Abstract
While adsorption is an age-old technique for the treatment of contaminated water, the involvement of magnetic phase separation is relatively new. Magnetism in adsorbents (or magnetic adsorbents) has eased the recovery of micro/nano-adsorbents post-adsorption. Magnetic adsorbents could lower the operation time/cost and improve the overall efficacy of the adsorption process. In this book, most of the chapters have highlighted the use of magnetic adsorbents in water remediation applications. This chapter is focused on the regeneration strategies and disposal assessment of magnetic adsorbents and eluents post-exhaustion. Moreover, the chapter has been updated with some accounts of repurposing strategies for exhausted magnetic adsorbents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
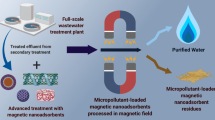
In general, adsorbents are judged based on their robustness and efficacy for pollutant uptake [1, 32]. Apart from the efficacy, ease of recyclability and the extent to which an adsorbent could be recycled are equally important, which are often ignored in the published works [26]. In wastewater treatment facilities, the cost of sludge processing and disposal is significantly higher than the cost of the adsorbent. Thus, the commercialization of magnetic adsorbents for wastewater treatment relies on the regeneration process and management of fully used adsorbents. Another feature that the studies lack is the environmental significance of the magnetic adsorbents-based treatment processes. The use of toxic chemicals or high energy input in the regeneration process needs to be criticized in the interest of achieving sustainability. At the same time, the disposal of toxic spent adsorbents in the landfills should be strictly monitored and guided by environmental norms. Reuse and disposal are two different aspects of waste management. Though regeneration of adsorbents is always an energy-intensive process, the choice of the regeneration process and the economic value of pollutants may balance the equation. Disposal is the process of discarding spent adsorbents either by burning in incinerations (applicable for organic adsorbent-pollutant systems) or in landfills. Since magnetic adsorbents cannot be incinerated, a landfill is considered the only viable option. In the subsequent sections, we have discussed different approaches for regeneration and disposal (Fig. 9.1).
9.2 Regeneration and Reuse of Spent Adsorbents
The regeneration of a spent magnetic adsorbent is a crucial process to cover the environmental and monetary cost of the adsorbent. An ideal regeneration process should revert the adsorption process and restore the physicochemical properties of the adsorbent. In the regeneration process, adsorbed pollutants are stripped out of the adsorbent and a limited number of adsorption sites are made available for the next cycle. The choice of the regeneration process is based on the physicochemical properties of pollutants, stability of adsorbent, and economic constraints. For heavy metal-loaded magnetic adsorbents, stripping agents (eluents) are recommendable. The eluents are either acid/base solutions or solutions of complexing agents, which could extract heavy metals into the solution phase [26]. The strength of eluents depends on the nature of adsorbate–adsorbent interactions, contact time, and temperature. For extracting organic pollutants from the spent adsorbent, the use of polar solvents like ethanol and methanol is recommendable [35]. In some studies, regeneration methods like thermal regeneration and ultrasonication have been reported, which are less tedious and suitable for stable adsorbents. The reusability of magnetic adsorbents for the removal of heavy metals and organic pollutants has been listed in Table 9.1. In general, the choice of using acid/base solutions for the desorption process is largely based on adsorbent–adsorbate interactions as a function of pH. In the pH-dependent adsorption profiles, the pH at which the pollutant uptake is the least is considered a suitable pH to desorb it. The adsorbed cations of heavy metals are stripped using strong acidic solutions due to the competitive nature of protons for the adsorption sites [39, 43, 44]. The use of strong acids for desorption lowers the structural stability of the adsorbent, which is due to the dissolution of magnetic nanoparticles. In some cases, where heavy metals are adsorbed via an ion-exchange mechanism, the use of electrolytic solution (KCl/NaCl) is preferred. The choice of using electrolyte solution for desorption of Tl(I) from magnetic Prussian blue derivative adsorbent was reported by Yeisy et al. [29]. The study reported the use of HNO3, NaOH, and KCl as three elution agents, where pH swing does not affect Tl(I) desorption (Fig. 2a). Though HNO3 could desorb 26% of the adsorbed Tl(I) through proton exchange, KCl was the best eluent with a 97% desorption efficacy (Fig. 2b). The oxyanions (arsenate and chromate) are usually desorbed with strong basic solutions (NaOH) [6, 25]. Chen et al. reported the use of 0.5 mol L–1 NaOH solution for the elution of chromate from spent polyethyleneimine-functionalized Fe3O4 [6]. The pH-dependent adsorption profile showed very low chromate uptake at pH 12, which made 0.5 mol L–1 NaOH solution, an ideal choice for the desorption studies. For this reason, the adsorbent was used for 20 cycles with a 13% loss in the uptake capacity after the 20th cycle.

Reproduced with permission from Elsevier (2021) [29]
a Tl(I) removal versus pH (right axis: ζ potential); b Tl(I) recovery percent versus Eluting solution for magnetic Prussian blue derivative adsorbent.
The method of pH swing is equally applicable to the stripping of organic pollutants from spent adsorbents. Since organic charged dyes are adsorbed by electrostatic interaction, pH variation using an acid/base could give a better desorption efficacy [42, 50]. Organic solvents like ethanol and methanol are also recommended as eluents either in the form of pure solvents [2] or acid/basic solvents [13] due to their high solubility in polar organic solvents. As compared to solvent stripping, thermal regeneration is a simplistic approach to eliminating organic pollutants from spent adsorbents. For a thermally stable adsorbent, heating at an elevated temperature could volatilize the adsorbed organic pollutants. Gupta et al. demonstrated the use of thermal regeneration of methylene blue-adsorbed CoFe2O4/graphene oxide by heating at 400 °C for 1 h in an inert atmosphere [15]. The nanocomposite adsorbent retained 80% of its dye uptake capacity even after the 4th cycle. In another study, the combined effect of pH variation and thermal regeneration was used to desorb rhodamine B from magnetic hollow carbon microspheres. The spent adsorbent was treated with 1.0 mol L–1 NaOH solution and heated at 400 °C for 1 h in an inert atmosphere. The method successfully regenerated the adsorbent with a 6% loss in the performance after the 6th cycle, which made it suitable for more adsorption–desorption cycles [30]. Thus, the development of regeneration strategies is largely dependent on the chemical/thermal stability of the adsorbent, the strength of interactions, the chemical state of pollutants, and so on. More information on the regeneration of adsorbents and recovery could be found in the published specialized reviews [14, 26, 34].
9.3 Disposal of Spent Stripping Solutions
The regeneration of spent adsorbents by solvent stripping methods generates large volumes of toxic organic/inorganic solutions, which need to be managed as per the rules set by the environmental protection agencies. So far, research works are the least concerned with the management of spent eluents. The most judicious way is the use of the same eluent for multiple cycles to produce solutions with a high concentration of heavy metals, which are like ore leachates. Heavy metals like copper, platinum, palladium, and nickel have a high monetary value due to their widespread use. The heavy metals from the spent solutions could be extracted by different metallurgical technologies like flotation with pyrometallurgy [38], solvometallurgy, and electrolysis [3]. Even multi-elemental solutions could be processed by varying the pH to extract precious metals [47]. Moreover, heavy metals are used as catalysts and co-catalysts in different industrial processes like catalytic cracking, syngas production, and Fischer–Tropsch process [4, 27]. These spent solutions can be converted to metal oxide catalysts by precipitation and thermal treatment, which will be an alternative way to convert waste into value-added products [10]. Many of the heavy metals (e.g., arsenate, chromate, and mercury) are costlier to recover than their market value but are highly toxic to living beings. Spent solutions, rich in such heavy metals are concentrated, encapsulated, and disposed of in well-maintained landfill sites [33]. Organic solvents used as dye-stripping agents like acetone, methanol, and ethanol have a low boiling point of 56, 65, and 78 °C, respectively. The dye-rich organic solvents could be regenerated by evaporation to recover the stripping solvents and dyes. Though the evaporation process is energy-intensive, the use of acetone as the stripping agent in combination with the concentrated solar radiation as the heating source could be a cost-cutting solution for the management of dye-rich spent solvents. Most of the suggested methods are yet to be reported in the literature in detail. The research work dealing with wastewater treatment is largely focused on answering whether the fabricated adsorbent can adsorb. If yes, then how much? Whether it could be reused and if yes, then for how many cycles? But it is expected that in the future studies, researchers will be more interested in exploring disposal strategies for exhausted eluents.
9.4 Disposal of Exhausted Adsorbents
After numerous cycles of adsorption-regeneration, the pollutant uptake capacity of an adsorbent reaches a point where it is no more economically feasible to reuse it. The spent adsorbent is replaced by a fresh one and the spent adsorbent is treated as a highly toxic solid waste. Dumping of these toxic exhausted adsorbents in open spaces poses severe risks to human health and the environment, especially in countries where waste segregation and disposal strategies are limited [5]. Even improper disposal of exhausted adsorbents in landfills or poor maintenance after disposal could generate the issue of secondary pollution of groundwater or soil [51]. Thus, it is critical to construct suitable waste-disposal mechanisms from the environmental perspective. However, the concept of finding an alternative application for a waste product is more valuable than finding a new path for disposal. Gupta et al. reported an alternative application of exhausted CuO after the H2S desulfurization process. The study reported better methylene blue photodegradation efficiency for exhausted materials compared to the fresh CuO (Fig. 9.3) [16]. A mesoporous silica (MCM-41) was developed for the removal of chromate from waste solutions. The Cr-loaded MCM-41 was calcined in air at 550 °C for 6 h to produce the Cr/MCM-41 catalyst, which was used for the catalytic degradation of methyl mercaptan (a volatile organosulfur compound with an obnoxious odor) [19]. The waste-to-wealth concept has been used for the conversion of spent Pb-loaded hydrochar to a carbonaceous anode for lithium-ion batteries [48].

Reproduced with permission from the American Chemical Society (2021) [16]
The exhausted CuO was repurposed for dye photodegradation as an environmentally benign route to provide value to the waste.
On a similar note, reports on the waste-to-wealth concept are available for spent magnetic adsorbents as well. A magnetic mesoporous silica/poly(m-aminothiophenol) nanocomposite was fabricated for Hg(II) removal [11]. The adsorbent showed a remarkable sorption capacity of 243 mg g−1 within 10 min of agitation. The adsorbent was reused for 5 cycles with a retention of 76% of its initial adsorption capacity. The spent adsorbent was repurposed as a catalyst for the conversion of phenylacetylene to acetophenone with a 97% yield. Further modifications were made to the material to develop a magnetic network polymer composite by introducing poly(m-aminothiophenol) and chitosan onto the magnetic nanoparticle using tannic acid as a cross-linking agent. The material possessed a Hg(II) removal capacity of 245.5 mg g−1 with excellent reusability and selectivity. Moreover, the exhausted adsorbent catalyzed the transformation of different phenylacetylene derivatives into corresponding acetophenone with ~ 98–99% yield [12]. In another study, the fabricated magnetic mesoporous silica/chitosan composite was studied for Hg(II) removal [20]. The adsorbent has an uptake capacity of 437.8 mg g−1 with the retention of 87% of its initial adsorption capacity after the 6th adsorption-regeneration cycle. The spent adsorbent was used as a thermal catalyst for the conversion of phenylacetylene to acetophenone with a yield of 98.3%.
Cruz et al. have reported a novel concept of turning waste into wealth on two fronts [7]. The natural organic matter (NOM)-rich water from a waterfall was used for the synthesis of CoFe2O4/NOM as an adsorbent for the removal of chromate. The adsorbent was reusable, where the adsorption performance was 82% in the 5th cycle as compared to 96% in the 1st cycle. The spent adsorbent was used as a catalyst for the reduction of 4-nitrophenol in the presence of NaBH4 as the reducing agent, where a conversion efficiency of 99.9% was recorded within 3 s. The catalyst was studied for two additional cycles, where the conversion efficiency was still 99.9% within 30 s. Apart from excellent reusability, the material showed insignificant leaching of chromium and iron in the solution phase. On the same concept, an adsorbent and subsequently a catalyst was developed using waste biomass [28]. Fir sawdust was used as a bio-sorbent for the adsorption of Cu(II) and Fe(III) ions. This metal-loaded biomass was pyrolyzed to yield Cu–Fe3O4–carbon material, which found application as a reusable catalyst for the reduction of 4-nitrophenol.
A low-cost Fe3O4/Carbon composite, developed for the adsorptive capture of chromate from wastewater, was repurposed as an anode material for potassium-ion batteries after chromate adsorption. The redox reaction between Cr(VI) and Cr(III) was responsible for the K-storage mechanism. Thus, more adsorption on chromate was favorable for improving the K-storage capacity of the material [31]. Zn–Al–La layered double hydroxide is an inorganic synthetic clay, which showed a high uptake behavior for amaranth (azo dye). After dye adsorption, the spent material was calcined (to form a carbonaceous composite material) and repurposed as a photocatalyst for the degradation of ibuprofen [41]. On the same line, spent magnetic adsorbents could be used as suitable photocatalysts for the degradation of organic pollutants. Specifically, transition heavy metals along with iron oxide in these adsorbents are excellent UV–visible responsive photocatalysts [17, 18]. Thus, spent magnetic adsorbents could be used for alternative applications of catalysis that comply with the principles of industrial symbiosis with extraordinary environmental benefits.
Spent adsorbents rich in heavy metals could be repurposed to produce ceramic materials, which works as an alternative method for stabilizing toxic metals and at the same time serves the purpose of construction material. One such example was the use of lime activated fly ash for adsorptive removal of heavy metals (Pb, Zn, and As) and subsequent valorization of exhausted adsorbent as an additive in construction material [23]. Rathore and Mondal have reported the stabilization of arsenic-loaded adsorbents namely, thermally treated laterite, acid–base treated laterite, and aluminum oxide/hydroxide nanoparticles as clay bricks. Brick properties like density, water absorption, shrinkage, compressive strength, and efflorescence complied with the Indian standards set for construction materials. Moreover, the maximum amount of arsenic in the brick leachate was well below the United State Environmental Protection Agency permissible limit of 5.0 mg L−1 [36, 37]. Though stabilization of spent magnetic adsorbent as clay bricks has not been studied, Verbinnen et al. developed a novel method to stabilize heavy metals and oxyanions forming elements (Cr, Ni, Cu, Zn, As, Cd, and Pb) by adsorbing it on zeolite- or perlite-supported magnetite. This was followed by mixing the spent adsorbent with industrial sludge (sludge/adsorbent ratio of 97/3) at 1100 °C for 0.5 h. Based on the study, the leaching of toxic metals from the ceramic was below the regulatory limits [46]. The production of lime and cement used for the solidification/stabilization of spent adsorbents before being dumped in landfills has a high carbon footprint due to CO2 release [22]. Stabilization of spent adsorbents as bricks and ceramics saves raw clay materials and lowers the environmental impact and cost of the landfill process.
9.5 Conclusion
Repurposing of spent adsorbents is a new concept, which needs to be explored more often to make the overall adsorption process, environmentally benign and affordable. In the literature, only a handful of research works have been dedicated to the cause. Nevertheless, the idea that has cropped up in these studies is worth reporting in the context of the environmental sustainability of the process. While the researchers are dedicated to exploring regeneration techniques, finding alternative applications for the exhausted adsorbents is highly lucrative and novel. With the enrichment of the literature with such studies in the future, it will be easier to judge the environmental applicability of the adsorption processes.
References
Abdel Maksoud MIA, Elgarahy AM, Farrell C, Al-Muhtaseb AH, Rooney DW, Osman AI (2020) Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord Chem Rev 403:213096. https://doi.org/10.1016/j.ccr.2019.213096
Arabkhani P, Asfaram A (2020) Development of a novel three-dimensional magnetic polymer aerogel as an efficient adsorbent for malachite green removal. J Hazard Mater 384:121394. https://doi.org/10.1016/j.jhazmat.2019.121394
Binnemans K, Jones PT (2017) Solvometallurgy: an emerging branch of extractive metallurgy. J Sustain Metall 3(3):570–600. https://doi.org/10.1007/s40831-017-0128-2
Biswas S, Pal A, Pal T (2020) Supported metal and metal oxide particles with proximity effect for catalysis. RSC Adv 10(58):35449–35472. https://doi.org/10.1039/D0RA06168A
Chaukura N, Gwenzi W, Tavengwa N, Manyuchi MM (2016) Biosorbents for the removal of synthetic organics and emerging pollutants: opportunities and challenges for developing countries. Environ Dev 19:84–89. https://doi.org/10.1016/j.envdev.2016.05.002
Chen B, Zhao X, Liu Y, Xu B, Pan X (2015) Highly stable and covalently functionalized magnetic nanoparticles by polyethyleneimine for Cr(vi) adsorption in aqueous solution. RSC Adv 5(2):1398–1405. https://doi.org/10.1039/C4RA10602D
Cruz DRS, Santos BTJ, Cunha GC, Romão LPC (2017) Green synthesis of a magnetic hybrid adsorbent (CoFe2O4/NOM): removal of chromium from industrial effluent and evaluation of the catalytic potential of recovered chromium ions. J Hazard Mater 334:76–85. https://doi.org/10.1016/j.jhazmat.2017.03.062
Dai K, Wang F, Jiang W, Chen Y, Mao J, Bao J (2017) Magnetic carbon microspheres as a reusable adsorbent for sulfonamide removal from water. Nanoscale Res Lett 12(1):528. https://doi.org/10.1186/s11671-017-2295-2
Deng Y, Ok YS, Mohan D, Pittman CU, Dou X (2019) Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ Res 169:434–444. https://doi.org/10.1016/j.envres.2018.11.035
Fan X, Song C, Lu X, Shi Y, Yang S, Zheng F, Huang Y, Liu K, Wang H, Li Q (2021) Separation and recovery of valuable metals from spent lithium-ion batteries via concentrated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2. J Alloys Compd 863:158775. https://doi.org/10.1016/j.jallcom.2021.158775
Fu Y, Sun Y, Chen Z, Ying S, Wang J, Hu J (2019) Functionalized magnetic mesoporous silica/poly(m-aminothiophenol) nanocomposite for Hg(II) rapid uptake and high catalytic activity of spent Hg(II) adsorbent. Sci Total Environ 691:664–674. https://doi.org/10.1016/j.scitotenv.2019.07.153
Fu Y, Sun Y, Zheng Y, Jiang J, Yang C, Wang J, Hu J (2021) New network polymer functionalized magnetic-mesoporous nanoparticle for rapid adsorption of Hg(II) and sequential efficient reutilization as a catalyst. Sep Purif Technol 259:118112. https://doi.org/10.1016/j.seppur.2020.118112
Ganesan V, Louis C, Damodaran SP (2018) Graphene oxide-wrapped magnetite nanoclusters: a recyclable functional hybrid for fast and highly efficient removal of organic dyes from wastewater. J Environ Chem Eng 6(2):2176–2190. https://doi.org/10.1016/j.jece.2018.03.026
Gkika DA, Mitropoulos AC, Kyzas GZ (2022) Why reuse spent adsorbents? The latest challenges and limitations. Sci Total Environ 822:153612. https://doi.org/10.1016/j.scitotenv.2022.153612
Gupta A, Viltres H, Gupta NK (2020) Sono-adsorption of organic dyes onto CoFe2O4/Graphene oxide nanocomposite. Surf Interfaces 20:100563. https://doi.org/10.1016/j.surfin.2020.100563
Gupta NK, Bae J, Kim KS (2021) From MOF-199 microrods to CuO nanoparticles for room-temperature desulfurization: regeneration and repurposing spent adsorbents as sustainable approaches. ACS Omega 6:25631–25641. https://doi.org/10.1021/acsomega.1c03712
Gupta NK, Ghaffari Y, Bae J, Kim KS (2020) Synthesis of coral-like α-Fe2O3 nanoparticles for dye degradation at neutral pH. J Mol Liq 301:112473. https://doi.org/10.1016/j.molliq.2020.112473
Gupta NK, Ghaffari Y, Kim S, Bae J, Kim KS, Saifuddin M (2020) Photocatalytic degradation of organic pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) nanoparticles at neutral pH. Sci Rep 10(1):4942. https://doi.org/10.1038/s41598-020-61930-2
He D, Zhang L, Zhao Y, Mei Y, Chen D, He S, Luo Y (2018) Recycling spent Cr adsorbents as catalyst for eliminating methylmercaptan. Environ Sci Technol 52(6):3669–3675. https://doi.org/10.1021/acs.est.7b06357
He H, Meng X, Yue Q, Yin W, Gao Y, Fang P, Shen L (2021) Thiol-ene click chemistry synthesis of a novel magnetic mesoporous silica/chitosan composite for selective Hg(II) capture and high catalytic activity of spent Hg(II) adsorbent. Chem Eng J 405:126743. https://doi.org/10.1016/j.cej.2020.126743
Islam MS, Choi WS, Nam B, Yoon C, Lee H-J (2017) Needle-like iron oxide@CaCO3 adsorbents for ultrafast removal of anionic and cationic heavy metal ions. Chem Eng J 307:208–219. https://doi.org/10.1016/j.cej.2016.08.079
Kajaste R, Hurme M (2016) Cement industry greenhouse gas emissions—management options and abatement cost. J Cleaner Prod 112:4041–4052. https://doi.org/10.1016/j.jclepro.2015.07.055
Karanac M, Đolić M, Veljović Đ, Rajaković-Ognjanović V, Veličković Z, Pavićević V, Marinković A (2018) The removal of Zn2+, Pb2+, and As(V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manage 78:366–378. https://doi.org/10.1016/j.wasman.2018.05.052
Kim E-J, Lee C-S, Chang Y-Y, Chang Y-S (2013) Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl Mater Interfaces 5(19):9628–9634. https://doi.org/10.1021/am402615m
Kumar ASK, Jiang S-J (2017) Synthesis of magnetically separable and recyclable magnetic nanoparticles decorated with β-cyclodextrin functionalized graphene oxide an excellent adsorption of As(V)/(III). J Mol Liq 237:387–401. https://doi.org/10.1016/j.molliq.2017.04.093
Lata S, Singh PK, Samadder SR (2015) Regeneration of adsorbents and recovery of heavy metals: a review. Int J Environ Sci Technol 12(4):1461–1478. https://doi.org/10.1007/s13762-014-0714-9
Lee DW, Yoo BR (2014) Advanced metal oxide (supported) catalysts: synthesis and applications. J Ind Eng Chem 20(6):3947–3959. https://doi.org/10.1016/j.jiec.2014.08.004
Liu W-J, Tian K, Jiang H, Yu H-Q (2014) Harvest of Cu NP anchored magnetic carbon materials from Fe/Cu preloaded biomass: their pyrolysis, characterization, and catalytic activity on aqueous reduction of 4-nitrophenol. Green Chem 16(9):4198. https://doi.org/10.1039/C4GC00599F
López YC, Ortega GA, Martínez MA, Reguera E (2021) Magnetic Prussian blue derivative like absorbent cages for an efficient thallium removal. J Cleaner Prod 283:124587. https://doi.org/10.1016/j.jclepro.2020.124587
Lu F, Huang C, You L, Wang J, Zhang Q (2017) Magnetic hollow carbon microspheres as a reusable adsorbent for rhodamine B removal. RSC Adv 7(38):23255–23264. https://doi.org/10.1039/C7RA03045B
Ma J, Liu C (2021) Turning waste into treasure: Reuse of contaminant-laden adsorbents (Cr(vi)-Fe3O4/C) as anodes with high potassium-storage capacity. J Colloid Interface Sci 582:1107–1115. https://doi.org/10.1016/j.jcis.2020.08.110
Mehta D, Mazumdar S, Singh SK (2015) Magnetic adsorbents for the treatment of water/wastewater—a review. J Water Process Eng 7:244–265. https://doi.org/10.1016/j.jwpe.2015.07.001
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142(1–2):1–53. https://doi.org/10.1016/j.jhazmat.2007.01.006
Patel H (2021) Review on solvent desorption study from exhausted adsorbent. J Saudi Chem Soc 25(8):101302. https://doi.org/10.1016/j.jscs.2021.101302
Paz R, Viltres H, López YC, Kumar Gupta N, Levya C (2021) Fabrication of magnetic cerium-organic framework-activated carbon composite for charged dye removal from aqueous solutions. J Mol Liq 337:116578. https://doi.org/10.1016/j.molliq.2021.116578
Rathore VK, Mondal P (2017) Stabilization of arsenic and fluoride bearing spent adsorbent in clay bricks: preparation, characterization and leaching studies. J Environ Manage 200:160–169. https://doi.org/10.1016/j.jenvman.2017.05.081
Rathore VK, Mondal P (2017) Competitive adsorption of arsenic and fluoride onto economically prepared aluminum oxide/hydroxide nanoparticles: multicomponent isotherms and spent adsorbent management. Ind Eng Chem Res 56(28):8081–8094. https://doi.org/10.1021/acs.iecr.7b01139
Ruismäki R, Rinne T, Dańczak A, Taskinen P, Serna-Guerrero R, Jokilaakso A (2020) Integrating flotation and pyrometallurgy for recovering graphite and valuable metals from battery scrap. Metals 10(5):680. https://doi.org/10.3390/met10050680
Safinejad A, Chamjangali MA, Goudarzi N, Bagherian G (2017) Synthesis and characterization of a new magnetic bio-adsorbent using walnut shell powder and its application in ultrasonic assisted removal of lead. J Environ Chem Eng 5(2):1429–1437. https://doi.org/10.1016/j.jece.2017.02.027
Sahoo JK, Paikra SK, Mishra M, Sahoo H (2019) Amine functionalized magnetic iron oxide nanoparticles: synthesis, antibacterial activity and rapid removal of Congo red dye. J Mol Liq 282:428–440. https://doi.org/10.1016/j.molliq.2019.03.033
Shen X, Zhu Z, Zhang H, Di G, Chen T, Qiu Y, Yin D (2020) Carbonaceous composite materials from calcination of azo dye-adsorbed layered double hydroxide with enhanced photocatalytic efficiency for removal of Ibuprofen in water. Environ Sci Eur 32(1):77. https://doi.org/10.1186/s12302-020-00351-4
Vahidhabanu S, Adeogun AI, Babu BR (2019) Biopolymer-Grafted, magnetically tuned halloysite nanotubes as efficient and recyclable spongelike adsorbents for anionic azo dye removal. ACS Omega 4(1):2425–2436. https://doi.org/10.1021/acsomega.8b02960
Venkateswarlu S, Yoon M (2015) Rapid removal of cadmium ions using green-synthesized Fe3O4 nanoparticles capped with diethyl-4-(4 amino-5-mercapto-4H-1,2,4-triazol-3-yl)phenyl phosphonate. RSC Adv 5(80):65444–65453. https://doi.org/10.1039/C5RA10628A
Venkateswarlu S, Yoon M (2015) Core–shell ferromagnetic nanorod based on amine polymer composite (Fe3O4@DAPF) for fast removal of Pb(II) from aqueous solutions. ACS Appl Mater Interfaces 7(45):25362–25372. https://doi.org/10.1021/acsami.5b07723
Venkateswarlu S, Yoon M (2015) Surfactant-free green synthesis of Fe3O4 nanoparticles capped with 3,4-dihydroxyphenethylcarbamodithioate: stable recyclable magnetic nanoparticles for the rapid and efficient removal of Hg(ii) ions from water. Dalton Trans 44(42):18427–18437. https://doi.org/10.1039/C5DT03155A
Verbinnen B, Block C, Van Caneghem J, Vandecasteele C (2015) Recycling of spent adsorbents for oxyanions and heavy metal ions in the production of ceramics. Waste Manage 45:407–411. https://doi.org/10.1016/j.wasman.2015.07.006
Yang C, Li J, Tan Q, Liu L, Dong Q (2017) Green process of metal recycling: coprocessing waste printed circuit boards and spent tin stripping solution. ACS Sustain Chem Eng 5(4):3524–3534. https://doi.org/10.1021/acssuschemeng.7b00245
Yu J, Tang T, Cheng F, Huang D, Martin JL, Brewer CE, Grimm RL, Zhou M, Luo H (2021) Exploring spent biomass-derived adsorbents as anodes for lithium ion batteries. Mater Today Energy 19:100580. https://doi.org/10.1016/j.mtener.2020.100580
Zhao F, Repo E, Sillanpää M, Meng Y, Yin D, Tang WZ (2015) Green synthesis of magnetic EDTA- and/or DTPA-cross-linked chitosan adsorbents for highly efficient removal of metals. Ind Eng Chem Res 54(4):1271–1281. https://doi.org/10.1021/ie503874x
Zhou Q, Li Z, Shuang C, Li A, Zhang M, Wang M (2012) Efficient removal of tetracycline by reusable magnetic microspheres with a high surface area. Chem Eng J 210:350–356. https://doi.org/10.1016/j.cej.2012.08.081
Zhu W, Yang X, He J, Wang X, Lu R, Zhang Z (2021) Investigation and systematic risk assessment in a typical contaminated site of hazardous waste treatment and disposal. Front Public Health 9:764788. https://doi.org/10.3389/fpubh.2021.764788
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gupta, N.K., Viltres, H., Leyva, C. (2024). A Perspective on Environmental and Disposal Assessment of Magnetic Sorbents. In: Sahoo, H., Sahoo, J.K. (eds) Iron Oxide-Based Nanocomposites and Nanoenzymes. Nanostructure Science and Technology. Springer, Cham. https://doi.org/10.1007/978-3-031-44599-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-44599-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44598-9
Online ISBN: 978-3-031-44599-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)