Abstract
In this chapter, we define malignant gliomas in accordance with the World Health Organization (WHO) classification for central nervous system tumors (CNS) as Glioblastoma, IDH-wildtype, CNS WHO grade 4; Astrocytoma, IDH-mutant, CNS WHO grade 3; and Astrocytoma, IDH-mutant CNS WHO grade 4. Malignant gliomas are aggressive and incurable tumors, but recent studies demonstrate improved survival with advanced surgical treatments and the addition of chemotherapy. The current treatment paradigm for most patients consists of maximal safe resection, followed by radiation and chemotherapy. Older patients have unique concerns. In this chapter, we discuss the importance of surgery to make a definitive diagnosis as well as post-operative approaches to treatment include radiation type and dosing, the role of tumor-treating fields and the dosing and type of chemotherapy. Tables that included specific treatment regimens as well as patient-facing language are included.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
WHO CNS Classification:
Glioblastoma, IDH-wildtype
Astrocytoma, IDH-mutant Grade 3 and 4
Clinical Scenario
A 58 year old right handed man came to medical attention due to a focal motor seizure of the left lower extremity. He was found to have a 3 × 3 cm right parieto-occipital homogeneously enhancing cystic mass. He was taken to the operating room where a gross total resection was achieved by right occipital craniotomy. Post-operatively, he had a left homonymous hemianopia. Pathology demonstrated glioblastoma, IDH-wild type, MGMT methylated, ATRX retained. He received chemoradiation to a total dose of 60 Gy over 30 fractions with concomitant temozolomide 75 mg/m2 days 1 through 42. He completed a total 6 cycles of adjuvant chemotherapy and chose not to wear tumor treating fields.
He developed distant disease recurrence 7 months after completion of treatment. Neuroimaging revealed a new left parietal lesion and the previously treated right occipital lesion was stable. Since that area was not the primary target of previous radiation, he was presented to tumor board to discuss the safety and feasibility of re-irradiation. A multi-disciplinary team decided to proceed with radiation at a dose of 40 Gy over 15 fractions.
Six months later, an MRI demonstrated further growth of the left parietal mass with increased enhancement and surrounding edema. Additionally, he had more clinical symptoms including hemiparesis, aphasia and intractable focal seizures. He underwent resection of the left temporo-parietal mass to relieve pressure, improve symptoms, treat seizures and to determine the extent to which this was recurrent tumor or necrosis. The pathology was consistent with tumor recurrence. He was planned for therapy with bevacizumab beginning 28 days after surgery, but developed a saddle pulmonary embolism requiring hospitalization and subsequent decline in performance status. As a result of progressive clinical decline, the patient elected to enroll in hospice care. He spent 5 months in hospice and passed away 28 months from his original diagnosis of glioblastoma.
Making the Diagnosis
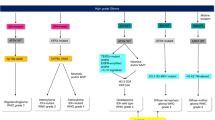
Gliomas arise from glial cells and neuronal precursors. They constitute 80% of all malignant primary brain and CNS tumors. Glioblastoma (GBM) is the most invasive, aggressive (grade 4) and common form. Patients can present with various symptoms including seizures, headaches, neurological deficits, and altered mental status. Magnetic Resonance Imaging (MRI) is the diagnostic modality of choice when a brain lesion is suspected. Computed Tomography (CT) scans are appropriate in emergent situations to evaluate for intracranial hemorrhage or hydrocephalus. While certain imaging characteristics are highly suggestive of GBM (heterogeneously enhancing expansile lesion), none are pathognomonic. In fact, many non-neoplastic processes can mimic gliomas, including multiple sclerosis, granulomatous diseases, infections, and radiation necrosis. Tissue diagnosis is essential to confirming the suspected diagnosis. Surgical approaches can range from a minimally invasive stereotactic biopsy to a craniotomy with gross total resection. A flowchart describing the diagnostic and treatment approach for these tumors is included in Fig. 1.1.
The current tenet of glioma surgery is to achieve maximal safe resection [1]. As diffusely infiltrating lesions, the oncologic concept of negative-margin resections applicable to other tumor types cannot be applied. Multiple studies over the past decades have demonstrated a survival benefit with gross total resection. Mathematical models applied to retrospective studies revealed a progressive improvement in survival with the extent of resection increasing between 78 and 98%. A systematic review and meta-analysis of the literature revealed a significant improvement in overall and progression-free survival with gross total resection compared to subtotal resection. In glioma surgery, the definition of gross total resection remains controversial. Achieving true gross total resection is impossible due to the far-reaching invasion of tumor cells into the normal brain parenchyma. Therefore, the consensus is that this terminology refers to the enhancing component. The controversy lies in the extent of resection of the T2 hyperintense portion. Subtotal resection and biopsy (open or stereotactic) are reserved for tumors in eloquent areas of the brain, for patients with a poor performance status or multiple medical co-morbidities and cannot medically tolerate resective surgery.
Once the importance of maximal safe resection was established, multiple surgical adjuncts promising to optimize efficacy were introduced [2]. These include intraoperative imaging modalities such as intraoperative ultrasound and intraoperative MRI. The quality of available data is at best moderate. However, all imaging modalities were found to improve the rate of gross total resection. Intraoperative ultrasound is inexpensive, readily available, easy to use and can localize small areas of residual that might not be visible to the naked eye. Intraoperative MRI on the other hand, requires an expensive infrastructure but can be very helpful in determining the need for further resection.
Fluorescence in brain tumor surgery was developed in the 1990s but its use became mainstream only recently. 5-Aminolevulinic acid (5-ALA) is an imaging agent used to detect glioma cells. It is given to patients orally 3 h prior to anesthesia induction at the dose of 20 mg/kg. 5-ALA causes accumulation of fluorescent porphyrin in tumor cells exclusively. These cells then emit a red-pink fluorescent light that is visible in the oculars of the microscope while the normal brain parenchyma appears in blue. This tool is especially valuable at the normal parenchyma/tumor interface. The use of 5-ALA has been shown to improve the ability of to achieve a gross total resection in a randomized study [3].
Another surgical adjunct is direct white matter stimulation. This technique is particularly important when resecting tumors in proximity to the corticospinal tract. During resection, the white matter fibers are directly stimulated at different amplitudes to elicit a motor evoked potential. Depending on the amplitude of the stimulation, a positive response indicates the presence of the corticospinal tract within a certain distance of the stimulus. Alternatively, awake surgery can be performed with cortical stimulation to minimize injury to eloquent areas. The combination of all 3 allows us to safely expand our resection beyond the contrast enhancing portion into what is defined as supramarginal resection.
Beyond cytoreduction, the role of surgery is to provide tissue for immunohistochemical and genetic analysis. The prognosis is heavily influenced by the genetics and molecular subtypes. Isocitrate dehydrogenase (IDH) mutation is ubiquitous in low grade gliomas. Malignant astrocytoma with IDH mutations are classified as either grade 3 or grade 4 astrocytomas, depending on histologic features and molecular signature. IDH-mutant infiltrative gliomas have a better prognosis than IDH-wild-type.
Based on the 2021 WHO Classification of Tumors, the diagnosis of glioblastoma is achieved in a high grade glioma that is IDH-wild-type [4]. For the purposes of this chapter, treatment recommendations apply to IDH-wild-type glioblastoma and can be extrapolated for treatment of IDH mutant grade 3 and grade 4 astrocytomas, for which there are few randomized trials to clearly define therapy. MGMT (O6-methylguanine-DNA methyltransferase) methylation status is a predictive biomarker that determines the response to temozolomide. MGMT is a DNA repair enzyme. It is particularly effective in repairing damage caused by alkylating agents and therefore confers a resistance to temozolomide. MGMT promoter gene methylation silences it and enhances response to temozolomide. IDH and MGMT status are only 2 of multiple mutations analyzed in GBM tissue. Once the genetic and molecular signatures of the tumor have been defined, the treatment paradigm, including clinical trial eligibility, are then determined.
Post-operative Treatment
Radiation
Glioblastoma is characterized by microscopic invasive disease within the brain parenchyma outside the tumor bulk, and most high grade glioma recurrences occur within 2 cm of the initial surgical resection margin. Adjuvant fractionated radiation therapy (RT) targeting this expected relapse field confers an overall survival benefit and comprises standard of care post-operative treatment, delivered concurrently with chemotherapy [5]. While there are several approaches to radiation therapy volume planning (EORTC vs RTOG recommendations), the accepted standard of care is the EORTC contouring approach. Radiation dosing of 60 Gy is delivered as 30 × 2 Gy fractions to the clinical target volumes (CTV), which is comprised of the gross tumor volume (GTV) + 2 cm. GTV encompasses the tumor resection cavity plus areas of residual T1 enhancement. Side effects most encountered include fatigue, cognitive decline, alopecia, and radiation dermatitis.
Elderly and frail patients may be considered for short course radiotherapy combined with chemotherapy. GBM survival decreases with advancing age and treatment is limited by toxic side effects and underlying coexisting conditions in the elderly [6], and patients over age 70 were excluded from the initial phase III study showing a benefit of combined chemoradiation versus radiotherapy alone using fractionated 60 Gy dosing [7]. Instead de-escalated treatment with hypofractionated radiation (40 Gy in 15 fractions) with concurrent and adjuvant temozolomide is a consideration in this population. Alternatively, elderly patients not fit for this strategy may be considered for hypofractionated radiotherapy or temozolomide monotherapy alone, with the latter treatment strategy more effective in patients with MGMT promoter methylation [8, 9].
Chemotherapy
Temozolomide (TMZ), a pro-drug alkylating agent which methylates DNA at the O6 position of guanine and which is able to penetrate the blood-brain barrier, is the current standard chemotherapy utilized in the adjuvant postoperative treatment of GBM [7, 10]. TMZ is delivered orally at a dose of 75 mg/m2 daily during concurrent radiotherapy. After completion of RT, TMZ is held for 4 weeks then resumed at 150 mg/m2 and subsequently escalated to 200 mg/m2 days 1–5 of every 28 days for a minimum of 6 months. No study has demonstrated benefit of adjuvant chemotherapy beyond 6 months, but it should be noted that in the CATNON study, 12 months of adjuvant therapy were given to individuals with anaplastic astrocytomas [11]. Analysis of the CATNON study also suggested that there may not be additional benefit for concurrent temozolomide in that population. Specific treatment regimens used in malignant gliomas are included in Table 1.1
.
Given the emetogenicity of TMZ use of ondansetron 8 mg, granisetron 1 mg or prochlorperazine 10 mg orally 30 min before each chemotherapy dose is recommended. During concurrent chemoradiotherapy weekly blood counts may be needed to monitor for cytopenias, and liver function testing monitored midway through radiation therapy and subsequently. Lymphopenia places patients at increased risk for Pneumocystis jirovecii pneumonia (PJP), and prophylaxis should be considered in patients still requiring corticosteroids. TMZ should be held for platelet count under 100,000 and ANC <1500/mL until count recovery.
In younger patients (age ≤ 70) with MGMT-methylated tumors and good performance status a combined lomustine/TMZ regimen concurrent with RT and adjuvant may be considered. This is based on randomized phase III data showing an improved overall survival compared to TMZ alone (48.1 months versus 31.4 months), however increased side effects were observed in the dual treatment arm. Younger fit patients may also be considered for a clinical trial up front.
Tumor Treating Fields
Tumor treating field (TTF) therapy is offered for frontline treatment of GBM in patients who tolerate this adjunctive modality. TTF technology consists of a portable medical device where electrodes are attached to the patient’s shaved scalp and transduce alternating electric fields at an optimal intensity and frequency for maximal tumor cell growth inhibition. TTF are felt to enact an antimitotic effect on the tumor hindering cell growth as their primary mechanism of action [12]. TTF are approved for use concurrent with monthly TMZ following completion of standard chemoradiation, and has proven to confer an overall survival benefit difference of nearly 5 months (20.9 months vs 16 months) compared with TMZ alone [13]. Side effects generally are mild and most prominently include a localized dermatitis under the placement of the electrodes on the scalp, which has been shown to respond well to topical steroid treatment [14].
Patients must wear these devices continuously for a goal 18 h per day, which has prompted concerns on the impact of quality of life (QoL). Questionnaires designed to assess QoL measures revealed no significant difference in global health status, emotional, social, physical, and cognitive functioning, as well as pain or leg weakness, and encouragingly patients receiving TTF had a significantly longer deterioration-free survival for several of these measures [15].
Symptom Management
Anti-epileptic therapy. Patients may present with seizures as their first symptom of GBM or experience seizures during their disease course, and approximately half of all GBM patients will be diagnosed with epilepsy during their disease [16]. Patients who develop seizures should be started on a single anti-seizure medication (ASM) for treatment using any first-line agent at the lowest effective doses. Typically levetiracetam is offered first line as this is well tolerated, though care should be used to monitor for potential neuropsychiatric side effects. While smaller studies have suggested an overall survival (OS) benefit with the use of valproate, a larger pooled analysis across four randomized trials found no difference in OS among patients on valproate as compared to other ASMs [17]. Patients who experience recurrent seizures while on therapy should have ASM levels monitored prior to dose escalation or consideration of adding a second drug. Seizure prophylaxis in a patient with no seizure history is generally not recommended, however perioperative prophylaxis may be considered with recommendations to taper and subsequently discontinue the ASM starting at 1–2 weeks post-operatively [18, 19].
Vasogenic edema management. Vasogenic edema results from local disruption of the blood-brain barrier from the tumor and is commonly encountered during disease management of patients with glioblastoma (see Chap. 10). Vasogenic edema appears on MRI as hypointense on T1-weighted images and hyperintense on T2-weighted images. Neurologic symptoms are variable however symptomatic patients require initiation of systemic steroids, with dexamethasone used as the standard agent, and clinical response should be monitored. Common starting doses of dexamethasone are 4–8 mg divided once or twice daily and subsequently a taper can be initiated once symptoms are stabilized. Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody can also be used as a steroid-sparing treatment for edema control, including in the management of edema related to radiation necrosis. Dosing is typically either 7.5 mg/kg every 3 weeks or 5 mg/kg every 2 weeks for four doses, with MRI monitoring mid-way through and at treatment completion.
Surveillance
After the initial concurrent chemoradiotherapy, it is standard practice to obtain a brain MRI with contrast 4–6 weeks following therapy completion. Thereafter, imaging is obtained every 2–4 months thereafter for monitoring assessment or earlier based on symptoms. The current criteria for imaging evaluation are based on the Response Assessment in Neuro-Oncology (RANO) Working Group, which includes guidelines for determination of progressive disease versus pseudoprogression,with progressive disease based on at least two sequential studies separated by 4 weeks and showing 25% or more increase in size or 40% or more increase in the total volume of the enhancing lesion [20, 21]. Moreover patients who are symptomatic or have tumors harboring MGMT promoter unmethylated status or IDH-wild-type are more likely to have true disease progression [22, 23]. Advanced imaging such as MRI perfusion and PET may not be widely available but can be helpful in differentiating pseudoprogression from true progression.
Treatment at Recurrence
After the determination of progressive disease and assessment of patient performance status, including a trial of steroids for treatment of symptomatic peritumoral edema if indicated, subsequent treatment can be considered for GBM. With both first and second recurrences, clinical trials should be considered. For patients with poor functional status or personal preference to not pursue additional therapy supportive care should be given. Patients with good functional status can be considered for reoperation with or without implantation of carmustine (Gliadel wafers) and/or re-irradiation if indicated. The role of laser thermal ablation in this setting is evolving. For patients in whom systemic therapy is being considered typical regimens are single-agent bevacizumab (10 mg/kg IV days 1, 15), single or combination nitrosourea-based regimens, or re-challenge with temozolomide (150–200 mg/m2 days 1–5 every 28 days). No agent has proven superiority to another or has demonstrated improved overall survival. Nitrosourea-based regimens typically consist of lomustine (CCNU) monotherapy (100–130 mg/m2 day 1, every 42 days), or in combinations such as procarbazine, CCNU and vincristine (PCV) (Procarbazine 100 mg/m2 PO on days 1–10, Lomustine 100 mg/m2 PO day 1, Vincristine 1.5 mg/m2 IV day 1, every 42 days).
Prognosis/Survivorship
The prognosis of patients with GBM is dependent upon age and functional status at diagnosis, as well as underlying genomic profile including presence of MGMT promoter methylation and IDH status, among others. The median overall survival of all GBM patients treated with standard combined TMZ and radiation is 14.6 months [24]. Table 1.2 includes patient-facing information including guidance related to prognosis. Moreover, patients on standard chemoRT who underwent a complete, partial, or biopsy only resection had median survivals of 18.8 months vs 13.5 months vs 9.4 months, respectively. Patients under age 50 had a median OS of 17.4 months versus 10.9 months for those over age 60. Patients with MGMT promoter methylation had a median OS of 23.4 months versus 12.6 months in the unmethylated group [24]. Astrocytoma, IDH-mutations are associated with improved overall survival as compared to glioblastoma, IDH-wildtype (27.4 versus 14 months, respectively) [25].
There is limited data on survivor care for patients with GBM given that most patients succumb to their disease in a short timeline. However, studies of long term GBM survivors have identified a need for the continued monitoring of recurrences, with multiple lines of chemotherapy necessary for disease relapse. Patients also often require continuation of anti-epileptic medications, monitoring of their neurocognitive decline, and other neurological sequelae including radiation necrosis, cerebrovascular accidents, hydrocephalus and VP shunting as well as dementia. This group of patients thus requires specialized Neuro-Oncologic care for their continued monitoring [26].
Trends and Future Directions
Glioblastoma is characterized by marked heterogeneity which underlies treatment resistance, parenchymal invasion and inevitable tumor recurrence. Clinical trials utilizing targeted therapies against known signaling aberrations within GBM have yet to demonstrate significant benefit for either up-front or salvage therapy [27]. The only FDA approved targeted therapy is bevacizumab for use in recurrent glioma. Trials so far of small molecule kinase inhibitors, antibodies, or antibody drug conjugates that target aberrant receptor tyrosine kinase signaling activity have not proven to yield a PFS or OS benefit. Additionally, the use of histone deacetylase inhibitors, PARP inhibitors, or IDH1mt inhibitors are under early investigation and the effectiveness is yet to be determined [27].
Immune therapies represent another promising treatment strategy, however to date there are no FDA-approved immune-based treatments. Investigations are underway exploring immune checkpoint inhibitors (ICI), vaccines, adoptive T-cell therapies, and viral therapy [28]. Of note a subset of TMZ treated glioblastomas display hypermutation signatures at tumor recurrence [29]. This has led to the proposal that these gliomas may respond to subsequent therapy with ICI, noting that high tumor mutational burden is predictive of response regardless of disease [30]. However, early trials to date have not shown significant anti-tumor efficacy of ICI therapy in recurrent GBM although it remains to be seen whether there is a subgroup of responders in follow-up analyses, and moreover, some evidence indicates ICI use in the neoadjuvant setting may lead to more consistent immune activation [31, 32]. Dendritic cell-based vaccines have been shown to yield durable responses in a minority of patients in clinical trials to date and studies remain ongoing [28]. Trials investigating CAR-T mostly have studied the IL-13Ra2, EGFRvIII and HER2 antigens, however with most patients not displaying significant tumor regression [28]. Likewise there has been a lack of durable response from viral directed anti-GBM therapy to date [33]. Further molecular studies to understand treatment response patterns are warranted. The current standard of care treatment for glioblastoma aims to prolong survival at best, though some patients have minimal benefit, dependent on their performance status and the tumor genetics. In the absence of any curative therapy, clinical trial participation is encouraged for all eligible patients with glioblastoma, with the hope of improving survival and quality of life over time.
References
Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–9.
Golub D, Hyde J, Dogra S, et al. Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis. J Neurosurg. 2020;1–15:484–98.
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. https://doi.org/10.1016/S1470-2045(06)70665-9. PMID: 16648043.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51. https://doi.org/10.1093/neuonc/noab106. PMID: 34185076; PMCID: PMC8328013.
Corso CD, Bindra RS, Mehta MP. The role of radiation in treating glioblastoma: here to stay. J Neurooncol. 2017;134(3):479–85.
Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39(4):350–7.
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus Temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–37.
van den Bent MJ, Tesileanu CMS, Wick W. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(6):813–23. https://doi.org/10.1016/S1470-2045(21)00090-5. Epub 2021 May 14. PMID: 34000245; PMCID: PMC8191233.
Margison GP, Santibanez Koref MF, Povey AC. Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis. 2002;17(6):483–7.
Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–88.
Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–95.
Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance Temozolomide vs maintenance Temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–16.
Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–202.
Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495–504.
Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34(1):98–102.
Happold C, Gorlia T, Chinot O, et al. Does valproic acid or Levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J Clin Oncol. 2016;34(7):731–9.
Tremont-Lukats IW, Ratilal BO, Armstrong T, Gilbert MR. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008;2008(2):CD004424.
Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the quality standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–93.
Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72.
Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–20.
Li H, Li J, Cheng G, Zhang J, Li X. IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg. 2016;151:31–6.
Rowe LS, Butman JA, Mackey M, et al. Differentiating pseudoprogression from true progression: analysis of radiographic, biologic, and clinical clues in GBM. J Neurooncol. 2018;139(1):145–52.
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4.
Peters KB, Woodring S, Affronti ML, et al. Long-term survivorship in adult primary glioblastoma: clinical and neurological outcomes of a large, single-center study. J Clin Oncol. 2014;32(15_suppl):9519.
Gupta SK, Kizilbash SH, Daniels DJ, Sarkaria JN. Editorial: targeted therapies for glioblastoma: a critical appraisal. Front Oncol. 2019;9:1216.
McGranahan T, Therkelsen KE, Ahmad S, Nagpal S. Current state of immunotherapy for treatment of glioblastoma. Curr Treat Options Oncol. 2019;20(3):24.
Daniel P, Sabri S, Chaddad A, et al. Temozolomide induced hypermutation in glioma: evolutionary mechanisms and therapeutic opportunities. Front Oncol. 2019;9:41.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1.
Caccese M, Indraccolo S, Zagonel V, Lombardi G. PD-1/PD-L1 immune-checkpoint inhibitors in glioblastoma: a concise review. Crit Rev Oncol Hematol. 2019;135:128–34.
Ito H, Nakashima H, Chiocca EA. Molecular responses to immune checkpoint blockade in glioblastoma. Nat Med. 2019;25(3):359–61.
Chiocca EA, Nassiri F, Wang J, Peruzzi P, Zadeh G. Viral and other therapies for recurrent glioblastoma: is a 24-month durable response unusual? Neuro Oncol. 2019;21(1):14–25.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ensign, S.F., Porter, A.B. (2023). Malignant Glioma. In: Mohile, N.A., Thomas, A.A. (eds) Brain Tumors. Springer, Cham. https://doi.org/10.1007/978-3-031-41413-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-41413-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-41412-1
Online ISBN: 978-3-031-41413-8
eBook Packages: MedicineMedicine (R0)