Abstract
The thermal stability and ageing properties of Vinamul 3161 poly(ethylene-co-vinyl acetate) and AS1160 polyester polyol resins have been investigated in support of shelf life assessment and also to identify storage conditions that may extend product life. These resins are typically used in the production of adhesives for specialised applications either as binders for filler particles or to minimise the relative movement of materials in multi-material assemblies. Our studies confirm that both these resins are susceptible to moisture and hydrolysis chemistry which potentially limits shelf life. The EVA resin readily accumulates acetic acid through hydrolysis of the pendent acetate groups which increases both the acidity (pH) and volatile outgassing characteristics of the material. The temperature sensitivity of pH combined with Arrhenius kinetics was used to identify a useful shelf life for EVA in conditions representative of normal storage conditions. In a separate set of experiments, relatively short-term thermally accelerated ageing studies have been carried out on AS1160 polyester polyol to investigate sensitivity to humidity, temperature and open/close ageing conditions. This material is hygroscopic, readily accumulates moisture and is susceptible to chain scission with molecular weight changes linked to the hydrolysis-esterification equilibrium. These changes do not however adversely impact adhesive bond strength allowing the resin to be potentially used significantly beyond the manufacturer recommended shelf life limit.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Characterisation
- Thermal stability
- Poly(ethylene-co-vinyl acetate)
- Polyol
- Accelerated ageing
- Bond strength
- Stability
- Adhesives
- Hydrolysis
- Chain scission
- Molecular weight
- Temperature sensitivity
- Humidity
- Shelf life
- Multi-material assemblies
- Esterification
- Acidity
- Product life
Introduction
For many modern products, long lifetimes (up to several decades) are advantageous, particularly for high consequence applications (e.g. nuclear energy, defence, space, etc.). There is also an increasing requirement for materials (resins, ingredients) and systems to last much longer than initially envisaged (>20 years) requiring programmes to be in place to support a life extension. In most cases, it is typically the organic materials that will age relatively faster than other materials (inorganic, metals, etc.), and therefore the chemical mechanisms and rates of reactions of these materials need to be understood in order to scientifically justify a life extension.
The technical challenge in high consequence defence-related multi-material assemblies is significant as materials must withstand complex and changing environments for decades that include combinations of heat, which may accelerate chemical reactions, radiation (alpha, beta, gamma and neutron) causing material degradation, mechanical loads (with reduced stiffness and changes in damping properties through life), varying degrees of oxygen and moisture (corrosion, hydrolysis) and volatile organics (degradation and corrosion reactions) [1]. The design of accelerated material ageing experiments to replicate service is therefore fraught with potential pitfalls.
Although temperature is the most common method of ageing materials, it is inappropriate when the issue is the radioactive degradation of any material. However, in practice temperature is a key parameter affecting chemical kinetics, and its use is normally based on the application of Arrhenius kinetics. In the absence of mechanistic information, the normal practice is to use the rule of thumb (the rate of reaction doubles per 10 °C rise) to identify a simulated age. However in practice, this is rarely true for many materials and multi-material assemblies and questions the validity of accelerated ageing trials based upon this rule of thumb. Therefore great caution is required in the use of Arrhenius kinetics in life prediction [2].
Another area of interest in the defence industry is the security of supply of key ingredients and the identification and qualification of new or replacement materials. It is increasingly difficult (and in many cases impossible) to purchase the exact same materials used in the production of early assemblies/materials. The preferred option for material supply (particularly the organic materials, with volatile constituents) in the defence industry is to have greater (in-house) control over the production of constituents and formulation of materials. All programmes to develop new or replacement materials require the delivery of assessments/tests to understand the ageing and chemical compatibility issues.
Commercially, EVA is the base polymer for some athletic shoes and also used in photovoltaic (PV) cells [3, 4]. Durability is of particular concern in the solar cells industry where EVA adhesive is used as an encapsulant for photovoltaic cells [5, 6]. On exposure to UV light, the material changes colour over a number of years, with potentially significant loss in power from the cell [7, 8].
In this paper, the key organic materials under consideration are poly(ethylene-co-vinyl acetate) (denoted EVA) and polyester polyol (denoted PL) resins. These resins are employed as adhesives for a number of specific applications either as binders for filler particles or to minimise the relative movement of materials in multi-material assemblies. A key area of interest is to understand shelf life and options for shelf life extension. Age-related changes to the adhesive could potentially impact the gas phase (resulting in corrosion of metals, sensitisation, etc.) and also influence system dynamics (e.g. de-bonding, compression set, etc.) with potential changes in system performance and/or safety [9]. We detail our initial studies on poly(ethylene-co-vinyl acetate) and AS1160 polyester polyol resins to understand thermal stability, useful shelf life and options for life extension. The general methodology, the analytical methods and the future strategy from an ageing and shelf life perspective are discussed.
Materials
Poly(ethylene-co-vinyl acetate) Resin
The poly(ethylene-co-vinyl acetate) resin used in this study (see Fig. 10.1) was adhesive grade Vinamul 3161 emulsion, batch number 2235/240211, and manufactured by Celanese in February 2011. The resin exhibits a glass transition temperature of approximately 3 °C, no melting point, and hardens on drying in air. The material was used as received and typically exhibits a pH of 5–7. The material is known to release acetic acid through hydrolysis of the acetate groups which alters the pH of the resin, and specification limits require a minimum pH of 4 (when stored at 20 °C), below which the resin cannot be used in the production of multi-material assemblies.
AS1160 Polyester Resin
Baycoll AS1160 is a highly branched polyester polyol (PL) consisting of four different constituents (see Fig. 10.2). The material typically has a low acid number, and a majority of end groups are hydroxyl that can react with a phenyl-based isocyanate to produce a polyurethane adhesive. Specification limits require the water content to be a maximum of 900 ppm and the hydroxyl concentration (based on ASTM D2849-69) to be 170 ± 10 mg/KOH g (expressed as KOH equivalent).
The maximum allowable manufacturer recommended shelf storage life for AS1160 is 6 years when stored at ambient conditions. It should be noted that the resin is no longer commercially available; however there is requirement to provide justification for the continued use of the available stock holding (which is beyond its manufacturer recommended 6-year shelf life) until a new replacement material is potentially sourced.
Test and Analysis
Ageing Studies on EVA Resin
The variation of resin pH as a function of time and temperatures was investigated to understand kinetics representative of the underlying chemical mechanism(s) that limits useful shelf life. Vinamul 3161 resin was aged in sealed containers and at four different temperatures (32, 45, 60, 75 °C) with the pH of the emulsion monitored at regular intervals using a Mettler Toledo SevenEasy™ pH metre. Temperature sensitivity was assessed by determining changes in pH, and the Arrhenius plot was used to predict the rate of change in pH at temperatures specific to shelf storage conditions (normally 20 °C).
Ageing Studies on AS1160 Resin
The experimental test matrix for studies on AS1160 resin is detailed in Table 10.1. These studies do not advocate studies in inert gas atmospheres as AS1160 is normally stored in air. To minimise diffusion effects/gradients between the bulk and surface of the material, samples were aged as thin samples with typically 30–40 g of material aged in 250 ml conical flasks at each temperature. The flask openings were covered with aluminium foil secured with metal wire. For experiments F1, F4 and F5, the aluminium foil was punctured with three small holes to ensure ageing in a non-sealed environment, enabling the exchange of volatiles/gases with the oven atmosphere. For experiments F2 and F3, ageing in a closed environment was simulated by heating AS1160 in a sealed conical flask.
As the sensitivity of AS1160 to humidity is not well understood, the experimental matrix incorporates a controlled humidity experiment (F4). The use of 60 °C combined with 20 % RH correlates to a dew point of 29 °C and simulates relatively humid conditions. Under these conditions, the rate of moisture diffusion through the resin is accelerated with temperature potentially degrading the material either through hydrolysis, chain scission or plasticisation effects.
Trials F2 and F3 were included to assess whether AS1160 is sensitive to the retention of volatile/gaseous species. In particular, a comparison of results between F3 and F5 will provide information on whether the retention of volatiles/gaseous species is important and whether this can alter the degradation chemistry and/or rate.
Gel Permeation Chromatography
For both resins, gel permeation chromatography (GPC) was employed to measure variation in molecular weight and molecular weight distributions. For the EVA resin, samples were dissolved in tetrahydrofuran (THF) and analysed using a Viscotek TDA GPC instrument calibrated against EasiCal poly(styrene) standards. The peaks observed from the EVA samples were within the range covered by the EasiCal poly(styrene) calibration standards used within this study.
For the AS1160 resin, triple detection and conventional calibration analyses were performed on a Viscotek TDA instrument. Acetone (HPLC grade) was used as the eluent with a flow rate of 1 ml/min. Two PL gel mixed-E 300 mm columns were used with an oven temperature of 35 °C. Calibration was performed using monodisperse poly(methyl methacrylate) standards, and three runs were performed per sample (concentration ~40 mg ml−1).
Moisture Content and Hydroxyl Functionality
The moisture levels in AS1160 were measured using Karl Fischer Coulometry, and the hydroxyl content was measured based on the standard ASTM D2849-69 procedure [10].
Bond Strength
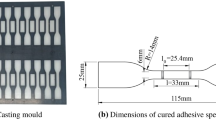
When the AS1160 resin is mixed with isocyanate, and cured for up to 24 h in dry (typically less than 15 % RH), a thixotropic polyurethane adhesive is formed. The adhesive is commercially used to bond metals, organic and inorganic materials, and is particularly useful for reducing the relative movement of components in multi-material assemblies. The performance of the polyurethane adhesive requires the ultimate tensile strength (UTS) to be a minimum of 15 MPa when measured using butt tensile specimens by ASTM Test Method D2095.
Results and Discussion
Shelf Life Assessment of EVA Resin
Poly(ethylene-co-vinyl acetate) resins are known to hydrolyse to the parent carboxylic acid and an alcohol. This mechanism increases the acidity of the resin, generates pendent hydroxyl functional groups on the EVA and ultimately limits the shelf life of the resin. Water, and/or hydroxyl groups in close proximity to the vinyl acetate groups, is thought to promote hydrolysis chemistry. The production of acetic acid is associated with an increase in acidity and a reduction in resin pH. Figure 10.3 shows pH versus time measured at a number of different temperatures. The sensitivity to temperature fits Arrhenius kinetics (see Fig. 10.4), and the resulting activation energy estimated from the slope is ~46 kJ/mol. The Arrhenius plot provides a route to the determination of the rate of change in resin acidity at temperatures representative of typical shelf storage conditions (20 °C) and ultimately leads to resin shelf life prediction based on our failure criteria that a resin exhibiting a pH of below 4 is deemed not suitable for use in manufacturing operations. This methodology predicts a pH reduction rate of less than 0.5 over 6 months with a typical resin shelf life estimated within 2–3 years. This prediction is, however, highly dependent on the health or age of the material (as determined by zero time pH data) when first prepared by the manufacturer.
Figure 10.5 shows the results of GPC characterisation of the uncured EVA resin as a function of age. The chromatograms suggest a relatively broad distribution with weight average molecular weight within the range of 500,000–600,000 g/mol (relative to polystyrene standards). In particular, ageing induces an increase in the low molecular weight peak (at 12.3 mL) relative to the high molecular weight shoulder (at 11.5 mL) with a general shift to the right-hand side (towards lower molecular weight). The shift towards relatively lower molecular weights is indicative of thermally induced oxidative chain scission of the EVA and is agreed with observations reported on similar materials by Jing Jin et al. [11]. To determine whether these age-related changes influence the key properties of the material as an adhesive, a series of mechanical property studies have been carried out and reported in our previous publication [12].
Vinamul 3161 EVA resin molecular weight as a function of resin storage temperature (over 4 months) as measured by GPC. The weight average and number average trends fit a power law relationship with accelerated ageing, inducing a gradual reduction in molecular weight through oxidative chain scission chemistry
AS1160 Resin Ageing Studies
Our ageing studies clearly show that AS1160 resin is relatively hygroscopic and readily accumulates moisture; see Figs. 10.6 and 10.7. All aged samples show elevated moisture levels which are above specification requirements (900 ppm) compared to zero time levels. This is particularly apparent for sample F4 which has been aged in relatively humid conditions.
The results in Table 10.1 show that ageing also potentially elevates the hydroxyl concentrations which are above specification limits (170 ± 10 mg/KOH g) compared to zero time values. However, it should be noted that the absorption of moisture may provide inaccurate hydroxyl concentration measurements due to the destruction of the esterification reagent and so the hydroxyl values should be treated with caution.
In addition, complex changes in GPC molecular weight were observed that could be related to the acid/alcohol versus ester/water equilibrium shown in Fig. 10.8. In the highest temperature ageing study (see Fig. 10.9), a significant molecular weight increase was noted, while the remainder broadly decreased. As expected, the sample aged under humid conditions had the largest initial decrease in molecular weight confirming the sensitivity of the material to hydrolysis. There are also clear differences in open to air and sealed ageing regimes (see Fig. 10.10) presumably because ageing in open conditions leads to loss of low molecular weight species.
With respect to real-time ageing, a significant decrease in M n (number average molecular weight) and a smaller decrease in M w (weight average molecular weight) during real-time ageing over 6 years are shown in Table 10.2. It is probable that relatively small increases in the population of low molecular weight oligomers have led to this decrease, since M n is strongly affected by low molecular weight components of the molecular weight distribution. However, overall most aged samples showed values (after 28 days) that are not too different to baseline zero time data and suggest that Baycoll AS1160 may be fairly resilient to thermal ageing although real-time ageing does appear to suggest more significant changes than that observed through accelerated ageing.
Our infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy did not show significant changes in the material, either in real-time ageing over a 6-year period or in accelerated ageing.
Polyurethane Adhesive Bond Strength
Although accelerated ageing suggests significant changes in resin moisture levels and complex changes in molecular weight, these changes do not appear to adversely affect adhesive bond strength which still remains well within specification limits (above 15 MPa); see Table 10.1. The bond strength remains well within specification limits even under harsh aggressive conditions, and it is unlikely that any moderate change to the polyol properties will decrease this strength to the point where it no longer satisfies the specification limits. However, a general observation is that the quality and reliability of the adhesive joints produced is found to decrease as the resin ages. This is presumably because the absorbed moisture leads to increased carbon dioxide production (through reaction with isocyanate) leading potentially to a more porous and poor-quality adhesive joint.
Overall, the accelerated ageing study did not reveal any significant changes in the mechanical properties of the adhesives even when unrealistic levels of water are present within the AS1160 resin. The resin is considered to be fairly resilient to thermal ageing, and the shelf life could be extended beyond the current manufacturer recommended 6-year limit, and storage in low humidity conditions is likely to promote shelf life.
Life Prediction Methodology and Future Direction
As demonstrated by the ageing studies reported in this paper, modern life assessment techniques often rely on the Arrhenius treatment of data collected at elevated temperatures in service representative regimes, to predict key properties at service or shelf storage temperatures. This issue arises largely because of the absence of analytical methods that can detect and track the very small changes occurring at service temperature. In addition, there is a need to better understand the failure criteria (or limits) representing the amount of age-related change that can be tolerated in order to understand the remaining service life of the material or component. The failure limits are often not known or understood at the individual material level or at the multi-material level, and engineering and/or physics assessments are often required to capture this information.
In most cases, the validation of predictions from accelerated ageing remains very difficult as real-time data from field trials remains very limited and there remains significant uncertainty on how to define the simulated age (i.e. artificially accelerated age) of multi-material assemblies.
To overcome some of these difficulties, the future research direction in the life assessment of materials is to develop and make full use (where possible) of ultrasensitive techniques (such as microcalorimetry or oxygen consumption). In the case of multi-material assemblies, a strategy to incorporate in situ diagnostic sensors (for chemical and physical properties) to track age-related events is often adopted. This strategy should enable the rate of age-related changes to be monitored in real time, providing advance warning of potential issues and reducing the reliance on accelerated ageing and Arrhenius-type treatments.
Conclusions
The ageing properties of Vinamul 3161 poly(ethylene-co-vinyl acetate) and AS1160 polyester polyol resins have been investigated in support of shelf life assessment and to identify storage conditions that may extend product life. The temperature sensitivity of pH, combined with Arrhenius kinetics, was used to predict changes for EVA in conditions representative of normal storage conditions. In separate experiments, relatively short-term thermally accelerated sensitivity studies have been carried out on the AS1160 resin to investigate variables such as humidity, temperature and open/close ageing conditions. The AS1160 resin is highly hygroscopic and readily accumulates moisture. This is potentially associated with complex changes in molecular weight, with ageing linked to the hydrolysis-ester formation equilibrium. However, these changes do not adversely impact adhesive bond strength, allowing the resin to be potentially used significantly beyond the manufacturer recommended shelf life limit.
References
Patel M, Skinner AR (2001) Polym Degrad Stab 73(3):399
Patel M, Soames M, Skinner ARS, Stephens TS (2004) Polym Degrad Stab 83(1):111
Martinez-Garcia A, Sanchez-Reche A (2003) J Adhes 79:525–547
Zanetti M, Camino G, Thomann R, Mulhaupt R (2001) Polymer 42:4501
Allen NS, Edge M, Rodriguez M, Liauw CM, Fontan R (2001) Polym Degrad Stab 71:1
Allen NS, Edge M, Rodriguez M, Liauw CM, Fontan R (2000) Polym Degrad Stab 68:363
Rimez B, Rahier H, Van Assche G, Artoos T, Biesemans M, Van Mele B (2008) Polym Degrad Stab 93(4):800–810
Létant SE, Plant DF, Wilson T, Alviso C, Read MSD, Maxwell RS (2011) Polym Degrad Stab 96:2019
Patel M, Bowditch M, Jones B, Netherton D, Khan N, Letant S, Maxwell RS, Birshall SA (2013) Polym Test 32:313
Standard ASTM D2849-69 replaced by ASTM D4274-05 (2005) Standard test methods for testing polyurethane raw materials: determination of hydroxyl numbers of polyols
Jing Jin, Shuangjun Chen, Jun Zhang (2010) Polym Degrad Stab 95:725
Mogon P, Simon P, Peter B, Mathew R, Paul M, Niaz K, Imran K, Nicola P, Sonia L, Von White II, Gregory LA (2013) Polym Test 32:785–793
Acknowledgments
The authors would like to thank Miss. J. Opie-Lovelace for the analytical support to this project.
This project also incorporates joint working agreements between AWE and Sandia National Laboratories (SNL). Sandia National Laboratories is a multiprogramme laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the US Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000.
© British Crown Owned Copyright 2013/AWE
Published with the permission of the Controller of Her Britannic Majesty’s Stationery Office.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Patel, M. et al. (2015). Shelf Life Assessment of Poly(ethylene-co-vinyl acetate) and Polyester Polyol Resins Used as Adhesives. In: White, C., Martin, J., Chapin, J. (eds) Service Life Prediction of Exterior Plastics. Springer, Cham. https://doi.org/10.1007/978-3-319-06034-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-06034-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06033-0
Online ISBN: 978-3-319-06034-7
eBook Packages: EngineeringEngineering (R0)