Abstract
The modern scientific community is very interested in using polymeric nanodielectrics in capacitors and other forms of improved energy storage devices. Hybridization of inorganic metal or metal oxide NPs as nanofillers with a high dielectric constant and the polymer as a matrix with a high breakdown strength is fundamental in fabricating polymer nanodielectric films. Findings from this study show, for example, that a higher NP filler concentration results in a higher dielectric permittivity and a lower dielectric loss in the nanodielectric film, making it an attractive candidate for use in the fabrication of high-voltage storage capacitors. This chapter provides a concise overview of the methods for preparing highly flexible polymer nano dielectrics with enhanced thermostability for the fabrication of electronic and microelectronic devices, including using various metal and metal oxide NPs as cores and a variety of polymer matrices as shells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Nowadays, there is a continuous depletion of energy sources like fossil fuels worldwide; hence there is a huge demand for the generation and storage of energy using renewable energy sources [1, 2]. Currently, most of the research development sectors focus on generating plenty of energy, particularly electrical energy using tidal, wind, and solar energy resources, etc. To store the generated electricity, there is a need for highly reliable and efficient energy storage devices such as capacitors [3,4,5]. In this regard, dielectric capacitors in energy storage devices are a better alternative than electrochemical capacitors and batteries because of their long lifetime, high power density with high-voltage tolerance capability, and higher cycling stability [6]. All these interesting characteristics associated with dielectric capacitors make them a promising material of choice for renewable energy storage and distribution systems [7]. Capacitors possessing high dielectric constants are much needed for cable insulation, electrocaloric cooling, etc. Marketed dielectrics are generally polymer or ceramic-based [8]. Though ceramic dielectrics have their advantages, it has some limitations, such as high density, low breakdown strength with poor flexibility, low-temperature tolerance capability, etc. [9, 10]. Thus, there is a need for another kind of material that will possess opposite characteristics to the traditionally used materials.
In this connection, the scientific community suggested polymeric dielectrics as an alternative energy storage device. The associated properties, like high-voltage endurance with dielectric strength and low resistance with less dielectric loss, made dielectric polymers an incomparable material of choice for a wide range of electrical insulation and energy storage applications [8,9,10,11]. As for wire and cable insulation, the polymer coating protects the internal shielding and conductors from mechanical, external moisture, ultraviolet (UV), and ozone damage [4, 5]. For tape and capacitor insulation, polymer films/sheets work as fundamental building blocks that provide electrical current handling capability and electrical charge build-up barriers [12]. Polymeric core-shell devices containing nanofillers, generally known as nanodielectrics are highly advantageous and quite appealing for energy storage purposes and application in capacitors [13]. After many hits and trials, it was signified that the performance of metal or metal oxide-based NPs as nanofiller is more enthusiastic. Due to the small particle size and the high surface area of NPs [14,15,16,17,18,19], the bonding between polymer matrix and NPs is quite superior. Polymers with a strong polarizing tendency and exceptional effectiveness, such as poly (vinylidene fluoride) (PVDF), PVDF-based copolymers and terpolymers, and linear polymers like polyetherimide (PEI) and poly(methyl methacrylate) (PMMA), were chosen as a matrix for this application [5,6,7].
Moreover, it was verified that, for metallic/polymer NP dielectrics, field electron emission from metallic NPs under a high field tends to decrease the dielectric breakdown strength and, thus, increase the electronic conduction [1,2,3]. The charming features associated with nanodielectrics include high energy density, high capacitance density, high voltage, high current handling capability, high thermal conductivity, high temperature, lightweight, and environmental reliability. The addition of nanofillers impressively increased the flexibility, dielectric constant, breakdown strength, and thermostability with low dielectric loss of the polymer matrix [20].
This chapter briefly summarizes the preparation and application of different metal or metal oxide-doped polymer-based nanodielectrics in different energy storage devices.
15.2 Application
In order to study its potential against HV insulating systems like HVDC cables, ZnO nanoparticles were selectively distributed on polyethylene (PE) mix and thermoelastic polymer (TPE) comprising polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene grafted maleic anhydride (SEBS-MA). The generated blended nanocomposites of PE/SEBS-MA/ZnO were analyzed and compared with a homopolymeric PE–ZnO nanocomposite for their mechanical and dielectric characteristics. Agglomeration of metal oxide NPs made them less compatible with the polyolefin matrix, leading to a compatibility problem. ZnO NPs were observed to be solely restricted inside the polymeric lattice and to be situated at the interface between SEBS-MA and PE, but in the case of SEBS-MA/ZnO and PE/SEBS-MA/ZnO, improved compatibility between SEBS-MA and the NPs occurred, resulting in good dispersion of the NPs. Additionally, PE/SEBS-MA/ZnO nanocomposites showed improved dielectric characteristics over PE/SEBS-MA nanocomposites because of the increased dispersion of ZnO NPs (unfilled blend). When compared with free PE/SEBS-MA, plane PE, and PE/ZnO nanocomposite, the blended nanomaterial showed reduced dielectric loss at higher power with temperatures 80 and 45 °C percent improved resistance to surface erosion value. Upon high loading of NPs, the blended nanomaterials exhibited decreased breakdown strength than unfilled material. Moreover, the blended nanocomposites displayed high mechanical flexibility with low reduction value due to the quality dispersion of the NPs (Figs. 15.1 and 15.2) [21].

Reprint and Copyright permission obtained from Elsevier publication [21]
SEM of unmodified PE–SEBS-MA (a), PE–SEBS-MA/1 (b), PE–SEBS-MA/5 (c), and PE–SEBS-MA/10 (d) composites.

Reprint and Copyright permission obtained from Elsevier publication [21]
Resistance to surface erosion through PE–ZnO as a function of PE–SEBS-MA–ZnO nanocomposites.
Go/Au (prepared by doping of Au on GO) and GO–Cu (prepared by doping of Cu over GO) integrated over poly vinylidene fluoride (PVDF)-based new and flexible nanocomposites were generated following the solution casting process. The presence of GO/Cu and GO/Au nanofillers increased the quantity of electroactive crystalline phases of PVDF. Low dielectric loss with high dielectric constant and a high content of electroactive phases made it a suitable candidate for fabricating over flexible high-performance nanodielectric materials like electronic devices and sensors. An increase in the concentration of the nanofillers leads to a rise in the electroactive phase due to the presence of electrostatic interactions between the nanofiller and CH2–CF2 dipoles of PVDF, as confirmed by FTIR spectra. Along with this, it did not display any absorption band related to the non-polar α phase in the FTIR spectrum. For PVDF filled with 1% GO/Au nanofiller, the maximum value of the electroactive phase content was found to be 95% which was around 2.5 times greater than neat PVDF, as demonstrated through FTIR. Compared with GO/Cu, GO/Au-based nanocomposites possessed an increased number of electroactive phases, which XRD determined. From capacitance, inductance, and LCR (resistance) measurement, nanomaterials exhibited increasing nanofiller content with high dielectric constant with low dielectric loss, which made it an interesting material of choice to fabricate over simple and flexible high-performance nanodielectric materials (Figs. 15.3, 15.4 and 15.5) [22].

Reprint and Copyright permission obtained from Elsevier publication [22]
FTIR spectra of neat PVDF and PVDF-based nanocomposites with different contents of GO–Au (a) and GO–Cu nanofiller (b).

Reprint and Copyright permission obtained from Elsevier publication [22]
XRD spectra of pristine PVDF and PVDF-based nanocomposites with different content of a GO–Au and b GO–Cu nanofiller.

Reprint and Copyright permission obtained from Elsevier publication [22]
Frequency dependence of dielectric constant and dielectric loss of pristine PVDF nanocomposites with different contents of GO–Au (a, c) and GO–Cu (b, d) nanofillers.
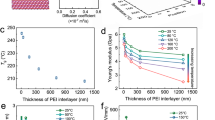
Dielectric polymeric nanocomposite matrices were prepared by the embedment of molybdenum disulfide (MoS2) and polydopamine (PDA)–encapsulated hydroxylated barium titanate (BTH) over [poly (arylene ether nitrile)] (PEN) matrix. The nanofillers, i.e., MoS2/PDA@BTH-1 were prepared through polydopamine deposition technology involving H-bonding interactions. The introduction of PDA contributed to the formation of nanocapacitor networks and multiple interfaces, enhancing interfacial polarization. Experimentally, it was found that the polymeric nanocomposite matrix exhibited a dielectric constant value of 17.3 at 1 kHz upon 15 wt% MoS2/PDA–BTH-1 loading. The matrix also maintained low dielectric loss at the meant time. The plausible mechanism of the synergistic effect of MoS2/PDA–BTH-1 upon the enhancement of the dielectric properties of the composite matrix was proposed, which was assumed that the dielectric constant value becomes stabilized upon reaching 158 °C as suggested by temperature-permittivity data. Thus, the developed film could be used in capacitors up to a temperature 150 °C, which was quite higher than that of most polymeric capacitors. The high-temperature tolerance nature and high dielectric constant (k) value of the developed material made it a demandable material for application in embedded devices as well as electrostatic capacitors (Figs. 15.6 and 15.7) [23].

Reprint and Copyright permission obtained from Elsevier publication [23]
Synthesis of MOS2–PDA–BTH-1 and MOS2–PDA–BTH-A–PEN nanocomposite film.

Reprint and Copyright permission obtained from Elsevier publication [23]
SEM and TEM micrographs of BTH (a, b), MOS2 (c, d), PDA@BTH (e, f), and MOS2–PDA–BTH-1 (g, h) particles.
Polymeric nanocomposite films containing carboxymethyl cellulose (CMC)-polyvinyl alcohol (PVA) (30/70 wt%) blend and Au NPs (0.36 wt%) as host polymer matrix were synthesized through the casting of aqueous solutions. The required Au NPs for synthesizing the polymeric matrix were successively generated from the leaf extract of Morus nigra (M. nigra). It was then irradiated under a nanosecond laser at a different time interval. The presence of functional groups in the CMC/PVA polymer interacted through H-bonding interactions, and simultaneously, the intensity of the spectral lines significantly increased upon irradiation and the addition of Au nanoparticles, as implied by FTIR. An increase in the absorbance intensity leading to a blue shift and appearance of SPK peak in the UV-Visible spectra confirmed the well dispersion of Au MPs upon the polymeric matrix. The TEM study confirmed that laser irradiation reduced the particle size and spherical shape of Au NPs. It exhibited high dielectric constant value and dielectric permittivity, making it a valuable material for further application in optoelectronic and electronic devices [24].
Polymeric nanocomposite films were developed by the incorporation of different weight percentages of ZnO NPs upon poly (vinyl pyrrolidone) (PVP) and poly (ethylene oxide) (PEO) blend matrix. The developed blended matrix was studied for its application in preparing next-generation microelectronic and optoelectronic devices. The structure and morphology of the nano polymeric film were studied using SEM, FTIR, XRD, etc. It was found that the incorporation of different amounts of ZnO NPs inside the polymer matrix significantly influenced the polymer-polymer, porous spherulite morphology, semicrystalline structure, and polymer-nanoparticle interactions of the materials. UV-Vis spectra were used to determine the optical properties such as optical band gap, refractive index, Urbach tail energy, and ZnO surface plasmon resonance energy. Increasing the concentration of ZnO in the polymeric film decreased the optical band gap. DRS spectroscopy was used to study its implication in the flexible nanodielectrics from 20 Hz to 1 MHz. The permittivity of the complex was found to increase linearly up to 3 wt% ZnO loading with respect to an increase in temperature, then a slight decrease in the value was noticed at 5%. However, with increased temperature, the electrical conductivity and the dielectric relaxation time obeyed the Arrhenius manner. Experimentally, it was observed that the dielectric constant of the film increased upon loading of ZnO nanoparticles [25].
Dispersion of Fe3O4 NPs was carried out in one step over polyethylene oxide (PEO)/chitosan (CS) based nanocomposite using laser ablation at different time intervals. The as-produced nanocomposite film containing PEO/CS/Fe3O4 was analyzed properly using XRD, SEM, TEM, FTIR, and UV-Visible spectroscopy. Also, the authors measured the nanofilm’s electrical conductivity and dielectric characteristics. The crystallinity of the nanocomposite film gets enhanced by the fabrication and well interaction of the PEO–CS matrix with Fe3O4 NPs. The average particle size of the Fe3O4 NPs generated under laser ablation was found to be 45 nm. FTIR spectra also confirmed the stable interaction present in the PEO–CS–Fe3O4 nanocomposite film. The appearance of the bright spots over the surface of the sample in the SEM image confirmed the successive dispersion of the NPs over the nanocomposite film. The optical characteristic of the generated material was confirmed by the appearance of an absorption peak at 218 nm, which shifted toward a higher wavelength region with respect to the rise in laser ablation time, also simultaneous decrease in the energy gap to 4.43 eV from 5.35 eV for direct transition and to 2.49 eV from 4.91 eV for indirect transition. It was also studied that the electrical conductivity of the sample increased with a simultaneous increase in Fe3O4 NPs. PEO–CS–Fe3O4 NPs achieved a high conductivity value of ∼8.47 × 10−6 S/cm at a laser ablation time of 20 min. The developed nanomaterial exhibited high electrical conductance, which can be applied in different electronics (Figs. 15.8 and 15.9) [26].

Reprint and Copyright permission obtained from Elsevier publication [26]
XRD spectra of PEO–CS blend and PEO–CS–Fe3O4 blend at various laser ablation times.

Reprint and Copyright permission obtained from Elsevier publication [26]
FTIR and UV-Vis spectra of PEO–CS and PEO–CS–Fe3O4 blend with various ablation times.
Dispersion of carbon fabricated Ni NPs was carried out over epoxy-based photoresist material (SU-8), doped over metal polymer composites (MPC), and used in-depth high-voltage dielectric characterization. The integration of MPC involved three stages: deagglomeration, surface functionalization, and dispersion in a polymeric matrix following the sonochemical process. In the end, the MPC films were deposited over silicon wafers through spin-coating and finally used to fabricate metal–insulator–semiconductor (MIS) capacitors. The dielectric permittivity was observed to be enhanced to approximately 116% with 0.32 loss at 5 kHz. The measured high-voltage dielectric properties indicated the 2e− field mechanism without electrical aging or dielectric breakdown less than ±0.38 MV/cm. The developed wafer-scale film fabrication process of MPCs was quite reliable and can be applied to manufacture pleasant miniaturization of high-voltage capacitors [27].
The increasing variety of uses for hybrid nanocomposites made from organic and inorganic elements has increased their popularity in recent years. The in-situ bulk polymerization method was utilized to synthesize [poly(methyl methacrylate)] (PMMA) and its binary and ternary nanocomposites using varying amounts of rGO and Fe2O3 NPs. PMMA; based ternary nanocomposites comprising 2:2 wt% of RGO:Fe2O3 NPs showed the greatest dielectric constant value up to 308 and also demonstrated a dielectric loss of 0.12 at 25 Hz, according to the results of the dielectric characteristics study conducted in the frequency range of 25 Hz to 1 MHz. The dielectric characteristics of ternary PMMA nanocomposites were predicted to be superior to those of binary nanocomposites containing only one interface, possibly as a result of the accumulation of a greater number of charges at the latter’s interface. The synergistic reduction in thermal resistance of together Fe2O3 NPs and rGO (2:2 wt%) compared with 2 wt% of PMMA–RGO and PMMA–Fe2O3-based binary nanocomposites at 1.04 W/m K and 0.98 W/m K, respectively, resulted in an increased thermal conductivity value of 2.04 W/m K in the ternary nanomaterials. Therefore, the ternary nanocomposite showed promise as a heat-control mechanism component. The nanomaterial spectra data indicated a robust interaction of the nanofillers with the PMMA matrix [28].
Cast synthesis was used to create bendable nanocomposite films using carboxymethyl cellulose (CMC), polyethylene oxide (PEO), and ZnO/GO NPs as nanofiller. Nanocomposite films were analyzed for their shape, crystallinity, and structure using XRD and FTIR spectra, which showed that ZnO/GO NPs interacted effectively with the PEO/CMC mixture. The optical properties, including Urbach energy and energy band gap of the nanocomposite and pure PEO/CMC blend, were measured by UV-Vis spectroscopy. It was studied that increasing the weight percentage of ZnO–GO NPs decreased the energy band gap with an increase in the Urbach energy. AC-impedance spectroscopy was used to compute the ionic conductivity of the polymer nanocomposites. For PEO–CMC composite film, the calculated ionic conductivity was found to be ∼10–9 S/cm, which increased to ∼10–7 S/cm by adding 8% ZnO–GO nanofiller at room temperature. Increasing the frequency increased the AC electrical conductivity; however, it reduced the values of ε′ and ε″ of PEO–CMC and ZnO–GO nanocomposites. Surprisingly, increasing the concentration of ZnO–GO NPs increased the ε′ and ε″ values of the nanocomposites. Hence, PEO/CMC nanocomposite films prepared by using 8 wt% ZnO–GO NPs with enhanced dielectric, optical, and electrical properties can further be employed for different industrial purposes like flexible energy storage devices, i.e., optoelectronic and electric devices [29].
(CMC/PAM)/Li4Ti5O12-based polymeric nanocomposite films were prepared by the fabrication of Li4Ti5O12 NPs with particle size <55 nm over a carboxymethyl cellulose/polyacrylamide (CMC–PAM) blend matrix involving a solution casting process. The optical, dielectric, structural, mechanical, and thermal properties of the nanocomposites were determined through various spectral studies. The existence of coordination and hydrogen bonds in the nanocomposite samples with increased CMC/PAM structural amorphous areas was revealed through FTIR and XRD. The absorption maximum of the nanocomposite samples was significantly enhanced by the reduction of the energy band gap which was determined by UV-Vis spectra. The inclusion of Li4Ti5O12 NPs, fine dispersion, and well interaction between the NPs and polymer enhanced the thermal stability of the CMC/PAM at a high temperature which was proved by the TGA study. Moreover, the addition of nanofillers improved the mechanical properties of the nanocomposite. Tuning of the dielectric parameters like modulus spectra and dielectric permittivity of the nanocomposite films can be achieved by tuning of Li4Ti5O12 NP content in the CMC/PAM blend which illustrates its novel applicability in the biodegradable polymer nanodielectrics. The advantageous features like the development of polymer electrolyte, optical band gap tuner, and controllable dielectric permittivity etc., associated with the nanocomposite made it a suitable material for application in high-density energy storing/advanced microelectronic devices [30].
Polymer-perovskite-based hybrid nanocomposites are highly demanding because their integration into electronic and optoelectronic devices enhances the physical and chemical properties of the target material. SrTiO3 NPs fabricated PVA–CMC-based nanocomposites were synthesized following the solution casting method. Increasing the content of SrTiO3 NPs decreased the degree of crystallinity of the nanocomposites, which was demonstrated through XRD, whereas it enhanced the optical characteristics of the material as studied by UV-Vis spectra. An FTIR spectrum was used to study the influence of the SrTiO3 nanoparticles upon the functional groups of the PVA–CMC hybrid system. SEM images confirmed a rise in the surface roughness with respect to the increase in the NP content. The authors also examined the dielectric dispersion and relaxation characteristics of the prepared PNC films, which signified that the polymeric nanocomposites possessed a high value of dielectric constant with a low dielectric loss than pure PVA–CMC blend. Thus, the observed outcomes depicted its further potential attribution in advanced optoelectronic devices [31].
Nanocomposites containing poly (methyl methacrylate) (PMMA) matrix and SnO2, ZnO, and TiO2 as nanofillers were designed and synthesized by a sonicated suspension of NPs in polymeric solutions following the casting process. DRS, XRD, and UV-Vis techniques characterized the prepared nanomaterial well. The successive formation of the nanocomposite with appropriate composition was confirmed by XRD and SEM studies. The dielectric permittivity of the PNC was also demonstrated. The band gap and the UV-Vis absorbance of the material were found to be tunable as characterized by UV-Vis. For a particular nanofiller concentration, maximum absorbance was displayed by PMMA/TiO2 films, and it was decreased for PMMA/SnO2 films. For equal PMMA/TiO2 composite composition, increasing the thickness of the material reduced the energy band gap with an enhancement of UV-Vis absorbance, as observed by the thickness study. For nanocomposites containing SnO2 and ZnO as nanofiller, the high dielectric permittivity value was found to be directly proportional to the concentration of the respective nanofillers; however, for TiO2 NP-containing films, a decrease in the permittivity value was noticed when compared with the host matrix film at 30 °C. Moreover, enhancing the experimental electric field frequency to 1 MHz from 20 Hz gradually decreased the permittivity of the nanocomposite. Hence, the associated versatile properties make the nanocomposite film a promising candidate for further application in the development of different advanced organoelectronic, nanodielectric insulators, and optoelectronic devices and to architect the coming generation of flexible electronic devices [32].
Polymeric nanocomposites (PNCs) are considered the key moiety present in the flexible type of advanced microelectronic devices based on polymer engineering and technology. A three-phase hybrid nanocomposite consisting of SiO2 NPs dispersed over PNC films of poly (vinylidene fluoride)/poly (ethylene oxide) (PVDF–PEO) blend was designed, synthesized, and characterized through various spectral methods. Experimentally, increasing the concentration of SiO2 NPs decreased the relative fraction of PVDF β-phase crystals and degree of crystallinity and simultaneously modified the spherulitic morphology of the PVDF/PEO blend matrix. Moreover, the loading of 5 wt% SiO2 nanofiller enhanced the dielectric permittivity and improved the dielectric constant at a frequency range from 20 Hz to 1 MHz. The tunable dielectric and structural properties made them a suitable candidate for successive application in appealing polymeric nanodielectrics, including organoelectronic and energy storage devices [33].
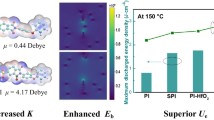
Flexible dielectric nanocomposites with excellent energy storage properties make it an alternative choice able material for its potential application in electrostatic capacitors. With an intention to eliminate the inconveniences stimulated by the interfacial polarization of two different components in the nanocomposite, authors prepared PVDF–HFP/HfO2–BT nanocomposites by the fabrication of HfO2–BT NPs over poly (vinylidene fluoride-co-hexafluoropropylene) film following solution casting process. Due to the high resistivity and moderate dielectric permittivity nature of the HfO2, it acts as a buffering barrier. Its inclusion in the nanocomposite reduces dielectric mismatch between the P (VDF-HFP) matrix and BT NPs. The addition of nanofiller substantially enhanced the energy density (Ue) and breakdown strength (Eb) of the nanocomposite. The maximum Ue value of 10.7 J/cm3 was exhibited by P (VDF-HFP)–HfO2–BT nanocomposites at 450 MV/m, around 36% and 59% greater than that of nanocomposite filled by only BT nanoparticles and also that of pristine P (VDF-HFP). Additionally, it possessed a charge–discharge efficacy of 72%, which was quite superior to the reported values. It is concluded that adding nanofillers containing a passivation layer might be an effective route to enhance the energy storage capacity of the flexible nanodielectrics [34].
Polymeric nanocomposites were prepared by the embedment of MWCNTs/Au NPs over PEO (polyethylene oxide) samples following the casting process. The interaction between the polymer and the nanoparticles was confirmed by FTIR spectra. Reduction in the crystallinity phase of PEO due to the incorporation of MWCNTS-Au NPs as well as the semicrystalline nature of the nanocomposite was confirmed through XRD. MWCNTs having a diameter of 10–25 nm and the spherical shape of the NPs with 2–25 nm particle size was predicted by TEM analysis. The absorption maximum shifted toward the red region, which indicated the decrease in the energy band gap with good reactivity between the polymer matrix and the nanofillers, as calculated by Tauc’s relation. An increase in the thermal stability of the nanocomposite was predicted by TGA analysis. Dielectric spectroscopy was used to measure the dielectric and electric spectra of the samples, which suggested its favorable application in the production of electroactive materials as well as for the preparation of electrical insulating polymeric nanodielectrics [35].
PEO/PMMA/MMT-based hybrid polymeric nanocomposite (HPNC) films were designed and synthesized by the dispersion of different composition ratios of organic (PEO and PMMA) and inorganic (MMT, montmorillonite) polymer blend matrices via solution-cast process followed by the melt-press method. The characteristics, properties, and structure of the polymer blend were analyzed by different spectral studies, i.e., SEM, XRD, DSC, dielectric spectroscopy, UV-Vis, etc. The authors also explored the influence of MMT nanofiller concentration and PEO/PMMA blend compositional ratio upon the degree of crystallinity, melting temperature of PEO crystallites, homogeneity, and variation in intercalated MMT structures, etc. It was demonstrated that the quantity of the constituents along with polymer–nanofiller, and polymer–polymer interphases in the HPNC and PB materials had a significant influence upon the UV-Vis absorbance with energy band gap, electrical conductivity, dielectric dispersion, and relaxation processes, and other optical parameters of the nanocomposites. Loading of MMT with PMMA blending enhanced the thermal stability of the PEO. It exhibited high dielectric permittivity in the lower frequency range, i.e., from 105 to 2 × 101 Hz, mostly due to the interfacial polarization that made them applicable in the flexible and tunable dielectrics [36].
5 wt% of different metal oxides (Al2O3, SnO2, TiO2, ZnO) NPs were used as nanofillers and were fabricated over PVDF/PEO (75/25 wt/wt%) blend considered as host to generate polymeric nanocomposite (PNC) films following casting process. The as-prepared nanocomposite films were successively characterized by XRD, FTIR, SEM, UV-Vis, DRS, etc. The influence of nanofillers upon the dielectric, optical, and structural properties of the polymeric blend was discussed. The presence of metal oxide nanoparticles significantly altered the spherulitic morphology of the polymer, created a huge number of micro- to nano-sized pores, and lowered the degree of crystallinity of the host matrix and also the β-phase content of the PVDF and crystalline phase of PEO. At ambient temperature, among the used metal oxides, Al2O3 NPs significantly enhanced the dielectric permittivity, whereas other nano inclusions altered the MWS relaxation and dielectric polarization processes of the PNC films in the frequency range from 20 Hz to 1 MHz. Additionally, Al2O3 NPs exhibited high electrical conduction, and a considerably decreased value was noticed for ZnO nanoparticles containing PNC films [37].
PEO–PMMA–SiO2 and PEO–PMMA–SnO2-based nanocomposite films were prepared by blending SiO2 and SnO2 nanofillers over PEO/PMMA polymeric matrix under ambient temperature. Experimentally, it was confirmed that, with respect to the increase in the concentration of the nanofillers and the applied electrical field frequency, the dielectric permittivity of the films gets decreased to 2.8 from 3.4. They also explored the effect of various NP sizes and dielectric constants on the dielectric permittivity of the film. The results suggested a relatively low nanodielectric loss with a dipolar relaxation at high frequency; however, the radiofrequency electrical conductivity of the HPNCs was increased linearly to 10−4 S/cm from ∼10−8 S/cm, and it was found to have not any connectivity with the concentration of the nanofiller. Hence, the film’s high dielectric and electrical properties made it a material of choice for the development of radiofrequency operative flexible-type electrical and electronic components and devices [38].
Hybrid nanocomposite films were prepared by the dispersion of the 5 wt% of different metal oxides NPs like Al2O3, ZnO, TiO2, or SnO2 over the PVDF/PEO blend matrix and were characterized by various spectral studies, including DSC and RF-IA. The degrees of the crystallinity of PVDF and PEO were measured over HPNC through DSC, which were found to be dependent upon the physical characteristics of metal oxide NPs. With respect to the increase in the radio wave electric field frequency to 109 Hz from 106 Hz, a nonlinear decrease in the dielectric permittivity of the medium was observed. Among the NPs, Al2O3 exhibited a high dielectric permittivity value. The alternating current of the medium displayed linear progress with the frequency variation and was found not to have been influenced by the concentration of the NPs. Hence, the high dielectric constant with permittivity value, high density, lower particle size, and high flexibility made it a promising material for application in different nanodielectric insulators and technologically advanced radio frequency devices [39]. Highly functional core-shell NPs in the polymer (PVDF) were prepared using a cost-effective process and applied as nanodielectrics in different energy storage devices. For this Al NPs were chosen as the core NP, and a thin layer of Al2O3 worked as a capping shell and provided electrical insulation to the prepared polymeric film. It also prevented the agglomeration of Al NPs. The resulting polymeric film exhibited high dielectric permittivity, low dielectric loss, and high energy storage capacity [40].
Nanocomposite films of PVP/PEO/MoO3 were prepared by casting MoO3 nanoplates over PVP/PEO. XRD revealed that the addition of MoO3 NPs upon the PVP/PEO blend increased the amorphous domain of the PNC films. The favorable interaction between PEO and PVP via H-bonding and the miscibility was confirmed by DFT/FTIR study. Also, it indicated a co-coordinative interaction existing between the C–O–C group of PEO/C=O group of PVP with MoO3 NPs. Increasing the amount of MoO3 NPs decreased the optical band gap of the PNC films and enhanced the electrical conductivity, as adding NPs increased the number of charge carriers. They also explored the dielectric dispersion and relaxation process of the PNC films, which were explained mechanistically using a barrier hopping model. The relaxation process followed a non-Debye one [41].
BCZT-P (VDF-HFP)-based polymeric nanocomposites were synthesized using different volume concentrations of BCZT nanofiller and studied their dielectric energy storage efficacy. Sol-gel process was followed for the synthesis of BCZT nanopowder through the citrate precursor process. XRD and TEM analysis analyzed the structural and morphological properties. To get superior ceramic interface coupling, the surface of the nanopowder was functionalized by a different aromatic ligand, naphthyl phosphate (NPh), which was validated through XPS and TGA analysis. The dielectric constant value of ~155 was estimated for surface passivated BCZT NPs through the slurry technique; however, due to higher innate surface conductivity, the dielectric permittivity of pristine BCZT nanopowder could not be measured. The PNC films were dispersed by ceramic fillers via the solution casting method, which was examined by SEM. Nano BCZT/PVDF-HFP films modified by NPh ligands exhibited maximum energy storage capacity at 5% filler concentration than untreated nano BCZT-P (VDF-HFP) and even pure polymer films. 8.5 J cm−3 was found as the maximum energy storage density value at 10% filler concentration for the PNC films of thickness 10 μm [42].
15.3 Conclusion and Future Perspective
In conclusion, this chapter puts the limelight on the significance of polymer nanodielectrics in the development of cost-effective and innovative high-voltage storage capacitors. Fabrication of metal or metal oxide NPs as nanofillers greatly increased the dielectric constant and dielectric permittivity of the polymeric film, which was rather difficult to attain with ceramic material. In some cases, the nanofiller functionalization was done to achieve high dispersion quality and to get better interaction between the polymer and the nanofiller. It was also found to enhance the breakdown strength of polymeric material with low dielectric loss, thus providing stability by increasing the energy density of the film. Though the results are quite satisfactory, the research work still needs more polish. Rather than using the chemical preparation method of PNC films, the scientific community should adopt a greener and atom economical approach to designing and developing PNC films. Also, rather than using the metal or metal oxide NPs to fabricate over PNC films, people should use the one-step functionalization of the polymer nanocomposite by a suitable functionalizing agent to attain flexibility and high dielectric constant value.
References
Dang Z-M, Yuan J-K, Yao S-H, Liao R-J (2013) Flexible nanodielectric materials with high permittivity for power energy storage. Adv Mater 25:6334–6365
Siwal SS, Zhang Q, Devi N, Thakur VK (2020) Carbon-based polymer nanocomposite for high-performance energy storage applications. Polymers 12:505–535
Zhang G, Li Q, Allahyarov E, Li Y, Zhu L (2021) Challenges and opportunities of polymer nanodielectrics for capacitive energy storage. ACS Appl Mater Interfaces 13:37939–37960
Tan DQ (2020) The search for enhanced dielectric strength of polymer-based dielectrics: a focused review on polymer nanocomposites. J Appl Polym Sci 137:49379
Cheng L, Chi X, Yan C, Xie D, Liu X, Wen Y, Liu W, Li S (2018) Polypropylene nanocomposite for power equipment: a review. IET Nanodielectrics 1:92–103
Barber P, Balasubramanian S, Anguchamy Y, Gong S, Wibowo A, Gao H, Ploehn HJ, zur Loye H-C (2009) Polymer composite and nanocomposite dielectric materials for pulse power energy storage. Materials 2:1697–1733
Hu J, Zhang S, Tang B (2021) 2D filler-reinforced polymer nanocomposite dielectrics for high-k dielectric and energy storage applications. Energy Storage Mater 34:260–281
Huang X, Sun B, Zhu Y, Li S, Jiang P (2019) High-k polymer nanocomposites with 1D filler for dielectric and energy storage applications. Prog Mater Sci 100:187–225
Zhang G, Allahyarov E, Zhu L (2018) Polymer nanodielectrics: current accomplishments and future challenges for electric energy storage. In: Nano/micro-structured materials for energy and biomedical applications. Springer, pp 1–48
Wei J, Zhu L (2020) Intrinsic polymer dielectrics for high energy density and low loss electric energy storage. Prog Polym Sci 106:101254
Pirzada BM, Sabir S (2018) Polymer-based nanocomposites for significantly enhanced dielectric properties and energy storage capability. In: Polymer-based nanocomposites for energy and environmental applications. Woodhead Publishing series in composites science and engineering, pp 131–183
Tan DQ (2020) Review of polymer-based nanodielectric exploration and film scale-up for advanced capacitors. Adv Func Mater 30:1808567
Luo H, Zhou X, Ellingfor C, Zhang Y, Chen S, Zhou K, Zhang D, Bowen CR, Wan C (2019) Interface design for high energy density polymer nanocomposites. Chem Soc Rev 48:4424–4465
Pal K, Chakroborty S, Panda P, Nath N, Soren S (2022) Environmental assessment of wastewater management via hybrid nanocomposite matrix implications—an organized review. Environ Sci Pollut Res 29:76626–76643
Panda P, Chakroborty S (2022) Optical sensor technology and its application in detecting environmental effluents: a review. J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2098480
Chakroborty S, Panda P (2022) Nanovaccinology against infectious disease. In: Nanovaccinology as targeted therapeutics. Wiley, pp 95–113
Panda P, Barik A, Unnamatla MVB, Chakroborty S (2021) Synthesis and antimicrobial abilities of metal oxide nanoparticles. In: Bio-manufactured nanomaterials. Springer, Cham, pp 41–58
Soren S, Panda P, Chakroborty S (2023) Nanotechnology in water and wastewater treatment. In: Agricultural and environmental nanotechnology. Springer, 127–143
Chakroborty S, Panda P, Unnamatla MVB, Chandra P, Varela-Guerreroc V (2023) Green nanotechnology research avenue in medicinal biology. In: Green nanoarchitectonics. Jenny Stanford Publishing, pp 167–195
Wu X, Chen X, Zhang QM, Tan DQ (2022) Advanced dielectric polymers for energy storage. Energy Storage Mater 44:29–47
Helal E, Pottier C, David E, Fréchette M, Demarquette NR (2018) Polyethylene/thermoplastic elastomer/zinc oxide nanocomposites for high voltage insulation applications: dielectric, mechanical and rheological behavior. Eur Polymer J 100:258–269
Fakhria P, Mahmood H, Jaleh B, Pegoretti A (2016) Improved electroactive phase content and dielectric properties of flexible PVDF nanocomposite films filled with Au- and Cu-doped graphene oxide hybrid nanofiller. Synth Met 220:653–660
Feng M, Li C, He M, Huang Y, Luo J (2020) Poly(arylene ether nitrile) ternary dielectric composites modulated via polydopamine-assisted BaTiO3 decorating MoS2 sheets. Ceram Int 46:19181–19190
Morsi MA, Asnag GM, Rajeh A, Awwad NS (2021) Nd:YAG nanosecond laser induced growth of Au nanoparticles within CMC/PVA matrix: multifunctional nanocomposites with tunable optical and electrical properties. Compos Commun 24:100662
Choudhary S (2018) Structural, optical, dielectric and electrical properties of (PEO–PVP)–ZnO nanocomposites. J Phys Chem Solids 121:196–209
Menazea AA, Ibrahium HA, Awwad NS, Moustapha ME, Farea MO, Bajaber MA (2022) Facile synthesis and high-performance dielectric properties of polyethylene oxide-chitosan-iron oxide nanocomposite for electrical applications. J Market Res 18:2273–2281
Alfonso MS, Lapeyronie C, Goubet M, Viala B, Tortai J-H (2021) Enhanced dielectric properties of epoxy-based photoresist nanocomposites using carbon-coated nickel nanoparticles for high voltage integrated capacitors. Compos Sci Technol 216:109063
Ul-Haq Y, Murtaza I, Mazhar S, Ullah R, Iqbal M, Zeeshan-ul-Huq, Qarni AA, Amin S (2020) Dielectric, thermal and mechanical properties of hybrid PMMA/RGO/Fe2O3 nanocomposites fabricated by in-situ polymerization. Ceram Int 46:5828–5840
Al-Harbi LM, Alsulami QA, Farea MO, Rajeh A (2023) Tuning optical, dielectric, and electrical properties of polyethylene oxide/carboxymethyl cellulose doped with mixed metal oxide nanoparticles for flexible electronic devices. J Mol Struct 1272:134244
Morsi MA, Abdelrazek EM, Ramadan RM, Elashmawi IS, Rajeh A (2022) Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polym Testing 114:107705
Al-Muntaser AA, Pashameah RA, Sharma K, Alzahrani E, Hameed ST, Mors MA (2022) Boosting of structural, optical, and dielectric properties of PVA/CMC polymer blend using SrTiO3 perovskite nanoparticles for advanced optoelectronic applications. Opt Mater 132:112799
Sengwa RJ, Dhatarwal P (2021) Polymer nanocomposites comprising PMMA matrix and ZnO, SnO2, and TiO2 nanofillers: a comparative study of structural, optical, and dielectric properties for multifunctional technological applications. Opt Mater 113:110837
Dhatarwal P, Sengwa RJ (2020) Tunable β-phase crystals, degree of crystallinity, and dielectric properties of three-phase PVDF/PEO/SiO2 hybrid polymer nanocomposites. Mater Res Bull 129:110901
Chen C, Xie Y, Liu J, Li J, Wei X, Zhang Z (2020) Enhanced energy storage capability of P(VDF-HFP) nanodielectrics by HfO2 passivation layer: preparation, performance and simulation. Compos Sci Technol 188:107968
Morsi MA, Rajeh A, Al-Muntaser AA (2019) Reinforcement of the optical, thermal and electrical properties of PEO based on MWCNTs/Au hybrid fillers: nanodielectric materials for organoelectronic devices. Compos B 173:106957
Sengwa RJ, Dhatarwal P (2022) Toward multifunctionality of PEO/PMMA/MMT hybrid polymer nanocomposites: promising morphological, nanostructural, thermal, broadband dielectric, and optical properties. J Phys Chem Solids 166:110708
Sengwa RJ, Dhatarwal P, Choudhary S (2020) A comparative study of different metal oxide nanoparticles dispersed PVDF/PEO blend matrix-based advanced multifunctional nanodielectrics for flexible electronic devices. Mater Today Commun 25:101380
Dhatarwal P, Sengwa RJ, Choudhary S (2022) Broadband radio frequency dielectric permittivity and electrical conductivity of dispersed tin oxide and silica nanoparticles in poly(ethylene oxide)/poly(methyl methacrylate) blend matrix-based nanocomposites for nanodielectric applications. J Macromol Sci 61:111–120
Sengwa RJ, Dhatarwal P (2022) Crystalline phases thermal behaviour and radio frequencies dielectric properties of PVDF/PEO/metal oxides hybrid polymer nanocomposite films. J Polym Res 5
Badi N, Mekala R, Herdandez FR (2013) Synthesis of Al–Al2O3/PVDF core shell nanodielectrics for energy storage applications. In: Technical proceedings of the 2013 clean technology conference and trade show. TechConnect Briefs, pp 330–333
Al-Muntaser AA, AlSaidi RAM, Sharma K, Alamri HR, Makhlouf MM (2022) Structural, optical, electrical, and DFT studies on polyvinyl pyrrolidone/polyethylene oxide polymer blend filled with MoO3 nanoplates for flexible energy-storage devices. Int J Energy Res 46:13832–13843
Sadhu SPP, Siddabattuni S, Muthukumar SV, Varma KBR (2018) Enhanced dielectric properties and energy storage density of surface engineered BCZT/PVDF-HFP nanodielectrics. J Mater Sci: Mater Electron 29:6174–6182
Acknowledgements
PP is thankful to the Department of Basic Sciences, RIE, Bhubaneswar, Odisha, India. SC is grateful to the School of Sciences, IES University, Bhopal, Madhya Pradesh, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Panda, P., Chakroborty, S., Modi, A., Moharana, S. (2024). Metal Oxide Nanofiller-Introduced Polymer-Based Nanocomposite Dielectrics for Advanced Energy Storage Devices. In: Moharana, S., Gregory, D.H., Mahaling, R.N. (eds) Emerging Nanodielectric Materials for Energy Storage. Nanostructure Science and Technology. Springer, Cham. https://doi.org/10.1007/978-3-031-40938-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-40938-7_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40937-0
Online ISBN: 978-3-031-40938-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)