Abstract
A state-of-the-art review of the Cenozoic fossil record from Western Amazonia is provided, based on literature and new data (regarding Paleogene native ungulates). It allows summarizing the evolution and dynamics of middle Eocene–Holocene mammalian guilds, at the level of species, families, and orders. Major gaps in the Western Amazonian mammal record occur in the pre-Lutetian and early Miocene intervals, and in the Pliocene epoch. Twenty-three orders, 89 families, and 320 species are recognized in the fossil record, widely dominated by eutherians from the middle Eocene onward. Probable Allotheria (Gondwanatheria) occur only in the earliest interval, whereas Metatheria and Eutheria are conspicuous components of any assemblage. Taxonomic diversity was probably fairly constant at the ordinal level (~12–14 orders in each time slice considered) and much more variable in terms of family and species richness: if most intervals are characterized by 40–50 co-occurring species and 19–31 co-occurring families, the early Miocene period illustrates a depauperate fauna (21 species, 17 families), strongly contrasting with the late Miocene climactic guild (82 species, 38 families). Recent mammalian taxonomic diversity from Western Amazonia (12 orders, 37 families, and 286 species) is at odds with all past intervals, as it encompasses only three orders of South American origin (Didelphimorphia, Cingulata, and Pilosa) but four North American immigrant orders (Artiodactyla, Perissodactyla, Carnivora, and Lagomorpha). In terms of taxonomic diversity, recent mammalian guilds are fully dominated by small-sized taxa (Chiroptera, Rodentia, and Primates). This overview also confirms the scarcity of large mammalian flesh-eaters in ancient Neotropical mammalian assemblages. The pattern and the timing of mammalian dispersals from northern landmasses into Western Amazonia are not elucidated yet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In spite of being intensively covered by either water or vegetation, Cenozoic deposits from Western Amazonia have yielded a wide range of fossil mammals, documenting three major lineages (Allotheria, Metatheria, and Eutheria). Most of these mammals are highly relevant to test major evolutionary and/or biogeographic hypotheses at either the Neotropical, South American, or Panamerican scale. Before the 1980s, specimens collected in Western Amazonian lowlands were primarily recovered as isolated or float remains, with poor stratigraphic constraints (e.g., Spillmann 1949; Willard 1966; Simpson and Paula-Couto 1981). Nevertheless, the fossil record has been widely substantiated in the meantime, thanks to considerable field efforts by international multidisciplinary teams, mostly focusing on Brazilian, Peruvian, and (to a lesser extent) Bolivian Amazonian lowlands (Fig. 1; see Antoine et al. 2016 for a review). In contrast, virtually no pre-Pleistocene mammal remain is known from Ecuadorian or Colombian Amazonian lowlands so far (Marshall et al. 1983; Webb and Rancy 1996).
This contribution aims at providing a state-of-the-art review of the Cenozoic mammalian record from Western Amazonia (mainly from Peru and Brazil), notably by incorporating new specimens of large Paleogene native ungulates (Figs. 2 and 3). Based on this Neotropical record, we summarize the evolution and dynamics of mammalian guilds in the same time and space at the ordinal, familiar, and specific levels (Fig. 4).
Stratigraphic chart of Cenozoic mammal-bearing localities from Western Amazonia mentioned in the text. CTA, localities from Contamana; Fm., Formation; MD, localities from the Upper Madre de Dios. Based on Simpson and Paula Couto (1981), Antoine et al. (2012, 2013, 2016), Marivaux et al. (2012), Ribeiro et al. (2013), and Tejada-Lara et al. (2015)
Mammalian remains from Paleogene deposits of Peruvian Amazonia. a-d Astrapotheriidae indet., upper canine in lingual (a), mesial (b), and labial views (c), and cross-section (d). Bagua-La Bocana, Amazonas, late Eocene. e-f Baguatherium jaureguii, tip of an unworn anterior tooth, in buccal view (e) and cross section (f). Bagua-La Bocana, Amazonas, late Eocene. g-i Pyrotherium macfaddeni, right m1 in lingual (g), occlusal (h), and labial views (i). Bellavista, San Martín, late Oligocene (Deseadan). The occlusal outline is reconstructed based on Shockey and Anaya Daza (2004). j-k Pyrotherium cf. macfaddeni, right m3 in occlusal (j) and labial views (k). Surface collection, Río Beu, Ucayali, supposedly late Oligocene (Deseadan) in age. l-n Uruguaytheriinae indet., right P4 in occlusal view (l) and i3 in lingual (m) and occlusal views (n). Surface collection, Río Beu, Ucayali, supposedly late Oligocene (Deseadan) in age. Scale bars = 2 cm (a–d) and 1 cm (e–n)
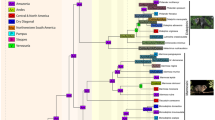
Mammalian taxonomic diversity in Western Amazonia from the middle Eocene onward. Evolution of diversity through time, at the ordinal, familial, and specific levels within Allotheria, Metatheria, and Eutheria. Time intervals considered coincide with standard ages of fossil-yielding localities: middle Eocene, late Eocene + early Oligocene (most localities are not constrained enough to split that interval), late Oligocene, early Miocene, middle Miocene, late Miocene, Pliocene, Pleistocene–Holocene, and Recent. Many families are monospecific, mostly due to sampling biases (low specimen number per locality). See Supplemental Material for taxonomic lists and counts. Scale bars = 10 taxa (grey or black: species; white: families). For recent orders, numbers denote family/species diversity based on Eisenberg and Redford (2000). The temporal extension of the Pebas Mega-Wetland System is provided by Salas-Gismondi et al. (2015)
Material and Methods
We have primarily taken into account time-constrained localities from the concerned area, further privileging plurispecific assemblages described in the literature and/or new ones uncovered by our team. Detailed information about the inferred age of all localities (hypothesized standard age and reference to a South American Land Mammal Age [SALMA]) and their mammalian content (faunal lists) can be found in Supplemental Material.
We have restricted our locality sample to Western Amazonian lowlands as observed today, i.e., matching the Amazon hydrographic basin (eastern Bolivia, western Brazil, southeastern Colombia, southeastern Ecuador, and eastern Peru) and bordered to the east by the Carauari Arch (Mora et al. 2010). We have included a few data from subandean zone low-altitude localities of Bolivia (Río Yapacani and Quebrada Saguayo; Marshall et al. 1983) and Peru (Bagua-La Bocana and Upper Madre de Dios; Fig. 2). Species-rich Andean localities, such as Salla (Bolivia, late Oligocene), Quebrada Honda (Bolivia, late middle Miocene), or La Venta (Colombia, late middle Miocene), were not taken into account in our study, as they fall outside Western Amazonian lowlands today, even though the concerned areas were probably part of a single biogeographic province by that time (e.g., Marshall et al. 1983; Croft 2007; Tejada-Lara et al. 2015).
Biases in species-richness and relative abundance may be due to taphonomic factors (e.g., hydrodynamics or predation) and result in the over-representation and better taxonomic assignment of micromammals with respect to meso- and mega-mammals, particularly perceptible for early time intervals, as it adds to a sampling bias (due to intensive screen-washing; e.g., Contamana and Santa Rosa localities; Antoine et al. 2016). On the other hand, large mammals are widely over-represented whereas micro- and meso-mammals are virtually absent from historical findings (e.g., Spillmann 1949; Willard 1966), especially in Pleistocene–Holocene fluvial deposits (e.g., Simpson and Paula-Couto 1981; Rancy 1991; Webb and Rancy 1996; Latrubesse and Rancy 1998), as they were merely resulting from surface collecting.
The evolution of mammalian guilds in Western Amazonia throughout the middle Eocene–Holocene interval is illustrated in Fig. 4. It was analyzed at the specific, familial, and higher levels (= order-group; for further information, see Antoine et al. 2016). Time intervals coincide mostly with successive sub-epochs or epochs (Gradstein et al. 2012): middle Eocene, late Eocene–early Oligocene, late Oligocene, early Miocene, middle Miocene, late Miocene, Pliocene (almost no attested record), and Pleistocene–Holocene. The concerned assemblages result from compiled taxonomic lists (either available in the literature or corresponding to original work), as detailed in the Supplemental Material.
Results
This section provides a group-based review of the Cenozoic mammalian record in Western Amazonia. To our knowledge, the earliest mammalian remain from Amazonian lowlands is an enamel fragment showing Hunter-Schreger bands originating from upper Paleocene marginal marine deposits in the Upper Madre de Dios, Peru (Mammalia indet., MD-85 locality; Louterbach et al. 2014). Identifiable remains range from the late middle Eocene (Pozo Formation [Fm.], Contamana, Peru; Figs. 1 and 2; Antoine et al. 2012, 2016) to the Holocene (e.g., Rancy 1991; Latrubesse and Rancy 1998).
Three major mammaliaform groups are recorded in the Cenozoic of Amazonian lowlands: (1) probable Allotheria (Gondwanatheria) occur only in Contamana (CTA-27, late middle Eocene; unidentified ?sudamericid) and in Santa Rosa (?late Eocene-early Oligocene; unnamed taxon of uncertain affinities), which would coincide with both the last occurrences of this enigmatic group in South America and the only ones in northern South America (Goin et al. 2004, 2012; Antoine et al. 2012, 2016). In contrast, (2) Metatheria and (3) Eutheria are conspicuous components of any corresponding mammalian assemblage from the middle Eocene onward (Fig. 4).
Among Metatheria, probable herpetotheriids (Rumiodon spp.) were identified in the middle Eocene–?early Oligocene interval in the CTA-27 locality at Contamana and Santa Rosa (Goin and Candela 2004; Antoine et al. 2016). Didelphimorphians (middle Eocene–late Miocene), documented by scarce dental remains, are either of uncertain affinities or referred to as marmosids (Marmosa (Micoureus) cf. laventica; MD-67, Upper Madre de Dios, middle Miocene; Antoine et al. 2012) and didelphids (unidentified didelphids and Didelphis solimoensis; Acre, late Miocene; Czaplewski 1996; Negri et al. 2010). Western Amazonia shelters 29 species of living didelphids (Eisenberg and Redford 2000). Sparassodonta remains are also scarce, small-sized (except the large canine from Fitzcarrald; Tejada-Lara et al. 2015), and requiring a taxonomic revision (four unidentified borhyaenoids are mentioned); the hathliacynids Patene campbelli and Sipalocyon sp. were recognized in Santa Rosa (late-Eocene-early Oligocene; Goin and Candela 2004) and in MD-67 (middle Miocene; Antoine et al. 2012; Fig. 4), respectively. Polydolopimorphians are abundant, small-sized, and quite diversified in the middle Eocene–early Oligocene interval (Pozo Fm. in Contamana, Santa Rosa locality; Figs. 2 and 4), with bonapartherioids-argyrolagoids (Wamradolops and close allies, plus Hondonadia pittmanae), and prepidolopids (Incadolops ucayali and two unidentified species; Goin and Candela 2004; Antoine et al. 2016). This range coincides with the climax of polydolopimorphians at middle and high latitudes (Goin et al. 2012). After this interval, a single late Miocene occurrence is attested for polydolopimorphians, corresponding to a lower molar of an argyrolagid recovered in CTA-44 (Mayoan SALMA; Antoine et al. 2016). Paucituberculatans are only recorded in the middle Eocene-early Miocene interval (Fig. 4; Supplemental Material). Santa Rosa and late middle Eocene localities from Contamana yielded palaeothentoids of uncertain affinities (Sasawatsu spp., Perulestes spp., and/or cf. Perulestes), whereas Deseadan SALMA localities from Contamana (Fig. 2) record abderitids (Abderites sp. and cf. Abderites), a possible palaeothentid, and a probable caenolestid (Goin and Candela 2004; Antoine et al. 2016). The only post-Paleogene paucituberculatan from Western Amazonia is an unidentified ?caenolestid in CTA-63 (early Miocene; Colhuehuapian–Santacrucian SALMAs; Antoine et al. 2016). Finally, two enigmatic metatherians were described in Santa Rosa (Wirunodon chanku and Kiruwamaq chisu; Goin and Candela 2004).
Eutherians have a far much better fossil record in Amazonian lowlands than allotherians and metatherians, in terms of both abundance and taxonomic diversity (Fig. 4; Supplemental Material). Fifteen orders are documented, either pertaining to South American natives (Cingulata and Pilosa, within Xenarthra; Notoungulata, Astrapotheria, Litopterna, and Pyrotheria among native ungulates), originating from remote landmasses (Rodentia, Chiroptera, and Primates), having aquatic habitats (Sirenia and Cetacea), or being North American invaders (Proboscidea, Artiodactyla, Perissodactyla, and Carnivora). Thus far, Lagomorpha have no fossil record in Western Amazonia.
Xenarthran remains consist of cingulates (Dasypodidae, Glyptodontidae, Pampatheriidae, and incertae sedis) and folivoran pilosans (mylodontoid and megatherioid sloths), whereas no vermilinguan pilosan is recorded (in contrast with recent times and with La Venta, middle Miocene of Colombia; Kay and Madden 1997). Cingulates are recognized in all intervals (Fig. 4), mainly on the basis of isolated and often fragmentary osteoderms, which in turn somewhat blur their taxonomic assignment (see discussion in Gaudin and Croft 2015). Dasypodids have a middle Eocene–Recent range, with conspicuous representatives such as Stegosimpsonia (late middle Eocene, Contamana; Antoine et al. 2016), Parastegosimpsonia (Santa Rosa; Ciancio et al. 2013), or Anadasypus (Acre, late Miocene; Ribeiro et al. 2013). Glyptodontids are first recorded in CTA-32 (late Oligocene; Fig. 2) with a close ally of Neoglyptatelus (Antoine et al. 2016) and they reach a climax by late middle Miocene times (Fitzcarrald; Supplemental Material; Tejada-Lara et al. 2015). Glyptodon and other glyptodontids (e.g., Hoplophorus and Sclerocalyptus) occur in Pleistocene localities from Brazil (Upper Juruá), Ecuador (Río Napo; Rancy 1991), and/or Peru (Río Ucayali) and isolated osteoderms are also recovered floating on river banks (e.g., Río Inuya, Peru; Willard 1966; Antoine et al. 2007). Pampatheriids have a fairly long and continuous range (late Oligocene–late Pleistocene). In particular, the unidentified pampatheriid from CTA-61 (late Oligocene, Deseadan SALMA; Fig. 2) may be among the earliest representatives of the family (Salas-Gismondi et al. 2011). Acre local fauna yields Scirrotherium carinatum (Góis et al. 2013 and references therein). Vassallia minuta occurs in Río Yapacani, Bolivia (Pliocene; Marshall et al. 1983). Pampatherium and Holmesina are recognized in Brazilian late Pleistocene deposits of Upper Juruá and Araras/Periquitos, respectively (Rancy 1991; Holanda et al. 2011; Góis et al. 2012). Other cingulates of uncertain affinities, such as Yuruatherium tropicalis (Santa Rosa; Ciancio et al. 2013) and Eocoleophorus glyptodontoides (CTA-29, Pozo Fm., late middle Eocene; Salas-Gismondi et al. 2011; Antoine et al. 2016) occur in Paleogene Peruvian localities (Supplemental Material). The latter species was previously restricted to the Oligocene locality of Tremembé, eastern Brazil; Oliveira et al. 1997) and the cingulate from CTA-29 may show closer affinities to a new taxon from the Paleogene fauna of Guabirotuba (southeastern Brazil; Sedor et al. in revision). The earliest sloths from Western Amazonia are unidentified late Oligocene mylodontoids (CTA-61; Antoine et al. 2016). Mylodontids occur throughout the post-Oligocene interval, with three associated genera in the late Miocene Acre assemblage (Urumacotherium, Pseudoprepotherium, and cf. Ranculcus; Ribeiro et al. 2013). Oreomylodon wegneri, Lestodon armatus, and Catonyx sp. (Scelidotherium sp. in Rancy 1991) are recognized in Pleistocene deposits of Upper Juruá (Rancy 1991; Latrubesse and Rancy 1998). The late Pleistocene Río Napo assemblage in Ecuador yields Mylodon sp. (e.g., Rancy 1991). Holanda et al. (2011) mentioned a close ally of Ocnotherium in the late Pleistocene Rio Madeira Fm. (Araras/Periquitos, Brazil). The mylodontid Octodontobradys (often assigned to Orophodontidae among Mylodontoidea) is recorded in the Acre local fauna and CTA-57 (early late Miocene; Fig. 2). An early diverging megatherioid, cf. Hapalops, occurs in the ?early-middle Miocene Talismã locality (Purus River, Amazonas, Brazil; Ribeiro et al. 2013). The earliest megatheriids from Amazonian lowlands are the planopsine cf. Planops (Talismã; Ribeiro et al. 2013) and the megatheriine Megathericulus sp. (Fitzcarrald, late middle Miocene; Pujos et al. 2013). Small-sized Megatherium species and the large-sized Eremotherium laurillardi are recorded in most Pleistocene lowland localities from Peru, Brazil, and Ecuador (Rancy 1991; Pujos and Salas 2004; Supplemental Material). Megalonychids have a middle Miocene–Recent range, with Pliomorphus, a close ally of Protomegalonyx in the late Miocene of Acre (Ribeiro et al. 2013), and representatives of Megalonyx and Ocnopus in the Pleistocene of Upper Juruá (Rancy 1991). A single nothrotheriine occurrence is attested in the late Miocene of Acre (De Iuliis et al. 2011), with a sub-complete skeleton of Mionothropus cartellei, previously considered as Holocene in age (=Nothropus priscus in Frailey 1986). All other Pleistocene nothrotheriine occurrences in Western Amazonia are unclear (e.g., Rancy 1991; Webb and Rancy 1996). Three species of tree sloths (Bradypodidae and Megalonychidae) live today in the studied area.
South American native ungulates are a conspicuous element of most inventoried faunas (Supplemental Material). Four orders out of five are documented (Notoungulata, Astrapotheria, Litopterna, and Pyrotheria; Fig. 4). Xenungulata are not known in Amazonian lowlands primarily because they are restricted to the Paleocene–early Eocene interval (Gelfo et al. 2008; Woodburne et al. 2014), lacking any mammalian record in the studied area. Nevertheless, xenungulates may have roamed these lowlands during early Paleogene times, as both “etayoids” and carodniids have an early Eocene record in nearby regions, with Etayoa bacatensis in Bogotá, Colombia (Villarroel 1987) and Carodnia inexpectans in northwestern Peru (Antoine et al. 2015). Notoungulata are the most diversified native ungulate order in Western Amazonian assemblages, with a late Miocene climax (Fig. 4). They span the middle Eocene–Pleistocene interval, with Typotheria (“Archaeohyracidae,” Interatheriidae, and Hegetotheriidae; middle Eocene–late Miocene) and Toxodontia (Toxodontidae, Leontinidae, and Notohippidae; middle Eocene–latest Pleistocene), the earliest records of which consist mainly of isolated teeth or tooth fragments (e.g., Antoine et al. 2012, 2016). “Archaeohyracids” are restricted to the late middle Eocene of Contamana (unidentified taxon, CTA-27; Antoine et al. 2012). Interatheriidae occur throughout the documented interval. They are mostly referred to as taxa of uncertain affinities (unidentified interatheriines in CTA-27, Santa Rosa, CTA-61, and CTA-57; Shockey and Anaya Daza 2004; Antoine et al. 2016); Miocochilius anamopodus is found in Fitzcarrald (Laventan; Tejada-Lara et al. 2015). The single occurrence of a hegetotheriid is Prohegetotherium? sp. from CTA-61 (Deseadan SALMA; Antoine et al. 2016). Toxodontia are much better represented, first by unidentified taxa (Contamana [Pozo Fm. localities], Santa Rosa, and La Bocana-Bagua; Shockey and Anaya Daza 2004; Antoine et al. 2016; this work), then by toxodontids, from Deseadan deposits (CTA-61) onward (Supplemental Material; MacFadden 2005). A systematic revision would be needed as multiple synonymies can be alleged among the Amazonian representatives of Pericotoxodon, Gyrinodon, Trigodon, Palaeotoxodon, Paratrigodon, Trigodonops, “Plesiotoxodon,” and Neotrigodon (e.g., Ribeiro et al. 2013). Dental remains referred to as Toxodon sp. and/or Mixotoxodon sp., and/or Trigodonops lopesi are recorded until the latest Pleistocene in Bolivian, Brazilian, and Peruvian Amazonia (Rancy 1991; MacFadden 2005; Holanda et al. 2011). Leontiniids and notohippids are recognized in Paleogene deposits of Santa Rosa (unidentified leontiniid and ?Eomorphippus, respectively; Shockey and Anaya Daza 2004), in the early-middle Miocene locality 28 from the Upper Juruá (Purperia cribatidens; Ribeiro et al. 2013), and in late Miocene Acre localities (unidentified notohippid; Ribeiro et al. 2013).
Two families are documented among Astrapotheria: Trigonostylopidae (?Trigonostylops in CTA-27, late middle Eocene; Antoine et al. 2012, 2016) and Astrapotheriidae (late Eocene–middle Miocene). A large-sized astrapotheriid occurs in Bagua-La Bocana (Figs. 1 and 2). This locality was described as being Oligocene, “pre-Deseadan” in age, by Salas et al. (2006). Yet, it may date back to the late Eocene instead (Mustersan SALMA), with new 40Ar/39Ar constraints between 36.47 ± 1.74 Ma and 36.15 ± 0.91 Ma for feldspars from volcanic ashes located 20 m below and 10 m above the vertebrate locality, respectively (see Supplemental Material). The corresponding astrapotheriid fossil is an upper canine with characteristic pear-like cross section, weak curvature, continuous enamel around the crown, and flat shearing surface (Fig. 3a–d). Although of greater dimensions (preserved length = 160 mm; cross section = 36x25 mm), its morphology is similar to that of Astraponotus from coeval deposits of Patagonia (e.g., Kramarz et al. 2011). It is also reminiscent of the upper canines of younger large astrapotheriids, such as Astrapotherium and Parastrapotherium (Kramarz and Bond 2008). Two isolated teeth of a large astrapotheriid were collected in the early 2000s as float specimens on a bank of the Río Beu, in the Santa Rosa Native Community, less than 2 km away from the Santa Rosa locality; Fig. 2). The first one is interpreted as a third lower incisor (bilobate and low-crowned; Fig. 3m–n); the second is a right P4 with a strong labial cingulum, a long and sagittally elongated protoloph, and a strongly constricted protocone (Fig. 3l). There is no fold on the ectoloph, metaloph, or hypocone. This combination of features points to a taxon of uruguaytheriine affinities, probably distinct from all known taxa (for a review, see Vallejo-Pareja et al. 2015). Other Western Amazonian occurrences consist of middle Miocene uruguaytheriines, such as Xenastrapotherium amazonense (“locality 28,” Upper Juruá, Brazil; e.g., Ribeiro et al. 2013), Xenastrapotherium sp., and Granastrapotherium cf. snorki (Fitzcarrald; Goillot et al. 2011; Tejada-Lara et al. 2015). The fragmentary canine of a late Miocene uruguaytheriine, described by Frailey (1986: 33) in Acre, was surface-collected on a “sand bar.” Accordingly, the persistence of astrapotheriids in the late Miocene of Amazonian lowlands is highly questionable (Fig. 4; Goillot et al. 2011; Ribeiro et al. 2013).
Litopterna occur throughout the middle Eocene–Pleistocene interval in Western Amazonia (Fig. 4). Paleogene remains are not identified accurately (Supplemental Material). Macraucheniids and proterotheriids are documented in the early middle Miocene of Talismã (unidentified proterotheriid; Bergqvist et al. 1998), in the late middle Miocene of Fitzcarrald (dental and postcranial remains of Theosodon sp., cf. Tetramerhinus sp., and unidentified taxa; Tejada-Lara et al. 2015), in the late Miocene river banks of Acre and Amazonas (cf. Cullinia sp.; Ribeiro et al. 2013), and in a Pleistocene terrace from the Upper Madre de Dios (MD-61 Top; Macrauchenia sp.: isolated phalanx).
Pyrotheria have a strict Paleogene record in Western Amazonia, as in other South American regions (Fig. 4; Billet et al. 2010). Griphodon peruvianus occurs in the Chicoca Red Beds, northeastern Peru, considered middle Eocene in age (Patterson 1977), but without stratigraphic constraint. A close ally of Griphodon is documented in the late middle Eocene of Contamana (tooth fragments; Antoine et al. 2016). Baguatherium jaureguii is restricted to Bagua-La Bocana (Salas et al. 2006), late Eocene in age (see above). The tip of an unworn anterior tooth was unearthed in 2010 at this locality (Fig. 3e–f). A medium-sized pyrothere, provisionally referred to as ?Propyrotherium sp., was described in the Upper Juruá local fauna (Paula Couto 1982), and ascribed to the early Oligocene by Ribeiro et al. (2013). The Deseadan genus Pyrotherium (e.g., Shockey and Anaya Daza 2004) is recognized for the first time in Amazonian lowlands, with the small-sized species P. macfaddeni in Bellavista, San Martín, Peru (TAR-11 locality, Chambira Fm.; right m1 excavated in-situ [Width = 33 mm; estimated Length = 35–37 mm]: Fig. 3g–i) and P. cf. macfaddeni in the Alto Río Beu, Ucayali (right m3 [Width = 38 mm; estimated Length = 42 mm]: Fig. 3j–k). Although found as float on a river bank and broken in two pieces, the latter specimen points to the presence of (upper) Oligocene deposits in the close vicinity of Santa Rosa. This is in full contradiction with the hypothesis of a long-ranging unconformity in the concerned area (Eocene–late Miocene “Ucayali Unconformity”; Frailey and Campbell 2004; Bond et al. 2015), and likely to support further a post-Eocene age for this locality (see also Kay 2015).
“Stratum 2” immigrants (rodents, bats, and primates; Simpson 1980) have a fairly contrasted fossil record in Western Amazonia (Fig. 4). With almost 160 species recognized (~98 fossil and 61 living species), Rodentia is the most diversified mammalian order in the region (Supplemental Material). They span the late middle Eocene–Recent interval and include notably the earliest rodents from South America (Contamana, Pozo Fm.; Antoine et al. 2012, 2016). Species-rich communities are documented in both Paleogene and Neogene localities, with up to 17 and 18 co-occurring fossil species, respectively (Santa Rosa and Acre local faunas; Supplemental Material). Early faunas mostly encompass stem caviomorphs (e.g., Canaanimys and Cachiyacuy; late middle Eocene, Contamana) and stem representatives of Octodontoidea (Eoespina in Contamana localities; Eodelphomys, Eosallamys, and Eosachacui in Santa Rosa) and Cavioidea (Eobranisamys in CTA-27 and CTA-29; Eoincamys, Eobranisamys, and Eopicure in Santa Rosa; Eoincamys in Upper Juruá; Supplemental Material). They also include a chinchilloid (unidentified taxon in CTA-29; Supplemental Material) and an erethizontoid (Eopululo wigmorei in Santa Rosa; Frailey and Campbell 2004). The earliest representatives of living families are documented in Santa Rosa (Eodelphomys: Echimyidae [spiny rats]; Frailey and Campbell 2004) and in Oligocene localities of Contamana (unidentified adelphomyine echimyids and erethizontids [New World porcupines] in CTA-32 and CTA-61; Antoine et al. 2016). Echimyidae are further represented by an unidentified eumysopine in Acre (Ribeiro et al. 2013). Among cavioids, Caviidae only occur in the middle–late Miocene interval, with Guiomys sp. in MD-67 (early middle Miocene; Antoine et al. 2013), Prodolichotis pridiana in Fitzcarrald (late middle Miocene; Tejada-Lara et al. 2015), an unidentified caviine in CTA-44 and CTA-43 (earliest late Miocene; Antoine et al. 2016), and an indisputable dolichotine in Acre (late Miocene; Kerber et al. in revision). Hydrochoeridae (capybaras) are represented by the late Miocene cardiomyine Caviodon sp. and the hydrochoerine Cardiatherium sp. in Acre (Kerber et al. in revision), by Neochoerus aff. sulcidens in the late Pleistocene of Araras/Periquitos, Brazil (Holanda et al. 2011), and by Hydrochoerus sp. in the Pleistocene/Holocene of Fitzcarrald (Río Inuya; Antoine et al. 2007; Supplemental Material). Unidentified dasyproctids (agoutis) and cuniculids (pacas) were recognized in CTA-44 (earliest late Miocene; Antoine et al. 2016) and in Acre deposits (Ribeiro et al. 2013), respectively. Vucetich and Verzi (2002) recognized a representative of Dasyprocta in Upper Juruá deposits, most likely Pleistocene in age. Dinomyids (pacarana and its kin; Chinchilloidea) are a conspicuous element of Neogene Amazonian faunas, as in other regions of South America (e.g., Vucetich et al. 1999). They are mostly represented by small-sized taxa, such as Scleromys or close allies in early and early middle Miocene localities (MD-61 and MD-67, Upper Madre de Dios; CTA-63 and CTA-45, Contamana; see discussion in Antoine et al. 2013). Medium- and large-sized dinomyids are prominent in late middle and late Miocene localities, with Drytomomys and Potamarchus in Fitzcarrald (Tejada-Lara et al. 2015), CTA-44 Top and CTA-43 (Antoine et al. 2016), with Simplimus? in CTA-57, and with Drytomomys spp., Potamarchus spp., Pseudopotamarchus villanuevai, Telicomys amazonensis, Tetrastylus sp., Gyrabrius sp., Simplimus sp., and Scleromys sp. among others in faunas from Acre and Amazonas (Ribeiro et al. 2013; Kerber et al. 2015; in revision). Large-sized neoepiblemids first occur in Fitzcarrald (late middle Miocene: Neoepiblema sp.; Tejada-Lara et al. 2015) and span the late Miocene interval in Contamana (CTA-44 Top and CTA-10; Supplemental Material) and Acre (Neoepiblema horridula and N. ambrosettianus; Negri et al. 2010), where they further co-occur with sheep- to cow-sized representatives of Phoberomys (P. burmeisteri, P. bordasi, and P. minima; Ribeiro et al. 2013). Erethizontidae are never abundant in the fossil record of Western Amazonia. In Neogene deposits, they are documented by few isolated teeth and restricted to MD-67 (cf. Microsteiromys; early middle Miocene; Antoine et al. 2013), CTA-43 (Steiromys? sp.; Antoine et al. 2016), and Acre local fauna (gen. et sp. indet., found float in Upper Juruá; Kerber et al. in revision).
Fossil bats span the middle Eocene–late Miocene interval, with isolated dental remains poorly constrained in terms of infra-familiar assignment (Fig. 4; Supplemental Material). Only Microchiroptera are documented. The earliest representatives are unidentified molossids, phyllostomids, and microbats in the Pozo Fm. (CTA-27 and CTA-66, late middle Eocene; Antoine et al. 2016), and an unidentified microbat in Santa Rosa (Czaplewski and Campbell 2004). The most diversified assemblage occurs in CTA-32 (late Oligocene), with an unidentified emballonurid, a small vespertilionoid, and a probable rhinolophoid (Antoine et al. 2016). Emballonurids are also recognized in CTA-63 and CTA-75 (early and earliest late Miocene, respectively; Supplemental Material). Unidentified late Miocene molossids are mentioned along the Río Purus, Peru (Czaplewski 1996) and in CTA-44 Top (Antoine et al. 2016). Czaplewski (1996) described Noctilio lacrimaelunaris in the late Miocene Acre River fauna, as being the first fossil representative of the recent fish-eating noctilionid genus. Today, Chiroptera is the most species-rich order in Western Amazonian, with 118 living species (Fig. 4; Eisenberg and Redford 2000).
Although widely diversified in the Neotropics today (33 species in Western Amazonia; Eisenberg and Redford 2000) and in the La Venta beds (late middle Miocene, Colombia; Kay and Madden 1997; Kay 2015), primates have a scarce fossil record in Amazonian lowlands, strikingly similar to that of bats (Fig. 4; Supplemental Material). The earliest representative is Perupithecus ucayaliensis from Santa Rosa (Bond et al. 2015), showing close affinities with the late Eocene oligopithecid Talahpithecus from Libya (Jaeger et al. 2010). This taxon is indisputably the earliest and most basal monkey from South America (Bond et al. 2015). Deseadan beds of Contamana have yielded a phalanx of a small primate of uncertain affinities (in CTA-32) and dental remains of a medium-sized soriacebine homunculid (in CTA-61; Antoine et al. 2016). Unquestionable crown platyrrhines occur during the Miocene epoch: the earliest remain is a talus referred to an unidentified callithrichine-sized cebine, from MD-61 (late early Miocene, Upper Madre de Dios; Marivaux et al. 2012). Cebidae are recognized in the late Miocene interval, with the large-sized Acrecebus fraileyi in Acre River fauna (Kay and Cozzuol 2006), but also with a capuchin-sized cebine and a marmoset-sized callitrichine in CTA-43 (Antoine et al. 2016). Upper Miocene Acre River deposits yield atelid remains, referred to as Solimoea acreensis and cf. Stirtonia sp. (Kay and Cozzuol 2006), whereas a talus of an unidentified atelid was recognized in the ?early middle Miocene Talismã locality (Upper Juruá; Bergqvist et al. 1998).
Sirenian fossil remains are also very scarce and poorly diversified (Fig. 4). A brachydont molar referable to an unidentified trichechine was unearthed in the early Miocene locality CTA-63 in Contamana (Antoine et al. 2016). Ribodon limbatus was recognized in Acre River deposits (deciduous tooth and fragmentary ribs; Frailey 1986; Negri et al. 2010). Specimens attributed to the extant species Trichechus inunguis and T. manatus were described from the late Pleistocene of Upper Juruá by Simpson and Paula-Couto (1981) and Rancy (1991). Today, only T. inunguis is recorded in Western Amazonia (Eisenberg and Redford 2000).
Fossil Cetacea are represented by odontocetes in the middle–late Miocene interval (Fig. 4). The corresponding cranio-mandibular and dental remains are referable to Recent families, such as Platanistidae (unidentified platanistid in Fitzcarrald: Bianucci et al. 2013; cf. Pomatodelphis bobengi in Acre River: Bocquentin et al. 2007) and Iniidae (Plicodontinia mourai in Upper Juruá and cf. Ischyrorhynchus in Acre; Bocquentin et al. 1990). Another iniid, Inia sp., occurs in the late Pleistocene Rio Madeira Fm. at Araras/Periquitos, Brazil (Holanda et al. 2011). A bulla of a delphinidan of uncertain affinities was described by Tejada-Lara et al. (2015) in the late middle Miocene Fitzcarrald fauna. Living Western Amazonian odontocetes are the iniids Inia geoffrensis and I. boliviensis and the delphinid Sotalia fluviatilis (Eisenberg and Redford 2000).
Fossil representatives of most North American migrant orders (i.e., Artiodactyla, Proboscidea, Perissodactyla, or Carnivora) have been described in Western Amazonia for more than one century, with proboscidean remains unearthed near the junction between Ríos Mayo and Huallaga, San Martín, Peru (Raimondi 1898). Except for procyonid carnivorans (late Pliocene of Venezuela and Colombia; Forasiepi et al. 2014), pre-Pleistocene occurrences of ‘Stratum 3 migrants’ sensu Simpson (1980) in northern South American lowlands are still controversial (e.g., Mothé et al. 2012; Gasparini et al. 2013 regarding proboscideans and tayassuid artiodactyls, respectively). To date, no fossil lagomorph has been mentioned, while Sylvilagus brasiliensis roams most of the concerned area today (Eisenberg and Redford 2000).
Among non-cetacean Artiodactyla, Tayassuidae (peccaries), Camelidae (llamas and vicuñas), and Cervidae (deers) have an indisputable fossil record in Western Amazonia. Tayassuid fossilized remains attributed to Tayassu pecari (Upper Juruá, Río Ucayali, and Río Napo; Rancy 1991) and Tayassu sp. (Río Inuya; Antoine et al. 2007) were found float on river banks and hypothetically referred to the Pleistocene epoch. Mostly based on dental specimens from the Harvey Bassler collection recovered decades ago along the Río Madre de Dios, Frailey and Campbell (2012) erected two new genera and species, Sylvochoerus woodburnei and Waldochoerus bassleri. The former taxon would be closely related to the recent genus Tayassu, and the latter to Pecari (Frailey and Campbell 2012). These remains are claimed to be late Miocene in age, which would somehow constrain the timing of the Great American Faunal Interchange (Montellano-Ballesteros et al. 2014; Carrillo et al. 2015), but their stratigraphic context is far from being unquestionable. To our knowledge, the only camelids identified from Western Amazonia are Vicugna sp. and Palaeolama sp., from late Pleistocene deposits of the Upper Juruá (Simpson and Paula-Couto 1981; Rancy 1991; Latrubesse and Rancy 1998). An unidentified cervid is mentioned in the late Pleistocene Araras/Periquitos assemblage, Rondonia, Brazil (Holanda et al. 2011). Lower jaws of cervids (?Odocoileus sp. and Mazama sp.), probably Pleistocene–Holocene in age, were found on the banks of the Ríos Inuya and Mapuya in Peru (Antoine et al. 2007). Surameryx acrensis was described by Prothero et al. (2014) as a dromomerycine palaeomerycid of North American affinities, late Miocene in age. The exact stratigraphic and geographic location of the holotype (and single specimen) is unknown: the lower jaw was found on a bank of the Acre River “between Cobija and Assis” (which is ~90 km) and its light patina, contrasting with the dark patina of specimens found in situ from Acre River Miocene beds, would point to a Pleistocene–Holocene age instead. Nevertheless, if the referral to dromomerycines is confirmed, it would imply a long ghost lineage for the subfamily in North, Central, or South America in either case.
Following the recent taxonomic revision by Mothé and Avilla (2015), Proboscidea are only represented by the Pleistocene gomphotheriid Notiomastodon platensis in Peruvian and Brazilian Amazonia (Supplemental Material). The anatomical features, taxonomic affinities, and stratigraphic age of the alleged late Miocene four-tusked gomphotheriid Amahuacatherium peruvium (Campbell et al. 2000) had been strongly challenged (e.g., Mothé and Avilla 2015): this gomphothere was two-tusked and it can be considered as a junior synonym of Notiomastodon platensis.
Perissodactyla are only represented by Tapiridae found in Pleistocene–Holocene deposits (Fig. 4; Supplemental Material). Most remains are jaw fragments and isolated teeth found on riverbanks of Peruvian Amazonia and assigned to Tapirus sp. (Río Inuya) or to Tapirus terrestris (Upper Juruá and Río Ucayali; Simpson and Paula-Couto 1981; Gasparini et al. 2013). Nevertheless, a new species of Tapirus (T. rondoniensis) was described based on a splendid skull unearthed in the late Pleistocene Araras/Periquitos fauna, Rondonia, Brazil (Holanda et al. 2011). Today, Tapirus is represented in Western Amazonia by T. terrestris and by T. kabomani, the status of which is disputed (see Voss et al. 2014 contra Cozzuol et al. 2013).
To our knowledge, fossil Carnivora from Western Amazonian lowlands include only Mustelidae (Supplemental Material). A mandible with p3 referred to a tayra (Eira sp.) was described by Rancy (1991) from the Pleistocene of Upper Juruá. A lutrine of unidentified affinities and age (?Pleistocene–Holocene) collected as float in the Río Inuya was mentioned by Antoine et al. (2007). Around 20 species of living carnivorans are recognized in Western Amazonia (canids, procyonids, mustelids, and felids; Eisenberg and Redford 2000).
Discussion and Conclusion
This work allowed for a synthetic survey of Cenozoic mammalian diversity in Western Amazonian lowlands: 23 orders (one, seven, and 15 among Allotheria, Metatheria, and Eutheria, respectively [i.e., 1/7/15]), 89 families [2/20/67], and 320 species [2/48/270] were recognized in the fossil record (Supplemental Material). Gondwanathere allotherians (of uncertain affinities) are restricted to the middle Eocene-?early Oligocene interval, whereas therian mammals (Metatheria and Eutheria) occur throughout the middle Eocene–Holocene interval in Amazonian lowlands (Fig. 4). For several groups (e.g., didelphimorphian marsupials, toxodontid notoungulates, non-potamarchine dinomyid rodents, octodontoid rodents, or tayassuids; Supplemental Material), a taxonomic revision would probably strongly alter the resulting outline, principally in lowering the related number of genera/species. Nevertheless, taking into account potential gaps in the fossil record (during the late Oligocene–middle Miocene and Pliocene intervals), taxonomic diversity was probably fairly constant at the ordinal level (around 12–14 orders in each interval; Fig. 4) throughout the documented interval. During most time slices, species diversity is also unchanged (40–51 species). The only exceptions are (1) the early Miocene depauperate assemblage (only 21 species), probably due to the existence of the long-lasting Pebas Mega-Wetland System (see Salas-Gismondi et al. (2015) and Antoine et al. (2016) for its influence on coeval vertebrate guilds) and, conversely, (2) the late Miocene climactic guild (85 species within 40 families). In that sense, recent mammalian taxonomic diversity from Western Amazonia (12 orders/37 families/286 species) is at odds with all past intervals, including the Pleistocene–Holocene, as it encompasses only three orders of South American origin (Didelphimorphia, Cingulata, and Pilosa) but four North American immigrant orders (Artiodactyla, Perissodactyla, Carnivora, and Lagomorpha): three orders became extinct during the late Pleistocene–Holocene interval, either native (Notoungulata and Litopterna) or of North American origin (Proboscidea). In terms of taxonomic diversity, recent mammalian guilds are dominated by small-sized taxa (Eisenberg and Redford 2000), such as Stratum-2 migrants (chiropterans, caviomorph rodents, and primates; Fig. 4) and non-caviomorph rodents (muroids and sciuroids) from northern landmasses.
Major gaps in the Western Amazonian fossil record correspond with the pre-Lutetian interval (Paleocene–middle Eocene: one specimen of uncertain affinities), the early Miocene (only 17 families encompassing 21 species), and the entire Pliocene epoch (one cingulate species; Fig. 4). Future field studies might focus on these intervals, in particular in order to unveil early mammals, to evaluate the influence of the Pebas System on mammalian evolutionary dynamics, and to better constrain the pattern and timing of the Great American Biotic Interchange(s), as highlighted by Carrillo et al. (2015).
Croft (2001) highlighted the scarcity of large-sized flesh-eaters in pre-GABI South American mammalian guilds, with the notable exception of La Venta (Colombia, late middle Miocene; Kay and Madden 1997). Middle Eocene–Holocene assemblages from Western Amazonia further support that statement, with (1) a strikingly low number of sparassodont marsupials, and (2) the virtual absence of post-GABI carnivorans (Supplemental Material). Crocodiles and birds were most likely top predators in these communities (e.g., Salas-Gismondi et al. 2015).
The pattern and timing of mammalian dispersals from northern landmasses into Western Amazonia are not elucidated yet, as most claimed pre-Pleistocene records of North American immigrants are not accurately constrained in terms of age (surface collections: tayassuid and “dromomerycine” artiodactyls; Frailey and Campbell 2012; Prothero et al. 2014) and/or highly challenged regarding their taxonomic affinities (e.g., the gomphotheriid proboscidean Amahuacatherium peruvium; Mothé and Avilla 2015). New geochemical methods performed on fossil dentine and/or enamel, using rare earth element uptakes as an indicator for quantifying relative diagenesis among mammalian orders (REE Index; MacFadden et al. 2010), could probably help disentangling such enigmas in the forthcoming years, in blueprinting these fossils, and recognizing their potential source levels (upper Miocene formations vs. Pleistocene terraces, likely to display highly distinctive REE indices).
References
Antoine P-O, Abello MA, Adnet S, Altamirano Sierra AJ, Baby P, Billet G, Boivin M, Calderon Y, Candela A, Chabin J, Corfu F, Croft DA, Ganerød M, Jaramillo C, Klaus S, Marivaux L, Navarrete RE, Orliac MJ, Parra F, Pérez ME, Pujos F, Rage J-C, Ravel A, Robinet C, Roddaz M, Tejada-Lara JV, Vélez-Juarbe J, Wesselingh FP, Salas-Gismondi R (2016) A 60 million-year Cenozoic history of western Amazonian ecosystems in Contamana, eastern Peru. Gondwana Res 31:30–59
Antoine P-O, Billet G, Salas-Gismondi R, Tejada Lara J, Baby, P, Brusset S, Espurt N (2015) A new Carodnia Simpson, 1935 (Mammalia, Xenungulata) from the early Eocene of northwestern Peru and a phylogeny of xenungulates at species level. J Mammalian Evol 22:129–140
Antoine P-O, Marivaux L, Croft, DA, Billet G, Ganerød M, Jaramillo C, Martin T, Orliac MJ, Tejada J, Altamirano AJ, Duranthon F, Fanjat G, Rousse S, Salas Gismondi R (2012) Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proc Roy Soc B-Biol Sci 279:1319–1326
Antoine P-O, Roddaz M, Brichau S, Tejada-Lara J, Salas Gismondi R, Altamirano A, Louterbach M, Lambs L, Otto T, Brusset S (2013) Middle Miocene vertebrates from the Amazonian Madre de Dios Subandean Zone, Perú. J S Am Earth Sci 42:91–102
Antoine P-O, Salas-Gismondi R, Baby P, Benammi M, Brusset S, De Franceschi D, Espurt N, Goillot C, Pujos F, Tejada J, Urbina M (2007) The middle Miocene (Laventan) Fitzcarrald Fauna, Amazonian Peru. In: Díaz-Martínez E, Rábano I (eds) Proceedings of the 4th EMPSLA, Cuad Mus Geominero 8:355–360
Bergqvist LP, Ribeiro AM, Bocquentin-Villanueva J (1998) Primata, Roedores e Litopternas do Mio/Plioceno da Amazõnia Sul-Ocidental (Formação Solimões, Bacia do Acre), Brasil. Geol Colomb 23:19–29
Bianucci G, Lambert O, Salas-Gismondi R, Tejada J, Pujos F, Urbina M, Antoine P-O (2013) A Miocene relative of the Ganges River dolphin from the Amazonian basin. J Vertebr Paleontol 33(3):741–745
Billet G, Orliac MJ, Antoine P-O, Jaramillo C (2010) New observations and reinterpretation of the enigmatic taxon Colombitherium (?Pyrotheria, Mammalia) from Colombia. Palaeontology 53:319–325
Bocquentin J, Filho JPS, Negri FR (1990) Neoepiblema acreensis, sp. n. (Mammalia, Rodentia) do Neogeno do Acre, Brasil. Bol Mus Paraense Emilio Goeldi 2:65–72
Bocquentin J, Negri FR, Melo J, Maciente A (2007) Novas ocorrências de Odontoceti (Mammalia, Cetacea) no Mioceno superior-Plioceno da Formaçao Solimões, Estado do Acre, Brasil. Anais do XX Congresso Brasileiro de Paleontologia 20:191
Bond M, Tejedor MF, Campbell KE, Chornogubsky L, Novo N, Goin FJ (2015) Eocene primates of South America and the African origins of New World monkeys. Nature 520:538–541
Campbell KE, Frailey CD, Romero-Pittman L (2000) The late Miocene gomphothere Amahuacatherium peruvium (Proboscidea: Gomphotheriidae) from Amazonian Peru: implications for the Great American faunal interchange. Bol Est Reg INGEMMET 23:1–152
Carrillo JD, Forasiepi A, Jaramillo C, Sánchez-Villagra MR (2015) Neotropical mammal diversity and the Great American Biotic Interchange: spatial and temporal variation in South America’s fossil record. Front Genet 5:451
Ciancio MR, Carlini AA, Campbell KE, Scillato-Yané GJ (2013) New Paleogene cingulates (Mammalia, Xenarthra) from Santa Rosa, Peru and their importance in the context of South American faunas. J Syst Paleontol 11(6):727–741
Cozzuol MA, Clozato CL, Holanda EC, Rodrigues FVHG., Nienow S, De Thoisy B, Redondo RAF, Santos FCR (2013) A new species of tapir from the Amazon. J Mammal 94:1331–1345
Croft DA (2001) Cenozoic environmental change in South America as indicated by mammalian body size distributions (cenograms). Divers distrib 7:271–287
Croft DA (2007) The middle Miocene (Laventan) Quebrada Honda Fauna, Southern Bolivia and a description of its Notoungulates. Palaeontology 50(1):277–303
Czaplewski NJ (1996) Opossums (Didelphidae) and bats (Noctilionidae and Molossidae) from the late Miocene of the Amazon Basin. J Mammal 77:84–94
Czaplewski NJ, Campbell KE (2004) A possible bat (Mammalia: Chiroptera) from the ?Eocene of Amazonian Peru. In: Campbell KE (ed) The Paleogene Mammalian Fauna of Santa Rosa, Amazonian Peru. Nat Hist Mus Los Angeles County Science Series 40:141–144
De Iuliis G, Gaudin TJ, Vicars MJ (2011) A new genus and species of nothrotheriid sloth (Xenarthra, Tardigrada, Nothrotheriidae) from the late Miocene (Huayquerian) of Peru. Palaeontology 54:171–205
Eisenberg JF, Redford KH (2000) Mammals of the Neotropics, Volume 3: Ecuador, Bolivia, Brazil (Vol. 3). University of Chicago Press, Chicago
Forasiepi AM, Soibelzon LH, Gomez CS, Sánchez R, Quiroz LI, Jaramillo C, Sánchez-Villagra MR (2014) Carnivorans at the Great American Biotic Interchange: new discoveries from the northern neotropics. Naturwissenschaften 101: 965–974
Frailey CD (1986) Late Miocene and Holocene mammals, exclusive of the Notoungulata, of the Rio Acre region, western Amazonia. Nat Hist Mus Los Angeles County Contrib in Science 374:1–46
Frailey CD, Campbell KE (2004) Paleogene rodents from Amazonian Peru: the Santa Rosa local fauna. In: Campbell, KE (ed), The Paleogene Mammalian Fauna of Santa Rosa, Amazonian Peru. Nat Hist Mus Los Angeles County Science Series 40:71–130
Frailey CD, Campbell KE (2012) Two new genera of peccaries (Mammalia, Artiodactyla, Tayassuidae) from upper Miocene deposits of the Amazon Basin. J Paleontol 86(5):852–877
Gasparini GM, Ferrero BS, Holanda EC (2013) Los tapires (Perissodactyla, Tapiridae) fósiles de América del Sur: aspectos sistemáticos, bioestratigráficos y biogeográficos. Actas del I Congreso Latinoamericano de Tapires y II Congreso Ecuatoriano de Mastozoología. Lugar: Puyo, Pastaza:136–137
Gaudin TJ, Croft DA (2015) Paleogene Xenarthra and the evolution of South American mammals. J Mammal 96(4):622–663
Gelfo J.N, López GM, Bond M (2008) A new Xenungulata (Mammalia) from the Paleocene of Patagonia, Argentina. J Paleontol 82(2):329–335
Goillot C, Antoine P-O, Tejada Lara J, Pujos F, Salas-Gismondi R (2011) Middle Miocene Uruguaytheriinae (Mammalia, Astrapotheria) from Peruvian Amazonia and a review of the astrapotheriid fossil record in northern South America. Geodiversitas 33(2):331–345
Goin FJ, Candela AM (2004) New Paleogene marsupials from the Amazon Basin of eastern Peru. In: Campbell KE (ed) The Paleogene Mammalian Fauna of Santa Rosa, Amazonian Peru. Nat Hist Mus Los Angeles County Science Series 40:15–60
Goin FJ, Tejedor MF, Chornogubsky L, López GM, Gelfo JN, Bond M, Woodburne M O, Gurovich Y, Reguero M (2012) Persistence of a Mesozoic, non-therian mammalian lineage (Gondwanatheria) in the mid-Paleogene of Patagonia. Naturwissenschaften 99:449–463
Goin FJ, Vieytes EC, Vucetich MG, Carlini AA, Bond M (2004) Enigmatic mammal from the Paleogene of Perú. In: Campbell KE (ed) The Paleogene Mammalian Fauna of Santa Rosa, Amazonian Peru. Nat Hist Mus Los Angeles County Sci Ser 40:145–153
Góis F, Scillato-Yané GJ, Carlini AA, Guilherme E (2013) A new species of Scirrotherium Edmund & Theodor, 1997 (Xenarthra, Cingulata, Pampatheriidae) from the late Miocene of South America. Alcheringa 37:177–188
Góis F, Scillato-Yané GJ, Carlini AA, Ubilla M (2012) Una nueva especie de Holmesina Simpson (Xenarthra, Cingulata, Pampatheriidae) del Pleistoceno de Rondônia, sudoeste de la Amazonia, Brasil. Rev Brasil Paleontol 15:211– 227
Gradstein FM, Ogg JG, Schmitz M, Ogg G (2012) The Geologic Time Scale 2012, 2-Volume Set. Elsevier, Amsterdam
Holanda EC, Ferigolo J, Ribeiro AM (2011) New Tapirus (Mammalia: Perissodactyla: Tapiridae) from the upper Pleistocene of Amazonia, Brazil. J Mammal 92(1):111–120
Jaeger J-J, Beard KC, Chaimanee Y, Salem M, Benammi M, Hlal O, Coster P, Bilal AA, Duringer P, Schuster M, Valentin X, Marandat B, Marivaux L, Métais G, Hammuda O, Brunet M (2010) Late middle Eocene epoch of Libya yields earliest known radiation of African anthropoids. Nature 467:1095–1098
Kay RF (2015) New World monkey origins. Science 347:1067–1068
Kay RF, Cozzuol MA (2006) New platyrrhine monkeys from the Solimões Formation (late Miocene, Acre State, Brazil). J Hum Evol 50:673–686
Kay RF, Madden, RH (1997) Paleogeography and paleoecology. In: Kay RF, Madden RH, Cifelli RL, Flynn JJ (eds) Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Smithsonian Institution Press, Washington D.C., pp 520–550
Kerber L, Negri FR, Ribeiro AM, Nasif N, Souza-Filho JP, Ferigolo J (in revision) Tropical fossil caviomorph rodents from the southwestern Brazilian Amazonia in the South American rodent fauna context: systematics, biochronology and paleobiogeography. J Mammal Evol
Kerber L, Negri FR, Ribeiro AM, Vucetich MG, De Souza-Filho JP (2015) Late Miocene potamarchine rodents from southwestern Amazonia, Brazil, with description of new taxa. Acta Palaeontol Polonica. doi: 10.4202/app.00091.2014
Kramarz AG, Bond M (2008) Revision of Parastrapotherium (Mammalia, Astrapotheria) and other Deseadan astrapotheres of Patagonia. Ameghiniana 45:537–551
Kramarz AG, Bond M, Forasiepi AM (2011) New remains of Astraponotus (Mammalia, Astrapotheria) and considerations on astrapothere cranial evolution. Palaeontol Z 85:185–200
Latrubesse EM, Rancy A (1998) The late Quaternary of the Upper Juruá River, Southwestern Amazonia, Brazil. In: Rabassa J, Salemme M (eds) Geology and Vertebrate Paleontology. Quaternary of South America and Antarctic Peninsula, Balkema, Rotterdam, v. 11, pp 27–46
Louterbach M, Roddaz M, Bailleul J, Antoine P-O, Adnet S, Kim JH, van Soelen E, Parra F, Gérard J, Calderon Y, Gagnaison C, Sinninghe Damsté JS, Baby P (2014) Evidences for a Paleocene marine incursion in Southern Amazonia (Madre de Dios Sub-Andean Zone, Peru). Palaeogeogr Palaeoclimatol Palaeoecol 414:451–471
MacFadden BJ (2005) Diet and habitat of toxodont megaherbivores (Mammalia, Notoungulata) from the late Quaternary of South and Central America. Quaternary Res 64:113–124
MacFadden BJ, DeSantis LR, Hochstein JL, Kamenov GD (2010) Physical properties, geochemistry, and diagenesis of xenarthran teeth: prospects for interpreting the paleoecology of extinct species. Palaeogeogr Palaeoclimatol Palaeoecol 291(3):180–189
Marivaux L, Salas-Gismondi R, Tejada J, Billet G, Louterbach M, Vink J, Bailleul J, Roddaz M, Antoine P-O (2012) A platyrrhine talus from the early Miocene of Peru (Amazonian Madre de Dios Sub-Andean Zone). J Hum Evol 63:696–703
Marshall LG, Hoffstetter R, Pascual R (1983) Mammals and stratigraphy: geochronology of the continental mammal-bearing Tertiary of South America. Palaeovertebrata Mém Spéc:1–93
Montellano-Ballesteros M, Rincón AD, Solorzano A (2014) Record of tayassuids in ?late Pliocene to Quaternary deposits in Venezuela. Rev Bras Paleontolog 17:169–182
Mora A, Baby P, Roddaz M, Parra M, Brusset S, Hermoza W, Espurt N (2010) Tectonic history of the Andes and sub-Andean zones: implications for the development of the Amazon drainage basin. In: Hoorn C, Wesselingh FP (eds) Amazonia, Landscape and Species Evolution: A Look into the Past. Blackwell-Wiley, Hoboken, pp 38–60
Mothé D, Avilla L (2015) Mythbusting evolutionary issues on South American Gomphotheriidae (Mammalia: Proboscidea). Quaternary Sci Rev 110:23–35
Mothé D, Avilla LS, Cozzuol M, Winck GR (2012) Taxonomic revision of the Quaternary gomphotheres (Mammalia: Proboscidea: Gomphotheriidae) from the South American lowlands. Quaternary Internatl 276:2–7
Negri FR, Bocquentin Villanueva J, Ferigolo J, Antoine P-O (2010) 16. A review of Tertiary mammal faunas and birds from western Amazonia. In: Hoorn C, Wesselingh FP (eds) Amazonia, Landscape and Species Evolution: A Look into the Past. Blackwell-Wiley, Hoboken, pp 245–258
Oliveira EV, Ribeiro AM, Bergqvist LP (1997) A new Oligocene cingulate (Mammalia, Xenarthra) from the Taubaté Basin, Brazil. An Acad Bras Ciênc 69:461–470
Patterson B (1977) A primitive pyrothere (Mammalia, Notoungulata) from the Early Tertiary of northwestern Venezuela. Fieldiana 33(22):397–422
Paula Couto C de (1982) Fossil mammals from the Cenozoic of Acre, Brazil. V. Notoungulata Nesodontinae, Toxodontinae and Haplodontheriinae, and Litopterna, Pyrotheria and Astrapotheria. Iheringia Sér Geol 7:5–43
Prothero DR, Campbell KE Jr, Beatty BL, Frailey CD (2014) New late Miocene dromomerycine artiodactyl from the Amazon basin: implications for interchange dynamics. J Vertebr Paleontol 88(3):434–443
Pujos F, Salas R (2004) A systematic reassessment and paleogeographic review of fossil Xenarthra from Peru. Bull Inst Fr Et And 33:331–377
Pujos F, Salas-Gismondi R, Baby G, Baby P, Goillot C, Tejada J, Antoine P-O (2013) Implication of the presence of Megathericulus (Xenarthra: Tardigrada: Megatheriidae) in the Laventan of Peruvian Amazonia. J Syst Palaeontol 11(8):973–991
Raimondi A (1898) Mandíbula inferior de “Mastodon andium” hallado en un terreno cercade la desembocadura del río Moyobamba al Huallaga. Bol Soc Geogr Lima 7(10):406–409
Rancy A (1991) Pleistocene mammals and paleoecology of the western Amazon. Unpublished PhD, University of Florida, Gainesville, 302 pp
Ribeiro AM, Madden RH, Negri FR, Kerber L, Hsiou AS, Rodrigues KA (2013) Mamíferos fósiles y biocronología en el suroeste de la Amazonia, Brasil. In: Brandoni D, Noriega JI (eds) El Neógeno de la Mesopotamia argentina. APA, Pub Esp 14:207–221
Salas R, Sánchez J, Chacaltana C (2006) A new pre-Deseadan pyrothere (Mammalia) from northern Peru and the wear facets of molariform teeth of Pyrotheria. J Vertebr Paleontol 26:760–769
Salas-Gismondi R, Tejada J, Antoine P-O (2011) Evidence on the tropical history of Paleogene Cingulata. IV Congreso Latinoamericano de Paleontología de Vertebrados, September 21–24, San Juan, Argentina
Salas-Gismondi R, Flynn JJ, Baby P, Tejada-Lara J, Wesselingh FP, Antoine P-O (2015) A Miocene hyperdiverse crocodylian community reveals peculiar trophic dynamics in proto-Amazonian mega-wetlands. Proc Roy Soc B-Biol Sci 282:20142490
Sedor FA, Oliveira EV, Silva DD, Fernandes LA, Cunha RF, Ribeiro AM, Dias EV (in revision) A new South American Paleogene land mammal fauna, Guabirotuba Formation (Southern Brazil). J Mammal Evol
Shockey BJ, Anaya Daza F (2004) Pyrotherium macfaddeni, sp. nov. (late Oligocene, Bolivia) and the pedal morphology of pyrotheres. J Vertebr Paleontol 24(2):481–488
Simpson GG (1980) Splendid Isolation. The Curious History of South American Mammals. Yale University Press, New Heaven and London, 275pp
Simpson GG, Paula-Couto C de (1981) Fossil mammals from the Cenozoic of Acre, Brazil III—Pleistocene Edentata Pilosa, Proboscidea, Sirenia, Perissodactyla and Artiodactyla. Iheringia 6:11–73
Spillmann F (1949) Contribución a la paleontología del Perú. Una mamífauna fósil de la región del Río Ucayali. Pub Mus Hist Nat “Javier Prado” 1:1–40
Tejada-Lara J, Salas-Gismondi R, Pujos F, Baby P, Benammi M, Brusset S, De Franceschi D, Espurt N, Urbina M, Antoine P-O (2015) Life in Protoamazonia: middle Miocene mammals from the Fitzcarrald arch (Peruvian Amazonia). Palaeontology 58(2):341–378
Vallejo-Pareja MC, Carrillo DJ, Moreno-Bernal JW, Pardo-Jaramillo M, Rodriguez-Gonzalez DM, Muñoz-Duran J (2015) Hilarcotherium castanedai, gen. et sp. nov., a new Miocene astrapothere (Mammalia, Astrapotheriidae) from the Upper Magdalena Valley, Colombia. J Vertebr Paleontol 35. doi: 10.1080/02724634.2014.903960
Villarroel C (1987) Caracteristicas y afinidades de Etayoa n. gen., tipo de una nueva familia de xenungulata (Mammalia) del Paleoceno medio de Colombia. Com Paleont Mus Montevideo 19:241–253
Voss RS, Helgen KM, Jansa SA (2014) Extraordinary claims require extraordinary evidence: a comment on Cozzuol et al. (2013). J Mammal 95: 893–898
Vucetich MG, Verzi DH (2002) First record of Dasyproctidae (Rodentia) in the Pleistocene of Argentina: paleoclimatic implications. Palaeogeogr Palaeoclimatol Palaeoecol 178:67–73
Vucetich GM, Verzi HD, Hartenberger JL (1999) Review and analysis of the radiation of the South American Hystricognathi (Mamalia, Rodentia) C R Acad Sci IIA 329:763–769
Webb SD, Rancy A (1996) Late Cenozoic evolution of the Neotropical mammal fauna. In: Jackson JBC, Budd AF, Coastes AG (eds) Evolution and Environment in Tropical America, University of Chicago Press, Chicago, pp 335–358
Willard B (1966) The Harvey Bassler Collection of Peruvian Fossils. Lehigh University, Bethlehem, 255 pp
Woodburne MO, Goin FJ, Bond M, Carlini AA, Gelfo JN, López GM, Iglesias A, Zimicz AN (2014) Paleogene land mammal faunas of South America; a response to global climatic changes and indigenous floral diversity. J Mammal Evol 21:1–73
Acknowledgments
We are grateful to anybody who has helped us in the field. Fieldwork was funded by the Toulouse University (SPAM Project), by the Paleo2 Program of the CNRS, by the Leakey Foundation, by the National Geographic Society (Grant #9679-15), and by the French Ministry of Foreign Affairs. This work was further supported by an “Investissements d’Avenir” grant managed by the Agence Nationale de la Recherche (CEBA, ANR-10-LABX-25-01). We are indebted to the Organizing Committee of the 4th International Palaeontological Congress, Mendoza, 2014 and to John R. Wible and Tim J. Gaudin for allowing us and publishing this work in the Journal of Mammalian Evolution. We warmly thank two anonymous reviewers and the Editor-in-Chief for their constructive remarks on a previous version of the manuscript, and for having provided invaluable references for the fossil record of southwestern Brazil. This is ISEM-2016-056-Sud publication.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 873 kb)
Rights and permissions
About this article
Cite this article
Antoine, PO., Salas-Gismondi, R., Pujos, F. et al. Western Amazonia as a Hotspot of Mammalian Biodiversity Throughout the Cenozoic. J Mammal Evol 24, 5–17 (2017). https://doi.org/10.1007/s10914-016-9333-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-016-9333-1