Abstract
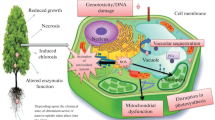
Abiotic stresses, which include high salt accumulation, drought, high temperature, heavy metal stress, light, lack of nutrients, radiation, and many others, pose a constant threat to plants living in an environment that is in a state of constant change. The productivity, as well as the quality of the crops, may be significantly reduced as a result of such stresses. It has been established that Cr is a human carcinogen that can enter the body of a person either through inhalation or the consumption of food products that are contaminated with Cr. Due to the hazardous consequences of the deposition of chromium in the environment, as well as the hazards that the metal may produce, both the Agency for Toxic Substances and Disease Registry and the United States Environmental Protection Agency categorize chromium as a major contaminant. As Cr is found in nature in several valence states, such as Cr3+ and Cr6+, it is possible to find it in several different valence states. Chromium (Cr) is a heavy metal that is known to produce reactive oxygen species (ROS), which are especially harmful to vegetation and need to be controlled to safeguard species against osmotic damage caused by high concentrations of Cr. One of the most dangerous and enduring types of Cr in the soil is Cr6+. Reactive oxygen species (ROS), which are produced as a result of Chromium, as well as some cellular and metabolic processes can be disrupted. Researchers who study plant genetics and transcriptional control have discovered that when plants are under Cr stress, various genes involved in detoxification are up-regulated, which confers tolerance on the plants. The higher production of reactive oxygen species (ROS) is an important indicator of the presence of such stresses at the molecular level. ROS are highly reactive in their natural state because they can interact with many different molecules and metabolites found within cells, which can ultimately result in irreversible metabolic dysfunction and death. As ROS were produced and scavenged in various structures of plant cells, the ROS-scavenging routes that arise from the various components of plant cells can also be integrated with the ROS-producing routes that are found in plant cells. New research on plants has demonstrated that extremely small concentrations of ROS may serve as chemical messengers and raise a plant’s sensitivity to abiotic and biotic stresses by regulating the activities of protective genes. Several studies have also demonstrated that plants with higher antioxidant levels, whether these antioxidants are inherent or induced, are more resilient to a range of environmental challenges. This phenomenon has been observed in both wild and cultivated plants. We aim to synthesise current findings on the role of ROS in abiotic stress tolerance in this chapter as well as the possible regulatory roles that ROS may play. In addition, We go over the improvements that have been made in the last several decades in terms of enhancing plants’ ability to withstand oxidative stress through the application of genetic engineering by various ROS detoxifying systems in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Abiotic stressors continue to be one of the most significant issues that limit agricultural yields in the world today. According to Rodriguez et al. (2005), Acquaah (2007), researchers have estimated that abiotic stresses directly account for more than fifty per cent of the decrease in yield. Several morphological, physiological, biochemical, and molecular changes in plants have been linked to abiotic stress, according to Wang et al. (2001), all of which negatively affect the growth and productivity of plants. However, it is worth noting that the punctuality and effectiveness of these responses may prove to be deciding factors in determining whether a given species is likely to be able to survive or not. Chromium (Cr) is a heavy metal that has oxidation numbers ranging from Chromium 2+ to Chromium 6+. In the modern periodic table, it is in the list of transition elements called VI-B (Abbas et al. 2018). Chromium is a hard metal and silver in colour. The atomic weight of this metal is 51.10 g/M and it has an atomic number of 24. On the list of the highest occurring metals on earth, this metal is ranked 21 on the list of its atomic number, density, and molecular weight (Acquaah 2007). It is estimated that the molecular weight of chrome is 51.10 g per million. There are two most stable forms of chromium found in nature, and they are Cr3+ and Cr6+ (Adejumo 2019). As the most toxic form of chromium, hexavalent chromium is considered to be the most toxic form because it is more water-soluble, mobile, as well as bioavailable than the other forms of chromium (Afonso et al. 2019). It is also a potentially powerful oxidizing agent. The oxygenated environment is capable of converting Cr3+ into Cr6+, and The elements involved in preserving the ideal ratio of various chromium forms include oxygen content, pH, complexing agents, and reducing agents (Agrawal et al. 2009). The mining of chromium has dramatically increased in recent years as a direct result of the material's growing demand across a variety of industrial sectors (Ahmed et al. 2010). The countries of Kazakhstan, South Africa, China, and India are the top four users of chromium anywhere in the world (Al Mahmud et al. 2017; Ali and Alqurainy 2006). The enterprises of tanning leather, metalworking, metal plating, copper alloys, ceramic glazes, timber protection, moisture corrosive environment suppression, heat-resistant masonry, pneumatically wood products, textile materials, as well as dyestuff, powders and acrylics, and paper and pulp making, are to blame for the excessive amount of chromium in the environment. Also, the high levels of Chromium in the environment are caused by things that people do, like dumping liquid and solid wastes that are contaminated with Chromium (Apel and Hirt 2004; Asada 1994; Asada and Takahashi 1987; Ashraf et al. 2017; Augustynowicz et al. 2020). There is a belief that the emissions of Cr from cooling towers of industries are an important source of Chromium (Augustynowicz et al. 2014; Balasaraswathi et al. 2017; Barbosa et al. 2007). Debris or Impurities rising from road banks also seems to be a significant source of Chromium. In agricultural land, the accumulation of more Chromium can adversely affect the growth and development of a plant on multiple levels, including at the organ, cellular, and even genetic levels, depending on the amount accumulated in the soil (Bhargava and Mishra 2018). A higher concentration of chromium in plants can cause a lot of damage to plants because of the induced reactive oxygen species (ROS), which are responsible for both cellular and extracellular damage caused by higher levels of chromium in plants (Blokhnia et al 2003). The ROS in plants have a distinct function: they serve as chemical messengers that trigger the activity of detection systems in response to stressors, which they do if they occur in large amounts and increase cellular injury, in which case they trigger the activity of the detection systems. This in turn can lead to the production of protective molecules that help the plant defend itself against damage and continue healthy growth. There is a direct relationship between the concentration of ROS in the plant's environment and these two functions. This, in turn, may enable the production of specific chemicals that are capable of providing the plant with extra protection against any potential harm. This will enable it to remain healthy and thrive. The greater the amount of ROS present in the air, the stronger the beneficial effects. The formation of reactive oxygen species (ROS) is a by-product of abiotic stress that can endanger tissues if they are allowed to persist for a long period due to the increased formation of abiotic stressors. Thus, by increasing the amount of ROS present in the air, the plant can better protect itself against the harm caused by abiotic stress, and ultimately, lead a healthier and more successful life. As a result, lipids and proteins may peroxide, nucleic acids will be damaged, enzymes will be inhibited, the programmed cell death pathway (PCD) will be activated, and then the cells will eventually die (Mittler 2002; Sharma and Dubey 2005, 2007). Oxidative stress is mostly a controlled process, and the outcome for the plant depends on the equilibrium between reactive oxygen species and antioxidative capacity. Even before abiotic stresses are allowed to continue for just a longer duration, the increased production of reactive oxygen species puts cells at risk (ROS) (Apel and Hirt 2004). Based on their mode of action, these defences can be categorised into either enzymatic antioxidants or non-enzymatic antioxidants to simplify their classification. According to Asada and Takahashi (1987), this antioxidant defence system offers proper protection against active oxygen and free radicals when it is operating under controlled conditions. On the other hand, when an organism is subjected to a stressful situation, the balance between the synthesis and scavenging of relative oxygen species may be disrupted, resulting in a response that is either optimum or less (Gill and Tuteja 2010). The enhanced antioxidant defence has been shown in multiple studies to be effective in the fight against oxidative stress brought on by different abiotic stressors. As the impact of increasingly harsh environmental conditions on crop production, breeders and researchers are facing a pressing issue of creating genes capable of enduring biological variations with the least amount of harm. Therefore, to understand the processes of plant regulation and defence, the first step is to acquire knowledge that will allow you to better understand them. Developing plants that have a higher potential for antioxidation presents an opportunity to develop plants that have a higher tolerance for abiotic stresses. The purpose of this section is to present our current understanding of how plants react to abiotic stressors, both from a physiological and molecular genetic perspective. There has been a particular focus on the physicochemical and non-enzymatic modulation of antioxidant defences under abiotic stress as well as the link between these mechanisms and abiotic stress tolerance.
5.2 Plants Produce ROS as a Result of Their Metabolism
Apel and Hirt (2004) found that reactive oxygen species (ROS) are continuously produced as a result of metabolic activity throughout all the compartments within the plant cells, notably chlorophyll, ATP, and mitochondria, as a result of metabolic activities throughout these compartments. Chloroplasts are the primary organelles in plants that produce reactive oxygen species (ROS). A chlorophyll triplet state may develop when there is an insufficient amount of energy absorbed during photosynthesis. By shifting its energy from this state to oxygen, it can produce a molecule of oxygen by transferring its excitation energy to it (Logan 2005). The electron transport chain (ETC) in photosynthesis makes \({\text{O}}_{{2}}^{ \cdot - }\) by reducing oxygen (Apel and Hirt 2004). Superoxide dismutase (SOD) then changes \({\text{O}}_{{2}}^{ cdot - }\)–H2O2 (Foyer and Noctor 2000). Reactive oxygen species in visible light are influenced by many physiological and environmental factors, such as a lack of water and access to bright light, which are important in the formation of reactive oxygen species. It appears that ribulose-1,5-bisphosphate carboxylase or oxygenase (RuBisCO) shows an increase in oxygenase activity when conditions prevent chloroplast CO2 fixation and that the generated glycolate is transported from chloroplasts to peroxisomes under these conditions (Takahashi and Murata 2008). According to Halliwell (2006), the production of hydrogen peroxide in peroxisomes requires glycolate oxidation, which is broken down by glycolate oxidase (GO) and the breakdown of lipids. In contrast, a tiny electron transport chain (ETC) at the level of the peroxisomal membrane and a reaction of xanthine oxidase (XO) within the organelle matrix are both necessary for the generation of O2 at the level of the peroxisomal membrane. It is important to note that both of these processes take place inside the organelle. It is using the ETC located in the cytoplasm of the plants that reactive oxygen species (ROS) are produced in plant tissues. A multi-complex dehydrogenase complex for the reduction of ubiquinone (Q) within the cell is composed of many small dehydrogenase units that work together. Complex I (NADH dehydrogenase) and the Q zone are likely to be responsible for the majority of ROS production in cells (Mller 2001; Blokhina et al. 2003). Although mitochondrial ROS production is significantly lower than that of chloroplasts, mitochondrial ROS regulate a variety of cellular processes, such as stress adaptation and programmed cell death (PCD), despite the fact that mitochondrial ROS are important regulators of the processes described above (Robson Vanlerberghe 2002). The primary enzyme in charge of producing hydrogen peroxide in glyoxysomes is aryl-CoA oxidase. Plasma membrane-bound NADPH oxidases (NADPHox) and peroxidases connected to cell walls serve as the primary suppliers of reactive oxygen and H2O2 generated by apoplastic enzymes (POX). It has been shown that these enzymes become active when they are exposed to different types of stress (Mittler 2002). The catalysis of some reactions which are used in detoxification is done by cytochromes in both the cytoplasm as well as in ER of plant cells are additional sources of reactive oxygen species (ROS) in plant cells (Urban et al. 1989).
5.3 The Effects of Chromium on Oxidative Stress in Plants
There is sufficient evidence to suggest that oxidative injury can be caused to plants when they are subjected to redox-active HM toxicity. Following HM absorption by carriers and movement to plant components, relative oxygen species production occurs. This is triggered either by the heavy metal redox process or by how an HM affects metabolism at a particular subcellular location. After HM is taken up by transporters and distributed to organelles, ROS is produced. Activation of plasma in a manner dependent on HM-membrane-localized NADPH oxidase is another enzyme that plays a role in the production of ROSHMs that is redox-active and facilitate redox processes in the cell include iron, copper, chromium, vanadium, and cobalt, as distinct from HMs that are physiologically non-redox-active, such as Zn2+ and Cd2+.They contribute to the generation of hydroxyl radicals from superoxide anion (H2O2) via the Haber-Weiss and Fenton reactions, which initiate the process of non-specific lipid oxidation. There is also one element that contributes to the specific increase of lipid peroxidation, and that is the activation of lipoxygenases (LOX) that are dependent on HM (Montillet et al. 2004). The formation of reactive oxygen species (ROS) by plants is a form of self-defence when they are exposed to hostile conditions (Byrne et al. 2017; Chakraborty and Pradhan 2011). There are several kinds of endogenous stress, but the most common is the accumulation of reactive oxygen species (ROS), which can lead to a reduction in plant growth and development (Chalapathi Rao and Reddy 2008). The plants produce a variety of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide anion (O2), singlet oxygen (\(^{{1}} {\text{O}}_{{2}}\)), and hydroxyl ion (HO−), peroxyl (RO−), alkoxyl (RO−), as well as organic hydroperoxide (ROOH) (Chandra 2004; Chen et al. 2003, 2017). Hydrogen peroxide is one of the most prevalent types of ROS in the environment. According to Conklin (1996), Cui (2017), De Tullio (2004), reactive oxygen species (ROS) are formed as a consequence of a range of metabolic processes occurring in mitochondria, peroxisomes, and chloroplasts. As mentioned above, ROS levels in plants are regulated by several mechanisms, including ROS production, enzymatic scavenging of ROS, and/or non-enzymatic scavenging of ROS (del Ro et al. 2006). As a result of exposure to the following metals, lead (Pb), aluminium (Al), nickel (Ni), cadmium (Cd), and chromium (Cr), there is a significant correlation between the generation and accumulation of reactive oxygen species (ROS) in the body (Dixon 2010; Elsawy et al. 2017; Eltayeb et al. 2006). The induced ROS were accumulated by a wide range of species of plants after they were exposed to either a harmful quantity of Cr or industrial wastes containing a toxic level of Cr. There is considerable evidence that the exposure of plants to chromium results in the formation of reactive oxygen species (ROS), which have a variety of physiological, metabolic, molecular, and morphological effects (Eltayeb et al. 2007). There is a possibility that Cr may interact directly with proteins, lipids, enzymes, and genetic material (DNA and/or RNA) to change physiological and biochemical processes, or it may trigger the accumulation of reactive oxygen species (ROS) within the organism (Fargasova 2012; Florea 2017; Foyer and Noctor 2000). In addition to damaging the membrane, Cr also destroys and inactivates genetic material, proteins, and enzymes, resulting in growth suppression by inhibiting cell division or triggering programmed cell death as a result of interactions with Cr (Fryer 1992; Gapper and Dolan 2006; Ghosh et al. 2017; Gielen et al. 2017). As a result of chromium-induced ROS, morphological changes are induced in a variety of plant tissues in an amount and tissue-specific manner. These morphological changes are irreversible and damage biomolecules of the plant except for DNA, cysteine, and methionine, which can be reconstituted (Hasanuzzaman et al. 2017). As a consequence, chromium-induced ROS are responsible for the destruction of biomolecules. Cr(VI) is reduced to Cr(III) by reactive oxygen species that are formed during the reduction process. The same happens with the Fenton reaction. The Fenton reaction is a catalytic reaction where Cr(III) has a higher catalytic efficiency than iron (Fe), copper (Cu), cobalt (Co), manganese (Mn), and zinc (Zn) (Kalve et al. 2011). There has been little investigation into the role of Cr in such reactions, and it has also been suggested that various other intermediates and variables may also be involved in the production of ROS as a result of Cr (Mittova et al. 2003; Mobin and Khan 2007). Several physiological, biochemical, molecular, and ultrastructural changes were caused by ROS, which acted as a mediator.
5.4 Non-enzymatic Antioxidants
5.4.1 Ascorbate
Ascorbate (AsA) is a vital antioxidant that is found in plant tissues. In higher plants, it is produced in the cytosol, primarily by the conversion of d-glucose to ascorbate, which is a major source of ascorbic acid. As a result of its ability to react with a wide range of reactive oxygen species (ROS), which include H2O2, O2·, and \(_{{1}} {\text{O}}^{{2}}\), it can exert its antioxidant activity. AsA, an electron donor with terminal properties, plays an essential role here by scavenging free radicals from the hydrophilic environment in which plants live. AsA is also an essential molecule in the antioxidant defence mechanism of plants, as it plays a vital role in reacting with ROS to preserve cellular integrity and prevent oxidative stress. In addition to this, it is capable of scavenging OH· at rates controlled by diffusion (Smirnoff 2000). APX uses the AsA-GSH cycle to produce MDHA by converting two molecules of AsA into the water through the reduction of H2O2, which is also the result of APX using two molecules of AsA to make H2O2. MDHA is a radical with a short half-life that degrades disproportionately into DHA and AsA as a result of its short half-life. As stated by Gapper and Dolan (2006), MDHAR or ferredoxin is responsible for catalyzing the reactions that occur within a chloroplast water-water cycle through the action of ferredoxin. It is common practice to use NADPH as an electron donor for a variety of purposes. In plant cells, AsA is the major reducing substrate that is used for the removal of H2O2 (Wu et al. 2007). In addition to the reduction of a-tocopherol, an antioxidant found in chloroplasts, AsA is also assumed to be necessary. According to Conklin et al. (1996) research on AsA in plants, this enzyme might play a role in the formation of the pigment zeaxanthin in plants, which protects against oxidative damage through the elimination of excess light energy from the thylakoid membranes. In addition, according to De Tullio (2004), ASA is also responsible for maintaining the reduced state of prosthetic metal ions, which, in turn, is responsible for the functioning of several antioxidant enzymes. Based on research conducted by Hasanuzzaman et al. (2011a, b), AsA plays a critical role in plant tolerance to abiotic stress. Exogenous administration of AsA reduces the damage caused by oxidative processes by affecting the activity of a large number of enzymes. In addition, it works synergistically with other antioxidants (Shalata and Neumann 2001). It has been shown that glutathione (GSH) is an antioxidant that acts as an antioxidant and is directly involved in the process of lowering the majority of ROS, according to Noctor and Foyer. Moreover, GSH plays a critical role in Foyer and Halliwell’s antioxidative defence system, as it plays a key role in the regeneration of other potential water-soluble antioxidants, such as AsA, through the AsA-GSH cycle, which promotes the regeneration of other water-soluble antioxidants. In doing so, it preserves a-tocopherol and zeaxanthin in a diminished state, which is how it indirectly protects membranes by preserving those two compounds in a diminished state. During times of stress, GSH acts to protect proteins from denaturation. This would otherwise result from the oxidation of the thiol groups in these proteins as a result of oxidative stress. GST and GPX both use GSH as a substrate for their enzymes, with both of them contributing to the elimination of ROS in the body (Noctor et al. 2002). Phytochelatins (PCs) are also produced by GSH, which has an affinity for HM and is transported as complexes into the vacuole, enabling plants to be somewhat resistant to HM if they accumulate enough phytochelatins. As a reduced sulfate, GSH is also a catalyst for the breakdown of xenobiotics and acts as a medium for the storage and transportation of this element (Srivalli and Khanna-Chopra 2008). A stress marker can be derived from the fact that glutathione plays a very significant role in the antioxidant defences of the body. The ratio of H2O2 reduced (GSH) to oxidized (GSSG) forms changes over the course of the breakdown of the gas. Several redox signalling pathways rely on this modification for their function (Li and Jin 2007). As a result of increased GSH levels, plants are protected from the damaging effects of oxidative stress. GSH functions as a redox sensor for environmental cues. Research has shown that by increasing the levels of GSH in the body, we are better able to cope with a range of abiotic stresses (Hasanuzzaman et al. 2011a).
5.4.2 Tocopherol
It has long been known that tocopherols are abundant in thylakoid membranes, which are also rich in polyunsaturated fatty acids (PUFAs) and are located close to ROS that is produced during photosynthesis (Fryer 1992). Consequently, these compounds are thought to play an important role in protecting thylakoid membranes from oxidative damage. Munne-Bosch and Alegre have found that tocopherol appears to have an important antioxidant role, based on circumstantial and correlative evidence. Additionally, the scientist suggests that tocopherol could be a critical component of the photoprotective system, underscoring its potential antioxidant potential. As a result of the conversion of lipid peroxyl radicals (LOO) to their corresponding hydroperoxides, tocopherols prevent lipid peroxidation from occurring. This action of tocopherols prevents the oxidation of lipids, thus protecting cellular membranes from damage. Tocopherols play an important role in reducing reactive oxygen species (mostly \(_{{1}} {\text{O}}^{{2}}\) and OH·) in the membranes of photosynthetic organisms in this way. By preventing lipid peroxidation, tocopherols protect the delicate cellular membranes from oxidative damage caused by reactive oxygen species, thus preserving photosynthetic organisms. Tocopherols are capable of physically quenching oxygen in chloroplasts. Tocopherols can donate electrons to the reactive oxygen species, neutralizing them and preventing them from damaging the cell membrane. This process is known as “quenching” and it is thought to be the primary mechanism by which tocopherols mitigate oxidative damage. According to Munné-Bosch, one molecule of a-tocopherol can deactivate as many as 120 molecules of oxygen in a single reaction. The fact that tocopherols are part of a complex signalling network that is regulated by reactive oxygen species (ROS), antioxidants, and plant hormones also make them an excellent pick for affecting cellular signalling in plants in a positive manner. This means that the presence of tocopherols can help reduce the damaging effects of ROS, which can interfere with the proper functioning of cells. Additionally, tocopherols can act as messengers that help regulate the expression of genes and other plant hormones, which can be beneficial for plant growth and development.
5.4.3 Components of an Enzyme
It should be noted that the enzymes involved in removing ROS are located in a variety of places within plant cells, and they work in concert together. There are several enzymes that are involved in the AsA-GSH cycle, as well as SOD, CAT, GPX, and GST, which are considered to be the most important antioxidant enzymes. These enzymes are responsible for removing ROS from the cell and ultimately preventing oxidative damage to the plant. They do this by using the molecules of ascorbate and glutathione, which are found in abundance in the cells, as well as other molecules like superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase. Together, these enzymes work to neutralize the ROS and prevent it from causing damage. Aside from AsA, GSH, and NADPH, four other enzymes play a significant role in the AsA-GSH cycle, which are known as APX, MDHAR, DHAR, and GR. The enzymes in this cycle, together with the other components of the cycle, play an important role in deactivating H2O2 and regenerating AsA and GSH through a series of cyclic processes. APX, MDHAR, DHAR and GR catalyze the redox reactions of the AsA-GSH cycle, and also reduce the amount of oxidizing agents, such as H2O2, in the cell. These enzymes also help to maintain the AsA and GSH levels in the cell, so they can be used to neutralize the harmful effects of free radicals and other toxic compounds.
5.4.4 Superoxide Dismutases (SOD)
In terms of protecting plant cells from reactive oxygen species (ROS), SODs are considered to be the first line of defence. To accomplish this, it catalyzes the dismutation of O2·. This results in the conversion of one molecule of O2· into H2O2 and the oxidation of another molecule of O2· into O2. In this way, the presence of O2· is removed from the system. It has been found that metal ions such as manganese (MnSOD), copper and zinc (Cu/ZnSOD), and iron are incorporated into SOD active sites and they are used as a classification of SODs (FeSOD). Although MnSOD can be found in the matrix of mitochondria and peroxisomes, Cu/ZnSOD can be observed in the cytosol and chloroplasts of higher plants, and FeSOD can be found in the chloroplasts of some higher plants, despite its location in mitochondria and peroxisomes (Scandalios 1993). Singh et al. (2008) have found that an increase in the activity of SODs can contribute to the mitigation of the effects of abiotic oxidative stress. MnSOD, Cu/ZnSOD, and FeSOD are all types of superoxide dismutase (SOD) enzymes that have different locations in the cell, but all have the same purpose of mitigating the effects of oxidative stress caused by abiotic stressors. Therefore, an increase in SOD activity can help protect the cell from these kinds of stressors.
5.4.5 Catalases (CAT)
Catalases are heme-containing tetrameric enzymes that are involved in the conversion of hydrogen peroxide to water and oxygen. Catalases are essential for many metabolic reactions, as they are extremely efficient in breaking down hydrogen peroxide, which can be harmful to cells. The catalase enzymes are responsible for protecting cells from oxidative damage by using hydrogen peroxide as a substrate and converting it into water and oxygen as a result, Sanchez-Casas and Klesseg (1994). Furthermore, catalase enzymes are widely distributed in organisms and are important in biochemical reactions such as photosynthesis and respiration, where they act as protective agents against oxidative damage. Catalases are found in peroxisomes, glyoxysomes, and other organelles that are connected to peroxisomes, as well as enzymes that produce H2O2 (Agarwal et al. 2009). According to Gill and Tuteja (2010), CAT plays a critical role in the removal of H2O2, which is generated in the peroxisome by oxidases that are involved in photorespiration, b-oxidation of fatty acids, and purine catabolism within the peroxisome. The reaction between CAT and various hydroperoxides has been demonstrated in addition to the reaction between CAT and molecular oxygen by Ali and Alqurainy (2006). It has been found that different patterns of response to different abiotic stressors and the level of CAT activity have been observed by Fujita.
5.4.6 AsA-GSH Cycle Enzymes
In mitochondria, chloroplasts, apoplasts, cytosols, and peroxisomes, the AsA-GSH cycle serves as the first line of defence against reactive oxygen species (ROS) present in mitochondria, chloroplasts, apoplasts, and cytosols. There are four enzymes involved in the AsA-GSH cycle, namely APX, MDHAR, DHAR, and GR, that are also implicated in the AsA-GSH cycle, along with AsA, GSH, and NADPH.
In the process of deactivating Molecular oxygen, these enzymes and the other components of the cycle work together to regenerate AsA and GSH and to regenerate Molecular oxygen through a series of cyclic processes. In this cycle, APX is responsible for catalyzing the reduction of H2O2–H2O, as well as generating monodehydroascorbate (MDHA), which is a precursor to vitamin C. It is then converted into ascorbic acid (AsA) through the action of NADPH-dependent MDHAR, or it can be converted nonenzymatically to ascorbic acid and dehydroascorbic acid (DHA) by nonenzymatic means. As such, the conversion of DHAA to AsA is of significant importance in the biosynthesis of ascorbic acid. Either DHA undergoes a hydrolysis process that cannot be reversed or it undergoes an oxidation process. With the help of DHAR, which utilizes GSH as a reductant, the 2,3-diketogulonic acid can either be converted back to AsA or 2,3-diketogulonic acid. By converting GSSG to GSH, Chen et al. (2003) demonstrate that GSSG will be produced, and GR will then take care of converting it back into GSH. Ascorbate peroxidase (APX) plays a major role in the removal of hydrogen peroxide from the ascorbate-glutathione (GSH) cycle in the first phase. Asada (1994) states that in higher plants this process may be the most critical stage in the process of removing reactive oxygen species and protecting cells from oxidative stress (Asada 1994). Heme-containing enzymes such as APXs play a very important role in the elimination of oxygen molecule H2 during the hydrological cycle as well as during the AsA-GSH cycle in the body. Several enzymes use AsA as a substrate and help in the transfer of electrons from AsA to H2O2 through these enzymes. There are five isoforms of the protein in the APX family, which results in different amounts of DHA and water. The chloroplast stroma soluble form (sAPX), mitochondrial form (mAPX), glyoxisome membrane form (gmAPX), and thylakoid (TapX).form are the four types. In addition to the cytosolic form of APX (CapX), there is also a cytosolic form of APX. In response to a wide range of diverse abiotic stress situations, plants show enhanced APX activity in response to a wide range of abiotic stress responses Hasanuzzaman and Fujita (2011).
5.4.7 Monodehydroascorbate Reductase (MDHAR) and Dehydroascorbate Reductase (DHAR)
AsA is engaged in a univalent oxidation process that leads to the formation of MDHA when it oxidizes univalent. This oxidation of AsA into MDHA is an essential step in the process of cellular energy production. It is imperative to keep in mind that if MDHA is not converted back to AsA by MDHAR, then MDHA will degrade on its own. Additionally, without MDHAR, the concentration of MDHA would increase, leading to inefficient use of energy and potential toxic effects. This will result in AsA and DHA if the process is not interrupted. AsA is then transformed into DHA by DHAR in a subsequent process, which also calls for GSH, in which DHA is regenerated into AsA by DHAR. Therefore, it is essential to ensure that MDHA is converted back to AsA through MDHAR and that the GSH-dependent regeneration of DHA from AsA is also maintained. To maintain the antioxidative capacity of AsA, rapid regeneration is essential. The regeneration of AsA during this cycle is mainly controlled by the activity of MDHAR that is NADPH-dependent. To regenerate AsA and maintain a low level of AsA in the body, this is crucial. An extensive range of crops was tested in this trial, including a variety of different varieties. A study published by Hossain et al. (2011) showed that MDAHR plays a key role in the regulation of oxidative stress tolerance as well as acclimation to environmental conditions. Despite this, there are few reports of Monodehydroascorbate reductase activity in other oxidative stress-related physiological processes. During oxidative stress, MDHAR and DHAR both play a crucial role in the regulation of the quantity of ascorbic acid (AsA) and the redox state of this substance (Eltayeb et al. 2007). This suggests that MDHAR, along with other enzymes in the antioxidant network, may be involved in the process of acclimation to environmental conditions, and it could be an important factor in the regulation of the ascorbic acid content of cells. It is also likely that MDHAR could play a role in the control of other physiological processes that are associated with oxidative stress. This indicates that MDHAR and DHAR are important in maintaining the AsA concentration, redox state and stress tolerance of an organism. Furthermore, this suggests that MDHAR influences the overall stress tolerance of organisms, which is important for the survival of organisms in their environment. Besides the dehydroascorbate reductase, which maintains the cellular redox state of AsA, the recycling system of AsA is also highly dependent on dehydroascorbate reductase, which regenerates AsA from its oxidized state (DHA) Martinez and Araya (2010). Thus, the ability to be able to withstand various abiotic stressors resulting in the generation of ROS is critical for the ability to survive there. In a study carried out by Hasanuzzaman et al. (2011a, b), it was found that different ROS-inducing stimuli had a positive effect on the level of DHAR activity.
5.4.8 Glutathione Reductase (GR)
There is a possibility that glutathione reductase (GR) may play an important role in the ascorbic acid-glutathione (GSH) cycle. Furthermore, it is also an important part of the body's defence mechanism against the damage caused by oxygen radicals and other reactive chemicals (ROS). In addition to increasing the cell's tolerance to stress, an increase in GR activity can have a significant effect on the oxidation and reduction states of the essential electron transport chain components. It is necessary for the preservation of the GSH pool because GR catalyzes the reduction of the disulfide links in GSSG in an NADPH-dependent manner (Chalapathi Rao and Reddy 2008). The enzyme acts as a reductant for GSH, a compound that plays an important role in a wide range of metabolic functions as well as antioxidative properties in plants. Thus, GR is the gene that controls a high ratio of GSH to GSSG in plant cells, which is not only required but also necessary for the pathway that removes hydrogen peroxide to accelerate, especially under a stressful situation (Pang and Wang 2010). So, GR ensures that plant cells always have a high ratio of GSH to GSSG. GR is an incredibly crucial aspect of plant development that determines how effectively plants will be able to cope with a variety of stresses because it ensures that the cell's antioxidant machinery is functioning properly and, as a result, provides resistance to stress (Hasanuzzaman et al. 2011a).
5.4.9 Glutathione Peroxidases
In this study, glutathione peroxidases (GPXs) will be investigated as they are enzymes that are known to protect plant cells from the detrimental effects of oxidative stress by decreasing the levels of hydrogen peroxide (H2O2) as well as organic and lipid peroxides (LOOHs). Additionally, GPXs have been found to reduce the potential for DNA damage, which can cause plant cells to be more susceptible to stress. The GSH family of enzymes is composed of a large number of isozymes. According to Kühn and Borchert, GPX is not only an integral part of the cellular metabolism that may be involved in the re-oxidation of membrane lipids, but it is also a repurposing defence against oxidative damage to the membrane. GPXs are found to reduce the potential for DNA damage by scavenging reactive oxygen species (ROS) and preventing the oxidation of membrane lipids. This helps protect the cell's membrane from oxidative damage and increases the cell’s ability to withstand stress. It was reported a few years ago that several GPX genes had been extracted from various plant species and that these genes were associated with H2O2 detoxification. Furthermore, GPX also functions as an oxidative signal transducer (Miao et al. 2006). Therefore, GPX plays a crucial role in both protecting the cell from oxidative damage and in promoting cellular stress tolerance.
5.4.10 Glutathione S-Transferases (GST)
As an enzyme that belongs to a domain that has been identified as being involved in catalyzing the conversion of electrophilic xenobiotic substrates into GSH, plant GSTs are versatile enzymes Dixon et al. (2010). Plant GSTs can recognize and modify a wide range of electrophilic xenobiotics, such as herbicides, pesticides and industrial pollutants, by catalyzing the transfer of the electrophilic group from the xenobiotic to the glutathione (GSH) molecule, which is a key component in the plant's defence mechanisms. As reported by Marrs (1996), GST isoenzymes account for about 1% of the total soluble protein of a plant. In the GSH metabolic pathway, these enzymes play an important role. GSTs help protect the plant from environmental damage caused by these xenobiotics by binding to them and detoxifying them. They also act as scavengers of reactive oxygen species, which are generated as a result of oxidative stress caused by these pollutants. This helps the plant to maintain its health by removing these potentially damaging molecules from its environment. According to Edwards et al. GSTs are proteins that catalyze the binding of numerous xenobiotics and their electrophilic metabolites to GSH. Among these xenobiotics are a wide range of pesticides, resulting in the formation of conjugates that are less hazardous and more readily soluble in water due to the reduction in hazardous properties. As a result, GSTs are a crucial defence mechanism for plants against pollutants, as they help reduce the toxicity of xenobiotics and facilitate their excretion from the plant’s cells. Furthermore, GSTs also help protect the plant’s cells from oxidative damage caused by reactive oxygen species. In a study by Gullner and Komives it was found that GST isoenzymes contain POX activity as well as their ability to catalyze the conjugation of electrophilic molecules to GSH. Abiotic stressors act in a variety of ways as effective inducers of GST activity in plants as a result of diverse abiotic factors. This is because GSTs can act as a scavenger for reactive oxygen species (ROS) like superoxide radicals and hydrogen peroxide, which are generated in large amounts in response to abiotic stress. GSTs can also conjugate these ROS to glutathione, a small peptide, thereby helping to reduce the ROS levels and protect the plant against oxidative damage. Hossain et al. (2011) found that plant GSTs play an important role in how plants adapt to different types of abiotic stress and provide plants with the ability to survive under stress (Tables 5.1 and 5.2).
5.5 Heavy Metal Stress Exposes Plants to a Range of Antioxidant Defence Mechanisms
To detoxify ROS, plants use both non-enzymatic antioxidants (AsA, GSH, a-tocopherol, and carotenoids) and enzymatic antioxidants (APX, SOD, CAT, GR, DHAR, MDHAR, GPX, and GST). It is this ROS-detoxifying antioxidant defence machinery in the plant that protects it from reactive oxygen species damage as well as repairing any damage it may sustain. There is a strong response from plant antioxidative mechanisms to HM exposure, but the direction in which the response takes place depends on the plant organ, plant species, HM utilized, and the intensity of HM stress. In an investigation conducted by Anjum et al. (2011), it was observed that both tolerant and sensitive cultivars of mung bean were found to have a significant decrease in AsA, the AsA/DHA ratio, GSH, and the GSH/GSSG ratio when treated with Cd (100 mg/kg soil). It should be noted that both the vulnerable variety as well as the resistant variety saw declines that were substantially less severe when compared to the vulnerable variety. Cd's stress tolerance is enhanced by the presence of AsA and GSH pools, which lends credence to the idea that these pools serve as protective mechanisms. Under the influence of high levels of HM stress, glutathione participates in bioreductive processes, in which it serves as a critical line of defence against reactive oxygen species (ROS). This aberrance is responsible for the loss of protection of cells from the negative effects of oxidative stress. Inhibiting metal transport into and out of cells, as well as chelating metal ions within cells, are two further methods for reducing metal toxicity. Since GSH is capable of directly scavenging metals, GSH may play a crucial role in HM tolerance and sequestration in a variety of ways (Wójcik and Tukiendorf 2011). It has been shown that the presence of additional GSH in rice plants under controlled conditions had a significant effect on the plants’ sensitivity to the effects of cadmium stress. It was discovered that the growth inhibition induced by Cd could be significantly alleviated by applying GSH exogenously to both genotypes and that the uptake of Cd could be significantly reduced by the application of GSH exogenously. On the other hand, Wójcik and Tukiendorf (2011) have discovered that the amount of endogenous GSH present naturally in wild-type Arabidopsis plants is sufficient for them to be resistant to Cd stress. As a result, this contradicts the conclusions that were reached by previous researchers. Plants that have a smaller amount of GSH are less susceptible to Cd, whereas plants that have a higher amount of GSH are less susceptible to Cd, and even become more hazardous as a result. To protect plants from the oxidative stress caused by toxic HMs, they need to increase the production of antioxidant enzymes, such as SOD, CAT, the enzymes of the AsA-GSH cycle (APX, MDHAR, and GR), as well as GST, and GPX. The combination of these biochemical properties serves as an indicator of how sensitive or resistant different plant species are to HMs on a species-by-species basis (Anjum et al. 2011). According to Gill et al. the increased tolerance to Cd can be attributed to the increased coordination between the antioxidative enzymes in response to Cd exposure. By working together, we can protect the machinery involved in the photosynthesis process. In the presence of an HM stress, the elimination of ROS is carried out by several enzymes that are sequentially and simultaneously activated, forming the enzymatic antioxidant system Gill et al. El-Beltagi et al. found that plants exposed to Cd stress exhibited significantly greater levels of antioxidant enzymes such as CAT, GST, and POX when compared to plants subjected to a control condition. CAT activity in the plant’s leaves was measured at a specific activity of 25 ppm of Cd when the CAT activity in the plant’s leaves was measured. This value was obtained when the concentration of Cd was raised to a certain level. The activity of CAT in both the leaf and root tissues was reduced when the Cd content was increased to 50 ppm, however, compared to 25 ppm Cd, the activity of CAT was not affected. There was a significant increase in the level of GST-specific activity in both the leaves and roots of plants when cd was used as a fertilizer discovered that the highest concentration of Cadmium, 50 ppm, caused GST activity to reach 459% in the leaves and 756% in the roots of the plants when compared to the control plants. As Dominguez et al. point out, plants have an excellent antioxidant system that allows them to develop normally despite being exposed to cadmium concentrations at the highest possible level. Despite adverse conditions, it has been demonstrated that it is possible to successfully establish resistance to the harmful effects of cadmium even under the most adverse conditions. This study found that activating enzymes that participate in the GPX, CAT, APX, and SOD pathways of the AsA-GSH cycle (APX, MDHAR, DHAR, and GR) was sufficient to inhibit ROS generation and oxidative damage caused by lower Cd concentrations (10 and 100 μM), but not the highest Cd concentration. The effect of this treatment was not enough to reduce the formation of ROS caused by Cd as well as the damage caused by high levels of Cd. Despite the activation of GR, the amount of ROS and oxidative stress that was produced by Cd (1 mM) was not sufficient to diminish the accumulation of ROS and oxidative stress. Anjum et al. (2011) demonstrated that the AsA-GSH cycle metabolism was protective in two different mung bean cultivars. Cd stress was applied to Pusa 9531, a Cd-resistant strain, and PS 16, a Cd-sensitive strain. Different redox states of AsA-GSH in plants treated with Cd, an increased level of asA-GSH-regenerating enzymes such as APX, MDHAR, DHAR, and GR, as well as other antioxidant enzymes such as SOD all strongly suggested overutilization of AsA-GSH in plants treated with Cd. There was a significant increase in lipid peroxidation and H2O2 content that was accompanied by a subsequent decrease in reduced ascorbic acid and glutathione pools, suggesting that, as a result of Cd toxicity, oxidative stress could be partially mitigated by a detoxification mechanism based on ascorbic acid and glutathione. Under stress, APX is a crucial component in the removal of H2O2 from the atmosphere, according to Gill et al. The amount of Cd used has a direct influence on the level of activity that APX produces. The same was true for both of the genotypes studied in this study. Hossain et al. found that Cd stress resulted in a significant increase in the amount of GSH and GSSG in the blood as well as a notable decline in the amount of AsA, as well as a sharp increase in the amount of H2O2 and MDA in the bloodstream. The activities of CAT, MDHAR, DHAR, and GR all significantly decreased in response to Cd stress at a concentration of 1 mM CdCl2 for 24 h. GPX GR, APX, DHAR, GPX, GR, GST, and CAT all increased in response to Cd stress at a concentration of 1 mM CdCl2. Exogenous application of betaine or proline increased GSH and AsA levels as well as the maintenance of a high GSH/GSSG ratio. According to Kachout et al. (2009), there is evidence that antioxidant machinery protects against oxidative stress caused by Cd. The antioxidant enzyme activities of Atriplex plants grown in soils contaminated with heavy metals (Cu, Ni, Pb, and Zn) were changed in plants grown in soils contaminated with heavy metals (Cu, Ni, Pb, and Zn). To get rid of the oxidative stress caused by too much copper, it may be very important for safflower plants to improve the activity of CAT, POX, and SOD enzymes. Several antioxidative mechanisms could be in place in rice seedlings to protect them from the oxidative damage caused by Pb, SOD, POX, and GR, which can play an important role in the protection of seedlings from Pb-induced oxidative damage, according to Verma and Dubey (2003). According to the researchers, the presence of antioxidative activity appears to play a very significant role in how Atriplex plants react to the HM stress that they are exposed to when they are subject to it. In the roots of rice seedlings that had been exposed to 1 mM Pb for 15 days, APX, GPX, and SOD levels were all increased in the roots of the plants. GR activities of the seedlings increased by approximately 128–196% when compared to those of the control seedlings. As a result of the treatment, however, CAT activities decreased as a result of a decrease in CAT activity. The amount of AsA that was present decreased in a dose-dependent manner under Pb stress, while the amount of DHA that was presently increased in a dose-dependent manner under Pb stress. Plants that had been treated with lead had remarkably higher levels of APX, CAT, SOD, GSSG as well as total glutathione than plants that had not been treated with lead (Qureshi et al. 2007). As a result of the addition of 500 mM Pb-acetate to the solution, SOD and APX activities increased dose-dependently, but CAT activities decreased (500 mM Pb-acetate solution). Singh et al. found that when exposed to As, the levels of ASA and GSH detected in the leaves of P. vittata were much higher than those found in P. ensiformis, and the ratios of AsA to DHA and GSH to GSSG were also much higher than those in P. ensiformis when exposed to As. Compared to the fronds of P. ensiformis, the leaves of P. vittata contained significantly more AsA and GSH. It is important to realize that the higher the level of reactive oxygen species (ROS) that P. ensiform is exposed to, the poorer its ability to scavenge them will be. The activity of APX and SOD decreased when As levels were low. CAT activity, on the other side, demonstrated a rising tendency when the levels of As were less than 1 mg/kg. The levels of antioxidant compounds in P. ensiformis are lower than average (AsA, GSH, and carotenoids) than in P. vittata. Gupta et al. (2009) discovered that the activity of SOD, GPX, and CAT all rose dramatically in two separate strains of Borrelia burgdorferi termed Varuna and Pusa Bold. Pusa Bold's higher antioxidant enzyme activity is probably responsible for the strain's increased tolerance. juncea in the presence of lower doses of A stress (50 mM). Ascorbic acid (40 mg/kg) can reduce the activity of SOD, APX, POX, and GR. This leads to a higher accumulation of reactive oxygen species (ROS), which in turn causes lipid peroxidation. Shri et al. (2009) found that the levels of various antioxidant enzymes and isozymes were increased with As exposure. Even though this interpretation contrasts with the findings of previous studies, which found that when As was present in rice leaves, there was a dramatic increase in the activity of both SOD and POX. Superoxide dismutase (SOD) is an enzyme family that includes such isoforms as GPX and APX. There was no evident catalase (CAT) enzyme induction. The treatment with Nickel resulted in a considerable increase in the activity of enzymes involved in the AsA-GSH cycle, including MDHAR, DHAR, and GR. Wang et al. discovered that the cotyledons, stems, and roots of Luffa cylindrica all showed significant increases in SOD, CAT, and GPX activities in other studies. They hypothesized that nickel treatment at varied doses could enhance the activity of these antioxidants, resulting in less oxidative damage caused by nickel-induced metal exposure and an increase in the plant's tolerance to nickel. A study conducted by Shanker et al. (2004) found that ROS-scavenging enzymes play an important role in numerous sections of plants when subjected to chromium (Cr) stress. In our study, we observed that a lower concentration of Cr did not result in any scavenging enzymes being induced because there was a regulated quantity of ROS formation. Furthermore, under Cr stress, the synergistic action of SOD and CAT was found to pivotal role in reducing the deleterious consequences of oxidative stress. This was attributed to both enzymes’ ability to scavenge H2O2 and O2·. As a result of its antioxidant defense system, Indian mustard (Brassica juncea) displayed an efficient metabolic defense and adaptive system when it was exposed to mercury (Hg)-induced oxidative stress. A higher concentration of Hg in the plant resulted in a more efficient development of an antioxidant defence system such as CAT (in particular) which was able to scavenge H2O2 more efficiently than the plant with a lower concentration of Hg. Therefore, Shiyab et al. (2009) found that there was a decreased level of H2O2 in those shoots as a result of this treatment.
5.6 Antioxidant Response
Cr toxicity in plants results in the generation of reactive oxygen species (ROS) via the Fenton and Haber-Weiss reactions (Montillet et al. 2004), which is then followed by altered antioxidant enzyme activity. The enhanced activity of antioxidant enzymes such as POD, catalase (CAT), APX, and SOD protects plants from reactive oxygen species (ROS) formed in response to chromium stress. When Bacillus thuringiensis oleracea, Zea mays, and Solanum lycopersicum were treated with Cr(VI), the roots and leaves had higher glutathione (GSH). These antioxidant enzymes disrupt the chain reaction of free radicals, either entirely stopping or considerably slowing the oxidation process (Munné-Bosch et al. 2009). Cr treatment has also been demonstrated to boost GSH production in Oryza sativa, Actinidia deliciosa (A. Chev.) C. F. Liang & A. R. Ferguson, Brassica napus, Salvinia natans, P. stratiotes, Salvinia rotundifolia, and Salvinia minima (Noctor et al. 2002). When subjected to Cr toxicity, however, Jatropha curcas showed a decrease in GSH activity (Panda 2007). Increased levels of glutathione reductase (GR), a key enzyme in the pathway that leads from ascorbate to glutathione, are seen as a response to Cr stress (Pandey et al. 2016). GR is a metal chelator and a ROS scavenger in addition to being a substrate for PC production. A recent study on Miscanthus sinensis found that when exposed to 0.50–1 mM Cr, 36 proteins involved in oxidative stress, metabolism, molecular chaperones, and other activities were over-expressed (Pang and Wang 2010).
5.7 Chromium-Mediated Alteration in the Enzymatic Antioxidant System
During the process of superoxide dismutase, superoxide, or O2, is converted into H2O2 as a result of superoxide dismutase. It has been developed by the plant an enzymatic antioxidant system that is highly sophisticated and well-organized to combat reactive oxygen species (ROS). As a result of a wide variety of stimuli, residual oxygen species (ROS) are generated in response to a variety of conditions, among them toxic concentrations of Cr Qureshi (2007). Ascorbate peroxidase (APX) and catalase (CAT) are enzymes that are capable of converting H2O2 into H2O2. To minimize the oxidative stress generated by Chromium, plants use an enzymatic antioxidant system composed of POD, SOD, APX, CAT, dehydroascorbate reductase (DHAR), and GR. It is also well known that plants use an enzymatic antioxidant system to combat oxidative stress. This system includes the enzymes POD, SOD, APX, CAT, dehydroascorbate reductase (DHAR), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), glutathione peroxidase (GPX), and glutathione S-transferase (GST). This system is in charge of regulating and scavenging reactive oxygen species caused by Cr.
5.8 Conclusion
It is undeniable that abiotic stress in international crop production has an undeniable significance because abiotic factors, collectively, are responsible for the majority of limitations that are placed on crop production around the world due to abiotic stress. Abiotic factors are responsible for the majority of the constraints placed on crop production as a result of abiotic factors. Thus, in the field of agriculture, it is essential to take additional steps to understand the molecular and physiological mechanisms that allow plants to tolerate abiotic stress and to discover how to plant stress tolerance can be increased to improve yield under drought conditions. Abiotic stress is one of the biggest threats to the agricultural production of a country, and as a result, it is possible to minimize this loss of agricultural production by utilizing knowledge of crop physiology and crop husbandry practices strategically. During the development, adaptation, and continuation of the existence of plants, reactive oxygen species (ROS), as well as their metabolism and detoxification, are crucial processes for their survival, development, and adaptation. It is possible to increase a plant's resistance to environmental stresses by artificially inducing the overexpression of novel isoforms of genes that code for ROS-detoxifying enzymes. There are some significant steps involved in the development of a plant's defence mechanism and regulatory mechanisms, such as the production of reactive oxygen species (ROS) and their subsequent removal through scavenging.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59
Acquaah G (2007) Principles of plant genetics and breeding. Blackwell, Oxford, p 385
Adejumo SA, Tiwari S, Thul S, Sarangi BK (2019) Evaluation of lead and chromium tolerance and accumulation level in Gomphrenacelosoides: a novel metal accumulator from lead acid battery waste contaminated site in Nigeria. Int J Phytoremediat 21:1341–1355
Afonso TF, Demarco CF, Pieniz S, Camargo FAO, Quadro MS, Andreazza R (2019) Potential of SolanumviarumDunal in use for phytoremediation of heavy metals to mining areas, southern Brazil. Environ Sci Pollut Res 26:24132–24142
Agrawal SB, Singh S, Agrawal M (2009) Ultraviolet-B induced changes in gene expression and antioxidants in plants. Adv Bot Res 52:48–86
Ahmed A, Hasnain A, Akhtar S, Hussain A, Abaid-Ullah YG, Wahid A, Mahmood S (2010) Antioxidant enzymes as bio-markers for copper tolerance in safflower (Carthamus tinctorius L.). Afr J Biotechnol 9:5441–5444
Al Mahmud J, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M (2017) Maleic acid assisted improvement of metal chelation and antioxidant metabolism confers chromium tolerance in Brassica juncea L. Ecotoxicol Environ Saf 144:216–226
Ali AA, Alqurainy F (2006) Activities of antioxidants in plants under environmental stress. In: Motohashi N (ed) The lutein-prevention and treatment for diseases. Transworld Research Network, Trivandrum, pp 187–256
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and Ascorbate-Glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defence systems in plants. CRC Press, Boca Raton, pp 77–104
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in chloroplasts. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition: topics in photosynthesis, vol 9. Elsevier, Amsterdam, pp 227–287
Ashraf A, Bibi I, Niazi NK, Ok YS, Murtaza G, Shahid M, Kunhikrishnan A, Li D, Mahmood T (2017) Chromium(VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int J Phytoremediation 19:605–613
Augustynowicz J, Wrobel P, Płachno BJ, Tylko G, Gajewski Z, Wegrzynek D (2014) Chromium distribution in shoots of macrophyte Callitrichecophocarpa Sendtn. Planta 239:1233–1242
Augustynowicz J, Sitek E, Bryniarski T, Baran A, Ostachowicz B, Urbańska-Stopa M, Szklarczyk M (2020) The use of Callitrichecophocarpa Sendtn. For the reclamation of Cr-contaminated freshwater habitat: benefits and limitations. Environ Sci Pollut Res 27:25510–25522
Balasaraswathi K, Jayaveni S, Sridevi J, Sujatha D, Aaron KP, Rose C (2017) Cr-induced cellular injury and necrosis in Glycine max L.: biochemical mechanism of oxidative damage in the chloroplast. Plant Physiol Biochem 118:653–666
Barbosa R, de Almeida A, Mielke M, Loguercio L, Mangabeira P, Gomes F (2007) A physiological analysis of Genipa americana L.: a potential phytoremediator tree for chromium polluted watersheds. Environ Exp Bot 61:264–271
Bhargava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosimicrobium sp isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf 147:102–109
Blokhnia O, Virolainen E, Fagerstedt V (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Byrne P, Taylor KG, Hudson-Edwards KA, Barrett JES (2017) Speciation and potential long-term behaviour of chromium in urban sediment particulates. J Soils Sediments 17:2666–2676
Chakraborty U, Pradhan D (2011) High temperature-induced oxidative stress in Lens culinaris, the role of antioxidants and amelioration of stress by chemical pre-treatments. J Plant Interact 6:43–52
Chalapathi Rao ASV, Reddy AR (2008) Glutathione reductase: a putative redox regulatory system in plant cells. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stresses in plants. Springer, Berlin/Heidelberg, pp 111–147
Chandra S, Chauhan LKS, Pande PN, Gupta SK (2004) Cytogenetic effects of leachates from tannery solid waste on the somatic cells of Vicia faba. Environ Toxicol 19:129–133
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Chen Q, Wu K, Tang Z, Guo Q, Guo X, Wang H (2017) Exogenous ethylene enhanced the cadmium resistance and changed the alkaloid biosynthesis in Catharanthus roseus seedlings. Acta Physiol Plant 39:267
Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic aciddefi client Arabidopsis mutant. Proc Natl Acad Sci USA 93:9970–9974
Cui W, Wang H, Song J, Cao X, Rogers HJ, Francis D, Jia C, Sun L, Hou M, Yang Y (2017) Cell cycle arrest mediated by Cd-induced DNA damage in Arabidopsis root tips. Ecotoxicol Environ Saf 145:569–574
De Tullio MC (2004) How does ascorbic acid prevent scurvy? A survey of the nonantioxidant functions of vitamin C. In: Asard H (ed) Vitamin C: its function and biochemistry in animals and plants. Garland Science/BIOS Scientific Publishers, London, New York, pp 176–190
del Río LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signalling. Plant Physiol 141:330–335
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338–350
El Fels L, Hafidi M, Silvestre J, Kallerhoff J, Merlina G, Pinelli E (2015) Efficiency of the co-composting process to remove genotoxicity from sewage sludge contaminated with hexavalent chromium. Ecol Eng 82:355–360
Elsawy EET, El-Hebeary MR, El Mahallawi ISE (2017) Effect of manganese, silicon and chromium additions on microstructure and wear characteristics of grey cast iron for sugar industries applications. Wear 390–391:113–124
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I (2006) Enhanced tolerance to ozone and drought in transgenic tobacco overexpressing dehydroascorbate reductase in the cytosol. Physiol Plant 127:57–65
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethene glycol stresses. Planta 225:1255–1264
Fargasova A (2012) Plants as models for chromium and nickel risk assessment. Ecotoxicology 21:1476–1483
Florea CD, Carcea I, Cimpoesu R, Toma SL, Sandu IG, Bejinariu C (2017) Experimental analysis of resistance to electrocorosion of a high chromium cast iron with applications in the vehicle industry. Rev Chim 68:2397–2401
Foyer C, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146:359–388
Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (a-tocopherol). Plant Cell Environ 15:381–392
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Ghosh I, Ghosh M, Mukherjee A (2017) Remediation of mine tailings and fly ash dumpsites: role of poaceae family members and aromatic grasses. In: Enhancing cleanup of environmental pollutants. Springer: Cham, Germany, pp 117–167
Gielen H, Vangronsveld J, Cuypers A (2017) Cd-induced Cu deficiency responses in Arabidopsis thaliana: are phytochelatins involved? Plant Cell Environ 40:390–400
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gupta M, Sharma P, Sarin NB, Sinha AK (2009) Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere 74:1201–1208
Halliwell B (2006) Reactive species and antioxidants, redox biology is the fundamental theme of aerobic life. Plant Physiol 141:312–322
Haokip N, Gupta A (2020) Phytoremediation of chromium and manganese by Ipomoea aquatica Forssk. from aqueous medium containing chromium-manganese mixtures in microcosms and mesocosms. Water Environ J
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defence and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res. https://doi.org/10.1007/s12011-011-8998-9
Hasanuzzaman M, Hossain MA, Fujita M (2011a) Selenium-induced up-regulation of the antioxidant defence and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res. https://doi.org/10.1007/s12011-011-8958-4
Hasanuzzaman M, Hossain MA, Fujita M (2011b) Nitric oxide modulates antioxidant defence and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. https://doi.org/10.1007/s11816-011-0189-9
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN, Fujita M (2017) Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci 8:115
Hossain MA, Hasanuzzaman M, Fujita M (2011) Coordinate induction of antioxidant defence and glyoxalase system by exogenous proline and glycine betaine is correlated with salt tolerance in mung bean. Front Agric China 5:1–14
Kachout SS, Mansoura AB, Leclerc JC, Mechergui R, Rejeb MN, Ouerghi Z (2009) Effects of heavy metals on antioxidant activities of Atriplex hortensis and A. rosea. J Food Agric Environ 7:938–945
Kalve S, Sarangi BK, Pandey RA, Chakrabarti T (2011) Arsenic and chromium hyperaccumulation by an ecotype of Pteris vittata–Prospective for phytoextraction from contaminated water and soil. Curr Sci 100:888–894
Labra M, Di Fabio T, Grassi F, Regondi SMG, Bracale M, Vannini C, Agradi E (2003) AFLP analysis as a biomarker of exposure to organic and inorganic genotoxic substances in plants. Chemosphere 52:1183–1188
Levizou E, Zanni AA, Antoniadis V (2018) Varying concentrations of soil chromium (VI) for the exploration of tolerance thresholds and phytoremediation potential of the oregano (Origanum vulgare). Environ Sci Pollut Res 26:14–23
Li JM, Jin H (2007) Regulation of brassinosteroid signaling. Trends Plant Sci 12:37–41
Logan B (2005) Reactive oxygen species and photosynthesis. In: Smirnoff N (ed) Antioxidants and reactive oxygen species in plants. Blackwell, Oxford, pp 250–267
Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158
Miadokova E, Duhova V, Vlckova V, Sladkova L, Sucha V, Vlcek D (1999) Genetic risk assessment of acid wastewater containing heavy metals. Gen Physiol Biophys 18:92–98
Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18:2749–2766
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Mondal NK, Nayek P (2020) Hexavalent chromium accumulation kinetics and physiological responses exhibited by Eichhornia sp. and Pistia sp. Int J Environ Sci Technol 17:1397–1410
Montillet JL, Cacas JL, Garnier L, Montané MH, Douki T, Bessoule JL, Polkowska-Kowalczyk L, Maciejewska U, Agnel JP, Vial A, Triantaphylidès C (2004) The upstream oxylipin profile of Arabidopsis thaliana: a tool to scan for oxidative stress. Plant J 40:439–451
Munné-Bosch S, Falanga V, Pateraki I, Lopez-Carbonell M, Cela J, Kanellis AK (2009) Physiological and molecular responses of the isoprenoid biosynthetic pathway in a drought-resistant Mediterranean shrub, Cistus creticus exposed to water deficit. J Plant Physiol 166:136–145
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53:1283–1304
Panda SK (2007) Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J Plant Physiol 164:1419–1428
Pandey B, Agrawal M, Singh S (2016) Ecological risk assessment of soil contamination by trace elements around the coal mining area. J Soils Sediments 16:159–168
Pang CH, Wang BS (2010) Role of ascorbate peroxidase and glutathione reductase in Ascorbate—Glutathione cycle and stress tolerance in plants. In: Anjum NA, Chan MT, Umar S (eds) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht, pp 91–112
Patnaik AR, Achary VMM, Panda BB (2013) Chromium(VI)-induced hormesis and genotoxicity are mediated through oxidative stress in root cells of Allium cepa L. Plant Growth Regul 71:157–170
Prado C, Pagano E, Prado F, Rosa M (2012) Detoxification of Cr(VI) in Salvinia minima is related to seasonal-induced changes of thiols, phenolics and antioxidative enzymes. J Hazard Mater 239:355–361
Qian XW (2004) Mutagenic effects of chromium trioxide on root tip cells of Vicia faba. J Zhejiang Univ Sci 5:1570–1576
Qureshi MI, Abdin MZ, Qadir S, Iqbal M (2007) Lead-induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol Plant 51:121–128
Ranieri E, Fratino U, Petruzzelli D, Borges AC (2013) A comparison between Phragmites australis and Helianthus annulus chromium phytoextraction. Water Air Soil Pollut 224:1–9
Robson CA, Vanlerberghe GC (2002) Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and independent pathways of programmed cell death. Plant Physiol 129:1908–1920
Rodríguez M, Canales E, Borrás-Hidalgo O (2005) Molecular aspects of abiotic stress in plants. Biotechnol Appl 22:1–10
Rodriguez E, Azevedo R, Fernandes P, Santos C (2011) Cr(VI) induces DNA damage, cell cycle arrest and polyploidization: a how cytometric and comet assay study in Pisum sativum. Chem Res Toxicol 24:1040–1047
Sajad MA, Khan MS, Bahadur S, Naeem A, Ali H, Batool F, Shuaib M, Batool S (2020) Evaluation of chromium phytoremediation potential of some plant species of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Acta Ecol Sin 40:158–165
Sánchez-Casas P, Klesseg DF (1994) A salicylic acid-binding activity and a salicylic acid-inhibitable catalase activity are present in a variety of plant species. Plant Physiol 106:1675–1679
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (Vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211
Shanker AK, Djanaguiraman M, Sudhagar R, Chandrashekar CN, Padmanabhan G (2004) Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek, cv CO4) roots. Plant Sci 166:1035–1043
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of the antioxidative defence system in growing rice seedlings exposed to toxic levels of aluminium. Plant Cell Rep 26:2027–2038
Shiyab S, Chen J, Han FX, Monts DL, Matta FB, Gu M, Su Y, Masad MA (2009) Mercury-induced oxidative stress in Indian mustard (Brassica juncea L.). Environ Toxicol 24:462–471
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Singh S, Khan NA, Nazar R, Anjum NA (2008) Photosynthetic traits and activities of antioxidant enzymes in black gram (Vigna mungo L. Hepper) under cadmium stress. Am J Plant Physiol 3:25–32
Sinha V, Pakshirajan K, Chaturvedi R (2014) Chromium(VI) accumulation and tolerance by tradescantia pallida: biochemical and antioxidant study. Appl Biochem Biotechnol 173:2297–2306
Sliwa-Cebula M, Kaszycki P, Kaczmarczyk A, Nosek M, Lis-Krzyścin A, Miszalski Z (2020) The common ice plant (Mesembryanthemum crystallinum L.)–Phytoremediation potential for cadmium and chromate-contaminated soils. Plants 9:1230
Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-faceted molecule. Curr Opin Plant Biol 3:229–235
Srivalli S, Khanna-Chopra R (2008) Role of glutathione in abiotic stress tolerance. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stress in plants. Springer, Berlin, Heidelberg, pp 207–225
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Taufikurahman T, Pradisa MAS, Amalia SG, Hutahaean GEM (2019) Phytoremediation of chromium (Cr) using Typha angustifolia L., Canna indica L. and Hydrocotyl eumbellata L. in surface flow system of constructed wetland. In: Proceedings of the IOP conference series: earth and environmental science, Ivano-Frankivsk, Ukraine, vol 308. IOP Publishing: Bristol, UK, p 012020
Truta E, Mihai C, Gherghel D, Vochita G (2014) Assessment of the cytogenetic damage induced by chromium short-term exposure in root tip meristems of barley seedlings. Water Air Soil Pollut 225:1933
Ullah R, Hadi F, Ahmad S, Jan AU, Rongliang Q (2019) Phytoremediation of lead and chromium contaminated soil improves with the endogenous phenolics and proline production in parthenium, Cannabis, Euphorbia, and Rumex Species. Water Air Soil Pollut 230:40
Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D (1989) Cloning, yeast expression and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J Biol Chem 272:19176–19186
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wang WX, Vinocur B, Shoseyov O, Altman A (2001) Biotechnology of plant osmotic stress tolerance: physiological and molecular considerations. Acta Hort 560:285–292
Wang C, Tan H, Li H, Xie Y, Liu H, Xu F, Xu H (2020) Mechanism study of Chromium influenced soil remediated by an uptake-detoxification system using hyperaccumulator, resistant microbe consortium, and nano iron complex. Environ Pollut 257:113558
Wójcik M, Tukiendorf A (2011) Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biol Plant 55:125–132
Wu G, Wei ZK, Shao HB (2007) The mutual responses of higher plants to the environment: physiological and microbiological aspects. Biointerfaces 59:113–119
Wu S, Hu Y, Zhang X, Sun Y, Wu Z, Li T, Lv J, Li J, Zhang J, Zheng L (2018) Chromium detoxification in arbuscular mycorrhizal symbiosis mediated by sulfur uptake and metabolism. Environ Exp Bot 147:43–52
Acknowledgements
We would like to acknowledge the department of agronomy at the Lovely Professional University, Phagwara, Punjab, India, for their consistent moral support and encouragement throughout the writing process.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sharma, M., Dey, S.R., Kumar, P. (2023). Antioxidant Defence: A Key Mechanism of Chromium Tolerance. In: Kumar, N., Walther, C., Gupta, D.K. (eds) Chromium in Plants and Environment. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-44029-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-44029-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44028-1
Online ISBN: 978-3-031-44029-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)