Abstract
The study and modeling of physical properties such as surface tension and interfacial tension are important factors in the formation and stability of fluid systems such as emulsions. The present work shows the experimental results for surface tension and interfacial tension measurements in water and/or oils systems in the presence of surfactants, using the pendant drop technique. Distilled water and straight-chain alkanes such as hexane, dodecane and hexadecane were used. The surfactants employed were sodium dodecyl sulphate (SDS) and sorbitan monooleate (SPAN 80), which are hydrophilic and lipophilic surfactants respectively. Some results show the dependence of surface tension or interfacial tension with respect to the surfactant concentration, other results were obtained by varying the temperature in a range from 20 to \(60\,{}^{\circ }\mathrm{{C}}\).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Surface Tension
- Sodium Dodecil Sulfate
- Interfacial Tension
- Surfactant Concentration
- Sodium Dodecyl Sulphate
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Research in surface physics is of relevance to many scientific areas such as Chemical Engineering, Materials Sciences, Physics, Electronics, among others. Surface Tension is one of the most widely used thermophysic property for surface characterization. Moreover, many industrial applications require (as part of their technological developments) the surface characterization of water–oil interfaces in the presence of other additives; this is the case of some processes in the Paint, Agrochemical, Oil and Cosmetics industries.
Several investigations have been carried out in the field of surface and interfacial tension of fluid systems. It is worth mentioning the work of Goebel and Lunkenheimer (1997), where surface tension between water and a series of n-alkanes (from pentane to hexadecane) were presented for a single temperature (\(22\,{}^{\circ }\mathrm{{C}}\)). In Zeppieri et al. (2001), surface tension between water and different alcanes (from hexane to dodecane) for a range of temperatures between \(10-60\,{}^{\circ }\mathrm{{C}}\) were reported. In turn, Rolo et al. (2002) reported surface tension for different kind of oils and binary mixtures of them.
The objective of this work is to analyse the experimental results obtained from surface tension measurements on different alcanes such as hexane, dodecane and hexadecane at different temperatures, as well as interfacial tension results between the aforementioned alcanes and water for different surfactant concentrations. The main purpose is to provide information that contribute to the study of emulsion type fluid systems, in particular, the surfactants SDS and Span 80 were selected due to their wide applicability in emulsions.
2 Methodology
In order to obtain surface and interfacial tension measurements an optical tensiometer was used. Image analysis of an emerging (pending) droplet was carried out so the determination of the surface parameters was achieved through the geometry of the drop and its relationship with surface.
The pendant drop technique allows for the determination of the surface or interfacial tension from distinct characteristics of the droplet profile. This calculation requires the solution of the Young-Laplace equation, which for the case of a pending drop can be expressed as:
where y is the vertical coordinate, \(\Delta \uprho \) is the density difference between both fluid phases, \(\mathrm{{R}}_{0}\) is the curvature radius of the drop bottom, H refers to its average curvature (which is a function of y) (Zeppieri et al. 2009). Figure 1 shows a sketch of the experimental setup used to obtain the measurements under controlled temperature conditions.
Different configurations for the dispersed phase injection may be used depending on the type of measurement, as shown in Fig. 2. For surface tension measurements a straight needle was used, while a curved “U” shaped immersed needle was selected for the interfacial tension measurements.
Once the droplet is fully developed for each case, Image Analysis was carried over in order to determine the curvature parameters, which complemented with the density differences determine the interfacial and surface tension. The fluid phases were deionized water, three types of oils: hexane, dodecane and hexadecane. The selected surfactants were: Sodium Dodecil Sulfate (SDS), which is a hydrophilic (affinity for water, i.e. soluble in water) surfactant; Sorbitan Monooleate (Span 80), which is a lipophilic (affinity for oil, i.e. soluble in oil) surfactant.
Next, Table 1 shows the corresponding values for density \(\uprho \), surface tension \(\sigma \) and interfacial tension between water–oil \(\upgamma \) reported in the literature for the different systems studied here Pardo et al. (2001); Vargaftik et al. (1983); Giner et al. (2007); Rolo et al. (2002) and Zeppieri et al. (2001).
For the experiments under different controlled temperatures, the fluid densities were selected from Pardo et al. (2001), as well as the corresponding values of the surface and interfacial tension were compared from Giner et al. (2007); Rolo et al. (2002); Zeppieri et al. (2009); Goebel and Lunkenheimer (1997).
The experimental results are presented in the next section. The values shown in the different figures correspond to averages of five independent experiments carried out under the same conditions. The error bars were determined from the sample standard deviation divided by the square root of the sample size.
3 Results
The first measurements correspond to surface tension in the range of temperature from 20 to \(60\,{}^{\circ }\mathrm{{C}}\). Figure 3 shows the graph of surface tension versus temperature for three different types of oil, such as hexane, dodecane, and hexadecane.
In Fig. 3 it can be seen that as the temperature increases, the surface tension decreases. The rate of change of surface tension with respect to the temperature is different for the three different types of oils that were studied. In the case of hexane a rate of change of \(-0.058\,\mathrm{{mN/m}}\,{}^{\circ }\mathrm{{C}}\) was obtained, while for dodecane the rate of change was \(-0.081\,\mathrm{{mN/m}}\,{}^{\circ }\mathrm{{C}}\), and for hexadecane the rate of change was \(-0.092\,\mathrm{{mN/m}}\,{}^{\circ }\mathrm{{C}}\). These results indicate that as the linear chain length increases, a larger slope in the surface tension is obtained as a function of temperature.
Giner et al. (2007) reported surface tension values only for the case of hexane, which are in good agreement with the results presented in this work. Values reported of surface tension with temperature for dodecane and hexadecane oils are limited.
Next, some results for the measurements of interfacial tension both in pure phases and also in the presence of hydrophilic and lipophilic surfactants are presented.
Figure 4 shows the graph of interfacial tension for variations in temperature, in this case for a water–hexane system. The range of temperature was from 22 to \(40\,{}^{\circ }\mathrm{{C}}\). As already mentioned in the preceding paragraphs, as the temperature increases the system tends to reduce its interfacial tension.
According to the results obtained the interfacial tension for the system hexane/water in the region studied here, it shows a dependence with the temperature as follows: \(\varvec{\upgamma } = -0.10665\,(\mathrm{{mN/m}}\,{}^{\circ }\mathrm{{C}}) \mathbf{T} + 53.2363\,(\mathrm{{mN/m}})\). Constructing the prediction interval (Walpole et al. 2012) that has a probability of 95 % for the interfacial tension measurements in a range from 22 to 40 \(^{\circ }\mathrm{{C}}\), in this region the interfacial tension data reported by Zeppieri et al. (2001) are contained into the prediction limits (see Fig. 4). For example, estimating the 95 % prediction interval for the interfacial tension in the case of \(30\,{}^{\circ }\mathrm{{C}}\) is \(49.8047 \le {\varvec{\upgamma }} (30\,{}^{\circ }\mathrm{{C}}) \le 50.2689\) in units of mN/m. For the same temperature, a value for interfacial tension of 49.96 mN/m \(\pm 0.04\) was reported by Zeppieri.
Now comparing both results obtained for the surface tension and interfacial tension in the case of hexane, the rate of change of interfacial tension with temperature is greater than the one obtained for surface tension. This result shows a greater sensitivity of the interfacial tension to temperature changes.
Next, let us present the results obtained from introducing surfactant molecules as a third component in the system. Figures 5 and 6 show the results of interfacial tension with hexadecane as the organic phase with a temperature set to \(25\,{}^{\circ }\mathrm{{C}}\).
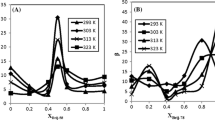
Figure 5 shows the results when an hydrophilic surfactant was used, in this case the SDS concentration was dissolved in water. Interfacial tension measurements were obtained for different SDS concentrations in a water–hexadecane system. The surfactant concentration of SDS was measured in terms of the critical micelle concentration (cmc) of SDS in water, which has a value of \(8\times 10^{-3}\) mol/L. The SDS concentration values that were used in this investigation were: 0.05, 0.1, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 all in units of cmc. The first result in the graph corresponds to the interfacial tension value without surfactant.
It is known that the main function of surfactant molecules is to decrease the surface tension or the interfacial tension in a fluid system, this is clearly showed in the graph. Also it can be noted that the greater variations in the interfacial tension occur at the lower surfactant concentrations. Thereafter, the interfacial tension values remain unchanged when a certain surfactant concentration is reached. This can be explained from the fact that these molecules saturate the interface once a given surfactant concentration is reached, and the surfactant excess no longer modifies the properties of the interface. The intersection value can be determined by the trend lines in the two regions of the interfacial tension, this point shows a threshold-value of approximately 0.18 cmc.
Figure 6 shows the results for the interfacial tension of water–hexadecane system at different concentrations of a lipophilic surfactant, in this case different concentrations of Span 80 were dissolved in oil. Due to the high viscosity of Span 80, the corresponding surfactant concentrations are usually reported in weight fraction or weight percent in the organic or continuous phase (%w/w). The values used for the interfacial tension measurements were as follows: 0.0 01, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 1.5, 2, 2.5 all in units of % w/w.
Again the effect of surfactant in the oil–water systems lowers the interfacial tension. The lower values for surfactant concentration produce greater variations in the interfacial tension measurements up to a given threshold value, and then the interfacial tension maintains a constant value. The intersection of the trend lines in the two regions of the interfacial tension, shows a threshold value of approximately 0.06 % w/w.
4 Conclusions
The technique of the pending drop was used to obtain surface and interfacial tension for water–oil systems at different temperatures in pure phase and in the present of surfactants. The threshold value that corresponds to the saturation was established, when an increase in surfactant concentration no longer causes a significant decrease in interfacial tension, both for the hydrophilic as well as the lipophilic surfactant.
References
Giner B, Villares A, Martín S, Artigas H, Lafuente C (2007) Study of the temperature dependence of surface tensions of some alkanol + hexane mixtures. J Chem Eng Data 52(5):1904–1907
Goebel A, Lunkenheimer K (1997) Interfacial tension of the water/n-alkane interface. Langmuir 13(2):369–372
Pardo JM, Tovar CA, González D, Carballo E, Romani L (2001) Thermophysical properties of the binary mixtures diethyl carbonate + (n-dodecane or n-tetradecane) at several temperature. J Chem Eng Data 46(2):212–216
Rolo LI, Caco AI, Queimada AJ, Marrucho IM, Coutinho JAP (2002) Surface tension of heptane, decane, hexadecane, eicosane, and some of their binary mixtures. J Chem Eng Data 47(6):1442–1445
Vargaftik NB, Volkov BN, Voljak LD (1983) International tables of surface tension of water. J Phys Chem Ref Data 12(3):817–820
Walpole RE, Myers RH, Myers SL, Ye K (2012) Chapter 11: Simple linear regression and correlation. In: Probability and statistics for engineers and scientists, 9th edn. Prentice Hall, New Jersey
Zeppieri S, Rodríguez J (2001) Interfacial tension of alkane + water systems. J Chem Eng Data 46(5):1086–1088
Zeppieri S, Ramírez Y, Molina J (2009) Estudio experimental de la tensión interfacial transitoria para sistemas alcanos-agua-tritón X-100 empleando la técnica de la gota emergente. Información Tecnológica 20(6):125–135
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cortés-Estrada, A.H., Ibarra-Bracamontes, L.A., Aguilar-Corona, A., Viramontes-Gamboa, G., Carbajal-De la Torre, G. (2014). Surface Tension and Interfacial Tension Measurements in Water-Surfactant-Oil Systems Using Pendant Drop Technique. In: Klapp, J., Medina, A. (eds) Experimental and Computational Fluid Mechanics. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-00116-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-00116-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00115-9
Online ISBN: 978-3-319-00116-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)