Abstract
Breast cancer is the most common neoplasm and the leading cause of cancer death in women worldwide, followed by cervical cancer. The process of developing anticancer drugs focuses on synthesis and biological testing. However, it is a process that requires a large investment of resources. In the present work, the visualization of their most stable structure, that is, the one that requires less energy for its conformation, and the visualization of their molecular orbitals have been carried out in the analysis of some drugs commonly used in breast cancer patients. To generate useful information for the design and study of potential analogs to treat breast cancer. To evaluate the feasibility and properties of drug candidates, DFT allows the theoretical prediction of pharmacological properties. The HOMO and LUMO are particularly important in the study of drugs because they are related to properties such as light absorption and emission, electron-donating or accepting capacity, and the formation of chemical bonds with other molecules. Pharmacological activity and the design of new compounds depend on these properties.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Breast cancer is a disease where cells in the breast tissue grow uncontrollably [1]. In 2020, the World Health Organization detected around 2 million new breast cancer cases, adding to an existing 7.8 million cases. This data confirms breast cancer is the most prevalent and common cancer worldwide [2, 3].
The breast is composed of three main parts: lobes that produce milk, ducts that carry milk to the nipple, and connective tissue that supports and surrounds all parts of the breast. Breast cancer has several subtypes, including invasive ductal carcinoma, invasive lobular carcinoma, and inflammatory breast carcinoma. Other subtypes are diagnosed based on molecular characteristics and specific markers found in cancer cells. The first subtype is HR-positive, which indicates the expression of hormone receptors, estrogen, and/or progesterone in the cancer cells. The second subtype is HER2-positive, where the cancer cells overexpress the HER2 receptor. Finally, triple-negative cancers are those that do not express hormone receptors (ER-/PR-) or overexpress HER2. Cancer treatment options depend on the tumor type and may include drugs, hormonal and biological therapies, surgery, and radiation therapy.
Biological testing and synthesis are the heart and ground of the cancer drug development process; however, it is a long process that requires over 15 years and more than 800 million dollars without guaranteeing the test’s success [4]. Therefore, integrating new technologies and reducing development times and costs would be necessary. An alternative option is computer-assisted drug design and testing which has proven to be a favorable implement in development cost and time [5]. Computational chemistry comprises the application of computation in the areas of chemistry, biology, and physics for the study of atoms, molecules, and macromolecules through modeling and representations in three dimensions [6, 7].
Specifically in breast cancer, computational chemistry has been used for the design of drug analogs to those already demonstrated to be effective. In a recent study, drug analogs were generated by modifying the functional groups of tamoxifen to improve the activity and selectivity of the drugs [8]. Using computer modeling techniques such as molecular dynamics, docking, and density functional theory (DFT), it has been possible to simulate molecular interactions and predict how a drug will interact with a specific protein or enzyme in the body. A review was published highlighting and demonstrating the efficacy of tucatinib and trastuzumab for HER2-positive breast cancer based on molecular docking results [11]. Also, another study in which a computational analysis of different candidate compounds for breast cancer was performed, obtained promising results for these compounds, designating them as good candidates for preclinical studies [10]. This saves time and resources by directly reducing the number of compounds that need to be synthesized and tested in laboratory experiments. In addition, computational science is used to predict the toxicity of compounds, helping to identify the potential side effects and risks associated with a drug before it is tested in animals or humans.
Although computational chemistry has been widely used in the study of drugs and their interactions with their therapeutic targets, including studies for breast cancer treatment, there is still a lack of physicochemical information on drugs that are effective against breast cancer and the chemical properties of their interactions with therapeutic targets. In the present work, the analysis of some drugs commonly used in breast cancer patients was performed, obtaining the visualization of their most stable structure and their molecular orbitals (HOMO-LUMO), to generate helpful information for the design and study of potential analogs drugs for the treatment of breast cancer using a computational chemistry methodology, reduce cost and investment time than the original drug design and study. To accomplish the objective of this study, a physicochemical characterization was performed, that is, to obtain chemical measurements to determine the properties of the drugs that are currently effective for breast cancer.
2 Methodology
The molecules used in this study were tamoxifen, anastrozole, lapatinib, tucatinib, capecitabine and isofosfamide. These are the most used molecules in the clinical treatment of the three types of cancer mentioned: HR, HER2, and triple negative. The full set of molecules shown in Fig. 1 was searched and downloaded from the open-access database PubChem, grouped according to the type of cancer being treated.
The first set of calculations consisted of a geometric optimization of all the structures. The aim is to obtain the most energy-stable molecular structure. We first obtained the structure by molecular dynamics using Tinker software with optimization parameters of structural convergence of energy 2x10–5 kcal/mol, force 0.5 kcal/mol/Å, and maximum displacement 1x10–5 Å with a maximum of 500 iterations. After the atoms are positioned at a given coordinate, Molecular Dynamics calculates each of these quantities. It then performs the same energy and force calculation by changing the position and torsion angle of each atom. These two measurements show that for some pairs of atoms, there is an increase/decrease in force and energy. The software then looks for combinations that will decrease both the force between the pairs of atoms and the total energy of the system.
The second set of calculations is performed with the most stable molecule obtained by molecular dynamics. This next level of theory is the Density Functional Theory (DFT) method. The DFT method in quantum chemistry uses electron density to calculate the properties and energies of compounds. It provides information on electronic states and energy transitions relevant to pharmacological activity. The Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) orbitals are of special interest at this level of theory as they indicate where a molecule can lose or give up electrons, and where it can accept electrons.
All calculations were performed on a desktop computer with an i7-processor with 32 GB of RAM and a dedicated video card Nvidia GTX-1080 Ti with 3584 CUDA cores.
3 Results and Discussion
The set of six molecules is shown in Table 1. The first row (molecules a) to f)) corresponds to the molecules assembled from the Lewis structure and optimized at the molecular dynamics level. The energetically optimized structures by DFT are shown in the second row (molecules g) to l)). In terms of distances, the atom-atom variations were less than 5 Å in absolute terms, while the energy decreased by more than 2 eV.
Since our molecules are in an isolated state, it is easy to see that they are different from the state they are in reality. This is because the drugs in clinical use are presented in a medium that would modify our ideal structure due to the different temperatures, pH, enzymes, proteins, and H2O that surround them and are not taken into account in the calculations. However, the molecules of the present work, after the mentioned energetic optimization, can be used to know each of their affinity and chemical selectivity towards therapeutic targets in a reliable way. The B3LYP functional we are considering has proven to be very powerful in the search for minimum energies in isolated molecules, so these structures can be used at this point to obtain their electronic structures and reliably compare them with each other. However, electronic quantities of interest, such as HOMO-LUMO, suffer from slight modifications in this isolation simulation compared to what would be expected in a real medium. It is the knowledge of the volumes of the HOMO-LUMO orbitals that allows us to move to the next point of analysis and use it for drug correlation.
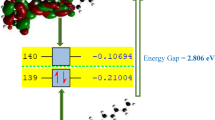
Table 2 shows the electronic structures of the 6 molecules energetically obtained at the level of DFT theory. The interactions between the drugs and the therapeutic target would be expected to occur in the volumes framed by the yellow and blue clouds in the images. The chemical properties listed would also depend on two factors, the position of the HOMO and the LUMO. On one hand, the HOMO of a molecule is associated with the most energetic electrons and thus represents the orbital with the greatest capacity to donate electrons. On the other hand, the LUMO is associated with the lower energy electrons and can act as an electron acceptor or acceptor orbital. These properties are relevant to the ability of the molecule to participate in chemical bonding and electron transfer reactions.
For tamoxifen, the chemical interactions would be at the ends of the benzene or phenyl rings (six carbon atoms). For anastrozole, the phenyl and triazole rings (two N and two C atoms) would be the sites of chemical interactions. For the remaining four drugs, the same trend is found: the HOMO-LUMO orbitals are found around the rings, which in these cases create more complex structures between more than one ring. For the six drugs, the volumes are not found in the other functional groups, such as methyl, amino, and cyan, so it would be expected that they do not intervene in the chemical bonds of the drugs. Thus, it is only in the part of the molecule formed by the rings that the anchoring points with enzymes or proteins present in the cancer process will occur. Non-covalent interactions such as hydrogen bonding, electrostatic interactions, and pi-pi stacking would be some of the crucial mechanisms for molecular recognition and therapeutic activity in chemically reactive zones defined by HOMO-LUMO volumes.
The HOMO orbital can only donate electrons to orbitals with a lower energy modulus (negative values closer to zero) that are present in the therapeutic targets. The tendency of the molecule to donate electrons increases as the energy level of the HOMO decreases. For example, tamoxifen, lapatinib, and tucatinib, which have energies closer to zero, can donate electrons to more molecules than anastrozole. LUMO can be thought of as the orbital that can accept electrons or participate in electron transfer reactions. It is an indicator of a molecule’s ability to accept electrons or act as an oxidant. The tendency of a molecule to accept electrons increases as the energy level of the LUMO decreases. Of our group of drugs, capecitabine has the highest oxidizing property and isofosfamide has the lowest. The difference in energy between the HOMO and LUMO, also known as the electronic gap, is also important. A smaller gap can indicate a higher reactivity and interaction capacity of a molecule.
As mentioned above, there are several computational studies of drugs used in cancer, however, the objectives of these studies are oriented to modify the properties to measure their efficacy, analyze interactions with other drugs or analyze the therapeutic activity of combined drugs, but there is no information available on the physicochemical characteristics of isolated drugs. An area of interest for this type of result is the development of targeted therapies since the drug and the therapeutic target must be known in detail to make the treatment more effective, however, a limitation to the present work would be that future cross-validations and comparisons with experimental data are important to support and verify the theoretical results. In addition, once the properties of the drugs are known, it is possible to perform molecular docking studies to determine their interactions with the therapeutic targets.
4 Conclusions
Cancer has impacted the scientific community since its beginnings, not only because of the complexity of its treatment, which does not always guarantee a complete cure but also because of all the social effects that the fight against this disease entails. One of the types of cancer that is currently most present in humanity is breast cancer, which impacts many women of all ages and backgrounds every year, and even if all risk factors are treated, only 30% of the probability of suffering from this cancer is reduced; even if it’s not present in the background of a lineage that precedes all patients. This motivates waves of fear and uncertainty in society, which at some point in their lives may be part of the statistics. This is why in modern times we find ourselves in the necessity of using all the tools and technology available to solve health problems as relevant as this one, to offer new effective and less invasive alternatives for breast cancer.
To accelerate drug discovery and development, improve efficiency, and reduce the costs associated with drug discovery and testing, computational science has provided powerful tools and methods. It is important to note, however, that computational science does not completely replace traditional methods. Rather, it is used in conjunction with them to enhance and complement the drug development process. It is important to note that DFT is used in conjunction with other experimental techniques and approaches, although it is a valuable tool in drug design. To ensure the efficacy and safety of the compounds being developed, drug design and development is a complex and multidisciplinary process that requires the integration of information and data from multiple sources. As mentioned before, knowing the drugs and properties that are currently effective against cancer provides relevant information for the search for analogous drugs or the design of new drugs with these same characteristics. HOMO and LUMO measurements in studies of molecules designed to treat breast cancer provide information about the reactivity, molecular interactions, and optical properties of the molecules that may be relevant to their therapeutic activity.
References
Organizacion Mundial de la Salud. Organizacion Mundial de la Salud. (2021). https://www.who.int/es/news-room/fact-sheets/detail/breast-cancer
Breast Cancer. Breast Cancer (2023). https://www.breastcancer.org/es/datos-estadisticas
Pashayan, N., Antoniou, A., Ivanus, U., Esserman, L., Easton, D., French, D., et al.: Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 17(11), 687–705 (2020)
Saldivar Gonzalez, F., Prieto Martinez, F., Medina, F.J.: Descubrimiento y desarrollo de farmacos: enfoque computacional. Educ. Quim. 28(1), 51–58 (2017)
Macalino, S.J., Gosu, V., Hong, S., Choi, S.: Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 38(9), 1686–1701 (2015)
Garofalo, M., Grazioso, G., Cavalli, A., Sgrignani, J.: How computational chemistry and drug delivery techniques can support the development of new anticancer drugs. Molecules 25(7), 1756 (2020)
Universidad Nacional Autonoma de Mexico. Naturalis: Quimica computacional. Boletin. Mexico: Universidad Nacional Autonoma de Mexico, Division de Ciencias Basicas
Landeros Martinez, L.: Modelado molecular de la funcionalizacion de nanodiamantes con Tamoxifeno dirigido a receptores del cancer mamario. Tesis doctoral. Chihuahua: Centro de Investigacion en Materiales Avanzados, S. C
Sirhan, Z., Thyagarajan, A., Sahu, R.: The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Military Med. Res. 9(1), 39 (2022)
Abdulrahman, H., Uzairu, A., Uba, S.: Computer modeling of some anti-breast cancer compounds. Struct. Chem. 32, 679–687 (2021)
Centro para el Control y la Prevencion de Enfermedades. ¿Que es el cancer de mama? (2022). https://www.cdc.gov/spanish/cancer/breast/basic_info/what-is-breast-cancer.htm. Accessed 06 July 2023
Palermo Picazo, J., Lassad Rosenthal, J., Juarez Aguilar, L., Medina Nuñez, C.A.: Cancer de mama: una vision general. Acta medica Grupo Angeles. 19(3), 356–360 (2021)
Abdulrahman, H., Uzairu, A., Uba, S.: QSAR, Ligand Based Design and Pharmacokinetic Studies of Parviflorons Derivates as Anti-Breast Cancer Drug Compound Against MCF-7 Cell Line. Chemistry Africa 4(1), 175 (2021)
Landeros, L., Glossman, D., Flores, N.: Interaction of tamoxifen analog with the pocket of some hormone receptors. A molecular docking and density functional theory study. Front. Chem 6, 293 (2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Stockton, A.M. et al. (2024). Drugs and Therapeutic Targets in Breast Cancer: Physicochemical Analysis by Computational Chemistry. In: Flores Cuautle, J.d.J.A., et al. XLVI Mexican Conference on Biomedical Engineering. CNIB 2023. IFMBE Proceedings, vol 96. Springer, Cham. https://doi.org/10.1007/978-3-031-46933-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-46933-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-46932-9
Online ISBN: 978-3-031-46933-6
eBook Packages: EngineeringEngineering (R0)