Abstract
The development of fingolimod, an unselective functional antagonist of the interactions between sphingosine 1 phosphate (S1P) and sphingosine 1 phosphate receptors (S1PRs), as the first oral therapy for multiple sclerosis (MS) has been a milestone. The parallel intensive research on the role of S1P, sphingosine kinases, and the five known S1PRs, their tissue distribution and expression in physiological and pathological conditions have led to a wide range of interesting findings. The initial focus of this research in the context of developing fingolimod as a treatment of MS has been on its immunological effects. The wide distribution and important roles of sphingosine, its metabolites, and their receptors in the central nervous system (CNS) in general, in myelin, and in all cell types of this organ have spurred interest to examine S1P and its five receptors in the brain as well. The present review will concentrate on the latter area and give a brief overview of what is known about S1P/S1PR interactions in the CNS in physiological and pathological conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple Sclerosis
- Experimental Autoimmune Encephalomyelitis
- Progressive Multifocal Leukoencephalopathy
- Retinal Nerve Fiber Layer Thickness
- Sphingosine Kinase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
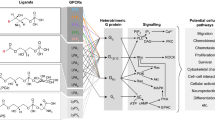
Sphingolipids, particularly sphingomyelin, are principal components of oligodendrocytes and myelin and of the CNS in general. Sphingomyelin is catalyzed to ceramide by sphingomyelinase, and ceramide metabolized further to sphingosine by ceramidase, which then can be phosphorylated by sphingosine kinases (SphK) to yield sphingosine-1-phosphate (S1P). The role of these metabolites was long enigmatic until the discovery of fingolimod, a first-in-class agonist of sphingosin-1 phosphate receptors (S1PRs). The parallel growing understanding of the functions of breakdown products of sphingomyelin, ceramide, and S1P, as signaling molecules upon binding to S1PRs, has shown that these molecules are involved in a wide range of functions of essentially all cells in our body via intracellular signaling in different pathways that are mediated by Rho-/Ras, phospholipase C, phosphatidylinositol-3 kinase, and Akt. Fingolimod binds to four of the five S1PRs, and among its pharmacological effects, immunomodulation and those on multiple CNS cells stand out. Based on the novel immunomodulatory mechanisms fingolimod was first explored as a treatment to prevent the rejection of allotransplants and then for multiple sclerosis (MS). In 2010, fingolimod was approved as the first oral treatment for MS. Already during later stage clinical development and in parallel animal model studies, it has been shown that fingolimod, besides its prominent effects on immune cell homing, has a number of mechanisms of action on CNS cells and the blood-brain barrier (BBB) (Brinkmann et al. 2004; Groves et al. 2013; Coelho et al. 2007; van Doorn et al. 2012), which are of interest not only in MS, but also in other neurodegenerative diseases including stroke (Kimura et al. 2008; Wei et al. 2011), brain/spinal cord injury (Lee et al. 2009), and glioma (Estrada-Bernal et al. 2012). S1PRs are expressed on endothelial cells at the BBB (van Doorn et al. 2012), on astrocytes (Sorensen et al. 2003; Wu et al. 2008), on neuronal cell populations and their progenitors (Kimura et al. 2007), on oligodendrocytes and their precursors (Jaillard et al. 2005), and on microglia (Kimura et al. 2007). These observations have spurred intensive further research. Currently, a series of small molecules and antibodies with more specific inhibitory profiles regarding S1PR subtype inhibition are being developed. The current state of knowledge about the role of S1P and S1PRs in the context of the CNS will be reviewed here. Since numerous excellent reviews have been written along the research in this field, a few are mentioned here for the interested reader (Groves et al. 2013; Brinkmann 2009; Chun and Hartung 2010).
2 Sphingosine-1 Phosphate Receptor Expression and Functions in the CNS
2.1 Neuronal Cell Populations
Following the discovery of the structural analog of S1P, fingolimod (FTY720), and its potent effects in MS, the role of S1P/S1PR interactions in CNS function and the expression of S1PRs on CNS cells and tissue received increasing interest. The involvement of S1P signaling in normal neural function became clear from in vivo knockout studies, from effects of fingolimod and other pharmacological inhibitors in various in vivo models, and also from in vitro studies with cultured cells (Kono et al. 2007; MacLennan et al. 2001; Ishii et al. 2002; Akahoshi et al. 2011; Edsall et al. 1997; Toman et al. 2004; Choi et al. 2011; Rau et al. 2011; Rossi et al. 2012; Callihan and Hooks 2012). Indirect evidence for a role of S1P/S1PRs in CNS function was derived from demonstration of sphingosine kinase 1 or −2 (SK1, SK2) expression in various cell types (Bryan et al. 2008) and their role in neural development (Mizugishi et al. 2005). Regarding the expression of S1PRs on CNS cells, since monoclonal antibodies (mabs) were not available until recently and are still not available for all subtypes, the/tissue-/cellular expression was examined mainly by nucleic acid-based methods (PCR, in situ hybridization), but recently also by immunohistochemistry (IHC) and western blotting using a S1PR-specific mab for staining autopsy specimens of brain tissue and parallel analysis by PCR (Brana et al. 2013; Nishimura et al. 2010). The five S1PRs are found in the developing and mature brain (Dubin et al. 2010), and constitutive knockout (ko) of S1PR1 causes a behavioral phenotype reminiscent of schizophrenia (Contos et al. 2002). S1PR1 is expressed on neuronal precursor cells and likely also mature neurons and has been found to affect neurogenesis, cell migration, and functions such a brain-derived neurotrophic factor (BDNF)-induced process extension (Chun and Hartung 2010; Deogracias et al. 2012). Fingolimod upregulates BDNF and hence probably contributes to tissue protection in EAE 31. The fact that S1PR1 is expressed preferentially and several fold higher in the gray compared to white matter hinted at prominent neuronal expression, but detailed examination of its cellular localization revealed expression in astrocytic foot processes (Nishimura et al. 2010). Further evidence for a role of S1P/S1PR signaling includes inhibition of amyloid production by cultured neurons upon exposure to FTY720-phosphate (FTY720-P) and a more selective S1PR1 agonist KRP203-P (Takasugi et al. 2013), the development of neural tube defects upon maternal ingestion of the mycotoxin fumonisin (Callihan et al. 2012), the S1P-mediated increase in glutamate release and expression of SK1 by hippocampal neurons (Kajimoto et al. 2007), the induction of neuronal precursor cell migration to areas of spinal cord injury via increased S1P and interaction with S1PR1 (Kimura et al. 2007), and by reduced neuronal cell death upon FTY720 treatment in stroke models (Hasegawa et al. 2010). Existing data thus documents a wide range of effects of S1P/S1PR signaling on neuronal precursor cells and mature neurons in different areas of the brain. Due to technical limitations, particularly the lack of mabs against all S1PRs, the expression of the different S1PRs on different cell types is, however, not yet clear. Developing a better understanding for the cellular distribution on neuronal cells during development of the healthy brain and during pathological conditions will be important.
2.2 Astrocytes
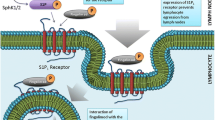
As already briefly mentioned above, S1PRs are widely expressed on astrocytes (Nishimura et al. 2010), a cell population that tightly interacts with neurons and endothelial cells of the BBB and is considered essential for many homeostatic processes within the brain but also tightly involved in neuroinflammation (Brinkmann 2007). Both in situ hybridization and conditional ko studies in animal models as well as data from intracerebral injection of FTY720 indicate that astrocytes are the major CNS cell type that is responsible for the beneficial effects of FTY720 in experimental autoimmune encephalomyelitis (EAE), the main animal model of MS (Choi et al. 2011; Miron et al. 2008; Wu et al. 2013). In vitro studies with human fetal astrocytes documented that FTY720 inhibits subsequent S1PR-mediated pERK1/2 signaling for a protracted period of time and thus desensitizes neuroinflammatory effects on astrocytes and their proliferation (Sorensen et al. 2003; Wu et al. 2013). Cultured murine cortical astrocytes express a wide range of lipid-activated receptors including the protease-activated receptors (PAR1-4), lysophosphatidic acid receptors (LPA1-3), and S1PR1, 3, 4, and 5, and each of these activates multiple downstream signaling pathways that participate in astrocyte proliferation and gliosis, Sorensen et al. 2003. Regarding S1PR subtypes on astrocytes, Rao et al. (2003) have demonstrated expression of S1P1, S1PR2, S1PR3, and S1PR5 with relatively higher expression of S1PR3 and S1PR1. In vitro or in vivo administration of S1P promotes the expression of glial fibrillary acidic protein (GFAP) and astrogliosis, but also astrocyte proliferation and migration (Chun and Hartung 2010). Particularly, S1PR3 appears to be involved in astrocyte proliferation and neurodegeneration during the terminal stages of Sandhoff’s disease (Wu et al. 2008). During treatment with FTY720, its metabolite FTY720P induces astrocyte migration through preferential binding to S1P1, while S1P binds to both S1P1 and S1P3, indicating that the profile of FTY720 could play a role for its therapeutic effects in MS (Mullershausen et al. 2007). Since glial proliferation is a general characteristic in many experimental models of neuroinflammation and neurodegeneration and also human neurodegenerative/inflammatory diseases, it is likely that S1P/S1PR-mediated functions play a major role during these pathological conditions.
2.3 Oligodendrocytes, Myelination, and Remyelination
Lipids and sphingomyelin, the precursor of S1P, are abundant in the CNS white matter and myelin. Further, myelin and oligodendrocytes (OLG) are the main targets of the immune system during MS, and demyelination and partial remyelination are characteristic of the disease (Noseworthy et al. 2000). In one pathological subtype of MS, i.e., in pattern III, preferential loss of myelin-associated glycoprotein (MAG) and metabolic alterations have been noted (Lucchinetti et al. 2000), and due to these histopathological findings and the efficacy of fingolimod, the role of S1P/S1PR interactions have been studied extensively in recent years in vitro in oligodendrocyte cultures from adult human brain (Miron et al. 2008, 2010) and from various developmental stages in animals (Jaillard et al. 2005), in oligodendrocyte precursor cell (OPC) models (Kim et al. 2011), in embryonic stem cell-derived OPCs (Bieberich 2011), in organotypic cerebellar slices (Miron et al. 2010), and in models of toxic (cuprizone, lysophosphatidyl-choline-induced, lysolecithin) demyelination (Miron et al. 2008, 2010; Kim et al. 2011; Jackson et al. 2011). Various S1PR subtypes are expressed by human and rodent OLGs, i.e., S1P5, S1P3, and S1P1 at decreasing levels (Terai et al. 2003; Yu et al. 2004; Miron et al. 2012). Upon interaction with S1P1 Gi/o-associated signaling is mediated via Rac1 and Ras GTPase activation, which affect membrane dynamics and survival (Jung et al. 2007; Spiegel and Milstien 2003), while S1P/S1P3 and -S1P5 interactions lead to G12/13-mediated RhoA GTPase activation (Jaillard et al. 2005; Toman et al. 2004). When OLGs from adult human brain were exposed to various concentrations of fingolimod in vitro, cyclical changes with sequential increase of membrane elaboration, retraction and recurring extension were observed at low doses (0.1–1 nmol/L), while the opposite sequence occurred at higher concentrations (10 nmol/L–1 μmol/L) (Miron et al. 2010). Further, membrane retraction could be reversed with a S1P3/S1P5 antagonist, suramine (Miron et al. 2008). In parallel, fingolimod prevented negative effects of serum and glucose withdrawal, which were again blocked by suramine (Miron et al. 2008). S1P5 (formerly Edg8) is expressed throughout oligodendrocyte differentiation, and in vitro experiments with 04-positive pre-oligodendrocytes as well as in S1P5 ko mice revealed that S1P/S1P5 interacions play a role in OLG process retraction and cell survival (Jaillard et al. 2005). Activation of S1P5 in rat neonatal cortex-derived OPCs in vitro inhibits the migration of these cells (Novgorodov et al. 2007). The systematic assessment of the effect of S1P and fingolimod on OLG differentiation from ES cell-derived neural precursor cells (NPCs) demonstrated the expression of S1P1 and protection from ceramide-induced apoptosis and preferential differentiation into the oligodendrocyte lineage upon S1P or fingolimod exposure (Bieberich 2011). When the effects of fingolimod were examined in demyelination models, i.e., in lysolecithin-induced demyelination in organotypic cerebellar slice cultures (Miron et al. 2010), in cuprizone-induced demyelination in vivo, and in mixed neural/glial aggregate cultures (Jackson et al. 2011), remyelination and increased process extension by OPCs and mature OLGs (Miron et al. 2008), reduced damage to OLGs, myelin, and axons (Kim et al. 2011), and increased de novo synthesis of myelin proteins (Jackson et al. 2011) were observed. Together, these data indicate not only the importance of S1P/S1PR signaling in oligodendrocytes and myelin, but also that pharmacological modulation of S1P represents an interesting way to modulate OPC and OLG biology during de- and remyelination.
2.4 Microglia
Microglia are the CNS-resident equivalent of monocytoid cells in the peripheral immune system (Benarroch 2013). Any type of damage and alteration of CNS cells such as for example, protein aggregate deposition, neuronal cell death, or damage of processes are sensed by microglia and lead to their activation (Benarroch 2013). Microglia serve a wide range of functions including phagocytosis, cytokine/inflammatory mediator release, antigen presentation, Fc receptor-mediated cell killing, and migration, and hence are the main mediators of neuroinflammatory processes, although astrocytes and innate and adaptive immune cells (T and B cells) that have entered the brain also participate (Goldmann and Prinz 2013). In MS, microglia activation is widespread, and it is currently believed that this cell population is activated already during the earlier relapsing-remitting phase of MS and plays a central role in secondary (SPMS) and primary chronic progressive disease (PPMS), i.e., during the phase (SPMS) or MS type that are characterized by increasing neurological deficit in the absence of relapses (Lassmann 2013). Despite the importance of microglia for MS and also for neurodegenerative disease, relatively little attention has been devoted to this cell type in the context of S1P/S1PR interactions and its pharmacological modulation by fingolimod. In the abovementioned model of lysophosphatidyl-choline-induced toxic demyelination, microglial ferritin, the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), nitric oxide metabolites, and apoptosis mediators (caspase 3 and −7) were all reduced by fingolimod and probably represent an important factor for increasing myelination (Jackson et al. 2011). In the lysolecithin-induced demyelination in cerebellar slice cultures, fingolimod treatment interestingly increased microglia numbers and also the expression of GFAP by astrocytes, however, the number of phagocytosing microglia remained unchanged (Miron et al. 2010). If analogies can be drawn from peripheral innate immune cells such as dendritic cells (DCs), which express all five of the S1PRs (Brinkmann 2009), it can be expected that S1P/S1PR signaling and modulation by S1P agonists exerts a wide range of effects on microglia (Brinkmann 2009).
2.5 Endothelial Cells and Blood–Brain Barrier
Maintaining brain homeostasis critically depends on the blood–brain (BBB) and blood-CSF barriers, which restrict access of components of the blood to the CNS (Engelhardt 2011), The BBB is composed of a complex cellular network including cerebrovascular endothelial cells with tight junctions, astrocytic foot processes and pericytes, which jointly restrict the paracellular and transcellular passage of molecules (Engelhardt 2011). The tight junctions together with membrane efflux pumps assure that this specialized barrier separates the CNS from potentially noxious substances within the blood and also blocks entry of immune cells (Cannon et al. 2012). In MS T and B lymphocytes as well as innate immune cells (macrophages and DCs) cross the BBB, and this transit involves a series of steps including interactions of α4-integrins, specifically very late antigen-4 (VLA-4), on activated immune cells with vascular cell adhesion molecule 1 (VCAM-1) on activated endothelial cells (Vajkoczy et al. 2001). This step is a central aspect during the formation of new inflammatory lesions in MS, and inhibition of binding of VLA-4 on activated T cells to VCAM-1 by the humanized monoclonal antibody natalizumab very efficiently blocks inflammatory disease activity and exacerbations of MS (Yednock et al. 1992; Polman et al. 2006). Other MS treatments such as interferon-β (IFN-β) have also been found to inhibit certain steps of BBB opening such as matrix metalloprotease activation (Waubant et al. 1999), although they are overall less efficient than natalizumab.
Understanding the effects of a novel drug on the BBB and the questions if it accesses the CNS compartment are therefore of high interest, and an important characteristic of fingolimod as a treatment of MS is the fact that it easily crosses the BBB due to its lipophilic nature (Meno-Tetang et al. 2006). In addition to its physicochemical characteristics, fingolimod inhibits P-glycoprotein, an ATP-driven efflux pump, which inhibits drug delivery through the BBB (Cannon et al. 2012). Studies of S1P5, which is relatively specifically expressed in the brain, in human brain tissue (autopsy material) and in vitro experiments using pharmacological modulation with fingolimod as S1P5 agonist as well as lentiviral knockdown of S1P5 in cultures of human brain endothelial cells demonstrated its involvement in BBB function (van Doorn et al. 2012). Fingolimod improved several aspects of BBB integrity and reduced the migration of inflammatory cells across endothelial cells (van Doorn et al. 2012a, b). Pharmacological modulation of S1PRs also affect other mechanisms involved in BBB function. Protein S, a vitamin K-dependent anticoagulant plasma protein, which is involved in maintaining BBB integrity during hypoxia-induced BBB damage, mediates its effects via the protein tyrosine kinase receptor Tyro3 and S1P1, and specific inhibition of S1P1 with the antagonist W146 blocked the protein S-mediated protection of ischemia-induced BBB opening (Zhu et al. 2010). During EAE, fingolimod enters the CNS compartment in a dose-dependent manner and preferentially accumulates in the white matter {Foster, 2007 #626}. Taken together, S1P and its interactions with specific S1PRs (e.g., S1P5 on endothelial cells) affect several aspects of BBB function, and functional S1PR antagonism appears to improve BBB integrity in the context of inflammatory processes.
3 Treating Multiple Sclerosis with the S1P Agonist Fingolimod
MS is considered a prototypic T cell-mediated autoimmune disease with a complex genetic background involving more than 100 quantitative trait loci conferring genetic risk (Beecham et al. 2013), but environmental factors also contribute to MS etiology (Ascherio and Munger 2008). MS pathogenesis involves the activation of autoimmune CD4+ T cells by molecular mimicry or other as yet incompletely understood mechanisms (Sospedra and Martin 2005), their entry into the CNS and initiation of inflammatory CNS lesions that result in damage of myelin, axons, and neurons, astrocyte activation and gliosis as well as complex metabolic alterations (Lassmann 2013). The inflammatory processes involve innate immune cells (DCs, macrophages, microglia), perturbations of astrocyte function, CD8+ T cells, and to various extents also antibody deposition and complement activation with the result of different patterns of CNS pathology (Lucchinetti et al. 2000). Permanent CNS tissue damage occurs already during the earliest stages of MS, but chronic inflammation, gradually increasing loss of axons/neurons, and incomplete remyelination characterize the later stages of MS. MS affects young adults between the ages of 20 and 40 years and women more than twice as often than men (Noseworthy et al. 2000). It is highly specific for the CNS, and the peripheral nervous system is almost never affected to a significant extent. MS can involve every functional system of the CNS including visual, motor, sensory, cerebellar, autonomic, and neurocognitive function, and consequently clinical signs and symptoms are very heterogeneous. Most patients initially present with periodic neurological deficits and a relapsing-remitting course (RRMS), which after 10–20 years often evolves into secondary progressive MS (SPMS) (Noseworthy et al. 2000). Relapses gradually disappear during SPMS, and neurological deficits and disability steadily increase during this stage. A minority of patients show primary progressive MS (PPMS). These patients worsen progressively from onset without relapses.
While many aspects of MS etiology and pathogenesis are still incompletely understood, the development of treatments for this disease has been remarkably successful during the last 20 years (Haghikia et al. 2013). Based on many lines of evidence that MS is an autoimmune disease affecting the CNS and that inflammation is the main cause of CNS tissue damage, several immunomodulatory or—suppressive treatments have been developed and approved for clinical use in RRMS. The injectable drugs IFN-β and a peptidic mixture, glatiramer-acetate (GA), which are both moderately effective, but very well tolerated have long been the mainstay of the treatment of RRMS until a humanized monoclonal antibody against VLA-4, natalizumab, was introduced in 2006 (Polman et al. 2006). The latter compound is considerably more effective than IFN-β and GA and usually well tolerated, but more than 400 patients developed a serious and often fatal complication, an opportunistic infection of the brain with the polyoma virus JC called progressive multifocal leukoencephalopathy (PML). The introduction of the oral S1P agonist fingolimod, which will be discussed in more detail below, was an important milestone in MS treatment since it was the first orally available compound with superior efficacy when compared to the injectable first-line drugs (IFN-β and GA) and an overall good safety profile (see below). Very recently, two additional oral drugs, teriflunomide (O’Connor et al. 2011) and dimethylfumarate (DMF) (Gold et al. 2012), and a humanized monoclonal antibody, anti-CD52 (alemtuzumab) (Coles et al. 2012) have been approved for the treatment of RRMS. Based on data from the large phase III trials all appear superior to IFN-β and GA, albeit only marginally in the case of teriflunomide. Among the oral drugs, DMF appears most active based on the clinical trial data, but direct comparisons with fingolimod or teriflunomide are not available. Alemtuzumab is more active than the oral compounds and probably comparable to natalizumab. Different from natalizumab, for which PML represents the most important liability regarding safety, alemtuzumab leads so secondary autoimmune diseases in a substantial fraction of patients and to an increased rate of infections probably due to the long-lasting lymphopenia. The long-term safety of the newer agents, i.e., teriflunomide, DMF, and alemtuzumab, remains to be determined. For a more detailed overview about MS treatments the reader is referred to special reviews of the topic.
As indicated above, the introduction of fingolimod as the first oral agent to treat MS has been a significant advance. Its development process has been summarized in excellent reviews elsewhere (Brinkmann 2009; Chun and Hartung 2010), and therefore we will focus here on the most important aspects in the context of S1P and the CNS. Drug development in MS usually involves proof-of-concept in the well established and widely used EAE model. Extrapolation from the EAE model to MS has been difficult, and in many cases promising findings in EAE did not hold up during clinical trials in MS from reasons such as differences between the immune systems of rodents and humans and others. There are, however, notable examples such as VLA-4 blockade by natalizumab and functional S1P antagonism by fingolimod, for which EAE data was fully confirmed in MS (Brinkmann 2009; Yednock et al. 1992). Fingolimod has been tested extensively both as prophylactic, i.e., prior to disease development, and therapeutic, i.e., given after disease onset, intervention in various chronic and relapsing-remitting EAE models (Fujino et al. 2003; Webb et al. 2004; Brinkmann 2009; Kataoka et al. 2005). Fingolimod treatment efficiently blocks disease activity in the EAE model, and a number of mechanisms, most importantly the trapping of CD4+ T helper 1 (Th1, secreting IFN-γ) and Th17 (secreting interleukin-17; IL-17) cells in lymph nodes, but also the stabilization of the BBB, and the abovementioned effects on astrocytes, oligodendrocytes and remyelination, and possibly also neurons, contribute to its efficacy as well [for review see (Brinkmann 2009)].
Following the highly promising data in the EAE model, transplant, and other animal models [for review see (Brinkmann 2009)], fingolimod was developed for the use as oral immunomodulatory agent for RRMS in a large phase IIb and—III program including multiple studies (Cohen et al. 2010; Kappos et al. 2010). Due to the highly positive results, fingolimod (Gilenya®, Novartis) was approved for RRMS in September 2010. In brief, the clinical trials showed the following: in a phase III randomized, placebo-controlled trial of fingolimod given as either 0.5 or 1.25 mg/d, the annualized relapse rate (ARR; primary outcome) was 0.18 with 0.5 mg fingolimod, 0.16 with 1.25 mg, and 0.4 with placebo (highly significant reduction) (Cohen et al. 2010; Kappos et al. 2010). Furthermore, a significant reduction with respect to cumulative probability of disability progression was observed with both doses, and other secondary outcomes (MRI) were also significantly improved (Kappos et al. 2010). In a second, similarly large phase III study intramuscularly injected IFN-β1a was compared with 0.5 and 1.25 mg/d fingolimod (Cohen et al. 2010), and the primary outcome (ARR) showed a significant reduction from 0.33 (placebo) to 0.16 (0.5 mg) and 0.20 (1.25 mg) fingolimod (Cohen et al. 2010). Secondary (MRI) outcomes supported these data, but there was no significant reduction of disability progression in the two verum groups (Cohen et al. 2010). Two fatal adverse events (disseminated varizella zoster virus (VZV) infection and herpes simplex (HSV1) encephalitis) were observed in the 1.25 mg dose, which was a main reason for later continuation of studies and filing for approval of the 0.5 mg dose, which is now in clinical use since September 2010 (for further details on adverse event profile, see below).
3.1 Immunomodulatory Effects
The immunomodulatory effects have been reviewed extensively elsewhere (Brinkmann et al. 2010) and therefore will only be briefly summarized here. The preclinical studies in EAE and later the clinical experience in MS with natalizumab, the anti-VLA-4 monoclonal antibody have highlighted the importance of keeping autoreactive T cells and other immune cells out of the brain. Therefore, other approaches including anti-LFA-1 monoclonal antibodies, small molecule VLA-4 inhibitors, and others were approached to achieve a similar outcome, and among these the oral S1P agonist fingolimod showed a promising profile due to oral availability and efficient modulation of lymphocyte migration/homing, which was expected to lead to a similar outcome, i.e., keeping autoreactive immune cells from gaining access to the CNS compartment. In brief, fingolimod interferes with a well-known sequence of events that occur during de novo activation of immune cells following antigen exposure in a peripheral organ, e.g., the skin, uptake and processing of antigen by organ-residing DCs and transport into regional lymph nodes (LN), where antigen is efficiently presented to T cells and results in T cell activation and proliferation (Matloubian et al. 2004). The containment of the latter step in LN leads to “trapping” of T lymphoctes and involves S1P/S1PR signaling and downregulation of S1P1 mRNA, which was shown by several approaches including mice with targeted deletion of S1P1 from hematopoietic cells resulting in S1P1-deficient thymocytes and T cells, in mice with genetic deletion of sphingosine kinase, which eliminated S1P, and by a series of other experimental strategies (Matloubian et al. 2004; Pappu et al. 2007; Brinkmann 2009). As a result, T cells cannot enter the LN cortical sinuses and consequently fail to enter the medullary sinuses and exit the LN via the subcapsular space and efferent lymph (Grigorova et al. 2009). Administration of fingolimod mimicked the situation in ko mice with targeted deletion of S1P1 in hematopoietic cells (Matloubian et al. 2004) and upon binding to S1P1 led to receptor internalization in LN T cells and subsequent ubiquitinylation and degradation in the proteasome (Oo et al. 2007, 2011). Interestingly, despite the efficient trapping of T cells in LN, viral immune responses (Brinkmann 2009; Pinschewer et al. 2000), CNS immune surveillance (Bartholomäus et al. 2008), and the development of thymocytes did not appear to be compromised although the egress of thymocytes and the homing to peripheral lymphoid structures were delayed (Metzler et al. 2008). Treatment with fingolimod primarily traps CCR7+ CD45RA+ naïve and CCR7+ CD45RA− central memory T cells (TCM) in LN, while CCR7− CD45RA+ and CCR7− CD45RA− effector memory T cells (TEM) remain relatively unaffected (Mehling et al. 2008). The recruitment into and regular passage through LN of naïve and central memory T cells via CCR7 play an important role in the relative subtype specificity of fingolimod for lymphocyte homing to LN. Functional testing of fingolimod-exposed T cells demonstrated reduced production of IL-2 and proliferation, but unperturbed release of the proinflammatory cytokine IFN-γ (Brinkmann et al. 2001; Mehling et al. 2008). Overall, the effects of fingolimod on S1P/S1P1 interactions preferentially affect CD4+ naïve and TCM T cells, but left the CD4+ TEM population and CD8+ cytolytic T cells functionally unperturbed (Brinkmann 2009).
Regarding the effects on T cell subsets, particular attention has been given to Th17 cells, which are defined by the production of IL-17 and IL-22 and the expression of the signature transcription factor RORγt (Sallusto et al. 2012). Th17 cells play a prominent role in the EAE model (Peters et al. 2011), but their role is less clear in MS (Lovett-Racke et al. 2011). Th17 cells can cross the BBB and kill neurons and contribute to CNS inflammation by recruiting other immune cells (Kebir et al. 2007). S1PR inhibition by fingolimod efficiently traps Th17 cells in LN and reduces their numbers to less than 5 % in the peripheral blood of MS patients (Hohlfeld et al. 2011) and also in CNS and PNS tissue in experimental models. Other immune cells also express S1PRs (Mehling et al. 2008) and are affected by treatment with fingolimod. B cells are retained in bone marrow and LNs, show reduced germinal center reaction, and upon vaccination with KLH and a pneumococcal vaccine a delayed production of specific IgG was observed (Sinha et al. 2009; Boulton et al. 2012). In fingolimod-treated MS patients, comparable vaccination efficacy was observed during influenza vaccination, and influenza-specific T cell numbers and IgM titers increased in both fingolimod- and placebo-treated patients (Mehling et al. 2008). DCs express all five S1PRs, but it is currently not clear to what extent the redistrubition between antigen-draining LN, where DC numbers drop, and peripheral tissues are mainly due to alterations in T cell homing and numbers or to effects on DCs (Brinkmann 2009). In macrophages, which express S1P1 and S1P2, fingolimod reduces the production of proinflammatory cytokines (Durafourt et al. 2011; Michaud et al. 2010). Monocytes are also affected and show a decrease in the peripheral blood, increased numbers in LN and bone marrow, and reduced expression of CD40 and TNF-α (Lewis et al. 2013). Regarding natural killer (NK) cells, another innate immune cell population that plays important roles during anti-viral defense and also in immunoregulation, e.g., during pregnancy, global NK cell numbers are not altered during fingolimod treatment in MS, but a subset of NK cells expressing CD56bright CD62L+ CCR7+ are reduced, probably by trapping in LN as it is observed for CCR7-expressing T cells (Johnson et al. 2011). NK cell effector functions (cytokine release, cytolysis) are not affected in fingolimod-treated MS patients, but the migratory properties of these cells are reduced (Johnson et al. 2011).
In summary, S1P modulation in vitro and in vivo has a broad range of effects on immune cells, and the data from experimental models as well as from fingolimod-treated MS patients indicate that the trapping of CCR7-expressing naïve and central memory T cells in LN and their drop in peripheral blood play a major role in reducing inflammatory disease activity and relapse rates in MS. Whether the inhibition of certain subpopulations of immune cells, e.g., the above mentioned CD56bright NK cells, which have been shown to be beneficial in the context of anti-CD25 blockade, another treatment approach in MS, and/or the effects on T and B cells and monocytes are involved in rare infectious adverse events (see below) remains to be determined.
3.2 Possible Neuroprotective Effects
Eight different immunomodulatory treatments are now available for the treatment of RRMS, but none for the chronic progressive diseases (SPMS, PPMS). Alterations in CNS tissue, which are often referred to as neurodegeneration, including axonal transections, neuronal loss, glial proliferation, de- and partial remyelination, and metabolic changes in neurons occur already during the earliest stages of MS and are the main causes of long-term disability and chronic progression. Hence, treatments that are neuro- or myelin-protective and prevent astrogliosis are urgently needed. Different from the reduction of new inflammatory lesions, which can be easily measured by decrease in contrast-enhancing or new T2-weighted MRI lesions in the brain (Stone et al. 1995), it is more difficult to document the influence of a treatment on the neurodegenerative aspects. Several measures have been proposed including the reduction of brain atrophy and brain volume loss, which are considerably higher in MS than in healthy individuals (0.5–1 % annual loss in MS patients vs. 0.1–0.2 % in controls), the reduction of lesions with signs of permanent tissue damage (so-called T1 holes), the improved recovery with respect to T1 hypointensity of new MRI lesions (Barkhof et al. 2009), and the reduction of retinal nerve fiber layer thickness loss, which is measured by optical coherence tomography (OCT) (Young et al. 2013). Each of these is considered useful to document neuroprotection, but measuring small changes accurately over time is technically demanding, and therefore it has remained difficult to document neuroprotective effects for a given treatment.
When considering the cellular/molecular aspects of neuroprotection, it can be defined as the lack of newly occurring damage or improvement of function and structural integrity of already damaged neurons and axons. Neuroprotection may involve many different direct, i.e., functions of neurons/axons themselves, or indirect mechanisms, i.e., functions of cells such as astroglia that metabolize excitatory neurotransmitters and/or provide trophic support such as astrocytes and oligodendrocytes. The reduction of autoimmune inflammatory mechanisms in the brain also results indirectly in neuroprotection, but will not be considered in the following brief summary of potential neuroprotective effects of S1P/S1PR interactions and treatment with functional S1PR antagonists such as fingolimod. As summarized above, several lines of evidence from in vitro experiments with various CNS cells types including neurons and their precursors, astrocytes, oligodendrocytes and OPCs, microglia, and cerebrovascular endothelial cells, but also in vivo animal models point at potentially beneficial effects in MS, EAE, and other CNS diseases (see below). Further, S1P1 and S1P3 are expressed at higher levels in MS brain tissue (van Doorn et al. 2010) indicating primary or secondary contributions of S1P/S1PR signaling during the pathologic processes in MS. Support for mechanisms of fingolimod that may result in neuroprotection stem from the phase III clinical trials, in which an attenuation of brain volume loss by MRI was observed after 2 years (Cohen et al. 2010). Another measure, i.e., persistent T1 hypointensities, so-called T1 holes, which indicate focal permanent CNS tissue damage and probably primarily the destruction of axons (Kappos et al. 2010), has also been explored. The accrual of T1 hypointense lesions and their volume was attenuated after 2 years in the FREEDOMS study, i.e., fingolimod versus placebo (Kappos et al. 2010), but no significant change was observed in the TRANSFORMS study, i.e., fingolimod versus IFN-β1a, after one year of treatment (Cohen et al. 2010). Together, these data indicate that modulation of S1P/S1PR interactions by fingolimod not only exerts indirect neuroprotective effects via the reduction of CNS inflammation, but may also protect axons/neurons and also myelin directly.
3.3 Adverse Event Profile
Balancing the risks versus the benefits has become more and more important with the introduction of more effective therapies in MS. While the previous first-line therapies, IFN-β and GA, are only moderately effective, 20 years of clinical experience have shown that they are very safe, and serious adverse events (AEs) are extremely rare. The newer treatments of MS such as natalizumab, alemtuzumab, but also fingolimod show increased clinical efficacy, but this comes at the price of more frequent and more serious side effects, among the latter particularly notable the occurrence of PML in natalizumab-treated patients (Kleinschmidt-DeMasters et al. 2012). Considering the importance of S1P/S1PR signaling in almost every cell and tissue and the potent immunomodulatory activity of functional S1P inhibition by fingolimod, it is not too surprising that a treatment with such pleiotropic effects has also resulted in a number of AEs. During clinical testing, total lymphocyte numbers drop to about 50 % of the normal threshold and in most individuals return to normal numbers within 45 days after treatment cessation after long-term therapy, however, some patients remain lymphopenic for prolonged periods of time from reasons that are not understood (Mehling et al. 2011). Lymphocytopenia was observed in 1 % (0.5 mg/d) and 4 % (1.25 mg/d) of fingolimod-treated patients in the TRANSFORMS study (Cohen et al. 2010) and in 3.5 % (0.5 mg/d) and 5.4 % (1.25 mg/d) in the FREEDOMS study (Kappos et al. 2010). Other notable AEs included bradycardia, arrhythmias and atrioventricular blocks, macular edema, epilepsia, a hint toward increased rates of malignancies or premalignancies, and abnormal liver enzymes. Probably as a consequence of the immunomodulatory effects, an increase in herpes virus infections, particularly those with varizella zoster virus (VZC) and herpes simplex virus type 1 (HSV1) were noted, while no general increase of infections was found. Two deaths, one from generalized VZV infection and one with HSV1 encephalitis, occurred in the TRANSFORMS study (Cohen et al. 2010), and increased reactivation of herpes viral infections have also been observed during postmarketing surveillance of fingolimod-treated MS patients. That the immune control of herpes viruses is reduced by fingolimod is supported by a recent study, which documented a reduction of VZV- and EBV-specific T cell reactivity and more frequent reactivation of latent VZV and EBV infection under fingolimod treatment (Ricklin et al. 2013). Furthermore, two cases of fatal hemophagocytic lymphohistiocytosis, which is characterized by a hyperinflammatory state due to uncontrolled T cell, macrophage, and histiocyte activation, accompanied by excessive cytokine production (Rosado and Kim 2013) have recently been reported as another immune system-related AE (Novartis, Adverse Even reporting). These occurred after 9 and 15 months fingolimod therapy with the approved dose of 0.5 mg/d, and both cases suffered from concomitant viral infections (Novartis, Adverse Even reporting). A case of hemorrhagic encephalitis with subsequent epilepsy (Leypoldt et al. 2009) and the occurrence of HSV1 encephalitis [(Cohen et al. 2010), own unpublished case] under fingolimod indicate that the CNS-related mechanisms of action may in certain, predisposed patients contribute to CNS side effects. A rigorous pharmacovigilance program has therefore been started, and several safety measures are taken. Periodic dermatological and ophthalmological examinations and cardiac monitoring are required during fingolimod treatment, and patients are instructed with respect to the possibility of herpes viral reactivations, however, it is currently not clear how patients at risk to develop these side effects can be identified prospectively. In the case of the reactivation of infections with herpes viruses probably a number of the immune effects of fingolimod, i.e., perturbed migration and LN homing of T cells, B cells, and DCs, changes in the composition of NK cells (Johnson et al. 2011) and other as yet unknown factors contribute. Further research in this direction to develop biomarkers and other means to define risk profiles are clearly needed. The fact that very specific infectious complications occur with specific drugs, e.g., PML under natalizumab and increased reactivation of herpes virus infections under fingolimod, probably also teach us important lessons, which components of the immune system play important roles during physiological immune control of these specific agents and how to avoid these complications in the future.
4 Potential Role of S1P and S1P Receptors in Models of Neurological Diseases and CNS Tumors
Fingolimod is, at the moment, the only approved S1P/S1PR-modulating agent, and MS is the only indication. There is, however, also promising data from using fingolimod or examining the role of S1P/S1PR interactions in models of other CNS diseases and from studies in the context of glioma. B cells that are deficient in S1PR expression fail to disseminate prion proteins (Mok et al. 2012), in animal model of heart failure interrupting TNF-α/S1P signaling inhibited vasoconstriction and improved cerebral blood flow (Yang et al. 2012), elevated S1P levels are involved in ethanol-induced neuroapoptotic effects in the developing brain (Chakraborty et al. 2012), and fingolimod reduces CNS inflammation and promotes functional recovery in a spinal cord injury model (Lee et al. 2009). Further, fingolimod phosphate blocks neurotoxicity induced by amyloid-β aggregation via release of brain-derived neurotrophic factor (BDNF) (Doi et al. 2013). Effects that might be beneficial in the context of stroke are that fingolimod treatment results in improvement of long-term outcome in stroke models primarily via its anti-inflammatory rather than direct effects on neurons (Wei et al. 2011), the contribution of S1P/S1PR signaling in hypoxic preconditioning via pathways involving hypoxia-inducible factor (HIF), sphingosine kinase- and CCL2 signaling-related signaling (Wacker et al. 2012). Neuromodulatory effects such as antinociception, hypothermia, catalepsy, and reduced locomotion are also at least in part mediated by S1PR-mediated (Sim-Selley et al. 2009).
Several lines of evidence show that S1P/S1PR interactions play a role in brain tumors and that fingolimod may be promising in glioblastoma. Fingolimod induces apoptotic death of glioblastoma stem cells (Estrada-Bernal et al. 2012), and S1P regulates the invasive growth of glioblastoma cells via urokinase plasminogen activating mechanisms (Young et al. 2009; Bryan et al. 2008).
Together, these preliminary data indicate that fingolimod or newer, more specific S1P/S1PR modulating agents may be useful in other CNS diseases such as stroke, Alzheimer`s and other neurodegenerative diseases, traumatic brain/spinal cord injury, and in glioblastoma.
5 Conclusions
Research on the role of S1P/S1PR interactions in many cells and tissues including the CNS and, in parallel, the development and approval of the functional S1P antagonist fingolimod as the first oral immunomodulatory treatment of MS have been very rewarding. These studies have not only led to many fundamental findings regarding basic biological mechanisms in health and disease, but also opened a new area of therapeutics, which may be effective not only in MS, but also a number of other conditions. Further research is ongoing with respect to modulating S1P/SP1R interactions more specifically than with fingolimod, but also with respect to mechanisms that are involved in overall rare, but sometimes serious side effects.
References
Akahoshi N, Ishizaki Y, Yasuda H, Murashima YL, Shinba T, Goto K, Himi T, Chun J, Ishii I (2011) Frequent spontaneous seizures followed by spatial working memory/anxiety deficits in mice lacking sphingosine 1-phosphate receptor 2. Epilepsy Behav 22:659–665
Ascherio A, Munger K (2008) Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol 28:17–28
Barkhof F, Calabresi PA, Miller DH, Reingold SC (2009) Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 5:256–266
Bartholomäus I, Schläger C, Brinkmann V, Wekerle H, Flügel A (2008) Intravital 2-photon imaging of encephalitogenic effector cells during fingolimod (FTY720) treatment of experimental autoimmune encephalomyelitis. World Congress on Treatment and Research in Multiple Sclerosis (ECTRIMS), Montreal. Poster P7
Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A et al (2013) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45:1353–1360
Benarroch EE (2013) Microglia: multiple roles in surveillance, circuit shaping, and response to injury. Neurology 81:1079–1088
Bieberich E (2011) There is more to a lipid than just being a fat: sphingolipid-guided differentiation of oligodendroglial lineage from embryonic stem cells. Neurochem Res 36:1601–1611
Boulton C, Meiser K, David OJ, Schmouder R (2012) Pharmacodynamic effects of steady-state fingolimod on antibody response in healthy volunteers: a 4-week, randomized, placebo-controlled, parallel-group, multiple-dose study. J Clin Pharmacol 52:1879–1890
Brana C, Frossard MJ, Pescini Gobert R, Martinier N, Boschert U, Seabrook TJ (2013) Immunohistochemical detection of sphingosine-1-phosphate receptor 1 and 5 in human multiple sclerosis lesions. Neuropathol Appl Neurobiol
Brinkmann V (2007) Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115:84–105
Brinkmann V (2009) FTY720 (fingolimod) in multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 158:1173–1182
Brinkmann V, Chen S, Feng L, Pinschewer D, Nikolova Z, Hof R (2001) FTY720 alters lymphocyte homing and protects allografts without inducing general immunosuppression. Transplant Proc 33:530–531
Brinkmann V, Cyster JG, Hla T (2004) FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant 4:1019–1025
Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9:883–897
Bryan L, Kordula T, Spiegel S, Milstien S (2008a) Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta 1781:459–466
Bryan L, Paugh BS, Kapitonov D, Wilczynska KM, Alvarez SM, Singh SK, Milstien S, Spiegel S, Kordula T (2008b) Sphingosine-1-phosphate and interleukin-1 independently regulate plasminogen activator inhibitor-1 and urokinase-type plasminogen activator receptor expression in glioblastoma cells: implications for invasiveness. Mol Cancer Res 6:1469–1477
Callihan P, Hooks SB (2012) Sphingosine-1-phosphate signaling in neural progenitors. Methods Mol Biol 874:193–200
Callihan P, Zitomer NC, Stoeling MV, Kennedy PC, Lynch KR, Riley RT, Hooks SB (2012) Distinct generation, pharmacology, and distribution of sphingosine 1-phosphate and dihydrosphingosine 1-phosphate in human neural progenitor cells. Neuropharmacology 62:988–996
Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS (2012) Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA 109:15930–15935
Chakraborty G, Saito M, Shah R, Mao RF, Vadasz C, Saito M (2012) Ethanol triggers sphingosine 1-phosphate elevation along with neuroapoptosis in the developing mouse brain. J Neurochem 121:806–817
Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G et al (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA 108:751–756
Chun J, Hartung HP (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33:91–101
Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C (2007) The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 323:626–635
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380:1829–1839
Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J (2002) Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol 22:6921–6929
Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, Barde YA (2012) Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci USA 109:14230–14235
Doi Y, Takeuchi H, Horiuchi H, Hanyu T, Kawanokuchi J, Jin S, Parajuli B, Sonobe Y, Mizuno T, Suzumura A (2013) Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLoS ONE 8:e61988
Dubin AE, Herr DR, Chun J (2010) Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J Neurosci 30:7300–7309
Durafourt BA, Lambert C, Johnson TA, Blain M, Bar-Or A, Antel JP (2011) Differential responses of human microglia and blood-derived myeloid cells to FTY720. J Neuroimmunol 230:10–16
Edsall LC, Pirianov GG, Spiegel S (1997) Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci 17:6952–6960
Engelhardt B (2011) Neuroscience. Blood-brain barrier differentiation. Science 334:1652–1653
Estrada-Bernal A, Palanichamy K, Ray Chaudhury A, van Brocklyn JR (2012) Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma. Neuro Oncol 14:405–415
Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, Li XK (2003) Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther 305:70–77
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI et al (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367:1098–1107
Goldmann T, Prinz M (2013) Role of microglia in CNS autoimmunity. Clin Dev Immunol 2013:208093
Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG (2009) Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol 10:58–65
Groves A, Kihara Y, Chun J (2013) Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 328:9–18
Haghikia A, Hohlfeld R, Gold R, Fugger L (2013) Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol Med 19:309–319
Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH (2010) Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 41:368–374
Hohlfeld R, Barkhof F, Polman C (2011) Future clinical challenges in multiple sclerosis: relevance to sphingosine 1-phosphate receptor modulator therapy. Neurology 76:S28–S37
Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J (2002) Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem 277:25152–25159
Jackson SJ, Giovannoni G, Baker D (2011) Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation 8:76
Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K et al (2005) Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci 25:1459–1469
Johnson TA, Evans BL, Durafourt BA, Blain M, Lapierre Y, Bar-Or A, Antel JP (2011) Reduction of the peripheral blood CD56(bright) NK lymphocyte subset in FTY720-treated multiple sclerosis patients. J Immunol 187:570–579
Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B (2007) Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia 55:1656–1667
Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S (2007) Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol 27:3429–3440
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, Chiba K (2005) FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol 2:439–448
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175
Kim HJ, Miron VE, Dukala D, Proia RL, Ludwin SK, Traka M, Antel JP, Soliven B (2011) Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J 25:1509–1518
Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, Kobayashi E, Hoshino Y, Yatomi Y, Sakata Y (2007) Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells 25:115–124
Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y (2008) Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke 39:3411–3417
Kleinschmidt-DeMasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T (2012) Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J Neuropathol Exp Neurol 71:604–617
Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T et al (2007) Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem 282:10690–10696
Lassmann H (2013) Multiple sclerosis: lessons from molecular neuropathology. Exp Neurol
Lee KD, Chow WN, Sato-Bigbee C, Graf MR, Graham RS, Colello RJ, Young HF, Mathern BE (2009) FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma 26:2335–2344
Lewis ND, Haxhinasto SA, Anderson SM, Stefanopoulos DE, Fogal SE, Adusumalli P, Desai SN, Patnaude LA, Lukas SM, Ryan KR et al (2013) Circulating monocytes are reduced by sphingosine-1-phosphate receptor modulators independently of S1P3. J Immunol 190:3533–3540
Leypoldt F, Munchau A, Moeller F, Bester M, Gerloff C, Heesen C (2009) Hemorrhaging focal encephalitis under fingolimod (FTY720) treatment: a case report. Neurology 72:1022–1024
Lovett-Racke AE, Yang Y, Racke MK (2011) Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta 1812:246–251
Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, Anderson KJ, Roper SN, Lee N (2001) An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci 14:203–209
Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355–360
Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, Kristofic C, Kuhle J, Lindberg RL, Kappos L (2008) FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 71:1261–1267
Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A (2011) Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology 76:S20–S27
Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ (2006) Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos 34:1480–1487
Metzler B, Gfeller P, Wieczorek G, Li J, Nuesslein-Hildesheim B, Katopodis A, Mueller M, Brinkmann V (2008) Modulation of T cell homeostasis and alloreactivity under continuous FTY720 exposure. Int Immunol 20:633–644
Michaud J, Im DS, Hla T (2010) Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol 184:1475–1483
Miron VE, Schubart A, Antel JP (2008a) Central nervous system-directed effects of FTY720 (fingolimod). J Neurol Sci 274:13–17
Miron VE, Hall JA, Kennedy TE, Soliven B, Antel JP (2008b) Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod. Am J Pathol 173:1143–1152
Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP (2010) Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 176:2682–2694
Miron VE, Durafourt BA, Antel JP, Kennedy TE (2012) Assessment of sphingosine-1-phosphate receptor expression and associated intracellular signaling cascades in primary cells of the human central nervous system. Methods Mol Biol 874:141–154
Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL (2005) Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25:11113–11121
Mok SW, Proia RL, Brinkmann V, Mabbott NA (2012) B cell-specific S1PR1 deficiency blocks prion dissemination between secondary lymphoid organs. J Immunol 188:5032–5040
Mullershausen F, Craveiro LM, Shin Y, Cortes-Cros M, Bassilana F, Osinde M, Wishart WL, Guerini D, Thallmair M, Schwab ME et al (2007) Phosphorylated FTY720 promotes astrocyte migration through sphingosine-1-phosphate receptors. J Neurochem 102:1151–1161
Nishimura H, Akiyama T, Irei I, Hamazaki S, Sadahira Y (2010) Cellular localization of sphingosine-1-phosphate receptor 1 expression in the human central nervous system. J Histochem Cytochem 58:847–856
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI (2007) Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J 21:1503–1514
O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A et al (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303
Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T (2007) Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem 282:9082–9089
Oo ML, Chang SH, Thangada S, Wu MT, Rezaul K, Blaho V, Hwang SI, Han DK, Hla T (2011) Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice. J Clin Invest 121:2290–2300
Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG et al (2007) Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316:295–298
Peters A, Lee Y, Kuchroo VK (2011) The many faces of Th17 cells. Curr Opin Immunol 23:702–706
Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM (2000) FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol 164:5761–5770
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Rao TS, Lariosa-Willingham KD, Lin FF, Palfreyman EL, Yu N, Chun J, Webb M (2003) Pharmacological characterization of lysophospholipid receptor signal transduction pathways in rat cerebrocortical astrocytes. Brain Res 990:182–194
Rau CR, Hein K, Sattler MB, Kretzschmar B, Hillgruber C, McRae BL, Diem R, Bahr M (2011) Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis. Am J Pathol 178:1770–1781
Ricklin ME, Lorscheider J, Waschbisch A, Paroz C, Mehta SK, Pierson DL, Kuhle J, Fischer-Barnicol B, Sprenger T, Lindberg RL et al (2013) T-cell response against varicella-zoster virus in fingolimod-treated MS patients. Neurology 81:174–181
Rosado FG, Kim AS (2013) Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol 139:713–727
Rossi S, Lo Giudice T, De Chiara V, Musella A, Studer V, Motta C, Bernardi G, Martino G, Furlan R, Martorana A et al (2012) Oral fingolimod rescues the functional deficits of synapses in experimental autoimmune encephalomyelitis. Br J Pharmacol 165:861–869
Sallusto F, Zielinski CE, Lanzavecchia A (2012) Human Th17 subsets. Eur J Immunol 42:2215–2220
Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, Satin LS, Welch SP, Selley DE (2009) Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J Neurochem 110:1191–1202
Sinha RK, Park C, Hwang IY, Davis MD, Kehrl JH (2009) B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity 30:434–446
Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, Traynelis SF, Hepler JR (2003) Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol 64:1199–1209
Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747
Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407
Stone LA, Smith ME, Albert PS, Bash CN, Maloni H, Frank JA, McFarland HF (1995) Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: relationship to course, gender, and age. Neurology 45:1122–1126
Takasugi N, Sasaki T, Ebinuma I, Osawa S, Isshiki H, Takeo K, Tomita T, Iwatsubo T (2013) FTY720/fingolimod, a sphingosine analogue, reduces amyloid-beta production in neurons. PLoS ONE 8:e64050
Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T (2003) Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience 116:1053–1062
Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, Bigbee JW, Spiegel S (2004) Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol 166:381–392
Vajkoczy P, Laschinger M, Engelhardt B (2001) Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest 108:557–565
van Doorn R, van Horssen J, Verzijl D, Witte M, Ronken E, van Het Hof B, Lakeman K, Dijkstra CD, van Der Valk P, Reijerkerk A et al (2010) Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 58:1465–1476
van Doorn R, Nijland PG, Dekker N, Witte ME, Lopes-Pinheiro MA, van het Hof B, Kooij G, Reijerkerk A, Dijkstra C, van van der Valk P et al (2012a) Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol 124:397–410
van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, van der Pol SM, Geerts D, van Horssen J, van der Valk P, van der Kam E et al (2012b) Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J Neuroinflammation 9:133
Wacker BK, Perfater JL, Gidday JM (2012) Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. J Neurochem 123:954–962
Waubant E, Goodkin DE, Gee L, Bacchetti P, Sloan R, Stewart T, Andersson PB, Stabler G, Miller K (1999) Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology 53:1397–1401
Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, Mandala S, Chun J, Rao TS (2004) Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol 153:108–121
Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V et al (2011) Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol 69:119–129
Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL (2008) Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet 17:2257–2264
Wu C, Leong SY, Moore CS, Cui QL, Gris P, Bernier LP, Johnson TA, Seguela P, Kennedy TE, Bar-Or A et al (2013) Dual effects of daily FTY720 on human astrocytes in vitro: relevance for neuroinflammation. J Neuroinflammation 10:41
Yang J, Noyan-Ashraf MH, Meissner A, Voigtlaender-Bolz J, Kroetsch JT, Foltz W, Jaffray D, Kapoor A, Momen A, Heximer SP et al (2012) Proximal cerebral arteries develop myogenic responsiveness in heart failure via tumor necrosis factor-alpha-dependent activation of sphingosine-1-phosphate signaling. Circulation 126:196–206
Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N (1992) Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356:63–66
Young N, Pearl DK, van Brocklyn JR (2009) Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol Cancer Res 7:23–32
Young KL, Brandt AU, Petzold A, Reitz LY, Lintze F, Paul F, Martin R, Schippling S (2013) Loss of retinal nerve fibre layer axons indicates white but not grey matter damage in early multiple sclerosis. Eur J Neurol 20:803–811
Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS (2004) Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia 45:17–27
Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV (2010) Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood 115:4963–4972
Acknowledgments
R. Martin, M. Sospedra and the Neuroimmunology and MS Resarch Section are supported by the Clinical Research Priority Program MS of the University Zurich.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Martin, R., Sospedra, M. (2014). Sphingosine-1 Phosphate and Central Nervous System. In: Oldstone, M., Rosen, H. (eds) Sphingosine-1-Phosphate Signaling in Immunology and Infectious Diseases. Current Topics in Microbiology and Immunology, vol 378. Springer, Cham. https://doi.org/10.1007/978-3-319-05879-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-05879-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05878-8
Online ISBN: 978-3-319-05879-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)