Abstract
Food security and increasing agricultural yields have become one of this century’s most essential and challenging topics. The global population is projected to reach 9.7 billion by 2050, so food production must increase significantly to meet the growing demand. Increasing agricultural yields is one of the ways to address the issue of food security. This can be achieved through various means, such as improving crop varieties, using better agricultural practices, and adopting advanced technologies such as precision agriculture and genetically modified crops. One of the ways to promote this is to improve understanding and activity within plants using electrical methods. This was the objective of the presented research. In this research, a hypothesis for signal conduction through the plant medium is suggested, modeled, and characterized. The results show that this approach could be included where the plant is used as the actual sensor, and changes in its internal activity indicate changes in the environment and the plant’s needs. It hereby allows the detection of water stress, different daylight conditions, and possibly future pathogenic attacks. Another new theoretical representation and approach were also presented and supported with various experimental methods showing that the plant’s physiological response and status can be derived from its electrical characteristics, similar to methods used in plant physiology studies. It paves the path for designing and applying new sensing technologies to promote plant monitoring and serve as an additional method in precision agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Plant impedance
- Spectroscopy
- Plant sensor system
- Plant electrical model
- Plant equivalent circuit
- Precision agriculture
1 Introduction
One of today’s most significant challenges is to ensure food security for a rapidly increasing global population while resources, like land and water, are limited. The United Nations Food and Agriculture Organization’s (UNFAO) latest report (Garlando et al. 2020) estimated that, by 2050, the world population will have reached over 10 billion. Simultaneously, trends like urbanization, economic changes, and migration will increase nutrition dependence on fruits and vegetables rather than cereal produce. Climate change threatens the limited natural resources needed for agricultural growth and production (Cervantes-Godoy et al. 2020).
The need for holistic solutions that involve a technological system approach is vital, according to the latest UNFAO report: “High-input, resource-intensive farming systems, which have caused massive deforestation, water scarcities, soil depletion and high levels of greenhouse gas emissions, cannot deliver sustainable food and agricultural production. Needed are innovative systems that protect and enhance the natural resource base while increasing productivity. Needed is a transformative process towards ‘holistic’ approaches, such as agroecology, agroforestry, climate-smart agriculture, and conservation agriculture, which also build upon indigenous and traditional knowledge. Technological improvements and drastic cuts in economy-wide and agricultural fossil fuel use would help address climate change and the intensification of natural hazards, which affect all ecosystems and every aspect of human life. Greater international collaboration is needed to prevent emerging threats to transboundary agriculture and food system, such as pests and diseases” (Garlando et al. 2020).
Providing food security, based on the availability of agricultural produce worldwide, has become of the highest significance. A few issues where closer crop monitoring will have an impact are as follows:
-
1.
The amount of available food or agricultural produce
-
2.
The nutritional level and quality of crops grown
-
3.
The management of available produce: maximizing the number of crops that reach the population while minimizing the amount of produce that goes to waste
One of the ways to increase agricultural yield, improve crop quality, and meet these increasing demands for agricultural produce, or “farm to fork,” is the incorporation of new technologies and monitoring methods into agriculture. This field has been named “precision agriculture.” Here, the combination of new accurate monitoring methods, i.e., technology-driven solutions, will allow data collection from areas, land, and when correctly used, leading to improved data analysis, decision-making, and crop management. These tasks depend on the quality and accuracy of the monitoring systems’ data. Technological solutions need to be researched and developed by combining knowledge and studies in agriculture, plant biology, and different engineering fields. One aspect of monitoring plants in agriculture is sensor technology. Here, the development of new methods to sense changes in plant well-being is needed. This research attempts to improve the understanding of electrical behavior in a plant by incorporating electrical sensing methods for monitoring. The development of such devices and approaches relies directly on the electronic and physical interpretation of the biological and chemical processes involved. Applying an interdisciplinary approach to developing new monitoring tools for precision agriculture will promote and improve monitoring methods, advancing food security throughout communities and crops.
We describe here a novel approach for a study of the plant in electronic terms, the plant network methods, sensor technologies, and an example of the electrical methodology that can be applied.
1.1 Internet of Things and Precision Agriculture
The Internet of Things describes a network in which different devices can be connected and exchange information. It can be used for monitoring and controlling an environment, machines, or a set of devices. Examples of available systems today are smart homes, intelligent cities, and smart cars. The ability to monitor and exchange information in the Internet of Things depends on sensing changes and transmitting this information into a network. Measurement of such changes relies both on network abilities and communication, but before that, on the ability to sense the device or specimen to be monitored.
This suggests that the existing sensing technology and ability to collect signals that can indicate a change and interpret and understand these signals is crucial for monitoring any specimen and creating an Internet of Things.
The increasing demand for agricultural produce corresponds with the growth in the worldwide population. Consequently, the ability to monitor, forecast, and affect plant well-being and health is incredibly significant (Bar-On et al. 2019a). Precision agriculture refers to incorporating technology into agriculture to improve all aspects, including crop monitoring and quality, development, use of resources such as water. Here, the ability to collect information from crops in the field and adapt their care quickly depends on the available sensor technologies and data interpretation for improved decision-making.
Combining these concepts suggests creating a Plant-Internet-of-Things. Plants (green plants, trees, fields, etc.) will be the monitored specimen in the network and will provide information on their status and receive information from the network. The concept is to address aspects in the development of an intelligent sensor system that will be interconnected with an external sensing system, similar to the methods of body channel communication, where the body serves as both the sensing interface and a medium for the signal transmission within the network (Lodi et al. 2020). The plant and its monitored responses will act as the sensing interface and a medium for signal transmission within the network created. The system will then be interfaced with the cloud via a propriety network. Such a network requires the use of a range of sensor technologies.
The sensor connected to the plant could combine a range of existing sensors with novel electronic sensors using concepts for both sensing and signal transmission within the plant. Today, various electrical measurement techniques exist for assessing plant well-being. Despite spanning a range of measurement methods and technologies, very few techniques offer either a direct assessment of the plant status or the overall status of the plant. For a comprehensive sensor system and network, an assessment indicating the status of the entire plant is needed. This status should be measured directly from the plant rather than a local measurement, showing a localized change in the plant or its surroundings. Therefore, this work aims to create a new technological aspect for direct plant monitoring using electrical measurements. A review of existing direct sensing methods is shown in Fig. 1.
1.2 Sensors in Agriculture
Many sensors are employed for monitoring in agriculture (Luvisi 2016). They span across many technologies alongside different parameters that are measured to determine crop status, treatment, health, and resource provisions. Here, we refer to different sensing areas: (a) indirect plant sensors and (b) direct plant sensors. While indirect sensors refer to different devices that collect data from the plant surrounding environment, and according to those readings, plant treatment is adapted. Usually, such sensors include temperature sensing, humidity, and soil moisture. These sensors are positioned in physical proximity to the plant to reflect environmental information closest to the plant experience. Direct plant sensors, also called functional sensors, collect a signal directly from the plant and use it to indicate its status. The novelty is that the plant itself is used as the sensor, while the electronic device “reads” its change in signaling due to the change in its status. A review of existing technologies, new developments, and gaps in the field is brought here, focusing on electronic measurement methods employed in the area and newer upcoming technologies. Data acquisition and analysis methods are currently emerging. These include direct plant measurements and methods for monitoring the plant environment (Mogili and Deepak 2018; Walter et al. 2017). This work aims to focus on the approach of sensing changes within the plant in a direct manner as a measure of plant physiological status and well-being. Different direct monitoring methods have been reported. Among these are sophisticated imaging and radar technologies used to monitor visual changes in crop status (Luvisi 2016; Zhang and Willison 1991). Others focus on root behavior, soil quality, and trunk health for tree stability assessments, using rather costly and not field-deployable tools (Yongzong et al. 2016; Sambuelli et al. 2003). Plant leaf changes are another monitored area. Here, temperature detection using thermocouples or capacitance measurements, imaging technology, and various electrical measurements are translated for plant treatment adaption (Repo et al. 2000; Volkov 2000; Volkov et al. 2016; Zhao et al. 2013). Sap flow monitoring has also been reported. It is considered as it represents the flow of nutrients toward the plant roots. However, it is plant-type-specific and seasonal.
In plant research, several electrical evaluations have been undertaken. The assessment of internal plant signaling among cells has been studied, showing action potentials and longer traveling signals called variation potentials (Volkov 2000; Volkov et al. 2016; Brown and Volkov 2006). These signals, however, indicate chemically induced changes or a response to local physical changes. Information on the overall plant status has not been reported. Attempts to electrically evaluate the response to plant trauma have been suggested, and it was concluded that local damage induces the generation and propagation of variation potentials. These potentials affect the physiological processes in plants (Repo et al. 2000; Zhao et al. 2013). Electrochemical and bioelectrochemical measurements of plants have also been carried out, showing that long-distance communication between plant tissue and cells can propagate rapidly with bioelectrochemical signals (Volkov et al. 2016). It has been suggested that the phloem is the carrier for these signals.
Sensors based on impedance measurements are commonly used for biological specimens, as impedance spectroscopy is a well-established method for material characterization. It is often used to evaluate changes in natural materials and structures. In plant research, it has been used to assess response at the cell level, differentiating between the cell membrane’s response or the cytoplasm’s vacuole, etc.
Plant tissue from different sections of the plant has also been evaluated for several phenomena, such as disease detection, fruit ripening, and the evaluation of frost response (Jócsák et al. 2019). Models used to interpret the spectrum data have generally been based on available models, with adaptations to the specific specimen studied. Jócsák et al. include many of these reports in their comprehensive review (Jócsák et al. 2019). In earlier years (the 1920s), the electrical impedance of wood was studied, although not in a living plant. Measurements were carried out on bulk wood. Initially, the resistivity was evaluated in DC (direct current). It was shown to be correlated to the wood moisture content (Stamm 1927). Later, an attempt was made to measure the wood’s AC (alternate current) response across various frequencies. The frequency range was limited, as these experiments were undertaken for the first time almost a century ago (Luyet 1932). Following these findings, limited literature has been published on electrical impedance spectroscopy measurements to characterize live plants.
1.3 Plant-Based Electrical Sensors
New crop-growing techniques are expanding. New approaches are available among growing greenhouse farms, including vertical farming, lighting technologies for indoor growth, hydroponics, and many more. Although these environments are well monitored and allow almost complete control of the growing conditions, they still rely on plant well-being measures derived from the environment, prior research, or product feedback (once we taste the tomato, we decide whether growing conditions should be adapted). Yet, there need to be more technology-orientated methods that monitor plant health directly from a growing plant to adjust its nutrient and conditions. This implies that early detection of plant physiological change, alongside the ability to adapt treatment before the crop is produced and distributed, may significantly improve overall agriculture yield. Furthermore, across outdoor farmlands, where it is impossible to control the environment and predict climate fluctuations, early detection of plant response, resulting in the ability to adapt irrigation and fertilization, would even more affect yield for the better (Fig. 2).
In this research, we suggest incorporating a sensing method that follows the behavior in the plant vascular cambium, mounted onto the plant stem, while observing the readings continuously and examining changes in induced external stress factors. The approach suggests exploring the plant’s basic anatomy and physiology and applying physics and engineering modeling methods and measurement techniques adapted to the plant structure. These applications are expected to allow a more rigorous quantitative assessment of plant physiological status, which can be adapted for a complete electronic system of sensors in the future.
The work aims to improve our ability to describe the plant structure and internal changes that indicate a physiological change in electronic terms. Once these terms are defined, they could be applied to any species of plants in the future, ranging from small shrubs to large trees, as the primary plant anatomical structure across the species is similar (Taiz and Zeiger 2010).
2 Impedance Spectroscopy for Plant Monitoring
Impedance is defined as the ability of a material to resist the flow of electric current through it. It can be measured across frequencies, yielding a response of the material or system to different excitations or using singular frequency measurements. Intrinsically, the impedance at different frequencies will estimate the ability of dipoles in the material to respond to the excitation. The magnitude of change and the response time will be incorporated into the term of impedance values. The response of the system or material across frequencies is named electrical impedance spectroscopy. Impedance spectroscopy is often used to characterize a material or a design and asses its frequency response. The measured frequency values are determined according to the studied specimen, expected physical affects, and measurement setup considerations. Impedance measurements are commonly used to assess change in a material due to an external change, shock, wear over time, and more. It is also often applied for sensing applications, which consider biological tissue or material changes. These detectable changes can often be used to assess a physiological phenomenon. As impedance spectroscopy has been found helpful for sensor applications in biology, it has also been applied in plant studies. A recent review (Jócsák et al. 2019) shows the different applications of impedance spectroscopy to plant studies. She mentions the different experimental work carried out and explains the use of frequency range, electrical circuit modeling, and parameter extraction. Additional work for plant monitoring based on impedance spectroscopy has also become available. Here is an attempt to present a few challenges to overcome for field implementation of impedance spectroscopy measurements in precision agriculture.
2.1 Coupling to the Plant and Electrodes
Electronically two approaches to coupling to the specimen are defined: galvanic and capacitive (or faradic). The galvanic coupling means the electrode is in direct contact with the measured material, creating a resistive junction between the electrode conducting material and the specimen. A capacitive coupling means that a junction layer that behaves capacitively is formed between the electrode-bearing material and the sample measured. An example can be taken from electrocardiogram (ECG) measurements, where an adhesive is used to couple the electrode to the patient’s skin. When the measurement results are extracted, the effect of the adhesive material and skin contact need to be extracted. This requires prior information regarding the geometry and dimensions of the contact and the material properties. This knowledge allows for extracting the actual signal being studied and obtaining data about a patient. The advantage of such coupling often means that direct contact may not be necessary or that invasive measures can be avoided. The electrode coupling and contact formation are significant for determining the signal measured and understanding the signal-to-noise ratios to determine whether an effect can be measured using a particular electrode contact configuration. When examining changes in a signal, as in sensor applications, the contributions from the surrounding setup are crucial to determining whether the change results from the electrode/contact interface or arises from an actual change in the measured specimen. The electrode configuration should determine the interface being measured. As in the literature, two-, three-, or four-point setups are suitable for different types of measurements. However, in each setup, the electrode specimen interface contributes to the overall measurement values. Therefore, the electrode interface and configuration need to be carefully tailored and adapted to the specimen monitored. An example can be taken from semiconductor interfaces used for microelectronics fabrication. Here, ohmic contacts are often formed between metals and semiconductors. Once the contact is characterized as ohmic, it behaves linearly, and its resistance can be estimated for different working regimes. In other cases, where a non-ohmic contact exists, the behavior must be considered part of the device functionality. In plant measurements, different electrode setups have been demonstrated. A four-probe measurement using capacitive coupling to the leaf as shown using graphene-based square electrodes mounted onto the leaf. Results showed that detection of induced water stress could be detected (Zheng et al. 2015). Another example is the electrochemical sensing setup, using a three-electrode setup, where the sensor device is mounted close to the leaf and can detect a change in gas composition near the stomatal opening. Another form of electrochemical sensing has been demonstrated within the plant stem, where the device and electrodes have been inserted into the plant stem and show the ability to measure sugar changes due to plant transpiration activity (Cervantes-Godoy et al. 2020). Other forms of electrodes have been used to measure fruit ripening; an example is the use of medically prevalent sticky electrode patches. These were attached to the fruit in a four-point configuration, and different ripening stages were detected. Frost detection in fruits has also been studied using four-point probe impedance measurements. The electrodes also seem to have been inserted into the specimen under test. More recent technology for flexible microneedle fabrication has also been demonstrated to measure impedance in plants, showing results similar to other electrode configurations. The coupling to the specimen under test is highly significant for electrical impedance spectroscopy measurement. The method relies on the specimen’s response to a signal applied across a frequency range. As the reaction is composed of resistive, capacitive, and possibly inductive parts, the actual electrode’s proximity, location, and interface can affect the results of the measurements. With advances in nanotechnology and material sciences, several electrode options are available to improve the electrode material and mounting to the plant. However, a better and more in-depth study of the electrode interface with the plant and the internal effects on a living plant is needed. In addition, geometrical considerations and mechanical stability need to be considered—also durability in harsher outdoor conditions—possibly feeling the plant anatomy in the electrode design and optimization.
2.2 System Design: Supporting Electronic Requirements
Continuous monitoring and easily mounted field devices are needed in precision agriculture. For such applications, embedded electronic circuits and devices need to be designed. While for continuous long-term measurements, additional considerations are required, such as, signal integrity over time and nondestructive connectivity to the plant being measured. The need for a compact system that allows continuous data collection and transfer to the cloud is clear.
Furthermore, it should consist of low-power electronics and be robust for outdoor conditions. A requirement would also be that it would be easy for untrained farming workers to support multiple plants or measurements simultaneously. Lab systems have been suggested to show prolonged continuous monitoring of tobacco plants (Garlando et al. 2022). The proposed method was multiplexed to measure various plants and combined with designated environmental sensors. However, a complete outdoor system is yet to be developed. Some of the challenges posed have been addressed for different biomedical devices, which with adaptions, may also offer solutions that could be applied to plants in precision agriculture. Yet for agricultural monitoring, the low cost of the devices is also a priority, as many of the food security issues span across the poorest countries, with low income and resources for technological development.
2.3 Data
Collection and interpretation of the signals acquired using impedance spectroscopy measurements are also needed. The specificity of the plant response to different environmental changes of disease is unknown and needs to be quantified. Furthermore, the sensitivity of the measured data also requires study in plant physiological terms, not only electronically. The question of how often measurements should be collected and according to what amount of data improved decision-making will occur is also unknown. Beyond these research questions also lies the technological aspect of collecting, analyzing, and interpreting the data using extensive data algorithmic methods alongside engineering and physiological understanding.
2.4 Biological Background
All seed plants have similar basic body plans. However, diversity is apparent. The vegetative body comprises three organs: leaf, stem, and root (see Fig. 3). The primary function of the leaf is photosynthesis, that of the stem, support and root, anchorage, and absorption of water and minerals. Leaves are attached to the stem at nodes, and the region of the stem between two nodes is termed the internode. An evolutionary difference exists between flowering and nonflowering plants. We do not take this into account in this work.
The stem consists of a vascular cambium, structured as a cylinder, and consists of supportive fiber cells and conduction vessels called the xylem and the phloem. These vessels conduct water and nutrients across the plant. The xylem provides a low-resistance pathway for water movement, thus reducing the pressure gradients needed to transport water from the soil to the leaves, while the phloem allows for nutrient flow from the leaves downwards. The stem growth is depicted in Fig. 4. Plant growth occurs from the center of the stem outwards, increasing the vascular cambium portion in the stem as the plant ages (Taiz and Zeiger 2010).
2.5 Electrical-Based Model
We chose a four-point configuration based on the following reasons. Impedance spectroscopy can be carried out using a different number of probes connected to the device:
-
A two-probe configuration is typical in cases where the specimen is expected to have linear behavior. The contact resistance between the probes and the measured sample can be assumed to be ohmic.
-
A three-probe configuration is suitable for interface interaction measurements, where one electrode can be used as a reference. This is commonly used for electrochemical measurements.
-
A four-point probe configuration allows the decoupling of the contact resistance of the behavior of the probe from the actual measurement of the device under the test itself. It is often used in cases where the contact interaction with the specimen is unknown or may change over time. It is also helpful in cases where the contact resistance may introduce high resistance values compared to the device under test.
The advantage of this configuration is that the voltage is sampled using different probes where the current is applied. Since the voltage is measured using a very high impedance operational amplifier, the effect of the contact impedance between the metal electrodes and the device under test is reduced. This is important since the exact characteristics of those contacts are often unknown. Four-point probe measurements are well known in the semiconductor industry for the characterization of different materials, from thin film dielectric layers to bulk metal lines and so on. The four-point probe configuration, both in direct and alternating currents, uses two probes to induce the current and the other two to measure the voltage drop across the specimen. The probes are often placed in a line, where the two outer probes are used for the current and the two inner probes for the voltage. In this manner, the current is forced across the specimen under test through a single set of leads (called “force”), while the voltage is measured through the second set (called “sense”). The voltage drop across the sense leads will be negligible, so the measured voltage is essentially the voltage across the specimen under test. This has several advantages over a two-probe configuration. The leads and connections resistances can be almost eliminated, allowing assessment of the actual contact resistances while providing more accurate and less noise-sensitive measurements.
There are two ways to connect to the device under test using a four-point measurement configuration. One is using four physical contacts to the device under test, or using only two.
The four physical contacts separate the “force” and “sense” connections. While with two physical contacts, each connected to a force and a sensor measurement. Therefore, a two-contact measurement requires specialized equipment and calibration due to current leakage. In our case, a four-point configuration was used to avoid calibration uncertainty and separate the current source and voltmeter. In addition, this also removes the uncertainty of current leakage via the sense leads (Keithley 2016). During this work, different equipment was used to collect impedance data. Initially, a manual impedance setup was devised. Here, a signal generator was used as the source for signal excitation at different frequencies (for example, Keysight waveform generator 33500B), the input was measured across a resistor, and the output was registered using an oscilloscope (Agilent MSOX2012A (Keithley 2016)). The response of the plant stem was calculated according to the difference in voltage drop across the output about the input. A schematic illustration of the setup is shown in Fig. 5.
Impedance measurement setup schematic diagram including the plant connected and the control system designed (Garlando et al. 2020)
2.6 Continuous Monitoring Systems
Continuous monitoring setups were devised during the research to acquire multiple ongoing readings of the plant across different intervals. The setups rely on an impedance analyzer that was controlled using dedicated software, which was developed during this work. Environment sensors and monitoring tools complemented these setups to study the plant status and response to different conditions comprehensively.
2.6.1 Setup 1—Lab Indoor Plant Monitoring System
An impedance analyzer (Keysight Technologies model 4294A (Keithley 2016)) was connected directly to the plant stem in a four-terminal setup. The analyzer was programmed and run continuously using a designated LabView© software interface. Measurements were conducted every 15 minutes, and data were logged and collected using an additional LabView© software. A diagram of the system can be seen in Fig. 6 (Bar-On et al. 2019a).
Sensor system block diagram (Bar-On et al. 2019a)
The system is described in detail in Bar-On et al. (2019a). In addition to the impedance analyzer setup, an electronic system with controlling software was devised. This system included electronic circuitry to support multiplexing of the impedance analyzer to measure two channels without affecting the accuracy of measurements. This enabled measuring two plants under similar environmental conditions. In addition to the multiplexer option, a set of external sensors were configured onto an embedded board, designed as a system for environmental monitoring. This setup included a range of commercial sensors, including a temperature and humidity sensor, a soil moisture gauge, and a light sensor. The software was developed to allow simultaneous data collection and synchronized readings from the impedance analyzer and the sensor system (Bar-On et al. 2019a).
The details for the system of sensors to collect information on the environmental status are described below.
The system allows continuous data collection of the surrounding plant environment and samples every 15 minutes. It is controlled and programmed using a Python interface, and the hardware is based on the Raspberry Pi© platform. It includes three generic sensors:
-
1.
HDC2080 (Texas Instruments Ltd)—temperature and relative air humidity sensor (Texas Instruments 2018)
-
2.
MAX44009 (Maxim Integrated Ltd)—ambient light sensor monitoring (Maxim Integrated 2011)
-
3.
200SS WATERMARK Sensor (Irrometer Ltd)—soil moisture monitoring (Irrometer 1978)
The sensors are all connected to the GPIO (General-Purpose Input Output) port of the Raspberry Pi© using the I2C (Inter-integrated Circuit) protocol (port #1). At the same time, the moisture sensor requires an analog-to-digital converter and a readout circuit. The circuit is constituted by the schematics and components recommended by the manufacturer, also using the I2C protocol and sharing the port with the previously mentioned sensors. The complete supporting circuit allows to control and collect data in a synchronized manner while having the readout from each sensor collected serially every 15 minutes and saved to a data file. A block diagram of the system is shown in Fig. 5. The circuit with the sensors connected, indicating the ports that are then connected to the Raspberry Pi, can be seen in Fig.7.
The designed environment sensor board including the direct plant electronics interface (see Bar-On et al. (2019a))
A graphic user interface that included plotting abilities and calibration of all the connected sensors and impedance measurements were also established (Fig. 8).
2.6.2 Field Outdoor System
An outdoor field system for continuous impedance monitoring was also devised. The plan was set up in an outdoor greenhouse in Tel Aviv, Israel. The setting allowed exposure to natural light and outdoor growing conditions while the temperature in the greenhouse was controlled. All other parameters were closely monitored as in standard commercial greenhouse growing facilities.
For the plant impedance monitoring, a Hioki IM3570 (Hioki Ltd n.d.) impedance analyzer was used in an analyzer mode. Measurements were taken at 500-mV RMS, while each measurement was averaged with a factor of 4. The frequency was swept logarithmically across 50 Hz to 4 MHz, collecting 801 points per sweep. Calibration, including cables, was completed before measurements. The electrodes were coupled galvanically to the plant by direct insertion into the plant stem at a distance of 5 cm (as described in Bar-on et al. 2019b). Au electrodes (0.5-mm diameter, approx. 4 cm in length) were inserted into the stem, ensuring direct contact with the vascular tissues inside the stem. Measurements were carried out continuously over a few days, and the results were logged and analyzed. Experiments were repeated across multiple plants, while they were each prolonged across 30–60 days at a time, and data were collected every 9–24 minutes, yielding experiment sets of over 10,000 measurements each.
2.6.3 Plant Choice
This study tested a few different plants, including tobacco, tomato, and the cherry tree. However, we chose to focus the research on tobacco plants. The plant type for this purpose was selected regarding the knowledge of the vascular structure, robustness, and the study of its genetic makeup. Nicotiana tabacum L. cv. Samsun-NN (tobacco) plants were a suitable match for this study. Young plants grown for 3–4 months were used, having a stem diameter of 0.7–1.1 cm (Fig. 9).
Plants were grown at different locations, providing growth conditions tailored per experiment.
2.6.4 Comparative Study
An assessment of the ability of impedance measurement to detect physiological change was completed in a comparative study manner.
A comparative study can be carried out by comparing the same sample to itself under the same conditions with controlled change across time. Such an experiment would be carried out multiple times across multiple specimens to have a correct experimental procedure. Another way is to compare two similar specimens in the same environment, under a different condition, keeping one in a control condition and the other in the test. Both these approaches were used during the research and experiments.
For example, this research completed a series of experiments to evaluate changes in impedance measurement values due to water stress. Initially, the plant was compared to its characteristics under normal hydration conditions, and once water stress causing dehydration was induced, values and changes in measurements were reevaluated. Next, a comparative study was done by multiplexing the measurement system and measuring two plants consecutively (with a difference of a few minutes between measurements so that this is almost simultaneous) in the same conditions.
Once this was achieved, the newly suggested measurement method was compared to well-known techniques commonly used in plant science to evaluate plant well-being and physiological state. The following section describes the different plant physiological measurements used.
3 Plant Physiological Monitoring
Plant monitoring in plant science and biology research is a well-established field. Only a few nondestructive measurement methods that estimate whole-plant status are available. One includes measuring plant water usage based on a continuous weight measurement technique. This method is called gravimetry, where weight changes are measured and attributed to changes in the monitored specimen. The estimated weight in gravimetry includes the overall plant weight, roots, stem and leaves, pot, and ground. These are counted multiple times, and the weight change across time can indicate plant water usage.
The system allows assessing a few terms that are used to determine the plant’s physiological behavior, which we define below:
-
(a)
Plant transpiration (transpiration rate)
-
(b)
Daily and across time
-
(c)
Volumetric water content
This method allows us to measure “whole-plant transpiration.” Transpiration defines the amount of water vapor loss from the plant and is usually measured across a single leaf or area; nowadays, using many high-precision weight measurements, overall plant water usage can be estimated to assess plant transpiration. Transpiration is a fundamental and highly complex process in the plant system. Ongoing gravimetric data collection provide information regarding overall plant transpiration activity, allowing us to study water use efficiency, improve the understanding of the physiological activity, and give a comprehensive view of the plant status. Therefore, this method was chosen as a comparative baseline for inspecting the electrical impedance spectroscopy data acquired during this research. To study changes in impedance values measured, plants were also monitored using this method simultaneously. This way, modifications could be compared to well-known effects and values and attributed to different physiological phenomena.
3.1 System Description—Gravimetric Measurements Using Plant Array
Whole-plant physiological performance was monitored with the functional phenotyping system. Plant array platform is described in detail. The system includes a weight sensory system with an accuracy of a few milligrams. In addition, it consists of a soil moisture sensor placed inside the pot as an indicator of changes in the soil water content. Next, the system is connected to a controlled irrigation system, where the plant can be hydrated according to experiment requirements. In addition to the local monitoring of the plant, the greenhouse environment is continuously monitored and maintained. This includes measurements of temperature, light condition, and air humidity. The soil moisture is continuously monitored as well using soil probes. The data collected by the system are logged into a soil–plant–atmosphere control software tool.
3.2 Physiological Studies Alongside Impedance Data
To establish whether the impedance data collected might indicate the plant status, a range of different conditions were tested experimentally during the research for the electrical plant response.
In plant physiology, the plant response to different situations, which can be induced externally, is known and can be expected. A simple example is water stress, i.e., once the plant lacks water resources, its leaves will wilt. Other stress conditions, such as lighting changes or disease, can also be induced.
3.2.1 Water Stress/Drought
Experiments were conducted to study the impedance measurements’ response to water stress situations. To accomplish this, plants were regularly watered, showing repetitive behavior across the days (watering saturation). Once These stable conditions were achieved study of different water stress cycles were completed by skipping specific watering time slots.
3.2.2 Light
Plant growth, development, and physiological regulation depend strongly on light. Therefore, exposure to changes in lighting and daylight hour fluctuations is monitored in plant research and taken into account for physiological change studies. While daylight fluctuations are present across the different seasons and light exposure of a plant can depend on its location and external factors, sometimes growth chambers are used. Here, a light that imitates the sun spectra is used, and daylight hours can be controlled. Throughout the experiments presented in this work, the systems included continuous light monitoring within each system (each sensor is described in the system setup description).
3.2.3 Soil
The ground where plants grow varies in different locations and areas worldwide. Due to these variations, different types of land have been studied and adapted for growing agricultural crops around the world. Every kind of ground has different attributes and is better suited for specific terrain, climate, and crop. In our case, two types of land were used: standard soil and coarse sand. While comparing the behavior of these two, and is known to have lower water retention than soil, this can be utilized while examining drought or weight changes.
3.3 Experimental Procedure Using the Gravimetric System
Wild-type Nicotiana tabacum seeds were sown in growing soil (Avin Ari Ltd., Israel). The plants were grown in fully controlled growth chamber equipment with “ELIXIA” lights of “Heliospectra” (ref) set on a long day (18/6 hours) with 500 PAR, and the temperature was controlled with AC (26/19). After 3 weeks, the plants were gently washed from the soil and transferred to the designated Plant Array 3.9 pot full of coarse sand of “Negev Industrial Minerals Ltd.,” Israel. Coarse sand is a highly homogeneous, inert medium; using this medium minimizes the noise caused by the soil absorption of water and nutrients (ref). An “EVA” foam sheet covered the pot’s top to minimize the evapotranspiration from the soil. The 3.9-L pot with the seedlings was transferred to the Plants Array room in the semicontrol greenhouse at the Institute for Cereal Crops Improvement, Tel Aviv University, for acclimation. AC controlled the greenhouse temperature (28–19), and natural light conditions were used; the weather station recorded the humidity, temperature, and light during the experiments. The Plant Array system is supplied with a fully controlled irrigation system. During the acclimation period (from 3 weeks to the start of the measurements), the plants were watered throughout the day to stabilize and form their root system. When the plants showed full acclimation determined by active growth and transpired more than 300 mL per day, we started irrigating only at night (21-02) at list 2 L to reach full saturation. After 2–3 days of night irrigation period, the experiment started. “Shaphir Nitrate Solutions” fertilization (4:2:6) of “Deshen Gat” was added to the irrigation to ensure healthy plant growth. When plants were in high turgor in the morning, four probes were galvanically coupled to one plant’s stem while the other was used as a control. The probes were made of noble metals to avoid corrosion effects. The two pairs of probes were placed at a distance of 5 cm from each other. The impedance measurement and the Plant Array systems were checked and synchronized to collect data continuously. To collect sufficient data, multiple rounds of experiments were run, each match including measurement under no-stress and stress water deficiency (drought) conditions. To measure the plant at control conditions (nonstressed), plants were irrigated to saturation (at list 2 L) and complete drain of the remaining water during the night (21-02). To measure the plants under stress conditions, the plant connected to the impedance was not irrigated overnight. After the plants showed stress phenotypically (low transpiration and withered leaves) and the soil probe showed low moisture, the plant was given recovery irrigation overnight for at least 3 days before the repetition of another stress cycle. Each experiment was run for approximately 3–4 weeks. A more detailed description of our model has been published in “Frontiers in Electronics” (Bar-On et al. 2021).
3.3.1 Setup 1—in Lab Monitoring—Turin, Italy
The results in the section have been published in IEEE over the research period (Bar-On et al. 2019a; Garlando et al. 2021). The indoor lab setup includes an impedance system alongside a set of environment sensors, which was established for this work. It allowed data collection across long periods, with different sampling rates. In addition to the impedance data collected, environmental data were also collected. A range of experiments were completed using two types of plants: tomato and tobacco (Fig. 10).
Nicotiana tabacum (tobacco) plants were grown in labs from seeds provided by the lab at the Faculty for Plant Science at Tel Aviv University (TAU); the monitored plants were grown for 3–4 months, reaching a stem diameter of 0.5–1 cm. In addition, as tomatoes are quickly grown in Italy, tomato plants bought for edible tomato growing were tested. Here, plants were small (about 50 cm in height), and the stem diameter was chosen to be similar to the used tobacco plants, yielding a measurement of 0.5–1 cm in diameter.
Initially, the system was set up to allow continuous measurements of a single plant. Later, the electronics allowing multiplexing the system to measure two plants consecutively was established. The system included (as described in Sect. 2.6) an impedance analyzer controlled using a dedicated LabView© software and a set of environmental sensors controlled using reliable electronics and controlled with Raspberry Pi© to run in a synchronized manner and log the data collected. Once continuous measurements of a single plant over time were completed and a sampling rate allowed the system to provide minimal noise during sampling, a multiplexer was added to collect data from two plants at a time. Here, a measurement was taken from each plant consecutively and continuously without affecting the quality of the data collected (i.e., parasitic impedances, signal averaging time on the impedance analyzer). Figure 11 shows this system setup with two plants connected for measurements.
Impedance measurements were carried out across time, and data were collected. The impedance analyzer was calibrated according to the manufacturer’s manual, allowing the establishment of impedance spectra of the plant stem. The measurements were set to measure across a frequency range of 40 Hz to 1 MHz. At the same time, in this domain, the impedance analyzer was fully calibrated with the additional multiplexing circuit and cables and connectors to the plant. Calibration included all wires except the actual probes in contact with the plant, as they were assumed to be perfect conductors (Au wires of approximately 4 cm in length and 0.6 mm in diameter were used). Voltage was set to 500 mV VRMS while averaged across 4 for each sweep.
This setup allowed data collection across a wide range of frequencies and time, so examining the results can be done in various ways. During this work, we presented the impedance data by looking at the impedance magnitude and impedance phase separately, as done in Bode plots (Agarwal and Lang 2005; Sze and Ng 2006); we found this useful for our study (other representations are available and presented briefly in this study).
Impedance spectra were initially collected, showing that they coincided with previous results. In addition, the orders of magnitude measured agree with our initial results (published in Bar-on et al. 2019b, using a manual setup for impedance measurements). The continuous manner of data collection allowed us to distinguish that a change in the measured impedance values of the plant occurs during the day. By dividing the day into three regions: morning 8–12 am, afternoon: 12–16, evening: and averaging the measurements taken every 4 minutes across these hours. We could distinguish that a shift in the measured curve exists, indicating the sensitivity of the size to the time of day (possibly corresponding to the plant’s daily cycle, daylight hours). An increase in impedance can be seen as the day proceeds across frequencies, while in phase, a shift is present, with decreasing absolute values of the angle.
Next, the behavior of the plant across several consecutive days was examined (shown in Fig. 13). Here, to examine the variation across frequencies and time, we examined the behavior at the centroid frequency. This data, for both impedance magnitude and phase, show that the measurement is sensitive to a daily trend in the plant. However, plant behavior under similar conditions across days is repetitive. These would require further studies but indicate that the collected data may be a significant monitor for the plant.
Next, using the multiplexer electronics, two plants were monitored simultaneously. This setup allowed us to carry out case studies in a comparative manner. Several experiments were carried out across 2–3 weeks each time (Fig. 12).
Impedance measurements across a day, showing the differences between different times (classified as morning, afternoon, and evening). Right: Impedance magnitude and phase values across different frequencies. (Similar results are presented in our publication (Bar-On et al. 2019a))
In the following example, the experiment was carried out across multiple days (up to 3 weeks at a time). Two tomato plants were monitored continuously, collecting impedance data at 15-minute intervals. Here, we present an example of measurements taken across 6 days, examining the response of the two plants: one to complete dehydration over time, which was started on day 6; the second plant, which was kept well hydrated with regular watering every day. The result can be seen in Fig. 22. In this experimental setup, it is to be noted that the plant’s watering was completed manually, using approximately 300 mL of tap water, at 17:00 each day. The daily trend is visible in both the impedance magnitude and phase (as in Fig. 21). A change due to hydration/dehydration is also present in both measures. It should be noted that irrigation was done manually and exposed to variations.
In addition to the impedance data, the sensor system collected environmental data simultaneously. The data acquired here were used comparatively. Presented below are impedance compared with data from a soil moisture sensor inserted into the pot plant soil and a light sensor.
Measurement of soil moisture is a well-known method for irrigation planning. Here, the soil moisture sensor was calibrated and inserted into the plant pot. For convenience, we inspect the time derivative of the soil moisture sensor reading to see a “spike” each time an irrigation event occurs (an example can be seen in Fig. 19). These values are shown alongside the impedance magnitude and phase across time, clearly showing a response in the plant impedance to these irrigation events (Fig. 13).
Impedance measurements shown at the centroid frequency across 6 days. The plant was manually watered once a day. The impedance magnitude (top) and phase (bottom) are shown separately across time. Both measures show a repetitive behavior across different days, indicating that the impedance data may follow the plant’s daily cycle trend
Examination of impedance data alongside external illumination conditions was also completed. A light sensor sensitive to visible light was connected in the system to provide indication to daylight hours and lighting fluctuations. Here, the light sensor was set up in the lab near the plants and a window exposing the outside lighting conditions. Here, we could see that the impedance values fluctuate qualitatively, with a similar trend to the daylight cycle. This indicates that the impedance measurement follows activity within the plant vascular cambium, which is known to respond directly to light exercise. This is shown in Figs. 14 and 15.
The results shown on the system established in the indoor lab conditions present the possibilities and sensitivities of the suggested impedance measurement of living plants. These results suggest that close monitoring and combination with environmental sensors can yield useful data for precision agriculture.
As the Department of Electronics and Telecommunications (DET) at the Politecnico di Torino lab has expertise in embedded electronics for sensor systems, the collaboration continued with a focus on developing additional multiplexing electronics, improvements, and adding sensors to monitor the surrounding environment while collecting data from plants in this lab.
In addition, we established that comparative studies must be carried out alongside well-known and established plant-monitoring methods in the plant physiological world. Here, we could better understand the data collected, compare it to commonly used forms, and plan experiments suited by plant biologists. This was set up and is shown in the next section.
3.3.2 Setup 2—Outdoor Greenhouse Monitoring—Tel-Aviv, Israel
The following experiments were conducted in Tel Aviv, Israel, in a greenhouse facility at the Cereal Research Institute. The greenhouse offers the ability to control and monitor plant growth conditions so that the temperature and humidity are controlled and monitored. In contrast, the plants are exposed to natural light conditions, as these greenhouses are situated outside. To carry out our impedance experiments, the impedance monitoring system was connected inside the greenhouse to collect data continuously across time. A significant advantage of this setup was that the greenhouse included a fully automated irrigation control system that allowed one to determine the exact irrigation conditions, such as timing, speed, water quality, and nutrients. To enhance the research, the chosen greenhouse included a state-of-the-art plant physiological monitoring system that offers high-end plant weight monitoring alongside electronic sensors (PlantArray©, DiTech Ltd.).
Multiple long-term experiments of a few weeks and up to 2 months were completed throughout the year. This provides information and consistency of the results across the different seasons of the year, accounting for changes in weather conditions, light temperatures, etc., as well as for multiple plant trials.
The results are structured to demonstrate the systems set up and connected, the specifications of the different experiments completed, and their effects. For each experiment, the tobacco plant was connected and stabilized on the weight system and connected to the impedance analyzer. The exact experimental procedure is explained in an earlier section. An example of the physical setup of a tobacco plant in the greenhouse attached to both impedance measurement and the gravimetric system for continuous monitoring can be seen in Figs. 16 and 17. Each experiment was set up to run for several weeks.
3.4 Impedance and Plant Physiological Response—Light, Daily Cycle, Water Stress
It is well known that activity in the plant, including transpiration and photosynthesis, depends on light conditions. The plant’s diurnal cycle depends on daylight hours and light exposure. To examine the response of the plant impedance spectra to a controlled light environment, the plant was kept fully hydrated, and constant intensity lighting was induced for a steady number of hours each day. The results show that, across the different frequencies, the plant impedance responds directly to the light changes.
Normal daily behavior—shown with the impedance response to muted response to different hydration amounts graphs to prepare: daily light, Transpiration Rate (TR), weight, etc.
4 Data Analysis
The suggested model was fitted to the experimental results, and an evaluation of the model’s accuracy across the frequency range was performed. The spectra were provided to the lumped element model using a standard least square fitting algorithm based on the impedance magnitude. An example of the fit and an experimentally obtained spectra are shown in Fig. 20. The fitting algorithm was derived from the mathematical representation of each component in the lumped element model presented. It was run using the Matlab® curve fitting toolbox. The measurement data were imported and organized into the program and then run sequentially using an appropriate algorithm that considers least-squares fitting. The fitting provides an estimate for each model coefficient and can be presented parametrically. Initial values and lower and upper bounds were used based on measured impedance values and expected model behavior, allowing model parameters to converge across measurements and frequencies. Thus, the fitting algorithm yielded a good fit for all experimentally obtained sets of impedance spectra, with a mean relative appropriate error of approximately 1.06% ± 0.12%. This error is well within the limits of experimental error and the error limits of the instruments used. The error across time, i.e., across different days measured, is shown in Figs. 16, 17, 18, 19, and 20, where minor deviations are visible (Fig. 21).
During data analysis, the following analysis has been done:
-
1.
Analysis of the impedance at prechosen representative frequencies.
-
2.
Spectral analysis using the dominant pole parameters as indicators.
-
3.
Fitting the spectrum, at each time point, to the physically based lumped element circuit model, using the model parameters as indicators. This analysis was also published by Bar-On et al. (2019a) and appeared in the section below.
Three analysis methods are presented, followed by a comparison to gravimetry. The results were analyzed from the plant’s electrical response each day. Across those days, a series of hydration–dehydration cycles were performed daily. A more extended dehydration period (about 24 hours) was applied every few days, followed by a return to the daily hydration/dehydration sequence. The more extended dehydration period was used to study the plant’s longer-term characterlike, such as post-dehydration recovery time. The results depended on the dehydration/hydration cycles and the ambient plant conditions, i.e., temperature, time of the day, humidity, and lighting. Therefore, the effect of dehydration/hydration cycles on the electrical measured parameters was studied at the same time daily where the temperature and lighting provide similar comparative conditions.
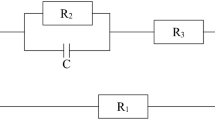
The lumped element modeling approach is commonly used to analyze a biologically based system using electronics. An equivalent circuit using the lumped element modeling has been suggested for this measurement setup (see Bar-On et al. (2019a)). A lumped element circuit approach attempts to consider the different physical components in the device under test and represent each of their contributions across the collection of frequencies used. The model parameter graphs across time are shown in Fig. 22. In our case, a group of resistors, constant phase elements, and capacitors have been arranged based on the measurement setup and known plant physiology. Due to the plant stem physiology, the circuit assumes current flow across a collection of channels. Each channel is represented by a resistor and a CPE (constant phase element), while the different channels are capacitively coupled. Inspection of each ingredient in such a circuit across time will indicate their contribution to conduction across the specimen and the sensitivity of the system to change. This allows us to assess the significance of different components and estimate the system response to induced physiological stress. The presented parameters of the lumped element circuit are grouped by type in each graph, i.e., resistive, capacitive, and CPEs. Inspecting the different parameters, we may notice different behaviors. The different resistive components all show a change due to the stress.
In addition, across the presented control period, they show slight fluctuation. These changes may, in the future, be correlated to daily changes within the plant. Yet all resistive components behave similarly. Looking into the capacitive and CPE details, all parameters show an absolute value change due to the water stress introduced. Yet the direction of the change differs. This may indicate the actual physical mechanism the parameter represents within the plant. The most significant change we observe using this method seemingly occurs in the first CPE component (CPE1) (Fig. 23).
The daily fluctuations are enhanced, while the response to stress (observed as a peak both in the middle and at the end) increases by order of magnitude. Furthermore, it clearly can be seen that after the stress, the baseline of values is shifted during recovery. In modeling a system as in the plant stem, a constant phase element represents transport and diffusion within the biological specimen and can be expected to show higher sensitivity to a physiological change such as the water stress tested here. Comparing the change observed across the resistive elements, it can be assumed that they are more responsive to changes in ion concentration and therefore indicate more minor deviations. A better understanding of these changes requires a combined study with known plant physiology measurement methods. In addition, these qualitative changes shown mean the added value of the presented continuous impedance measurement method, as it allows for data sensitivity to more than a single change across the plant. However, the lumped element approach with different parameters requires further study of the relations between the parameters and their physical meaning. The ability to detect variations across the different components is apparent.
References
Agarwal A, Lang J (2005) Foundations of analog and digital electronic circuits. Elsevier
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis: a new hypothesis. Differentiation 24(1–3):203–208
Bar-On L, Peradotto S, Sanginario A, Ros PM, Shacham-Diamand Y, Demarchi D (2019a) In-vivo monitoring for electrical expression of plant living parameters by an impedance lab system. In: 2019 26th IEEE international conference on electronics, circuits, and systems (ICECS), pp 178–180
Bar-on L, Jog A, Shacham-Diamand Y (2019b) Four point probe electrical spectroscopy based system for plant monitoring. In: 2019 IEEE international symposium on circuits and systems (ISCAS), pp 1–5
Bar-On L, Garlando U, Sophocleous M, Jog A, Motto Ros P, Sade N, Avni A, Shacham-Diamand Y, Demarchi D (2021) Electrical modelling of in-vivo impedance spectroscopy of Nicotiana tabacum plants. Front Electronics 2. https://doi.org/10.3389/felec.2021.753145
Beck CB, Schmid R, Rothwell GW (1982) Stelar morphology and the primary vascular system of seed plants. Bot Rev 48(4):691–815
Brown CL, Volkov AG (2006) Plant electrophysiology. Springer, Berlin, Heidelberg
Cervantes-Godoy D et al (2020) Technical Report 4, 2014. UNICEF FAO. The State of Food Security and Nutrition in the World. Technical report
Garlando U, Bar-On L, Ros PM, Sanginario A, Peradotto S, Shacham-Diamand Y, Avni A, Martina M, Demarchi D (2020) Towards optimal green plant irrigation: watering and body electrical impedance. In: 2020 IEEE international symposium on circuits and systems (ISCAS), pp 1–5
Garlando U, Bar-On L, Ros PM, Sanginario A, Calvo S, Martina M, Avni A, Shacham-Diamand Y, Demarchi D (2021) Analysis of in vivo plant stem impedance variations in relation with external conditions daily cycle. In: 2021 IEEE international symposium on circuits and systems (ISCAS), pp 1–5
Garlando U, Calvo S, Barezzi M, Sanginario A, Ros PM, Demarchi D (2022) Ask the plants directly: understanding plant needs using electrical impedance measurements. Comput Electron Agric 193:106707
Georgia Tech Biological Science. Sugar transport in plants: phloem. Biology 1520
Hioki Ltd. Hioki Impedance Analyzer IM3570 (n.d.)
Irrometer (1978) Model 200SS WATERMARK Sensor WATERMARK Soil Moisture Sensor â MODEL 200SS
Jócsák I, Végvári G, Vozáry E (2019) Electrical impedance measurement on plants: a review with some insights to other fields. Theor Exp Plant Physiol 31(3):359–375
Keithley (2016) Low-level measurements handbook, pp vi, I–vi, 5
Lodi MB, Curreli N, Fanti A, Cuccu C, Pani D, Sanginario A, Spanu A et al (2020) A periodic transmission line model for body channel communication. IEEE Access 8:160099–160115
Luvisi A (2016) Electronic identification technology for agriculture, plant, and food. A review. Agron Sustain Dev 36:13
Luyet BJ (1932) Variation of the electric resistance of plant tissues for alternating currents of different frequencies during death. J Gen Physiol 15
Maxim Integrated (2011) MAX44009 industry s lowest-power ambient light sensor. p 1–20
Mogili UR, Deepak BBVL (2018) Review on application of drone systems in precision agriculture. Procedia Comput Sci 133:502–509
Repo T, Zhang G, Ryyppö A, Rikala R (2000) The electrical impedance spectroscopy of scots pine (Pinus sylvestris L.) shoots in relation to cold acclimation. J Exp Bot 51(353):2095–2107
Ros PM, Macrelli E, Sanginario A, Demarchi D (2017) Intra-plant communication system feasibility study. 62(11):2732
Ros PM, Macrelli E, Sanginario A, Shacham-Diamand Y, Demarchi D (2019) Electronic system for signal transmission inside green plant body. In: 2019 IEEE international symposium on circuits and systems (ISCAS), pp 1–5
Sack L, Tyree MT (2005) Leaf hydraulics and its implications in plant structure and function. In: Vascular transport in plants. Academic Press, pp 93–114
Sambuelli L, Socco LV, Godio A (2003) Ultrasonic, electric, and radar measurements for living trees assessment. Boll Geofis Teor Appl 44(3–4):253–279. Leonardo, (1)
Stamm AJ (1927) The electrical resistance of wood as a measure of its moisture content. Ind Eng Chem 19(9):1021–1025
Sze SM, Ng KK (2006) Physics of semiconductor devices. Wiley, New-Jersey
Taiz L, Zeiger E (2010) Plant physiology, vol 1. Sinauer Associates, Inc., Publishers, Sunderland
Texas Instruments. HDC2080 low power humidity and temperature digital sensor. 2018
Volkov AG (2000) Green plants: electrochemical interfaces. J Electroanal Chem 483(1):150–156
Volkov AG, Nyasani EK, Tuckett C, Blockmon AL, Reedus J, Volkova MI (2016) Bioelectrochemistry cyclic voltammetry of apple fruits: memristors in vivo. Bioelectrochemistry 112:9–15
Walter A, Finger R, Huber R, Buchmann N (2017) Smart farming is key to developing sustainable agriculture. Proc Natl Acad Sci 114(24):6148–6150
Yongzong L, Yongguang H, Asante EA, Kumi F, Li P-p (2016) Design of capacitance measurement module for determining the critical cold temperature of tea leaves. Sens Bio-Sens Res., Elsevier 11:26–32
Zhang J, Willison M (1991) Electrical impedance analysis in plant tissues: a double Shell model. J Exp Bot 42(11):1465–1475
Zhao DJ, Wang ZY, Li J, Wen X, Liu A, Huang L, Wang XD, Hou RF, Wang C (2013) Recording extracellular signals in plants: a modeling and experimental study. Math Comput Model 58(3–4):556–563
Zheng L, Wang Z, Sun H, Zhang M, Li M (2015) Real-time evaluation of corn leaf water content based on the electrical property of the leaf. Comput Electron Agric 112:102–109
Zimmermann MH, Jeje AA (1981) Vessel-length distribution in stems of some American woody plants. Can J Bot 59(10):1882–1892
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bar-On, L. et al. (2024). Plant-Based Electrical Impedance Spectroscopy for Plant Health Monitoring. In: Priyadarshan, P.M., Jain, S.M., Penna, S., Al-Khayri, J.M. (eds) Digital Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-031-43548-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-43548-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43547-8
Online ISBN: 978-3-031-43548-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)