Abstract
This study was conducted to determine the concentration of toxic metals in soil, forage and blood plasma of lactating and non-lactating buffaloes in the district Jhang, Punjab, Pakistan. Soil samples were collected from varying distances from the road side. Plasma separation was achieved by centrifugation. The concentration of arsenic (As), selenium (Se), cadmium (Cd), chromium (Cr), iron (Fe), zinc (Zn), copper (Cu) and cobalt (Co) were determined by using Atomic Absorption Spectrophotometer. The results of the study showed that the mean As, Se and Cd concentrations in soil samples were lower while Cr, Fe, Zn, Cu and Co were higher than the official guidelines. In plasma samples, mean concentration values of Co, Zn, Fe, Cd, Se and As were lower while Cu and Cr were higher than the recommended concentrations. According to the results of the study there was no potential exposure of toxicity in buffaloes of the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
As natural components of ecosystems, metals and their allied compounds are present in all organisms, plants and soils, though in small quantities (Ugulu 2015c). Both natural and anthropogenic processes result in distribution of metals in environment (Dogan et al. 2014a). Metals are removed naturally through biogeochemical cycles, but human activities have contributed to enormous quantities in environment even exceeding the rate of removal (Carneiro et al. 2016). Therefore, it is necessary to monitor their environmental levels and changes in ecosystems (Ugulu et al. 2012; Dogan et al. 2014b). The use of direct methods, in which the concentration of elements or their compounds is determined in air, soil and water, does not fully reflect the degree of the threat to humans and animals (Durkan et al. 2011; Ugulu 2015a; Ugulu et al. 2016). For this reason, measurements of chemical residues in tissues of animals are of growing importance for environmental studies. Biological methods that include bioindicators are used to define the effect of harmful substances on living organisms (Ahmad et al. 2018).
Environmental researches often use animals as indicators of the degree of environmental pollution with toxic metals. Identification of toxic metal concentrations in the organs of animals provides an indirect measure of environmental pollution while making it possible to determine the extent of exposure of animals to these elements in each area (Wieczorek-Dabrowska et al. 2013). Livestock are among the common animals used for bioindication/biomonitoring of environmental pollution in land ecosystems. Their biomonitoring results mainly from relatively long life-span, huge availability of basic data and relatively simple sampling procedure (Unver et al. 2015).
Livestock is one of the major sub-sectors of agriculture in Pakistan. It contributes 12% to the gross domestic product and provides milk, meat, leather and manure. Rural communities are heavily dependent on this sector (Chandio et al. 2016). The nutritional needs of livestock are fulfilled by fodder crops and minerals in soil. Forage analysis indicates the level of microminerals in soil (McDowell 2003). However, animal tissue, such as liver and kidney, and blood samples are better indicators of potential toxicity than forage due to diet selection by grazing animals (Khan et al. 2009). Plants and animals concentrate potentially toxic metals over time; trace metals in soil in are assimilated by plants up to toxic levels and are then ingested by livestock and may cause damage to both animals and human health (Ahmad et al. 2010). These metals can cause adverse effects even at relatively low concentrations (Dogan et al. 2010; Ugulu 2015b).
In general, the aims of monitoring the animals include data collection to: (1) estimate human health risks; (2) determine contamination of the food chain; (3) define the levels of environmental contamination; (4) identify negative effects on animals; and (5) assess spatial or temporal trends in pollution levels (Carneiro et al. 2016). Present study was conducted to determine the concentration of potentially toxic metals in soil, forage and blood plasma samples of buffaloes in district Jhang, Pakistan. The main purpose was to evaluate how the levels of potentially toxic metals present in the soil affect the levels of metals in forage and blood plasma of buffaloes that consume vegetation (forage) of that area.
Materials and Methods
The study was undertaken in Shorkot, Jhang City, Pakistan located on the east bank of Chenab River (31.30677 N, 72.32814 E). Jhang City is characterized by extreme climate and the temperature is generally hot despite significant variations between summer and winter. In the plain the mean June temperature is 35 °C, while the mean January temperature is 12°C.
The pastures in the study area had shown a diversity of forages of several species. At the time of survey dominant forages species were Zea mays, Sorghum bicolor, Pennisetum glaucum and other grasses. Mostly the animals of Shorkot livestock were foraged on the mentioned forages. They were fertilized the soil with 25 kg urea/ha.
Thirty-six samples of soil and forage each of grazing animals were taken in November 2014. Soil samples were taken at 5, 10, 15 and 20 m distance from the road at a surface depth of 20 cm. At the same site from where soil samples were taken 36 samples of different forages which were mostly feed by animals were collected. The forage samples were collected from the height of 3–6 cm from ground to stimulate the grazing behavior of animals. Constant weight was achieved after drying out the samples. Heparin (anticoagulant) containing plastic syringes were used to draw blood (15 mL) from the jugular vein. Plasma separation was achieved by centrifugation.
Twenty healthy buffaloes were randomly selected for blood sampling at the livestock station. Two cohorts were made (non-lactating buffaloes and lactating) of ten buffaloes each. All animals were of 4–5 years and with an average weight of 70–100 kg. Each animal had a specific number imprinted clearly on animal’s body. 15 mL of blood samples were taken out from these 20 animals through syringes and few drops of heparin added to avoid from blood clotting.
In preparation process, air dried samples of soil were further dried in oven for 72 h then grinded soil samples with the help of grinder. Then the powdered soil samples pass through the mash having size of 1 mm. Then 1 g of dry and powdered soil transferred to digestion flask which was covered with 8 mL of H2SO4. After this, mixture of soil and acid heated for 30 min. Further 10 mL of H2SO4 added and heated until residue became bright. After this, 4 mL of H2O2 was added and again heated the mixture. At the end, the solution was diluted with distilled water up to 20 mL and kept in the glass tubes until analysis of metals.
Air dried samples of forage were further dried in an oven for 72 h then grinded with the help of grinder to pass the grinded forage through a mash having size of 1 mm. 1 g of forage samples were digested with H2SO4 and H2O2 (4:2) at the temperature of 250°C for 3–4 h until solution become colorless and thick white fumes appeared in the flask. The contents of flask were washed with pure distilled water, filtered through filter paper and samples were dilute up to 50 mL.
The heparinized blood was centrifuged. Then 2 mL of blood plasma were mixed with 2 mL of H2SO4 and the mixture of sample was allowed to stand overnight for digestion purpose. The mixture of sample was digested by heating it at 120°C until all the organic matter was dissolved. 2 mL of H2O2 was added to enhance the process of digestion by oxidation process. The digested samples were cooled and dilute the digested samples up to 50 mL with ultrapure water and stored in glass tubes till analysis.
The concentrations of heavy metals were determined by using Atomic Absorption Spectrophotometer (AAS) (Shimadzu double beam AA-6300 and Perkin Elmer Aanalyst 400). The detection limits of AAS is given in Table 1. As and Se were determined with the very sensitive hydride technique. The following operating conditions were used for As and Se analysis (Table 2).
To make sure the stability of results, the quality assurance was done by measurements of natural matrix certified reference material (CRM-1570) and the measurements of duplicates for each batch of samples. To avoid contamination, the samples were handled cautiously. During the study, double distilled water was used, and glass ware was cleaned methodically. For verification of investigative measures, a study was done by homogenizing the analyzed samples with a varying quantity of standard solution of each metal. The results showed the certified value for each metal within ± 2%.
Applied to examine the proportion of concentration of metals in plants to that in corresponding soil. It was used to evaluate the uptake of metals from soil and their bioaccumulation in vegetable using the following formula:
And similarly, bioaccumulation factor (BAF) from forage to blood plasma:
Results and Discussion
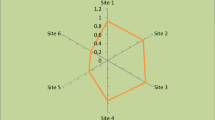
The accumulation levels of the metals in 36 soil samples collected from 5, 10, 15 and 20 m distance from the road of Shorkot City are as follows: The contents of As, Se, Cd, Cr, Fe, Zn, Cu and Co ranged from 12.01 to 14.89, 0.70 to 0.94, 1.97 to 2.18, 0.18 to 0.22, 17.22 to 20.14, 1.84 to 2.08, 2.35 to 3.89, and 6.57 to 8.14 mg/kg, respectively (Fig. 1).
Maximum permissible limits (MPL) of the As, Se, Cd, Cr, Fe, Zn, Cu and Co accumulation in soil were reported as 20, 3, 3, 100, 50,000, 300, 100 and 50 mg/kg, respectively by WHO, FAO, Standard Guidelines in Europe (Chiroma et al. 2014) and USEPA (2002). The values of metals in the present study were lower than these MPL in soil samples.
In other studies, in Jhang City, Khan et al. (2016a) identified the As, Se, Cd, Cr, Fe, Zn, Cu and Co values between 28.17–30.80, 1.81–2.02, 11.28–15.80, 0.17–0.20, 20.22–25.11, 8.09–8.67, 15.65–18.43 and 17.18–21.13 mg/kg in soil samples contaminated from sewage water. Khan et al. (2016b) defined the As, Se, Cd, Cr, Fe, Zn, Cu and Co values between 22.13–22.76, 1.96–2.16, 16.29–18.99, 0.14–0.16, 19.92–20.50, 7.96–8.11, 15.98–20.26 and 18.12–19.65 mg/kg in soil samples irrigated with domestic wastewater. When three studies in the same city are compared, accumulations for all trace metals in the soil samples gathered from the roadside were higher than the samples contaminated with sewage water. However, the levels of all metals except for Cr in soil samples irrigated with domestic wastewater higher than the values in samples gathered from the roadside. This result an indication that domestic wastewaters are quite hazardous pollutants.
When the recent studies in other cities of Punjab-Pakistan are examined; Ahmad et al. (2016a) conducted a study in contaminated water irrigated agricultural sites of Sargodha and detected the As, Se, Fe, Cu and Zn values between 3.52–4.19, 1.81–2.51, 6.04–6.77, 2.79–4.13 and 2.35–3.54 mg/kg respectively in soil samples. While As and Fe values in the present study were higher than the findings of this, Se, Cu and Zn values were lower. This result may be originated from the differences in the metal resources and soil composition of the study area.
Ahmad et al. (2016b) performed a study in the peri-urban areas of Khushab and defined the As, Se, Cd, Cr, Fe, Zn, Cu and Co values ranged from 42.24 to 47.17, 1.70 to 2.76, 8.65 to 20.30, 0.84 to 0.94, 34.09 to 42.05, 4.33 to 6.40, 3.31 to 3.69 and 13.4 to 19.7 mg/kg, respectively in soil samples. As compared to these results with the present study, it was seen that the metal accumulation in the soil of peri-urban areas of Khushab were higher than the samples collected from roadside of Jhang. On the other hand, Ahmad et al. (2016c) studied on the long-term wastewater-irrigated agricultural land of Sargodha and determined the As, Se, Cr, Fe, Zn, Cu and Co values between 56.1–59.3, 2.44–3.33, 0.07–0.09, 42.9–48.4, 6.71–9.96, 4.02–4.66 and 14.3–26.2, in soil samples. Similar to Khushab, potentially toxic metal concentrations except for Cr in the wastewater-irrigated agricultural land of Sargodha were higher than Jhang the study area of the present study.
The potentially toxic metal accumulations in forage samples collected from where soil samples were taken are as follows: The contents of As, Se, Cd, Cr, Fe, Zn, Cu and Co ranged from 1.82 to 1.91, 0.16 to 0.18, 0.21 to 0.23, 2.03 to 2.10, 22.13 to 26.08, 31.80 to 38.04, 10.41 to 11.31, and 0.13 to 0.15 mg/kg, respectively (Fig. 2).
The MPL of the As, Se, Cd, Cr, Fe, Zn, Cu and Co accumulation in plants were reported as 7, 0.1, 0.1, 3, 425, 100, 100 and 50 mg/kg, respectively by WHO, FAO, Standard Guidelines in Europe (Chiroma et al. 2014), NRC (1996) and USEPA (2002). The range values of metals in the present study were lower than these MPL in forage samples.
Khan et al. (2016a, b) were conducted their studies in Jhang City. Khan et al. (2016a) determined the As, Se, Cd, Cr, Fe, Zn, Cu and Co values in forage samples between 9.15–11.20, 0.54–0.55, 0.27–0.48, 10.48–10.53, 39.39–42.94, 55.71–60.27, 20.55–23.51 and 0.48–0.49 mg/kg, respectively in Abelmoschus esculentus samples as contaminated vegetable from sewage water. Khan et al. (2016b) defined the As, Se, Cd, Cr, Fe, Zn, Cu and Co values varied from 9.71 to 12.70, 0.45 to 0.77, 0.31 to 0.35, 11.94 to 12.66, 38.96 to 41.14, 53.63 to 41.13, 18.69 to 19.38 and 0.57 to 0.73 mg/kg, respectively in Cucurbita maxima samples irrigated with domestic wastewater. When three studies in the same city are compared, accumulations for all metals in the forage samples (Z. mays, S. bicolor, P. glaucum and other grasses) collected from the roadside were lower than the A. esculentus samples contaminated with sewage water and the C. maxima samples irrigated with domestic wastewater.
When the recent studies examining the accumulation of potentially toxic metals in plant and vegetable samples in other cities of Punjab-Pakistan are examined; Ahmad et al. (2016a) detected the As, Se, Fe, Cu and Zn values between 2.07–3.68, 0.25–0.45, 32.61–42.63, 10.43–15.65 and 30.83–38.17 mg/kg in Coriandrum sativum samples grown in contaminated water irrigated agricultural sites of Sargodha. Ahmad et al. (2016b) conducted a study in the peri-urban areas of Khushab and defined the As, Se, Cd, Cr, Fe, Zn, Cu and Co values ranged from 3.45 to 4.63, 0.50 to 0.51, 0.66 to 0.75, 1.32 to 1.42, 30.28 to 37.63, 39.12 to 55.27, 14.55 to 18.65 and 0.73 to 0.89 mg/kg in Daucus carota samples. Ahmad et al. (2016c) determined the As, Se, Cr, Fe, Zn, Cu and Co values varied from 4.06 to 8.82, 0.72 to 0.83, 15.4 to 29.3, 50.1 to 78.4, 61.2 to 74.7, 23.9 to 28.9 and 0.64 to 1.16 mg/kg in Solanum melongena samples grown in a long-term wastewater-irrigated agricultural land of Sargodha. The accumulations reported in these studies for all metals were higher than this study except for Cr values presented by Ahmad et al. (2016b). This result may be due to the different working areas, as well as the accumulation properties of the plants used in the studies.
The potentially toxic metal accumulations in blood plasma samples of buffaloes are as follows: The contents of As, Se, Cd, Cr, Fe, Zn, Cu and Co are between 0.91–0.94, 0.02, 0.081–0.082, 0.34–0.36, 4.89–5.71, 0.62–0.63, 0.85–0.88, and 0.19–0.21 mg/L, respectively (Fig. 3).
For As accumulation in blood plasma, the values determined in the present study were lower as compared to the toxic value of 0.1 mg/kg suggested by Puis (1981). However, the mean As values as a result of present investigation was higher than reported by Kincaid and Hodgson (1989). In terms of Se accumulation, the mean concentrations of blood plasma were similar in both lactating and non-lactating samples. Concentration of Se detected during present study was lower than the critical concentration of 0.03 mg/L as demonstrated by McDowell (2003).
The present Cd values were within the critical limit of 0.1 mg/L (WHO 1983). Gowda et al. (2003) reported lower Cd level in blood plasma as compared to present study. For Cr accumulation in blood, the concentration of Cr was higher than normal value of 0.34 mg/L as suggested by Christensen et al. (1993). Relatively higher concentration was found in non-lactating buffaloes.
In blood plasma samples, the mean Fe concentrations in blood plasma samples were lower than the critical value 225 mg/L suggested by NRC (1996). On the other hand, the mean Zn concentrations in blood plasma samples remained relatively similar in both buffalo categories. McDowell (2003) reported a critical value of 0.80 mg/L for Zn level in blood plasma samples. The Zn values in the present study were lower than the value recognized by McDowell (2003).
The Cu concentrations were greater than the recommended value of 0.65 mg/L reported by McDowell (2003). The values found during present study were lower than the value previously reported by Bhardwaj (1998). The values exceeded the critical level studied by Khan et al. (2009).
However, all means of blood plasma Co level were lower than the critical value 0.25 mg/L recorded by McDowell (2003). In the present study, standard concentration of Co was similar to the concentration previously suggested by Rehman et al. (1998) in Sudan.
BAF ≤ 1 demonstrate that plant can just retain however not collect toxic metals; when a BAF > 1, it demonstrates that plant can accumulate the metals from soil. According to the distance from the roadside, among different at four areas, the maximum BAF was noticed for Zn and the minimum for Co. The order of BAF at the four distances were: Zn > Cr > Cu > Fe > Se > As > Cd > Co (Table 3). Various ability of different vegetables to accumulate potentially toxic metals from soil is analyzed by BAF (Amlan et al. 2012). The highest BAF was observed for Zn in the forage samples in the present study, suggesting that Zn is more bioavailable. Present values of BAF for Fe, Zn and Cd were higher and the values of Co, Cr, Cu and Se were lower than the values observed by Ahmad et al. (2016c) in Pakistan.
According to the buffalo categories, among lactating and non-lactating buffaloes, the maximum BAF was noticed for Zn and the minimum for Co. The order of BAF at the four distances were: Zn > Cu > Se > Cr > Fe > Cd > As > Co (Table 4). Ahmad et al. (2018) determined the BAF values for As, Se, Fe and Co varied from 0.46 to 0.57, 0.12 to 0.31, 0.03 to 0.04 and 1.72 to 2.04 in blood plasma of horse samples grown in Central Punjab, Pakistan. All BAF values presented in this study were higher than this values except for Co. Metal absorption might be affected by forage and animal varieties, age, climatic conditions, soil nature and pH (Carneiro et al. 2016). Metal concentrations in forages were used to calculate BAF, which indicated that among examined metals Co was the least absorbed by buffaloes.
References
Ahmad K, Ejaz A, Khan ZI et al (2010) Evaluation of dynamics of iron and manganese from pasture to buffaloes: a case study of rural livestock farms. Pak J Bot 42:3415–3421
Ahmad K, Ashfaq A, Khan ZI et al (2016a) Health risk assessment of heavy metals and metalloids via dietary intake of a potential vegetable grown in contaminated water irrigated agricultural sites of Sargodha, Pakistan. Hum Ecol Risk Assess 22(3):597–610
Ahmad K, Khan ZI, Yasmin S et al (2016b) Contamination of soil and carrots irrigated with different sources of water in Punjab, Pakistan. Environ Earth Sci 75:426
Ahmad K, Khan ZI, Ashfaq A et al (2016c) Contamination and accumulation of heavy metals in brinjal (Solanum melongena L.) grown in a long-term wastewater-irrigated agricultural land of Sargodha, Pakistan. Fresenius Environ Bull 25(7):2404–2410
Ahmad K, Nawaz K, Khan ZI et al (2018) Effect of diverse regimes of irrigation on metals accumulation in wheat crop: an assessment-dire need of the day. Fresenius Environ Bull 27(2):846–855
Amlan K, Ghosh MA, Bhatt HP et al (2012) Effect of long-term application of treated sewage water on heavy metal accumulation in vegetables grown in Northern India. Environ Monit Assess 184:1025–1036
Bhardwaj RL (1998) Age correlated histoenzymic distribution of acetyl cholinesterase and non-specific esterase in the ovary of Indian buffalo. Ind J Anim Sci 68:448–449
Carneiro M, Colaço B, Colaço J et al (2016) Biomonitoring of metals and metalloids with raptors from Portugal and Spain: a review. Environ Rev 24:63–83
Chandio AA, Yuansheng J, Magsi H (2016) Agricultural sub-sectors performance: an analysis of sector-wise share in agriculture GDP of Pakistan. Int J Econ Financ 8(2):156–162
Chiroma TM, Ebewele RO, Hymore FK (2014) Comparative assessment of heavy metal levels in soil, vegetables and urban grey waste water used for irrigation in Yola and Kano. Int Refer J Eng Sci 3:1–9
Christensen JM, Holst E, Bonde JP, Knudsen L (1993) Determination of chromium in blood and serum—evaluation of quality control procedures and estimation of reference values in Danish subjects. Sci Tot Environ 132:11–25
Dogan Y, Ugulu I, Baslar S (2010) Turkish red pine as a biomonitor: a comperative study of the accumulation of trace elements in needles and barks. Ekoloji 19(75):88–96
Dogan Y, Baslar S, Ugulu I (2014a) A study on detecting heavy metal accumulation through biomonitoring: content of trace elements in plants at Mount Kazdagi in Turkey. Appl Ecol Environ Res 12(3):627–636
Dogan Y, Unver MC, Ugulu I, Calis M, Durkan N (2014b) Heavy metal accumulation in the bark and leaves of Juglans regia planted in Artvin City, Turkey. Biotech Biotechnol Equip 28(4):643–649. https://doi.org/10.1080/13102818.2014.947076
Durkan N, Ugulu I, Unver MC, Dogan Y, Baslar S (2011) Concentrations of trace elements aluminum, boron, cobalt and tin in various wild edible mushroom species from Buyuk Menderes River Basin of Turkey by ICP-OES. Trace Elem Electrolyte 28(4):242–248. https://doi.org/10.5414/TEX01198
Gowda NKS, Malathi VS, Jash S, Roy KS (2003) Status of pollutants and trace elements in water, soil, vegetation and dairy animals in industrial area of Bangalore. Ind J Dairy Sci 56:86–90
Khan ZI, Ahmad K, Ashraf M, Valeem EE, McDowell LR (2009) Mineral status of forage and its relationship with that of plasma of farm animals in southern Punjab. Pak J Bot 41:67–72
Khan ZI, Ahmad K, Ashraf M et al (2016a) Risk assessment of heavy metal toxicity through contaminated vegetable from sewage water: Implications for populace health. Hum Ecol Risk Assess 22(2):302–311
Khan ZI, Ahmad K, Ashraf M et al (2016b) Risk assessment of heavy metal and metalloid toxicity through a contaminated vegetable from wastewater irrigated area: a case study for a site-specific risk assessment in Jhang, Pakistan. Hum Ecol Risk Assess 22(1):86–98
Kincaid RL, Hodgson AS (1989) Relationship of selenium concentrations in blood of calves to blood selenium of the dam and supplemental selenium. J Dairy Sci 72:259–263
McDowell LR (2003) Minerals in animal and human nutrition. Elsevier Science, Amsterdam
National Research Council (NRC) (1996) Nutrient requirements of beef cattle. National Academy Press, Washington, DC
Puis R (1981) Veterinary trace mineral deficiency and toxicity information. Canada Department of Agriculture. Publication 5139. Ottawa, Canada
Rehman A, Kinacid MM, Elzubejr PI (1998) Mineral deficiencies in grazing cattle in Kardofan and Darfur regions in the eastern Sudan. Trop Anim Health Prod 30:123–135
Ugulu I (2015a) A quantitative investigation on recycling attitudes of gifted/talented students. Biotech Biotechnol Equip 29:20–26. https://doi.org/10.1080/13102818.2015.1047168
Ugulu I (2015b) Development and validation of an instrument for assessing attitudes of high school students about recycling. Environ Educ Res 21(6):916–942. https://doi.org/10.1080/13504622.2014.923381
Ugulu I (2015c) Determination of heavy metal accumulation in plant samples by spectrometric techniques in Turkey. Appl Spectros Rev 50(2):113–151. https://doi.org/10.1080/05704928.2014.935981
Ugulu I, Dogan Y, Baslar S, Varol O (2012) Biomonitoring of trace element accumulation in plants growing at Murat Mountain. Int J Environ Sci Tech 9:527–534
Ugulu I, Unver MC, Dogan Y (2016) Determination and comparison of heavy metal accumulation level of Ficus carica bark and leaf samples in Artvin, Turkey. Oxid Commun 39(1):765–775
Unver MC, Ugulu I, Durkan N, Baslar S, Dogan Y (2015) Heavy metal contents of Malva sylvestris sold as edible greens in the local markets of Izmir. Ekoloji 24(96):13–25
USEPA (US Environmental Protection Agency) (2002) Exposure factors handbook. Volume II-food ingestion factors. Office of Research and Development, Washington, DC
WHO Study Group (1983) Recommended health-based limits in occupational exposure to heavy metals. World Health Organization, Geneva
Wieczorek-Dabrowska M, Tomza-Marciniak A, Pilarczyk B, Balicka-Ramisz A (2013) Roe and red deer as bioindicators of heavy metals contamination in north-western Poland. Chem Ecol 29(2):100–110
Acknowledgements
Laboratory facilities are provided by High Tech Lab University of Sargodha. The authors also thank all the supporters for suggestions and comments for the improvement of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, Z.I., Ugulu, I., Umar, S. et al. Potential Toxic Metal Accumulation in Soil, Forage and Blood Plasma of Buffaloes Sampled from Jhang, Pakistan. Bull Environ Contam Toxicol 101, 235–242 (2018). https://doi.org/10.1007/s00128-018-2353-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2353-1