Abstract
Global warming is caused mainly by the excessive use of fossil fuels (coal, oil, diesel, gasoline, etc.) that emit millions of tons of pollutants into the environment. Besides, the fact that these fossil fuels are nonrenewable resources promotes the research in cleaner energy sources. In this chapter are presented two different technologies that could be introduced in the sugarcane industry to generate electricity and other kinds of clean fuel (producer gas and hydrogen); the case of hydrogen production by ethanol steam reforming and biomass gasification, which appear like promising technologies for energy generation in the sugarcane industry. Currently, most hydrogen is obtained from natural gas through a process known as reforming. Other technologic alternatives that may improve the supply of energy to the sugarcane industry is the use of biomass gasifiers in association with cogeneration system utilizing combined cycles to produce simultaneously electricity and heat, a technology known as Biomass Integrated Gasification/Gas Turbine Combined Cycle (BIG/GTCC). Cogeneration, has been accepted by different industries and has gained great application in the sugarcane industry, where the thermic and electric demands are favorable to use this type of energy system. The main fuel used in the process is sugarcane bagasse which is a by-product of sugar and ethanol production processes; the obtained energy is used in the form of mechanical power, electric power, and saturated steam in the processes. The surplus electricity can be sold. Technical, economical, and ecological analyses were performed for introduction of hydrogen production and BIG/GTCC in the sugarcane industry, using bagasse as fuel, in order to identify the better scenarios for electricity and heat generation. The introduction of these technologies will engender innovations in the sugarcane industry and will promote the sector development and as main results will increase electricity production with an economic and ecologic sustainable approach.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
18.1 Introduction
The gradual increase in energy demand and environmental pollution caused by combustion processes of fossil fuels, has made necessary the development of new technologies employing alternative fuels, thus facilitating the reduction of dependence on fossil fuels oil, natural gas, and coal. Generally, the combustion process of fossil fuels produce greenhouse gases such as CO2, NO x , and others that increases the temperature. Energy consumption, mainly in developed countries, has reached incredible limits, which has led, combined with other factors, the increase in global warming, with large worldwide implications. Biomass is a renewable resource that plays a substantial role in the sustainable energy future. Currently, the sensitivities to environmental issues and energy security have led to the promotion of the use of endogenous renewable energy sources. Biomass as an energy source covers 10 % (50 EJ) of the global primary energy source (IEA 2009). Sugarcane is cultivated in more than 80 countries and the by-products obtained from the sugar production process represent a great biomass potential. The harvest of sugarcane in the producing countries is about 1.2 Gt and potentially its residue can be used for electric power production of 300 TW h y−1 (Filippis et al. 2004). Besides, nearly 95 % of hydrogen is produced from fossil-based materials, with steam reforming of methane being the most used and usually the most economical option. However, in this process, carbon is transformed into CO2 and released into the atmosphere, leading to global climate change (Navarro et al. 2005); thus the main interest is focusing on alternative methods for the production of hydrogen from renewable energy sources. These processes are being investigated as long-term solutions, while generation of hydrogen from biomass has been recognized as a more feasible option for the near-term solution due to its renewable and carbon-neutral nature (Yang et al. 2006). In this chapter are presented two different technologies that could be introduced in the sugarcane industry to generate electricity and other kind of clean fuel (producer gas and hydrogen); specifically hydrogen production by ethanol steam reforming and biomass gasification, which are promising technologies for energy generation in the sugarcane industry.
18.2 Incorporation of Biohydrogen Production in Sugarcane Industry
Fuel Cell (FC) appears like a promising alternative technology for energy generation, since it is any efficient system that consists of an electrochemistry process. In this process, water, electricity, and heat are generated through the combination of hydrogen and oxygen (Silveira et al. 2009). Hydrogen can be produced from a variety of sources including water and biomass (Silveira et al. 2008). Currently, most hydrogen is made from natural gas through a process known as reforming.
18.2.1 The Steam Reforming Process
For hydrogen production, several technologies can be used. Steam reforming is one of the most common installed in chemical industries. The reforming process efficiency is a function of physical–chemical properties of feedstock, thermodynamic conditions (temperature and pressure of reaction), technical configurations of reformer (dimensions and catalysts), and feedstock and water flows. The reformer to be used depends on the fuel cell, which will use the reforming products. The fuel cell technology determines the hydrogen purity required. Steam reforming occurs in the presence of a catalyst, the syngas produced includes hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), among others. Some arrangements to minimize various reactions that can contribute to decrease the hydrogen production are necessary. Since this reaction is endothermic, heat from external sources is necessary. To minimize losses, several products of steam reforming, like the nonreacted fraction of reactants, might be utilized to heat up the reactants (Souza et al. 2006).
18.2.1.1 Ethanol Reforming Reactions
Souza et al. (2006) indicated that this way of reforming can be described through the following main reactions:
-
Global reaction. Ethanol reacts with steam in an endothermic reaction, taking place in the production of carbon dioxide and hydrogen, as shown in Eq. 18.1:
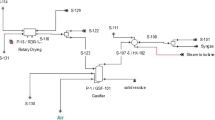
$$ {\text{C}}_{2} {\text{H}}_{5} {\text{OH}} + 3\,{\text{H}}_{2} {\text{O}} \to 2\,{\text{CO}}_{2} + 6\,{\text{H}}_{2} $$(18.1)Figure 18.1 shows inlet and outlet flows of ethanol steam reforming process, and Fig. 18.2 shows the prototype developed in São Paulo State University, by Energetic Systems Optimization Group (www.feg.unesp.br/gose).
Fig. 18.1 Inlet and outlet flows of ethanol steam reforming process (Silveira et al. 2009)
-
Ethanol steam reforming reaction. Equation 18.2 shows the reaction where the production of carbon monoxide and hydrogen occurs:
$$ {\text{C}}_{2} {\text{H}}_{5} {\text{OH}} + {\text{H}}_{2} {\text{O}} \to 2\,{\text{CO}} + 4\,{\text{H}}_{2} $$(18.2) -
Water Gas Shift Reaction. Since carbon monoxide damages fuel cell catalyst, an additional process is necessary to remove it. The Water Gas Shift Reaction (Eq. 18.3), is exothermic, reversible, and occurs at lower temperatures than the forming reaction:
$$ {\text{CO}} + {\text{H}}_{2} {\text{O}} \to {\text{CO}}_{2} + {\text{H}}_{2} $$(18.3) -
Methanation. Several chemical reactions occur simultaneously. Equation 18.4 shows methane production from carbon monoxide:
$$ {\text{CO}} + 3\,{\text{H}}_{2} \to {\text{CH}}_{4} + {\text{H}}_{2} {\text{O}} $$(18.4) -
Bouduard Reaction. This reaction (Eq. 18.5) describes carbon production from carbon monoxide decomposition:
$$ 2\,{\text{CO}} \to {\text{CO}}_{2} + {\text{C}} $$(18.5)
18.2.1.2 Hydrogen Production in Sugarcane Industry
Brazil has the largest and most successful biofuel programs in the world, involving production of ethanol from sugarcane and has the world’s first sustainable biofuel economy. Together, Brazil and the United States lead the industrial world in global ethanol production, accounting for 70 % of the world’s production (Silveira et al. 2009). The Brazilian sugarcane-based industry is far more efficient than the corn-based industry of USA. In the near future, the sugarcane industry of Brazil could be modified according to our purpose, as shown in Fig. 18.3. In this case, in addition to the production of sugar and ethanol, the Brazilian sugarcane industry will be able to produce biohydrogen.
The goal is innovation in the sugarcane industry production chain through incorporation of hydrogen production process by steam reforming of ethanol.
It is proposed to incorporate ethanol steam reforming to the traditional sugarcane industry process which is composed of extraction, juice treatment, evaporation, cooking, fermentation, and distillation to produce ethanol and sugar as well as electricity generation through cogeneration system, as shown on Fig. 18.3. These processes are described below:
-
Extraction. In this step the cane is cleaned and milled. The milling consists in breaking the hard structure of the cane and grinding it. To increase the amount of juice, water is added. The bagasse obtained from extraction is used in the boiler of the cogeneration system, as shown in Fig. 18.4 (Pellegrine 2009).
Fig. 18.4 Cogeneration system (Bernardo 2013)
-
Juice treatment. The juice is first strained to remove large particles. Then it is treated with chemical substances to modify the pH, coagulate the colloidal material (greases, proteins, etc.), and precipitate certain impurities (organic acids, sulfates, etc.). The purification process is chosen according to the sugar type that is desired to produce. After the addition of chemical substances, the mixture is heated with water vapor in high pressure. The insoluble particulate mass (mud) is separated by decantation (Silva 2010). Clarified juice goes to the evaporators without additional treatment. The mud is filtered and the filter cake is washed with water.
-
Sugar production. According to Castro (2001) the sugar production involves two steps: evaporation and cooking, as described below.
-
Evaporation. In this step the clarified juice is concentrated. First the juice is passed through heat exchangers to preheat and then to the evaporator stations, typically a series of five evaporators called multiple-effect evaporators. The concentrated juice (syrup) follows to the cooking step (Silva 2010).
-
Cooking. The syrup goes through the second phase of concentration until it takes the consistency of honey and begins to form sugar crystals. Once crystallization is complete, the massecuite is centrifuged and the crystallized sugar and honey are separated. The crystals obtained are of good quality and the syrup returns to the crystallization process. The end honey, or molasses, can be used as raw material for the fermentation of ethanol. The crystal sugar obtained goes through the refining process, where it is transformed into amorphous sugar, aiming to improve the purification and composition (Silva 2010).
Pellegrine (2009) advocates two stages for alcohol obtaining: fermentation and distillation.
-
Fermentation. The mud is diluted to correct the concentration and transferred to vats where the fermentation process takes place. In this stage are added nutrients, antiseptic, and yeast, mainly responsible for fermentation. After that, wine is obtained, which goes to the distillation process (Pellegrine 2009).
-
Distillation. The wine is directed to a decanter and after that to centrifuge where the yeast wine is obtained. It is transferred to a wine reservoir where the alcohol is separated through distillation processes (Silva 2010).
The cogeneration system is shown in Fig. 18.4.
In 18.4, after juice extraction, bagasse is directed to the boilers where it is burned. The steam from boiler goes to the steam turbine, which is connected to the electricity generator. In self-sufficient power plants, the surplus electricity can be sold to grid. As a result of the incorporation and the new configuration of the sugarcane industry will be produced hydrogen in addition to sugar, ethanol, and electricity.
18.3 Incorporation of Biomass Gasification in Sugarcane Industry
Traditionally, sugar mills use bagasse and cane trash with high moisture content as fuel for low pressure boilers to generate steam, using a conventional condensing–extraction steam-turbine (CEST) technology to provide the plant with heat, electricity, and mechanical power. The recent years have seen more modern systems for burning bagasse in suspension that allow to raise the steam pressure and temperature for the purpose of obtaining a higher electric power cycle cogeneration. The plant thermal efficiency is usually in the 15–30 % range, consequently the size of conventional combined heat and power generation plants from bagasse have been limited by these low efficiencies and the amount of fuel within an economical transportation radius.
The BIG/GTCC technology has been identified by several authors (Babu 1995; Larson et al. 2001) as an advanced technology with the potential to be cost-competitive with CEST technology using the biomass by-products of sugarcane processing as fuel, while dramatically increasing the electricity generated per unit of sugarcane processed. This type of technology does not require a large investment demand and can be inserted into the production process of ethanol (Sánchez Prieto and Nebra 2001).
18.3.1 Biomass Gasification Process
Gasification is a thermochemical process in which a carbonaceous substrate is transformed into a fuel gas, through a number of reactions that take place at high temperature in the presence of a gasifying agent (air, oxygen, and/or water vapor). The gasification process includes the following steps:
-
Drying is an endothermic process and to achieve acceptable efficiencies the maximum amount of moisture in the solid is limited between 20 and 30 % by weight. The drying process begins at temperatures below 100 °C and can be expressed by the following reaction:
$$ \text{Biomass}_{{\text{wet}}} +\text{Heat} \leftrightarrow \text{Biomass}_{{\text{dry}}} + \text{ H}_{\text{2}} \text{O}_{{\text{(g)}}} $$(18.6) -
Pyrolysis is an endothermic process, consists of biomass thermal degradation, and is developed at temperatures between 200 and 600 °C. The pyrolysis products are carbon, condensable gases (light and heavy hydrocarbons), and noncondensable gases (methane, water vapor, carbon monoxide, hydrogen, and carbon dioxide). The reaction can be represented as
$$ \text{Biomass}_{{\text{dry}}} +\text{Heat } \leftrightarrow \text{Char} + {\text{CO}} + {\text{CO}}_{\text{2}} + \text{H}_{\text{2}} + \text{C}_{\text{2}} \text{H}_{\text{4}} + \text{Tar} $$(18.7) -
Oxidation. The oxidation step is important from the energy point of view, since it is the exothermic reaction that releases the energy required to develop the gasification process. The reaction represented by this phase would be:
$$ \text{Pyrolisis}\;\text{products} + {\text{O}}_{\text{2}} \leftrightarrow \text{CO}_{\text{2}} + \text{CO} + {\text{H}}_{\text{2}} \text{O}_{{\text{(g)}}} + \text{Heat} $$(18.8) -
Reduction. The reduction step begins to develop significantly when the solid reaches a temperature around 700 °C. Thus, the char reacts with water vapor, carbon dioxide, and hydrogen, and gases react together to produce the final gas mixture, obtained as a result of the following reactions:
$$ {\text{Char}} + {\text{H}}_{ 2} {\text{O}} + {\text{O}}_{ 2} \leftrightarrow {\text{CO}}_{2} + {\text{Heat}} + {\text{Ash}} $$(18.9)$$ \text{Char} + {\text{Heat}} + {\text{CO}}_{{2}} \leftrightarrow 2 \text{CO} + {\text{Ash}} $$(18.10)$$ \text{Char} + {\text{H}}_{{2}} \text{O} + {\text{Heat}} \leftrightarrow \text{CO} + {\text{H}}_{{2}} + \text{Ash} $$(18.11)
The producer gas is the principal product of gasification, and its lower heating value (LHV) varies depending on the composition of biomass and the gasifying agent employee. Using air as the gasifying agent, the LHV of the producer gas is in the range between 4 and 6 MJ/Nm3 and using water vapor or oxygen the LHV is between 8 and 20 MJ/Nm3 (Reed et al. 2005).
18.3.2 Gasification in Fluidized Bed Reactors
Fluidized bed reactors are those in which the gasifying agent circulates inside them at a rate such that a bed is in a state of fluidization, existing inside the gasifier several conditions that intensify the transfer of energy and material between the fuel and gas. There are two main categories within these types of gasifiers: bubbling and circulating. In the bubbling fluidized beds, the fluidizing velocity–gasifying agent is sufficiently low as there is no significant movement of solid. By contrast, in the circulating fluidized bed, the velocity of the agent is much higher resulting in a solids circulation. This solid is recirculated to the reactor by the use of a cyclone return system to the gasifier. The main advantages of fluidized beds include better control of temperature and reaction rates, high specific capacity, potential scaling to larger sizes, and adaptation to changes of biomass. On the contrary, show moderate–high tars and particulates levels in the exhaust gas and the fuel conversion are not as high as in the fixed bed gasifiers. A comparison between bubbling and circulating fluidized bed gasifiers is shown in Table 18.1 (Williams et al. 1995).
18.3.3 Biomass Gasification Technology for Cogeneration of Heat and Power
Cogeneration study can be divided into three different types of cycles. The conventional cycle in which steam is used at low pressure and temperature and the steam and power generated is just enough for own consumption of the plant. A second cycle is the advanced cycle with similar configuration to conventional cycle but operating with a higher pressure and temperature, which results in significantly greater generation of electricity than the needs of the sugar factory; the excess energy can be sold to external consumers. The third cycle is the BIG/GTCC and also generates an excess of electricity. The combined cycle system is the simplest scheme used for cogeneration, shown in Fig. 18.5. It employs a gas turbine, a heat recovery steam generator without supplementary firing, and steam-turbine (Silveira et al. 2006).
18.3.4 Sugarcane Bagasse as Biomass
There are several studies on the use of sugarcane bagasse as fuel in gasification processes. Olivares (1995) studied different types of bagasse with the objective of introducing it as fuel in a fluidized bed gasifier. Bagasse is a material with high fiber content and low density and has an extensive range of sizes. It exits the sugar production process with a moisture content of approximately 50 % (wet basis); for this reason, a pre-treatment process is necessary that includes drying, crushing, and others in order to improve their properties and facilitate the feeding process to fluidized bed reactors.
One of the principal parameters to evaluate the quality of bagasse is the moisture content because the more humid bagasse will decrease its lower calorific value (LHV), and therefore it will has less available energy for the same amount of fuel.
Table 18.2 shows the main physical and chemical properties of sugarcane bagasse reported by Jenkins et al. (1998). Bagasse is classified as a fuel with high reactivity due to its high content of volatiles and low ash content, making it a good feedstock for gasification.
18.3.5 Combined Cycle Associated with a Fluidized Bed Gasifier
In a combined cycle, the fuel combustion provides the mechanical energy to the electric generator and the exit gases from the combustion are directed to a heat recovery steam generator to produce steam; this steam will drive a steam turbine that will be linked the other electricity generator. It is generally employed in this type of cycle-to-cycle Brayton combination with a Rankine cycle type (Diniz et al. 2013). To have the possibility of entering the gasifier in this cycle, there must be previous drying since the sugarcane bagasse has relative humidity around 50 % in natura (Olivares et al. 1995). It is also necessary to clean the producer gas generated, since it contains a load of particulate and tar, as shown in Fig. 18.6.
Research has shown the potential of BIG/GTCC-based systems to be competitive with, if not superior to, conventional combustion power plants because of their higher efficiency, superior environmental performance, and competitive cost (Reed et al. 2005). However, much of the advancements are still under research and development. BIG/GTCC is a combination of two leading technologies: gasification and gas turbine combined cycle. The gasification portion of the BIG/GTCC plant produces a clean gas which fuels the gas turbine. For this system, the gasification stage is carried out in a fluidized bed. Typical operating temperature of a fluidized bed is 800–850 °C. Air is blown through the bed at a sufficient velocity to keep the bed materials in a state of suspension. The fuel particles are introduced at the bottom of the reactor, very quickly mixed with the bed material, and almost instantaneously heated up to the bed temperature and hence the subsequent producer gas generation. After the producer gas has left the fluidized bed chamber, it goes through a cleaning unit. The gas after the cleaner unit is then led to a boost compressor that compresses it to the gas turbine combustion chamber pressure conditions. The exhaust heat from the combustion turbine is recovered in the heat recovery steam generator to produce steam. This steam then passes through a steam turbine to power another generator, which produces more electricity. The combined cycle is more efficient than conventional power generating systems because it reuses waste heat to produce more electricity (Okure et al. 2006).
18.3.5.1 Energy Analysis of the Integration of Gasification of Bagasse in the Sugar and Alcohol Sector
The energy analysis comprises the study based on the first law of thermodynamics, the law of conservation of energy. This type of study is universally valid and is used in the mass and energy balances in the gasifier and other components of the BIG/GTCC system. For realization of this energy, analysis is determined of the operating parameters and efficiencies of the process and its components. However, only the use of the first law of thermodynamics proves to be insufficient in subsequent economic evaluation of an energy system, because it does not estimate the amount of energy available for conversion into work or power. Given this, the study based on only the first law of thermodynamics gives us an incomplete analysis of the potential energy of a system.
18.3.5.2 Exergetic Analysis of Integration of Gasification of Bagasse in the Sugar and Alcohol Sector
Given the limitations of the first law in formulating the quality and quantity of useful energy in a system, the concept of Exergy was created from the second law of thermodynamics. According to Tuna (1999), Exergy is that portion of noble energy that can be completely converted into work reversibly. However, the exergy can be defined as the maximum useful work that can be obtained by an energy carrier (Tsatsaronis 1993). The exergy inefficiency of a system consists in a destruction of exergy associated with irreversibilities. The irreversibility in a system can be decomposed into internal irreversibility, known as the Second Law of Thermodynamics as destruction of energy and external irreversibility, which is the exergy loss to the environment, developing out of the control volume selected for thermodynamic analysis (Valero et al. 2011). The maximum improvement in exergy efficiency for a process or system is obviously determined when the exergy loss or irreversibility is minimized, the latter being determined by the following equation (Sozen et al. 2002; Utlu et al. 2006):
Exergy analysis or even availability analysis is then drawn in this way to achieve the goal of a more effective use of energy resource as it enables the location, cause, and true magnitude of waste and loss. Such information can be used in the design of efficient energy systems and to increase the performance of existing systems. Exergy analysis also provides a broader view of the problem under consideration, avoiding conclusions based purely on the application of the first law of thermodynamics. Tuna (1999) emphasizes that the analysis of first and second law are not competing, but complementary, and together contribute to a consistent assessment of the thermal system.
18.4 Economic Analysis
In both technologies the methodology to make the economic analysis is similar and is based on engineering economics calculations developed by Silva (2010), who considered the sugarcane industry producing hydrogen using ethanol and gasification of electricity generated in the gas turbine and steam turbine by use of producer gas from bagasse gasification. In order to reach this proposal, an economic analysis based on the investment of the hydrogen production system and BIG/GTCC system were developed considering the input costs, operating cost, maintenance cost, operation period, interest rate, and annuity factor.
The global equation for hydrogen cost is shown in the following equation:
The annual cost of obtaining electricity (Cel), US$/kWh, for each selected system is given as
where:
k is the amortization period or pay-back, given in years. \( C_{{{\text{H}}_{2}}} \)—Hydrogen production cost (US$/kWh); \( C_{\text{el}} \)—Electricity production cost (US$/kWh); Invref—Reference investment for hydrogen production (×104 US$); f—Annuity factor (1/year); H—Equivalent period of operation (h/year); \( E_{{{\text{H}}_{2}}} \)—Energy provided by Hydrogen (kW); C op—Operational cost (US$/kWh); C man—Maintenance cost (US$/kWh).
Operational cost using bagasse as fuel is shown in Eq. 18.18, and the operational cost using electricity is shown in Eq. 18.19. According to Kothari et al. (2008), the maintenance cost of steam reformer was estimated as 3 % of investment.
where:
- E fuel :
-
Energy provided by sugarcane bagasse (kW);
- C fuel :
-
Fuel cost (sugarcane bagasse) (US$/kWh);
- E EtOH :
-
Energy provided by ethanol (kW);
- C EtOH :
-
Ethanol cost (US$/kWh);
- E Elet :
-
Electricity consumed by reformer (kW);
- C Elet :
-
Electricity cost (US$/kWh).
The investment cost (acquisition cost of equipment, installation cost) to produce steam covers the cost of system gas turbine (compressor, combustion chamber, gas turbine electric generator, and other accessories), the heat recovery steam generator is considered as separate module. Thus, the following equation is used to calculate the investment to be made:
For investment cost of heat recovery steam generator (Ivcr) without supplemental fuel burning is used Eq. 18.21, defined as the technique of Boehn (1987) according to the steam production in (kg/h), with a multiplicative factor of 10 % related to the cost of installation of the boiler recovery and valid for production values higher than 800 kg/h and less than 4000 kg/h.
The equation final investment cost becomes:
where:
C—Equipment cost for an interest capacity S; m—Incidence factor indicating the economics scale (0.5–1.0); Cr—Equipment cost for a reference capacity Sr.
Silva (2010) has adapted the reference investment for steam reforming process with hydrogen production range of 1 up to 1500 (Nm3/h), resulting in Eq. 18.24
The expected annual revenue is calculated as the sum of earnings or annual benefits due to the installation of a system (Silveira and Tuna 2003, 2004).
18.5 Ecological Analysis
At present, practically all known forms of energy production have some kind of interference in the environment. Due to this fact using biomass gasification combined with a cogeneration system is a set of recommended alternative energy, from the environmental point of view.
18.5.1 Ecological Efficiency
The ecological efficiency analysis is based on calculations of equivalent carbon dioxide [(CO2) e ], and pollution indicator (Πg) for determining the ecological efficiency of the process of hydrogen production by ethanol steam reforming, and for the BIG/GTCC system.
18.5.2 Determination of Equivalent Carbon Dioxide (CO2e)
The equivalent carbon dioxide depends on the emission of SO2, NOx, and PM, and can be determined using Eq. 18.25.
18.5.3 Determination of Pollution Indicator \( \left( {\varPi_{P} } \right) \)
The pollution indicator \( \left( {\Pi_{P} } \right) \) is the ratio between the amount of CO2e in kg and the power supplied by the producer gas and for the hydrogen production it is shown in Eq. 18.26 (Silveira et al. 2012).
18.5.4 Determination of Ecological Efficiency \( \left( \varepsilon \right) \)
Ecological efficiency is defined as an indicator for evaluating the performance of a particular system, considering the emissions of pollutants burning 1 kg of fuel. Their values vary between 0 and 1, where the higher the vicinity of 0 means the greater the environmental impact, and if it is proximate to 1, indicates that it is nonpolluting (zero environmental impact). The ecological efficiency can be determined using Eq. 18.27 (Silveira et al. 2012).
18.5.5 Calculation of CO2 Emissions from Combustion Process of Sugarcane Bagasse
The CO2 emissions from 1 kg of fuel can be calculated according to Eq. (18.28).
where:
\( M_{\text{CO}_{2}}\)—CO2 emissions (\( \text{kg}_{\text{CO}_{2}}\)/kgfuel); Molar mass of fuel (bagasse) (kg/kgmol).
The molar mass of bagasse can be determined based on the elemental composition (Table 18.2). Therefore, the molar mass of bagasse can be calculated through Eq. 18.27.
18.6 Conclusions
Hydrogen, the principal energy carrier to fuel cells, can be produced through various ways, but ethanol steam reforming is an alternative to guarantee the volume of production necessary in the Brazilian case. The integration or association of hydrogen production with sugar industry, can certainly put Brazil in a good classification in the “Hydrogen Era,” in the near future. Similarly in terms of ecological efficiency, the fluidized bed gasifier operating with bagasse is an environmentally friendly way, with high ecological efficiency to produce energy in the sugarcane industry. This technology proves that this type of combine cycle is an excellent alternative to the traditional electric power generation technology, based on the Rankine cycle, used in this industry for electricity and heat generation. Thus, these technologies can be inserted with energy and environmental gains in the sugarcane industry.
References
Babu S (1995) Thermal gasification of biomass technology development: end of task report for 1992 to 1994. Biomass Bioenergy 9(1–5):271–285
Bernardo A (2013) Participation of sugar cane mills in power generation of the country could be six times greater. GalileuMagazine. http://revistagalileu.globo.com/Revista/Common/0,,ERT326727-18537,00.html
Boehm FR (1987) Design analysis of thermal systems. Wiley, New York, p 173
Castro HF (2001) Sugar industry. Industrial chemical processes II. Handout 2, Lorena College of Engineering, Sao Paulo University (USP), Portuguese
Diniz PT, Silveira JL, Tuna CE, Lamas WQ (2013) Energetic, ecologic and fluid-dynamic analysis of a fluidized bed gasifier operating with sugar cane bagasse. Applied Thermal Engineering, Accepted Manuscript, 15 March (in press)
Filippis P, Borgianni C, Paolucci M, Pochetti F (2004) Gasification process of Cuban bagasse in two-stage reator. Biomass Bioenergy 27:247–252
IEA Bioenergy Report (2009). www.ieabioenergy.com
Jenkins BM, Baxter LL, Miles TR Jr, (1998) Miles TR combustion properties of biomass. Fuel Process Technol 54:17–46
Kothari R, Buddhi D, Sawhney RL (2008) Comparison of environmental and economic aspects of various hydrogen production methods. Renew Sustain Energy Rev 12:553–563
Larson ED, Robert HW, M. Regis, LV Leal (2001) A review of biomass integrated-gasifier/gas turbine combined cycle technology and its application in sugarcane industries, with an analysis for Cuba. Energy Sustain Dev 5(1):54–76
Navarro RM, Álvarez Galván MC, Cruz Sánchez Sánchez M, Rosa F, Fierro JLG (2005) Production of hydrogen by oxidative reforming of ethanol over Pt catalysts supported on Al 2O3Al2O3 modified with Ce and La. App Catal B Environ 55:229–241
Okure MAE, Musinguzi WB, Nabacwa BM, Babangira G, Arineitwe NJ, Okou RA (2006) Novel combined heat and power (CHP) cycle based on gasification of bagasse. In: International conference on advances in engineering and technology, pp 465–472
Olivares E, Lora ES, Cortez LAB (1995) Constructive features, operation and sizing of fluidized-bed gasifiers for biomass. Energy Sustain Dev 2(4):52–57
Pellegrine LF (2009) Thermo-economic-environmental analysis and optimization applied to the production combined of sugar, Ethanol and electricity. Ph. D. thesis, Sao Paulo University (USP), São Paulo. Portuguese
Reed GP, Paterson NP, Zhuo Y, Dugwell DR, Kandiyoti R (2005) Trace element distribution in sewage sludge gasification: source and temperature effects. Energy Fuels 19:298–304
Sánchez Prieto MGS, Carril TP, Nebra SA (2001) “Análise do custo exergético do sistema de geração de vapor da usina Cruz Alta”, Anais do XVI Congresso Nacional de Engenharia Mecânica, vol 4, Uberlândia, pp 196–205
Silva ME (2010) Experimental analysis of ethanol steam reforming: technical, economical and ecological aspects. Ph. D. thesis. Sao Paulo State University, Guaratinguetá. Portuguese
Silveira JL, Braga LB, de Souza ACC, Antunes JS, Zanzi R (2009) The benefits of ethanol use for hydrogen production in urban transportation. Renew Sustain Energy Rev 13:2525–2534
Silveira JL, Souza ACC, Silva ME (2008) Thermodynamic analysis of direct steam reforming of ethanol in molten carbonate fuel cell. J Fuel Cell Sci Technol 5:21012(1–6)
Silveira J, Tuna C (2004) Thermoeconomic analysis method for optimization of combined heat and power systems part II. Prog Energy Combust Sci 30:673–678
Silveira JL, Beyene A, Leal EM, Antunes J S, Okada D (2006) Thermoeconomic analysis of a cogeneration system of a university campus. Appl Therm Eng 22:1471–1483
Silveira JL, Tuna CE (2003) Thermoeconomic analysis method for optimization of combined heat and power system. Part I. Prog Energy Combust Sci 29:479–485
Silveira JL, Lamas WQ, Tuna CE, Villela IAC, Miro LS (2012) Ecological efficiency and thermoeconomic analysis of a cogeneration system at a hospital. Renew Sustain Energy Rev 16(5):2894–2906
Souza ACC, Silveira JL, Sosa MI (2006) Physical-chemical and thermodynamic analyses of ethanol steam reforming for hydrogen production. J Fuel Cell Sci Technol 3:1–6
Sözen A, Altıparmak D, Usta A (2002) Development and testing of a prototype of absorption heat pump system operated by solar energy. Appl Therm Eng 22(16):1847–1859
Tsatsaronis G (1993) Thermoeconomic analysis and optimization of energy systems. Prog Energy Combust Sci 19:227–257
Tuna CE (1999) Um método de análise exergoeconomica para a otimização de sistemas energéticos, Tese de Doutorado, Faculdade de Engenharia de Guaratinguetá. Universidade Estadual Paulista–UNESP, p 150
Utlu Z, Hepbasli A (2006) Estimating the energy and exergy utilization efficiencies for the residential–commercial sector: an application. Energy Policy 34(10):1097–1110
Valero AL, Valero A (2011) A prediction of the exergy loss of the world’s mineral reserves in the 21st century. Energy 36(4):1848–1854
Williams RH, Larson ED, Katofsky RE, Chen J (1995) Methanol and hydrogen from biomass for transportation. Energy Sustain Dev 1(5):18–34
Yang Y, Ma J, Wu F (2006) Production of hydrogen by steam reforming of ethanol over a Ni/ZnO catalyst. Int J Hydrogen Energy 31:877–882
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Silveira, J.L. et al. (2014). Technological Advancements in Biohydrogen Production and Bagasse Gasification Process in the Sugarcane Industry with Regard to Brazilian Conditions. In: da Silva, S., Chandel, A. (eds) Biofuels in Brazil. Springer, Cham. https://doi.org/10.1007/978-3-319-05020-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-05020-1_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05019-5
Online ISBN: 978-3-319-05020-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)