Abstract
Chromium as an essential nutrient in livestock nutrition has been reported in early 1960s. Earlier it has been known more as heavy material which is potential bio accumulative toxin of daily production systems. More frequently the Chromium is seen in the trivalent and hexavalent structures though other oxidation states (from −2 to +6) also exist. Among the different oxidation states in which chromium is found in living organisms, trivalent chromium (Cr3+) is the most stable and is contemplated to be a highly safe form of chromium. In India there is very poor documentation available regarding the heavy metal toxicity of daily feeds. In the field study carried out a total of 142 soil, 142 water, 608 dairy ration components and 790 plasma samples of crossbred cattle were collected and screened for chromium content. The chromium concentration in soil was below the permissible limit. The dry forages like paddy straw and groundnut straw indicated Chromium to a considerable extent (<1.6 Mg/Kg). While all the green forages (except Sweet Sudan grass) did not contain chromium in detectable concentration. Highest chromium concentration was found in particularly mineral mixtures and to a lesser extent in concentrate ingredients such as rice bran, wheat bran homemade concentrate mixtures and compounded cattle feeds. Different categories of crossbred cattle under investigation indicated that, Plasma chromium concentration of anoestrous cows, pregnant cows and pregnant heifers, observed to contain higher (P < 0.01) levels, followed by anoestrous heifers while lowest in lactating cows. The study indicated that there are no toxic levels of chromium in soil and dairy feeds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Mineral status of the livestock can be assessed by the sampling of forages fed to the animals and the soil in which they are cultivated. In many developing countries (including India) soil, plant and animal systems were not thoroughly examined because of several intricacies. Acquiring the information on the mineral status of the livestock, understanding of the interrelationships of various inorganic minerals in the forenamed system is essential for obtaining augmented livestock productivity. Both essential and nonessential elements can be toxic when levels exceed a certain threshold. Excessive deposition of toxic heavy metals particularly in the soil is a consequence of human activities which is often referred as heavy metal contamination. When these heavy metals enter the food chain it is highly hazardous to the nature and organisms in the environment. The concentration of essential and contaminant heavy metals such as chromium are potential bio-accumulative toxins in production system of livestock because the soils serve as long term sinks for such toxic metals. They are primarily absorbed on to organic matter of soil, followed by different forms of humified natural organic matter which also receive a larger concentration. Apart from this, metal oxides especially iron and manganese oxides, clay minerals etc. also incorporated with them (Li et al. 2005). The presence of high concentrations of manganese in the soil, as well as alkaline pH levels, may affect oxidation processes. When the forages are grown in such polluted environment, toxic minerals are absorbed from the soil under specific soil conditions as well as from the metal which is deposited on the surfaces of the plants. In addition, excess use of fertilizers with heavy metals used in the agriculture is the additional sources for heavy metal accumulation. Heavy metals exhibit a different physical and chemical properties based on which their mobility pattern in the soil takes place. Generally, some low amount of losses takes place as a consequence of absorption by the crops grown in that area, soil erosion and leaching (Aldrich et al. 2002). The soil productivity is diminished when there is long term accumulation of heavy metals in agricultural soil, which in turn inhibit the populations of microbes and fauna and that may create a risk to animal, human and health of ecosystem.
Among the essential trace minerals Chromium (Cr) is also listed which having importance biological system. Similar to many other minerals, Cr also plays several important roles in the metabolism of living organisms (Tokalioglu et al. 2000; Tokaliglu and Kartal 2005). A variety of metabolism related roles are played by Cr in both animals as well as plants (McDowell 2003). In man and laboratory animals Chromium has been considered as an essential trace element (Anonymous 1997), that comes from anthropogenic sources. It is seen in different oxidative conditions, but the three and six oxidation states are most commonly found in nature. The hexavalent form of Cr is an oxidizing mineral which is employed in most of the processes related to industry such as electroplating, welding and chromate painting. Trivalent chromium is considered to be essential to carry out normal carbohydrate, lipid, protein metabolism and the maintenance of nucleic acid (NA) structural integrity. Biologically active form of Chromium is found as part of an oligopeptide—chromodulin—potentiating the effect of insulin by facilitating insulin binding to receptors at the cell surface (Amata 2013). In carbohydrate and lipid metabolism the trivalent form of Cr is considered to be the most essential (NRC 2001). The importance of Chromium in the organism is related to the functionality of insulin, as a cofactor (Cr3+ is a component of glucose tolerance factor-GTF) it enhances the activity of insulin (Pechova and Pavlata 2007). The important role played by Cr3+ ions in enhancing insulin activity is by activation of the insulin receptor tyrosine kinase, and also it potentiates it by three-fold.
Because of ubiquitous nature of Cr it exists in air, water, soil and biological materials, and hence the supplementation is generally not considered essential. Through limited studies available, Cr supplementation in swine production reported to have beneficial responses but in dairy cattle not that encouraging (European Commission 2003). In spite of the fact that in most of animal species Cr is relatively harmless but, ingestion of high dose (30–40 mg/kg of B. wt./day) of Cr in dairy calves has resulted in toxicity (European Commission 2003).
Despite of the fact that Cr plays substantial role of in various physiological processes in ruminants, information on evaluation of mineral status of grazing ruminants, is quite limited, especially pertaining to the availability of Cr in the biological system of animals. The Chromium concentration required in feeds of livestock ranges between 0.3 and 1.6 mg/kg (Anonymous 1997) which is generally higher than the available Cr to livestock through livestock feeds. When Chromium levels exceed than these range which are deemed to be not only toxic to livestock but also awkwardly influence the reproductive potential of ruminants (McDowell 2003; McDowell and Arthington 2005). In view of the importance of this element to livestock, the premier focus of the present investigation was set to authenticate the practicability of broad feeding pattern for free grazing ruminants rather than appraising the actual daily ingestion of Cr for animals.
In the assessment of mineral status of livestock, sampling of forages offered to livestock and soil used for cultivation these forage plants is the chief activity involved. The sample and sampling procedure have the greatest value which need to be collected from soil, plant and biological tissues of animal in question and also depends upon the mineral to be determined from these variables used for assessment (McDowell 1985). Present investigation was planned in Chittoor district of Andhra Pradesh in India, in order to determine the chromium levels in soil (grazing and cultivated), drinking water, concentrate feeds, fodders (green/dry) as well as in plasma of crossbred dairy cattle.
15.2 Materials and Methods
15.2.1 Samples Collection
The study was conducted at Chittoor district (13.2172°N, 79.1003°E) in Andhra Pradesh state (India), which is classified under southern agro- climatic zone of Andhra Pradesh state. The district comprises of four revenue divisions and 66 revenue mandals. One village from each mandal was selected, which were geographically located apart in direction and a total sixty-six mandals of the district in which sampling procedure was carried out.
A minimum of two soil (grazing and cultivated land) samples from each mandal were collected (total of 142 soil samples). From four different sites within the pasture/ grazing land, samples of soil were collected randomly. From the surface of the soil a minimum 15–20 cm depth was considered to collect the soil samples. Initially in the sampling area, surface litter was removed from the surface of the sampling point before taking the soil sample. Zigzag sampling design was followed, using a spade a depth of 20 cm (i.e. from the surface of soil to 20 cm beneath the soil surface) was cut and a thin portion of soil with about 5 cm thick was collected. Then this segment of soil was placed in pail and mixed with hands. A sub-sample of 300–500 g from the bulk soil sample was collected. This sample was placed in a plastic sample bag which contained label on the outside. Soon after the soil samples arrive at the laboratory, air drying at room temperature (25–35 °C) was carried out. Once soil samples were air dried, they were sieved by using a 2 mm sieve. All samples of soil collected were dried in the oven at appropriate temperature (100 ± 5 °C) until the constant dry weights were obtained.
With regard to water sampling, a minimum of two water (bores, canals, wells and river streams etc.) samples were collected (total of 142 samples) by composite sample method into a clean sterile collection bottle. During the preplanned time/after prefixed flow, smaller samples were collected in succession and mixed in the same container. When the samples were obtained by compositing the integrated samples of equal volume across the cross section were taken from different places at different depths-with equal flow, so that the overall composition of a stream has been represented. In larger water sources, because of the requirement of large number of samples, it was determined by section of a stream, along with depth and cross section where the water constitution will be constant.
The forage/fodder samples were collected at random from four different locations within the grazing area and pasture land which were being used for feeding of cattle. All forage/fodder samples collected were dried in hot air oven at appropriate temperature (80 °C) until constant dry weights are observed. Different feeds and fodders (Green and dry) offered (total 528) to the crossbred dairy cattle, by the farmers at the household level were also collected from each farmer in the study area. After collection all the representative samples of feeds and fodders, were subjected for drying in forced draft oven (at 80 °C for 24 h), subsequently pulverized to pass through 2 mm size sieve and were stored plastic sample bags in moisture free condition, till further analysis.
From each village a minimum of 10 crossbred dairy cattle in diverse physiological phases (lactating cows, pregnant cows, pregnant heifers, cows in anoestrous condition and heifers in anoestrous condition) were considered for collection of blood samples. The jugular vein was punctured in an aseptic manner and blood was drawn and transferred in to heparinized blood collection vials (a total of 790 samples). After collection the blood samples were subjected for centrifugation process at 3000 rpm for about 20 min so that plasma was separated. After collection plasma samples were stored in deep freeze, (at −20 °C) for further analysis.
15.2.2 Processing of Samples
Soil sample (0.5 g) was dissolved in 0.1 N HCl and then a pinch of charcoal was added to prepare mineral extract. Then it was filtered by using no. 1 Whatman filter paper. Then diluted with double glass distilled water to get appropriate concentration for analysis (Singh et al. 1999). When water sample (45 ml) was digested, 5 ml of concentrated nitric acid was used. Similarly digesting 0.5 g of feed/fodder sample 15 ml of nitric acid was used. Whereas 2 ml of plasma sample digested with 15 ml of nitric acid. The micro wave sample digester (CEM Mars X-press) was used in the digestion process of all samples. All the digested samples were filtered through Whatman filter paper no. 1 to obtain mineral extract and dilution was carried out with double glass distilled water to get the suitable concentration. The Chromium was estimated in the mineral extract prepared after digestion of the samples, in an atomic absorption spectrophotometer (Perkin Elmer, Avanta PM-A-6287), with 0.1 ppm detection limits. For the determination of chromium monoelement hollow cathode lamp was employed. Initially the calibration of AAS was carried out by following the recommendations of instrument manufacturers. Absorbance reading was plotted on the Y-axis and that of concentration of different standard solutions of Cr mineral on X-axis for building up a standard curve was prepared by versus. Then, the AAS reading of sample of interest was plotted on the standard curve, and then concentration of Cr metal was calculated in the sample.
15.2.3 Statistical Analysis
The data generated on the Cr content of the samples (Viz. soil, water, feed/fodder and plasma) were subjected for statistical analysis with the help of SPSS software. Each variable was worked out with a one-way analysis of variance (ANOVA).
15.3 Results and Discussion
15.3.1 Soil
The results of soil analysis (Table 15.1) revealed the average chromium concentration was 4.08 ppm (observed the range from 2.4 to 6.2 ppm). The detected range of chromium in all the soil samples collected found to be less than the permissible ceiling of 100 ppm (Misra and Mani 1978) and within the reported range by Devasena et al. (2012). There was a variation in soil Cr content due to the season has been reported. Low amount of Cr was reported during December as compared to high concentration during January and during the entire study period which observed to be in the range of 0.006–0.007 mg/kg (Khan et al. 2010c). Contrary to this higher soil Cr content reported in some earlier studies (Hodgson 1990). It was opinioned that, soil Cr concentration was significantly influenced by the time of sampling. Elevated levels of Cr in soil samples was found during the October (1st sampling) and lower amount of soil Cr was found during the April (4th sampling) as reported by Danish et al. (2014). Besides, chromium found abundantly to a much greater degree in soil as compared to the crops (Underwood and Suttle 1999). In the present study the low Cr level was observed, for which the alkaline soil pH (7.30–7.63) could be a reason. Higher exchangeable Cr takes place in the acidic soil than in the alkaline soil which has been evidenced in several reports (Xu et al. 2020). Presence of ferrous oxide and manganese oxide in the soil aggravate the deposition of heavy metal (Li et al. 2005). In agricultural soils the major routes of heavy metal load predominantly include atmospheric deposition, sewage sludge, animal manures, agrochemicals and inorganic fertilizers. While the losses takes place due to the uptake by cultivation of crops or livestock products, soil leaching and erosion. (Nicholson et al. 1999).
15.3.2 Water
The analysis of water for pH indicated a faintly alkaline range from 7.33 to 7.69. In all water samples (142) analyzed, chromium was not in the detectable concentration. Water quality in addition to availability is extremely important for animal health and productivity. Even low concentrations of water contaminants effect animal performance when consumed over a long period (NRC 2001). In nature Hexavalent form of chromium happens commonly. Water solubility is more for Hexavalent chromium compounds as compared to trivalent chromium compounds. During the process of disinfection of water oxidization of trivalent chromium to hexavalent chromium takes place. In drinking water the amount of acquired hexavalent chromium for which the primary source is from oxidation of naturally occurring chromium present in combustible geologic formations. Chromium compounds have been released to the various locations of the environment by means of effusion, defective storage and due to inappropriate industrial waste discarding practices. In water the Chromium compounds either trivalent or hexavalent chromium are very pertinacious (USEPA 2011).
Present results indicated that, there was no contamination of chromium from other sources into either soil or water. As per the guidelines of World Health Organization (WHO 2022) the maximum total permissible chromium content for drinking water is 0.05 mg/L. At present, there are no standard integrated regulations for chromium in drinking water for individual livestock species (WHO 2022). The United States Environmental Protection Agency (USEPA) has set a maximum contaminant level of 100 ppb or 0.1 mg/L (USEPA 2011) in drinking water and it is the regulatory authority for setting the maximum permissible level of total chromium in standards of drinking water.
15.3.3 Forages/Roughages
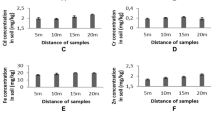
Paddy straw (0.16 ppm) and groundnut straw (0.49 ppm) contained chromium to a considerable extent among the dry roughages analyzed (Table 15.2). Out of dry forage samples (153) analyzed, paddy straw (88.3%) and groundnut straw (80.63%) samples contained chromium and in the others types of dry roughages it was not in the range that could be detected. In case of green forages (among the 175 samples), all samples of SSG-898 contained (0.03 to 0.29 ppm) chromium, while in other fodder (138) and tree foliage samples (20) analyzed the Cr concentration was not in the range that could be detected. The Cr concentration in the forage reported by Danish et al. (2014) ranged from 2.9–4.0 mg/kg and was greater than the range observed in the present study. It has been reported that different plant parts contain variable amount of Cr (Anderson et al. 1990). While relatively low chromium concentration (0.002–0.0025 mg/kg) of forages was reported by Khan et al. (2010c) which was not toxic for animals being reared. Chromium enters the food chain of living organisms when the cell walls of plants passively take up Cr3+ from the soil, through cation transport/exchange sites. The quality and availability of pastures in semi‐arid areas is substantially affected in dry season due to scant rainfall patterns, when more rainfall is experienced improvements in availability and quality of grasses are seen. But forages are observed to be acutely deficient as compared to the requirement for growth and development of ruminant livestock which is suggesting the need for supplementation. The Cr concentration of fodder/forage samples in the present evaluation was found to be variable as compared to the reported values in literature but was not in the toxic range (Devasena et al. 2012).
15.3.4 Feed Ingredients
In case of concentrates a total of 200 samples analysed out of which 12% contained chromium in the detectable range. Among the rice bran (0.22 ppm) samples 80 and 44% of wheat bran (0.17 ppm) samples contained chromium to a considerable extent. But in gram chuni, cereal grains (Maize and Bajra grain), different protein supplements (Groundnut cake, Soybean meal and Sun flower meal, Homemade concentrate mixture) chromium was not detected. Compounded cattle feed which was commercially available in the market found to contain 0.51 ppm chromium and 57% of the samples were detected with chromium. Islam et al. (2016) reported higher levels of chromium (1.64–1.71 ppm) in commercial broiler feed. Considerably lower chromium concentration was observed than the NRC (2005) set permissible limit (500 ppm or μg/g). Whereas in the present investigation the Cr concentrations noticed are indicating lower concentration than the values reported by Li et al. (2005). However, higher Chromium concentration in protein meal feed (10–218 mg/Kg) was reported by Jothi et al. (2016). This therefore was suggestive that, regular screening for detection of heavy metals needed for protection from harmful effects.
In the present study 20% of the home made concentrate mixtures shown to contain the chromium in the range of 0.21–0.28 ppm. These compounded/homemade concentrate mixtures contained chromium because the brans often form the major component of the concentrate mixtures (prepared by the farmers at their home and commercially prepared compounded feeds which are marketed being used by the farmers in this area), since during the analysis brans were detected with considerably higher concentration of chromium could be the reason.
Accurately measuring naturally occurring Cr in feed ingredients is difficult because of Cr contamination that can occur during harvesting, processing, and collection of samples as well as laboratory analysis of Cr. Grinding whole grain samples, de-oiled meals, by products like brans and chunies in Wiley mill which pass through a stainless steel screen greatly increased analyzed Cr concentrations. Appreciable amount of Cr found in the feed ingredients emerge as a result of contamination from soil as well as due to metal proximity in the process of cultivation, harvesting of crops, procedure of feed ingredients preparation or combination of both (Spears et al. 2017).
15.3.5 Mineral Mixtures
In case of mineral mixtures 71.4% were containing chromium in the detectable level with average content of 28.1 ppm (18.4–49.4 ppm) which indicated moderately higher concentration of chromium as compared to the other feed ingredients analysed. Higher levels of Cr observed in the feed stuffs might be due to the mixing of these feed ingredients in processors and stainless steel containers (which particularly contain 18% Cr), may give rise to increased level of Cr in feed stuffs as a result of contamination. As per NRC (2001), acceptable normal level of chromium in the rations of livestock is 1.6 mg/Kg and that of upper limit for toxicity of Cr is 1000–3000 mg/Kg. Present research results suggested that, the Cr levels observed with regard to most of feed/fodder samples were quite lower than the typical values reported (Li et al. 2005). As per the research data available, maximum threshold limit of the Cr concentration was not available but a concentration range between 0.03 and 1.0 mg/kg was suggested in majority of studies. Chromium reported in the salt-range of Pakistan was in the range of 0.156–0.285 mg/g (Khan et al. 2010a, b). Whereas lower concentration (from 0.0003 to 0.0006 mg/L) was observed in central Punjab of Pakistan (Raj et al. 2006). Although there is no evidence of unfavorable effect of higher Cr concentration in forage and blood plasma, still the concentration can be decreased with the plants that absorb reduced quantity of chromium. Moreover, supplementation of area specific minerals and minerals mixture with specific combination in the livestock is suggested in view to conserve the environment and safeguard the human health.
The cycling of many heavy metals through the dairy food chain occurs which is possibly to be restricted by the soil–plant barrier in a closed system (Chaney 1990). The soil acts as barrier which limits transmission of metals in to the food chain by different chemical processes which might have been involved in the regulation of the bioavailability of the metals. The soil cation exchange capacity is the principle component, which is governed in turn, by other soil chemical properties such as pH, salinity, macro nutrients, micro nutrients like different minerals and their concentration (Alloway 1995). At times the soils are subjected for alterations, due to the addition of bio-solid wastes or manures because of theses specific properties in the modification the bio-availability of the metals will be diverged (Chaney 1990). Similarly even the certain barriers present in plant restricts transmission of certain heavy metals into the food chain, in view of the fact that metals are phytotoxic because of which considerable yield reduction occurs before the crop would results in risk of metal overdosing during the ingestion by live-stock in their lifetime.
15.3.6 Animals
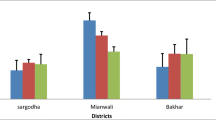
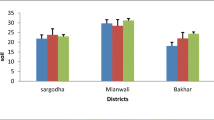
Chromium concentration determined in the plasma of various categories of crossbred cattle summarized in Table 15.3. The outcome of the experimentation indicated that cows in pregnancy, pregnant heifers and cows in nonproductive condition were significantly (P < 0.01) higher (ranged from 0.31 to 0.33 ppm) in contrast to heifers in nonproductive stage (0.27 ppm) and cows which were lactating (0.15 ppm). The variations observed were remarkable (P < 0.05) in plasma concentration amongst the animals of diverse physiological categories and in between the animals belonging to four revenue divisions which can be attributed to differences in feeding practices adopted by the farmers (Devasena et al. 2012). The animals in the district were fed by paddy straw and groundnut straw to the major extent where as green fodder offered to a very limited extent. The supply of concentrate feeds was limited to those animals which are in productive condition (Lactating animals). In addition to this, when the animals are reared under grazing system, annual ingestion of about 600 kg of soil in dairy cows has been reported in many parts of the world (Healy 1974), which might be another contributing factor for the plasma levels of the minerals though the levels are considerably less in most of the feeds. The plasma Cr content varied between 3.5 and 4.3 mg/L. The variability in the concentration of Cr is presumed to be due to the divergent sources of blood collection (Young/growing animals, lactating dairy animals and non productive/dry animals) which might influence the amount of Cr level as well as there might be some synergy between time of collection in a year and source of blood collection which exemplify the concentration level.
There were reports which indicated that higher blood Cr levels were found during February and lower amounts during December (Danish et al. 2014). The higher blood Cr concentration was observed than the suggested value by different authors (Khan et al. 2010a, b; Raj et al. 2006). The required range for Cr concentration in livestock is 0.3–1.6 mg/kg (Khan et al. 2010a, b) and the variability do exist which might be there due to the type of condition of animals (young growing calves, lactating dairy cows and dry/pregnant cows) in which the blood was examined. In spite of the detrimental effect of higher Cr concentration in blood plasma, which is a result of higher Cr in forage that are fed to the livestock, yet it can be decreased by growing the plants that absorb less quantity of chromium. Limited information is available about the Cr concentrations in feeds and fodders fed to the livestock and even barely known regarding Cr bioavailability from feedstuffs which are commonly offered to livestock at field level (Bryan et al. 2004). Moreover, it was suggested that supplementation of area specific minerals mixture in rations of livestock would be beneficial which not only conserve the environment but also safeguard human health (Danish et al. 2014).
In spite of the fact that recycling of several heavy metals through the dairy food chain occurs which is possibly to be restricted by different variables such as cation exchange capacity of soil, salinity, pH, phytotoxicity of the metal etc. In dairy system it is essential that the sources of that particular metal should be recognized for effective prevention of such complications connected with accumulation in soil. The reproductive potential of ruminants may badly be affected due to the elevated concentrations which are toxic to livestock (Raj et al. 2006). Present study revealed that the analysis of samples of soil, feeds, fodders and biological samples of animals carried out to determine the chromium content revealed to contain lower than the permissible levels (NRC 2001) which suggested that, chances of cumulative toxicity are less.
15.4 Conclusion
At present times, alarming level of heavy metal pollution in livestock feeds and fodders is most obvious throughout the world. Present research results on determination of chromium reflected existence of nontoxic levels in soil, water and dairy feeds and fodders. The chromium concentration in plasma of crossbred cattle of different age and physiological groups was lower than the permissible levels (NRC 2001) which indicated that, chances of cumulative toxicity are less.
References
Aldrich AP, Kistler D, Sigg L (2002) Specifications of Cu and Zn in drainage water from agricultural soils. Environ Sci Technol 36:4824–4830
Alloway BJ (1995) Soil processes and the behaviour of metals. 11–37 in Heavy metals in soils. 2nd edn. B. J. Alloway, ed. Kluwer Academic Publ., Dordrecht, The Netherlands
Amata IA (2013) Chromium in livestock nutrition: a review. Glob Adv Res J Agricult Sci 2(12):289–306 (Special Anniversary Review Issue). ISSN: 2315-5094. http://garj.org/garjas/index.htm
Anderson RA, Polansky MM, Bryden NA, Kanary JJ, Mertz W (1990) Seventh international symposium on trace elements in man and animals, Dubroobnik, Yugoslavia
Anonymous (1997) The role of chromium in animal nutrition. National Academy Press, Washington, DC
Bryan MA, Socha MT, Tomlinson DJ (2004) Supplementing intensively grazed late-gestation and early-lactation dairy cattle with chromium. J Dairy Sci 87:4269–4277
Chaney RL (1990) 20 years of land application research. Biocycle 31:54–59
Danish M, Ahmad N, Sharif I, Shahzad MN, Rizvi SS, Nazar MF (2014) Inter-relationshipof Soil-Forage-Plasma, and Milk Chromium: a case study in an arid Region of Pakistan. J Environ Anal Toxicol 4(3):21
Devasena B, Ramana JV, Prasad PE, Sudheer S, Prasad JR (2012) Chromium concentration in soil, feeds and plasma of animals in Chittoor district of Andhra Pradesh. Indian J Anim Nutr 29:384–387
European Commission (2003) Opinion of the scientific committee on animal nutrition on undesirable substances in feed. European Commission, Health and Consumer Protection Directorate, Brussels, Belgium
Healy WB (1974) Ingested soil as source of elements to grazing animals. In: Hockstra WG, Suttle JW, Gouther HE, Mertz W (eds) Trace element metabolism in animals-2. University Park Press, Baltimore, USA, pp 448–450
Hodgson J (1990) Grazing management: science into practice. Longman Scientific and Technical, Wiley, New York
Islam MM, Kabir SML, Sarker YA, Sikder MMH, Islam SKS, Akhter AHMT, Hossain MM (2016) Risk assessment of chromium levels in broiler feeds and meats from selected farms of Bangladesh. Bangladesh J Vet Med 14(2):131–134
Jothi JS, Yeasmin N, Anka IZ, Hashem S (2016) Chromium and lead contamination in commercial poultry feeds of Bangladesh. Int J Agril Res Innov Tech 6(2):57–60
Khan ZI, Ahmad K, Raza N, Al-Qurainy F, Ashraf M (2010a) Assessment of chromium concentrations in soil-plant-animal continuum: possible risk for grazing cattle. Pak J Bot 42:3409–3414
Khan ZI, Ashraf M, Mukhtar MK, Raza N, Ahmad K, Akram NA (2010b) A study on the transfer of iron in soil–plant–animal continuum under semi-arid environmental conditions in Sargodha, Pakistan. Biol Trace Element Res. https://doi.org/10.1007/s12011-010-8799-6
Khan ZI, Ahmad K, Raza N, Al-Qurainy F, Ashraf M, Hussain A (2010c) Assessment of chromium concentrations in soil-plant-animal continuum: possible risk for grazing cattle. Pak J Bot 42(5):3409–3414
Li Y, Mc Crory DF, Powell JM, Saam H, Jackson-Smith D (2005) A survey of selected heavy metal concentrations in dairy feeds. J Dairy Sci 88:2911–2922
McDowell LR (1985) Nutrition of grazing ruminants in warm climates. Academic Press, San Diego
McDowell LR (2003) Minerals in animal and human nutrition, 2nd edn. Amsterdam. Univ, Florida
McDowell LR, Arthington D (2005) Minerals for grazing ruminants in tropical regions. Extension bulletin. Animal Science Department, University of Florida, USA
Misra SG, Mani D (1978) Soil pollution. Ashish Publishing House, New Delhi
Nicholson FA, Chambers BJ, Williams JR, Unwin RJ (1999) Heavy metal contents of livestock feeds and animal manures in England and Wales. Biores Technol 70:23–31
NRC (2001) Nutrient requirements of dairy cattle. National Academy press, Washington, DC
NRC (2005) Mineral tolerance of domestic animals, National Research Council 2005. National Academy press, Washington, DC
Pechova A, Pavlata L (2007) Chromium as an essential nutrient: a review. Vet Med 52(1):1–18
Raj BG, Patnaik MC, Babu SP, Kalakumar B, Singh MV (2006) Heavy metal contaminants in water-soil-plant-animal continuum due to pollution of Musi river around Hyderabad in India. Indian J Anim Sci 76:131–133
Singh D, Chhonkar PK, Pandey RN (1999) Soil, plant, water analysis. A methods manual. ICAR, New Delhi
Spears JW, Lioyd KE, Krafka K (2017) Chromium concentrations in ruminant feed ingredients. J Dairy Sci 100(5):3584–3590
Tokalioglu S, Kart S, Gunes S (2000) Determinations of heavy metals in soil extract and plant tissues at around of a zinc smelter. Int J Environ Anal Chem 80:2010–2017
Tokaliglu S, Kartal S (2005) Determination of Cu, Pb, Cd, Ni, Cr, Co, Mn, Fe, and Zn in algae and vegetable samples using wet and dry ashing procedures. Trace Elem Electr 22:169–173
Underwood EJ, Suttle NF (1999) The mineral nutrition of livestock, 3rd edn. Midlothian, UK, pp 283–392
USEPA (2011) Basic information about chromium in drinking water. http://water.epa.gov/drink/contaminants/basicinformation/chromium.cfm
WHO (2022) Guidelines for drinking-water quality. World Health Organization, Geneva
Xu T, Nan F, Jiang X, Tang Y, Zeng Y, Zhang W, Shi B (2020) Effect of soil pH on the transport, fractionation, and oxidation of chromium (III). Ecotoxicol Environ Saf 195:110459
Acknowledgements
The authors highly acknowledge the financial support provided by a grant from the ICAR, New Delhi, for this study. The authors wish to thank the Andhra Pradesh State Animal Husbandry Department officers for their help in collecting the samples during the survey and SVVU, Titupati, Andhra Pradesh, for the support rendered to execute this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Devasena, B., Ramana, J.V. (2023). Chromium Profile in Soil, Fodders and Plasma of Crossbreed Cattle. In: Kumar, N., Walther, C., Gupta, D.K. (eds) Chromium in Plants and Environment. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-44029-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-44029-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44028-1
Online ISBN: 978-3-031-44029-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)