Abstract
In the present study, hexavalent chromium (5, 10 and 30 mg/L) phytoaccumulation by two free floating macrophytes, Eichhornia sp. and Pistia sp., was investigated in a greenhouse. The results revealed higher accumulation of chromium by Eichhornia sp. at 30 mg/L Cr solution. However, Pistia sp. showed highest accumulation at intermediate chromium solution of 10 mg/L. Pigment data indicated higher reduction of chlorophyll for Pistia sp. compared to Eichhornia sp. Both the tested species showed gradual reduction of both chlorophyll-a and chlorophyll-b significantly with increasing metal concentration from 5 to 30 mg/L. However, chlorophyll stability index data indicated higher chlorophyll stability index at higher Cr concentrations in case of both the macrophytes. On the other hand, lipid peroxidation in the form of malondialdehyde concentration was observed to increase with increase in chromium load for both the tested species. Almost similar results were recorded in the enzyme analysis data. Study results revealed that all the studied enzymes are highly sensitive toward chromium. However, catalase activity showed the highest sensitivity. Chromium bioaccumulation kinetics study revealed that only Pistia sp. is more suited with pseudo-first-order (0.910) and pseudo-second-order (0.665) kinetics equation compared to Eichhornia sp. New root development was observed only for Eichhornia sp. during the third day of incubation. The wet biomass of both the macrophytes showed gradual reduction in chromium solutions of increasing concentrations. Therefore, it may be concluded that Eichhornia sp. and Pistia sp. may be effectively used in remediation of Cr(VI) contaminated aquatic bodies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals have density equal to or higher than 5 g/cm3 (Nies 1999). Different heavy metals such as cadmium (Cd), lead (Pb), chromium (Cr) are constantly discharged into the environment through industrial and anthropogenic activities (RoyChowdhury et al. 2017). All the heavy metals at higher concentration levels have negative impact on the plant community (Fan et al. 2017). Among the various heavy metals, chromium has been specially studied by toxicologists (Medda and Mondal 2017). Chromium enters the environment through two main paths, as natural ferrochromite (Fe2Cr2O4) and in the form of other minerals in the earth’s crust. It mainly exists in two stable oxidation states: Cr(III) and Cr(VI) (Gil-Cardeza et al. 2014). Cr(VI), mainly originated from anthropogenic sources, is found in water as HCrO4−, Cr2O72− and CrO42−, which are highly mobile anions. The dominant forms of Cr(III) are mainly cationic CrOH2+aq, Cr(OH)2−aq or neutral Cr(OH)3. Previous data suggest that Cr(III) is less toxic and less absorbed by the plants than Cr(VI) (Dhal et al. 2013). On the other hand, the hexavalent chromium is considered as a potent mutagenic and teratogenic metal due to its high penetration power in the cell membranes and subsequent interaction with protein and nucleic acid (Mishra and Bharagava 2016). Also, hexavalent chromium has strong oxidizing capacity at lower pH (Kota and Stasicka 2000).
Heavy metal stress always leads to the generation of reactive oxygen species (ROS) which are detrimental to all plants (Singh et al. 2017). Lipid peroxidation is another major consequence of heavy metal stress in plants (Goswami and Das 2016). Every plant system has auto scavenging of ROS which protects the plants from oxidative damage (Singh et al. 2017). The antioxidant enzymes are catalase (CAT), peroxidase (POD) and ascorbic acid oxidase (AAO), which play an important role in scavenging reactive oxygen species (Rusina et al. 2004). Every antioxidant has a distinct function toward detoxification of ROS such as CAT and POD which can reduce H2O2 to water and oxygen (Sharma and Dietz 2009). Similarly ascorbic acid oxidase enzyme catalyzes the oxidation of ascorbic acid to dehydroascorbic acid (Shimada and Ko 2008).
Therefore, it is of utmost importance to control and check the introduction of Cr(VI) into the environment. The only possible way is through the development of remediation strategies (Gil-Cardeza et al. 2014). The previous literature highlighted one common strategy for reduction of toxicity through conversion of Cr(VI) to Cr(III) (Sagar et al. 2012). Amin et al. (2015) and Sheoran and Sheoran (2006) suggested that some specific mechanisms that restrict the mobility of element like Cr in plants body and subsequently protect the plants from toxicity. It is also documented that some aquatic plants can hyperaccumulate the metals in their roots in comparison with other parts (Yabanli et al. 2014). However, traditional management techniques are also available such as chemical precipitation, reverse osmosis, coagulation, adsorption, reverse osmosis. But, all these methods have serious limitations and may also not be economically viable or environment friendly. To overcome these difficulties, a very popular method called phytoremediation has been introduced (Rahman and Hasegawa 2011). Phytoremediation is a green technology which is absolutely plant based (Torok and Dransfield 2017; Thijs et al. 2017). The different types of phytoremediation are phytoextraction, phytostabilization, phytovolatilization, phyto-transformation and rhizofiltration (Vamerali et al. 2010). Among these, phytoextraction is a technique in which plants take up pollutants from the soil or aquatic bodies and store them in their body parts including vacuoles with the help of phytochelators (Kontoghiorghe et al. 2015).
Macrophytes are vegetative and photosynthetic organisms and can comfortably grow in the water bodies. They have an important role in the aquatic ecosystem owing to their capability to act as phytoremediating agents through accumulation of both inorganic and organic pollutants (Lin et al. 2018; Wang et al. 2018).They are found throughout the world and constitute an important constituent of wetlands. Very recently Fariasa et al. (2018) highlighted that macrophytes can acts as bioindicators of heavy metal pollution. Different varieties of macrophytes are available in our locality such as Eichhornia crassipes, Lemna minor L., Pistia stratiotes L. Among them, some are emergent, floating-leaved, and some submerged, and free floating. Out of the different varieties of macrophytes, water hyacinth (Eichhornia crassipes) is the most noxious aquatic vegetative organism because of its very fast growth rate, high pollution resistance and enormous nutrient absorption capacity (Swarnalatha and Radhakrishnan 2015). Pistia stratiotes is another abundant macrophyte, also known as water cabbage or water lettuce, etc. This macrophyte has enormous capability toward absorption of heavy metals from contaminated aquatic bodies (Vesely et al. 2011).
The present study has highlighted the potentiality of Eichhornia sp. and Pistia sp. toward phytoremediation of hexavalent chromium with variation of incubation time. Additionally, kinetics of Cr(VI) accumulation, growth parameters and biochemical analysis including enzyme studies have been undertaken. The present study was conducted at the Department of Environmental Science (both inside (laboratory) and outside (at the greenhouse) during the period from November to February 2017.
Materials and methods
Plant material and experimental design
The aquatic macrophytes used for the removal of Chromium from synthetic medium were Eichhornia sp. and Pistia sp. The aquatic macrophytes, with an average weight of 20 g, were collected from a stagnant water body in Golapbag, Burdwan University Campus (87°50′ 53.71″ E and 23°15′ 19.68″ N), and washed thoroughly with distilled water to remove the particles adhering to the plants. All the macrophytes were sterilized according to Tiwari et al. (2012) and maintained as stock cultures on Pirson–Seidel nutrient medium (Pirson and Seidel 1950). Each treatment was run in triplicate. pH of all the treatment sets was adjusted to 6.5 ± 0.1 throughout the entire incubation period. All the treatment sets were incubated under a 15-h light/9-h dark photoperiod (1500LX) using light-emitting diodes (LEDs) (2 red and 1 blue).
Stock chromium solution
Stock of chromium solution was prepared by dissolving 0.283 g anhydrous potassium dichromate (K2Cr2O7) in distilled water and diluted to 1000 mL. The intermediate concentration was prepared by proper dilution method. The pH of the medium was adjusted by using 1(N) HCl and 1(N) NaOH.
Standard chromium solution
From stock chromium solution, 100 mL was taken and diluted to 1000 mL with distilled water.
Growth parameters
According to the ISO 20079 test protocol (2004), the growth of Eichhornia sp. and Pistia sp’s was determined measuring new root growth, root length, fresh weight (FW) and dry weight (DW). Plants were surface-dried between layers of paper towels and then dried at 80 °C (Cedergreen et al. 2007) up to constant weight. Relative growth rate (RGR) was calculated from the following equation with the measured parameter × (FW) and the start of the test (t0) for each replicate separately: \({\text{RGR}} = \frac{{(\ln x_{{t_{1} }} - \ln x_{{t_{o} }} )}}{{t_{1} - t_{o} }}.\)
Dry-to-fresh weight ratio (DW/FW) was determined according to calculation: dry weight (g)/fresh weight (g).
Biochemical parameters
Photosynthetic pigment
Estimation of total chlorophyll and carotenoids content from plant material (Arnon 1949). For the estimation of chlorophyll content, Arnon’s (1949) method was employed. Chlorophyll is extracted in 80% acetone, and the absorption at 645, 652 and 663 nm is read in spectrophotometer (PerkinElmer Lambda 35).
Using the absorption coefficient, the amount of chlorophyll is calculated.
Chlorophyll Stability Index (CSI)
The CSI was calculated in stressed and control plants according to the following formula (Sairam et al. 2008).
Enzyme antioxidants and protein content
After 10 days of incubation, three antioxidant enzymes including catalase (CAT) (EC 1.11.1.6), peroxidase (POD) (EC 1.11.1.7) and ascorbic acid oxidase (AAO) (EC 1.10.3.3) and total protein in leaves were evaluated by using spectrophotometer (PerkinElmer Lambda 35). The fresh leaves along with 0.05 M phosphate buffer were ground in a mortar and pestle and filtered through four layers of muslin cloth followed by centrifugation at 12,000 rpm for 10 min in cold centrifuge (REMI C24). Finally, this extract was used for estimation of CAT, POD and AAO according to Zhang (1992). Estimation of total protein was done by following the method of Lowry et al. (1951) using bovine serum albumin as the standard protein, and the results were expressed as mg g−1 F.W.
Lipid Peroxidation content
The level of lipid peroxidation was measured on the basis of malondialdehyde (MDA) formation as demonstrated by Heath and Packer (1968). Plant material (0.5 g) was ground with 5 mL (0.1%) trichloroacetic acid (TCA) and centrifuged at 11,000 rpm for 5 min. After centrifugation, 1 mL of supernatant liquid was mixed with 4 mL (0.5%) thiobarbituric acid (TBA) which was prepared with 20% TCA. The entire mixture was then heated at 95 °C for 30 min and quickly frozen by placing the mixture container in ice bath followed by centrifugation (Remi R24) at 11,000 rpm for 15 min. The absorbance at 532 and 600 nm was measured using UV–Vis spectrophotometer (PerkinElmer Lambda 35).
Percentage of metal accumulation and accumulation capacity
The culture mediums were centrifuged at 5000 rpm for 10 min at room temperature at different time intervals (1–10 days) followed by filtration through cellulose acetate filter (0.45 µm), and the filtrate was used for estimation of total Cr(VI) by atomic absorption spectrometry (AAS, Agilent). The percentage of Cr(VI) removal was calculated by the following formula (Zhou et al. 2012):
where C0 and Cf are initial and final Cr(VI) concentration (mg/L), respectively. The uptake capacities of the two macrophytes were also measured by applying the following equation (Yang et al. 2015):
where V is volume of the experimental solution and M is the weight (g) of macrophyte at the end of the experiment.
Modeling
The modeling procedure of chromium ion bioaccumulation data was based on the chromium mass balance where the relation between the biomass growth and the metal concentration reduction from the hydroponic media against time was quantified. Chromium ion bioaccumulation from hydroponic media by using living aquatic macrophytes Eichhornia sp. and Pistia sp. has been described by applying four non-structural kinetic models (Mondal et al. 2014), and the accumulated metal, maximum capacity and rate constants of bioaccumulation process were estimated. These adsorption models resemble the Langmuir-type irreversible, pseudo-first and second interaction models, respectively. It is a great challenge from a mathematical point of view to consider the mass transfer mechanisms involved in the heavy metal removal process by living macrophytes. The mechanistically classical models of enzymatic and adsorption kinetics have been used. Hence, we have followed the non-living biosorbent action-based adsorption kinetic classical modeling.
where C (in mg Cr L−1) is the chromium concentration in the liquid phase in time; r (in mg Cr g−1 day−1) is the chromium bioaccumulation action rate by aquatic plant; q (in mg Cr g−1) is the macrophyte-accumulated chromium concentration; qmax (in mg Cr g−1) is the macrophyte-accumulated maximum chromium content constant; k1 (in l mg−1 Cr day−1), k2 (in day−1) and k3 (in g mg−1 Cr day−1) are the bioaccumulation rate constants; and kb (in day−1) is the chromium desorption rate constant only for irreversible kinetic model.
Quality control study
Both precision and accuracy of analysis can be achieved through repeated measurement by following the standard method (SRM 1570) for heavy metals. These results were recorded ± 2% of the certified value. The instrument was calibrated after fine determination/readings. Overall quality control measures were taken to assess the contamination level and reliability of data.
Statistical Analysis
All the replicated data were analyzed statistically by one-way ANOVA and Duncan’s multiple range test (DMRT). For statistical interpretation of the observed tabulated data, Panse and Sukhatme (1967), together with Gomez and Gomez (1984), were consulted.
Results and discussion
Cr uptake modeling
The Cr(VI) accumulation data clearly indicate that gradual reduction of accumulation with increasing incubation time for Eichhornia sp. (Fig. 1a). However, almost an opposite trend was recorded for Pistia sp. especially with higher incubation time (Fig. 1b). The chromium bioaccumulation from a nutrient medium by living aquatic macrophytes Eichhornia sp. and Pistia sp. has been described by three non-structural kinetics model, but instead of the adsorb rate determination, the accumulated metal maximum capacity and rate constant of bioaccumulation were estimated. These models resemble the Langmuir-type irreversible, pseudo-first-order and pseudo-second-order models, respectively. Indeed, there are several mass transfer mechanisms involved in the heavy metal removal process by living microorganisms, which cause great difficulties for the modeling of the whole process (Mishra and Tripathi 2009).
A mechanistically classical model of enzymatic and adsorption kinetics has been used, in order to represent the heavy metal bioaccumulation kinetic for living organism. In this work, the non-living biosorbent action-based adsorption kinetic classical modeling was followed (Eqs. 1–3).
The entire rate constants and goodness of fit value are presented in Table 1. From Table 1 it is clear that only Pistia sp. showed very good fitness with both pseudo-first-order (p < 0.01) and pseudo-second-order (p < 0.05) kinetic equation with very high R2 values at lower (5 mg/L) and higher (30 mg/L) concentration of Cr(VI), respectively. On the other hand, none of the macrophyte showed good fitness with irreversible kinetic equation. Almost similar lead (Pb) accumulation by living aquatic macrophyte, Salvinia auriculata, was reported by Espinoza-Quiñones et al. (2009). They also stated that the non-structural kinetic models have shown good agreement with the lead uptake experimental data in all the investigated cases. The aquatic macrophytes have exceptional potentiality toward accumulation of heavy metals in their body parts (Reznia et al. 2016). It was reported that they can accumulate up to 1,00,000 times greater than the amount of associated aqueous substance (Muthusaravanan et al. 2018). Aquatic macrophyte can exhibit their metal accumulation mechanism through phytoaccumulation (Kamal et al. 2004), phytostabilization (Berti and Cunningham 2000), phytodegradation (Newman and Reynolds 2004), phytovolatilization (Zayed et al. 2000).
Growth and development
Biomass of plant is a valuable tool for characterizing the growth performance of heavy metal stressed plants (Malik et al. 2019). Growth patterns of the two studied macrophytes were understood from the wet biomass of the macrophytes. Study results revealed that wet biomass of Eichhornia sp. decreased with increase in number of days of incubation with 5 mg/L chromium solution. However, at 10 mg/L, wet biomass increased linearly up to 5 days of incubation (Table 2). Similar incremental picture of biomass was recorded with 30 mg/L chromium solution, but after third day of incubation, wet biomass showed almost constant value (Table 2). The shunted growth perhaps due to chromium interference in aquaporins or by altering the membrane permeability (Malar et al. 2015) or due to the impairment in cell division (Vecchia et al. 2005). On the other hand, Pistia sp. does not show any biomass growth in all the studied solution concentrations (Table 3). Several polynomial type functions and segment curves in different growing phase were recorded (figure not provided). Therefore, from this phytoremediation study, it may be easily concluded that Eichhornia sp. is a better for chromium accumulator than Pistia sp. Again from root growth data, it is clear that none of the concentration of chromium is suitable for the root growth of Eichhornia sp. except concentration 5.0 mg/L. Almost similar results were recorded for Pistia sp. also. Heavy metals have remarkable effects on root length of plants and it is well documented by the earlier researchers (Dey and Mondal 2016; Tang et al. 2001). Prasad et al. (2001) highlighted in their research that 88.78% reduction in biomass was achieved at 5 mg/L Cr solution for Eichhornia sp. Similar reduction in biomass was also reported by Chen et al. (2001), and they demonstrated that root weight and root length of wheat were affected by a concentration of 20 mg Cr (VI) kg−1 soil as K2Cr2O7. Currently, Malik et al. (2019) also supported that heavy metal can prevent the overall growth and development of plants through cellular damage.
Observation of medium pH
Throughout the incubation period of 1–10 days, the pH of the hydroponic medium was measured and the results are depicted in Tables 2 and 3. From Table 2, it is found that for Eichhornia sp. at 5 mg/L Cr solution, pH ranges from 6.53 to 7.82. Almost similar incremental patterns were recorded for other treatments also with slight deviation at 30 mg/L. On the other hand, Pistia sp. showed much lower pH during first day of incubation. However, after third day of incubation, there is remarkable change in pH (Table 3).
Enzyme study
The main toxicity of heavy metal is due to the production of reactive oxygen species which may causes oxidative stress in plants and also hamper the grain yield (Shahid et al. 2014). To overcome such stress conditions, plants inherently developed innate enzymatic defense mechanism (Hassanein et al. 2012) through generation of enzymes and subsequently control the toxic effects of free radicals (Chen et al. 2015). In the present study, it has been found that the activity of antioxidant enzymes of peroxidase, catalase and ascorbate increases with increase in incubation time period for all the studied concentration of Eichhornia sp. However, enzymatic activity of Pistia sp. initially decreased from first to third day of incubation, but after that increased at 5 days of inoculation and again decreased at 10 days of incubation (Table 4). The variations of peroxidase (EC 1.11.1.7) activity showed almost similar trends as catalase (EC 1.11.1.6) for Eichhornia sp., but Pistia sp. showed initial increment up to 5 days of incubation followed by decrease at 10 days of incubation except for 5 mg/L or solution (Table 4). On the other hand, ascorbate (EC 1.11.1.4) concentration did not show any such decremental trend with metal concentrations and days of incubation. For both Eichhornia sp. and Pistia sp., the activity of antioxidant (peroxidase and catalase) remarkably varied in all concentration of Cr(VI) salt, which is probably due to generation of ROS inside the plant (Tauqeer et al. 2016). This over expression of antioxidant enzyme will act as a valuable indicator for the survival of heavy metal accumulator plants (Hibiba et al. 2015).
Therefore, this enzymatic variation of plants under the influence of heavy metal stress may be a good marker of metal pollution in aquatic bodies (Hibiba et al. 2015). The fluctuation is the result of changes in lipid peroxidation (Sarwar et al. 2017). MDA content increases with increasing concentration of Cr VI salt solution at 5 mg/L. But with increasing incubation time, no such trend was recorded for Eichhornia sp. Almost similar enzymatic study was reported by Singh et al. (2016) for weed plants under metal stress condition. Very recently, Dalo et al. (2019) recorded the enhanced level of MDA under Ni-Fe stress in plants (Phragmites australis).
Root length and number of new roots
In phytoremediation study, the study of number of roots and root length is indispensable (Shanker et al. 2005). The present study results clearly revealed that root length of both Eichhornia sp. and Pistia sp. decreased with increasing concentration of chromium (Fig. 2a and b). Decrease in root growth is a well-documented phenomenon under metal stress condition (Mondal et al. 2013, 2015). However, toxicity of different heavy metals is different. Prasad et al. (2001) evaluated the toxicity of different heavy metals as Cd > Cr > Pb. They also highlighted that root length was more affected by Cr than by other heavy metals studied. The present study also highlighted that variation of root length is also concentration dependent with respect to control (Fig. 2a and b). This is possible due to destruction of root cells under chromium stress (Saddiqe et al. 2015). Results also demonstrated that at 5 mg/L Cr solution, Eichhornia sp. showed new root growth (figure not supplied). However, at higher concentrations (> 5 mg/L) no new root growth was recorded for both Eichhornia sp. and Pistia sp. Our study is in accordance with Panda and Patra (2000), and they demonstrated that 1 µM of Cr solution increases the root length in seedling growing under nitrogen nutrition levels. Another interesting observation was recorded during the growth period that the population of root hair steadily decreased with increasing Cr concentration (figure not provided) and growth of root hair almost stopped at 5 mg/L. Almost, identical result was reported by Suseela et al. (2002) through scanning electron microscopic study of root affected by Cr. They also examined the pitch and cortical tissue layer and root hair through microscopic study.
Pigment level
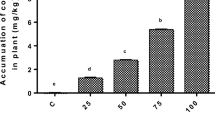
Both Eichhornia sp. and Pistia sp. showed gradual decrease in pigment concentration in the form of Chl-a, Chl-b and total Chl (Table 5). However, carotenoids showed initial increment when Cr concentration changed from 5 to 10 mg/L, but after that, pigment concentration decreased at 30 mg/L (Table 3). Chromium is such a heavy metal which can directly interfere in photosynthesis, activation of various functional enzymes, inadequate supply of sunlight energy required for conversion of ADP to ATP and electron transport (Clijsters and Van Assche 1985). Previous researches highlighted the negative impact of chromium on small plants, but very limited information is available on higher plants (Medda and Mondal 2017). Chromium can inhibit photosynthesis which is probably due to disorganization of chloroplast ultra-structure (Mondal et al. 2015) and disruption in electron transport through photo-phosphorylation process (Bishnoi et al. 1993). Zeid (2001) observed that in peas, Cr at higher concentrations decreased photosynthesis drastically. The previous study also highlighted that not only Cr has negative effect on green plants, but also other metals such as Cd on wheat (Rizwan et al. 2015) and Cu on wheat (Keller et al. 2015), Cd on Brassica napus (Ehsan et al. 2014). Jarvis and Bielmyer-Fraser (2015) demonstrated that at higher metal concentration, higher metal accumulation occurred which subsequently causes the degradation of green pigment in leaves.
Results also revealed that Chl-a/b ratio increased with increase in the incubation period for both Eichhornia sp. and Pistia sp. (Table 5). However, Chl-a-to-Chl-b ratio is absolutely random at different concentration of Cr (Table 5). The decrease in the Chl-a/b ratio (Shanker 2003) under Cr stress is possibly due to reduction of size of the peripheral part of the antenna complex. The level of carotenoid under different concentrations of Cr(VI) solution is presented in Table 5.
Chlorophyll Stability Index (CSI)
Chlorophyll stability index (CSI) value gradually decreases with increase in the incubation period and strength of chromium solution for both the macrophytes (Table 6). However, degree of decomposition is different for different macrophytes. After 24 h, the reduction of CSI for Eichhornia sp. is 7.6%, 16.5% and 25.63% at 5, 10, 30 mg/L Cr(VI) solution, respectively (Table 5). But, during the same time interval Pistia sp. exhibited much higher level of CSI reduction as 18.6%, 28.1% and 34.2% at 5, 10, 30 mg/L Cr(VI) solutions, respectively (Table 6). The degradation of chlorophyll under the influence of heavy metal was well documented by the previous researchers (Hashem et al. 2015; Dey and Mondal 2016). Heavy metals such as Cd, Cr, Pb directly interfere with the chlorophyll synthesis either through direct inhibition of enzymatic synthesis or by causing deficiency of an essential nutrient (Meers et al. 2010). In another study conducted by Bajpai and Preti (2012), it was reported that CSI could be a viable method toward understanding of metal stress. Similarly, Begum et al. (2012) reported that CSI should be a good indicator for understanding the status of chlorophyll content under stress condition.
Protein level
Protein is a good indicator of oxidative heavy metal stress in plants (Plata et al. 2009). Protein level under the influence of different concentration of Cr(VI) of both Eichhornia sp. and Pistia sp. is presented in Table 7. From Table 7, it is clear that both the macrophytes exhibit a declining trend of protein level with increase in the concentration of Cr(VI) and different periods of incubation. In comparison, Eichhornia sp. showed much higher level of protein reduction than Pistia sp. This is probably due to the fact that heavy metals always cause stress which generate reactive oxygen species (ROS) and these ROS lead to membrane damage and cell death (Shahid et al. 2014). Almost similar results were reported by Mondal et al. (2015) while recording interference of Hg in mungbean. On the other hand, Tauqeer et al. 2016 and Aldoobie and Beltagi (2013) reported exactly opposite results; that is, protein content increases under lower level of metal stress. However, at higher level of metal stress, protein level decreases which suggest that plant can tolerate up to a certain level of metal.
Conclusion
Plant-mediated remediation of the contaminated water seems to be a good alternative in the long term. Many aquatic plant species have been tested for their potentiality toward bioaccumulation of toxic heavy metals. However, very few macrophytes have shown the ability to accumulate chromium from aqueous medium. The present study shows that both Eichhornia sp. and Pistia sp. effectively accumulate chromium from aquatic medium, but Eichhornia sp. showed higher chromium accumulation at higher concentrations than Pistia sp. Biochemical analysis suggests that both pigment and CSI decrease with increasing chromium concentration, but malondialdehyde concentration increases with increasing chromium concentration for both the tested species. Among the studied enzymes, catalase showed the highest sensitivity toward chromium. On the other hand, chromium bioaccumulation kinetic study revealed that both pseudo-first-order and pseudo-second-order kinetics are better fitted for Pistia sp. than Eichhornia sp. Based on this study, it may be concluded that both Eichhornia sp. and Pistia sp. can be good candidates for phytoremediation of Cr(VI) from contaminated water. Finally, we have to take special care during disposal of used plants. This time we recommend that the used plants should be used for generation of energy and the ash may be utilized in the cement or bricks industry.
Abbreviations
- CSI:
-
Chlorophyll stability index
- Chl:
-
Chlorophyll
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- POD:
-
Peroxidase
- AAO:
-
Ascorbic acid oxidase
- MDA:
-
Malondialdehyde
- TCA:
-
Trichloroacetic acid
- ROS:
-
Reactive oxygen species
- LED:
-
Light emitting diodes
- TBA:
-
Thiobarbituric acid
- AAS:
-
Atomic absorption spectrometry
- DW:
-
Dry weight
- FW:
-
Fresh weight
- RGR:
-
Relative growth rate
- EC:
-
Enzyme commission
- ANOVA:
-
Analysis of variance
- DMRT:
-
Duncan’s multiple range test
References
Aldoobie NF, Beltagi MS (2013) Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. Afr J Biotechnol 12:4614–4622
Amin R, Edraki M, Mulligan DR, Gultom TH (2015) Chromium and nickel accumulation in the macrophytes of the Kawasi wetland on Island, North Maluku Province, Indonesia. Aust J Bot 63(7):549–553
Arnon DI (1949) Copper enzymes in isolated chloroplast. Polyphenol oxidase in beta vulgaris. Plant Physiol 24:1–15
Bajpai R, Preti DK (2012) Accumulation of toxic effect of arsenic and other heavy metals in acontaminated area of West Bengal, India, in the lichane Pyxinecocees (Sw.) NY1. Ecotoxicol Environ Saf 83:63–70
Begum MK, Alam MR, Islam MS, Arefin MS (2012) Effect of water stress on physiological characters and juice quality of sugarcane. Sugar Tech 14(2):161–167
Berti WR, Cunningham SD (2000) Phytostabilization of metals. In: Phytoremediation toxic met using plants to clean up environ, pp 71–88
Bishnoi NR, Dua A, Gupta VK, Sawhney SK (1993) Effect of chromium on seed germination, seedling growth and yield of peas. Agric Ecosyst Environ 47:47–57
Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO (2007) The occurrence of hormesis in plants and algae. Dose-response 5:150–162
Chen NC, Kanazawa S, Horiguchi T, Chen NC (2001) Effect of chromium on some enzyme activities in the wheat rhizosphere. Soil Microorg 55:3–10
Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z et al (2015) Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci Rep 5:13554
Clijsters H, Van Assche F (1985) Inhibition of photosynthesis by heavy metals. Photosynth Res 7:31–40
Dalo E, Sadikaj R, Sahiti H (2019) Assessment of accumulation of heavy metals and lipid peroxidation in common reed (Phragmites australis) in the Albanian Part of Lake Ohrid. J Ecol Eng 20(4):114–120. https://doi.org/10.12911/22998993/102795
Dey U, Mondal NK (2016) Ultrastructural deformation of plant cell under heavy metal stress in Gram seedlings. Cogent Environ Sci 2:1196472
Dhal B, Thatoi H, Das N, Pandey B (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 250:272–291
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Safety 106:164–172
Espinoza-Quiñones FR, Módenes AN, Thomé LP, Palácio SM, Trigueros DEG, Oliveira AP, Szymanski N (2009) Study of the bioaccumulation kinetic of lead by living aquatic macrophyte Salvinia auriculata. Chem Eng J 150(2–3):316–322
Fan Y, Zhu T, Li M, He J, Huang R (2017) Heavy metal contamination in soil and brown rice and human health risk assessment near three mining areas in central China. J Healthc Eng 2017:4124302. https://doi.org/10.1155/2017/4124302
Fariasa DR, Hurdb CL, Eriksenc RS, Macleod CK (2018) Macrophytes as bioindicators of heavy metal pollution in estuarine and coastal environments. Mar Pollut Bull 128:175–184
Gil-Cardeza ML, Ferri A, Cornejo P, Gomez E (2014) Distribution of chromium species in a Cr-polluted soil: presence of Cr(III) in glomalin related protein fraction. Sci Total Environ 493:828–833
Gomez KA, Gomez AA (1984) Statistical procedures for agriculture research, 2nd edn. Wiley, New York
Goswami S, Das S (2016) Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotoxicol Environ Saf 126:211–218
Hashem A, Abd_Allah EF, Alqarawi AA, Aldubise A, Egamberdieva D (2015) Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J Plant Int 10(1):230–242
Hassanein RA, Hashem HA, Khalil RR (2012) Stigmasterol treatment increases salt stress tolerance of faba bean plants by enhancing antioxidant systems. Plant Osmics J 5:476–485
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hibiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defence system and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res Int 22:1534–1544
ISO 20079 (2004) Water quality—determination of the toxic effect of water constituents and waste water to duckweed (Lemna minor)—duckweed growth inhibition test. International Standard ISO 20079, Geneva
Jarvis TA, Bielmyer-Fraser GK (2015) Accumulation and effects of metal mixtures in two seaweed species. Comp Biochem Physiol Part C 171:28–33
Kamal M, Ghaly AE, Mahmoud N, Côté R (2004) Phytoaccumulation of heavy metals by aquatic plants. Environ Int 29:1029–1039. https://doi.org/10.1016/S0160-4120(03)00091-6
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics under Cu stress. Planta 241:847–860
Kontoghiorghe C, Kolnagou A, Kontoghiorghes GJ (2015) Phytochelators intended for clinical use in iron overload, other diseases of iron imbalance and free radical pathology. Molecules 20(11):20841–20872. https://doi.org/10.3390/molecules201119725
Kota J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107(3):263–283. https://doi.org/10.1016/S0269-7491(99)00168-2
Lin L, Chen F, Wang J, Liao M, Lv X, Wang Z, Li H, Deng Q, Xia H, Liang D, Yi Tang, Wang X, Lai Y, Ren W (2018) Effects of living hyperaccumulator plants and their straws on the growth and cadmium accumulation of Cyphomandra betacea seedlings. Ecotoxicol Environ Saf 155:109–116
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Malar S, Sahi SV, Favas PJC, Venkatachalam P (2015) Assessment of mercury heavy metal toxicity-induced physiochemical and molecular changes in Sesbania grandiflora L. Int J Environ Sci Technol 12:3273–3282
Malik B, Pirzadah TB, Tahir I, Rehman RU (2019) Growth and physiological responses in chicory towards mercury induced in vitro oxidative stress. Plant Physiol Rep. https://doi.org/10.1007/s40502-019-00442-2
Medda S, Mondal NK (2017) Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Ann Agrar Sci 15(3):396–401
Meers E, Van-Slycken S, Adria-Ensen K, Ruttens A, Vangrons-Veld J, Witters G, Thewys N, Tack TF (2010) The use of bioenergy crops (Zea mays) for phytoattenuation of heavy metals on moderately contaminated soils: a field experiment. Chemosphere 78(1):35–41
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 34(1):1–32. https://doi.org/10.1080/10590501.2015.1096883
Mishra VK, Tripathi BD (2009) Accumulation of chromium and zinc from aqueous solutions using water hyacinth. J Hazard Mater 164:1059–1063
Mondal NK, Das C, Roy S, Datta JK, Banerjee A (2013) Effect of varying cadmium stress on chickpea (Cicer arietinum L) seedlings: an ultrastructural study. Ann Environ Sci 7:59–70
Mondal NK, Bhaumik R, Dey U, Pal KC, Das C, Datta JK (2014) Flouride remediation using floating macrophytes. Commun Plant Sci 4:23–33
Mondal NK, Das C, Datta JK (2015) Effect of mercury on seedling growth, nodulation and ultrastructural deformation of Vigna radiata (L) Wilczek. Environ Monitor Assess 187(5):1–14
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad Mu, Prakashmaran JV, Gayathri V, Omar K, Duaij A (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359. https://doi.org/10.1007/s10311-018-0762-3
Newman LA, Reynolds CM (2004) Phytodegradation of organic compounds. Curr Opin Biotechnol 15:225–230. https://doi.org/10.1016/j.copbio.2004.04.006
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51(6):730–750
Panda SK, Patra HK (2000) Does Cr III produces oxidative damage excised wheat leaves. J Plant Biol 27(2):105–110
Panse VG, Sukhatme PV (1967) Statistical methods for agricultural workers. ICAR, New Delhi, pp 97–123
Pirson A, Seidel F (1950) Cell metabolism and physiology in Lemna minor root deprived of potassium and calcium, in German (Zell- und stoffwechselphysiologiche Untersuchungen an der Wurzel von Lemna minor unter besonderer Berücksichtigung von Kaliumund Calciummangel). Planta 38:431–473
Plata JS, Villasante CO, Flores-Caceres ML, Escobar C, del Campo FF, Hernandez LE (2009) Differential alterations of antioxidant defenses as bio-indicators of mercury and cadmium toxicity in Alfalfa. Chemosphere 77:946–954
Prasad MNV, Malec P, Waloszek A, Bojko M, Strzalka K (2001) Physiological responses of Lemna trisulca L. (duckweed) to cadmium and copper bioaccumulation. Plant Sci 161:881–889
Rahman MA, Hasegawa H (2011) Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere 83(5):633–646. https://doi.org/10.1016/j.chemosphere.2011.02.045
Reznia S, Taib SM, Md Dim MF, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazards Mater 318:587–599
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2015) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427. https://doi.org/10.1007/s11356-015-5351-4
RoyChowdhury A, Sarkar D, Deng Y, Datta R (2017) Assessment of soil and water contamination at the tab-simco coal mine: a case study. Mine Water Environ 36:248–254. https://doi.org/10.1007/s10230-016-0401-9
Rusina Y, Kaloyan N, Christov L, Petrova P (2004) Antioxidative enzymes in barley plants subjected to soil flooding. Environ Exp Bot 51:93–101
Saddiqe Z, Farooq A, Khan F et al (2015) Effect of Chromium(VI) on physical growth and biochemical parameters of Wheat (Triticum aestivum L.) seedlings. Biologia (Pakistan) 61(2):219–226
Sagar S, Dwivedi A, Yadav S, Tripathi M, Kaistha SD (2012) Hexavalent chromium reduction and plant growth promotion by Staphylococcus arlettae Strain Cr11. Chemosphere 86(8):847–852
Sairam RK, Deshmukh PS, Shukla DS (2008) Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agronomy Crop Sci 178(3):171–178
Sarwar N, Imran M, Shaheen MR, Ishaq W, Kamran A, Matloob A, Rehimb A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shahid ML, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol 232:1–44
Shanker AK (2003) Physiological, biochemical and molecular aspects of chromium toxicity and tolerance in selected crops and tree species. Ph.D. Thesis, Tamil Nadu Agricultural University, Coimbatore
Shanker AK, Cervantes C, Lozatavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753. https://doi.org/10.1016/j.envint.2005.02.003
Sharma SS, Dietz K-J (2009) The relationship between metal toxicity and cellular redox balance. Trends Plant Sci 14:43–50
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetland: a critical review. Miner Eng 19(2):105–116
Shimada Y, Ko S (2008) Ascorbic acid and Ascorbic acid oxidase in vegetables. Chugokuen J 7:7–10
Singh NK, Raghubanshi AS, Upadhyay AK, Rai UN (2016) Arsenic and other heavy metal accumulation in plants and algae growing naturally in contaminated area of West Bengal, India. Ecotoxicol Environ Safety 130:224–233
Singh H, Verma A, Kumar M, Sharma R, Gupta R, Kaur M, Negi M, Sharma SK (2017) Phytoremediation: a green technology to clean up the sites with low and moderate level of heavy metals. Austin Biochem 2(2):1012
Suseela MR, Sinha S, Singh S, Saxena R (2002) Accumulation of chromium and scanning electron microscopic studies in Scirpus lacustris L. treated with metal and tannery effluent. Bull Environ Contam Toxicol 68:540–548
Swarnalatha K, Radhakrishnan B (2015) Studies on removal of Zinc and Chromium from aqueous solutions using water Hyacinth. Pollution 1:193–202
Tang SR, Wilke BM, Brooks RR, Tang SR (2001) Heavy-metal uptake by Metal tolerant Elsholtzia haichinesis and Commelina communis from China. Commun Soil Sci Plant Anal 32(5–6):895–905
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoextraction of heavy metals by Alternanthera beltzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Thijs S, Sillen W, Weyens N, Vangronsveld J (2017) Phytoremediation: state-of-the-art and a key role for the plant microbiome in future trends and research prospects. Int J Phytoremediation 19(1):23–38
Tiwari S, Arya A, Kumar S (2012) Standardizing sterilization protocol and establishment of callus culture of sugarcane for enhanced plant regeneration in vitro. Res J Bot 7(1):1–7
Torok B, Dransfield T (2017) Green chemistry: an inclusive approach, 1st edn. Elsevier, Amsterdam, pp 359–373. https://doi.org/10.1016/B978-0-12-809270-5.00015-7
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land: a review. Environ Chem Lett 8(1):1–17
Vecchia FD, Larocca N, Moro I, Defaveri S, Andreoli C, Rascio N (2005) Morphogenetic, ultra structural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci 168:329–338
Vesely T, Tlustoˇs P, Száková J (2011) The use of water lettuce (Pistia stratiotes L.) for rhizofiltration of a highly polluted solution by cadmium and lead. Int J Phytoremediation 13:859–872
Wang L, Lina H, Dong Y, He Y (2018) Effects of cropping patterns of four plants on the phytoremediation of vanadium-containing synthetic wastewater. Ecotoxicol Environ Saf 115:27–34
Yabanli M, Yozukmaz A, Sel F (2014) Heavy metal accumulation in the leaves, stem and root of the invasive submerged macrophyte Myriophyllum spicatum L. (Haloragaceae): an example of Kadin Creek (Mugla, Turkey). Braz Arch Biol Technol 57:434–440
Yang J, Cao J, Xing G, Yuau H (2015) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and Zinc by Oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol 175:537–544
Zayed A, Pilon-Smits E, de Souza M et al (2000) Remediation of selenium polluted soils and waters by phytovolatilization. In: Terry N, Bañuelos GS (eds) Phytoremediation of contaminated soil and water. CRC Pr, Boca Raton, FL, pp 61–83
Zeid I-M (2001) Responses of Phaseolus vulgaris to chromium and cobalt treatments. Biol Plant 44:111–115
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Zhou GJ, Peng FQ, Zhaug LJ, Ying GG (2012) Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scemedesmus obliquels. Environ Sci Pollut Res 19(7):2918–2929
Acknowledgement
The authors are thankful to all faculty members and non-teaching staff of the Department of Environmental Science, University of Burdwan, West Bengal, India, for providing infrastructural facilities and active moral support toward completion of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article is original and contains unpublished material. The corresponding author confirms that all of the other authors have read and approved the manuscript and no ethical issues involved.
Additional information
Editorial responsibility: Abhishek RoyChowdhury.
Rights and permissions
About this article
Cite this article
Mondal, N.K., Nayek, P. Hexavalent chromium accumulation kinetics and physiological responses exhibited by Eichhornia sp. and Pistia sp.. Int. J. Environ. Sci. Technol. 17, 1397–1410 (2020). https://doi.org/10.1007/s13762-019-02418-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02418-z