Abstract
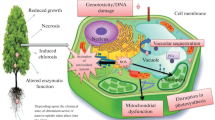
Accumulative contamination of soil with chromium-containing compounds generates many adverse effects in plants. The toxic effect is manifested in the retardation of plant development, lower green biomass, morphological defects, and poorer flower quality and crop yield. Stimulation of reactive oxygen species (ROS) synthesis followed by oxidative stress development is considered to underlie the harmful effects of both Cr6+ and Cr3+. Since a high intensity oxidative stress may cause the inhibition of photosynthetic mechanisms and oxidative modifications of cellular molecules, plants up-regulate various antioxidant and non-antioxidant defense mechanisms to avoid oxidation, maintain low stable-state ROS levels and repair damages that have already occurred in response to Cr(IV) exposure. These mechanisms include: (i) the antioxidant enzymes that directly scavenge ROS; (ii) the non-enzymatic antioxidant low molecular mass molecules (vitamins, terpenoids, phenolics, etc.) which are able to scavenge ROS and repair oxidized molecules, and some of them (ascorbic acid, glutathione) are used as substrates by antioxidant enzymes, in particular in so-called ascorbate-glutathione pathway; (iii) thioredoxins and glutaredoxins to restore oxidized proteins; (iv) metal-binding proteins such as phytochelatins and metallothioneins which sequestrate toxic metals in the specific plant compartments (vacuoles). In this review, we analyze current data on the involvement of redox regulators, particularly Rap2.4a and NPR1, in the regulation of adaptive antioxidant response of plants under Cr exposure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
Metals with variable valence, such as chromium, iron, copper, cobalt, nickel, etc., have a wide practical application in various branches of industry. At the same time, they are one of the most widespread pollutants of the environment, because they are intensively released with industrial emissions and domestic waste (Uddin 2017). Metal retention in soil poses a serious danger to soil organisms, including agricultural plants, reducing their quality and crop yield (Alengebawy et al. 2021; Saud et al. 2022; Wyszkowska et al. 2023). Chromium(Cr) is a particularly dangerous metal pollutant for all organisms, from microorganisms to humans (Kubrak et al. 2010; Vasylkiv et al. 2010; Stambulska et al. 2018; Saud et al. 2022). Chromium can exist in eleven valence states, but the main valence states of Cr in the soil are Cr3+ and Cr6+. Hexavalent Cr compounds are widely used in industry. In particular, Cr6+ compounds are used in the metallurgy (e.g., for the production of different steels, metal materials with high resistance to physical and chemical factors) as well as in the pigment production, leather tanning, etc. (Pellerin and Booker 2000; Shahid et al. 2017). Hexavalent chromium emitted into the atmosphere is airborne for some time in the form of particles or dust. As sediments, it contaminates soil and water. Rain precipitates Cr6+ particles from the atmosphere, depositing them in the soil, or they can be spread by wind for long distances (Yu et al. 2014; Tumolo et al. 2020). Emissions from diesel vehicles are the main source of Cr air pollution, as Cr is a component of diesel fuel (Wang et al. 2003; Yu et al. 2014). It is generally accepted that Cr6+ is more toxic than Cr3+. However, many studies showed that both Cr6+ and Cr3+ can increase dramatically the amount of so-called reactive oxygen species (ROS), triggering oxidative stress in different organisms with various harmful effects (Lushchak et al. 2009; Vasylkiv et al. 2010; Sawicka et al. 2021). Mutagenic and genotoxic effects of Cr6+ and Cr3+ ions are largely a result of ROS-induced damage to DNA (Figgitt et al. 2010; Novotnik et al. 2016; Sawicka et al. 2021).
Plants are able to absorb chromium from the soil and, as a result, excessive accumulation of chromium is detrimental for plants, causing defects in growth and development up to plant death. Chromium toxicity in plants is associated with its inhibitory effect on a number of metabolic processes, in particular photosynthesis, chlorophyll, and protein biosynthesis, as well as with DNA damage (López-Bucio et al. 2015; Stambulska and Bayliak 2020; Wakeel et al. 2020; Kumar et al. 2022; Saud et al. 2022; Wyszkowska et al. 2023).
Unlike animals, which can leave the contaminated area, plants are immobile, so they have evolved powerful defense systems to withstand pollution. In response to chromium exposure, many plants up-regulate a battery of enzymatic and non-enzymatic defenses to sequester chromium, keeping redox balance, preventing oxidative damage and repair damaged biomolecules (Anjum et al. 2017; Stambulska et al. 2018; Stambulska and Bayliak 2020; Kumar et al. 2022). If the capacity of defense systems is not enough to overcome the increase in ROS levels and treat the damage, chromium-induced damage to biomolecules can be significantly enhanced, which is manifested in severe oxidative stress with the corresponding detrimental consequences for plants. It should be noted that chromium toxicity is reduced in the presence of associative or symbiotic microorganisms, e.g. nitrogen-fixing rhizobia, which are able to accumulate heavy and transition metals and thus reduce metal uptake by plants (Stambulska et al. 2018; Sharma et al. 2022).
This chapter summarizes the available information on the mechanisms of toxicity of Cr6+ ions in plants, focusing on their involvement in the generation of ROS and cellular and tissue oxidative injuries. Studies point out that plants with more powerful antioxidant systems are more resistant to soil chromium contamination. Therefore, we have also analyzed in detail the plant antioxidant compounds contributing to prevention and neutralization of Cr toxic effects. The main molecular players of plant responses to redox perturbations are also discussed.
16.2 Reactive Oxygen Species and Reactive Nitrogen Species as Components of Plant Immunity and Energy Metabolism
In living organisms, reactive oxygen (ROS) and nitrogen (RNS) species are products of incomplete reduction of oxygen and nitrogen and are generated as primary products or either byproducts of basic metabolism. In plants, ROS and RNS as side products of metabolism are generated in plastids, mitochondria, peroxisomes, apoplast, and cytosol (Gupta and Kaiser 2010; Luis and Río 2013; Del Rio and Lopez-Huertas 2016; Mittler 2017; Qi et al. 2017). Intracellular ROS/RNS homeostasis is rigorously managed to sustain proper cellular functions by a wide range of non-enzymatic (vitamins (A, E, K) organic acids, glutathione, ionic metals (Fe2+, Cu2+, Zn2+, and Mn2+), phenolic and terpenoid compounds), and enzymatic scavengers (superoxide dismutase (SOD), catalase (CAT), enzymes engaged in the reduction-oxidation of non-enzymatic antioxidants (glutathione and ascorbic acid), amine oxidase, etc.) (Chaudiere and Ferrari-Iliou 1999, Apel and Hirt 2004; Choudhury et al. 2017; Mur et al. 2013; Cadet and Davies 2017; Samardzic and Rodgers 2017).
The main ROS formed by the plant cells are the typical for aerobic organisms and include hydrogen peroxide (H2O2), hydroxyl radicals (OH·), superoxide anions (\({\text{O}}_{{2}}^{ \cdot - }\)), and singlet oxygen (\(^{{1}} {\text{O}}_{{2}}\)). They can be signaling molecules in plants, controlling various physiological processes—differentiation, senescence and death of the cells, pathogen defense, acclimation, and abiotic stress survival (Foyer and Noctor 2005; Gechev et al. 2006; Di Meo et al. 2016; Dunnill et al. 2017).
In photosynthesizing chloroplasts of green organs, three main ROS-generated processes can be distinguished (Fig. 16.1). First one is ROS generation in photosystem II via overexcitation of chlorophyll (Fryer et al. 2003). The Mehler reaction in photosystem I, in terms of which O2 accepts one electron converting to \({\text{O}}_{{2}}^{ \cdot - }\), is a second process leading to ROS generation (Badger et al. 2000; Ort and Baker 2002). The third process is related to the Rubisco by the substantial production of H2O2 by glycolate oxidase (Douce and Neuburger 1999). Glycolytic acid also undergoes oxidation in peroxisomes, and this is accompanied by H2O2 release. Even under favorable conditions, a certain amount of oxygen formed in chloroplasts is metabolized into ROS (Asada 1999). One of the major producers for H2O2 are peroxisomes (Noctor et al. 2002). Peroxisomal membrane NADPH oxidase and peroxisomal matrix xanthine oxidase produce \({\text{O}}_{{2}}^{ \cdot - }\) radicals that are quickly reduced to H2O2 (Del Rio and Lopez-Huertas 2016). Enzymes, such as superoxide dismutase and different oxidases (sarcosine, flavin, sulfite copper amine ones) can produce H2O2 in peroxisomes (Gilroy et al. 2016; Hasanuzzaman et al. 2020a, b). While in animals, mitochondria are the main producer of ROS, in plants chloroplasts do this function. The rate of chloroplast ROS formation is 20 times higher than mitochondrial one (Foyer and Noctor 2003). At low levels, ROS are responsible for the coordinated functioning of cellular organelles, cellular homeostasis, and adaptability to rapidly changing external conditions (Gechev et al. 2006). Mitochondrial respiratory complexes I and III are the major sites for the emergence of ROS, superoxide, and H2O2, but the factors that lead to the intensification of ROS production in mitochondria are mostly unknown (Rhoads et al. 2006). In apoplast, oxalate oxidase, amine oxidase, and peroxidases can produce ROS (You and Chan 2015; Mittler 2017; Qi et al. 2017).
Schematic overview of the ROS generating sites in the plant cell and their activity in various cellular components. The model is based on results described by various authors (Karuppanapandian et al. 2011; Hasanuzzaman et al. 2020a, b; Mandal et al. 2022). In chloroplasts, during photosynthesis, sunlight energy is captured and transferred to photosystem I (PS1) and photosystem II (PS2), where superoxide radical (\({\text{O}}_{{2}}^{ \cdot - }\)) can be formed. Then \({\text{O}}_{{2}}^{ \cdot - }\) is converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Under excess light conditions, PS2 is able to generate singlet oxygen (\(^{{1}} {\text{O}}_{{2}}\)). The glycolate (CLO), produced in chloroplasts, moves to peroxisomes, where it is oxidized by glycolate oxidase (GOX) with formation of H2O2. In peroxisomes, H2O2 can also be formed from O2 by xanthine and flavin oxidases (XO and FOX, respectively) coupled to SOD. In the mitochondrial electron transport chain, \({\text{O}}_{{2}}^{ \cdot - }\) production is likely to occur in complex I—the flavoprotein region of NADH dehydrogenase segment reduces O2 into superoxide \({\text{O}}_{{2}}^{ \cdot - }\). Acyl-CoA oxidase (ACO), GOX, peroxidases (POX), and SOD are the primary enzymes responsible for \({\text{O}}_{{2}}^{ \cdot - }\) and H2O2 generation in glyoxysomes. The endoplasmic reticulum-mediated ROS generation takes place by means of cytochrome P450. Cell-wall-associated peroxidase and SOD are the main sources of \({\text{O}}_{{2}}^{ \cdot - }\) and H2O2 in apoplast of plant cells. In the presence of redox-active metals, the extremely reactive hydroxyl radical (·OH) can be formed from H2O2 through the Fenton reaction (F). Other abbreviations used: CAT, catalase; GLO, glycolate; GLT, glycerate; Gly, glycine; PG, phosphoglycolate; RBP, ribulose 1,5-bisphosphate; Ser, serine; SG-P, sugar-phosphates
In addition, the excessive production of ROS is an effective component of the plant immunity under pathogenic infections. In plant–pathogen interactions, the major site of elevated ROS levels is the apoplast (Qi et al. 2017). NADPH oxidase produces of ROS (\({\text{O}}_{{2}}^{ \cdot - }\) and H2O2) and thus provides innate plant immunity. Also, the redox networks of cells (peroxiredoxins, thioredoxins, glutaredoxins) affect very strongly ROS levels. The enhanced ROS production reduces bacterial and fungal growth (Suzuki et al. 2011; Mittler et al. 2011). The increasing concentration of ROS can cause increase in synthesis of phytoalexins and other secondary metabolites which have defensive roles (Thoma et al. 2003). ROS may limit the spread of pathogens or play a signaling function (Mur et al. 2008).
Reactive nitrogen species (·NO, ONOO–, ·NO2), like ROS, are signal molecules in plants. S-nitrosation and nitration are the main RNS-modifying mechanisms of plant cell behavior (Kharma et al. 2019; Corpas and Palma 2020; Corpas et al. 2021). S-Nitrosation is the reaction between ·NO and cysteine, giving S–N=O bound. This mechanism is important for posttranslational modification of proteins and ·NO signal transmission. As a result of alterations in the functional activity of proteins, changes also occur in the proteome of the cells (Corpas and Palma 2020). For example, S-nitrosoglutathione GS–N=O) is obtained as a result of glutathione S-nitrosation. The irreversible transformation of GS–N=O into oxidized glutathione (GSSG) occurs with the participation of S-nitrosoglutathione reductase (GSNOR) (Corpas et al. 2021). Then glutathione reductase catalyzes NADPH-dependent reduction of GSSG to GSH (Fig. 16.2).
S-nitrosylation of reduced form of glutathione (GSH) in plant cells. More details in the text. GSNO, S-nitrosoglutathione; GSNOR, S-nitrosoglutathione reductase; GSSG, oxidized form of glutathione; GR, glutathione reductase; Pr-SH, thiol-containing proteins; Pr-SNO, S-nitroso-containing proteins; NADH, nicotinamide adenine dinucleotide (reduced form); NAD+, nicotinamide adenine dinucleotide (oxidized form); NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); NADP+, nicotinamide adenine dinucleotide phosphate (oxidized form)
S-nitrosoglutathione reductase determines cellular concentrations of GSNO (Feechan et al. 2005), since this metabolite is a stable supply of ·NO in plant cells. Nitric oxide performs immune functions, since cellular ·NO concentrations enhance salicylic acid levels (SA, plant immune activator) followed by the increased expression of SA-dependent genes (Feechan et al. 2005; Loake and Grant 2007). Salicylic acid modulates the activity of proteins (SA binding protein 3, SABP3) that perform immune functions in plants (Slaymaker et al. 2002; Kumar and Klessig 2003). Therefore, ·NO is a key regulator of SA-dependent immune responses against pathogens.
Nitration is an addition of a nitro group (–NO2) to different biomolecules, especially proteins. The result of this type of protein nitration is a formation of nitrated amino acid residues (e.g., tyrosine, cysteine, tryptophan, methionine). These chemical modifications entail a loss of functional activity of the entire protein molecule (Corpas and Palma 2020). In addition, the interaction of metal ions with ·NO (metal nitrosylation) can occur in metalloproteins. Such an interaction can also lead to changes in protein metabolic activity (Corpas and Palma 2020). Reactive nitrogen species react also with fatty acids. Nitro-fatty acids act as messenger molecules, that modulate gene expression during developmental processes and stress events (Di Fino et al. 2021; Kolbert et al. 2019; Medrano-Macías et al. 2022).
16.3 Antioxidant System of Plants: Overview
As described above, ROS/RNS and other reactive species are continually being formed in plants as by-products of photophosphorylation and respiration. Reactive species are highly damaging molecules that rapidly oxidize protein and lipid molecules, nitrogen bases in DNA, exerting a strong damaging effect on individual organelles, cells, organs and, the plant organism as a whole. Therefore, cells have evolved sophisticated defense mechanisms to neutralize ROS/RNS. At the same time, complete neutralization of ROS/RNS does not occur, as they perform signaling functions within a single cell and at a distance. ROS/RNS regulate some cell functions and processes such as cell division, regeneration, organogenesis, cell death, immune responses, resistance to UV radiation, etc. Temporary increase of ROS/RNS levels is a component of plant immune defense against disease agents (viruses, microbes, fungi, parasites), mechanical injuries, and physical stressors (water deficit, temperature changes, etc.) (Hasanuzzaman et al. 2020a, b; Mandal et al. 2022). Thus, the cell strictly regulates the synthesis and degradation of ROS in accordance with its needs, so that the level of reactive forms allows for the effective performance of their regulatory functions and the amount of oxidative damage caused by them is minimized. Maintenance of low stationary ROS/RNS concentrations is achieved through the regulation of systems that produce reactive species, as well as systems that scavenge reactive species and restore /degrade oxidatively damaged biomolecules.
In plant cells, the system that protects against damaging effects of ROS/RNS is multilevel and includes: (1) antioxidant enzymes that are able directly to neutralize ROS/RNS—superoxide dismutases and various peroxidases; (2) ascorbate–glutathione cycle enzymes, destroying H2O2 and utilizing NADPH as a donor to reduce oxidized glutathione; (3) enzymes involved in NADPH generation (e.g., ferredoxin-NADP-reductase); (4) non-enzymatic low molecular mass antioxidants, such as redox-active vitamins, glutathione, phenolic compounds, terpenoids etc.; (5) enzymes that reduce peroxides and thiol-containing compounds (glutathione reductase, thioredoxin reductase, etc.); (6) enzymes involved in detoxification of xenobiotics (e.g., glutathione-S-transferase); (7) peptides/proteins capable of binding metals via SH-groups (phytohelatins and metallothioneins); (8) heat shock proteins (HSPs) involved in formation of the native protein structure (folding) and restoring native structure of denatured proteins (Cobbett and Goldsbrough 2002; Michalak 2006; Anjum et al. 2017; Kapoor et al. 2019; Jedelská et al. 2020; Stambulska and Bayliak 2020; Fedenko et al. 2022). Since ROS and RNS are synthesized in different organelles and cytosol of the plant cell, a sophisticated network of defense mechanisms is present not only in the cytosol but also in the organelles of the plant cell, especially in mitochondria, chloroplasts, and peroxisomes (Geigenberger et al. 2017; Noctor et al. 2018; Martí et al. 2020). Also, the composition of the antioxidant system is slightly distinct in different parts of the plant (roots, flowers, fruits, leaves).
16.4 Low-Molecular Mass Antioxidants
16.4.1 Tocopherols
Tocopherols (collectively called vitamin E) are a lipophilic compounds that are synthesized in thylakoid membranes of plastids; therefore, they are found mostly in the green plant organs. There are four isomers of tocopherol (α, β, γ and δ) (Szewczyk et al. 2021). According to their antioxidant activity, the α isomer is the most active and δ one has the lowest antioxidant activity. The antioxidant activity is related to degree of methylation (Dumanović et al. 2021). Chromanol ring of tocopherols possesses the ability easily to donate elections to reduce oxidized molecules that determines the antioxidative effects of this vitamin. Due to their structure, tocopherols and tocotrienols are incorporated into the phospholipid bilayer of cell membranes, where they perform both antioxidant and structural functions (Szewczyk et al. 2021). The membrane of chloroplasts contains predominantly α-tocopherol; therefore, green parts of the plant are rich in α-tocopherol. At the same time, seeds contain mainly γ-tocopherol (Dumanović et al. 2021). In chloroplasts, tocopherols protect membrane lipids against oxidation by trapping singlet oxygen \(^{{1}} {\text{O}}_{{2}}\), thus ensuring the normal functioning of photosystem II. The 1O2 scavenging activity of tocopherols is extremely effective: in vitro it was calculated that more than 100 molecules of \(^{{1}} {\text{O}}_{{2}}\) can be neutralized by one molecule of α-tocopherol until the latter degrades (Gill and Tuteja 2010). Alpha-tocopherol also interrupts lipid peroxidation in plasma and intracellular membranes, reacting with the lipid radicals (LO·, L· and LOO·), which arise from the oxidation of polyunsaturated fatty acids at the membrane-water surface (Dumanović et al. 2021). By giving protons to a radical molecule, tocopherol is converting to a tocopheroxyl radical, which is not reactive enough to initiate membrane peroxidation by itself (Das and Roychoudhury 2014). In model plant Arabidopsis thaliana, inactivation of genes, encoding enzymes of tocopherol biosynthesis, led to a strong oxidative state and lowered content of photosynthetic pigments in green plant parts (Semchuk et al. 2009, 2011). In plants, alpha-tocopherol was also found to perform protective functions contributing to the tolerance to salt stress (Semchuk et al. 2012), high/low temperature (Bergmüller et al. 2003), and xenobiotics (Semchuk et al. 2011).
16.4.2 Ascorbic Acid
Ascorbic acid, or ascorbate, is a common compound found in all cellular compartments. The concentration of ascorbate (AsA) is the highest in cytosol but is also substantial in chloroplasts (Smirnoff and Wheeler 2000). Under physiological conditions, AsA is implicated in photosynthesis serving a cofactor of enzymes (including those involved in the synthesis of phytotohormones and phenolic compounds) and in the control of the cell growth. Some fruits (e.g. black currant, pepper) show a high concentration of ascorbic acid, which may be the result of its compartmentalization and stabilization in acidic vacuoles (Smirnoff and Wheeler 2000).
Glucose, synthetized in chloroplasts during photosynthesis, is a precursor of ascorbic acid. Metabolic conversion of glucose occurs mostly via formation of GDP-mannose first and then L-galactose, which is oxidized to L-galactono-1.4-lactone with conversion of the latter to AsA by specific dehydrogenase located in mitochondrial membrane (Siendones et al. 1999). Plant mitochondria are involved in both de novo synthesis of AsA and the regeneration of oxidized forms of AsA such as dehydroascorbic acid (Gill and Tuteja 2010). Ascorbate is considered to be the most powerful ROS scavenger due to its ability to easily give electrons and protons. It is able to directly react with \({\text{O}}_{{2}}^{ \cdot - }\), H2O2 and ·OH, converting them to non-radical molecules, thus contributing to maintenance of membrane integrity. In addition, ascorbate regenerates α-tocopherol by reducing its oxidized form—tocopheroxyl radical. Moreover, ascorbate is a component of the ascorbate-glutathione cycle, described in more detail below. In reduced form, AsA maintains the activity of enzymes containing transition metal ions as prosthetic groups, preventing the oxidation of these metal ions (Gill and Tuteja 2010). Important function of ascorbate is serving as cofactor for several peroxidases and other enzymes like methionine sulfoxide reductases (Noctor et al. 2018). AsA-dependent enzymes are called ascorbate peroxidases (APXs). They are polygenic group of proteins. In particular, there was identified eight genes encoding APXs in Arabidopsis (Narendra et al. 2006) and 11 APX genes in poplar tree (Leng et al. 2021).
Plants with higher ascorbate content demonstrated the enhanced tolerance to stressors whose action leads to oxidative stress (Ding et al. 2009; Akram et al. 2017). Exposure to heavy metals was found to decrease ascorbate levels with increasing content of its oxidized forms (dehydroascorbic acid) (Gill and Tuteja 2010) In particular, Cd exposure decreased ascorbate levels in Cucumis sativus chloroplasts (Zhang et al. 2002), leaves of A. thaliana (Skórzyńska-Polit et al. 2003) and pea (Rodríguez-Serrano et al. 2006), roots and nodules of Glycine max (Balestrasse et al. 2008). Exposure to hexavalent chromium decreased ascorbate levels in tomato roots, while the addition of exogenous ascorbate improved tolerance of tomatoes to Gr (Al-Huqail et al. 2020). Decrease in ascorbate levels under Cr and Cd exposure was shown to be connected with up-regulation of APXs which use AsA a substrate to counteract a toxicant-stimulated ROS production (Sinha et al. 2018).
16.4.3 Glutathione
Glutathione (GSH) is a tripeptide (L-γ-glutamyl-L-cysteinylglycine) found in all living organisms, including plants. It is synthesized by γ-glutamylcysteine synthetase and glutathione synthetase (Lushchak 2012). In plants, glutathione synthesis occurs in the chloroplasts and cytosol and then GSH can be recruited also in other organelles, including nucleus (Dumanović et al. 2021). In plant cells, 85–90% of GSH is cytosolic (Kosakivska et al. 2021). Glutathione has two forms in the cell, an reduced one (GSH) and an oxidized one formed by disulfide binding of two molecules of glutathione (GSSG) (Noctor et al. 2018). In the cell, glutathione is largely present as GSH, when GSSG consists of about 1% of the total content (Lushchak 2012). For the cell, it is important the GSH/GSSG ratio which determines whole redox status of the cell. In plants, excessive ROS production may cause oxidation of GSH and decease in GSH/GSSG levels indicating more oxidative state of the cell (Queval et al. 2011).
Glutathione has a variety of functions in the cell; in particular, as we mentioned above, it is a key maintainer of redox cellular state in the range of values essential for adequate functioning of redox-sensitive signaling pathways controlling processes connected with growth and aging (seed development, formation of organs and fruits, senescence) (Noctor et al. 2018). This is evidenced by growth retardation and higher vulnerability to oxidant compounds in A. thaliana mutants, defective in glutathione synthesis (Cobbett and Goldsbrough 2002). As an antioxidant, GSH can directly react with ROS such as hydroxyl radical (HO·) or peroxynitrite (ONOO−) (Lushchak 2012) Also, GSH is involved in eliminating peroxides (H2O2, organic peroxides) with the help of appropriate enzymes and is involved in the regeneration of the oxidized form of AsA, which is formed during H2O2 reduction (Foyer and Halliwell 1976). In detail, ascorbate-glutathione cycle is described in the section below. Glutathione is also able to conjugate with molecules having free SH-groups with forming respective disulfides in a reaction called S-glutathionylation (Lushchak 2012). So, GSH reacts with cysteine-rich proteins. This process is reversible and affects the protein function. In particular, S-glutathionylation inhibited the activity of the chloroplastic glyceraldehyde-3-phosphate dehydrogenase, an enzyme of the Calvin-Benson cycle in plants (Zaffagnini et al. 2007). S-glutathionylation is accepted to be a cellular protective strategy to protect cysteine residues in proteins against irreversible oxidation (Lushchak 2012). GSH is a substrate of glutathione-S-transferases to conjugate and eliminate different xenobiotics (Lushchak 2012). Also, GSH can interact with certain metal ions, especially cadmium, copper, zinc, mercury, arsenic, and lead (Lushchak 2012; Kosakivska et al. 2021). Chelating Cu2+ ions by GSH prevented their participating in the Fenton and Haber-Weiss reactions. In addition, GSH seems to be involved in Cr6+ reduction to Cr3+ that is considered as a way to decrease chromium toxicity (Lushchak 2012). However, some studies proposed that CSH participates in detoxification of heavy metals not directly but as a precursor molecule for phytochelatins (Cobbett and Goldsbrough 2002; Kosakivska et al. 2021).
Short term treatment of certain plants (maize, tomato and cauliflower) with Cr6+ increased GSH levels in the leaves and roots (di Toppi et al. 2002). Enhanced GSH levels were also reported in sorghum (Sorghum bicolor L.) treated with Cr6+ (Kumar et al. 2019). Some studies report that supplementation with exogenous glutathione improved plant growth and tolerance to Cr toxicity (Zeng et al. 2012; Sharma et al. 2020; Wen et al. 2022). The addition of exogenous GSH improved the growth of soybean and rice exposed to chromium and increased antioxidant capacity in the treated plants (Zeng et al. 2012; Wen et al. 2022). Treatment with exogenous glutathione resulted in chromium accumulation in the roots of plants rather than in the green part. This indicates that glutathione compartmentalizes chromium ions in plants, thus reducing chromium toxicity (Zeng et al. 2012; Wen et al. 2022).
16.4.4 Carotenoids
Carotenoids are isoprenoid polymeric compounds with diverse roles in plant metabolism, including those related to confer resistance to oxidative stress. The known carotenoids include lutein, β-carotene, zeaxanthin, lycopene, etc. (Pérez-Gálvez et al. 2020). As photosynthetic pigments, carotenoids absorb light at 400–50 nm and transfer it to chlorophyll (Havaux et al. 1998). Secondly, they protect the photosynthetic apparatus by quenching singlet oxygen \(^{{1}} {\text{O}}_{{2}}\) and ·OH formed during light stage of photosynthesis (antioxidant function) (Stahl and Sies 2003). Antioxidant function of carotenoids also include prevention of the formation of \(^{{1}} {\text{O}}_{{2}}\) by reacting with excited chlorophyll (Chl∗) and breakdown of the chain reactions of lipid peroxidation (Das and Roychoudhury 2014). Thirdly, they as lipid-soluble molecules are components of the photosystem I and provide stability of the proteins of the photosynthetic complex and the thylakoid membrane as a whole (structural role) (Gill and Tuteja 2010). In Capsicum annuum, exposure to Cr-containing compounds was reported to result in increased carotenoid levels (Sharma et al. 2020). This suggests that carotenoids can contribute to Cr tolerance in plants.
16.4.5 Phenolic Compounds
Phenol compounds are ubiquitous in plant kingdom. Up to now, there are identified over eight thousand phenolic compounds in plants. Depending on the structure, phenolic compounds are divided into several classes: simple phenols, phenolic acids (gallic acid, caffeic acid, ferulic acid. etc.) stilbenes (e.g., resveratrol), lignans, coumarins, and flavonoids (including simple flavonoids (e.g., quercetin, luteolin, apigenin, catechins) and condensed tannins (Dai and Mumper 2010). Phenols are secondary metabolites in plants and are synthetized from intermediates of carbohydrate metabolism. In the cell, phenols are mainly accumulated in vacuoles, as well as in apoplast. Phenolic compounds confer pigmentation of flowers and fruits, germination of pollen, defense against UV radiation and different pathogens (Das and Roychoudhury 2014).
Plant phenols are well-known as powerful antioxidants. Antioxidant properties of plant phenols include:
-
(i)
direct scavenging of free radicals (superoxide radical anion \({\text{O}}_{{2}}^{ \cdot - }\), hydroxyl radical ·OH, peroxynitrite ONOO−);
-
(ii)
inhibition of lipid peroxidation through interaction with peroxide radicals (RO·, ROO·) and reduction of hydroperoxides (ROOH);
-
(iii)
quenching of excited molecules (e.g., singlet oxygen \(^{{1}} {\text{O}}_{{2}}\));
-
(iv)
chelation of transition metal ions through aromatic hydroxyl groups (prevention of the Fenton reaction);
-
(v)
reduction of oxidized low molecular weight antioxidants (e.g., tocopheroxyl radical);
-
(vi)
direst binding followed modulation of pro-/antioxidant enzymes, as well as the impact on cellular signaling pathways at biochemical and molecular level (Rice-Evans et al. 1997; Michalak 2006; Dai and Mumper 2010; Bayliak et al. 2016b; Fedenko et al. 2022).
Similarly, to other non-enzymatic antioxidants (e.g., ascorbic acid and carotenoids), plant phenols may also demonstrate pro-oxidant properties, which depend on many external factors—pH, oxygen availability and presence of transition metals (Dai and Mumper 2010; Bayliak et al. 2016a, b). Exposure to heavy metals, including Cr6+, leads to increased levels of phenolic compounds in the treated plants, suggesting a defensive role of phenolics against Cr toxicity (Michalak 2006; Stambulska and Bayliak 2020; Alsherif et al. 2022).
16.5 Protein Non-enzymatic Antioxidants
16.5.1 Metal-Binding Proteins
In animals and fungi, heavy metals (HM) induce the increase in content of metal-binding proteins so-called metallothioneins (Zhou and Goldsbrough 1994). Metallothioneins are small Cys-rich proteins that binding HM make them metabolically inactive, conferring heavy metal tolerance. Like animals and fungi, plants possess several metal-binding proteins, which function to sequestrate metals with variable valence (Cobbett and Goldsbrough 2002; Pal and Rai 2010). Among the plant metal-binding proteins, the phytochelatins (PCs) and metallothioneins (MTs) are the best described (Cobbett and Goldsbrough 2002). PCs are enzymatically synthesized peptides, whereas MTs are proteins. PCs are a polymer based on glutathione monomers. The main role of PCs is to detoxify xenobiotic metals (Ag, Cd, Hg) and maintain essential metal homeostasis (Cu, Zn, Se, Ni) (Cobbett and Goldsbrough 2002; Kosakivska et al. 2021). It should be noted that overexpression of phytochelatin synthase gene confers contrast tolerance to HM in transgenic A. thaliana, in particular, both enhanced cadmium (Cd) tolerance and Cd hypersensitivity was observed dependently on experimental conditions (Wang et al. 2012). Also, phytochelatin synthase had contrasting effects on cadmium and arsenic accumulation in rice grains dependently on experimental conditions (Uraguchi et al. 2017).
Plant MTs are low molecular mass proteins, rich in cysteine residues and are capable of binding mono-or divalent metal ions, in particular Cu ions (Domènech et al. 2006). Moreover, a correlation between MT gene expression and copper tolerance has been observed in A. thaliana (Murphy and Taiz 1995). Plants contain several types of MT which are distinguished by their structure and subcellular localization (MT1-MT4) (Kosakivska et al. 2021). In sorghum, Cr stress was shown to induce MT transcription but did not induce PC synthesis, suggesting a role of MT in Cr detoxification (Panda and Choudhury 2005). On contrary, another study showed that the PC content was significantly up-regulated in green parts of plants exposed to Cr6+. Also, plants with higher PC content accumulated chromium predominantly in roots and cell walls, that was proposed to be a mechanism improving plant tolerance to Cr (Saud et al. 2022).
16.5.2 Heat Shock Proteins
Heat shock proteins (HSPs), termed also chaperons, are ubiquitous in plant and animal cells. They were originally identified as proteins whose levels increase in the response to heat stress (Vierling 1991; Park and Seo 2015). To date, it has been established that the functions of HSPs are much broader. The integrity of cells is maintained by heat shock proteins which participate in correct formatting of native structure of de novo synthesized proteins, restoration of structure of misfolded or denatured proteins, preventing aggregation of damaged proteins and directing to degradation of the proteins which cannot be repaired under different stresses (Vierling 1991; Park and Seo 2015; Waters and Vierling 2020). Increased HSP90 was found in rice seedlings which were exposed to Cr6+ (Zeng et al. 2014). It was shown that copper, cadmium and chromium induced the synthesis of HSPs in A. thaliana (Cui et al. 2019; Appiah et al. 2021).
16.6 Enzymatic Antioxidant Defense
16.6.1 Thioredoxins and Glutaredoxins
Thioredoxins (Trx) and glutaredoxins (Grx) belong to families of small oxidoreductases, containing SH groups in the active site, that enable the reduction of disulfide intramolecular bridges in a specific set of proteins (Meyer et al. 2012). Thus, like glutathione, these proteins help to maintaining the more reduced state of the cell. Proteomic approaches identified the putative Trx and Grx target proteins, which are components of many physiological processes and stress adaptive responses in plants (Meyer et al. 2012; Martí et al. 2020). Transgenic plants defective in Trx and Grx allow to identify that these redox proteins are components combating with pathogen and are induced by pathways mediated by phytohormones (Meyer et al. 2008, 2012).
In plants, the thioredoxin and glutaredoxin families include a large number of isoforms. Thus, at least 20 genes encoding thioredoxins and about 50 genes encoding glutaredoxins are identified in A. thaliana (Gelhaye et al. 2005; Meyer et al. 2008; Martí et al. 2020). Thioredoxins and glutaredoxins are classified depending on their primary structure and subcellular localization. By reducing intramolecular disulfide bonds in target proteins, Trx and Grx are oxidized themselves, forming intramolecular disulfides. Plant cells use different ways to reduce disulfide bounds to free thiol forms in the oxidized Trx/Grx. In chloroplasts, oxidized Trx can be reduced by a ferredoxin-thioredoxin reductase, whereas NADPH-dependent thioredoxin reductases catalyze reduction of Trx in mitochondria, nucleus, and cytoplasm (Smiri et al. 2010; Jedelská et al. 2020). Oxidized Grx can be reduced by glutathione (Meyer et al. 2008). Besides that, Grx is able to reduce oxidized Trx (Gelhaye et al. 2005). Diversity of metabolic pathways of regeneration of oxidized thioredoxins identified emphasizes the important role of these proteins in protection against stressors, especially oxidants (Gelhaye et al. 2005; Jedelská et al. 2020). Knock-out mutants in specific chloroplastic Trx showed defects in chloroplast structure and were more susceptible to stressors associated with increased ROS generation (Noctor et al. 2018). Heavy metals were found to affect thioredoxin reductase/thioreoxin system (Smiri et al. 2010, 2013). Moreover, it was shown that Trx possesses metal-chelating property, in particular regarding Cd2+ (Smiri and El Ghoul 2012).
16.6.2 Antioxidant Enzymes
Enzymatic antioxidant defense system includes of several antioxidant enzymes such as superoxide dismutases, catalases, and peroxidases (glutathione-ascorbate-dependent, GPx and APX, and non-specific for substrates ones, POX); enzymes of GSH and AsA redox metabolism—glutathione reductase (GR), glutathione S-transferase (GST), monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR).
Superoxide dismutase (SOD) is one of the most effective enzymes for protecting plant cells from \({\text{O}}_{{2}}^{ \cdot - }\) toxicity. Three main types of this metalloenzyme are described in plants—Cu, Zn-SOD, Mn-SOD, and Fe-SOD ones. The SOD isoforms differ in protein structure and subcellular distribution. Fe-SOD is located in plastids, Mn-SOD is in mitochondria, apoplast, and peroxisomes, and Cu, Zn-SOD occurs in peroxisomes, cytosol, and plastids (Pilon et al. 2011; Miller 2012). By catalyzing of \({\text{O}}_{{2}}^{ \cdot - }\) dismutation to H2O2, SODs reduce the probability of oxidizing effects of the superoxide radical and the likelihood of its interaction with transition metal ions with the appearance of ·OH (Gill and Tuteja 2010).
Catalase is the heme-containing oligomeric enzyme, which catalyzes disproportionation of the product of the SOD reaction H2O2 to water and oxygen. In this reaction, one molecule of H2O2 is a donor of elections and the second is the electron acceptor. Catalase (CAT) activity has been detected in mitochondria, peroxisomes, and cytosol of different plants (Sofo et al. 2015). Catalase isozymes exhibit developmental stages and tissue specificity in plants. The one CAT molecule can convert 6 million H2O2 molecules per minute (Mehla et al. 2017). This is the maximum rate of substrate transformation into a product among antioxidant enzymes.
Hydrogen peroxide can be also scavenged by different peroxidases, which use H2O2 for reduction of different substrates. Phenol peroxidase (POX) can generate phenoxyl radical (PhO·) by oxidizing phenols with concomitant H2O2 reduction to water, and two molecules of H2O are formed in this reaction. The polyphenol oxidase (PPO) found in the chloroplast thylakoid membrane can then interact with peroxidase, which helps to scavenge ROS. In PPO-catalyzed reaction, reduced phenols are oxidized to the quinone QA and H2O (Boeckx et al. 2015).
Glutathione peroxidase (GPX) is the non-heme peroxidase family and protects cells from oxidative damage by reducing of the lipid hydroperoxides (LOOH) and H2O2 into non-toxic products or H2O (Bela et al. 2015). GPX has been found in many cellular compartments (Koua et al. 2009). Currently, the functions of plant GPXs, which contain Cys residues in their active sites, are not completely understood. In stress conditions, GPXs can maintain H2O2 homeostasis, modify nuclear signaling proteins, and contribute to crosstalk between different signaling pathways (immune responses to pathogens, H2O2-mediated and the abscisic acid-mediated signaling). The plant GPXs also contribute to hormone-stimulated growth of main and lateral roots, plant regeneration, flower- and seed development, development of leaves (Bela et al. 2015).
Glutathione reductase (GR) is a protein that reduces of GSSG to the sulphydryl form GSH (Hasanuzzaman et al. 2017). In higher plants, three types of GR are predominantly localized in chloroplasts (about 80% of total CR activity), but its small amount is also found in peroxisomes, mitochondria, and cytosol (Edwards et al. 1990). In plants, maintaining a high ratio GSH/GSSG by chloroplastic GR is necessary important for normal oxygenic photosynthesis (Kornyeyev et al. 2003). Different GR types are activated by specific abiotic signals that ensure the specificity of the plant responses to stressors (Stevens et al. 1997).
Glutathione S-transferases (GST) are group of detoxification enzymes regulating different functions in plant cells such as the elimination of toxic substances, mitigation of oxidative stress, tyrosine degradation and hormone transport (Oakley 2011; Dong et al. 2016). Some GSTs can also scavenge toxic lipid hydroperoxides. GSTs catalyze conjugating of glutathione with metals/metalloids to reduce their toxicity (Dixon et al. 2011). In plant development, glutathione S-transferases also have functions, comprising formation of ion channels, modulation of regulatory kinases, and the glutathionylation of proteins (Dixon et al. 2010). GSTs are found in mitochondria, cytosol, and microsomes. In contrast to mitochondrial and cytosolic forms of GSTs, microsomal GSTs are identified as the integral membrane proteins that participate in eicosanoid and glutathione metabolism. Expression of GSTs was shown can be induced by fungal elicitors and pathogen attack, cold, drought, hormone treatments, phosphate starvation, heavy metals, salt, and wounding (Kim et al. 2011; Chen et al. 2013; Xu et al. 2015; Shukla et al. 2015; Jia et al. 2016).
Ascorbate peroxidase (APX) is a heme-containing peroxidase which scavenges H2O2 in the presence of ascorbate (Hasanuzzaman et al. 2019). APX has several isoforms with different cellular localization—cytosolic (cAPX), mitochondrial (mitAPX), chloroplastic (chAPX), and peroxisomal/glyoxisomal (mAPX) ones (Del Rio and Lopez-Huertas 2016). All APX isoforms participate in detoxifying H2O2 with oxidation of AsA to monodehydroascorbate (MDHA) and then to dehydroascorbate (DHA) (Fig. 16.3).
The glutathione-ascorbate cycle in the chloroplasts (Foyer-Halliwell-Asada pathway). APX, ascorbate peroxidase; AsA, ascorbate; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; GSH, reduced form of glutathione; GSSG, oxidized form of glutathione; GR, glutathione reductase; MDHA, monodehydroascorbate; MDHAR, monodehydroascorbate reductase
Monodehydroascorbate reductase (MDHAR) is a NADH/NADPH-dependent FAD-containing enzyme (Fig. 16.3). MDHAR is involved in AsA regeneration from MDHA and the phenoxyl radical reduction (Hasanuzzaman et al. 2019). Isoforms of this enzyme are in different plant cell components like chloroplasts, mitochondria, peroxisomes, glyoxysomes, and cytosol (Leterrier and Cagnac 2018).
Dehydroascorbate reductase (DHAR) can regenerate AsA by the oxidation of GSH to GSSG (Das et al. 2015). This reaction proceeds by a ping-pong mechanism, in which dehydroascorbate connects to the free reduced form of DHAR with the binding of GSH (Fig. 16.3).
DHAR can also be involved in the regulation of normal plant growth and development (Ding et al. 2020). Increased DHAR activity may be one of the mechanisms that regulate the regeneration of AsA from DHA under stress conditions accompanied by increased ROS formation (Locato et al. 2009). Therefore, increasing AsA content may restrict the detrimental effects of ROS accumulation (Wang et al. 2010).
16.7 Toxicity of Heavy Metals in Plants: Overview
Industrial waste, fertilizers, smelting, and wastewater discharges cause leaching of heavy metals (HMs) such as copper (Cu), manganese (Mn), lead (Pb), nickel (Ni), cadmium (Cd), cobalt (Co), iron (Fe), chromium (Cr), zinc (Zn), silver (Ag), arsenic (As), and platinum (Pt) into soil or groundwater (Gupta and Ali 2002; Dağhan and Ozturk 2015; Basheer 2018a). Plants need for their growth and development in small amounts some HMs (Co, Cu, Fe, Mn, Mo, Ni, V, and Zn). However, excessive amounts of these elements can become harmful to them. For example, Zn and Mn in excessive amounts impair the growth of plants and compete with Fe. The Fe2+ excess in cells participates in the Fenton reaction by forming ·OH (Shanmugam et al. 2011). HMs chelate by low molecular mass compounds (GSH, AsA, cysteine or others) and sequestrate in plant vacuoles. Other HMs (Pb, Cd, Hg, and Cr) are toxic for plants because of intensive protein denaturation and DNA damage. Most HMs are immobile, although some of them can be taken up by root system via endocytosis, diffusion, or through metal transporters (Krzesłowska 2011; Ali et al. 2015a, 2017; Basheer 2018b). The plant species accumulate HMs at different rates. Heavy metals impair virtually all molecular and biochemical processes in plants causing growth defects, root browning, chlorosis, and death (Gupta and Ali 2002; Dağhan and Ozturk 2015).
Heavy metals can bind to proteins, leading to inactivation or denaturation of the latter. The binding of HMs to membrane proteins disrupts the integrity of membranes, which is mainly reflected in the disruption of the transport of various substances. Ultimately, this leads to impaired photosynthesis and ion homeostasis (Hossain et al. 2012). The result of HMs stress is the overproduction of ROS including ·OH, \({\text{O}}_{{2}}^{ \cdot - }\), OONO−, \(^{{1}} {\text{O}}_{{2}}\), ·NO, H2O2, hypochlorous acid (HOCl), and then resulting in oxidative stress (Rascio and Navari-Izzo 2011). This process is considered one of the primary mechanisms of HM toxicity in plants (Lushchak 2011; Sharma et al. 2019). Cu, Cd, Ni, Cr, and As ions exceeding permissible limits in plant cells lead to increased ROS amounts (Husak 2015; Shahzad et al. 2018). Depending on the bioactivity, HMs can be grouped into redox-active (Cr, Cu, Fe) and non-redox-active (Cd, Hg, Ni, Zn). Redox active metals may be oxidized in Haber-Weiss or Fenton reactions yielding highly dangerous free radicals having the capacity to produce oxidative injuries in plant cells. This results in excessive ROS generation and then disturbs the balance between prooxidant and antioxidant levels. Regarding redox-inactive metals, they form covalent bonds with sulfhydryl groups of protein.
All plant responses of HMs toxicity can be broadly classified as those that ensure the acquisition of tolerance to HMs or those reducing the uptake of metals (Krzesłowska 2011). The detoxification of HMs can occur by the chelation of metals and their transportation in the plant vacuoles. Under these conditions, the plant activates the synthesis of some stress-related proteins to prevent and cope with adverse effects of HMs (Ghori et al. 2019).
One of the most important pathways involved in plant abiotic stress is that mediated by the mitogen-activated protein kinases (MAPK) (Jonak et al. 2002). These phosphorylating cascades are also stimulated by hormones and control pathways of cell proliferation/differentiation, and expression of various stress-related genes. In plant cells, the MAPKs are diverse, and can interplay to synergetic action or inhibit the action of each other.
In the next sections, we discuss the toxic effects caused by chromium compounds and analyze the plants responses to the Cr stress.
16.8 Oxidative Stress as a Mechanism of Cr Toxicity
Chromium is used in different industries like electroplating, metallurgy, production of paints, pigments and paper, tanning, and manufacturing different chemical compounds (Ghani 2011). This large-scale application of Cr is a major cause of contamination of soil and water by its highly toxic valence state Cr6+ in the forms of chromate (\({\text{CrO}}_{{4}}^{{{2} - }}\)), dichromate (\({\text{Cr}}_{{2}} {\text{O}}_{{7}}^{{{2} - }}\)) and oxide (CrO3). Toxic forms of Cr can generate H2O2, \({\text{O}}_{{2}}^{ \cdot - }\), ·OH, cause plant growth retardation, chlorosis, and roots destruction (Shanker et al. 2003a; Ozturk et al. 2015). The negative effects of Cr ions on plant photosynthetic systems, mineral and water metabolism have also shown (Stambulska et al. 2018).
In plant species, all toxic effects of Cr can be divided into four types. The first, Cr damages mitochondria and affects the synthesis of essential photosynthetic pigments, especially chlorophylls (Boonyapookana et al. 2002). The second mechanism is the increasing production of GSH and AsA or the increase in antioxidant enzyme activities as a direct response to Cr-induced ROS production to avoid significant oxidation of cellular components (Shanker et al. 2003a). Chromium ions can interact with iron-porphyrin in the active center of CAT, inactivating the enzyme (Sharma et al. 2003). A decrease in catalase activity may be one of the ways facilitating ROS increase in Cr-exposed plants and aggravating damaging effects of Cr. Cr can also inhibit other antioxidant enzymes such as GPX, GR, POX, and APX (Adrees et al. 2015; Ali et al. 2015b). The third, Cr induces the production of phytochelatins and histidine as secondary metabolites. These metabolites contribute toward Cr stress tolerance (Schmfger 2001). The fourth mechanism is the DNA damage (Shanker et al. 2003b). Most heavy metals interact directly with DNA, but Cr realizes its genotoxicity via intensive ROS production (Fang et al. 2014). Cr6+ can produce inter-DNA strand cross-links and nucleotide strand breaks (Fang et al. 2014; Meng et al. 2017). Chromium-DNA adducts are considered to be the primary cause of Cr6+-induced mutagenicity (Fang et al. 2014).
Chromium toxicity involves the damage of cell membranes via the induction of lipid peroxidation, as well as the degradation of genetic material, and inactivation of enzymes, which results in the activation of programmed cell death (Zhang et al. 2016; Wakeel et al. 2019). An increase in malondialdehyde content was observed with a sharp increase in ROS upon chromium treatment (Ali et al. 2015a; Adrees et al. 2015). Increased malondialdehyde level is a marker of lipid oxidation; therefore, its accumulation leads to the deterioration of membrane permeability (Rahman et al. 2010).
High Cr concentration disturbs the photosynthetic process. Cr-induced chloroplast ultrastructural changes, alterations in auto-fluorescence of chloroplast, or binding of Cr to cytochrome groups were established (Shahid et al. 2017; Balasaraswathi et al. 2017). As a result, it disturbs CO2 fixation and electron transport in photosystems I and 2, with inhibiting of respective enzymes (Shahid et al. 2017; Wakeel et al. 2020). Furthermore, high Cr levels mediate elevated ROS synthesis in chloroplasts, being involved in the suppression of photosynthesis (Rodriguez et al. 2011; Wakeel et al. 2020). Delta-aminolaevulinic acid dehydratase can be inhibited by the Cr accumulation in chloroplasts, which leads to a decrease in the content of chlorophyll (Yildiz et al. 2012; Paul 2016).
Chromium also affects the activities of many enzymes, since Cr is able to interact with their catalytic site, thus leading to the alteration in enzymatic activity (Yadav 2010). In plant roots, Cr inhibits such enzymes as nitrate reductase (Stambulska et al. 2018) and Fe-reductase (Barton et al. 2000). In the mitochondrial respiratory chain, Cr6+ can replace Cu and Fe ions in prosthetic groups of metal-containing proteins such as cytochrome oxidase (DalCorso 2012; Singh et al. 2013). The binding of Cr to complex IV may be the reason of the inhibition of cytochrome oxidase activity. It seems that Cr binds to cytochrome a3 (Dixit et al. 2002). Studies suggest that the plasma membrane NADPH oxidase (NOX) can produce ROS in association with Cr (Potocký et al. 2012; Weyemi and Dupuy 2012). Under these conditions, NOX, consuming cytosolic NADPH, produces \({\text{O}}_{{2}}^{ \cdot - }\), which is quickly converted to H2O2 (Pourrut et al. 2008).
Chromium detoxification in plants occurs by certain mechanisms (Zayed and Terry 2003). The first is a reduction of Cr6+–Cr3+ due to the formation of intermediate forms, Cr5+ and Cr4+ (Fig. 16.4). ROS can produce during this reduction and Fenton reaction. In the Fenton reaction, the catalytic power of Cr3+ is greater than iron, copper, or other metal ions (Shahid et al. 2017).
Reactions of reduction and oxidation of chromium ions in plant cells. Cr6+ can actively enter the cells through channels for the transfer of the isoelectric andisostructural anions, such as \({\text{SO}}_{{4}}^{{{2} - }}\) and \({\text{HPO}}_{{4}}^{{{2} - }}\) channels. In the cell, glutathione (GSH), ascorbate (AsA) or cysteine (Cys) reduce Cr6+ to Cr5+. The glutathione-derived thiyl radical (GS·) can further react with other thiol molecules (RSH) in oxygenated tissues to give the superoxide radical (\({\text{O}}_{{2}}^{ \cdot - }\)). Cr5+ can also be reduced by GSH or AsA to Cr4+ and then to Cr3+. Once formed, Cr5+, Cr4+ or Cr3+ species alter the DNA conformation followed by formation of Cr-DNA adducts. However, Cr3+-DNA adducts play the dominant role in the chromium mutagenicity
Cr4+ and Cr5+ ions which occur during radical oxidation of Cr3+ are catalytically active and can generate the hydroxyl radical (Fig. 16.4). However, the Cr involvement in this reaction is not well studied (Strile et al. 2003). Next mechanism is the Cr immobilization in the cell wall and vacuoles (Han et al. 2004). Furthermore, Cr3+ can form highly stable complexes with glutathione, carbohydrates, NADH, FADH2, and these complexes are stored in cell vacuole of plants (Lushchak 2012; Babula et al. 2008). Phytochelatins (PCs) and metallothioneins (MTs) are a very important for chromium detoxification in plants (Cobbett 2000; Cobbett and Goldsbrough 2002). However, their role in chromium detoxification in plants is not fully studied. Root exudates containing organic acids like citric, malic, aspartic, oxalic or carboxylic acids can form complexes with inorganic Cr, making them available for plant uptake (James and Bartlett 1983; Srivastava et al. 1999). However, organic acids in Cr detoxification in plants are studied not enough.
16.9 Redox-Sensitive Mechanisms of Plant Adaptive Response to Cr
As mentioned above, Cr toxicity is largely due to its ability to increase ROS production and facilitate non-enzymatic oxidation of cellular components. In response to the Cr-induced increase in ROS levels, plants up-regulate a protective battery of enzymatic and non-enzymatic molecules that help to combat with redox imbalance, repair existing oxidative damage and prevent new ones under stress. It was demonstrated that high antioxidant capacity predicts high HM tolerance (Alsherif et al. 2022). Studies indicate that in plants chromium treatment induces many antioxidant and related enzymes, non-enzymatic small antioxidants (glutathione, ascorbic acid, etc.), metal-binding enzymes, and repair components (Smiri et al. 2010; Stambulska et al. 2018; Saud et al. 2022). These biochemical markers were proposed to be indicators of resistance degree of plants to complex HM contamination (Alsherif et al. 2022). Molecular mechanisms of the up-regulation of defense mechanisms under Cr stress are studied scare, but it seems ROS are key participants in these signaling pathways.
Data suggest that molecular response to Cr is regulated at different levels—transcription of certain required genes, translation, and posttranslational modification. Posttranslational modifications ensure an immediately (“fast”) response of the cell to the stimulus to stop initiation of adverse effects. These modifications include covalent changes in protein structure (e.g., phosphorylation-dephosphorylation, oxidation-reduction, association-dissociation of subunits) that affect protein function. In parallel, transcriptional (“slow”) responses are triggered via multicomponent signaling cascades. ROS/RNS are proposed to be mediator of both “fast” and “slow” stress responses. Moreover, overlapping of two systems—“fast” and “slow” allows better adaptation of plants to stress challenges. Fast short-term signaling mechanisms are utilize the present set of proteins for defense, while a “slow” response includes the activation of expression of genes encoding defensive proteins (Lushchak 2011).
Various stresses, including heavy metals, stimulate processes that increase in different ROS levels. This increase is detected directly by redox-sensitive transcription factors (TFs) and specific sensors or act as mediators in a number of signaling pathways leading to global metabolic reprogramming (Foyer and Noctor 2013; He et al. 2018; Moore et al. 2016). Plant hormones, such as salicylic acid (SA) act through ROS-related mechanisms (Lushchak 2011; Foyer and Noctor 2013). Changes that will take place in the cell and the whole plant are determined by the type of ROS formed and the site of their subcellular generation (Foyer and Noctor 2013; Gadjev et al. 2006; Willems et al. 2016). Furthermore, elevated ROS specifically change the set of genes that are transcribed in a time-dependent manner. According to the conducted transcriptomic meta-analysis, the time of exposure to increased ROS might be a determinant in the pattern of gene expression. In a later period, the initial specific stress may cause more general changes in gene expression (Willems et al. 2016).
The direct effects of ROS, e.g. H2O2, on target proteins, involve the oxidation of sulfur-containing amino acids—cysteine and methionine. Thiol-groups of cysteine are first oxidized to sulfenic acid (Cys-SOH), which reacts rapidly with other thio-containing molecules, especially GSH. Such alterations protect protein cysteines from further irreversible oxidation to sulfuric (SO2H) or sulfonic (SO3H) acid (He et al. 2018; Martí et al. 2020). Among proteins, which undergo Cys-modification, are metabolic enzymes (e.g., chloroplastic glyceraldehyde-3-phosphate dehydrogenase), antioxidant oxidoreductases (thioredoxins, glutaredoxins), signaling proteins, such as protein kinases, phosphatases, and transcription factors (Lushchak 2012; He et al. 2018; Martí et al. 2020).
Under non-stressful conditions, many transcriptional factors (TFs) are in an inactive form in the cytoplasm, but oxidative stress activates them followed by their translocation to the nucleus. Here, we will review the effects of redox perturbations on gene expression in nucleus with a focus on two plant stress responses that are regulated by the redox-sensitive proteins NPR1/TGA and Rap2.4a.
The NPR1 protein (the non-expressor of the pathogenesis-related gene 1) is the main transcriptional coactivator for pathogenesis-related genes (PR) and other genes induced by salicylic acid. This protein also regulates SA-mediated suppression jasmonic acid signaling (Lushchak 2011; He et al. 2018). Under physiological conditions, NPR1 is the cytosolic protein in the form of oxidized oligomer with intermolecular disulfide bridges between Cys82 and Cys216 (Fig. 16.5a) (Mou et al. 2003). Nitric oxide (·NO) may cause S-nitrosylation of Cys156 facilitating the formation of disulfide bonds between NPR1 monomers to form inactive oligomers. In response to pathogens or other stressors, plant hormone SA triggers thioredoxin-dependent S–S bond repair in oligomeric NPR1 and its dissociation into NPR1monomers, which are active form of the protein (Tada et al. 2008). The active NPR1 protein is translocated to the nucleus, where it interacts with the TGAs proteins, which are basic domain/leucine zipper TFs (Després et al. 2003). The complex NPR1/TGA binds to promotors of genes containing specific nucleotide sequence, namely TGACG sequence, leading to activation of transcription of stress-protective genes (Lushchak 2011; He et al. 2018). It should be noted that SA is able to directly bind to NRP1 leading to conformational changes in NPR1 (Wu et al. 2012). Plants with inactivated NPR1 showed poor systemic acquired resistance which is mediated by SA (Dong et al. 2001). Six TGA transcription factors were identified to cooperate with NPR1. The dominant role of TGA2, TGA3, TGA5, and TGA6 have been proved (Zhang et al. 2003; Kesarwani et al. 2007). It is proposed that HM due to H2O2-induced production may stimulate SA/NRP1 signaling to increase plant tolerance (Dutta et al. 2018).
Redox-changes in the structure can modulate the affinity of transcription factors towards DNA. This mechanism is well studied in transcriptional factor Rap2.4A, which is encoded by the At1g36060 gene. The Rap2.4A protein is able to bind the promoter element of the specific genes in redox-dependent manner (Fig. 16.5b) (He et al. 2018). In particular, it was found that the activation of the 2-Cys peroxiredoxin-A gene in A. thaliana is controlled by Rap2.4A. The use of Rap2.4a mutant lines demonstrates that this protein regulates tolerance to stressors by increasing photosynthetic ability and defense capacity of chloroplasts (Shaikhali et al. 2008; Rudnik et al. 2017). In particular, Rap2.4A controls expression of ZAT10 gene, which encodes a transcription factor activating expression of cytosolic ascorbate peroxidase 2 APX 2 (Shaikhali et al. 2008). The ability of RAP2.4A to interact with DNA depends on the degree of oxidation Cys residues. Cys at positions 113, 286, and 302 was shown to determine the functional activity of Rap2.4a (Lushchak 2011). When these cysteine residues are oxidized, intermolecular disulfide bonds are formed, which results in the formation of a homodimer of Rap2.4A, which is its active form to induce transcription (He et al. 2018). The homodimer Rap2.4A moves to the nucleus and binds to target genes. It is assumed that before binding, the protein undergoes reduction by disulfide bridges, but the mechanism of this reduction is hypothetical. Only a moderate ROS increase leads to the Rap2.4a activation, when severe oxidative stress cause inactivation of this protein (Shaikhali et al. 2008). This is likely due to the fact that with increased ROS levels, there are formed aggregates of oxidized Rap2.4a protein that do not have DNA-binding activity. Thus, Rap2.4a serves as a highly sensitive and precisely controllable redox system that provides an adaptive response to small changes in the redox state, which can be a useful strategy to prevent the development of significant oxidative damage in the plant cell. It is not currently known whether Rap2.4a is involved in tolerance to heavy metals. However, its role as an inducer of antioxidant defense suggests that it may be involved in protection against metal toxicity. Detailed molecular redox mechanisms that provide chromium tolerance require further research.
16.10 Conclusions and Perspectives
Toxicity of heavy metals (HM) is the topic which is intensively studied. In this context, it is important to know how plants cope with and tolerate HMs, especially with regards to agricultural plants. Contamination of agricultural plants with HM has a direct effect on human health. This review summarizes available information on the relationship between chromium toxicity and ROS/RNS generation in plant cells. The analysis of literature data indicates that chromium causes disturbances in the redox balance through a number of mechanisms—directly inactivating protective proteins, disrupting the functioning of electron transport systems, destroying membrane integrity. In chloroplasts, Cr inhibits electron transport, reduces CO2 fixation and glucose synthesis, causing drying, chlorosis, necrosis, and wilting of leaves. Plant cells up-regulate complex antioxidant non-enzymatic and enzymatic mechanisms that help the cells to adapt their metabolism to Cr stress. However, the mechanisms of Cr-induced toxicity at the biochemical and molecular levels still require deeper investigation. Molecular mechanism underling Cr tolerance is still poorly understood, and this is an avenue for future research.
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- Grx:
-
Glutaredoxin
- HM:
-
Heavy metal
- HSP:
-
Heat shock protein
- MT:
-
Metallothionein
- NPR1:
-
Non-expressor of the pathogenesis-related gene
- PC:
-
Phytochelatin
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- TF:
-
Transcription factor
- TGA:
-
TGACG sequence-specific binding protein
- Trx:
-
Thioredoxin
References
Adrees M, Ali S, Iqbal M, Bharwana SA, Siddiqi Z, Farid M, Ali Q, Saeed R, Rizwan M (2015) Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol Environ Saf 122:1–8. https://doi.org/10.1016/j.ecoenv.2015.07.003
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:613. https://doi.org/10.3389/fpls.2017.00613
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang M-Q (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9:42. https://doi.org/10.3390/toxics9030042
Al-Huqail AA, Ali HM, Kushwaha BK, AL-Huqail AA, Singh VP, Siddiqui MH (2020) Ascorbic acid is essential for inducing chromium (VI) toxicity tolerance in tomato roots. J Biotechnol 322:66–73. https://doi.org/10.1016/j.jbiotec.2020.07.011
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015a) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609. https://doi.org/10.1007/s11356-015-4271-7
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015b) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22:10669–10678. https://doi.org/10.1007/s11356-015-4193-4
Ali I, Suhail M, Basheer AA (2017) Advanced spiral periodic classification of the elements. Chem Int 3:220–224. https://doi.org/10.5281/zenodo.1473118
Alsherif EA, Al-Shaikh TM, Almaghrabi O, AbdElgawad H (2022) High redox status as the basis for heavy metal tolerance of Sesuvium portulacastrum L. inhabiting contaminated soil in Jeddah, Saudi Arabia. Antioxidants 11:19. https://doi.org/10.3390/antiox11010019
Anjum SA, Ashraf U, Khan I, Tanveer M, Shahid M, Shakoor A, Wang L (2017) Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27:262–273. https://doi.org/10.1016/S1002-0160(17)60315-1
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Appiah C, Yang Z-F, He J, Wang Y, Zhou J, Xu W-Z, Nie G, Zhu Y-Q (2021) Genome-wide identification of Hsp90 gene family in perennial ryegrass and expression analysis under various abiotic stresses. Plants 10:2509. https://doi.org/10.3390/plants10112509
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. https://doi.org/10.1146/annurev.arplant.50.1.601
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6(4):189–213. https://doi.org/10.1007/s10311-008-0159-9
Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubsico oxygenase. Philos Trans R Soc Lond B: Biol Sci 355:1433–1445. https://doi.org/10.1098/rstb.2000.0704
Balasaraswathi K, Jayaveni S, Sridevi J, Sujatha D, Aaron KP, Rose C (2017) Cr-induced cellular injury and necrosis in Glycine max L.: biochemical mechanism of oxidative damage in chloroplast. Plant Physiol Biochem 118:653–666. https://doi.org/10.1016/j.plaphy.2017.08.001
Balestrasse KB, Yannarelli GG, Noriega GO, Batlle A, Tomaro ML (2008) Heme oxygenase and catalase gene expression in nodules and roots of soybean plants subjected to cadmium stress. Biometals. Int J Role Met Ions Biol Biochem Med 21:433–441. https://doi.org/10.1007/s10534-008-9132-0
Barton LL, Johnson GV, O’Nan AG, Wagener BM (2000) Inhibition of ferric chelate reductase in alfalfa roots by cobalt, nickel, chromium, and copper. J Plant Nutr 23(11–12):1833–1845. https://doi.org/10.1080/01904160009382146
Basheer AA (2018a) Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century. Chirality 30(4):402–406. https://doi.org/10.1002/chir.22808
Basheer AA (2018b) New generation nano-adsorbents for the removal of emerging contaminants in water. J Mol Liq 261:583–593. https://doi.org/10.1016/j.molliq.2018.04.021
Bayliak MM, Burdyliuk NI, Lushchak VI (2016a) Effects of pH on antioxidant and prooxidant properties of common medicinal herbs. Open Life Sci. 11:298–307. https://doi.org/10.1515/biol-2016-0040
Bayliak MM, Burdylyuk NI, Lushchak VI (2016b) Quercetin increases stress resistance in the yeast Saccharomyces cerevisiae not only as an antioxidant. Ann Microbiol 66:569–576. https://doi.org/10.1007/s13213-015-1136-8
Bela K, Horváth E, Gallé Á, Szabados L, Tari I, Csiszár J (2015) Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol 176:192–201. https://doi.org/10.1016/j.jplph.2014.12.014
Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in gamma-tocopherol methyltransferase. Plant Mol Biol 52:1181–1190. https://doi.org/10.1023/b:plan.0000004307.62398.91
Boeckx T, Winters AL, Webb KJ, Kingston-Smith AH (2015) Polyphenol oxidase in leaves: is there any significance to the chloroplastic localization? J Exp Bot 66:3571–3579. https://doi.org/10.1093/jxb/erv141
Boonyapookana B, Upatham ES, Kruatrachue M, Pokethitiyook P, Singhakaew S (2002) Phytoaccumulation and phytotoxicity of cadmium and chromium in duckweed Wolfa globosa. Int J Phytoremed 4:87–100. https://doi.org/10.1080/15226510208500075
Cadet J, Davies KJA (2017) Oxidative DNA damage & repair: an introduction. Free Radic Biol Med 107:2–12. https://doi.org/10.1016/j.freeradbiomed.2017.03.030
Chaudiere J, Ferrari-Iliou R (1999) Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol 37:949–962. https://doi.org/10.1016/s0278-6915(99)00090-3
Chen I, Chiu MH, Cheng SF, Hsu YH, Tsai CH (2013) The glutathione transferase of Nicotiana benthamiana NbGSTU4 plays a role in regulating the early replication of Bamboo mosaic virus. New Phytol 199:749–757. https://doi.org/10.1111/nph.12304
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867. https://doi.org/10.1111/tpj.13299
Cobbett C (2000) Phytochelatins and their role in heavy metal detoxification. Plant Physiol 123:463–469. https://doi.org/10.1104/pp.123.3.825
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182. https://doi.org/10.1146/annurev.arplant.53.100301.135154
Corpas FJ, Palma JM (2020) Assessing nitric oxide (NO) in higher plants: an outline. Nitrogen 1:12–20. https://doi.org/10.3390/nitrogen1010003
Corpas FJ, González-Gordo S, Palma JM (2021) Protein nitration: a connecting bridge between nitric oxide (NO) and plant stress. Plant Stress. 2:100026. https://doi.org/10.1016/j.stress.2021.100026
Cui Y, Wang M, Yin X, Xu G, Song S, Li M, Liu K, Xia X (2019) OsMSR3, a small heat shock protein, confers enhanced tolerance to copper stress in Arabidopsis thaliana. Int J Mol Sci 20:6096. https://doi.org/10.3390/ijms20236096
Dağhan H, Ozturk M (2015) Soil pollution in Turkey and remediation methods. In: Hakeem K, Sabir M, Ozturk M, Mermut A (eds) Soil remediation and plants: prospects and challenges. Elsevier, New York, pp 287–312
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352. https://doi.org/10.3390/molecules15107313
DalCorso G (2012) Heavy metal toxicity in plants. In: plants and heavy metals, springer, briefs in molecular science, Dordrecht, Netherlands, pp 1–25
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53. https://doi.org/10.3389/fenvs.2014.00053
Das P, Nutan KK, Singla-Pareek SL, Pareek A (2015) Oxidative environment and redox homeostasis in plants: dissecting out significant contribution of major cellular organelles. Front Environ Sci 2:70. https://doi.org/10.3389/fenvs.2014.00070
Del Rio LA, Lopez-Huertas E (2016) ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol 57:1364–1376. https://doi.org/10.1093/pcp/pcw076
Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15:2181–2191. https://doi.org/10.1105/tpc.012849
Di Fino L, Arruebarrena Di Palma A, Perk EA, García-Mata C, Schopfer FJ, Laxalt AM (2021) Nitro-fatty acids: electrophilic signaling molecules in plant physiology. Planta 254:120. https://doi.org/10.1007/s00425-021-03777-z
Di Meo S, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med Cell Longev 2016. https://doi.org/10.1155/2016/1245049
di Toppi LS, Fossati F, Musetti R, Mikerezi I, Favali MA (2002) Effects of Hexavalent Chromium on Maize, Tomato, and Cauliflower Plants. J Plant Nutr 25:701–717. https://doi.org/10.1081/PLN-120002953
Ding S, Lu Q, Zhang Y, Yang Z, Wen X, Zhang L, Lu C (2009) Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol Biol 69:577–592. https://doi.org/10.1007/s11103-008-9440-3
Ding H, Wang B, Han Y, Li S (2020) The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J Exp Bot 71:3405–3416. https://doi.org/10.1093/jxb/eraa107
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv: Azad) root mitochondria. Plant Cell Env 25:687–693. https://doi.org/10.1046/j.1365-3040.2002.00843.x
Dixon DP, Sellars JD, Edwards R (2011) The Arabidopsis phi class glutathione transferase AtGSTF2: binding and regulation by biologically active heterocyclic ligands. Biochem J 438:63–70. https://doi.org/10.1042/BJ20101884
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338–350. https://doi.org/10.1016/j.phytochem.2009.12.012
Domènech J, Mir G, Huguet G, Capdevila M, Molinas M, Atrian S (2006) Plant metallothionein domains: functional insight into physiological metal binding and protein folding. Biochimie 88:583–593. https://doi.org/10.1016/j.biochi.2005.11.002
Dong X, Li X, Zhang Y, Fan W, Kinkema M, Clarke J (2001) Regulation of systemic acquired resistance by NPR1 and its partners. Novartis Found Symp 236:165–173; discussion 173–175. https://doi.org/10.1002/9780470515778.ch12
Dong Y, Li C, Zhang Y, He Q, Daud MK, Chen J, Zhu S (2016) Glutathione S-transferase gene family in Gossypium raimondii and G. arboreum: comparative genomic study and their expression under salt stress. Front Plant Sci 7:139. https://doi.org/10.3389/fpls.2016.00139
Douce R, Neuburger M (1999) Biochemical dissection of photorespiration. Curr Opin Plant Biol 2:214–222. https://doi.org/10.1016/S1369-5266(99)80038-7
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:552969. https://doi.org/10.3389/fpls.2020.552969
Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Georgopoulos NT (2017) Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J 14:89–96. https://doi.org/10.1111/iwj.12557
Dutta S, Mitra M, Agarwal P, Mahapatra K, De S, Sett U, Roy S (2018) Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal Behav 13:e1460048. https://doi.org/10.1080/15592324.2018.1460048
Edwards EA, Rawsthorne S, Mullineaux PM (1990) Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 180:278–284. https://doi.org/10.1007/BF00194008
Fang Z, Zhao M, Zhen H, Chen L, Shi P, Huang Z (2014) Genotoxicity of tri- and hexavalent chromium compounds in vivo and their modes of action on DNA damage in vitro. Plos One 9:e103194. https://doi.org/10.1371/journal.pone.0103194
Fedenko VS, Landi M, Shemet SA (2022) Metallophenolomics: a novel integrated approach to study complexation of plant phenolics with metal/metalloid ions. Int J Mol Sci 23:11370. https://doi.org/10.3390/ijms231911370
Feechan A, Kwon E, Yun BW, Wang Y, Pallas JA, Loake GJ (2005) A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci 102:8054–8059. https://doi.org/10.1073/pnas.0501456102
Figgitt M, Newson R, Leslie IJ, Fisher J, Ingham E, Case CP (2010) The genotoxicity of physiological concentrations of chromium (Cr(III) and Cr(VI)) and cobalt (Co(II)): an in vitro study. Mutat Res 688:53–61. https://doi.org/10.1016/j.mrfmmm.2010.03.008
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/BF00386001
Foyer H, Noctor G (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364. https://doi.org/10.1034/j.1399-3054.2003.00223.x
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: a reevaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071. https://doi.org/10.1111/j.1365-3040.2005.01327.x
Foyer CH, Noctor G (2013) Redox signaling in plants. Antioxid Redox Signal 18:2087–2090. https://doi.org/10.1089/ars.2013.5278
Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33:691–705. https://doi.org/10.1046/j.1365-313x.2003.01656.x
Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141:436–445. https://doi.org/10.1104/pp.106.078717
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101. https://doi.org/10.1002/bies.20493
Geigenberger P, Thormählen I, Daloso DM, Fernie AR (2017) The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci 22:249–262. https://doi.org/10.1016/j.tplants.2016.12.008
Gelhaye E, Rouhier N, Navrot N, Jacquot JP (2005) The plant thioredoxin system. Cell Mol Life Sci CMLS 62:24–35. https://doi.org/10.1007/s00018-004-4296-4
Ghani A (2011) Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egypt Acad J Biol Sci 2:9–15. https://doi.org/10.21608/eajbsh.2011.17007
Ghori N-H, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16(3):1807–1828. https://doi.org/10.1007/s13762-019-02215-8
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpinski S (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171:1606–1615. https://doi.org/10.1104/pp.16.00434
Gupta VK, Ali I (2002) Encyclopedia of surface and colloid science. Marcel Dekker, New York, pp 136–166
Gupta KJ, Kaiser WM (2010) Production and scavenging of nitric oxide by barley root mitochondria. Plant Cell Physiol 51:576–584. https://doi.org/10.1093/pcp/pcq022
Han FX, Sridhar BBM, Monts DL, Su Y (2004) Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea. New Phytol 162(2):489–499. https://doi.org/10.1111/j.1469-8137.2004.01027.x
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M (2017) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18:200. https://doi.org/10.3390/ijms18010200
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384. https://doi.org/10.3390/antiox8090384
Hasanuzzaman M, Bhuyan BMHM, Parvin K, Bhuiyan TF, Anee TI, Nahar K, Hossen MdSh, Zulfiqar F, Alam MdM, Fujita M (2020a) Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Int J Mol Sci 21:8695. https://doi.org/10.3390/ijms21228695
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020b) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Havaux M, Tardy F, Lemoine Y (1998) Photosynthetic light-harvesting function of carotenoids in higher-plant leaves exposed to high light irradiances. Planta 205:242–250
He H, Van Breusegem F, Mhamdi A (2018) Redox-dependent control of nuclear transcription in plants. J Exp Bot 69:3359–3372. https://doi.org/10.1093/jxb/ery130
Hossain MA, Piyatida P, Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxifcation of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37. https://doi.org/10.1155/2012/872875
Husak VV (2015) Copper and copper-containing pesticides: metabolism, toxicity and oxidative stress. J Vasyl Stefanyk Precarpathian Natl Univ 2(1):39–51. https://doi.org/10.15330/jpnu.2.1.38-50
James BR, Barlett RJ (1983) Behavior of chromium in soils. VI. Interactions between oxidation and reduction and organic complexation. J Environ Qual 12:173–176. https://doi.org/10.2134/jeq1983.00472425001200020004x
Jedelská T, Luhová L, Petřivalský M (2020) Thioredoxins: emerging players in the regulation of protein S-nitrosation in plants. Plants 9:1426. https://doi.org/10.3390/plants9111426
Jia B, Sun M, Sun X, Li R, Wang Z, Wu J, Wei Z, DuanMu H, Xiao J, Zhu Y (2016) Overexpression of GsGSTU13 and SCMRP in Medicago sativa confers increased salt–alkaline tolerance and methionine content. Physiol Plant 156:176–189. https://doi.org/10.1111/ppl.12350
Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5:415–424. https://doi.org/10.1016/s1369-5266(02)00285-6
Kapoor D, Singh S, Kumar V, Romero R, Prasad R, Singh J (2019) Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 19:100182. https://doi.org/10.1016/j.plgene.2019.100182
Karuppanapandian T, Moon JC, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. AJCS 5(6):709–725
Kesarwani M, Yoo J, Dong X (2007) Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol 144:336–346. https://doi.org/10.1104/pp.106.095299
Kharma A, Grman M, Misak A, Domínguez-Álvarez E, Nasim MJ, Ondrias K, Chovanec M, Jacob C (2019) Inorganic polysulfides and related reactive sulfur–selenium species from the perspective of chemistry. Molecules 24:1359. https://doi.org/10.3390/molecules24071359
Kim SI, Andaya VC, Tai TH (2011) Cold sensitivity in rice (Oryza sativa L.) is strongly correlated with a naturally occurring I99V mutation in the multifunctional glutathione transferase isoenzyme GSTZ2. Biochem J 435:373–380. https://doi.org/10.1042/BJ20101610
Kolbert Z, Barroso JB, Brouquisse R, Corpas FJ, Gupta KJ, Lindermayr C, Loake GJ, Palma JM, Petrivalský M, Wendehenne D, Hancock JT (2019) A Forty year journey: the generation and roles of NO in plants. Nitric Oxide 93:53–70. https://doi.org/10.1016/j.niox.2019.09.006
Kornyeyev D, Logan BA, Payton P, Allen RD, Holaday AS (2003) Elevated chloroplastic glutathione reductase activities decrease chilling-induced photoinhibition by increasing rates of photochemistry, but not thermal energy dissipation, in transgenic cotton. Funct Plant Biol 30:101–110. https://doi.org/10.1071/FP02144
Kosakivska IV, Babenko LM, Romanenko KO, Korotka IY, Potters G (2021) Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol Int 45:258–272. https://doi.org/10.1002/cbin.11503
Koua D, Cerutti L, Falquet L, Sigrist CJ, Theiler G, Hulo N, Dunand C (2009) PeroxiBase: a database with new tools for peroxidase family classification. Nucleic Acids Res 37:261–266. https://doi.org/10.1093/nar/gkn680
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33(1):35–51. https://doi.org/10.1007/s11738-010-0581-z
Kubrak OI, Lushchak OV, Lushchak JV, Torous IM, Storey JM, Storey KB, Lushchak VI (2010) Chromium effects on free radical processes in goldfish tissues: comparison of Cr(III) and Cr(VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol C Toxicol Pharmacol 152:360–370. https://doi.org/10.1016/j.cbpc.2010.06.003
Kumar D, Klessig DF (2003) High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proc Natl Acad Sci 100:16101–16106. https://doi.org/10.1073/pnas.0307162100
Kumar P, Tokas J, Singal HR (2019) Amelioration of chromium VI toxicity in Sorghum (Sorghum bicolor L.) using glycine betaine. Sci Rep 9:16020. https://doi.org/10.1038/s41598-019-52479-w
Kumar S, Wang M, Fahad S, Qayyum A, Chen Y, Zhu G (2022) Chromium induces toxicity at different phenotypic, physiological, biochemical, and ultrastructural levels in sweet potato (Ipomoea batatas L.) plants. Int J Mol Sci 23:13496. https://doi.org/10.3390/ijms232113496
Leng X, Wang H, Zhang S, Qu C, Yang C, Xu Z, Liu G (2021) Identification and Characterization of the APX Gene family and its expression pattern under phytohormone treatment and abiotic stress in Populus trichocarpa. Genes 12:334. https://doi.org/10.3390/genes12030334
Leterrier M, Cagnac O (2018) Function of the various MDAR isoforms in higher plants. In: Gupta D, Palma J, Corpas F (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham, Switzerland, pp 83–94
Loake G, Grant M (2007) Salicylic acid in plant defence—The players and protagonists. Curr Opin Plant Biol 10:466–472. https://doi.org/10.1016/j.pbi.2007.08.008
Locato V, de Pinto MC, De Gara L (2009) Different involvement of the mitocondiral, plastidal and cytosolic ascorbate-glutathione redox enzymes in heat shock responses. Phyiol Plantarum 135:296–306. https://doi.org/10.1111/j.1399-3054.2008.01195.x
López-Bucio J, Ortiz-Castro R, Ruíz-Herrera LF, Juárez CV, Hernández-Madrigal F, Carreón-Abud Y, Martínez-Trujillo M (2015) Chromate induces adventitious root formation via auxin signalling and SOLITARY-ROOT/IAA14 gene function in Arabidopsis thaliana. Biometals 28:353–365. https://doi.org/10.1007/s10534-015-9838-8
Luis A, Río D (2013) Peroxisomes and their key role in cellular signalling and metabolism. Springer, Dordrecht, The Netherlands
Lushchak OV, Kubrak OI, Torous IM, Nazarchuk TY, Storey KB, Lushchak VI (2009) Trivalent chromium induces oxidative stress in goldfish brain. Chemosphere 75:56–62. https://doi.org/10.1016/j.chemosphere.2008.11.052
Lushchak VI (2011) Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp Biochem Physiol C Toxicol Pharmacol 153:175–190. https://doi.org/10.1016/j.cbpc.2010.10.004
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012:736837. https://doi.org/10.1155/2012/736837
Mandal M, Sarkar M, Khan A, Biswas M, Masi A, Rakwal R, Agrawal GK, Srivastava A, Sarkar A (2022) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants–maintenance of structural individuality and functional blend. Adv Redox Res 5:100039. https://doi.org/10.1016/j.arres.2022.100039
Martí MC, Jiménez A, Sevilla F (2020) Thioredoxin network in plant mitochondria: cysteine s-posttranslational modifications and stress conditions. Front Plant Sci 11:571288. https://doi.org/10.3389/fpls.2020.571288
Medrano-Macías J, Flores-Gallegos AC, Nava-Reyna E, Morales I, Tortella G, Solís-Gaona S, Benavides-Mendoza A (2022) Reactive oxygen, nitrogen, and sulfur species (RONSS) as a metabolic cluster for signaling and biostimulation of plants: an overview. Plants 11:3203. https://doi.org/10.3390/plants11233203
Mehla N, Sindhi V, Josula D, Bisht P, Wani SH (2017) An introduction to antioxidants and their roles in plant stress tolerance. In: Khan MIR, Khan NA (eds) Reactive oxygen species and antioxidant systems in plants: role and regulation under abiotic stress. Springer, Singapore, pp 1–23
Meng X, Fang Z, Wei H, Yang Z, Huang Z (2017) Genotoxicity investigation of tri-and hexavalent chromium using a yeast SUP4-o mutation assay. Carcinog Teratog Mutagen 29:65–69. https://doi.org/10.3969/j.issn.1004-616x.2017.01.013
Meyer Y, Siala W, Bashandy T, Riondet C, Vignols F, Reichheld JP (2008) Glutaredoxins and thioredoxins in plants. Biochim Biophys Acta 1783:589–600. https://doi.org/10.1016/j.bbamcr.2007.10.017
Meyer Y, Belin C, Delorme-Hinoux V, Reichheld J-P, Riondet C (2012) Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxid Redox Signal 17:1124–1160. https://doi.org/10.1089/ars.2011.4327
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Miller AF (2012) Superoxide dismutases: ancient enzymes and new insights. FEBS Lett 586:585–595. https://doi.org/10.1016/j.febslet.2011.10.048
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti BV, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16(6):300–309. https://doi.org/10.1016/j.tplants.2011.03.007
Moore M, Gossmann N, Dietz K-J (2016) Redox regulation of cytosolic translation in plants. Trends Plant Sci 21:388–397. https://doi.org/10.1016/j.tplants.2015.11.004
Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113:935–944. https://doi.org/10.1016/s0092-8674(03)00429-x
Mur LAJ, Kenton P, Lloyd AJ, Ougham H, Prats E (2008) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59:501–520. https://doi.org/10.1093/jxb/erm239
Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJ, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5:pls052. https://doi.org/10.1093/aobpla/pls052
Murphy A, Taiz L (1995) Comparison of metallothionein gene expression and nonprotein thiols in ten Arabidopsis ecotypes. Correlat Copper Toleran Plant Physiol 109:945–954. https://doi.org/10.1104/pp.109.3.945
Narendra S, Venkataramani S, Shen G, Wang J, Pasapula V, Lin Y, Kornyeyev D, Holaday AS, Zhang H (2006) The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J Exp Bot 57:3033–3042. https://doi.org/10.1093/jxb/erl060
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer C (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89:841–850. https://doi.org/10.1093/aob/mcf096
Noctor G, Reichheld J-P, Foyer CH (2018) ROS-related redox regulation and signaling in plants. In: Seminars in cell & developmental biology Redox signalling in development and regeneration, vol 80, pp 3–12. https://doi.org/10.1016/j.semcdb.2017.07.013
Novotnik B, Ščančar J, Milačič R, Filipič M, Žegura B (2016) Cytotoxic and genotoxic potential of Cr(VI), Cr(III)-nitrate and Cr(III)-EDTA complex in human hepatoma (HepG2) cells. Chemosphere 154:124–131. https://doi.org/10.1016/j.chemosphere.2016.03.118
Oakley AJ (2011) Glutathione transferases: a structural perspective. Drug Metab Rev 43:138–151. https://doi.org/10.3109/03602532.2011.558093
Ort DR, Baker N (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198. https://doi.org/10.1016/s1369-5266(02)00259-5
Ozturk M, Ashraf M, Aksoy A, Ahmad MSA (2015) Plants, pollutants and remediation. Springer, New York
Pal R, Rai JPN (2010) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160:945–963. https://doi.org/10.1007/s12010-009-8565-4
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–102. https://doi.org/10.1590/S1677-04202005000100008
Park C-J, Seo Y-S (2015) Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J 31:323–333. https://doi.org/10.5423/PPJ.RW.08.2015.0150
Paul AK (2016) Evaluation of chromate reductase activity in the cell-free culture filtrate of Arthrobacter sp. SUK 1201 isolated from chromite mine overburden. Chemosphere 156:69–75. https://doi.org/10.1016/j.chemosphere.2016.04.101
Pellerin C, Booker SM (2000) Reflections on hexavalent chromium: health hazards of an industrial heavyweight. Environ Health Perspect 108:A402–A407
Pérez-Gálvez A, Viera I, Roca M (2020) Carotenoids and chlorophylls as antioxidants. Antioxidants 9:505. https://doi.org/10.3390/antiox9060505
Pilon M, Ravet K, Tapken W (2011) The biogenesis and physiological function of chloroplast superoxide dismutases. Biochim Biophys Acta 1807:989–998. https://doi.org/10.1016/j.bbabio.2010.11.002
Potocký M, Pejchar P, Gutkowska M, Jiménez-Quesada MJ, Potocká A, de Dios Alché J, Kost B, Žárský V (2012) NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipidsand Rac/Rop GTPases. J Plant Physiol 169:1654–1663. https://doi.org/10.1016/j.jplph.2012.05.014
Pourrut B, Perchet G, Silvestre J, Cecchi M, Guiresse M, Pinelli E (2008) Potential role of NADPH-oxidasein early steps of lead-induced oxidative burst in Vicia faba roots. J Plant Physiol 165:571–579. https://doi.org/10.1016/j.jplph.2007.07.016
Qi J, Wang J, Gong Z, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38:92–100. https://doi.org/10.1016/j.pbi.2017.04.022
Queval G, Jaillard D, Zechmann B, Noctor G (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34:21–32. https://doi.org/10.1111/j.1365-3040.2010.02222.x
Rahman MM, Rahman MM, Islam KS, Chongling Y (2010) Effect of chromium stress on antioxidative enzymes and malondialdehyde content activities in leaves and roots of mangrove seedlings Kandelia candel (L.) druce. J Environ Sci 26:171–179
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Rhoads DM, Umbach AL, Subbaiah CC, Siedow TN (2006) Mitochondrial reactive oxygen species. Contribution of oxidative stress and interorganellar signaling. Plant Physiol 141:357–366. https://doi.org/10.1104/pp.106.079129
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159. https://doi.org/10.1016/S1360-1385(97)01018-2
Rodriguez E, Azevedo R, Fernandes P, Santos C (2011) Cr(VI) Induces DNA damage, cell cycle arrest and polyploidization: a how cytometric and comet assay Study in Pisum sativum. Chem Res Toxicol 24:1040–1047. https://doi.org/10.1021/tx2001465
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544. https://doi.org/10.1111/j.1365-3040.2006.01531.x
Rudnik R, Bulcha JT, Reifschneider E, Ellersiek U, Baier M (2017) Specificity versus redundancy in the RAP2.4 transcription factor family of Arabidopsis thaliana: transcriptional regulation of genes for chloroplast peroxidases. BMC Plant Biol. 17:144. https://doi.org/10.1186/s12870-017-1092-5
Samardzic K, Rodgers KJ (2017) Oxidised protein metabolism: recent insights. Biol Chem 398:1165–1175. https://doi.org/10.1515/hsz-2017-0124
Saud S, Wang D, Fahad S, Javed T, Jaremko M, Abdelsalam NR, Ghareeb RY (2022) The impact of chromium ion stress on plant growth, developmental physiology, and molecular regulation. Front Plant Sci 13:994785. https://doi.org/10.3389/fpls.2022.994785
Sawicka E, Jurkowska K, Piwowar A (2021) Chromium (III) and chromium (VI) as important players in the induction of genotoxicity—Current view. Ann Agric Environ Med AAEM 28:1–10. https://doi.org/10.26444/aaem/118228
Schmfger MEV (2001) Phytochelatins: complexation of metals and metalloids, studies on the phytochelatin synthase. Ph.D. thesis. Munich University of Technology (TUM), Munich
Semchuk NM, Lushchak OV, Falk J, Krupinska K, Lushchak VI (2009) Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol Biochem 47:384–390. https://doi.org/10.1016/j.plaphy.2009.01.009
Semchuk NM, Vasylyk IV, Kubrak OI, Lushchak VI (2011) Effect of sodium nitroprusside and S-nitrosoglutathione on pigment content and antioxidant system of tocopherol-deficient plants of Arabidopsis thaliana. Ukr. Biokhimichnyi Zhurnal 83:69–79
Semchuk NM, Vasylyk YV, Lushchak OV, Lushchak VI (2012) Effect of short-term salt stress on oxidative stress markers and antioxidant enzymes activity in tocopherol-deficient Arabidopsis thaliana plants. Ukr Biokhimichnyi Zhurnal 84:41–48
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533. https://doi.org/10.1016/j.chemosphere.2017.03.074
Shahzad B, Tanveer M, Rehman A, Cheema SA, Fahad S, Rehman S, Sharma A (2018) Nickel: whether toxic or essential for plants and environment: a review. Plant Physiol Biochem 132:641–651. https://doi.org/10.1016/j.plaphy.2018.10.014
Shaikhali J, Heiber I, Seidel T, Ströher E, Hiltscher H, Birkmann S, Dietz K-J, Baier M (2008) The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol 8:48. https://doi.org/10.1186/1471-2229-8-48
Shanker AK, Djanaguiraman M, Pathmanabhan G, Sudhagar R, Avudainayagam S (2003a) Uptake and phytoaccumulation of chromium by selected tree species. In: Proceedings of the international conference on water and environment held in Bhopal, India
Shanker AK, Sudhagar R, Pathmanabhan G (2003b) Growth phytochelatin SH and antioxidative response of sunflower as affected by chromium speciation. In: 2nd international congress of plant physiology on sustainable plant productivity under changing environment, New Delhi
Shanmugam V, Lo JC, Wu CL, Wang SL, Lai CC, Connolly EL, Huang J-L, Yeh K-C (2011) Differential expression and regulation of iron regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana—The role in zinc tolerance. New Phytol 190:125–137. https://doi.org/10.1111/j.1469-8137.2010.03606.x
Sharma D, Sharma C, Tripathi R (2003) Phytotoxic lesions of chromium in maize. Chemosphere 51:63–68. https://doi.org/10.1016/s0045-6535(01)00325-3
Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Sidhu GPS, Bali AS, Handa N, Kapoor D, Yadav P, Khanna K (2019) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 38:1–23. https://doi.org/10.1007/s00344-019-10018-x
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D (2020) Chromium bioaccumulation and its impacts on plants: an overview. Plants 9:100. https://doi.org/10.3390/plants9010100
Sharma P, Chouhan R, Bakshi P, Gandhi SG, Kaur R, Sharma A, Bhardwaj R (2022) Amelioration of Chromium-induced oxidative stress by combined treatment of selected plant-growth-promoting rhizobacteria and earthworms via modulating the expression of genes related to reactive oxygen species metabolism in Brassica juncea. Front Microbiol 13:802512. https://doi.org/10.3389/fmicb.2022.802512
Shukla T, Kumar S, Khare R, Tripathi RD, Trivedi PK (2015) Natural variations in expression of regulatory and detoxification related genes under limiting phosphate and arsenate stress in Arabidopsis thaliana. Front Plant Sci 6:e898. https://doi.org/10.3389/fpls.2015.00898
Siendones E, González-Reyes JA, Santos-Ocaña C, Navas P, Córdoba F (1999) Biosynthesis of ascorbic acid in kidney bean l-galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol 120:907–912
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254. https://doi.org/10.1007/s10311-013-0407-5
Sinha V, Pakshirajan K, Chaturvedi R (2018) Chromium tolerance, bioaccumulation and localization in plants: an overview. J Environ Manag 206:715–730. https://doi.org/10.1016/j.jenvman.2017.10.033
Skórzyńska-Polit E, Dra¸ żkiewicz M, Krupa Z (2003) The activity of the antioxidative system in cadmium-treated Arabidopsis thaliana. Biol Plant 47:71–78. https://doi.org/10.1023/A:1027332915500
Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF (2002) The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci USA 99:11640–11645. https://doi.org/10.1073/pnas.182427699
Smiri M, Chaoui A, Ferjani EE (2010) Interaction between heavy metals and thiol-linked redox reactions in germination. Pak J Biol Sci 13:877–883. https://doi.org/10.3923/pjbs.2010.877.883
Smiri M, El Ghoul J (2012) Role for plant thioredoxin in Cd2+ chelation. Int J Veg Sci 18:93–105. https://doi.org/10.1080/19315260.2011.579692
Smiri M, Jelali N, El Ghoul J (2013) Cadmium affects the NADP-thioredoxin reductase/thioredoxin system in germinating pea seeds. J Plant Interact 8:125–133. https://doi.org/10.1080/17429145.2012.689865
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19:267–290. https://doi.org/10.1080/07352680091139231
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578. https://doi.org/10.3390/ijms160613561
Srivastava S, Srivastava S, Prakash S, Srivastava MM (1999) Fate of trivalent chromium in presence of organic acids. Chem Spec Bioavail 10:147–150. https://doi.org/10.3184/095422998782775763
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Aspects Med 24:345–351. https://doi.org/10.1016/s0098-2997(03)00030-x
Stambulska UY, Bayliak MM, Lushchak VI (2018) Chromium(VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. BioMed Res Int 2018:8031213. https://doi.org/10.1155/2018/8031213
Stambulska UY, Bayliak MM (2020) Legume-Rhizobium symbiosis: secondary metabolites, free radical processes, and effects of heavy metals. In: Mérillon J-M, Ramawat KG (eds) Co-evolution of secondary metabolites, reference series in phytochemistry. Springer International Publishing, Cham, pp 291–322. https://doi.org/10.1007/978-3-319-96397-6_43
Stevens RG, Creissen GP, Mullineaux PM (1997) Cloning and characterization of a cytosolic glutathione reductase cDNA from pea (Pisum sativum L.) and its expression in response to stress. Plant Mol Biol 35:641–654. https://doi.org/10.1023/a:1005858120232
Strile M, Kolar J, Selih VS, Kocar D, Pihlar B (2003) A comparative study of several transition metals in Fenton like reaction system at circum neutral pH. Acta Chin Slov 50:619–632
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14(6):691–699. https://doi.org/10.1016/j.pbi.2011.07.014
Szewczyk K, Chojnacka A, Górnicka M (2021) Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int J Mol Sci 22:6222. https://doi.org/10.3390/ijms22126222
Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321:952–956. https://doi.org/10.1126/science.1156970
Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ (2003) Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J 34:363–375. https://doi.org/10.1046/j.1365-313x.2003.01730.x
Tumolo M, Ancona V, De Paola D, Losacco D, Campanale C, Massarelli C, Uricchio VF (2020) Chromium pollution in european water, sources, health risk, and remediation strategies: an overview. Int J Environ Res Public Health 17:5438. https://doi.org/10.3390/ijerph17155438
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462. https://doi.org/10.1016/j.cej.2016.09.029
Uraguchi S, Tanaka N, Hofmann C, Abiko K, Ohkama-Ohtsu N, Weber M, Kamiya T, Sone Y, Nakamura R, Takanezawa Y, Kiyono M, Fujiwara T, Clemens S (2017) Phytochelatin synthase has contrasting effects on cadmium and arsenic accumulation in rice grains. Plant Cell Physiol 58:1730–1742. https://doi.org/10.1093/pcp/pcx114
Vasylkiv OY, Kubrak OI, Storey KB, Lushchak VI (2010) Cytotoxicity of chromium ions may be connected with induction of oxidative stress. Chemosphere 80:1044–1049. https://doi.org/10.1016/j.chemosphere.2010.05.023
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42:579–620. https://doi.org/10.1146/annurev.pp.42.060191.003051
Wakeel A, Ali I, Wu M, Kkan AR, Jan M, Ali A, Liu Y, Ge S, Wu J, Gan Y (2019) Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ Exp Bot 161:166–179. https://doi.org/10.1016/j.envexpbot.2018.09.004
Wakeel A, Xu M, Gan Y (2020) Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int J Mol Sci 21:728. https://doi.org/10.3390/ijms21030728
Wang Y-F, Huang K-L, Li C-T, Mi H-H, Luo J-H, Tsai P-J (2003) Emissions of fuel metals content from a diesel vehicle engine. Atmos Environ 37:4637–4643. https://doi.org/10.1016/j.atmosenv.2003.07.007
Wang Z, Zhang L, Xiao Y, Chen W, Tang K (2010) Increased vitamin c content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Int Plant Biol 52(4):400–409. https://doi.org/10.1111/j.1744-7909.2010.00921.x
Wang F, Wang Z, Zhu C (2012) Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim Biophys 44:886–893. https://doi.org/10.1093/abbs/gms073
Waters ER, Vierling E (2020) Plant small heat shock proteins—Evolutionary and functional diversity. New Phytol 227:24–37. https://doi.org/10.1111/nph.16536
Wen K, Li X, Huang R, Nian H (2022) Application of exogenous glutathione decreases chromium translocation and alleviates its toxicity in soybean (Glycine max L.). Ecotoxicol Environ Saf 234:113405. https://doi.org/10.1016/j.ecoenv.2022.113405
Weyemi U, Dupuy C (2012) The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat Res 751:77–81. https://doi.org/10.1016/j.mrrev.2012.04.002
Willems P, Mhamdi A, Stael S, Storme V, Kerchev P, Noctor G, Gevaert K, Van Breusegem F (2016) The ROS wheel: refining ROS transcriptional footprints. Plant Physiol 171:1720–1733. https://doi.org/10.1104/pp.16.00420
Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1:639–647. https://doi.org/10.1016/j.celrep.2012.05.008
Wyszkowska J, Borowik A, Zaborowska M, Kucharski J (2023) Sensitivity of Zea mays and soil microorganisms to the toxic effect of chromium (VI). Int J Mol Sci 24:178. https://doi.org/10.3390/ijms24010178
Xu J, Xing X-J, Tian Y-S, Peng R-H, Xue Y, Zhao W, Yao Q-H (2015) Transgenic Arabidopsis plants expressing tomato glutathione S-transferase showed enhanced resistance to salt and drought stress. Plos One 10:e0136960. https://doi.org/10.1371/journal.pone.0136960
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76(2):167–179
Yildiz M, Terzi H, Cenkci S, Yildiz B (2012) Chromium(VI)-induced alterations in 2-D protein profiles and antioxidant defence systems of barley cultivars. Hacet J Biol Chem 40:257–265
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092. https://doi.org/10.3389/fpls.2015.01092
Yu CH, Huang L, Shin JY, Artigas F, Fan Z (Tina) (2014) Characterization of concentration, particle size distribution, and contributing factors to ambient hexavalent chromium in an area with multiple emission sources. Atmosp Environ (1994) 94:701–708
Zaffagnini M, Michelet L, Marchand C, Sparla F, Decottignies P, Le Maréchal P, Miginiac-Maslow M, Noctor G, Trost P, Lemaire SD (2007) The thioredoxin-independent isoform of chloroplastic glyceraldehyde-3-phosphate dehydrogenase is selectively regulated by glutathionylation. FEBS J 274:212–226. https://doi.org/10.1111/j.1742-4658.2006.05577.x
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249(1):139–156. https://doi.org/10.1023/A:1022504826342
Zeng F, Qiu B, Wu X, Niu S, Wu F, Zhang G (2012) Glutathione-mediated alleviation of chromium toxicity in rice plants. Biol Trace Elem Res 148:255–263. https://doi.org/10.1007/s12011-012-9362-4
Zeng F, Wu X, Qiu B, Wu F, Jiang L, Zhang G (2014) Physiological and proteomic alterations in rice (Oryza sativa L.) seedlings under hexavalent chromium stress. Planta 240:291–308. https://doi.org/10.1007/s00425-014-2077-3
Zhang F, Shi W, Jin Z, Shen Z (2002) Response of antioxidative enzymes in cucumber chloroplasts to cadmium toxicity. J Plant Nutr 26:1779–1788
Zhang Y, Tessaro MJ, Lassner M, Li X (2003) Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15:2647–2653. https://doi.org/10.1105/tpc.014894
Zhang M, Smith JAC, Harberd NP, Jiang C (2016) The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol Biol 91:651–659. https://doi.org/10.1007/s11103-016-0488-1
Zhou J, Goldsbrough PB (1994) Functional homologs of fungal metallothionein genes from Arabidopsis. Plant Cell 6:875–884. https://doi.org/10.1105/tpc.6.6.875
Acknowledgements
This work was supported by a grant from Ministry of Education and Science of Ukraine (#0122U000894).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Husak, V., Bayliak, M. (2023). Molecular Mechanisms of Chromium Tolerance in Plants: A Key Role of Antioxidant Defense. In: Kumar, N., Walther, C., Gupta, D.K. (eds) Chromium in Plants and Environment. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-44029-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-44029-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44028-1
Online ISBN: 978-3-031-44029-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)