Abstract
Stream salmonids inhabit areas impacted by aerial, terrestrial, and underwater sounds which make up the holo-soundscape. Components of the holo-soundscape include sounds from biological sources (biophony), natural sources (geophony), and human activities (anthropophony). Here we review and synthesize the limited research on freshwater soundscapes as they pertain to stream-dwelling salmonids and suggest that holo-soundscape characteristic differences among habitats and along stream-order gradients likely play a role in salmonid ecology. We suggest that the holo-soundscape interacts with other biotic and abiotic attributes of habitats and has the potential for both indirect and direct effects. Direct effects occur when sounds are perceived by, or have a physiological impact on a given species, while indirect effects impact a species by affecting other species, or other components of its habitat. The role of the holo-soundscape in the ecology of salmonids and the potential for direct and indirect impacts of anthropophony have rarely been considered and represent an area for future research. To do this, simultaneous aerial and underwater recording should be incorporated in research programs. Finally, there is a critical need for documentation of salmonid hearing ability and sound production at all ontogenetic stages.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Freshwater soundscapes
- Sound production
- Ambient sounds
- Anthropogenic noise
- Passive acoustics
- Fish behavior
- Fish ecology
- Habitat selection
1 Introduction
Salmonids are important members of both marine and freshwater ecosystems throughout the northern hemisphere with 223 recognized species with three subfamlies: Coregoninae (88 spp), Salmoninae (121 spp), and Thymallinae (14 spp) (Nelson 2016). Some species of salmonid spend their entire lives in fresh water (e.g., Oncorhynchus clarkii), while other species spend a portion of their lives in the marine environment (e.g., Oncorhynchus tshawytscha) and others exhibit a mix of these life strategies (e.g., Oncorhynchus nerka) (Pavlov and Savvaitova 2008). However, freshwater habitats represent spawning and rearing habitats for all salmonids, with the majority spawning in stream habitats. For example, in North America, 78% of salmonids have their primary spawning habitat listed as streams, with an additional 17% that can spawn in streams (Willson 1997). Even though salmonids have a strong reliance on stream habitats for spawning, the soundscape in stream habitats and the role it plays in the ecology of salmonids is not well understood. The soundscape of streams is a complex interaction among aerial, terrestial, and underwater soundscapes constituting a “holo-soundscape” for the habitat (Fig. 1; Rountree et al. 2020).
Illustration of some of the holo-soundscape components found within stream-dwelling salmonid habitats that contribute to direct or indirect effects: micro-habitat specific (turbulence and bubbles at riffles and falls, movement of falling logs and submerged vegetation, gas seeps); movement and vocalizations of predators (birds, bears, humans, and other mammals); movement and vocalization of conspecifics and other fishes (redd cutting, air-movement sounds, jumps and splashes, catfish barks); movement and vocalization of other aquatic organism (insects, crayfish, turtles, frog): invasive noises (traffic, planes, boats, fishing)
To begin understanding the role of the holo-soundscape to salmonids, first we must understand the components that make it up (Fig. 1). A soundscape is the ambient acoustic environment (intensity and frequency composition) an animal is exposed to in its specific habitat (terrestrial or underwater) in time and space which encompasses sounds produced by geological (geophony), biological (biophony), and anthropogenic (anthropophony) sources (Pijanowski et al. 2011; ISO 2017). The geophony is made up of many types of “natural” sounds that characterize a habitat or location, such as wind, rain, and surf. An often-overlooked component are sounds produced by the effects of moving water on objects such as pebbles, logs, and plant matter (Fig. 1). Similarly, the biophony is composed of natural sounds that characterize a habitat and that are produced by vertebrates such as fish, turtles, amphibians, birds, and mammals, but also includes sounds made by invertebrates such as insects. In contrast, the anthropophony is composed of sounds from human-made sources that are invasive to the habitat, such as, but not limited, to sounds from boats, traffic, trains, and construction (reviewed in Duarte et al. 2021), hereafter referred to as noise. Noise from the human voice, and human movements can be considered anthropophony or biophony depending on the specific circumstances, but like other human activities, can have an impact (Fig. 1).
The first recognition of the potential importance of the soundscape to salmonid fishes dates back to 1969 (Stober 1969), but unfortunately, the freshwater soundscape of stream-dwelling salmonids has yet to receive significant attention (Table 1). In fact, freshwater soundscapes in general have only recently received attention from the scientific community (see reviews in: Gammell and O’Brien 2013; Linke et al. 2018; Rountree et al. 2019, 2020; Decker et al. 2020; Desjonquères et al. 2020). Much of the focus to date has been on the effects of anthropogenic noise on specific species (Mickle and Higgs 2018), description of sound production by specific species (reviewed in Rountree et al. 2018), or quantification of ambient sound levels (see review in Rountree et al. 2020). Only a handful of studies have attempted to describe the overall soundscape composition of, or ecological importance to, freshwater habitats in temperate regions within the geographic range of salmonids (Table 1).
Here, we argue that the holo-soundscape is an important defining characteristic of aquatic habitats and the ecological niches of resident and transient biota. We start by outlining and synthesizing the current state of research on all freshwater soundscapes since there is limited information on each individual habitat. Due to the inconsistent naming of freshwater habitats in the literature, we have grouped habitats together into broad categories. We will be grouping lotic habitats (ponds and lakes) together and arbitrarily grouping lentic habitats into two categories: smaller habitats that are higher order (stream/creek/brook/run) and relatively large rivers (main stem rivers) that are lower order.
Next, we discuss the few studies on the hearing abilities and sound production in salmonids to understand the direct and indirect effects of a changing freshwater soundscape. To do this, we first briefly discuss sound and how it is detected by fishes. All sounds have two components: sound pressure and particle motion. Sound pressure is created by the compression and expansion of water (or other media such as air) and propagates as a pressure wave, while particle motion is the oscillation of individual particles due to the pressure wave and is a measure of particle displacement (ISO 2017). Although recently published reviews on fish hearing have highlighted the importance of particle motion (Popper and Hawkins 2018, 2019), most freshwater soundscape studies to date have relied on pressure measurements because of the difficulty of measuring particle motion in the field and lack of widely available and affordable detectors. While we recognize the importance of measuring particle motion in future freshwater soundscape studies, here we focus on the current state of the literature that has been sound pressure dominated.
Lastly, we address the potential role the holo-soundscape plays in the ecology of salmonids in streams. Like other attributes of habitats, sounds can have both direct and indirect effects on a given species. For example, a sound can adversely affect a study species that does not perceive the sound itself, by adversely affecting its prey. We suggest that a more holistic consideration of soundscapes is needed to understand their role in salmonid ecology. For this reason, we define “noise” as any sound that alters the natural soundscape regardless of how it may be perceived by a given study species.
2 Ambient Sound
One component of the soundscape of particular importance is the ambient sound. Ambient sound is defined as the background sound when no individually recognizable sounds are observed (Amoser and Ladich 2010). Ambient sound is typically measured as the sound pressure level (SPL) in decibels (dB) relative to a standard such as 1 μPa (underwater) and over a specified frequency range (see Hawkins and Popper 2014; Merchant et al. 2015 for a review of methodologies). In most cases, it should be understood SPL values are the received values, i.e., the level at the location of the hydrophone, and not the source level. However, only a handful of studies have made comparisons of ambient sound levels among different freshwater habitat types (Stober 1969; Amoser and Ladich 2010; Wysocki et al. 2007; Bolgan et al. 2018; Kacem et al. 2020; Rountree et al. 2020, Table 1).
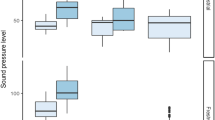
Previous studies have reported ambient sound levels from lake (Bolgan et al. 2016b, c; Putland and Mensinger 2019a; Rountree et al. 2020), pond (Desjonquéres et al. 2015; Rountree et al. 2020), and river/stream (Tonolla et al. 2010, 2011; Vračar and Mijić 2011; Desjonquères et al. 2018; Kacem et al. 2020; Rountree et al. 2020) habitats. Studies conducted in small lakes and ponds indicated these habitats are relatively quiet with low SPL values (Desjonquéres et al. 2015; Putland and Mensinger 2019a). In Minnesota, small lakes (<7 km2 surface area) were found to have broadband (100–12,000 Hz) sound pressure levels that ranged from 60 to 78 dB re 1 μPa in the summer then the SPL dropped to 51–65 dB re 1 μPa in the winter months (Putland and Mensinger 2019a). Another study examined sounds produced in temperate ponds in France (Desjonquéres et al. 2015), but no sound pressure metrics were reported. In comparison, river habitats tended to have higher SPL values compared to lakes and ponds (Amoser and Ladich 2010; Bolgan et al. 2018; Rountree et al. 2020). Rountree et al. (2020) compared three broad habitat types: stream/creek/brook, pond/lake, and river, and no significant differences among mean total SPL were detected, but a significant difference in sound level spectra was observed. Rivers demonstrated the highest SPL and ponds and lakes the lowest SPL, except at frequencies below 500 Hz where brooks, streams, and creeks demonstrated the highest SPL values (Fig. 2). Similarly, the Danube River exhibited sound pressure levels (LLeq, 60s) between 80 and 138 dB re 1 μPa (0.005–20 kHz) depending on which section of the river the readings were taken from (Amoser and Ladich 2010). In a study conducted on large rivers in Europe, the highest mean spectral energy was at lower frequencies (20–40 Hz), then SPLs continually decreased up to the maximum frequency recorded (10 kHz: Vračar and Mijić 2011). Similarly, when examining the power spectral density plot for a lock chamber on the Mississippi River, ambient sound was higher for lower frequencies (10–1000 Hz) at 80–100 dB re 1 μPa2/Hz compared to frequencies above 1000 Hz which were <80 dB re 1 μPa2/Hz (Putland and Mensinger 2019b). In contrast, Bolgan et al. (2018) documented higher SPL values in a lake (110 ± 1.4 dB) compared to a river (87 ± 0.6 dB) but this difference was attributed to anthropogenic factors.
Comparison of ambient sound spectra among three habitat types in relation to the known hearing ability of salmonid fishes. Inset is the mean (SE) total ambient sound levels (24 kHz bandwidth) by habitat. Adapted from Rountree et al. (2020)
Different river sections can also influence the SPL within a single river (Tonolla et al. 2011; Desjonquères et al. 2018; Kacem et al. 2020). Rivers are classified by river order, with smaller order values given to headwater tributaries and order values increasing as the river flows toward the lowlands (Zaimes and Emanuel 2014). Within one stream in Canada, six different habitat types were identified, and SPL generally rose with increasing river order, while water velocity, water depth, and habitat type were found to be the most important hydrological components to impact SPL values within the stream (Kacem et al. 2020). Additionally, Desjonquères et al. (2018) documented changes in acoustic communities that were significantly correlated to lateral connectivity on a flood plain on the River Rhone, but the mechanism could not be evaluated. In addition, during 5.5 min drift surveys of five river systems, Tonolla et al. (2011) found significant differences between median SPL values in all octave bands by river segment, indicating SPL trends are conserved across rivers. Another study compared ambient sound in locations in one river with changes in sound composition observed, but no SPL values were reported (Anderson et al. 2008).
Rivers are also separated by habitat type with increasing water movement: pools are areas of deeper waters with slow moving water, runs are areas with moderate current and constant depths, riffles are shallow areas of fast-moving water, and step pools are sections of steep drops followed by a pool. Although studies documenting the likely unique holo-soundscapes of these habitats are limited, a few have noted differences in ambient sound. Generally, river sections with stagnant waters (e.g., pools) have SPL values below 100 dB re 1 μPa compared to sections with fast-moving waters (e.g., rapids: Fig. 1) that are above 110 dB re 1 μPa (Wysocki et al. 2007), with some faster moving environments having SPL values 20–30 dB above low flow environments (Tonolla et al. 2010). Finally, step pools have the highest SPL of all river habitat types, with recorded SPL as high as 150 dB re 1 μPa (Tonolla et al. 2010).
Frequency composition of freshwater ambient sound also differed by water flow rates and river habitat type. Stagnant sections contained the highest energy in low frequencies (< 100 Hz) followed by a fast decline between 100 and 800 Hz, while fast-moving sections also had most energy in low frequencies (<100 Hz) but energy declined only to rise again after 500 Hz (Wysocki et al. 2007; Tonolla et al. 2011). Likewise, small streams have a similar acoustic profile to fast-moving sections of large rivers (Holt and Johnston 2015). Ambient sound was highest at 43 Hz (~80 dB re 1 μPa) then again at 581–1140 Hz (~66 dB re 1 μPa), creating a window between 170 and 450 Hz for biological sounds (Wysocki et al. 2007; Holt and Johnston 2015). The highest SPL occurred at low frequencies for all riverine habitats, but values increased with flows and habitat type by up to 20 dB (Tonolla et al. 2011). Even though flows increase sound pressure levels (up to 13 dB at 125 Hz), the mid frequency window of lower SPL remains, creating acoustic space for animals to communicate (Tonolla et al. 2011).
3 Geophony
Sounds produced by natural processes such as weather, water flow, mechanical movements of wood, rocks and vegetation, and gas seeps constitute the geophony (Fig. 1). Rain can increase ambient sound conditions by 10 dB depending on the rate and size of droplets (Bom 1969; Nystuen 1986) and has been indicated as a main factor influencing broadband sound levels in shallow waters of a freshwater lake (Bolgan et al. 2016b). Wind is another important factor, ranging from only influencing frequencies below 500 Hz to the entire spectrum from large gusts (Karaconstantis et al. 2020). For example, in Lough Na Fooey (Ireland), wind speed and direction were connected to broadband sound levels, with wind speed more important at deeper depths while both wind speed and direction were important for shallow environments (Bolgan et al. 2016b). Wind was also connected to the significant difference observed between seasons in a lake in Minnesota; lakes are ice covered in the winter, and wind no longer impacts the soundscape (Putland and Mensinger 2019a). Other studies in marine systems have documented wind increasing ambient sound levels. An increase in wind speed from 2.5 to 17.5 m/s can increase ambient sound by 17 dB at depths of 258 m (Ødegaard et al. 2019), and shallower depths are more impacted in lower frequencies (<400 Hz). Noise that is generated by wind is predictable in marine environments (Cauchy et al. 2018), and likely the same is true for freshwater systems.
Unfortunately, little attention has been paid to other types of sounds that contribute to the geophony but may play important roles in stream ecology (Fig. 1). Noise created from ice in freshwater habitats has only been minimally accounted for in the literature. Peak frequency for ice-cracking noise was between 400 and 600 Hz and increased hourly SPLrms by around 6 dB (Martin and Cott 2016) in a lake in northern Canada and in an Arctic lake ice cracking produced SPLrms values >130 dB re 1 μPa (0–22 kHz: Mann et al. 2009). While not describing ice noise, another study documented a drop in SPL values in the winter months due to ice covering lakes (Putland and Mensinger 2019a). Each micro-habitat within streams and rivers (still pools, rushing water, rapids, small and large waterfalls) likely have unique acoustic signatures just from the geophony. For example, areas with faster moving water will have movement of sediment (rocks, pebbles) that creates sounds, with the fast-moving current moving larger sediment and resulting in increased geophony sounds. Also, areas surrounded by forests will have lots of woody debris that will make noise in the current from water splashing and the logs creaking. Riffles and small waterfalls created by rocks and woody debris create turbulent noise from bubbles and falling water (Fig. 1). Areas with submerged and emergent vegetation create unique sound signatures from their movements brushing against each other and effects on water flow. In addition to unique ambient sound characteristics due to the geophony, habitat related differences in the biota likely produce different biophonic signatures contributing to habitat-specific holo-soundscape signatures.
4 Biophony
Biological sounds add to the soundscape as well and lead to variation among locations depending on species composition (Fig. 1). Sounds produced in different freshwater habitats vary by location and time of day (Desjonquéres et al. 2015, 2018; Karaconstantis et al. 2020; Rountree et al. 2020). Desjonquéres et al. (2015) documented that the sounds produced in ponds varied each day and different sounds were produced in different ponds. A similar trend was observed on a floodplain where the acoustic community at each site was highly variable and site specific, with sites only having 15% of the same sounds produced (Desjonquères et al. 2018). The acoustic differences were correlated with differences in the macroinvertebrate communities sampled at each site (Desjonquères et al. 2018). Likewise, spatial and temporal variation in river sounds was linked to diel patterns in fish and insect activity, with fish most active during the day and insects starting to call at dusk and increasing in activity until midnight in the Einasleigh River, Australia (Karaconstantis et al. 2020). However, the opposite was observed in a survey of multiple freshwater habitats in North America, where insect sounds (see an example in Fig. 3a) composed the majority of biological sounds produced during the day while fish sounds were the most dominant at night (Rountree et al. 2020). Air movement sounds (examples in Fig. 4) produced by various fish species dominated sounds produced at night and were most prevalent in deeper pond, lake, and river habitats; however, insect sounds dominated in shallower, fast-moving habitats (brook/creek: Rountree et al. 2020). In the Hudson River, a similar trend was observed, with biological sound produced mainly by fish increasing at dusk (Anderson et al. 2008). Biological sounds can have a significant impact on the soundscape, sometimes increasing the sound pressure level by over 10 dB when calls are present (Martin and Cott 2016).
Examples of biological and anthropogenic sounds recorded under water in freshwater habitats. (a) Catfish (red box) and insect sounds (orange box), (b) herring gull sound, (c) car crossing a bridge, (d) lawn mower, (e) human walking on shore with low frequency footfalls (orange box) and higher frequency noise when stepping on gravel (red boxes), (f) fishing fly-line hitting water. Yellow line denotes the upper limit of known hearing in salmonids
Comparison of air movement related sounds produced by four species of salmonids. (a) Brook trout: quiet surface event followed by snitch sound, (b) Brown trout: quiet surface event followed by two vFRTs, (c) Rainbow trout: loud splash followed by a gurgle sound and two snitches, (d) Atlantic salmon: loud jump followed by a moan. Yellow line denotes upper limit of known hearing in salmonids
Underwater soundscapes are not only influenced by sounds produced under water; aerial and terrestrial sounds can also be detected. Shallow systems, like smaller rivers and streams, are strongly influenced by aerial and terrestrial sounds because some sound energy penetrates to shallow depths or is transmitted through the sediment. Sounds made by terrestrial predators (bears, otters, humans, eagles, etc.: Fig. 1) can also sometimes be transmitted into the underwater soundscape and thus contribute to the holo-soundscape. For example, the sounds of a human walking along the shore can be detected under water (Fig. 3e). Additionally, Rountree et al. (2020) reported that bird sounds occurred in 5–15% of recordings depending on habitat type, with one example being herring gull (Larus argentatus) calls (Fig. 3b). Sounds of fish splashing or jumping, ducks landing and taking off, and sounds made by aquatic and terrestrial mammals can all contribute to the soundscape (Fig. 1) but are poorly studied.
5 Anthropophony
Human generated noise has been documented as an important component of the soundscape in marine environments but has only recently been examined in freshwater habitats (Table 1). In various freshwater habitats, anthropogenic noise was the dominant noise source based on relative time (92% day, 88% night) but with different composition depending on time of day (Rountree et al. 2020). During the day, boating activities comprise the highest proportion of anthropogenic noise detected (Rountree et al. 2020) and can influence the SPL in freshwater habitats. In small lakes, boats have shown an increased power spectra density across all frequencies (100–12,000 Hz) by greater than 10 dB re 1 μPa (Putland and Mensinger 2019a). Similarly, outboard motors from boats increased noise levels in a lake by 10–40 dB re 1 μPa (0.005–20 kHz) and a powerboat race created significantly different noise levels compared to ambient conditions (Amoser et al. 2004). Large rivers can also be heavily impacted by boating, with recreational boats increasing the broadband SPL (200–5000 Hz) by a maximum of 35 dB and commercial vessels with a maximum of 40 dB during a single transit by a hydrophone (Putland and Mensinger 2019b). Additionally, boat wakes can increase ambient sound levels at 8 kHz in lakes as they break at the shoreline, and at 5–6 kHz for trailing waves (Stober 1969).
Aerial anthropogenic noise is also a problem for fish in freshwater habitats (Kuehne et al. 2013; Holt and Johnston 2015; Erbe et al. 2018) and contribute to the sound composition during both day and night (Rountree et al. 2020). Traffic noise can be detected underwater (Fig. 1, and an example in Fig. 3c) and have been documented to increase low frequencies (<475 Hz) above ambient sound pressure levels (Holt and Johnston 2015), while being the most numerous sound detected during both day and night recordings (Rountree et al. 2020). Additionally, airplanes passing overhead (Fig. 1) can be heard under water (e.g., Rountree et al. 2020), which can be problematic near airports where airplanes are frequently landing and taking off (Erbe et al. 2018). In the Canning River by the Perth Airport (Australia), planes landing were detected for 30–40 s and increased broadband noise below 3 kHz, with the highest increase below 300 Hz (Erbe et al. 2018). Other important types of anthropogenic noise including trains, shoreline construction, and shoreline activities such as lawn mowing (example Fig. 3d) have been documented (Rountree et al. 2020). The sound of humans walking along the shore or in the water (example Fig. 3e) and fishing activity (Fig. 3f) can also be detected (Marley et al. 2016; Rountree et al. 2020). Overall, aerial anthropogenic noise sources show strong correlation with elevated noise levels (0–8 kHz) under water and have been linked to the level of urbanization (Kuehne et al. 2013). As an accumulation of anthropophonic sounds, noise levels have been shown to have a strong impact on the biophony (Rountree et al. 2020).
While anthropogenic noise is present in most freshwater habitats, composition varies by habitat and river order (Rountree et al. 2020). In lower order locations like brooks and creeks, traffic sounds were detected most often, and this pattern was consistent during both day and night. In comparison, boat noise was not present in brooks or creeks but dominated all other habitats (Rountree et al. 2020). Additionally, regions of rivers that are closer to marine systems (tidal zones) had significantly more boat noise compared to nontidal zones, with boat noise occurring 31% of the time compared to 2% of the time (Rountree et al. 2020). Limited research has occurred on the differences in anthropogenic noise based on river habitat or order, but this is a key component that needs to be evaluated further.
6 Salmonid Hearing Abilities
Fishes hear through three otoliths located in semi-circular canals located inside their inner ear (Popper and Lu 2000). Otoliths are composed of calcium carbonate and move in response to the displacement (particle motion and pressure) created near a sound source, which moves the cilia of sensory hair cells and triggers an electrical impulse to be sent to the brain (Popper and Fay 1973). However, to be able to detect higher frequencies (>1000 Hz) or detect sound further from a source, additional specialized structures are required (Popper et al. 2003). These specialized structures increase sensitivity by connecting the swim bladder to the inner ear, which allows for pressure changes to be transferred. The two main ways this is achieved are through Weberian ossicles (Diogo 2009) or anterior extensions of the swim bladder (Fletcher and Crawford 2001). Additionally, fish are also able to detect low frequency sounds (<400 Hz) through the lateral line (Higgs and Radford 2013).
Hearing abilities in fishes are typically broken down into two categories, hearing generalists and hearing specialists (which have specialized structures to increase hearing range). Salmonids are hearing generalists with no specialized structures. The hearing ability of most salmonids has not been evaluated, but from the few that have (4 out of 223 species), we can estimate that the hearing abilities are similar across the family. Atlantic salmon (Salmo salar) had their hearing evaluated for both particle motion and sound pressure and were found to have a hearing range of 100–580 Hz, with highest sensitivity to 160 Hz (Hawkins and Johnstone 1978). Hawkins and Johnstone (1978) also found that Atlantic salmon are more sensitive to particle motion compared to sound pressure. When the speaker was moved outside of the exposure tank (low particle motion), the hearing abilities documented were dramatically different from the hearing abilities when the speaker was within the tank (high particle motion), suggesting that particle motion may be the dominant sound component in salmonid hearing. However, Atlantic salmon could still detect the sounds produced though the sound pressure component but required a higher decibel level to invoke a response. Similarly, broad whitefish (Coregonus nasus) had both components tested for their hearing abilities and had peak sensitivity at 200 Hz (106 dB) and were least sensitive at 800 Hz (133 dB), but sensitivity started to increase again at 1600 Hz (123 dB), the highest frequency evaluated (Mann et al. 2007). However, broad whitefish are again more sensitive to particle motion, even though pressure and particle motion could not be separated (Mann et al. 2007).
Other studies of salmonid hearing are primarily based on sound pressure rather than particle motion. Chinook salmon (Oncorhynchus tshawytscha) exhibit maximum sensitivity between 100 and 300 Hz at under 110 dB re 1 μPa and are able to detect sounds of up to 1000 Hz at a higher decibel level (130–150 dB re 1 μPa); however, hearing was not evaluated above 1000 Hz (Oxman et al. 2007). Similarly, European whitefish (Coregonus lavaretus) demonstrated peak sensitivity at 300 Hz with a maximum frequency of 800 Hz, while sensitivity above 800 Hz could not be identified (Amoser et al. 2004). Likely salmonids can only effectively detect lower frequencies (<300 Hz), but are sensitive to both the sound pressure level and the particle motion components of a sound source, with the latter being more important.
7 Salmonid Sound Production
Sound production has been documented in 15 species of salmonids (Table 2): European whitefish Coregonus lavaretus (Dubois and Dziedzic 1989), cutthroat trout Oncorhynchus clarkii (Stober 1969), pink salmon O. gorbuscha (Kuznetsov 2009), chum salmon O. keta (Kuznetsov 2009), coho salmon O. kisutch (Neproshin 1972), rainbow trout O. mykiss (Rountree et al. 2018), sockeye salmon O. nerka (Neproshin 1972), Chinook salmon (Neproshin 1972), Atlantic salmon (Rountree et al. 2018), brown trout Salmo trutta (Rountree et al. 2018), Arctic char Salvelinus alpinus alpinus (Bolgan et al. 2016a), brook trout Salvelinus fontinalis (Rountree et al. 2018), Dolly Varden Salvelinus malma (Neproshin 1972), lake trout Salvelinus namaycush (Johnson et al. 2018), and grayling Thymallus thymallus (Persat and Zakharia 1992). However, much of this is based on limited observations or is anecdotal information (Table 2). Air movement sounds (sometimes referred to as pneumatic sounds) are the most common sound type in salmonids (Rountree et al. 2018). Air movement sounds are highly variable, often species-specific, and are produced by internal air movement between the gas bladder and other anatomical structures, and sometimes by external air release through the anus, pneumatic duct, operculum, or mouth. Other common sound types include percussion (jaw snapping), sounds produced by splashing or jumping during air gulping (Stober 1969; Bolgan et al. 2016a; Rountree et al. 2018) which may also be species-specific (Rountree et al. 2018), and sounds produced during redd cutting (Stober 1969; Satou et al. 1994; Moore and Waring 1999).
Air movement sounds are produced in association with air gulping and occur in a sequence including the rise, air gulp, dive, and resumption of activity (Stober 1969; Rountree et al. 2018). Most sounds are produced after the fish has returned to pre-rise activity. Examples of air movement sounds produced by four species of salmonids include fast repetitive ticks (FRTs), very fast repetitive ticks (vFRTs), chirps, moans, whistles, and gurgles (Fig. 4). Some air movement sounds are from gas release out of the anus or gills but there are also sounds produced through internal movement into the pneumatic duct (Neproshin and Kulikova 1975), but all air movement sounds are associated with air gulping at the surface (see Fig. 12 in Rountree et al. 2018). Chum and pink salmon produce air movement sounds and the resonance frequency of their sounds are associated with their swim bladder morphology (Kuznetsov 2009). Arctic char have been documented to produce air gulps (pulse trains of broadband sounds) and snaps (short high frequency sounds) associated with air gasping behaviors and bubble release, but also have sounds (FRTs) that could not be linked with air exchange behaviors (Bolgan et al. 2016a). Fast repetitive ticks (FRTs) produced by Arctic char were infrequent and consisted of repetitive, short (98–107 ms) ticks (690–760 Hz: Bolgan et al. 2016a). Other salmonids have been documented to produce a similar sound to FRTs, but have ticks occurring much closer together, known as a very fast repetitive tick (vFRT).
Multivariate analysis of air movement sounds among four species of salmonids demonstrated they are species-specific although there was strong overlap in characteristics of individual sound parameters (e.g., peak frequency and duration; Rountree et al. 2018). Each species produced multiple sound types. Brook (Fig. 4a), brown (Fig. 4b), and rainbow trout all produced vFRT sounds, while Atlantic salmon did not (Rountree et al. 2018). In addition, brook trout produce a snitch sound at a peak frequency of 4617 Hz, while brown trout produce a chirp like sound that had a peak frequency of 4760 Hz, and rainbow trout produce a “gurgle” sound that had a peak frequency of 2409 Hz (Fig. 4c). Atlantic salmon also produced a lower frequency gurgle sound (748 Hz) and a unique “moan” sound (943 Hz, Fig. 4d). Air gulping behavior and associated sounds also differed among the four species. Brook and brown trout tended to make little splash or noise when gulping air (Fig. 4a, b), while rainbow trout and Atlantic salmon tended to make loud splash or jumping sounds (Fig. 4c, d). Additionally, brown trout also occasionally produce bubble sounds at a lower peak frequency of 1031 Hz (Rountree et al. 2018).
Other potential air movement sounds have been documented in coho and Chinook salmon that sound like a whistle (up to 6000 Hz), while low frequency knocks (100–500 Hz) were observed in Dolly Varden, sockeye, coho, and Chinook salmon, but only sockeye salmon produced high frequency (100–1600 Hz) knocks (Neproshin 1972). However, peak frequency or behaviors associated with these sounds were not reported. Air movement sounds have also been documented to show a diel pattern in a variety of species with differences in the pattern depending on the species. Pink and chum salmon sound production increases at dawn and dusk (Kuznetsov 2009), but lake trout increase only at night (Johnson et al. 2018). Similarly, brown trout also demonstrated a diel pattern with peak sound production at dusk (Rountree et al. 2018).
Other common sounds include substrate thrashing (e.g., redd building: Stober 1969; Moore and Waring 1999; Satou et al. 1987, 1991, 1994), jaw snapping (Neproshin and Kulikova 1975; Bolgan et al. 2016a), and hydrodynamic sounds (Neproshin and Kulikova 1975). Sounds produced through redd building (example Fig. 5) are also thought to serve a behavioral purpose by potentially priming the females for gamete release (Moore and Waring 1999) and could be vital for reproduction in salmonids. Cutthroat trout have been documented to produce sounds associated with behaviors like digging redds (Fig. 1) at frequencies between 700 and 2000 Hz (Stober 1969). Likewise, Arctic char produce sounds associated with gravel movement during courtship, with interactions like chasing and biting linked with sediment sounds (Bolgan et al. 2016a). Spawning grounds for grayling were also acoustically sampled, and spawning activity (gravel excavation) was detected at up to five meters away with a frequency range of up to 40 kHz (Persat and Zakharia 1992). Additionally, some other types of sounds have been documented to be associated with spawning. Lake trout produce “growls” (20–100 Hz) while spawning which do not occur at other times (Johnson et al. 2018).
Some species of salmon have been documented producing clicking or scraping sounds that are likely attributed to jaw movements (Neproshin and Kulikova 1975). Scraping sounds could be linked with movement of the tongue rubbing against the teeth, while clicking noises produced by snapping the jaw shut can be detected at 85–165 dB, with the upper end occurring infrequently (Neproshin and Kulikova 1975). Similarly, Johnson et al. (2018) found snaps (170 Hz) were produced with jaw movements and/or nudging in lake trout, and Bolgan et al. (2016a) documented clicks associated with mouth closing behaviors in Arctic char. European whitefish have been documented to produce stridulation noises (100–300 Hz) produced during contact between males and females during courtship (Dubois and Dziedzic 1989). Cutthroat trout produce thump sounds (150 Hz) associated with tail-flip behaviors (Stober 1969). Lastly, splashing and jumping sounds made by salmonids when gulping air prior to production of air movement sounds were found to be species-specific and ranged from barely detectable sounds in brook trout, loud splashes in rainbow trout, to noisy jumping in Atlantic salmon (Fig. 4: Rountree et al. 2018).
Due to their hearing abilities, salmonids might not be able to detect some of their own sounds, which makes researchers suspect the sounds may be incidental. However, some air movement sounds have sufficient energy in the low frequencies to be potentially detectable by salmonids (Fig. 4, Rountree et al. 2018). In addition, if the sounds are detectable with hydrophones, they may serve as markers for species identification in passive acoustics monitoring regardless of why or how they are produced (Rountree et al. 2018). Such sounds can also contribute to the holo-soundscape with the potential to be recognized by other species and predators. Studies of Atlantic (Clupea harengus) and Pacific herring (Clupea pallasii) suggest that air movement sounds may be socially mediated and function in schooling and/or predator avoidance behavior (Wilson et al. 2004). Similar behaviors have been hypothesized for salmonids (Neproshin and Kulikova 1975; Rountree et al. 2018). Even though few salmonids have been evaluated for sound production, it can be assumed that since they are all physostomous (connection between swim bladder and external environment) there is a potential for many other species to exhibit air movement sounds.
Sounds produced by other salmonids might not be the only acoustic signals to which salmonids may be paying attention, sounds produced by prey and predators might also be important. In various species of fish, sounds produced by prey have invoked a behavioral response in the predator. Holt and Johnston (2011) found cyprinid fishes are attracted to a speaker (UW-30, Lubell Labs) playing rock shuffling sounds over white noise. The response declined with repeated playback indicating the fish were maybe expecting a prey item near the sound, and when they did not find one, they stopped moving towards the sound (Holt and Johnston 2011). This study demonstrated that cyprinids are able to forage using acoustic signals from their prey, and that in low visibility areas they might rely on acoustic signals even more. Another study on piranhas (Serrasalmus spp.) documented a similar trend; piranhas were observed to attack prey that were moving and splashing at the surface more often than silent prey (Markl 1972). For salmonids, one main prey source in streams are insects, however, sounds produced by aquatic insects are well above the known hearing range (Fig. 3a), so it is not likely that salmonids can use these sounds to locate invertebrate prey. Similarly, it is not known if salmonids can detect sounds produced by insect movement and other activities.
Multiple species of salmonids have been documented to alter their behavior (e.g., startle response, dive deeper) when exposed to visual predators, aerial (Stober 1969; Gotceitas and Godin 1991; Miyamoto 2016) or under water (Gregory 1993), as well as when chemical cues of predation are present (Miyamoto 2016), so it is likely that hearing sounds from predators or conspecifics could elicit an antipredator response. Sounds produced by predators walking along shore (Fig. 3e) are within the known hearing range for salmonids and could serve as another cue that predators are present (Fig. 1). In cutthroat trout, tail-flips produce thump sounds (150 Hz) that were only observed when aerial predators were present and could be part of an antipredator response (Stober 1969). Stober (1969) also suggested only one individual made the thump noise but others responded suggesting they could be used sound as a warning for the entire school. Additionally, differences in surface behaviors associated with air movement sounds (splashing at surface vs being quiet) could be related to predator avoidance in areas with heavy terrestrial predators, where silently gulping air at the surface would be an advantage (Fig. 1).
Sounds produced by predators could also be important to salmonids (Figs. 1 and 3). Some are above the known hearing range of salmonids, like herring gull sounds (Fig. 3b) and other bird species (peak frequency: 2800 Hz Rountree et al. 2020), but there is more overlap with sounds produced by other fish species (average peak frequency: 700 Hz Rountree et al. 2020) and the peak hearing range in salmonids. For example, catfish sounds are well within the hearing range of salmonids (Fig. 3a). While it is unclear if salmonids can hear many of these sounds, future research should examine how these sounds might be important for salmonids and their antipredator and foraging behaviors in streams, based on indirect as well as direct effects.
Another interesting theory that has not been fully evaluated is the impact of different river soundscapes on navigation and homing in salmonids. This idea was first proposed by Stober (1969) but has taken a back seat to other signals important for homing (e.g., chemicals, magnetic). Salmon can potentially use these differences in SPL and frequency composition to identify locations for building redds or site-specific breeding, resting, and foraging locations (Kacem et al. 2020). Additionally, river order could be important and changes in the holo-soundscape could aid in deciding how far to move upriver before selecting a breeding location. Previous literature has also suggested that redd building sounds could be important for reproduction (Moore and Waring 1999); however, redd building sounds are generally above the known hearing range of salmonids (Fig. 5). The impact the acoustic environment plays in salmonid homing and reproduction remains unknown, but future research should evaluate this topic to fully understand its importance.
Ambient and biological sounds may not be the only sound sources influencing salmon behavior in freshwater systems. When the noise spectra from a powerboat race was compared to audiograms from fish species, significant overlap between peak sensitivity and highest noise levels was observed, and it was demonstrated that boat noise should be detectable by fish species in close range regardless of hearing ability (Amoser et al. 2004). Vessels that pass nesting sites for another hearing generalist (oyster toadfish, Opsanus tau) were detectable at a peak SPL of between 117 and 123 dB re 1 μPa when the vessel SPL was corrected for their hearing abilities (Sprague et al. 2016). Additionally, boat activities have been known to alter behavior (Jacobsen et al. 2014) and induce a stress response in fish of varying hearing abilities (Wysocki et al. 2006). European perch (Perca fluviatilis), which has a similar hearing range as salmonids (100–1000 Hz), demonstrated increased swimming speed when boat noise was present but did not change their spatial distribution in a lake (Jacobsen et al. 2014). Similarly, European perch displayed increased cortisol when exposed to playback of ship noise. The increase in cortisol was also observed in other freshwater species with elevated hearing abilities, demonstrating that the stress response observed was consistent regardless of the species hearing abilities (Wysocki et al. 2006). However, boat noise differs greatly depending on the boat type and activity (Rountree et al. 2020). While a running boat creates noise largely above the hearing range of salmonids (mean peak frequency 875 Hz, max peak frequency 4266 Hz), noise from a boat at idle strongly overlaps salmonid hearing (mean peak frequency 435 Hz, max peak frequency 1406 Hz; Rountree et al. 2020). Moreover, while running boat noise tends to be transitory (short duration), idling boat noise is more chronic (long duration; Rountree et al. 2020). To our knowledge, there has been no research on the impacts of boat noise on salmonids. In contrast, pile driving impacts have been observed in a marine system (Feist et al. 1992); juvenile pink and chum salmon demonstrated movement away from pile driving activities when sounds were 25 dB above ambient making them audible to the salmon (Feist et al. 1992). Similarly, pile driving can cause physiological effects. Chinook salmon exposed to pile driving sounds in a lab had significant tissue damage and sometimes experienced organ hemorrhage, depending on the sound exposure level (Halvorsen et al. 2012). Understanding the impacts of boat noise on salmonids is crucial and should be evaluated in future studies in both marine and freshwater systems.
Anthropogenic noise could also influence sound production in salmonids. Holt and Johnston (2015) found that traffic sounds resulted in significant masking of blacktail shiner (Cyprinella venusta) knocks (160–630 Hz) and growls (100–315 Hz) up to 12 km from a bridge at 108 Hz. Similarly, traffic noises from a bridge (Fig. 3c) show that most of the acoustical energy recorded is within the known hearing range for salmonids (peak frequency: 225 Hz: Rountree et al. 2020) and could have a similar masking effect in streams (Figs. 1 and 3c). Noise created by boats has also been documented to mask sound production in a variety of species in marine systems (Vasconcelos et al. 2007; Codarin et al. 2009; Luczkovich et al. 2016). Additionally, humans walking (Fig. 3e) along the shore strongly overlaps with documented peak frequency for salmonid hearing (100–300 Hz), and other human activities like mowing lawns (Fig. 3d) and fishing lines hitting the water (Fig. 3f) overlap with the upper range of their hearing abilities. These anthropogenic activities could be influencing behaviors and survival in streams, but no research has yet been conducted on this topic.
Increased noise levels can also change antipredator behaviors in fish and could vary depending on hearing abilities. Three-spined stickleback (Gasterosteus aculeatus) responded faster to a visual predator when noise levels were elevated, but European minnow (Phoxinus phoxinus) did not change their behavior with additional noise (Voellmy et al. 2014). Predator–prey interactions may also play a role in what type of response a species might exhibit to a noise disturbance. European roach (Rutilus rutilus), perch and pike (Esox lucius) were all exposed to boat noise, and each displayed varying reactions that could be linked to their antipredator behaviors (Jacobsen et al. 2014). European perch have also been shown to respond to predators by fleeing to the bottom and using structure as a refuge (Christensen and Persson 1993). When exposed to boat noise perch increased their swimming speed for a short period (1 h), which could indicate they were relocating to a deep refuge and then remaining still (Jacobsen et al. 2014). Increases in anthropogenic activities could also affect predator–prey interactions by allowing one species to exploit increased noise levels to forage more without increasing the risk of being preyed upon themselves, creating an acoustic refuge (Roca et al. 2020). Additionally, anthropogenic sounds have been documented to mask communication in other freshwater fishes (Holt and Johnston 2015), so there could be a similar impact on salmonids if their sounds are used to communicate. Some species (cutthroat trout: Stober 1969) have already been documented to produce sounds in association with predator avoidance and these sounds could serve as a warning to others, if these signals are masked it could result in increased predation risk and decreased survival.
Passive acoustic monitoring could provide another option for understanding population dynamics of salmonid species. Since most salmonids have consistent spawning grounds, an underwater hydrophone could be placed in close proximity and be used to monitor numbers and species that are returning to various habitats. Passive acoustics has been proposed as an option for monitoring invasive species in freshwater habitats (Rountree and Juanes 2017) and could be an option for many salmonid species of concern. However, before this can be possible more research into acoustics in freshwater environments and the sounds produced by salmonid species needs to be documented.
8 Synthesis: The Holo-Soundscape
The time salmonids spend in freshwater habitats could be influenced by the sounds produced in both underwater and terrestrial environments (holo-soundscape) which generate a unique environment (Fig. 1). However, little research has been dedicated to understanding the holo-soundscape and its role in ecological habitat identification and niche development for salmonids and freshwater fish in general. Within a river there are multiple microhabitats that likely have different acoustic signatures (Kacem et al. 2020), and stream order (Strahler 1957; Shreve 1966) is an important factor in creating these different signatures (Fig. 6). Habitat characteristics, environmental conditions, and predation risks form gradients along the stream order (e.g., Platts 1979; Barila et al. 1981; Rountree and Able 2007), as do holo-soundscape characteristics, yet the role of interactions between these phenomena on the ecology of stream-dwelling salmonids is not known. As an organism moves down a river from lower order streams (headwaters) to higher orders, elevation and substrate size decrease along with increased depth and volume of water moving through a section, and these factors are important for creating the acoustic environments in the different order streams (Fig. 6). For example, propagation of sound depends on the wavelength of the sound and depth of the water and therefore is affected by stream order. The cut-off depth is the depth at which sound of a given frequency (hence wavelength) will not transmit beyond the source (Au and Hastings 2008). Particle motion, on the other hand, could be increased in shallow habitats due to sound pressure being converted to particle motion at the surface since air is more elastic compared to the water (Popper and Hawkins 2018). Sediment type also affects the cut-off depth and particle motion. In marine systems, at a depth of 10 m, the lowest frequency that can be detected under ideal conditions ranges from 30 to 200 Hz from a rocky to soft bottom type, while at 1 m the range is 300–2000 Hz (Au and Hastings 2008). Additionally, particle motion is affected by different sediments in the river. Hard bottom substrate can reduce particle motion by stopping movement between individual particles (Hawkins et al. 2020) compared to soft bottoms where particle motion is expanded into sediment (Popper and Hawkins 2018). So, habitat characteristics could be very important for salmonids as their hearing is most sensitive at frequencies between 100 and 300 Hz, with particle motion the more crucial component, and suggests a mismatch between hearing sensitivity and habitat selection in salmonids since many stream-dwelling salmonids live in habitats too shallow for sounds of those frequencies to propagate.
Schematic of impact of stream order on the holo-soundscape and salmonid habitat and niche characteristics (numbers indicate stream order). Gradients are formed along the stream order in habitat characteristics, anthropogenic noise impacts, and sources of predation risk. Sound pressure and particle motion properties are also strongly influenced by these gradients
These different habitat signatures could be aiding in homing, like Stober (1969) first suggested, as salmon need to be able to locate optimal locations within a river to mate, rest, or forage. Often these are different habitats with different holo-soundscape characteristics (e.g., noisy fast-flow spawning sites vs quiet deep-pool resting and foraging sites). One study examined a link between salmonid densities and acoustic properties (Kacem et al. 2020). They found more brook trout present when SPL (>100 dB) values were elevated within their best hearing range (100–300 Hz) in pools and riffles but not glide or cascade habitats in a stream in Canada (Kacem et al. 2020). The presence of more salmon in regions of elevated SPL could be indicating salmonids are choosing to be in a certain location based on the acoustic signature which could be serving as a proxy for habitat quality (e.g., increased food availability due to higher flows) as suggested by Kacem et al. 2020. However, if locations in the river are too noisy, foraging and finding a mate could be significantly hindered, so salmon could be using sound to find the best acoustical environment. Salmon could also be using these acoustic cues to detect areas of a lower predation risk in streams (Fig. 1). In shallower environments, there is a higher risk of predation from avian and terrestrial predators, but if salmon can locate a deeper pool within a section of a river, that habitat would provide a safe haven as well as shelter from the current (Fig. 6).
The holo-soundscape is not only important for salmonids but also could be important for predators of salmonids (Fig. 1). One specific sound that could be important is redd building sounds (Fig. 1). To build an effective redd, salmon need to move gravel around which produces sound (Fig. 5) well above their hearing range but is within the range for many predators. These sounds could cue in underwater predators to locations with salmon and eggs, which if disturbed would decrease fecundity. Furthermore, terrestrial predators like bears (Fig. 1) and otters could likewise use the sounds of salmon jumping and splashing (Fig. 4c, d) to know where good places are to hunt. As it remains unclear if predators are homing in on specific habitats based on the soundscape signature to locate prey, future research should examine this knowledge gap.
Depending on the importance of the acoustic environment to salmonids, anthropogenic activities could alter survival in streams. Anthropogenic activities have been documented to substantially impact various fish species, and salmon are running out of locations that are free of human disturbances. Depending on the location within a river there are different anthropogenic noise sources, with lower order streams and rivers having more traffic and other aerial sounds (e.g., airplanes: Fig. 1). Higher order rivers and tidal regions have more boating activities and their associated sounds as well as increased urbanization since many large cities are located on the water (Fig. 6). Since salmonids are already limited to few suitable acoustic environments in streams, noise could have significant impacts on their abilities to locate these “quieter” regions of the river that are optimal for reproduction and survival. The high impact of anthropogenic activities in streams and rivers suggests that reducing human impacts in these locations is a crucial conservation concern to protect salmonid populations.
In our review, we focused on the adult life stage for salmonids due to a lack of research on other ontogenetic stages. To our knowledge, no research has been published on hearing or sound production in juvenile salmonids. In other marine species, hearing ability has been documented in larval fish as small as 9 mm, and sensitivity to sounds increases with size (Wright et al. 2011). Sound has also been shown to be important for settlement in coral reef fishes (Radford et al. 2011), such that larval and juvenile stages of salmon could also be using sound cues from an early age, but more research into this topic is required.
9 Next Steps
Passive acoustic monitoring could provide another option for understanding population dynamics of salmonid species. Since most salmonids have consistent spawning grounds, an underwater hydrophone could be placed in close proximity and be used to monitor species returning to various habitats. However, before this is possible more research into acoustics in freshwater environments and the sounds produced by each species needs to be documented. Luckily, these are relatively simple to accomplish. Underwater hydrophones are inexpensive and can record autonomously in a diverse range of habitats to understand the acoustics (Rountree et al. 2006; Chapuis et al. 2021; Lamont et al. 2022). Hydrophones are also compact and easy to transport to remote locations away from the influences of human activities. Each hydrophone can be deployed for short or long periods to help understand the influences of geophony, biophony, and anthropophony. However, we contend that the holo-soundscape is of critical importance and research should attempt to record both underwater and aerial sounds whenever possible. Building a library of sounds produced by salmonids can also be incorporated into already existing research projects without having to add much more work. Many salmonids have at least some locations where they are reared in hatcheries or housed in local aquariums. These locations provide an opportunity to record species and see the different sounds produced, but also come with some issues. Hatcheries and other facilities that house fish in captivity are noisy, with an assortment of pumps and other equipment, so recording fish vocalizations in these environments can be quite challenging and results in low signal-to-noise ratios (Riera et al. 2018). Additionally, rearing under these high noise environments can significantly affect the hearing abilities of these fish (Caiger et al. 2012), which could impact sound production and survival. If recordings are made in these environments, care should be taken to reduce background noise as much as possible. Sound production can also be recorded in the river if the underwater hydrophone is paired with video or real-time observations on species near the hydrophone. The use of acoustic arrays in conjunction with video or human observations has recently been used to validate sound production in marine fishes (Mouy et al. 2018) and hold promise in freshwater systems.
In addition to collecting more data on the ambient sound pressure levels in freshwater habitats, the inclusion of the particle motion component of sound is required to truly understand the holo-soundscape in freshwater systems. Many fishes and invertebrates are more sensitive to particle motion compared to sound pressure, but to date no information has been collected on particle motion when describing freshwater soundscapes likely due to the complexity of shallow habitats. The best way to measure particle motion is through an accelerometer, but accelerometers are not only sensitive to particles moving from sounds but all movement, so they do not work in a flow field that has continuously moving water (Popper and Hawkins 2018). Accelerometers are also not as readily available in comparison to hydrophones used to collect sound pressure levels, making it challenging for researchers to collect necessary data. Currently, technology does not exist to effectively measure particle motion as it relates to the soundscape (Miksis-Olds et al. 2018) outside the lab or other controlled settings, but hopefully as new technology is developed, particle motion will become a standard component of holo-soundscape analysis.
Understanding the holo-soundscape represents a new frontier for researching the ecology of salmonids in freshwater habitats but will require substantial research to fully evaluate. Classification of holo-soundscape characteristics along stream order and unique freshwater habitats will allow for detailed descriptions of the acoustic environments of these habitats. Then these different acoustic environments can be used to understand if salmonids and their predators are using acoustic signatures for niche specialization. Research should also continue to evaluate sound production and hearing in salmonids, as linking the acoustic environment they are choosing to their hearing abilities and sounds they produce will aid in understanding salmonids behavior in freshwater habitats. Salmonids are important species around the world, and understanding the acoustic environments they are exposed to and their contribution to the holo-soundscape will add to our understanding of their behavior, ecology, and conservation in freshwater habitats.
References
Amoser S, Ladich F (2005) Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J Exp Biol 208(18):3533–3542. https://doi.org/10.1242/jeb.01809
Amoser S, Ladich F (2010) Year-round variability of ambient noise in temperate freshwater habitats and its implications for fishes. Aquat Sci 72(3):371–378. https://doi.org/10.1007/s00027-010-0136-9
Amoser S, Wysocki LE, Ladich F (2004) Noise emission during the first powerboat race in an Alpine lake and potential impact on fish communities. J Acoust Soc Am 116(6):3789–3797. https://doi.org/10.1121/1.1808219
Anderson KA, Rountree RA, Juanes F (2008) Soniferous fishes in the Hudson River. Trans Am Fish Soc 137(2):616–626. https://doi.org/10.1577/t05-220.1
Au WWL, Hastings MC (2008) Principles of marine bioacoustics. Springer, New York
Barila TY, Williams RD, Stauffer JR (1981) The influence of stream order and selected stream bed parameters on fish diversity in Raystown Branch, Susquehanna River drainage, Pennsylvania. J Appl Ecol 18:125–131
Bolgan M, O’Brien J, Rountree RA, Gammell M (2016a) Does the Arctic charr Salvelinus alpinus produce sounds in a captive setting? J Fish Biol 89(3):1857–1865. https://doi.org/10.1111/jfb.13067
Bolgan M, O’Brien J, Winfield IJ, Gammell M (2016b) An investigation of inland water soundscapes: Which sonic sources influence acoustic levels? Proc Meet Acoust 27(1):070004. https://doi.org/10.1121/2.0000260
Bolgan M, Chorazyczewska E, Winfield IJ, Codarin A, O'Brien J, Gammell M (2016c) First observations of anthropogenic underwater noise in a large multi-use lake. Journal Limnol 75(3):644-651
Bolgan M, Brien JO, Chorazyczewska E, Winfield IJ, Gammell M, Bolgan M, Brien JO, Chorazyczewska E, Winfield IJ (2018) The soundscape of Arctic Charr spawning grounds in lotic and lentic environments: can passive acoustic monitoring be used to detect spawning activities? Bioacoustics 27:57–85. https://doi.org/10.1080/09524622.2017.1286262
Bom N (1969) Effect of rain on underwater noise level. J Acoust Soc Am 45(1):150–156. https://doi.org/10.1121/1.1911351
Caiger PE, Montgomery JC, Radford CA (2012) Chronic low-intensity noise exposure affects the hearing thresholds of juvenile snapper. Mar Ecol Prog Ser 466:225–232. https://doi.org/10.3354/meps09933
Cauchy P, Heywood KJ, Merchant ND, Queste BY, Testor P (2018) Wind speed measured from underwater gliders using passive acoustics. J Atmos Ocean Technol 35:2305–2321. https://doi.org/10.1175/JTECH-D-17-0209.1
Chapuis L, Williams B, Gordon TAC, Simpson SD (2021) Low-cost action cameras offer potential for widespread acoustic monitoring of marine ecosystems. Ecol Indic 129(July):107957. https://doi.org/10.1016/j.ecolind.2021.107957
Christensen B, Persson L (1993) Species-specific antipredatory behaviours: effects on prey choice in different habitats. Behav Ecol Sociobiol 32:1–9
Codarin A, Wysocki LE, Ladich F, Picciulin M (2009) Effects of ambient and boat noise on hearing and communication in three fish species living in a marine protected area (Miramare, Italy). Mar Pollut Bull 58(12):1880–1887. https://doi.org/10.1016/j.marpolbul.2009.07.011
Cott PA, Mann DA, Higgs DM, Johnston TA, Gunn JM (2012) Assessing disturbance from under-ice noise on fishes in boreal lakes. In: Popper AN, Hawkins A (eds) The effects of noise on aquatic life. Springer, New York, pp 363–366
Decker E, Parker B, Linke S, Capon S, Sheldon F (2020) Singing streams: Describing freshwater soundscapes with the help of acoustic indices. Ecol Evol 10(11):4979–4989. https://doi.org/10.1002/ece3.6251
Desjonquéres C, Rybak F, Depraetere M, Gasc A, Le Viol I, Pavoine S, Sueur J (2015) First description of underwater acoustic diversity in three temperate ponds. PeerJ 2015(11):1–16. https://doi.org/10.7717/peerj.1393
Desjonquères C, Rybak F, Castella E, Llusia D, Sueur J (2018) Acoustic communities reflects lateral hydrological connectivity in riverine floodplain similarly to macroinvertebrate communities. Sci Rep 8(1):1–11. https://doi.org/10.1038/s41598-018-31798-4
Desjonquères C, Gifford T, Linke S (2020) Passive acoustic monitoring as a potential tool to survey animal and ecosystem processes in freshwater environments. Freshw Biol 65(1):7–19. https://doi.org/10.1111/fwb.13356
Diogo R (2009) Origin, evolution and homologies of the Weberian apparatus: a new insight. J Morphol 27(2):333–354
Duarte CM, Chapuis L, Collin SP, Costa DP, Devassy RP, Eguiluz VM, Erbe C, Gordon TAC, Halpern BS, Harding HR, Havlik MN, Meekan M, Merchant ND, Miksis-Olds JL, Parsons M, Predragovic M, Radford AN, Radford CA, Simpson SD, Slabbekoorn H, Staaterman E, Van Opzeeland IC, Winderen J, Zhang X, Juanes F (2021) The soundscape of the Anthropocene ocean. Science 371(6529). https://doi.org/10.1126/science.aba4658
Dubois J, Dziedzic A (1989) Underwater sound detection applied to aquatic ethology: some results on coregonids and charr spawning sites in two subalpine lakes. Rev des Sci l’Eau 2:847–858
Erbe C, Williams R, Parsons M, Parsons SK, Hendrawan IG, Iwan IM (2018) Underwater noise from airplanes : An overlooked source of ocean noise. Mar Pollut Bull 137:656–661. https://doi.org/10.1016/j.marpolbul.2018.10.064
Feist BE, Anderson JJ, Miyamoto R (1992) Potential impacts of pile driving on juvenile pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon behavior and distribution. University of Washington
Fletcher LB, Crawford JD (2001) Acoustic detection by sound-producing fishes (Mormyridae): the role of gas-filled tympanic bladders. J Exp Biol 204(2):175–183
Gammell MP, O’Brien JM (2013) Acoustic communication in aquatic animals: All quiet on the freshwater front? Aquat Conserv Mar Freshw Ecosyst 23(3):363–365. https://doi.org/10.1002/aqc.2356
Gotceitas V, Godin JJ (1991) Foraging under the risk of predation in juvenile Atlantic salmon (Salmo salar L.): effects of social status and hunger. Behav Ecol Sociobiol 29:255–261
Gregory RS (1993) Effect of turbidity on the predator avoidance behaviour of juvenile chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 50:241–246
Halvorsen MB, Casper BM, Woodley CM et al (2012) Threshold for onset of injury in chinook salmon from exposure to impulsive pile driving sounds. PLoS One 7(6):e38968
Hawkins AD, Johnstone ADF (1978) The hearing of the Atlantic Salmon, Salmo salar. J Fish Biol 13(6):655–673. https://doi.org/10.1111/j.1095-8649.1978.tb03480.x
Hawkins A, Popper A (2014) Assessing the impacts of underwater sounds on fishes and other forms of marine life. Acoust Today 10(2):30–41
Hawkins AD, Johnson C, Popper AN (2020) How to set sound exposure criteria for fishes. J Acoust Soc Am 147(3):1762–1777
Higgs DM, Radford CA (2013) The contribution of the lateral line to ‘hearing’ in fish. J Exp Biol 216(8):1484–1490
Holt DE, Johnston CE (2011) Can you hear the dinner bell ? Response of cyprinid fishes to environmental acoustic cues. Anim Behav 82(3):529–534. https://doi.org/10.1016/j.anbehav.2011.06.004
Holt DE, Johnston CE (2015) Traffic noise masks acoustic signals of freshwater stream fish. Biol Conserv 187:27–33. https://doi.org/10.1016/j.biocon.2015.04.004
International Organization for Standardization (ISO) (2017) Underwater acoustics-terminology
Jacobsen L, Baktoft H, Jepsen N, Aarestrup K, Berg S, Skov C (2014) Effect of boat noise and angling on lake fish behaviour. J Fish Biol 84(6):1768–1780. https://doi.org/10.1111/jfb.12395
Johnson NS, Higgs D, Binder TR, Ellen Marsden J, Buchinger T, Brege L, Bruning T, Farha S, Krueger CC (2018) Evidence of sound production by spawning lake trout (Salvelinus namaycush) in lakes huron and champlain. Can J Fish Aquat Sci 75(3):429–438. https://doi.org/10.1139/cjfas-2016-0511
Kacem Z, Rodríguez MA, Roca IT, Proulx R (2020) The riverscape meets the soundscape: acoustic cues and habitat use by brook trout in a small stream. Can J Fish Aquat Sci 77(6):991–999. https://doi.org/10.1139/cjfas-2019-0311
Karaconstantis C, Desjonqueres C, Gifford T, Linke S (2020) Spatio-temporal heterogeneity in river sounds: Disentangling micro- and macro-variation in a chain of waterholes. Freshw Biol 65(1):96–106
Kuehne LM, Padgham BL, Olden JD (2013) The soundscapes of lakes across an urbanization gradient. PLoS One 8(2):e55661. https://doi.org/10.1371/journal.pone.0055661
Kuznetsov MY (2009) Traits of acoustic signalization and generation of sounds by some schooling physostomous fish. Acoust Phys 55(6):866–875. https://doi.org/10.1134/S1063771009060219
Lamont TAC, Chapuis L, Williams B, Dines S, Gridley T, Frainer G, Fearey J, Maulana PB, Prasetya ME, Jompa J, Smith DJ, Simpson SD (2022) HydroMoth: Testing a prototype low-cost acoustic recorder for aquatic environments. Remote Sens Ecol Conserv. https://doi.org/10.1002/rse2.249
Linke S, Gifford T, Desjonquères C, Tonolla D, Aubin T, Barclay L, Karaconstantis C, Kennard MJ, Rybak F, Sueur J (2018) Freshwater ecoacoustics as a tool for continuous ecosystem monitoring. Front Ecol Environ 16(4):231–238. https://doi.org/10.1002/fee.1779
Lomask MR, Saenger RA (1960) Ambient noise in a deep inland lake. J Acoust Soc Am 32(7):878–883. https://doi.org/10.1121/1.1908245
Luczkovich JJ, Krahforst CS, Kelly KE, Sprague MW (2016) The Lombard effect in fishes: How boat noise impacts oyster toadfish vocalization amplitudes in natural experiments. Proc Meet Acoust 27:010035. https://doi.org/10.1121/2.0000340
Lugli M (2010) Sounds of shallow water fishes pitch within the quiet window of the habitat ambient noise. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196(6):439–451. https://doi.org/10.1007/s00359-010-0528-2
Lugli M, Fine ML (2003) Acoustic communication in two freshwater gobies: Ambient noise and short-range propagation in shallow streams. J Acoust Soc Am 114(1):512–521. https://doi.org/10.1121/1.1577561
Lugli M, Fine ML (2007) Stream ambient noise, spectrum and propagation of sounds in the goby Padogobius martensii: Sound pressure and particle velocity. J Acoust Soc Am 122(5):2881. https://doi.org/10.1121/1.2783113
Mann DA, Cott PA, Hanna BW, Popper AN (2007) Hearing in eight species of northern Canadian freshwater fishes. J Fish Biol 70(1):109–120. https://doi.org/10.1111/j.1095-8649.2006.01279.x
Mann D, Cott P, Horne B (2009) Under-ice noise generated from diamond exploration in a Canadian sub-arctic lake and potential impacts on fishes. J Acoust Soc Am 126(5):2215–2222. https://doi.org/10.1121/1.3203865
Markl HV (1972) Aggression und Beuteverhalten bei Piranhas (Serrasalminae, Characidae). Z Tierpsychol 30:190–216
Marley SA, Erbe C, Salgado-Kent CP (2016) Underwater sound in an urban Estuarine river: sound sources, soundscape contribution, and temporal variability. Acoust Aust 44(1):171–186. https://doi.org/10.1007/s40857-015-0038-z
Martin BS, Cott PA (2016) The under-ice soundscape in Great Slave Lake near the city of Yellowknife, Northwest Territories, Canada. J Great Lakes Res 42(2):248–255. https://doi.org/10.1016/j.jglr.2015.09.012
Martin SB, Popper AN (2016) Short- and long-term monitoring of underwater sound levels in the Hudson River (New York, USA). J Acoust Soc Am 139(4):1886–1897. https://doi.org/10.1121/1.4944876
Merchant ND, Fristrup KM, Johnson MP, Tyack PL, Witt MJ, Blondel P, Parks SE (2015) Measuring acoustic habitats. Methods Ecol Evol 6(3):257–265. https://doi.org/10.1111/2041-210X.12330
Mickle MF, Higgs DM (2018) Integrating techniques: A review of the effects of anthropogenic noise on freshwater fish. Can J Fish Aquat Sci 75(9):1534–1541. https://doi.org/10.1139/cjfas-2017-0245
Miksis-Olds JL, Martin B, Tyack PL (2018) Exploring the ocean through soundscapes. Acoust Today 14(1):26–34
Miyamoto K (2016) Effect of visual and chemical stimuli on predator avoidance behavior in juvenile masu salmon Oncorhynchus masou. Aquacult Sci 64(1):43–51
Moore A, Waring CP (1999) Reproductive priming in mature male Atlantic salmon parr exposed to the sound of redd cutting. J Fish Biol 55(4):884–887. https://doi.org/10.1006/jfbi.1999.1034
Morgan L (2014) A passive acoustic and experimental study of juvenile blue catfish, Ictalurus furcatus, sound production and agnostic behavior in the tidal freshwater James river. Virginia Commonwealth University
Mouy X, Rountree R, Juanes F, Dosso SE (2018) Cataloging fish sounds in the wild using combined acoustic and video recordings. J Acoust Soc Am 143(5):EL333–EL339. https://doi.org/10.1121/1.5037359
Nelson JS (2016) Fishes of the world, 5th edn. Wiley, Hoboken
Neproshin AY (1972) Some physical characteristic ofsound in Pacific salmons. Zool Zhurnal 51:1025–1030
Neproshin AY, Kulikova NI (1975) Sound-producing organs in Salmonids. J Ichthyol 15:481–485
Nystuen JA (1986) Rainfall measurements using underwater ambient noise Rainfall measurements using underwater ambient noise. J Acoust Soc Am 79(4):972–982. https://doi.org/10.1121/1.393695
Ødegaard L, Pedersen G, Johnsen E (2019) Underwater noise from wind at the High North Love Ocean Observatory. In: UACE conference proceedings, pp 359–366
Oxman DS, Barnett-Johnson R, Smith ME, Coffin A, Miller DL, Josephson R, Popper AN (2007) The effect of vaterite deposition on sound reception, otolith morphology, and inner ear sensory epithelia in hatchery-reared Chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 64(11):1469–1478. https://doi.org/10.1139/F07-106
Pavlov DS, Savvaitova KA (2008) On the problem of ratio of anadromy and residence in Salmonids (Salmonidae). J Ichthyol 49(9):778–791
Persat H, Zakharia ME (1992) The detection of reproductive activity of the grayling Thymallus thymallus (L. 1758) by passive listening. Arch für Hydrobiol 123(4):469–477
Phillips MJ (1989) The feeding sounds of rainbow trout, Salmo gairdneri Richardson. J Fish Biol 35(4):589–592. https://doi.org/10.1111/j.1095-8649.1989.tb03008.x
Pijanowski BC, Farina A, Gage SH, Dumyahn SL, Krause BL (2011) What is soundscape ecology? An introduction and overview of an emerging new science. Landsc Ecol 26(9):1213–1232. https://doi.org/10.1007/s10980-011-9600-8
Platts WS (1979) Relationships among stream order, fish populations, and aquatic geomorphology in an Idaho river drainage. Fisheries 4(2):5–9
Popper AN, Fay RR (1973) Sound detection and processing by fish: a critical review. J Acoust Soc Am 53:1515–1529
Popper AN, Hawkins AD (2018) The importance of particle motion to fishes and invertebrates. J Acoust Soc Am 143(1):470–486
Popper AN, Hawkins AD (2019) An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J Fish Biol 94(4):692–713
Popper AN, Lu Z (2000) Structure–function relationships in fish otolith organs. Fish Res 46(1-3):15–25
Popper AN, Fay RR, Platt C, Sand O (2003) Sound detection mechanisms and capabilities of teleost fishes. In: Collin SP, Marshall NJ (eds) Sensory processing in aquatic environments. Springer, New York, pp 3–38
Putland RL, Mensinger AF (2019a) Exploring the soundscape of small freshwater lakes. Ecol Inform 55:101018. https://doi.org/10.1016/j.ecoinf.2019.101018
Putland RL, Mensinger AF (2019b) The effect of sound produced by vessel passage at a navigational lock on freshwater fishes. Proc Meet Acoust 5ENAL 37(1):070004
Radford CA, Stanley JA, Simpson SD, Jeffs AG (2011) Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30(2):295–305. https://doi.org/10.1007/s00338-010-0710-6
Riera A, Rountree RA, Pine MK, Juanes F (2018) Sounds of Arctic cod (Boreogadus saida) in captivity: A preliminary description. J Acoust Soc Am 143(5):EL317–EL321. https://doi.org/10.1121/1.5035162
Roca IT, Magnan P, Proulx R (2020) Use of acoustic refuges by freshwater fish: Theoretical framework and empirical data in a three-species trophic system. Freshw Biol 65(1):45–54. https://doi.org/10.1111/fwb.13077
Roh H-S, Sutin A, Bunin B (2008) Determination of acoustic attenuation in the Hudson River Estuary by means of ship noise observations. J Acoust Soc Am 123(6):EL139–EL143. https://doi.org/10.1121/1.2908404
Rountree RA, Able KW (2007) Spatial and temporal habitat use patterns for salt marsh nekton: implications for functions. Aquat Ecol 41(1):25–45
Rountree RA, Juanes F (2017) Potential of passive acoustic recording for monitoring invasive species: freshwater drum invasion of the Hudson River via the New York canal system. Biol Invasions 19(7):2075–2088. https://doi.org/10.1007/s10530-017-1419-z
Rountree RA, Gilmore RG, Goudey CA, Hawkins AD, Luczkovich JJ, Mann DA (2006) Listening to fish: applications of passive acoustics to fisheries science. Fisheries 31(9):433–446
Rountree RA, Juanes F, Bolgan M (2018) Air movement sound production by alewife, white sucker, and four salmonid fishes suggests the phenomenon is widespread among freshwater fishes. PLoS One 13(9):e0204247
Rountree RA, Bolgan M, Juanes F (2019) How can we understand freshwater soundscapes without fish sound descriptions? Fisheries 44(3):137–143. https://doi.org/10.1002/fsh.10190
Rountree RA, Juanes F, Bolgan M (2020) Temperate freshwater soundscapes: A cacophony of undescribed biological sounds now threatened by anthropogenic noise. PLoS One 15(3). https://doi.org/10.1371/journal.pone.0221842
Satou M, Takeuchi H, Takei K, Hasegawa T, Okumoto N, Ueda K (1987) Involvement of vibrational and visual cues in eliciting spawning behaviour in male Hime salmon (Landlocked Red Salmon, Oncorhynchus nerka). Anim Behav 35:1556–1584
Satou M, Shiraishi A, Matsushima T, Okumoto N (1991) Vibrational communication during spawning behavior in the himé salmon (landlocked red salmon, Oncorhynchus nerka). J Comp Physiol A 168(4):417–428. https://doi.org/10.1007/BF00199602
Satou M, Takeuchi HA, Takei K, Hasegawa T, Matsushima T, Okumoto N (1994) Characterization of vibrational and visual signals which elicit spawning behavior in the male himé salmon (landlocked red salmon, Oncorhynchus nerka). J Comp Physiol A 174(5):527–537. https://doi.org/10.1007/BF00217372
Seppänen J, Nieminen M (2004) Measurements and descriptions of underwater noise in Finland. Geophysica 40(1–2):23–38
Shreve RL (1966) Statistical law of stream numbers. J Geol 74(1):17–37
Sprague MW, Krahforst CS, Luczkovich JJ (2016) Noise propagation from vessel channels into nearby fish nesting sites in very shallow water. Proc Meet Acoust 4ENAL 27(1):010012
Stober J (1969) Underwater noise spectra , fish sounds and response to low frequencies of cutthroat trout (Salmo clarki) with reference to orientation and homing in Yellowstone Lake. Trans Am Fish Soc 98(4):652–663
Strahler AN (1957) Quantitative analysis of watershed geomorphology. EOS 38(6):913–920
Straight CA, Freeman BJ, Freeman MC (2014) Passive acoustic monitoring to detect spawning in large-bodied catostomids. Trans Am Fish Soc 143(3):595–605. https://doi.org/10.1080/00028487.2014.880737
Tonolla D, Acuña V, Lorang MS, Heutschi K, Tockner K (2010) A field-based investigation to examine underwater soundscapes of five common river habitats. Hydrol Process 24(22):3146–3156. https://doi.org/10.1002/hyp.7730
Tonolla D, Lorang MS, Heutschi K, Gotschalk CC, Tockner K (2011) Characterization of spatial heterogeneity in underwater soundscapes at the river segment scale. Limnol Oceanogr 56(6):2319–2333. https://doi.org/10.4319/lo.2011.56.6.2319
Vasconcelos RO, Amorim MCP, Ladich F (2007) Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. J Exp Biol 210(12):2104–2112. https://doi.org/10.1242/jeb.004317
Voellmy IK, Purser J, Simpson SD, Radford AN (2014) Increased noise levels have different impacts on the anti-predator behaviour of two sympatric fish species. PLoS One 9(7):e102946
Vračar MS, Mijić M (2011) Ambient noise in large rivers (L). J Acoust Soc Am 130(4):1787–1791. https://doi.org/10.1121/1.3628666
Willson MF (1997) Variation in salmonid life histories: patterns and perspectives. Res. Pap. PNW-RP-498. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR. 50 p
Wilson B, Batty RS, Dill LM (2004) Pacific and Atlantic herring produce burst pulse sounds. Proc R Soc B Biol Sci 271:95–97. https://doi.org/10.1098/rsbl.2003.0107
Wright KJ, Higgs DM, Leis JM (2011) Ontogenetic and interspecific variation in hearing ability in marine fish larvae. Mar Ecol Prog Ser 424:1–13. https://doi.org/10.3354/meps09004
Wysocki LE, Dittami JP, Ladich F (2006) Ship noise and cortisol secretion in European freshwater fishes. Biol Conserv 128(4):501–508
Wysocki LE, Amoser S, Ladich F (2007) Diversity in ambient noise in European freshwater habitats: Noise levels, spectral profiles, and impact on fishes. J Acoust Soc Am 121(5):2559–2566. https://doi.org/10.1121/1.2713661
Zaimes GN, Emanuel R (2014) Stream processes
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply
About this chapter
Cite this chapter
Murchy, K.A., Rountree, R.A., Juanes, F. (2024). The Role of the Soundscape in the Behavioral Ecology of Stream-Dwelling Salmonids. In: Lobon-Cervia, J., Budy, P., Gresswell, R. (eds) Advances in the Ecology of Stream-Dwelling Salmonids. Fish & Fisheries Series, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-031-44389-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-44389-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44388-6
Online ISBN: 978-3-031-44389-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)