Abstract
The process by which successive groups using the same resources occupy the same geographic area through time is frequently attributed to competition. Several authors have argued that competitive displacement was the cause of the decline and extinction of Sparassodonta, due to the introduction of carnivorans into South America about 8–7 Ma, although this view has been recently criticized. The diversity of Sparassodonta was low relative to that of Carnivora throughout the Cenozoic. The greatest peak in sparassodontan diversity was during the early Miocene (Santacrucian), with 11 species. After the late Miocene (Huayquerian), sparassodont diversity decreased and the group became extinct in the mid-Pliocene (~3 Ma, Chapadmalalan). In the late Miocene–mid Pliocene (Huayquerian–Chapadmalalan), the fossil record shows that sparassodonts and carnivorans overlapped. During this time, carnivoran diversity consisted of four or fewer species; thereafter, it expanded to more than 20 species in the early–Middle Pleistocene (Ensenadan). Initially, Carnivora was represented by middle-sized, omnivorous species, with large omnivores first represented in the mid-Pliocene (Chapadmalalan). By contrast, over this period, Sparassodonta was represented by both large and small hypercarnivores and a single large omnivorous species. We review hypotheses of replacement using the available information and perform new analyses to test the effect of sampling bias, ecological overlap between clades, and the relevance of environmental and faunistic changes for the evolution of sparassodonts. From this review of the fossil record, it is suggested that stochastic mechanisms other than competitive displacement may have caused the decline and extinction of Sparassodonta, possibly as part of a larger faunistic turnover related to multicausal biological and physical factors. Similarly, at the Pleistocene/Holocene boundary, an extinction event affected large mammals in South America, including large carnivorans, in the context of a multicausal event that involved human presence as well as collateral factors.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Sparassodonta
- Carnivora
- Competitive displacement
- Ecological replacement
- Faunistic turnover
- Multicausal event
6.1 Introduction
The ecological interaction between species is one of the drivers of macroevolutionary processes. Using the analogy of the Red Queen in Through the Looking Glass, Van Valen (1973) proposed that organisms are in a constant evolutionary battle to out-compete competitors. In this context, ecological competition refers to the situation in which a local population of one species reduces the rate of expansion of another (Sepkoski 2001). Competitive displacement (competitive exclusion or active displacement, sensu Krause 1986) in the fossil record concerns contexts in which one taxon wanes while other waxes—the so-called “double wedge” pattern (Sepkoski 2001)—with the consequent extinction of one of the species involved. Relevant studies include classical ecological pairs such as gastropods and brachiopods (Gould and Calloway 1980; Sepkoski 2001), basal archosauromorphs and dinosaurs (Brusatte et al. 2008; Langer et al. 2009), multituberculates and rodents (Krause 1986), creodonts and carnivorans (Van Valkenburgh 1999; Friscia and Van Valkenburgh 2010), and different clades of carnivorans (Silvestro et al. 2015).
In South America, the decline and extinction of the native mammalian predators (Sparassodonta) was classically interpreted in relation to the arrival of placental carnivorans (Carnivora), during the late Miocene–Pliocene (e.g., Simpson 1950, 1969, 1971, 1980; Patterson and Pascual 1972; Savage 1977; Werdelin 1987; Wang et al. 2008). However, this hypothesis has been questioned and even rejected (e.g., Marshall 1977, 1978; Reig 1981; Bond 1986; Pascual and Bond 1986; Goin 1989, 1995; Ortiz Jaureguizar 1989, 2001; Marshall and Cifelli 1990; Alberdi et al. 1995; Forasiepi et al. 2007; Forasiepi 2009; Prevosti et al. 2013; Zimicz 2014; López-Aguirre et al. 2017).
Different models have been applied to try to understand the evolution of two convergent clades occupying the same geographic area at the same time. The simplest model to predict the possibility of competitive displacement is linear decrease in diversity and/or abundance of one taxon, associated with increase in diversity and/or abundance in another (Benton 1983; Krause 1986; Van Valkenburgh 1999). Other predictions have coupled logistic functions describing local population sizes of competing species in local environments (Sepkoski 1996, 2001; Sepkoski et al. 2000). Under this model, both groups increase their diversity/abundance until a threshold is reached; subsequently, the out-competed taxon declines. Another model implies that both groups persist for a long time until an external perturbation occurs, when the diversity/abundance of one of them decreases in favor of the other (“incumbent replacement” sensu Rosenzweig and McCord 1991). Recently, new approaches have been made with Bayesian analyses (e.g., Silvestro et al. 2015; Pires et al. 2015).
Similarly, environmental changes have been largely discussed in relation to the extinction processes of different mammalian groups. In examples that have been studied, such as the “Grande Coupure” at the Eocene/Oligocene boundary in Eurasia, not one but several taxonomic groups are affected, triggering a faunistic turnover. In South America, this complex has been identified in relation to the cooling of Patagonia also at the Eocene/Oligocene boundary (Goin et al. 2010, 2012), or Andean orogeny and Southern cone aridification by the mid-Miocene (Pascual and Ortiz Jaureguizar 1990) (Chap. 2). In a similar context, our last revision (Prevosti et al. 2013) suggested that the extinction of Sparassodonta was related to environmental and faunistic changes occurring between the middle Miocene and the Pliocene. In this chapter, we review the pattern of evolution of carnivores in SA and evaluate the hypothesis of competitive displacement and the role of environmental factors in faunal turnover.
6.2 Changes in Predator Diversity: The Last Sparassodonts and First Carnivorans in South America
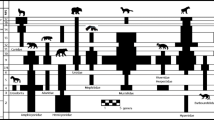
Our revised dataset produced diversity curves very similar to ones presented earlier (Prevosti et al. 2013; see also Chaps. 3–5; Fig. 6.1). During the earliest 20 million years of the Cenozoic, until the middle Eocene (Casamayoran), sparassodont taxonomic diversity and ecological disparity were low. It is worth mentioning that the phylogenetic position of the early Paleocene (Tiupampan) metatherians as sparassodonts is still inconclusive (Chap. 3); however, as a means of expressing the idea that the group was indeed present (e.g., de Muizon 1994, 1998; de Muizon et al. 1997, 2015), we include one Tiupampan metatherian for our analyses. The subsequent Peligran Age has provided only one isolated astragalus that resembles in size and shape of a fox-like borhyaenid (Goin et al. 2002). Unquestioned sparassodonts were present by the early Eocene (Itaboraian Age), as demonstrated by Patene simpsoni. The first peak in diversity is recorded by the middle Eocene (Casamayoran) with nine species, but this number may be biased due to averaging of faunal associations (Chap. 5). No or little record exists for the Mustersan–Tinguirirican, Friasian–Colloncuran, or Mayoan. The major peak in sparassodont diversity is recorded in the Santacrucian with 11 species (Fig. 6.2), followed by a decrease after the Huayquerian (Fig. 6.3) until their last records in the Chapadmalalan. Carnivoran diversity is low during the late Miocene–Pliocene, with a notable increase in the early–Middle Pleistocene (Ensenadan) (Fig. 6.4). The addition of Lazarus taxa to the analysis helps to reduce the effects of apparent declines and missing records, but it does not completely eliminate biases (Chap. 5).
Artist’s portrayal of a landscape during the early Miocene (Santacrucian Age) in Patagonia. On the left, crouching Borhyaena (Sparassodonta) waiting to ambush a group of protherotheriids, Thoatherium (Litopterna), also chased by a terror bird, Phorusrhacos (Phorusrhacidae). In the background, Astrapotherium (Astrapotheria). Artist Jorge Blanco
Artist’s portrayal of a landscape during the late Miocene (Huayquerian Age) in Pampa. In the foreground, left to right the carnivores Cyonasua (Carnivora) and Thylacosmilus (Sparassodonta). The center of the image is dominated by Argentavis (Theratornithidae), the largest extinct flying bird, with a ca 7-m wingspan. In the distant left background, three hoplophoriine glyptodonts (Glyptodontidae) and a group of Xotodon (Notoungulata). Artist Jorge Blanco
Seven late Miocene (Huayquerian Age; Fig. 6.3) sparassodont species were included in our analysis: Stylocynus paranensis, Borhyaenidium altiplanicus, Borhyaenidium musteloides, Notictis ortizi, and Thylacosmilus atrox, as well as Eutemnodus americanus (MACN-A 4975, 3990, 3991) and Borhyaena sp. (MACN-PV 13207), represented by fragmentary material. The alpha taxonomy of the last two taxa remains uncertain, but they nonetheless record the presence of at least one more large-sized hypercarnivorous taxon in the late Miocene of southern SA.
Late Miocene–early Pliocene carnivorans were represented by procyonids (Cyonasua and Chapalmalania). Other lineages (mustelids, foxes) were represented only since the Vorohuean (Prevosti and Soibelzon 2012; Prevosti et al. 2013). All living groups (as well as certain fossil ones) have known Pleistocene representatives. As was discussed in Chap. 4, the presence of felids (i.e., “Felis” pumoides, Smilodontidion riggii) and a skunk (i.e., Conepatus altiramus) is not supported for the Pliocene, but in some of our analyses, we explore the potential impact of these dubious Pliocene records for our interpretations.
6.3 Diet, Body Size, and the Evolution of South American Carnivore Faunas
In view of the estimates based on RGA index and body masses, we consider that sparassodonts were mostly hypercarnivores, with the curve of hypercarnivory mostly corresponding to taxonomic diversity (Tables 6.1, 6.2, 6.3 and 6.4). There is only one mesocarnivore taxon in the Deseadan (Pharsophorus tenax), while omnivores have low diversity throughout and are mostly restricted to the Paleogene, with the exception of the Huayquerian and Laventan (Stylocynus paranensis and Hondadelphys fieldsi, respectively; Table 6.1). Body mass estimates indicate that middle-sized sparassodonts were few and restricted to the Mustersan, Colhuehuapian, Laventan, and Chasicoan (Table 6.1). The opposing extremes, small and large sparassodonts, were common, and their abundance follows the diversity curve. Body mass and RGA values together indicate that sparassodonts were small and omnivorous until the Casamayoran, when large body sizes and hypercarnivory were also the most frequent categories. The largest body size (~200 kg, Proborhyaena gigantea) was recorded for the Deseadan, but other large taxa (~100 kg, Thylacosmilus) managed to persist until the late Miocene–Pliocene. Median body size reached ca. 20 kg in the Deseadan and remained near or above this value until the extinction of the group. Median size dropped to ca. 6 kg in the Santacrucian when a high diversity of small taxa was present (Prevosti et al. 2012; Ercoli et al. 2014), but recovered to ca. 13 kg in the Chasicoan (Table 6.3). Relative grinding area (RGA) index range is greatest in the Casamayoran (range ~0.7–0), followed by the Huayquerian (range ~0.6–0); while it is ~0.3 for most other ages (Table 6.3). In the Huayquerian, middle-sized hypercarnivores were not in evidence but small and large hypercarnivores were present; no mesocarnivore has been recorded, and there was only a single large omnivore. In the Montehermosan and Chapadmalalan, only hypercarnivores in the small and large size ranges remained (Table 6.4).
During the late Miocene–Pliocene, the ecological disparity of SA carnivorans was limited (Prevosti and Soibelzon 2012; Prevosti et al. 2013; Chap. 4). Broad disparity in body size (0.23–900 kg) and diet (RGA between 0 and >1) existed from the Ensenadan onward (Tables 6.1 and 6.2; the drop observed in the Bonaerian is due to fossil record bias; see Chap. 5). New body size estimates classify Cyonasua spp. as a middle-sized omnivore (contra small omnivore in Prevosti and Soibelzon 2012; Prevosti et al. 2013). Large omnivores (Chapalmalania spp.) were present during the Chapadmalalan, and small omnivores and hypercarnivores (Lycalopex cultridens and Galictis sorgentinii, respectively) in the Vorohuean (Tables 6.1 and 6.2, Chap. 4). After the Lujanian, large hypercarnivores became fewer as a consequence of the Late Pleistocene–Holocene extinctions (see below).
As in the case of diversity, biases in the fossil record (Chap. 5) also affect the distribution of ecological types. The inclusion of Lazarus taxa does not modify the general pattern, but it certainly affects interpretation of the carnivoran fossil record for the Bonaerian, making it more similar to the Lujanian and Ensenadan (Table 6.2).
To compare and explore ecological variation within each age, we performed a principal component analysis on the correlation matrix, with body size and diet classes analyzed per age (Fig. 6.5). The first axis organizes taxonomically more diverse faunas (Lujanian, Ensenadan, Platan) to the right and less diverse ones (e.g., Bonaerian, Marplatan Subages) to the left. The second axis orders the faunas by the relative number of large hypercarnivores (positively correlated with axis 2 scores) and mesocarnivores, omnivores, and medium-sized taxa (negatively correlated with axis 2 scores). Ensenadan, Santacrucian, and Casamayoran Ages have the highest scores on this axis, while Chasicoan, Huayquerian, and Platan are at the opposite extreme (Fig. 6.5). The position of certain intervals (e.g., Bonaerian, Itaboraian, and Marplatan Subages), lying at the negative extreme of axis 1 and the middle of axis 2, is clearly conditioned by their low diversity, something that could be partially explained by biases in the fossil record. This is clear for the Bonaerian, because the inclusion of Lazarus taxa generates a similar morphospace, but this unit is positive for axis 1 and axis 2 and thus more similar to the Lujanian and Ensenadan. Other ages with low diversities do not change significantly when Lazarus taxa are included, and this suggests that the low taxonomic diversity and ecological diversity of some intervals (e.g., Marplatan Subages and the Chapadmalalan in particular) is a real pattern. Other methods (e.g., ghost lineages, Bayesian, capture–recapture; e.g., Liow and Finarelli 2014; Silvestro et al. 2015; Pires et al. 2015; Finarelli and Liow 2016) should be considered to further evaluate diversity and ecological characterization.
First two principal components of the analysis of South American Ages, based on the abundance of each body size and diet class excluding a or excluding or including b Lazarus Taxa. TIUP Tiupampan; PELI Peligran; ITAB Itaboraian; RIOC Riochican; CASA Casamayoran; MUST Mustersan; DESE Deseadan; COLH Colhuehuapian; SANT Santacrucian; FRIA Friasian; COLL Colloncuran; LAVE Laventan; MAYO Mayoan; CHAS Chasicoan; HUAY Huayquerian; MONT Montehermosan; CHAP Chapadmalalan; BaLob Barrancalobian; VOR Vorohuean; Sand Sanandresian; ENSE Ensenadan; BONA Bonaerian; LUJA Lujanian; PLAT Platan; gra large size; med middle size; sma small size; hip hypercarnivores; meso mesocarnivores; omni omnivores. Green lines represent the loading of each ecological variable
Finally, we explored the ecological “disparity” (i.e., the area occupied by a morphospace, as well as, the morphospace area divided by the number of taxa) of well-sampled ages (Table 6.5) using a morphospace generated by body size and RGA (Fig. 6.6), and measuring spatial distribution using the software Past 2.17c (nearest neighbor distance with Donnelly edge correction; Clark and Evans 1954; Hammer et al. 2001; Hammer 2016). We judged disparity according to the density of the distribution of taxa. Our first measurement evaluates whether the taxa are distributed in clustered, random, or ordered (or over-dispersed) pattern. Unlike studies involving extant faunas, our analysis includes faunas at a continental scale, over intervals of a few thousands to millions of years. Averaging may generate false clustering if different taxa with similar ecological types are included. On the other hand, bias in fossil preservation could mask spatial distribution patterns, something that we try to minimize by analyzing only well-sampled faunas (see Chap. 5).
Distribution of mammalian carnivores in the morphospace generated by body size (BM, in kg) and the relative grinding area (RGA) index of lower carnassial. Red circle, sparassodonts; blue triangles, carnivorans; green rhombus, dubious Pliocene carnivorans (Conepatus altiramus, “Felis” pumoides, and Smilodontidion riggii)
Unexpectedly, and because of data averaging, all the analyzed faunas showed significant ordered (or over-dispersed) distribution patterns (nearest neighbor distance well above 1) congruent with a well-structured guild pattern in which separation in body size and diet (RGA) acts to minimize competition between taxa. Ercoli et al. (2014) found the same pattern for the Santa Cruz Fm. (Santacrucian) using a similar approach, but including also locomotor habits, which minimized predator overlap. Unfortunately, the data are insufficient to include this ecological aspect in our analyses. The inclusion of inferences concerning carnivoran locomotion in Pleistocene and extant taxa may minimize overlap (Chap. 4).
Ecological disparity (Table 6.5) increases in the Deseadan due to a significant increase in the maximum body size, and in the Huayquerian by an increase in the range of RGA as well as body size (Table 6.5). In the Chapadmalalan, Ensenadan, and Lujanian, the ecological disparity of the associations was broader, but during the Platan, a decrease occurred, caused by the extinction of the largest carnivorans at the end of the Lujanian. The standardization of the area of the morphospace by the number of taxa provided a similar result, but the Platan possessed a lower disparity than the Chapadmalalan, and the Huayquerian lower than the Deseadan (Table 6.5). The use of covered morphospace for each Age as a disparity measurement gives a similar pattern.
In summary, the analyzed faunas, whether consisting of sparassodonts, carnivorans, or in combination, revealed a well-structured guild organization within the morphospace defined by diet and body mass, suggesting low intraguild competition (see also Prevosti and Vizcaíno 2006; Prevosti and Martin 2013; Ercoli et al. 2014). These faunas become more diverse from the late Miocene at least, due to the appearance of small and large omnivores as well as hypercarnivores (including saber-toothed sparassodonts), but especially in the Pleistocene (saber-toothed cats, giant bears, large hypercarnivorous canids, as well as other living ecological carnivoran types) (see Chap. 4).
The inclusion of “Felis” pumoides, Smilodontidion riggii, and Conepatus altiramus in these Chapadmalalan does not modify the results, which show a good ecological separation among predators (Fig. 6.6; Table 6.5).
6.4 Carnivorans Versus Sparassodonts: Competition?
Several authors have suggested that carnivorans competed with sparassodonts, and that the former’s immigration to South America during the GABI caused the latter’s extinction (e.g., Simpson 1950, 1969, 1971, 1980; Patterson and Pascual 1972; Savage 1977; Werdelin 1987; Wang et al. 2008). This interpretation was informed by the ecological equivalence thought to exist between these clades using general descriptions of their anatomy (e.g., Marshall 1977, 1978, 1979, 1981), but does not consider direct quantitative analysis nor possible overlap in time of key clades in both groups. However, competition hypothesis was questioned by different authors (Marshall 1977, 1978; Reig 1981; Bond 1986; Pascual and Bond 1986; Goin 1989, 1995; Ortiz Jaureguizar 1989, 2001; Marshall and Cifelli 1990; Alberdi et al. 1995; Forasiepi et al. 2007; Forasiepi 2009; Prevosti et al. 2013; Zimicz 2014).
Diet, locomotion, and body mass have been the major paleoecological features analyzed for sparassodonts, with inferences primarily based on qualitative comparisons between marsupials and carnivorans. Based on dental features, hathliacynids and some borhyaenoids (Prothylacynus patagonicus and Lycopsis torresi) were considered to have been predominantly omnivorous (Marshall 1977, 1978, 1979, 1981). Hathliacynids were compared with didelphids, mustelids, or canids, while Prothylacynus patagonicus and Lycopsis torresi were compared with ursids and procyonids. Large borhyaenids (Borhyaena tuberata, Acrocyon sectorius, and Arctodictis munizi) were compared with canids and felids, being possibly able to break bones, and thus resembling living scavengers (Marshall 1977, 1978; Argot 2004a; Forasiepi et al. 2004). Thylacosmilidae, at the time represented only by Thylacosmilus atrox, was compared with saber-toothed cats (Machairodontinae; Patterson and Pascual 1972; Marshall 1976, 1977, 1978), due to the hypertrophy of the upper canines and associated cranial features. More recently, based on the shape of the skull and jaw, T. atrox was compared with nimravids (Barbourofelis; Prevosti et al. 2010).
More recently, statistical inferences are consistent with idea that about 90% of sparassodonts were narrowly hypercarnivorous (Wroe et al. 2004; Zimicz 2012, 2014; Prevosti et al. 2013; López-Aguirre et al. 2017; Table 3.2). Even the molar structure of hathliacynids and some borhyaenoids with small talonids (e.g., Pseudothylacynus, Lycopsis, Prothylacynus, and Pseudolycopsis) are within the range of living hypercarnivores. This feature is more evident in proborhyaenids (e.g., Arminiheringia, Callistoe, Proborhyaena), borhyaenids (e.g., Australohyaena, Arctodictis, Borhyaena), and Thylacosmilus, all of which virtually lack talonids and thus resemble hypercarnivorous Felidae and Nimravidae (Table 3.2). The hypercarnivorous association of sparassodonts contrasts with modern and past carnivoran communities, which are constituted by a larger proportion of omnivores and mesocarnivores (e.g., Van Valkenburgh 1999, 2007).
Reduced disparity in dental morphology within Sparassodonta in comparison to carnivorans was explained by the presence of phylogenetic constraints associated with the pattern of tooth replacement in metatherians (Werdelin 1987; see Goswami et al. 2011 for a different view). During ontogeny, lower molars erupt successively and occupy the mechanically optimal position at the middle of the jaw, until the mandible reaches adult size and the m4 assumes the most favorable site. Consequently, during development, each lower molar functions as a carnassial, at least temporarily. This form of carnassial specialization overtook specializations for other activities, such as grinding and crushing food in a typical mortar-and-pestle arrangement, as in the case of the m2–m3 in Carnivora (Werdelin 1987). However, the dentition of some sparassodonts exemplifies different evolutionary strategies to circumvent this plausible phylogenetic constraint. For example, it has been suggested that the last upper premolar of thylacosmilids (i.e., Thylacosmilus and Patagosmilus) is in fact the deciduous premolar at this locus, retained in the adult dentition (Goin and Pascual 1987; Forasiepi and Carlini 2010). This unusual ontogenetic condition, together with the hypselodont–hypertrophied upper canine, is an example of heterochronic shifts that circumvent possible constraints, increasing the morphological disparity of taxa via developmental mechanisms (Forasiepi and Sánchez-Villagra 2014).
Broad talonids represent the plesiomorphic condition for Sparassodonta. Consequently, current phylogenies tend to recover sparassodonts with broad talonids (e.g., Patene simpsoni, Hondadelphys fieldsi, Stylocynus paranensis) in a basal position in the tree (Forasiepi 2009; Engelman and Croft 2014; Forasiepi et al. 2015; Suarez et al. 2015). The late Miocene (Huayquerian) Stylocynus paranensis has been classically compared with omnivorous taxa, such as ursids and procyonids (Marshall 1978); however, cusps and crests are sharper in this species than in those carnivorans (e.g., Marshall 1979; Babot and Ortiz 2008). As a result, RGA values are lower in Stylocynus than in Cyonasua spp. which were contemporaneous in age (Tables 6.1 and 6.2; Fig. 6.6).
The postcranial anatomy of sparassodonts (Argot 2003a, b, 2004a, b, c; Prevosti et al. 2012; Ercoli et al. 2012, 2014) was quite generalized compared to carnivorans, allowing for unspecialized forms of arboreal (e.g., Pseudonotictis), scansorial (e.g., Cladosictis, Prothylacynus), and terrestrial activity (e.g., Thylacosmilus, Borhyaena), but without marked adaptations for cursoriality, as in canids or felids. The combination of a specialized hypercarnivorous dentition and a generalized postcranium is not commonly found among carnivorans, with the exception of some mustelids (e.g., wolverine) and some viverrids (e.g., civet).
Our revised dataset accords with the analysis of Prevosti et al. (2013), especially with regard to the temporal overlap between Sparassodonta and Carnivora during the Huayquerian–Chapadmalalan (Fig. 6.6). Our new body size estimates for Cyonasua (12–15 kg) place it among medium-sized carnivorans rather than small ones, as classified by Prevosti et al. (2013), but this change does not increase the ecological overlap with sparassodonts (Fig. 6.6). Cyonasua spp. remain as omnivores in our classification, which means that their only potential Huayquerian competitor would have been Stylocynus (with a body mass of 31 kg; Chaps. 3 and 4). However, note that based on actualistic evidence (Dickman 1986; Palomares and Caro 1999; Donadio and Buskirk 2006; Oliveira and Pereira 2014), the larger sparassodont would have been dominant in competitive situations with the smaller carnivoran. A similar conclusion has been recently suggested by López-Aguirre et al. (2017) based on a slightly different database averaged to generic level, and multiple regression and beta diversity analyses.
Another possibility is that competitive displacement between these carnivore clades might be masked by the imperfections of the fossil record, which do not allow us to ecologically differentiate sparassodonts and carnivorans with inferred similar habits (Chap. 5). We cannot completely dismiss the possibility that future records will reveal that sparassodonts become extinct later in time than the mid-Pliocene, or, alternatively, that large hypercarnivorous or omnivorous carnivorans appeared earlier in South America. Here we test the following propositions: (1) The sampling effort has affected recognition of apparent ecological overlap during the late Miocene–Pliocene (see Chaps. 5 and 2) the inclusion of doubtfully Pliocene taxa (“Felis” pumoides, Smilodontidion riggii, and Conepatus altiramus) affects interpretation of first arrivals of these carnivoran ecological types.
In Chapter 5, we recognized important biases in the SA fossil record. In order to test the possibility that the biases are obscuring the diversity and ecological patterning between sparassodonts and carnivorans, we compared differences in the abundance of observed ecological classes (a combination of diet and body size) in each clade during the Huayquerian–Chapadmalalan (i.e., large hypercarnivores, small hypercarnivores, medium omnivores, large omnivores), using a randomly generated ratio to obtain the probability that an observed instance is larger than the random estimate. This was performed twice, once using the proportion of the number of localities in each age, and once using the number of specimens, up to the size of each resampling. The observed abundance difference between sparassodonts and carnivorans in the large hypercarnivore category is highly significant (p = 0.0001) for the Huayquerian and Chapadmalalan, but not for the Montehermosan (p > 0.05). This means that the randomly expected difference is lower than the observed one. When the Santacrucian fauna is used for the resampling, the observed difference in the number of specimens of large hypercarnivores is significant for the Huayquerian (p < 0.0135) and Chapadmalalan (p < 0.005107). The observed difference for medium omnivores was always lower than randomly expected (p < 0.01). Similar results were obtained for small hypercarnivores, except for the Chapadmalalan when Huayquerian or Friasian–Chasicoan faunas were used in the resampling (with both sample proxies), and for the Huayquerian when Friasian–Chasicoan faunas were used in the resampling (using the number of localities), with a non-significant pattern (p > 0.05). We use the same procedure to test how the limited fossil record of the Marplatan Subages could mask the overlap between large and small hypercarnivores (the ecological types represented by the last sparassodonts) due to potential persistence of sparassodonts or to an earlier immigration of large hypercarnivorous carnivorans during the Marplatan. The Montehermosan includes very few localities; consequently, the random expectation regarding the abundance of large hypercarnivores among sparassodonts versus carnivorans is mostly not significantly different from the observed absence of them in the Marplatan Subages. The only exceptions are the Vorohuean and Sanandresian when the Friasian–Colloncuran faunas are used in the resampling; in these cases, values are significantly lower (p = 0.03). On the other hand, the random difference between small hypercarnivores is significantly greater (p = 0.02) than the observed one.
With regard to the inclusion of dubious carnivorans in the Chapadmalalan (two large hypercarnivores and one small omnivore), the difference between small omnivores is not significant, except when the Friasian–Chasicoan faunas are used in the resampling. In this case, the difference is significantly smaller than the random expectation (p < 0.005). By contrast, the large hypercarnivore difference is highly significantly larger than the random expectation (p = 0.0001), except when the Santacrucian and number of localities are used. However, the exclusion of only one of the dubious large hypercarnivores results in a significantly larger value (p = 0.0344). This is relevant because even if a Pliocene age is accepted for “Felis” pumoides, it is not possible to allocate it in any specific age within the Pliocene, Chapadmalalan, or otherwise.
These results, in combination with arguments and analyses presented above and in Chapter 5, suggest that competition between carnivorans and sparassodonts in the Huayquerian–Chapadmalalan was absent, because the observed difference of hypercarnivores is significantly larger than random expectation. A similar conclusion can be made for large omnivores in the Huayquerian. One caveat is that faunal structure during the late Miocene–Pliocene is different from that of the Pleistocene and older Neogene epochs. The results obtained for some ecological classes (e.g., between-clade differences significantly larger than random for medium omnivores and/or small hypercarnivores) are congruent with this possibility (Prevosti and Soibelzon 2012; Prevosti et al. 2013). Although it is difficult to unambiguously predict the effect of this caveat, if the inferred late Miocene–Pliocene absence of hypercarnivore carnivorans and limited sparassodont diversity accurately mirrors past occupancy of the predator guild, our sense of an absence of competition is only strengthened.
Another relevant result of these analyses is that Ages with very small sample sizes (either in number of specimens or localities; e.g., Montehermosan and Marplatan Subages) provide only ambiguous or non-significant results and thus do not permit rejection of between-clade competition. This is especially true for the Marplatan, where low sampling may mask an overlap of sparassodont and carnivoran hypercarnivores. Nonetheless, as discussed in Chap. 5, the available evidence is insufficient to support the coexistence of these morphotypes and is in fact more congruent with absence of competition.
If the presence of the three dubious carnivoran taxa record in the Pliocene is eventually confirmed, judging from actualistic ecological studies of intraguild competition (Dickman 1986; Palomares and Caro 1999; Donadio and Buskirk 2006; Oliveira and Pereira 2014), Smilodontidion riggii (=Smilodon sp.) is the only taxon that could have actively displaced Thylacosmilus. However, these sabertooths did not share the same morphotype or body mass (Wroe et al. 2013), and this difference might have influenced their prey selection and predatory habits (Chap. 3). In turn, “Felis” pumoides was comparable in size to a small puma (Puma concolor) and thus much smaller than Thylacosmilus.
Summing up, data and analyses are more congruent with the absence of competition between sparassodonts and carnivorans. More fieldworks and additional analyses using new approaches to test competition, clade diversification, and sampling effort (Liow and Finnarelli 2014; Pires et al. 2015; Silvestro et al. 2015; Finarelli and Liow 2016) potentially will provide new insights to test this interpretation.
6.5 Terror Birds and Carnivorous Didelphimorphian: Other Cases of Competition?
Alternative competitive exclusion hypotheses affecting sparassodonts include the possible role of “terror birds” (Phorusrhacidae; Marshall 1977, 1978, partim; Marshall and Cifelli 1990; see also Croft 2006) and didelphimorphian marsupials with carnivorous dentitions (Marshall 1977, 1978, partim; Goin 1989; Goin and Pardiñas 1996).
Terror birds were flightless, cursorial predators that were present in South America during most of the Cenozoic and included small to gigantic forms (Degrange et al. 2012). The group also reached North America at the end of the Neogene and was represented in the Pliocene by Titanis walleri (MacFadden et al. 2007).
Appraisal of the diversity of Phorusrhacidae in South America (Alvarenga and Höfling 2003; Degrange et al. 2012) indicates that the number of species was low during the Cenozoic, with peaks of only four species during Deseadan, Santacrucian, and Huayquerian time (Fig. 6.1). The latest records with reliable stratigraphic data are from the Chapadmalalan (Degrange et al. 2015), but material from putative Pleistocene beds have been found in Uruguay (Tambussi et al. 1999; Agnolin 2009). The most recent of these seemingly include a phorusrhacid from the Late Pleistocene (Lujanian) (Alvarenga et al. 2009; Jones et al. 2016).
Marshall and Cifelli (1990; see also Marshall 1977, 1978) suggested that terror birds may have displaced large sparassodonts during the Paleogene, resulting in the disappearance of proborhyaenids. Yet later, during the late Miocene–early Pliocene when savannas, pampas, and open environments expanded, advantages for cursorial carnivores like terror birds should have been even greater. However, biochron data for phorusrhacids and diversity peaks are roughly similar to those of sparassodonts (Fig. 6.1), indicating that competition was unlikely between these clades and, minimally, that niches were at least partially segregated (see also Marshall 1978; Bond and Pascual 1983; Argot 2004b; Prevosti et al. 2013; Ercoli et al. 2014).
Birds represent the second major group of terrestrial predators in the early Miocene Santacrucian vertebrate association, with four phorusrhacids, one cariamid, one anatid, and two falconids (Degrange 2012; Degrange et al. 2012). The largest species was the probable scavenger Brontornis burmeisteri, an anseriform estimated to have weighed more than 300 kg (Tonni 1977). Among “terror birds,” the largest phorusrhacid was Phorusrhacos longissimus, about 100 kg and an active predator on large prey (Fig. 6.2). It possessed a rigid cranium, high bite force, and stocky neck and limbs (Degrange 2012; Degrange et al. 2012). Smaller phorusrhacids were represented by Patagornis marshi (30 kg), Psilopterus lemonei (10 kg), and Psilopterus bachmanni (4.5 kg). Comparing the body sizes of phorusrhacids and sparassodonts, Patagornis marshi is within the range of Acrocyon sectorius and Prothylacynus patagonicus. If the ecological impact of body size (translated into prey size) can be taken as a comparable proxy between birds and mammals, potential competition becomes a possibility (Ercoli et al. 2014). However, long term competition (Croft 2001, 2006) and locomotory differences would have favored the adoption of different predatory strategies on the part of these taxa (Ercoli et al. 2014).
Sparassodonts were a group that failed to develop a strict cursorial morphotype. While bone-crackers and other carnivores are commonly ecologically associated with other more efficient predators that partially consume and abandon the carcasses (Viranta 1996; Argot 2004b), this latter role could have been occupied by phorusrhacids. Consequently, it seems highly probable that these terrestrial birds occupied an ecological niche different from that of the sparassodonts (Argot 2004b; Ercoli et al. 2014; López-Aguirre et al. 2017), and that consequently the phorusrhacids did not force their extinction.
Similarly, other non-mammalian predators were in a minority in terrestrial ecosystems. The diversity of sebecid crocodiles and madtsoiid snakes was low during the Cenozoic (Gasparini 1996; Albino 1996; Paolillo and Linares 2007; Riff et al. 2010), and their extinction preceded that of sparassodonts (Fig. 6.1). During the late Miocene–Pleistocene, several different carnivorous morphotypes developed within didelphimorphian marsupials, some of which are still represented in the living fauna. These were distributed in two main groups: Sparassocynidae (Hesperocynus dolgopolae, Sparassocynus bahiai, S. derivatus, S. heterotopicus) and Didelphidae (Didelphis albiventris, D. crucialis, D. reigi, D. solimoensis, Hyperdidelphys dimartinoi, H. inexpectata, D. parvula, D. pattersoni, Lestodelphys halli, Lutreolina crassicaudata, L. tracheia, Thylatheridium cristatum, T. chapadmalensis, T. hudsoni, T. pascuali, Thylophorops chapadmalensis, Th. lorenzinii, and Th. perplanus) (Zimicz 2014). Sparassocynidae is an extinct family of carnivorous didelphimorphians (<1 kg in body mass; Zimicz 2014). Didelphidae includes omnivorous to carnivorous forms. The latter filled the size range from small (body sizes of a few grams) to medium (sizes up to about 10 kg; Zimicz 2014). The last hathliacynids (Borhyaenidium altiplanicus, B. musteloides, B. riggsi, Notocynus hermosicus, Notictis ortizi) overlapped with some carnivorous didelphimorphians during the Huayquerian–Chapadmalalan (Sparassocynidae, Didelphis, Hyperdidelphys, Lutreolina, Thylateridium, Thylophorops). This temporal and body size overlap, together with the terrestrial to scansorial habits of several extinct carnivorous didelphids, suggested a possible competitive interaction between small- and medium-sized hathliacynids and the carnivorous didelphimorphians (Marshall 1977, 1978, partim; Goin 1989; Goin and Pardiñas 1996; Forasiepi 2009). However, mesocarnivorous and hypocarnivorous, but not hypercarnivorous, diets have been inferred for the didelphimorphians occurring within the period of temporal overlap (Zimicz 2014). These differences point to the existence of a potential niche segregation between these two metatherian groups, without competitive displacement (Prevosti et al. 2013) or passive replacement (Zimicz 2014).
Correlation analysis (Spearman correlation coefficient) of the diversity of sparassodonts versus that of phorusrhacids, sebecids, or madtsoiid snakes, individually and in combination, support these interpretations, because most correlations are not significant (p > 0.05). The exception is total diversity of non-mammalian predators (R = 0.60, p < 0.05). However, the correlation is positive rather than negative, as would be expected in a competitive exclusion scenario. There are only three Ages in which sparassodonts and carnivorous didelphids overlap (Huayquerian, Montehermosan, and Chapadmalalan), making correlation analysis irrelevant, but in any case the observed data do not show a clear pattern that could be seen to support competition between sparassodonts and carnivorous didelphimorphians (Prevosti et al. 2013; Zimicz 2014).
6.6 Paleoenvironments and Faunal Changes: Do These Correlate?
The relevance of environmental changes and their impact on terrestrial mammalian communities in connection with the demise of sparassodonts occasionally has been addressed (see also Marshall 1977, 1978, partim; Marshall and Cifelli 1990, partim; Forasiepi et al. 2007; Prevosti et al. 2013). As documents in Chapter 2 (see also Pascual and Odreman Rivas 1971; Patterson and Pascual 1972; Pascual et al. 1985; Pascual and Ortiz Jaureguizar 1990; Zachos et al. 2001; Barreda and Palazzesi 2007; Barreda et al. 2008; Dozo et al. 2010), Neogene global climate change affected South America. In the middle Miocene–Recent, nonlinear but gradual global temperature decrease has been the rule, correlated with the establishment of permanent ice sheets in western Antarctica and the onset of glaciation in high-latitude Northern Hemisphere (Zachos et al. 2001). Rejuvenation of Andean orogeny during the Neogene clearly modified prevailing conditions in the physiography, climate, and biota of South America. Marine transgression, lacustrine development, and widespread fluvial incision would have affected previous biogeographic patterns during the middle and late Miocene (Campbell et al. 2006; Cozzuol 2006; Latrubesse et al. 2007; Marengo 2015).
For some regions of South America (e.g., Patagonia), there is evidence of the onset of desertification, continuing up to the present. This process featured replacement of forest by more open and xerophytic vegetation in the middle–late Miocene; in particular, open dry forest, composed of Schinus (pepper trees), Prosopis (algarrobos), and Celtis (nettle trees) with shrubs of Ephedraceae and Asteraceae, is known for the late Miocene (Barreda and Palazzesi 2007; Barreda et al. 2008; Dozo et al. 2010; Fig. 6.8). Floras featuring mixtures of C3–C4 species have been recovered from localities situated between 21° and 35°S in Bolivia and Argentina, suggesting the existence of extensive grasslands by 8 Ma (MacFadden et al. 1996) throughout a large part of South America.
In the Neogene, the observed climatic and floral changes in Patagonia also impacted mammalian paleocommunities, which experienced important compositional changes, including the extinction of some native lineages (Pascual and Odreman Rivas 1971; Patterson and Pascual 1972; Pascual et al. 1985; Pascual and Ortiz Jaureguizar 1990). Several South American “native ungulates” (e.g., Astrapotheria, Leontinidae, Adianthidae, Notohippidae) became extinct during the middle Miocene, although Toxodontidae and certain xenarthran taxa experienced a radiation (Megalonychidae, Megatheriidae, and Mylodontidae; Marshall and Cifelli 1990). On the whole, “native ungulates” experienced a continuous decline from the middle Miocene onward, with a steep reduction in the Pliocene and final disappearance during the Pleistocene–Holocene (Marshall and Cifelli 1990; Bond et al. 1995). In the Pampean Region, where the vertebrate fossil record for the late Miocene–Quaternary is relatively good, a faunal turnover has been detected for the mid-Pliocene (e.g., Kraglievich 1952; Tonni et al. 1992). Some authors have argued that this was related to environmental changes triggered by Andean orogeny rather than competition with North American immigrants (e.g., Ortiz Jaureguizar et al. 1995; Cione and Tonni 2001), but a more recent hypothesis suggests that a meteor impact in the Pampean Region during the late Chapadmalalan (ca. 3.3 Ma) drove this faunistic change (Schultz et al. 1998; Vizcaíno et al. 2004).
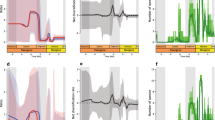
In this context, the decrease in sparassodontan diversity and their extinction in the mid-Pliocene appear to be part of a more general phenomenon of widespread faunal turnover independent of clade affiliation, life history, or major adaptations (Prevosti et al. 2013; Fig. 6.7). Sparassodonts, especially larger ones with a dietary specialization toward hypercarnivory, may have been more vulnerable to extinction than non-hypercarnivorous species (cf. Van Valkenburgh et al. 2004). Additionally, if sparassodonts had imperfect homeothermy and lactation limited to the rainy season, as do living marsupials (McNab 1986, 2005, 2008; Green 1997; Krockenberger 2006), the decrease in temperature and increase in aridity during the late Miocene when xerophytic vegetation and open environments were established in South America (Barreda and Palazzesi 2007; Barreda et al. 2008; see Chap. 2) might have influenced the decline of the group. This is a plausible argument, but it is unclear whether extrapolation from living marsupials to sparassodonts is justified.
South American Ages, sparassodont (blue solid line) and carnivoran (red broken line) diversity, temperature curve, climatic, environmental, and tectonic events (Modified from Prevosti et al. 2013). Colored boxes correspond to different floral changes (darker is older). Antarctic ice sheets: Dashed bar represents minimal ice (<than 50% of present ice volume), while gray represents full glaciation (>50% of present ice volume; Zachos et al. 2001)
To test the potential role of climatic and biological factors in the evolution of sparassodonts, we performed simple correlation tests (Spearman coefficient) between global temperature (median, minimum, maximum, and range of δ18O for each age, taken from Zachos et al. 2001; Table 6.6) against a range of variables including systematic diversity, estimated body mass, body size abundance, dietary classes, and median, minimum, and maximum RGA of lower carnassials (Chaps. 3 and 4; Tables 6.1, 6.2, 6.3 and 6.4). For a limited set of faunas, we also included the median, minimum, and maximum body size of medium and large prey species (Table 6.6). Lazarus taxa are alternately included or excluded from the analysis of systematic diversity, body size abundance, climate, and dietary classes.
By including Lazarus taxa, we try to ameliorate bias in the fossil record. In contrast, the inclusion of ages lacking sparassodont fossils or Lazarus taxa explores the possibility that these absences are real—a hard assumption to accept—given that ghost lineages indicate that the taxa must have existed, even if their fossils are (so far) unknown. Due to the large number of correlations, we adjust p values using the False Discovery Rate procedure (Benjamin and Hochberg 1995).
Total diversity of sparassodonts is positively correlated with temperature (Spearman R between 0.40 and 0.53, p < 0.05). However, it is only when Lazarus taxa are included that this correlation is significant at the p level indicated by the FDR correction (p = 0.0075), when minimal temperature shows a correlation of 0.53. Also, when Lazarus taxa are included, the abundance of omnivores and small taxa shows a positive correlation with temperature (Spearman R 0.63–0.67 and 0.54–0.59, respectively, p < 0.0075), but when ages lacking omnivores and/or small taxa are excluded, these correlations become non-significant even at p = 0.05. The same pattern is observed when Lazarus taxa are omitted, but in this case the correlation is lower (Spearman R 0.41–0.48, only significant at p = 0.05). Body mass and RGA reveal a contrasting pattern, especially when Lazarus taxa are included, with the first variable being negatively correlated (Spearman R 0.50–0.82) and the second positively correlated (Spearman R 0.50–0.74) with temperature. Most of these correlations are significant at the p level established by the FDR correction (p = 0.0075). The same pattern was found when Lazarus taxa are excluded, but few correlations are significant after FDR correction (e.g., median body mass against median and minimum temperatures).
These correlations imply a possible connection between temperature change and sparassodont evolution and extinction during the Cenozoic. Diversity, RGA, and abundance of omnivores and small taxa all increase with rising global temperature, while body size (median and maximum) increases with declining temperature. The similar response of RGA and omnivore abundance is consistent with this picture, since increase in the first variable indicates that sparassodont faunas are less carnivorous (or, alternatively, more omnivorous).
In relation to prey body mass distribution, the only significant correlations are between range and maximum prey body size, as well as and range and maximum sparassodont body mass, with or without Lazarus taxa or using the FDR correction. Maximum sparassodont body size has the largest correlation (Spearman R 0.94–0.95, p < 0.006). Range of sparassodont body mass also is positively correlated (Spearman R 0.82), but is only marginally significant (p = 0.049).
Our results are congruent with the analyses of Zimicz (2012, see also Goin et al. 2016). They also detected a positive relationship between diversity and omnivore abundance and temperature, but no correlation between body mass and temperature (Zimicz 2012). In our results, the increase of body size with temperature decrease could be seen as another example of the Bergmann and/or Cope “rules,” but this hypothesis should be tested in a phylogenetic context (e.g., Gould and MacFadden 2004). On the other hand, maximum prey size apparently influenced maximum sparassodont body size, since a highly positive relationship was detected, at least in regard to the faunas included in this analysis. Maximum prey body size apparently decreased in the Barrancalobian (from 3 tons in the Chapadmalalan to ca. 1.5 tons), though this could be partly due to a bias present in this Age. Later prey body size experienced an increase to ca. 4 tons in the Vorohuean and to ca. 7.5 tons in the Sanandresian–Lujanian, when the largest body size is achieved (Table 6.6). Thus, if sparassodonts did not become extinct in the Chapadmalalan, we would expect an increase in their maximum body size after the Vorohuean. But several factors could modify this expectation, including a threshold in body size above which prey are invulnerable to predation and, consequently, irrelevant to sparassodont evolution. The large increment in prey body mass observed since the Sanandresian is generated by the immigration of gomphotheres, a lineage, and morphotype that did not evolve together with sparassodonts. On the other hand, several native lineages (“native ungulates”, glyptodonts, ground sloths) increase their body mass in the Pleistocene, the largest being the ground sloth Megatherium americanum that had a body mass of ca. 4 tons (Vizcaíno et al. 2012), 1 ton larger than the maximum recorded in the Chapadmalalan. Thus, even excluding gomphotheres, at least a modest increase in maximum sparassodont body size should be expected if other factors were not involved.
Inclusion of Lazarus taxa significantly affected correlations. This may be taken as an indication that the use of methods correcting for bias in the fossil record might recover more relationships between sparassodont diversity and variables concerned with environmental and faunistic factors. Reduction of global temperatures during the late Miocene–Pleistocene may have had a negative impact on sparassodonts, probably in correlation with the decline and extinction of South American native ungulate lineages. Obviously, these results, based as they are on elementary correlation analyses, should be taken as preliminary working hypotheses that should be tested with more robust methods and a wider faunistic sample, including ones that control for bias (e.g., Liow and Finarelli 2014; Silvestro et al. 2015; Pires et al. 2015; Finarelli and Liow 2016).
6.7 The Extinction of Sparassodonta: Competition with Incoming Carnivorans, Environmental and Faunal Changes, or a Combination Thereof?
Our data and analyses support the hypothesis that there was no competition between sparassodonts and carnivorans inhabiting South America in the late Miocene–Pliocene, because they did not overlap in ecological space. Dubious records of some carnivorans in the Pliocene do not meaningfully support ecological competition between these clades. Other evidence points to environmental as well as more general change in the South American mammalian fauna forced the decline and demise of sparassodonts in the Pliocene. The available data are robust for the decline of sparassodonts since the Huayquerian, but for the final loss of this clade, the information is more ambiguous since Marplatan is not a well-sampled Age and/or has a poorer fossil record.
A similar conclusion has been recently supported by López-Aguirre et al. (2017), using a multivariate statistical approach. In their view, the extinction of the Sparassodonta is related to faunal changes and ecological interaction with other non-predator mammals. The authors provides less support to environmental changes (climate and Andean orogeny); however, the proxies used in their study to record Andean rising only considered one section of the Andes, and they use a global climatic scale that does not track changes at local scale.
We think that the hypothesis that Sparassodonta became extinct due to environmental and faunal changes has the epistemological advantage of being more vulnerable to falsification than its alternatives, such as interclade competition. We hope that additional targeted fieldwork and the application of more refined methodologies will provide useful tests (e.g., Liow and Finarelli 2014; Silvestro et al. 2015; Pires et al. 2015; Finarelli and Liow 2016).
6.8 Carnivoran Impact on Pleistocene Faunas: Giant Makers?
The immigration of placental predators to SA during the GABI impacted on the communities of native prey, in some cases triggering their extinction (e.g., Marshall 1988). If correct, this illustrates on trophic interactions intervening and modulating the dynamics of macroevolutionary processes. In this sense, Faurby and Svenning (2016) have recently demonstrated that the asymmetry in the exchange of mammals during the GABI, with NA clades more successful in SA, could be explained by differential susceptibility to predation pressure between animals from the two continents. But, does another biological response exist against the arrival of several carnivoran lineages into SA during the early Pleistocene? In this regard, Soibelzon and colleagues (Soibelzon et al. 2009, 2012; Zurita et al. 2010) argued that the presence of large carnivorans produced an adaptive response in several native herbivores due to predation pressure created by the newcomers. Gigantism observed in several lineages during the Pleistocene (e.g., glyptodonts, ground sloths, toxodontids, and macrauchenids), all of whom had body sizes larger than 1 ton (Vizcaíno et al. 2012; Fariña et al. 2013), behavioral changes (e.g., subterranean dwelling; Soibelzon et al. 2009, 2012), and anatomical changes (e.g., thicker carapace in some large glyptodonts; Zurita et al. 2010) could be correlated with defenses against wholly new types of predators. These hypotheses were proposed to explain the coincident occurrence in the SA fossil record of the first large carnivorans and megaherbivores in the Ensenadan.
Another scenario explains the existence of a continent with a diverse community of large mammals and megamammals with few large predators. This could have given ecological space to the new Pleistocene carnivoran immigrants. This scenario is congruent with the interpretation that the largest Pleistocene carnivorans (e.g., Arctotherium spp., Smilodon populator, Theriodictis platensis, Protocyon spp., and “Canis” gezi) apparently had their origins in South America (Figueirido and Soibelzon 2009; Soibelzon and Schubert 2011; Prevosti and Soibelzon 2012; Mitchell et al. 2016).
These two hypotheses are not mutually exclusive. South America hosted large mammals and megamammals during the Pleistocene, prior to the arrival of the large carnivorans (Vizcaíno et al. 2012), which could have provided ecological space for the development of large-bodied predators. In turn, the pressure exerted by these predators could have favored larger body sizes among herbivores, in an arm-race-like scenario (cf. Van Valen 1973; Dawkins and Krebs 1979). Alternatively, this faunistic change and increasing body mass could be governed by climatical–environmental changes (cf. Barnosky 2001; Raia et al. 2005).
Utilization of caves or dens in SA terrestrial environments by autochthonous mammals predated the arrival of large carnivorans (De Elorriaga and Visconti 2002; Genise et al. 2013; Cardonatto et al. 2016), but substantial enlargement of such dwellings postarrival clearly reflects an increase in body sizes. Large caves of up to 1.5 m width, attributed to ground sloths, armadillos, glyptodonts, or mesotheriids, are frequently encountered in late Miocene rocks (e.g., Cerro Azul Formation: De Elorriaga and Visconti 2002; Cardonatto et al. 2016). Quaternary paleocaves surpassed this value, attaining in some cases as much as ca. 2 m in width (Vizcaíno et al. 2001; Dondas et al. 2009; see also Fariña et al. 2013).
In 1996, Fariña and collaborators proposed the hypothesis that the Late Pleistocene South American faunas were not in balance, with a larger biomass of herbivores than predators (Fariña 1996; Fariña et al. 2014; see also Fariña et al. 2013; Fig. 6.8). This conclusion was reached using allometric relationships among living mammals in relation to their estimated body masses. However, the methodology was criticized, because it did not include comparative methods to constrain phylogenetic effects in calculating allometric equations, adjustments for taphonomic biases produced by time averaging of associations, or variability observed in population density or biomass in living communities (Prevosti and Vizcaíno 2006; see also Prevosti and Pereira 2014). Based on these criticisms, another scenario was proposed: If the Late Pleistocene supported a large biomass of herbivores, then it could have also supported a large density of predators (Prevosti and Vizcaíno 2006; Fig. 6.8). In fact, in a more recent analysis, Segura et al. (2016) suggested that the structure of the Late Pleistocene faunas of Buenos Aires (Argentina) and Uruguay resembles modern faunas.
Schematic representation of the predator–prey “imbalance” hypothesis of Fariña (1996) (a) and the alternative hypothesis proposed by Prevosti and Vizcaíno (2006) under which the high diversity of large mammals and megamammals supported a great abundance of predators (b). From Prevosti and Vizcaíno (2006)
Prevosti and Vizcaíno (2006) also investigated prey–predator relationships by means of allometric equations (and, more recently, by stable isotope analyses; Prevosti and Martin 2013; Bocherens et al. 2016) and discussed intraguild relationships. The largest hypercarnivore occurring in SA Late Pleistocene associations was Smilodon populator. This taxon is the most common carnivoran found in fossil sites of this age, and its presence probably generated a top-down negative cascade affecting other predators (Palomares and Caro 1999; Donadio and Buskirk 2006; Oliveira and Pereira 2014), with the possible exception of Arctotherium angustidens, whose body mass could exceeded 1 ton (Chap. 4).
6.9 Carnivoran Extinctions During the Pliocene–Holocene: Intraguild Competition, Human Impact, or Another Cause Entirely?
An important change in carnivoran faunas occurred between the late Pliocene (Vorohuean) and the early Pleistocene (Ensenadan), when the procyonid “heralds” of the GABI became extinct and the carnivoran contingent of the “legions” of the GABI appeared. The extinction of the large bear-like Chapalmalania at the end of the Pliocene (Vorohuean) was suggested to be a consequence of the immigration and radiation of bears in the Pleistocene, ca. 1.8 Ma (Kraglievich and Olazábal 1959; Prevosti and Pereira 2014). However, the coexistence of these clades remains unconfirmed, with a gap of ca. 0.8 ka in the first appearances of these two groups in South America (Table 4.1). A recent study based on ancient DNA suggested that the origin of large scavenging bears was an independent development in North and South America (Arctodus and Arctotherium), facilitated by the absence or limited number of other large mammalian scavengers (Mitchell et al. 2016). A parallel exists in the extinction of Cyonasua during the early Pleistocene and the presence of new carnivorans in the Pleistocene (Soibelzon 2011), albeit with insufficient information to exclude other possibilities (e.g., environmental and faunal changes; Prevosti and Pereira 2014).
A number of extinctions occurred at the end of the Ensenadan (ca. 0.5 Ma) when 12 taxa disappeared (e.g., Theriodictis platensis, Protocyon scagliarum, “Canis” gezi, Lycalopex cultridens, Arctotherium angustidens, Cyonasua spp., Lyncodon bosei, Stipanicicia pettorutii, Galictis hennigi, Smilodon gracilis, Homotherium venezuelensis, “Felis” vorohuensis; Prevosti and Soibelzon 2012). This event affected large and small hypercarnivores and omnivores (Prevosti and Soibelzon 2012). The degree to which these losses were actually coordinated in time is not well understood; possibly, their apparently shared end-Ensenadan disappearance date may be an artifact of analytic averaging of South American Land Mammal Ages and the effect of taphonomic biases. Last appearance records for L. bosei and Cy. meranii are dated to ca. 1 Ma (Soibelzon et al. 2008), indicating that they were possibly extinct before other Ensenadan carnivorans. In addition, the Bonaerian Age has a poor fossil record which may fail to include some Ensenadan carnivorans (Chap. 5). The recent extension of the time span of Lycalopex ensenadensis and Conepatus mercedensis into the Lujanian (Chap. 4) supports this interpretation. The end-Ensenadan extinction is roughly coincident with the establishment of wider climatic cycles of 100 ka, with colder glacials and warmer interglacials since 0.9 Ma (Rutter et al. 2012) and could be related to the faunistic changes observed between this Age and the Bonaerian–Lujanian.
The Late Pleistocene–Holocene transition incorporates another mammalian extinction event that also included large carnivorans, spawning a diverse set of hypotheses over the last decades. These include environmental changes, human hunting, introduced pathogens, meteoric impact, and the combination of all or any of the above (Martin and Wright 1967; MacPhee and Marx 1997; Cione et al. 2003, 2009; Borrero 2009; Lima Ribeiro and Diniz Filho 2013; Prado et al. 2015; Surovell et al. 2015; Villavicencio et al. 2016; Metcalf et al. 2016; Bartlett et al. 2016). In South America, this extinction occurred between 12 and 7 ka BP, with the Pampean Region showing the latest completion date (Cione et al. 2003, 2009; Steele and Politis 2009; Prado et al. 2015; Villavicencio et al. 2016; Metcalf et al. 2016; Politis et al. 2016). South American carnivorans followed a similar pattern to other mammals, in that large mammals and megamammals became extinct, and most large carnivorans disappeared (i.e., saber-toothed cats, large hypercarnivorous canids, bears; Table 4.1; Fig. 6.9), leaving the puma Puma concolor, jaguar Panthera onca, and spectacled bear Tremarctos ornatus as the only living large carnivores on the continent (Cione et al. 2003, 2009; Prevosti and Soibelzon 2012). Small carnivorans that became extinct in the Late Pleistocene (Lycalopex ensenadensis and Conepatus mercedensis) could have disappeared before the end of the epoch (Chap. 4).
Taxon dates (calibrated 14C dates BP) of terrestrial extinct South American carnivorans (black dots), presence of humans in South America, and paleoclimate proxy (West Antarctic δ18O). LIA little ice age; MWP medieval warm period; WAIS West Antarctic ice sheet. East Antarctic refers to Vostok core, and temperature variation to past minus present temperature (°C). Modified from Metcalf et al. (2016)
The disappearance of the large mammals and megamammals would have impacted the larger carnivorans, resulting in their mutual extinction (Prevosti and Vizcaíno 2006; Prevosti 2006; Prevosti and Martin 2013; cf. Van Valkenburgh et al. 2015). Isotopic analysis of material of Smilodon populator and Protocyon troglodytes from the Late Pleistocene of the Pampean Region indicated that they hunted in more open environments than the jaguar and could have suffered from the warmer and wetter climate of the Holocene (Bocherens et al. 2016). Recently, Villavicencio et al. (2016) considered that large carnivorans became extinct before large megamammals in southern Patagonia, due to the appearance of humans. However, when doubling the number of radiometric dates for megamammals in the region, Metcalf et al. (2016) did not recover such a time-shift. These authors calibrated the extinction for megamammals in Patagonia at ~12.3 ka, correlated with an abrupt warming phase, environmental changes, and human occupation. In this context, large carnivorans could have gone extinct through direct persecution by humans (cf. Villavicencio et al. 2016) or because humans caused a decline or demise of carnivoran prey (Cione et al. 2009). In the latter case, the competitive displacement and extinction of large carnivorans could have triggered a cascade of secondary extinctions in the ecosystems (cf. Cione et al. 2009). Accordingly, Whitney Smith (2009) proposed the “Second Order Predation Theory,” that humans reduced predator populations, leading to a dramatic increase in megaherbivore populations, with a consequent environmental exhaustion and faunal extinction.
Some authors have claimed that the use of domestic dogs could also have contributed to the extinction of these populations under stress, as already hypothesized for Europe and North America (Fiedel 2005; Shipman 2015). However, in South America, the record of domestic dogs dates between 7.5 and 4.5 ka and it is later than the time of the megafaunal extinction (Prates et al. 2010), suggesting that it was not involved in this extinction event.
Unfortunately, there is insufficient evidence from the Late Pleistocene–early Holocene faunas of South America to identify the exact sequence of events (cf. Prevosti and Pereira 2014). Progress has been made with the recent large number of dates published by Metcalf et al. (2016), revealing the simultaneity of the extinction of several megamammals and large predators in southern Patagonia. A conjunction of factors, such as an abrupt change in the climatic and environmental conditions and human presence (along any number of vectors, including hunting pressure, decreasing habitat range, diseases, importation of exotic animals), could have pushed larger mammals to a tipping point leading to their sudden extinction (MacPhee and Marx 1997; Cione et al. 2009; Van Valkenburgh et al. 2015; Metcalf et al. 2016; Fig. 6.9). In this context, the independent role of large carnivores is not clear or discernible from the available data.
After the Late Pleistocene–Holocene extinction, the carnivoran communities of South America survived nearly intact, at least in species composition at a continental scale, except for the extinction of the Dusicyon lineage. The last representatives of this genus were the Malvinas fox D. australis, which was hunted to extinction during the late nineteenth century, and D. avus, which lived on the continent. It disappeared during the last five hundred years, probably due to a combination of environmental change and human impact (Prevosti et al. 2015; Fig. 6.9).
References
Agnolin F (2009) Sistemática y Filogenia de las Aves Fororracoideas (Gruiformes, Cariamae). Fundación de Historia Natural Félix de Azara, Buenos Aires
Alberdi MT, Ortiz Jaureguizar E, Prado JL (1995) Evolución de las comunidades de mamíferos del Cenozoico superior de la Provincia de Buenos Aires, Argentina. Rev Esp Paleontol 10:30–36
Albino AM (1996) The South American fossil Squamata (Reptilia: Lepidosauria). In: Arratia G (ed) Contributions of Southern South America to vertebrate paleontology. Verlag Dr. Friedrich Pfeil, München, pp 185–202
Alvarenga HMF, Höfling E (2003) Systematic revision of the Phorusrhacidae (Aves: Ralliformes). Pap Avulsos Zool 43:55–91
Alvarenga H, Washington J, Rinderknecht A (2009) The youngest record of phorusrhacid birds (Aves, Phorusrhacidae) from the Late Pleistocene of Uruguay. Neues Jahrb Geol Paläontol Abh 256:229–234
Argot C (2003a) Functional adaptations of the postcranial skeleton of two Miocene borhyaenoids (Mammalia, Metatheria) Borhyaena and Prothylacynus, from South America. Palaeontology 46:1213–1267
Argot C (2003b) Postcranial functional adaptations in the South American Miocene borhyaenoids (Mammalia, Metatheria): Cladosictis, Pseudonotictis, and Sipalocyon. Alcheringa 27:303–356
Argot C (2004a) Functional-adaptative analysis of the postcranial skeleton of a Laventan borhyaenoid, Lycopsis longirostris (Marsupialia, Mammalia). J Vertebr Paleontol 24:689–708
Argot C (2004b) Evolution of South American mammalian predators (Borhyaenoidea): anatomical and palaeobiological implications. Zool J Linn Soc 140:487–521
Argot C (2004c) Functional-adaptive features and paleobiologic implications of the postcranial skeleton of the late Miocene sabretooth borhyaenoid, Thylacosmilus atrox (Metatheria). Alcheringa 28:229–266
Babot MJ, Ortiz PE (2008) Primer registro de Borhyaenoidea (Mammalia, Metatheria, Sparassodonta) en la provincia de Tucumán (Formación India Muerta, Grupo Choromoro; Mioceno tardío). Acta Geol Lilloana 21:34–48
Barnosky AD (2001) Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the Northern Rocky Mountains. J Vert Pal 21:172–185
Barreda V, Palazzesi L (2007) Patagonian vegetation turnovers during the Paleogene-early Neogene: origin of arid-adapted floras. Bot Rev 73:31–50
Barreda V, Guler V, Palazzesi L (2008) Late Miocene continental and marine palynological assemblages from Patagonia. Dev Quat Sci 11:343–350
Bartlett L, Bartlett DR, Williams GW, Prescott AB, Rhys EG, Eriksson A, Valdes PJ, Singarayer JS, Manica A (2016) Robustness despite uncertainty: regional climate data reveal the dominant role of humans in explaining global extinctions of late Quaternary megafauna. Ecography 39:152–161
Benjamin Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300
Benton MJ (1983) Large-scale replacements in the history of life. Nature 302:16–17
Bocherens H, Cotte M, Bonini R, Scian D, Straccia P, Soibelzon L, Prevosti FJ (2016) Paleobiology of sabretooth cat Smilodon populator in the Pampean Region (Buenos Aires Province, Argentina) around the Last Glacial Maximum: Insights from carbon and nitrogen stable isotopes in bone collagen. Palaeogeogr Palaeoclimatol Palaeoecol 449:463–474
Bond M (1986) Los carnívoros terrestres fósiles de Argentina: resumen de su historia. Actas IV Congr Arg Paleontol Bioest 2:167–171
Bond M, Pascual R (1983) Nuevos y elocuentes restos craneanos de Proborhyaena gigantea Ameghino 1897 (Marsupialia, Borhyanidae, Proborhyaeninae) de la edad Deseadense. Un ejemplo de coevolución. Ameghiniana 20:47–60
Bond M, Cerdeño E, López G (1995) Los ungulados nativos de América del Sur. In: Alberdi MT, Leone G, Tonni EP (eds) Monografías del Museo Nacional de Ciencias Naturales, Madrid, pp 259–275
Borrero LA (2009) The elusive evidence: the archeological record of the South American extinct megafauna. In: Haynes G (ed) American megafaunal extinctions at the end of the Pleistocene, pp 145–168
Brusatte S, Benton M, Ruta M, Lloyd G (2008) Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321:1485–1488
Campbell KE, Frailey CD, Romero-Pittman L (2006) The Pan-Amazonian Ucayali peneplain, late Neogene sedimentation in Amazonia, and the birth of the modern Amazon River system. Palaeogeog Palaeoclimatol Palaeoecol 239:166–219
Cardonatto MC, Melchor RN, Mendoza Belmontes FR, Montalvo CI (2016) Large mammal burrows in late Miocene calcareous paleosols from central Argentina. In: Fourth International Congress on Ichnology, Indanha-a-Nova (Portugal), Abstracts
Cassini GH, Cerdeño E, Villafañe AL, Muñoz NA (2012) Paleobiology of Santacrucian native ungulates (Meridiungulata: Astrapotheria, Litopterna and Notoungulata). In: Vizcaíno SF, Kay RF, Bargo MS (eds) Early Miocene Paleobiology in Patagonia. Cambridge University Press, Cambridge, pp 243–286
Cione AL, Tonni EP (2001) Correlation of Pliocene to Holocene southern South American and European vertebrate-bearing units. Boll Soc Paleontol Ital 40:1–7
Cione AL, Tonni EP, Soibelzon LH (2003) The Broken Zig-Zag: late Cenozoic large mammal and turtle extinction in South America. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 5:1–19
Cione AL, Tonni EP, Soibelzon LH (2009) Did humans cause large mammal Late Pleistocene-Holocene extinction in South America in a context of shrinking open areas? In: Haynes G (ed) American megafaunal extinctions at the end of the Pleistocene. Springer Publishers, Vertebrate Paleobiology and Paleontology Series, pp 125–144
Clark PJ, Evans FC (1954) Generalization of a Nearest Neighbormeasure of dispersion for use in K dimensions. Ecology 60:316–317
Cozzuol MA (2006) The Acre vertebrate fauna: age, diversity, and geography. J South Am Earth Sci 21:185–203
Croft DA (2001) Cenozoic environmental change in South America as indicated by mammalian body size distributions (cenograms). Divers Distrib 2:271–287
Croft DA (2006) Do marsupials make good predators? Insights from predator-prey diversity ratios. Evol Ecol Res 8:1193–1214
Dawkins R, Krebs JR (1979) Arms races between and within species. Proc R Soc 205(1161):489–511
Degrange F (2012) Morfología del cráneo y complejo apendicular posterior de aves fororracoideas: implicancias en la dieta y modo de vida. Unpublished PhD thesis, Universidad Nacional de La Plata, La Plata.
Degrange FJ, Noriega JI, Areta JI (2012) Diversity and paleobiology of the Santacrucian birds. In: Vizcaíno SF, Kay RF, Bargo MS (eds) Early Miocene paleobiology in Patagonia. Cambridge University Press, Cambridge, pp 138–155
Degrange FJ, Tambussi CP, Taglioretti ML, Dondas A, Scaglia F (2015) A new Mesembriornithinae (Aves, Phorusrhacidae) provides new insights into the phylogeny and sensory capabilities of terror birds. J Vertebr Paleontol 35(2):e912656. doi:10.1080/02724634.2014.912656
de Muizon C (1994) A new carnivorous marsupial from the Palaeocene of Bolivia and the problem of marsupial monophyly. Nature 370:208–211
de Muizon C (1998) Mayulestes ferox, a borhyaenoid (Metatheria, Mammalia) from the early Palaeocene of Bolivia. Phylogenetic and palaeobiologic implications. Geodiversitas 20:19–142
de Muizon C, Cifelli RL, Céspedes Paz R (1997) The origin of the dog-like borhyaenoid marsupials of South America. Nature 389:486–489
de Muizon C, Billet G, Argot C, Ladevèze S, Goussard F (2015) Alcidedorbignya inopinata, a basal pantodont (Placentalia, Mammalia) from the early Palaeocene of Bolivia: anatomy, phylogeny and palaeobiology. Geodiversitas 37:397–634
Dickman CR (1986) An experimental study of competition between two species of dasyurid marsupials. Ecol Monog 56:221–241
Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in Carnivora. Am Nat 167:524–536
Dondas A, Isla FI, Carballido J (2009) Paleocaves exhumed from the Miramar Formation (Ensenadan Stage-age). Quat Int 210:44–50
Dozo MT, Bouza P, Monti A, Palazzesi L, Barreda V, Massaferro G, Scasso R, Tambussi C (2010) late Miocene continental biota in northeastern Patagonia (Península Valdés, Chubut, Argentina). Palaeogeogr Palaeoclimatol Palaeoecol 297:100–109
De Elorriaga EE, Visconti G (2002) Crotovinas atribuibles a grandes mamíferos del Cenozoico en el sureste de la Provincia de La Pampa. 9° Reunión Argentina de Sedimentología, Resúmenes 1:63
Engelman RK, Croft DA (2014) A new species of small-bodied sparassodont (Mammalia: Metatheria) from the middle Miocene locality of Quebrada Honda, Bolivia. J Vert Paleontol 34:672–688
Ercoli MD, Prevosti FJ, Álvarez A (2012) Form and function within a phylogenetic framework: locomotory habits of extant predators and some Miocene Sparassodonta (Metatheria). Zool J Linn Soc 165:224–251
Ercoli MD, Prevosti FJ, Forasiepi AM (2014) The structure of the mammalian predator guild in the Santa Cruz Formation (late early Miocene), Patagonia, Argentina. J Mammal Evol 21:369–381
Fariña RA (1996) Trophic relationships among Lujanian mammals. Evol Theo 11:125–134
Fariña RA, Vizcaíno SF, De Iuliis G (2013) Megafauna. Giant Beasts of Pleistocene South America. Indiana University Press, Bloomington, p 416
Fariña RA, Czerwonogora ADA, Giacomo MDI (2014) Splendid oddness: revisiting the curious trophic relationships of South American Pleistocene mammals and their abundance. An Acad Bras Cienc 86:311–331
Faurby S, Svenning, J-C (2016) The asymmetry in the Great American Biotic Interchange in mammals is consistent with differential susceptibility to mammalian predation. Global Ecology and Biogeography 25:1443–1453
Fiedel SJ (2005) Man’s best friend—mammoth’s worst enemy? A speculative essay on the role of dogs in Paleoindian colonization and megafaunal extinction. World Archaeol 37(1):11–25
Figueirido B, Soibelzon LH (2009) Inferring paleoecology in extinct tremarctine bears (Carnivora, Ursidae) via geometric morphometrics. Lethaia 43:209–222
Finarelli JA, Liow LH (2016) Diversification histories for North American and Eurasian carnivorans. Biol J Linn 118(1):26–38
Forasiepi AM (2009) Osteology of Arctodictis sinclairi (Mammalia, Metatheria, Sparassodonta) and phylogeny of Cenozoic metatherian carnivores from South America. Monogr Mus Argent Cienc Nat 6:1–174
Forasiepi AM, Carlini AA (2010) New thylacosmilid (Mammalia, Metatheria, Sparassodonta) from the Miocene of Patagonia, Argentina. Zootaxa 2552:55–68
Forasiepi AM, Goin FJ, Tauber AA (2004) Las especies de Arctodictis Mercerat, 1891 (Metatheria, Borhyaenidae), grandes metaterios carnívoros del Mioceno de América del Sur. Rev Esp Paleontol 19:1–22
Forasiepi AM, Martinelli AG, Goin FJ (2007) Revisión taxonómica de Parahyaenodon argentinus Ameghino y sus implicancias en el conocimiento de los grandes mamíferos carnívoros del Mio-Plioceno de América del Sur. Ameghiniana 44:143–159
Forasiepi AM, Sánchez-Villagra MR (2014) Heterochrony, dental ontogenetic diversity and the circumvention of constraints in marsupial mammals and extinct relatives. Paleobiology 40:222–237
Forasiepi AM, Babot MJ, Zimicz N (2015) Australohyaena antiqua (Mammalia, Metatheria, Sparassodonta), a large predator from the late Oligocene of Patagonia, Argentina. J Syst Palaeontol 13:503–525
Forasiepi AM, Macphee RDE, Hernández Del Pino S, Schmidt GI, Amson E, Grohe C (2016) Exceptional skull of Huayqueriana (Mammalia, Litopterna, Macraucheniidae) from the late Miocene of Argentina: anatomy, systematics, and paleobiological implications. Bulletin of the American Museum of Natural History 404:1–76
Friscia A VanValkenburgh B (2010) Ecomorphology of North American Eocene carnivores: evidence for competition between carnivorans and creodonts. In: Goswami A, Friscia A (eds) Carnivoran evolution: new views on phylogeny, form, and function. Cambridge University Press, Cambridge and New York, pp 311–341
Gasparini Z (1996) Biogeographic evolution of the South American crocodilians. Münchner Geowiss Abh 30:159–184
Genise JF, Melchor RN, Sánchez MV, González MG (2013) Attaichnus kuenzelii revisited: a Miocene record of fungus-growing ants from Argentina. Palaeogeogr Palaeoclimatol Palaeoecol 386:349–363
Goin FJ (1989) Late Cenozoic South American marsupial and placental carnivores: changes in predator-prey evolution. V Internatl Ther Congr, Abstracts: 271–272
Goin FJ (1995) Los marsupiales. In: Alberdi MT, Leone G, Tonni EP (eds) Evolución biológica y climática de la Región Pampeana durante los últimos cinco millones de años. Un ensayo de correlación con el Mediterráneo Occidental, Monografías del Museo Nacional de Ciencias Naturales, Madrid, pp 165–179
Goin FJ, Pascual R (1987) News on the biology and taxonomy of the marsupials Thylacosmilidae (late Tertiary of Argentina). Acad Nac Cienc Exactas Fis Nat B Aires 39:219–246
Goin FJ, Pardiñas UFJ (1996) Revisión de las especies del género Hyperdidelphys Ameghino, 1904 (Mammalia, Marsupialia, Didelphidae), su significado filogenético, estratigráfico y adaptativo en el Neógeno del Cono Sur Sudamericano. Estudios Geol 52:327–359
Goin FJ, Forasiepi AM, Candela AM, Ortiz-Jaureguizar E, Pascual R, Archer M, Godthelp H, Muirhead J, Augee M, Hand S, Wroe S (2002) Earliest Paleocene Marsupials from Patagonia. In: First International Palaeontological Congress. Sydney, Abstracts
Goin FJ, Abello MA, Chornogubsky L (2010) Middle tertiary marsupials from central Patagonia (early Oligocene of Gran Barranca): understanding South America’s Grande Coupure. In: Madden RH, Carlini AA, Vucetich MG, Kay RF (eds) The paleontology of Gran Barranca: evolution and environmental change through the middle Cenozoic of Patagonia. Cambridge University Press, New York, pp 71–107
Goin FJ, Gelfo NF, Chornogubsky L, Woodburne MO, Martin T (2012) Origins, radiations, and distribution of South American Mammals: from greenhouse to icehouse worlds. In: Patterson BD, Costa LP (eds) Bones, clones, and biomes: an 80-million year history of Recent Neotropical mammals. University of Chicago Press, Chicago, pp 20–50
Goin FJ, Woodburne MO, Zimicz AN, Martin GM, Chornogubsky L (2016) A brief history of South American metatherians, evolutionary contexts and intercontinental dispersals. Springer, Dordrecht
Goswami A, Milne N, Wroe S (2011) Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proc R Soc B 278:1831–1839
Gould S, Calloway C (1980) Clams and brachiopods; ships that pass in the night. Paleobiology 6:383–396
Gould GC, McFadden BJ (2004) Gigantism, dwarfism, and Cope’s Rule: “Nothing in evolution makes sense without a phylogeny”. Bull Am Mus Natl Hist 285:219–237
Green B (1997) Field energetics and water flux in marsupials. In: Saunders NR, Hinds LA (eds) Marsupial biology: recent research, new perspectives. University of New South Wales Press, Sidney, pp 143–162
Hammer O (2016) Past 3.0. http://folk.uio.no/ohammer/past
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Pal Elect 4(1):9 pp
Jones W, Rinderknecht A, Montenegro F, Alvarenga H, Ubilla M (2016) New evidences of terror birds (Cariamae, Phorusrhacidae) from the Late Pleistocene of Uruguay. In: 9th international meeting of the society of Avian paleontology and evolution, abstracts, vol 1, p 16
Kraglievich JL (1952) El perfil geológico de Chapadmalal y Miramar. Resumen Preliminar. Rev Mus Mun Cs Nat y Trad Mar del Plata 1:8–37
Kraglievich JL, Olazábal AG (1959) Los prociónidos extinguidos del género Chapalmalania Ameghino. Rev Mus Argent Cienc Nat, Zool 6:1–59
Krause DW (1986) Competitive exclusion and taxonomic displacement in the fossil record: the case of rodents and multituberculates in North America. In: Flanagan KM, Lillegraven JA (eds) Vertebrates, phylogeny and philosophy. University of Wyoming Contributions to Geology, Special Paper 3:119–130
Krockenberger A (2006) Lactation. In: Armati P, Dickman C, Hume I (eds) Marsupials. Cambridge University Press, Cambridge, pp 108–136
Langer M, Ezcurra M, Bittencourt J, Novas F (2009) The origin and early evolution of dinosaurs. Biol Rev 85:55–110
Latrubesse EM, da Silva SAF, Cozzuol M, Absy ML (2007) Late Miocene continental sedimentation in southwestern Amazonia and its regional significance: biotic and geological evidence. J South Am Earth Sci 23:61–80
Lima-Ribeiro MS, Diniz-Filho JAF (2013) American megafaunal extinctions and human arrival: improved evaluation using a meta-analytical approach. Quat Int 299:38–52
Liow LH, Finarelli JA (2014) A dynamic global equilibrium in carnivoran diversification over 20 million years. Proc R Soc B 281:20132312
López-Aguirre C, Archer M, Hand SJ, Laffan SW (2017). Extinction of South American sparassodontans (Metatheria): environmental fluctuations or complex ecological processes? Palaeontology 60:91–115
MacFadden BJ, Cerling TE, Prado J (1996) Cenozoic terrestrial ecosystem evolution in Argentina: evidence from carbon isotopes of fossil mammal teeth. Palaios 11:319–327
MacFadden BJ, Labs-Hochstein J, Hulbert RC (2007) Revised age of the late Neogene terror bird (Titanis) in North America during the Great American Interchange. Geology 35(2):123–126
MacPhee RDE, Marx PA (1997) The 40,000-year plague: humans, hyperdisease, and first-contact extinctions. Smithsonian Institution Press, Washington, DC, pp 169–217
Marengo H (2015) Neogene micropaleontology and stratigraphy of Argentina. The Chaco-Paranense basin and the Península de Valdés. Springer, Dordrecht
Marshall LG (1976) Evolution of the Thylacosmilidae, extinct sabertooth marsupials of South America. PaleoBios 23:1–30
Marshall LG (1977) Evolution of the carnivorous adaptive zone in South America. In: Hecht MK, Goody PC, Hecht BM (eds) Major patters in vertebrate evolution. Plenum Press, New York, pp 709–722
Marshall LG (1978) Evolution of the Borhyaenidae, extinct South American predaceous marsupials. Univ Calif Publ Geol Sci 117:1–89
Marshall LG (1979) Review of the Prothylacyninae, an extinct subfamily of South American “dog–like” marsupials. Fieldiana. Geol [ns] 3:1–50
Marshall LG (1981) Review of the Hathlyacyninae, an extinct subfamily of South American “dog–like” marsupials. Fieldiana. Geol [ns] 7:1–120
Marshall LG, Cifelli RL (1990) Analysis of changing diversity patterns in Cenozoic Land Mammal Age faunas, South America. Palaeovertebrata 19:169–210
Martin PS, Wright HEJ (eds) (1967) Pleistocene extinctions: the search for a cause. Yale University Press, New Haven
McNab BK (1986) Food habits, energetics and the reproduction of marsupials. J Zool 208:595–614
McNab BK (2005) Uniformity in the basal metabolic rate of marsupials: its causes and consequences. Rev Chil Hist Nat 78:183–219
McNab BK (2008) An analysis of the factors that influence the level and scaling of mammalian BMR. Comp Biochem Physiol A 151:5–28
Metcalf JL, Turney C, Barnett R, Martin F, Bray SC, Vilstrup JT, Orlando L, Salas-Gismondi R, Loponte D, Medina M, De Nigris M, Civalero T, Fernández PM, Gasco A, Duran V, Seymour KL, Otaola C, Gil A, Paunero R, Prevosti FJ, Bradshaw CJA, Wheeler JC, Borrero L, Austin JJ, Cooper A (2016) Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the Last Deglaciation. Sci Adv 2:e1501682
Mitchell KJ, Bray SC, Bover P, Soibelzon L, Schubert BW, Prevosti FJ, Prieto A, Martin F, Austin JJ, Cooper A (2016) Ancient mitochondrial DNA reveals convergent evolution of giant short-faced bears (Tremarctinae) in North and South America. Biol Let 12:20160062
Oliveira TG, Pereira JA (2014) Intraguild predation and interspecific killing as structuring forces of Carnivoran Communities in South America. J Mam Evol 21(4):427–436
Ortiz Jaureguizar E (1989) Analysis of the compositional changes of the South American mammal fauna during the Miocene-Pliocene (Panaraucanian Faunistic Cycle). V Internatl Ther Congr Abstracts 277–278
Ortiz Jaureguizar E (2001) Cambios en la diversidad de los mamíferos sudamericanos durante el lapso Mioceno Superior-Holoceno: el caso pampeano. In: Meléndez G, Herrera Z, Delvene G, Azanza B (eds) Los fósiles y la paleogeografía. Publicaciones del SEPAZ, Univ Zaragoza 5:397–403
Ortiz Jaureguizar E, Prado JL, Alberdi MT (1995) Análisis de las comunidades de mamíferos continentales del Plio-Pleistoceno de la región pampeana y su comparación con la del área de mediterráneo occidental. In: Alberdi MT, Leone G, Tonni EP (eds) Evolución biológica y climática de la Región Pampeana durante los últimos cinco millones de años. Un ensayo de correlación con el Mediterráneo Occidental. Monografías del Museo Nacional de Ciencias Naturales, Madrid, pp 385–406
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153:493–508
Paolillo A, Linares OJ (2007) Nuevos cocodrilos Sebecosuchia del Cenozoico Suramericano (Mesosuchia: Crocodylia). Paleobiología Neotropical 3:1–25
Pascual R, Odreman Rivas OE (1971) Evolución de las comunidades de vertebrados del Terciario Argentino. Los aspectos paleozoogeográficos y paleoclimáticos relacionados. Ameghiniana 8:372–412
Pascual R, Bond M (1986) Evolución de los marsupiales cenozoicos de Argentina. Actas IV Congr Arg Paleontol y Bioest 2:143–150
Pascual R, Ortiz Jaureguizar E (1990) Evolving climates and mammal faunas in Cenozoic South American. J Human Evol 19:23–60
Pascual R, Vucetich MG, Scillato-Yané GJ, Bond M (1985) Main pathways of mammalian diversification in South America. In: Stehli FG, Webb SD (eds) The Great American Biotic Interchange. Plenum Press, New York, pp 219–247
Patterson B, Pascual R (1972) The fossil mammal fauna of South America. In: Keast A, Erk FC, Glass B (eds) Evolution, mammals and Southern continents. State University of New York Press, New York, pp 274–309
Pires MM, Silvestro D, Quental TB (2015) Continental faunal exchange and the asymmetrical radiation of carnivores. Proc R Soc B 282:20151952. doi:10.1098/rspb.2015.1952
Politis GG, Gutiérrez MA, Rafuse DJ, Blasi A (2016) The arrival of Homo sapiens into the Southern Cone at 14,000 years ago. PLoS ONE 11(9):e0162870. doi:10.1371/journal.pone.0162870
Prado JL, Martínez-Maza C, Alberdi MT (2015) Megafauna extinction in South America: a new chronology for the Argentine Pampas. Palaeogeogr Palaeoclimatol Palaeoecol 425:41–49
Prates L, Prevosti FJ, Berón M (2010) First records of prehispanic dogs in Southern South America (Pampa, Patagonia, Argentina). Curr Anthropol 51:273–280
Prevosti FJ (2006) Grandes cánidos (Carnivora, Canidae) del Cuaternario de la Republica Argentina: sistemática, filogenia, bioestratigrafíay paleoecología. Unpublished PhD thesis, Universidad Nacional de La Plata, La Plata, 506 pp
Prevosti FJ, Vizcaíno S (2006) The carnivore guild of the late Pleistocene of Argentina: Paleoecology and carnivore richness. Acta Palaeontol Pol 51:407–422
Prevosti FJ, Soibelzon LH (2012) The evolution of South American carnivore fauna: a paleontological perspective. In: Patterson B, Costa LP (eds) Bones, clones and biomes: the history and geography of recent Neotropical mammals. University Chicago Press, Chicago, pp 102–122
Prevosti FJ, Martin FM (2013) Paleoecology of the mammalian predator guild of the southern patagonia during the latest Pleistocene: ecomorphology, stable isotopes, and taphonomy. Quatern Int 305:74–84
Prevosti FJ, Pereira JA (2014) Community structure of South American Carnivores in the past and present. J Mam Evol 21(4):363–368
Prevosti FJ, Turazzini GF, Chemisquy MA (2010) Morfología craneana en tigres dientes de sable: alometría, función y filogenia. Ameghiniana 47:239–256
Prevosti FJ, Forasiepi AM, Ercoli MD, Turazzini GF (2012) Paleoecology of the mammalian carnivores (Metatheria, Sparassodonta) of the Santa Cruz Formation (late early Miocene). In: Vizcaíno SF, Kay RF, Bargo MS (eds) early Miocene paleobiology in Patagonia. Cambridge University Press, Cambridge, pp 173–193
Prevosti FJ, Forasiepi A, Zimicz N (2013) The evolution of the Cenozoic terrestrial mammalian predator guild in South America: competition or replacement? J Mammal Evol 20:3–21
Prevosti FJ, Ramírez MA, Martin F, Udrizar Sauthier DE, Carrera M (2015) Extinctions in near time: new radiocarbon dates point to a very recent disappearance of the South American fox Dusicyon avus (Carnivora: Canidae) Biol J Linn Soc 116:704–720
Raia P, Piras P, Kotsakis T (2005) Turnover pulse or Red Queen? Evidence from the large mammal communities during the Plio-Pleistocene of Italy. Palaeogeogr Palaeoclimatol Palaeoecol 221:293–312
Reig OA (1981) Teoría del origen y desarrollo de la fauna de mamíferos de América del Sur. Monographiae Naturae 1:1–162
Reguero MA, Candela A, Cassini G (2010) Hypsodonty and Body Size in rodent-like notoungulates. The Paleontology of Gran Barranca: Evolution and Environmental Change through the Middle Cenozoic of Patagonia. New York, Cambridge University Press, 1–448
Riff D, Romano PSR, Ribeiro Oliveira G, Aguilera AO (2010) Neogene crocodile and turtle fauna. In: Hoorn C, Wesselingh FP (eds) Northern South America Amazonia, landscape and species evolution: a look into the past. Blackwell Publishing, Oxford, pp 259–280
Rosenzweig ML, McCord RD (1991) Incumbent replacement: evidence for long-term evolutionary progress. Paleobiology 17:202–213
Rutter N, Coronato A, Helmens K et al (2012) Glaciations in North and South America from the Miocene to the Last Glacial Maximum. Comparisons, linkages and uncertainties. Springer, Dordrecht
Savage RJG (1977) Evolution in carnivorous mammals. Palaeontology 20:237–271
Schultz PH, Zarate M, Hames W, Camilión C, King J (1998) A 3.3 Ma impact in Argentina and possible consequences. Science 282:2061–2063
Segura V, Cassini GH, Prevosti FJ (2016) Three-dimensional cranial ontogeny in pantherines (Panthera leo, P. onca, P. pardus, P. tigris; Carnivora: Felidae). Biol J Linnean. doi:10.1111/bij.12888
Sepkoski JJ Jr (1996) Patterns of Phanerozoic extinctions: a perspective from global databases. In: Walliser H (ed) Global events and event stratigraphy. Springer, Berlin, pp 35–52
Sepkoski JJ Jr (2001) Competition in evolution. In: Briggs DEG, Crowther PR (eds) Paleobiology II. Blackwell Sciences, Oxford, pp 171–175
Sepkoski JJ Jr, McKinney FK, Lidgard S (2000) Competitive displacement among post-Paleozoic cyclostome and cheilostome bryozoans. Paleobiology 26:7–18
Shipman P (2015) The Invaders: how humans and their Dogs Drove Neanderthals to Extinction. Harvard university Press, Cambridge (Massachusetts), pp 1–266
Silvestro D, Antonelli A, Salamin N, Quental TB (2015) The role of clade competition in the diversification of North American canids. PNAS 112(28):8684–8689
Simpson GG (1950) History of the fauna of Latin America. Am Scientist 38:361–389
Simpson GG (1969) South American mammals. In: Fitkau EJ, Illies J, Klinge H, Schwabe GH, Sioli H (eds) Biogeography and ecology in South America. Dr. W Junk Publishers, The Hague, pp 876–909
Simpson GG (1971) The evolution of marsupials in South America. An Acad Bras Cs 43:103–118
Simpson GG (1980) Splendid isolation. The curious history of South American mammals. Yale University Press, New Haven
Soibelzon LH (2011) First description of milk teeth of fossil South American procyonid from the lower Chapadmalalan (late Miocene-early Pliocene) of “Farola Monte Hermoso”, Argentina: paleoecological considerations. Palaontologische Z 85:83–89
Soibelzon LH, Schubert BW (2011) The largest known bear, Arctotherium angustidens, from the early Pleistocene pampean region of Argentina: with a discussion of size and diet trends in bears. J Paleontol 85:69–75
Soibelzon E, Gasparini GM, Zurita AE, Soibelzon LH (2008) Análisis faunístico de vertebrados de las toscas del Río de La Plata (Buenos Aires, Argentina): un yacimiento paleontológico en desaparición. Rev Mus Arg Cs Nat 10(2):291–308
Soibelzon LH, Pomi LH, Tonni EP, Rodriguez S, Dondas A (2009) First report of a South American short-faced bears’ den (Arctotherium angustidens): palaeobiological and palaeoecological implications. Alcheringa 33(3):211–222
Soibelzon LH, Zamorano M, Scillato-Yané GJ, Soibelzon E, Tonni EP (2012) Un glyptodontidae de gran tamaño en el holoceno temprano de la región pampeana, argentina. Rev Bras Paleontol 15(1):105–112
Steele J, Politis G (2009) AMS 14C dating of early human occupation of southern South America. J Arch Sci 36(2):419–429
Suarez C, Forasiepi AM, Goin FJ, Jaramillo C (2015) Insights into the Neotropics prior to the Great American Biotic Interchange: new evidence of mammalian predators from the Miocene of Northern Colombia. J Vert Paleontol. doi:10.1080/02724634.2015.1029581
Surovell TA, Pelton SR, Anderson-Sprecher R, Myers AD (2015) Test of Martin’s overkill hypothesis using radiocarbon dates on extinct megafauna. PNAS 113(4):886–891
Tambussi C, Ubilla M, Perea D (1999) The youngest large carnassials bird (Phorusrhacidae, Phorusrhacinae) from South America (Pliocene–early Pleistocene of Uruguay). J Vertebr Paleontol 19:404–406
Tonni EP (1977) El rol ecológico de algunas aves fororracoideas. Ameghiniana 14:316
Tonni EP, Alberdi MT, Prado JL, Bargo MS, Cione AL (1992) Changes of mammal assemblages in the Pampean Region (Argentina) and their relation with the Plio-Pleistocene boundary. Palaeogeogr Palaeoclimatol Palaeoecol 95:179–194
Van Valen L (1973) A new evolutionary law. Evol Theor 1:1–30
Van Valkenburgh B (1999) Major patterns in the history of carnivorous mammals. Ann Rev Earth Plan Sci 27:463–493
Van Valkenburgh B (2007) Déjà vu: the evolution of feeding morphologies in the Carnivora. Integr Comp Biol 47:147–163
Van Valkenburgh B, Wang X, Damuth J (2004) Cope’s rule, hypercarnivory, and extinction in North American canids. Science 306:101–104
Van Valkenburgh B, Hayward MW, Ripple WJ, Meloro C, Roth VL (2015) The impact of large terrestrial carnivores on Pleistocene ecosystems. PNAS 113(4):862–867
Villafañe AL, Ortiz-Jaureguizar E, Bond M (2006) Cambios en la riqueza taxonómica y en las tasas de primera y última aparición de los Proterotheriidae (Mammalia, Litopterna) durante el Cenozoico. Estudios Geológicos 62:155–166
Villavicencio NA, Lindsey EL, Martin FM, Borrero LA, Moreno PI, Marshall CR, Barnosky AD (2016) Combination of humans, climate, and vegetation change triggered late Quaternary megafauna extinction in the Última Esperanza region, southern Patagonia, Chile. Ecography 39:125–140
Viranta S (1996) European Miocene Amphicyonidae—taxonomy, systematics and ecology. Acta Zool Fen 204:1–61
Vizcaíno SF, Bargo M, Dondas A (2001) Pleistocene burrows in the Mar del Plata area (Argentina) and their probable builders. Acta Pal Pol 46(2):289–301
Vizcaíno SF, Fariña RA, Zárate MA, Bargo MS, Schultz P (2004) Palaeoecological implications of the mid-Pliocene faunal turnover in the Pampean Region (Argentina). Palaeogeogr Palaeoclimatol Palaeoecol 213:101–113
Vizcaíno SF, Cassini GH, Toledo N, Bargo MS (2012) On the evolution of large size in Mammalian Herbivores of Cenozoic faunas of Southern South America. In: Patterson B, Costa LP (eds) Bones, clones and biomes: the history and geography of Recent Neotropical mammals. University Chicago Press, Chicago, pp 76–101
Wang X, Tedford R, Antón M (2008) Dogs: their fossil relatives and evolutionary history. Columbia University Press, Chichester
Werdelin L (1987) Jaw geometry and molar morphology in marsupial carnivores: analysis of a constraint and its macroevolutionary consequences. Paleobiology 13:342–350
Whitney Smith E (2009) The second-order predation hypothesis of pleistocene extinctions: a system dynamics model. VDM Verlag Dr. Müller, Saarbrücken, pp 1–156
Wroe S, Argot C, Dickman C (2004) On the rarity of big fierce carnivores and primacy of isolation and area: tracking large mammalian carnivore diversity on two isolated continents. Proc Royal Soc Lond 271:1203–1211
Wroe S, Chamoli U, Parr WCH (2013) Comparative Biomechanical Modeling of Metatherian and Placental Saber-Tooths: A Different Kind of Bite for an Extreme Pouched Predator. PLoS One 8:8–16
Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292:686–693
Zimicz AN (2012) Ecomorfología de los marsupiales paleógenos de América del Sur. Unpublished Ph.D. thesis, Universidad Nacional La Plata
Zimicz AN (2014) Avoiding competition: the ecological history of metatherian carnivores. J Mamm Evol 21:383–393
Zurita AE, Soibelzon L, Soibelzon E, Gasparini GM, Cenizo M, Arzani H (2010) Accessory protection structures in Glyptodon Owen (Xenarthra, Cingulata, Glyptodontidae). Ann Pal 96(1):1–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Prevosti, F., Forasiepi, A.M. (2018). Evolution and Biological Context of South American Mammalian Carnivores During the Cenozoic and the Biological Context. In: Evolution of South American Mammalian Predators During the Cenozoic: Paleobiogeographic and Paleoenvironmental Contingencies. Springer Geology. Springer, Cham. https://doi.org/10.1007/978-3-319-03701-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-03701-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03700-4
Online ISBN: 978-3-319-03701-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)