib KB (1979) Urodynamic evaluation as neurologic test of sacral cord function. Urology 13:682–687
Snooks SJ, Swash M (1984) Abnormalities of the innervation of the urethral striated sphincter musculature in incontinence. Br J Urol 56:401–405
Blaivas JG, Zayed AA, Labib KB (1981) The bulbocavernosus reflex in urology: a prospective study of 299 patients. J Urol 126:197–199
Podnar S (2007) Neurophysiology of the neurogenic lower urinary tract disorders. Clin Neurophysiol 118:1423–1437
Lefaucheur JP (2006) Neurophysiological testing in anorectal disorders. Muscle Nerve 33:324–333
Podnar S, Mrkaić M (2002) Predictive power of motor unit potential parameters in anal sphincter electromyography. Muscle Nerve 26:389–394
Podnar S (2003) Electromyography of the anal sphincter: which muscle to examine? Muscle Nerve 28:377–379
Opsomer RJ, Caramia MD, Zarola F, Pesce F, Rossini PM (1989) Neurophysiological evaluation of central-peripheral sensory and motor pudendal fibres. Electroencephalogr Clin Neurophysiol 74:260–270
Jost WH, Schimrigk K (1994) Magnetic stimulation of the pudendal nerve. Dis Colon Rectum 37:697–699
Pelliccioni G, Scarpino O, Piloni V (1997) Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral magnetic stimulation: normative data. J Neurol Sci 149:69–72
Acknowledgments
The authors are especially indebted to Miss Luana Rosa, technician in neurophysiology, for her technical assistance and skillfulness.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pelliccioni, G., Piloni, V., Sabbatini, D. et al. Sex differences in pudendal somatosensory evoked potentials. Tech Coloproctol 18, 565–569 (2014). https://doi.org/10.1007/s10151-013-1105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-013-1105-9