Abstract
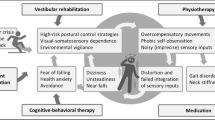
The term persistent postural-perceptual dizziness (PPPD) was introduced into the International Classification of Vestibular Disorders in 2017, but conditions reminiscent of it were described in medical literature dating back to the 1870s. After it was defined, clinical epidemiologic studies found it to be one of the most common diagnoses in neurotologic practice, accounting for 20% of all patients referred for specialty consultations for dizziness. PPPD manifests with chronic symptoms of dizziness, unsteadiness, or nonspinning vertigo lasting at least 3 months. These core symptoms are exacerbated by upright posture, active or passive motion of the self, and exposure to environments with complex or moving visual stimuli. PPPD may be precipitated by acute or episodic, peripheral or central neuro-otologic disorders, other medical conditions, or acute psychological distress that disrupts spatial orientation or balance functioning. PPPD is diagnosed by identifying its key symptoms and exacerbating factors in patients’ histories. Physical examinations and diagnostic testing may yield evidence about precipitating or coexisting conditions. Mechanistic research has uncovered pathophysiologic processes that alter control of posture and gait, disrupt spatial orientation, and change the activity and connectivity of brain networks that support these functions in patients with PPPD. Treatment studies dating back to predecessors of PPPD, but confirmed in studies of PPPD itself, showed substantial clinical benefits from individualized vestibular rehabilitation, serotonin reuptake inhibitors, and skill-building psychotherapies, alone or in combination. Thus, PPPD is a common, readily recognizable, and treatable vestibular disorder that involves alterations in the functioning of brain networks that control locomotion and spatial orientation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic dizziness
- Functional dizziness
- Postural control
- Psychotherapy
- Serotonin reuptake inhibitors
- Vestibular rehabilitation
- Visual dependence
Introduction
Persistent postural-perceptual dizziness (PPPD) was introduced into the medical nomenclature in 2017 when its diagnostic criteria were enumerated by an expert panel chartered by the Bárány Society for the International Classification of Vestibular Disorders (ICVD) [1]. A narrative definition was included in the 11th edition of the International Classification of Diseases (ICD-11), which was finalized on January 1, 2022, by the World Health Organization [2]. The core symptoms of PPPD are swaying or rocking (nonspinning) vertigo, unsteadiness when standing or sitting upright, and nonvertiginous dizziness that patients often describe as heavy-, light-, foggy-, or swimmy-headed sensations (Table 12.1, criterion A). Some patients also describe subtle illusions that fixed objects in the environment are not quite still. These symptoms are present most hours of the day and most days of the week. Patients may experience breaks in symptoms for a few hours to a few days, but otherwise, symptoms are quite persistent and may debilitate affected individuals for years. Various activities and environmental exposures exacerbate symptoms of PPPD, including upright posture, active and passive movement, and exposure to environments with complex or moving visual stimuli (Table 12.1, criterion B). A factor analysis of common activities associated with these provocations found that a factor encompassing visual stimuli was more sensitive and specific for PPPD than factors related to upright posture/walking and other movements [3]. This was consistent with unpublished data presented to members of the Bárány Society during the development of the definition of PPPD (for an abstract, please see [4]. PPPD may be precipitated by peripheral or central vestibular disorders, other medical conditions, and periods of psychological distress (Table 12.1, criterion C). Four studies examined the relative prevalence of these triggering events. Two were cross-sectional investigations of patients diagnosed with chronic subjective dizziness (CSD), a closely related predecessor of PPPD [5, 6]. A third was a retrospective study of patients meeting the criteria for PPPD abstracted from a large research database at a tertiary center [7]. The fourth was a retrospective review of clinical experience with the disorder over a 5-year period [8]. Disorders causing structural deficits of the peripheral or central vestibular system (e.g., benign paroxysmal positional vertigo, unilateral peripheral vestibulopathies, stroke) trigger PPPD in 21–25% of patients. Other central nervous system precipitants included vestibular migraine (11–25% of patients), mild traumatic brain injury (3–15%), and dysautonomias (1–7%). Medical conditions, such as paroxysmal cardiac dysrhythmias and metabolic illnesses, precipitated 3–11% of cases. Active psychiatric disorders, particularly panic and generalized anxiety disorders, were identified in 20–25% of patients in three of the studies [5,6,7]. The fourth found a notably higher rate of 42% [8]. Although the precipitating conditions identified in these studies appear to be quite disparate, they share an ability to trigger vertigo, unsteadiness, or dizziness or disrupt normal balance function, which engenders subsequent shifts in control of locomotion and spatial orientation that maintain PPPD (see section “Pathophysiologic Mechanisms for details). To merit a diagnosis of PPPD, patients must experience distressing or impairing levels of symptoms that warrant clinical attention (Table 12.1, criterion D). The final diagnostic criterion (Table 12.1, criterion E) is sometimes interpreted to mean that PPPD is a diagnosis of exclusion, which is not correct. The actual purpose of this criterion is to ensure that clinicians and investigators carefully consider patients’ entire clinical presentations when making final determinations about the presence or absence of PPPD, either alone or in conjunction with other illnesses [1, 9, 10].
The term PPPD is relatively new, but German physicians and others in Europe and the USA engaged in lively debates in the late 1800s about the causes of spatial disorientation, altered locomotion, and anxiety reported by patients exposed to the chaotic marketplaces of nineteenth-century village squares (see Ref. [11] for review). Their descriptions of patients’ symptoms, otologic precipitants, and associated psychological factors were remarkably prescient, given the data that are continuing to emerge about the interplay of these variables in patients with PPPD. One hundred years after those initial observations, the clinical conditions of phobic postural vertigo (PPV) [12], space motion discomfort [13], visual vertigo (VV) [14], and CSD [15] informed the details of the diagnostic criteria of PPPD [1]. After its definition was published in 2017, PPPD superseded CSD, a term that is no longer used. Some clinicians and researchers retained PPV for a clinical condition equivalent to PPPD plus additional phobic features [1, 9]. Space motion discomfort and VV, which were renamed visually induced dizziness in the ICVD [16], remained complex symptoms, not independent diagnoses. They form part of criterion B of PPPD (Table 12.1) but also occur during attacks of vestibular migraine and as sequelae of acute peripheral or central vestibular lesions apart from PPPD [13, 14].
Epidemiology of PPPD
Since the publication of the definition of PPPD, two large clinical epidemiologic studies and one smaller investigation conducted in university neurology clinics in China (N = 9200) [17], Korea (N = 21,267) [18], and Croatia (N = 147) [19] reported a remarkably consistent point prevalence of PPPD of about 20% (range 19.0–21.8%) among patients referred for evaluation of vestibular symptoms. A similar investigation in a general internal medicine clinic in Japan (N = 229) found a 14.4% point prevalence of PPPD among patients with dizziness [20]. In a study from a multidisciplinary clinic in Canada designed specifically for patients with chronic dizziness (N = 292), PPPD was the sole diagnosis of 9.2% of referred individuals and co-existed with other illnesses in an additional 44.2% of patients [21], meaning that PPPD was a factor in the morbidity of about one-half of all patients presenting to that specialized dizziness center. It is likely that many patients with PPPD have previously received no definitive diagnosis. For example, a cross-sectional investigation from a tertiary neurotology clinic in the USA conducted just prior to the publication of the definition of PPPD reported no identifiable diagnoses in 25% of patients [22]. The experience of the author’s clinical team was similar, with a 25% gap in undiagnosed patients prior to 2010 [5]. After incorporating processes to identify PPPD and vestibular migraine [23] into the diagnostic workflow, however, the number of patients whose diagnosis could not be determined with reasonable certainty declined to <2%. This illustrates the positive effect that the introduction of PPPD into the diagnostic nomenclature has had on the fields of neuro-otology and vestibular medicine, offering hope for many patients with chronic dizziness who previously received no specific diagnosis or recommendations for effective treatment.
In the aforementioned clinical epidemiologic studies of PPPD [17,18,19], the average ages of patients were in the mid-50s (range 53–59 years), and two-thirds of affected individuals were women. These findings were consistent with previous reports on PPV [24] and CSD [6]. PPPD also affects children and adolescents. Clinical epidemiologic data from a tertiary pediatric balance clinic in the USA (N = 1021) showed that 7.3% of patients seeking consultation were diagnosed with PPPD [25]. They had an average age of 14.6 years (range 8–22 years) and a female predominance (83%), which was higher than among adults.
There have not yet been any prospective studies that investigated the incidence of PPPD following potentially triggering events, but 10 prospective and retrospective investigations totaling 552 patients predating 2017 reported rates of chronic PPPD-like dizziness ranging from 7.5% to 53% (weighted mean of 26.3%) following acute vestibular syndromes, mostly vestibular neuritis, and benign paroxysmal positional vertigo [26]. A more recent study suggested that the incidence of PPPD could be at the lower end of that range among patients who received adequate diagnostic assessments within several weeks of the onset of vestibular symptoms. In a retrospective review of the medical records of 155 patients who presented to a tertiary neurotology center in Japan within 90 days of new-onset vestibular symptoms between January 2019 and December 2020, [27] found that only 8 (5.2%) patients developed PPPD over 18 months of follow-up, 7 of whom met all criteria for the diagnosis at the time of their initial evaluation except the required 3-month duration of illness. On initial presentation, another 70 (45.2%) patients had elevated scores on the Niigata PPPD Questionnaire, a 12-item self-report that measures sensitivity to activities and exposures within the domains of criterion B of PPPD [3], but they did not develop PPPD during the follow-up period. Thus, symptoms of space motion discomfort and visually induced dizziness were common within the first 3 months after the onset of acute vestibular symptoms, with a prevalence of about 50%, but only patients who reported substantive early sensitivities across all three domains of criterion B of PPPD, namely upright posture/walking, self-motion, and exposure to complex or moving visual stimuli, were at high risk of developing the disorder. If verified in future investigations, these results offer promise for early identification of patients at risk for PPPD, who could then be triaged for potentially preventative interventions. That would have substantial public health benefits given the unfavorable descriptions of long-term outcomes previously reported for patients with PPV or CSD, many of whom experienced waxing and waning vestibular and balance symptoms lasting for years, complicated by rates of comorbid anxiety or depressive disorders approaching 75% [28, 29].
Making the Diagnosis of PPPD
The key to diagnosing PPPD is identifying its primary symptoms and exacerbating factors in patients’ clinical histories, along with evidence of likely precipitating illnesses (i.e., Table 12.1, criteria A–C). Additional data gathered from physical examinations, laboratory testing, or neuroimaging may be useful for identifying precipitants (Table 12.1, criterion C) and determining if PPPD is the best diagnosis for patients’ symptoms, either alone or co-existing with other conditions (Table 12.1, criterion E). Patients who report only nonspecific dizziness or do not otherwise fulfill all criteria in Table 12.1 should not receive a diagnosis of PPPD. In situations of diagnostic uncertainty, a period of prospective monitoring of patients’ symptoms may be necessary to reach final diagnostic impressions [10].
The initial clinical course of PPPD may follow three trajectories (Table 12.1, criterion C). When it develops after an acute vestibular syndrome such as a unilateral peripheral vestibulopathy (e.g., vestibular neuritis), the symptoms of PPPD typically begin as the acute illness resolves, often without an asymptomatic interval, as found by [30]. When the precipitant is an episodic vestibular syndrome such as benign paroxysmal positional vertigo, vestibular migraine, or panic disorder, symptoms of PPPD may begin in full after an initial attack or occur intermittently at first and then consolidate with longer and more severe symptoms occurring as attacks of the precipitating illness continue. Subsequent attacks would then be superimposed on the chronic symptoms of PPPD. The most difficult clinical scenario (also the least common) is when the precipitant is a chronic illness that begins insidiously, such as a degenerative neurologic or otologic disorder, a gradually worsening generalized anxiety disorder, or the slowly increasing symptoms of postural orthostatic tachycardia syndrome. In such cases, hypersensitivity to visual stimuli may be the best indicator of the onset of coexisting PPPD given the fact that visually induced dizziness is less likely to be caused by chronic vestibular or other precipitating conditions than exacerbations of symptoms with head motion or upright posture, especially when patients are stationary [1, 9, 10].
There are no diagnostic tests for PPPD, but scores on two self-reported questionnaires may help identify patients with the disorder. In a retrospective study of patients examined within 3 months of new-onset vestibular symptoms, a score of 27 or higher on the Niigata PPPD Questionnaire [3] was highly sensitive (sensitivity = 0.88) but not very specific (specificity = 0.52) for identifying those at risk of developing PPPD [30]. In a retrospective study of patients with chronic vestibular symptoms, a score greater than 60 on the Dizziness Handicap Inventory [31] was highly specific (specificity = 0.88) for the presence of functional or psychiatric vestibular disorders, particularly PPPD, whereas a score of 30 or less was highly specific (specificity = 0.98) for their absence [32]. Hopefully, future validation studies will refine the utility of these questionnaires as diagnostic aids for PPPD and as measures for tracking treatment outcomes.
Differential Diagnosis of PPPD
The differential diagnosis of PPPD is described in detail in the defining document of the disorder [6], which is available online, free of charge, with all other publications of the ICVD (https://www.iospress.com/jvr-icvd). The most important concept in working through the differential diagnosis of chronic vestibular symptoms is to reach a final diagnostic impression that properly accounts for patients’ active symptoms, whether that is a single diagnosis such as PPPD, another chronic disorder, or a combination of illnesses [9, 10]. Pitfalls include attributing current symptoms to past illnesses (e.g., a previous acute vestibular syndrome that has resolved), focusing on episodic conditions to explain chronic symptoms (e.g., diagnosing Meniere’s disease alone in patients reporting persistent visual motion hypersensitivity), and failing to recognize commonly coexisting problems (e.g., PPPD and vestibular migraine). As outlined in Table 12.2, when PPPD is triggered by an acute vestibular syndrome, the diagnostic challenge is to determine if the acute syndrome has resolved completely or if the patient has compensated fully for any residual peripheral or central vestibular deficits. When PPPD is triggered by episodic vestibular syndrome, the same concern about residual structural deficits applies (e.g., stepwise loss of peripheral function from Meniere’s disease leading to a chronically uncompensated peripheral vestibulopathy). In addition, clinicians must recognize that a clinical course of episodic flares of vestibular symptoms superimposed on a background of persistent dizziness and motion sensitivity suggests the presence of an ongoing episodic vestibular syndrome coexisting with PPPD. In patients with other chronic vestibular syndromes, the diagnostic challenge is to determine if PPPD has developed as a comorbid condition. The presence of visually induced dizziness, especially when patients are stationary, is the most important clinical clue because other causes of chronic vestibular and balance symptoms do not produce hypersensitivity to complex or moving visual stimuli when patients are still. Finally, PPPD does not cause major alterations of gait, such as near falls or falls, so the presence of these symptoms and signs warrants assessment for structural, metabolic, and functional gait disorders and causes of syncope.
Pathophysiological Mechanisms of PPPD
Initial hypotheses about the putative pathophysiologic mechanisms of PPPD were derived from studies of its four predecessors and older investigations of patients with chronic dizziness following acute vestibular syndromes. Since its definition was published in 2017, however, data have emerged about PPPD, itself, from investigations of its clinical phenomenology, associated physiological and psychological variables, and alterations in brain functioning on neuroimaging [5, 9, 10, 33]. This evidence is converging around five processes: (1) possible predisposition by an anxiety diathesis, (2) promotion by acute psychological responses consisting of increased body vigilance and worrisome perceptions about the chronicity of vestibular symptoms and perpetuation by the parallel processes of (3) stiffened postural control and (4) visual dependence that fail to reset after precipitating events resolve or remit and appear to degrade spatial cognition, all of which are associated with (5) altered activity and connectivity of key networks in the brain involving the vestibular cortex, visual cortex, and hippocampus.
-
1.
Anxiety diathesis (trait and state variables). In two cross-sectional studies, patients with CSD had significantly higher levels of neurotic personality traits, measured by the NEO-PI standardized personality inventory, than comparison groups of patients with other vestibular disorders [34, 35] and healthy controls [34]. A cross-sectional investigation of patients with PPPD also found increased neuroticism [36]. Obsessive-compulsive personality traits were part of the defining characteristics of PPV [12], and an older study of patients with chronic PPPD-like dizziness described contributions from dependent personality traits [37]. In contrast to the apparent vulnerability to chronic dizziness associated with these anxiety-related personality traits, a prospective study found that patients with higher resilience and sense of coherence, which are personality traits linked to lower anxiety-related distress, were less likely to develop chronic dizziness as a sequela of acute or episodic vestibular disorders [38]. Thus, studies conducted in four countries on three continents over the past 30 years seem to have identified anxiety-related personality traits as a universal predisposing factor for developing PPPD-like chronic dizziness. However, unpublished data from the largest sample of patients with PPPD yet studied was presented at the 2022 Bárány Society meeting and did not show elevated levels of neuroticism or abnormal scores of any other personality traits on the NEO-PI compared to population norms among patients with PPPD (Korean Balance Society Multicenter Working Group 2020. The characteristics of persistent postural perceptual dizziness in Korea. XXXI Bárány Society Congress, Madrid, Spain, May 2022, poster FP1225).
Published data on the relationship between state anxiety and chronic dizziness is also mixed, with some studies finding an increased risk for chronic dizziness after acute vestibular syndromes in patients with pre-existing personal or family histories of anxiety disorders [39,40,41], but others not [37, 42]. One observational study suggested that preexisting anxiety disorders increase the risk of heightened anxiety over the long term in patients who develop chronic dizziness following acute vestibular illnesses, thereby worsening overall morbidity but not predisposing to the development of chronic dizziness itself [41].
-
2.
Acute body vigilance and negative perceptions of illness. Vertigo is highly anxiety-provoking, particularly for patients who experience it for the first time [43]. Prospective studies conducted prior to 2017 found that high anxiety occurring during and immediately after acute vestibular syndromes (e.g., vestibular neuritis or benign paroxysmal positional vertigo) was a stronger predictor of developing chronic PPPD-like dizziness than the severity of labyrinthine deficits [40, 44]. A series of studies showed that the forms of acute anxiety most strongly associated with persistent dizziness [40, 42, 44] and PPPD [45] were heightened body vigilance (i.e., conscious attention to the somatic sensations of dizziness and unsteadiness) and negative illness perceptions (e.g., worrisome thoughts about causes, consequences, and controllability of vestibular and balance symptoms); the latter was also associated with the severity of dizziness-related handicap in patients with vestibular symptoms [46].
-
3.
Altered control of stance and gait. A fairly consistent picture of changes in control of locomotion has emerged from studies of patients with PPV, CSD, and PPPD. Patients with PPV [47] and CSD [48] adopted a stiffer stance by co-contracting lower leg muscles, mediated by a lower threshold than normal individuals for engaging closed-loop feedback for postural control [49]. Normally, a quiet stance is maintained through an open-loop process driven primarily by vestibular inputs and spinal reflexes. Closed-loop control that fully engages visual and proprioceptive processes is typically utilized in more demanding circumstances [49]. Thus, patients exerted more control effort and consciously paid more attention to their balance than was necessary to maintain a routine stance. Under modestly demanding conditions (standing on foam), the sway patterns of patients with PPV approximated those of normal individuals as both groups engaged closed-loop feedback and attentional processes needed for the more challenging task [50, 51]. Stiffening of lower body control may come at the cost of reduced overall postural stability with increased upper body sway, especially in demanding situations. This was demonstrated in a portion of patients with CSD during static posturography [48] and in nearly all patients with PPPD on conditions 5 and 6 of the Sensory Organization Test [52]. Changes in gait also were measured in patients with PPV, who manifested slower mean gait speed, shorter mean stride length, a widened base of support, and a fractional increase in the duration of two-footed support during walking compared to normal individuals [53].
-
4.
Visual dependence. Spatial orientation is derived from the multisensory integration of vestibular, visual, and somatosensory information. Visual dependence refers to the tendency to rely most strongly on visual inputs, even if they are not the most accurate information available. Visual dependence is often measured by asking individuals to align a rotating bar or line on a screen with their perceptions of the earth’s vertical (i.e., the subjective visual vertical). Tests that confound this task with tilted or rotating background scenes, such as the Rod and Frame or Rod and Disk Tests, are commonly used in vestibular research. Given that visually induced dizziness may be the most sensitive and specific element of criterion B for PPPD [3], it is not surprising that studies have linked visual dependence to chronic dizziness and PPPD. In a prospective study, a combination of high body vigilance and visual dependence measured by the Rod and Disk Test within 48 h of the onset of acute vestibular neuritis predicted persistent PPPD-like dizziness rather than asymptomatic recovery at 6-month follow-up [42]. A cross-sectional study of patients with PPPD found that they performed poorer than healthy controls on another measure of visual dependence, the functional head impulse test, in which participants must identify the direction of optotypes centered in a matrix of rotating dots while making head impulses [54].
-
5.
Changes in brain activity and connectivity. A recent review by Indovina and colleagues [33] summarized the results of neuroimaging studies conducted worldwide on patients with PPPD, CSD, PPV, and VV. Six of these investigations used task-related functional magnetic resonance imaging (fMRI) to compare patients to healthy controls. One study used sound-evoked vestibular stimulation in patients with CSD [55]. Four visual motion stimuli were used, two in patients with PPPD [56, 57] and one each in patients with PPV [27, 58]. The latter also used caloric irrigation [58]. Four fMRI investigations used resting state protocols to compare patients with VV [59] or PPPD [60,61,62] to healthy controls. One single-photon emission computerized tomography study compared patients with PPPD to healthy controls [63]. Three studies investigated brain structure in patients versus healthy controls; one in patients with PPPD [64] using surface-based morphometry and one each in patients with PPV [27] and PPPD [65] using voxel-based morphometry. When pooled together despite the different patient populations and neuroimaging methods [33], the results of these studies indicated that patients with PPPD have decreased local activity and functional connectivity in multimodal vestibular cortical areas (e.g., right posterior insula, parietal operculum, and surrounding regions), which is potentially related structurally to reduced cortical folding and gray-matter volumes in those areas. In contrast, connectivity between the prefrontal cortex, which regulates attentional and emotional responses to external stimuli, and primary visual and motor regions appears to be increased in PPPD, possibly modulated by state anxiety and neuroticism. These imaging findings complement the physiological and psychological mechanisms listed above.
In a task-related fMRI study measuring brain responses to a standardized picture set designed to evoke positive versus neutral versus negative emotions, women with PPPD activated brain regions associated with visuospatial processing (parahippocampal gyrus, intraparietal sulcus) in response to negatively valenced stimuli compared to women who had recovered from acute vestibular syndromes who activated anxiety-related areas (amygdala, orbitofrontal cortex) as do normal individuals [66]. This suggests that patients with PPPD were more attuned to the spatial elements of the visual stimuli than their emotional content.
Additional Alterations in Functioning
Two studies suggested that patients with PPPD may have compromised spatial navigation abilities and reduced mental sharpness, which are common complaints reported by individuals following the onset of the disorder. In one investigation, patients with PPPD performed worse on the virtual Morris Water Maze Test, a test of spatial navigation and memory, than patients with either unilateral vestibular deficits or healthy controls [67]. In another study, patients with PPPD scored worse than patients with vestibular migraine, Menière’s disease, and benign paroxysmal positional vertigo on the Cognitive Failure Questionnaire, a self-report instrument that measures momentary cognitive slips, absent-mindedness, and inattentiveness. Their scores also were lower than population norms [68]. One possible explanation for these findings is that the unnecessary attention that patients with PPPD pay to sensations of dizziness and conscious control of posture diverts cognitive resources from the broader tasks of spatial orientation and other daily activities.
Treatment
There have been no large-scale, randomized, controlled trials of any treatment for PPPD. Case series, open-label studies, and small controlled trials of treatments for the predecessors of PPPD, including PPV, CSD, and VV, found support for three treatments: (1) vestibular habituation, (2) serotonin reuptake inhibitors, and (3) cognitive behavioral therapy, either alone or in combination (see Ref. [9, 10] and Staab 2000 for reviews). After the diagnostic criteria for PPPD were formulated, several studies tested the efficacy of these treatments in patients specifically diagnosed with PPPD.
-
1.
Vestibular habituation. Vestibular rehabilitation was developed in the 1990s to treat patients with chronic dizziness [69]. The descriptions of patients included in those early studies suggest that many would have met the criteria for PPPD. In a retrospective review and telephone follow-up of 26 patients diagnosed with PPPD using a draft version of the ICVD criteria, Thompson et al. [70] found that nearly all patients valued education about the condition provided by experienced physical therapists, and 14 (56%) reported clinically significant benefits from individualized, self-paced, home-based vestibular exercise programs. Improvements in tolerance for self-motion were greater than tolerance for complex or moving visual stimuli. In a prospective study of 60 patients diagnosed with PPPD per final ICVD criteria, Nada et al. [71] reported a mean reduction of Dizziness Handicap Inventory (DHI) scores from 55 (high moderate) to 36 (low moderate); 27 (45%) patients achieved scores less than 30 (mild) after 6 weeks of therapist-directed vestibular rehabilitation.
-
2.
Medication. In seven studies of patients with chronic dizziness conducted before 2017, investigators reported significant improvements in PPPD-like symptoms using selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) ([9, 10]). All six commercially available SSRIs (fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, and escitalopram), and two of the five available SNRIs (venlafaxine and milnacipran) were included in at least one of those studies. These medications had roughly equal benefits and tolerability, with about 65% of all patients and 85% of patients who completed at least 8 weeks of treatment demonstrating clinically meaningful reductions in symptoms, for example, 50% decreases in DHI scores from moderate (31–59) to mild (0–30) ranges. Two studies reported the results of SSRI treatment in patients diagnosed specifically with PPPD. In a retrospective review of the outcomes of 197 patients with PPPD treated over a 3-year period, [72] reported results that mirrored the older studies, with 65% of all patients being much improved or very much improved after treatment with SSRIs (mostly escitalopram) with or without adjunctive low-dose benzodiazepines (mostly clonazepam). In an 8-week prospective study, Yu et al. [73] reported a reduction in mean DHI scores from 54 (moderate) to 26 (mild) in 45 patients with PPPD treated with sertraline alone and a decrease from 54 (moderate) to 15 (mild) in 46 patients treated with sertraline plus cognitive behavior therapy, with the latter group needing lower doses of medication. Table 12.3 lists the dosing strategies for two SSRIs (sertraline and escitalopram) and one SNRI (venlafaxine) that appeared most often in published studies and are used most frequently by the author’s clinical team.
-
3.
Psychotherapy. Early studies of cognitive behavioral therapy produced nonsustained benefits for patients with PPV, but later studies were much more promising for patients with CSD (see Ref. [10] for review). In addition to the aforementioned study that showed significant benefits from adding cognitive behavioral therapy to sertraline for patients with PPPD [73], Waterston and colleagues [8] reported results from a retrospective review of patients with PPPD that they managed in their practices over a 5-year period. They achieved a mean reduction in DHI scores from 50 (moderate) to 24 (mild) in 150 patients who completed a full course of cognitive behavioral therapy. In a prospective study of 27 patients with PPPD, Kumabara et al. [74] showed that a 6-week treatment program combining acceptance and commitment therapy (a spinoff of cognitive behavioral therapy) with vestibular rehabilitation had a large effect on mean Dizziness Handicap Scores, achieving a reduction from 49 (moderate) before treatment to 26 (mild) at 6-month follow-up.
Taken together, these investigations demonstrated that the three treatments developed for predecessors of PPPD, namely physical therapy with vestibular habituation, pharmacotherapy with SSRIs and SNRIs, and psychotherapy with cognitive behavioral techniques, also benefited patients with PPPD. All treatments reduced dizziness-related handicaps from moderate levels (i.e., interfering with daily activities) to mild levels (i.e., nagging but not impairing) for most patients. They also appeared to have synergistic effects when combined. Indeed, Axer et al. [75] reported that individualized combinations of these three modalities introduced during a week-long intensive outpatient program produced sustained benefits for 305 patients with PPPD at 6-month follow-up.
Conclusion
Formal diagnostic criteria for PPPD were published in 2017 and are now included in both the International Classification of Vestibular Disorders and the International Classification of Diseases, 11th edition. The diagnosis of PPPD may be new, but descriptions of patients struggling with similar syndromes date back at least 150 years. The prevalence of PPPD in the general population is not yet known, but clinical epidemiologic studies in university- and hospital-based neurology centers found that 20% of patients referred for dizziness had PPPD, making it the most common cause of chronic dizziness in tertiary care and one of the top three diagnoses identified in adults seeking specialty consultation for vestibular symptoms, along with benign paroxysmal positional vertigo and vestibular migraine. A bespoke measure of PPPD symptoms was created (the Niigata PPPD questionnaire), with early results bringing hope that it may be a helpful tool for diagnosing PPPD and tracking the results of treatment. Data emerging from an expanding array of physiological, psychological, and neuroimaging investigations suggest that alterations in the functioning of locomotor control systems (i.e., stiffened stance and gait) and multimodality spatial orientation systems (i.e., visual dependence) plus reduced activity and connectivity in brain networks that support these processes form the pathophysiological mechanisms underlying PPPD. The efficacy of treatment strategies first developed for the precursors of PPPD is now supported by the first few retrospective and prospective studies of patients with PPPD itself. Though still lacking data from fully powered, randomized controlled trials, current evidence supports the use of individualized interventions with vestibular rehabilitation, SSRIs or SNRIs, and cognitive behavioral therapy, alone or in combination, to reduce the morbidity of this highly prevalent disorder.
References
Staab JP, Eckhardt-Henn A, Horii A, Jacob R, Strupp M, Brandt T, Bronstein A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Barany Society. J Vestib Res. 2017;27(4):191–208.
World Health Organization. ICD-11 for mortality and morbidity statistics (Version 02/2022). AB32.0 Persistent Postural-Perceptual Dizziness. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/2005792829. Accessed 20 Aug 2022.
Yagi C, Morita Y, Kitazawa M, Nonomura Y, Yamagishi T, Ohshima S, Izumi S, Takahashi K, Horii A. A validated questionnaire to assess the severity of persistent postural-perceptual dizziness (PPPD): the Niigata PPPD Questionnaire (NPQ). Otol Neurotol. 2019;40:e747–52.
Staab J, Eggers S, Neff B, Shepard N, Goulson A, Carlson M. Validation of a clinical syndrome of persistent dizziness and unsteadiness. J Vestib Res. 2010;20(3-4):172.
Staab JP. Behavioural neuro-otology. In: Bronstein AM, editor. Oxford textbook of vertigo and imbalance. 2nd ed. Oxford, UK: Oxford University Press; 2013. p. 333–46.
Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170–6.
Habs M, Strobl R, Grill E, Dieterich M, Becker-Bense S. Primary or secondary chronic functional dizziness: does it make a difference? A DizzyReg study in 356 patients. J Neurol. 2020;267:212–22.
Waterston J, Chen L, Mahony K, Gencarelli J, Stuart G. Persistent postural-perceptual dizziness: precipitating conditions, co-morbidities and treatment with cognitive behavioral therapy. Front Neurol. 2011;12:795516.
Dieterich M, Staab JP. Functional dizziness: from phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness. Curr Opin Neurol. 2017;30(1):107–13.
Staab JP. Persistent postural-perceptual dizziness. Semin Neurol. 2020;40(1):130–7.
Balaban CD, Jacob RG. Background and history of the interface between anxiety and vertigo. J Anxiety Disord. 2001;15:27–51.
Brandt T, Dieterich M. Phobischer Attacken Schwankschwindel, ein neues Syndrom? Munch Med Wschr. 1986;28:247–50.
Jacob RG, Lilienfeld SO, Furman JMR, Durrant JD, Turner SM. Panic disorder with vestibular dysfunction: further clinical observation and description of space and motion phobic stimuli. J Anxiety Disord. 1989;3:117–30.
Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psychiatry. 1995;59:472–6.
Staab JP, Ruckenstein MJ, Amsterdam JD. A prospective trial of sertraline for chronic subjective dizziness. Laryngoscope. 2004;114(9):1637–41.
Bisdorff A, von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1–13.
Xue H, Chong Y, Jiang ZD, Liu ZL, Ding L, Yang SL, Wang L, Xiang WP. Etiological analysis on patients with vertigo or dizziness. Zhonghua Yi Xue Za Zhi. 2018;98(16):1227–30.
Kim HJ, Lee JO, Choi JY, Kim J. Etiologic distribution of dizziness and vertigo in a referral-based dizziness clinic in South Korea. J Neurol. 2020;267:2252–9.
Adamec I, Meaški SJ, Skorić MK, Jažić K, Crnošija L, Milivojević I, Habek M. Persistent postural-perceptual dizziness: clinical and neurophysiological study. J Clin Neurosci. 2020;72:26–30.
Ishizuka K, Shikino K, Yamauchi Y, Yanagita Y, Yokokawa D, Ikegami A, Tsukamoto T, Noda K, Uehara T, Ikusaka M. The clinical key features of persistent postural perceptual dizziness in the general medicine outpatient setting: a case series study of 33 patients. Intern Med. 2020;59(22):2857–62.
Staibano P, Lelli D, Tse D. A retrospective analysis of two tertiary care dizziness clinics: a multidisciplinary chronic dizziness clinic and an acute dizziness clinic. J Otolaryngol Head Neck Surg. 2019;48:11.
Muelleman T, Shew M, Subbarayan R, Shum A, Sykes K, Staecker H, Lin J. Epidemiology of dizzy patient population in a neurotology clinic and predictors of peripheral etiology. Otol Neurotol. 2017;38(6):870–5.
Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, Bisdorff A, Versino M, Evers S, Newman-Toker D. Vestibular migraine: diagnostic criteria. Consensus document of the Bárány Society and the International Headache Society. J Vestib Res. 2012;22(4):167–72.
Brandt T. Phobic postural vertigo. Neurology. 1996;46:1515–9.
Wang A, Fleischman KM, Kawai K, Corcoran M, Brodsky JR. Persistent postural-perceptual dizziness in children and adolescents. Otol Neurotol. 2021;42(8):e1093–100.
Trinidade A, Cabreira V, Goebel JA, Staab JP, Kaski D, Stone J. Predictors of persistent postural-perceptual dizziness (PPPD) precipitated by peripheral vestibular disorders: a systematic review. J Neurol Neurosurg Psychiatry. 2023; https://doi.org/10.1136/jnnp-2022-330196.
Popp P, Zu Eulenburg P, Stephan T, Bögle R, Habs M, Henningsen P, Feuerecker R, Dieterich M. Cortical alterations in phobic postural vertigo—a multimodal imaging approach. Ann Clin Transl Neurol. 2018;5:717–29.
Huppert D, Strupp M, Rettinger N, Hecht J, Brandt T. Phobic postural vertigo – a long-term follow-up (5 to 15 years) of 106 patients. J Neurol. 2005;252:564–9.
Staab JP. Chronic subjective dizziness. Continuum (Minneap Minn). 2012;18:1118–41.
Kabaya K, Tamai H, Okajima A, Minakata T, Kondo M, Nakayama M, Iwasaki S. Presence of exacerbating factors of persistent perceptual-postural dizziness in patients with vestibular symptoms at initial presentation. Laryngoscope Investig Otolaryngol. 2021;7(2):499–505.
Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. JAMA Otolaryngol Head Neck Surg. 1990;116:424–7.
Graham MK, Staab JP, Lohse CM, McCaslin DL. A comparison of dizziness handicap inventory scores by categories of vestibular diagnoses. Otol Neurotol. 2021;42(1):129–36.
Indovina I, Passamonti L, Mucci V, Chiarella G, Lacquaniti F, Staab JP. Brain correlates of persistent posturalperceptual dizziness: a review of neuroimaging studies. J Clin Med. 2021;10(18):4274.
Chiarella G, Petrolo C, Riccelli R, Giofrè L, Olivadese G, Gioacchini FM, Scarpa A, Cassandro E, Passamonti L. Chronic subjective dizziness: analysis of underlying personality factors. J Vestib Res. 2016;26(4):403–8.
Staab JP, Rohe DE, Eggers SD, Shepard NT. Anxious, introverted personality traits in patients with chronic subjective dizziness. J Psychosom Res. 2014;76(1):80–3.
Yan Z, Cui L, Yu T, Liang H, Wang Y, Chen C. Analysis of the characteristics of persistent postural-perceptual dizziness: a clinical-based study in China. Int J Audiol. 2016;56:1–5.
Godemann F, Koffroth C, Neu P, Heuser I. Why does vertigo become chronic after neuropathia vestibularis? Psychosom Med. 2004;66(5):783–7.
Tschan R, Best C, Beutel ME, Knebel A, Wiltink J, Dieterich M, Eckhardt-Henn A. Patients’ psychological well-being and resilient coping protect from secondary somatoform vertigo and dizziness (SVD) 1 year after vestibular disease. J Neurol. 2011;258:104–12.
Best C, Tschan R, Eckhardt-Henn A, Dieterich M. Who is at risk for ongoing dizziness and psychological strain after a vestibular disorder? Neuroscience. 2009;164:1579–87.
Heinrichs N, Edler C, Eskens S, Mielczarek MM, Moschner C. Predicting continued dizziness after an acute peripheral vestibular disorder. Psychosom Med. 2007;69:700–7.
Staab JP, Ruckenstein MJ. Chronic dizziness and anxiety: effect of course of illness on treatment outcome. Arch Otolaryngol Head Neck Surg. 2005;131(8):675–9.
Cousins S, Kaski D, Cutfield N, Arshad Q, Ahmad H, Gresty MA, Seemungal BM, Golding J, Bronstein AM. Predictors of clinical recovery from vestibular neuritis: a prospective study. Ann Clin Transl Neurol. 2017;4:340–6.
Pollak L, Klein C, Rafael S, Vera K, Rabey JM. Anxiety in the first attack of vertigo. Otolaryngol Head Neck Surg. 2003;128:829–34.
Godemann F, Siefert K, Hantschke-Bruggemann M, Neu P, Seidl R, Ströhle A. What accounts for vertigo one year after neuritis vestibularis—anxiety or a dysfunctional vestibular organ? J Psychiatr Res. 2005;39:529–34.
Trinidade A, Harman P, Stone J, Staab JP, Goebel JA. Assessment of potential risk factors for the development of persistent postural-perceptual dizziness: a case-control pilot study. Front Neurol. 2021;21(11):601883.
Wolf J, Sattel H, Limburg K, Lahmann C. From illness perceptions to illness reality? Perceived consequences and emotional representations relate to handicap in patients with vertigo and dizziness. J Psychosom Res. 2020;130:109934.
Krafczyk S, Schlamp V, Dieterich M, et al. Increased body sway at 3.5–8 Hz in patients with phobic postural vertigo. Neurosci Lett. 1999;259:149–52.
Ödman M, Maire R. Chronic subjective dizziness. Acta Otolaryngol. 2018;128:1085–8.
Wuehr M, Pradhan C, Novozhilov S, Krafczyk S, Brandt T, Jahn K, Schniepp R. Inadequate interaction between open- and closed-loop postural control in phobic postural vertigo. J Neurol. 2013;260(5):1314–23.
Querner V, Krafczyk S, Dieterich M, Brandt T. Patients with somatoform phobic postural vertigo: the more difficult the balance task, the better the balance performance. Neurosci Lett. 2000;285:21–4.
Schniepp R, Wuehr M, Pradhan C, Novozhilov S, Krafczyk S, Brandt T, Jahn K. Nonlinear variability of body sway in patients with phobic postural vertigo. Front Neurol. 2013;4:115. https://doi.org/10.3389/fneur.2013.00115.
McCaslin DL, Shepard NT, Hollman JH, Staab JP. Characterization of postural sway in patients with persistent postural-perceptual dizziness (PPPD) using wearable motion sensors. Otol Neurotol. 2022;43(2):e243–51.
Schniepp R, Wuehr M, Huth S, Pradhan C, Brandt T, Jahn K. Gait characteristics of patients with phobic postural vertigo: effects of fear of falling, attention, and visual input. J Neurol. 2014;261:738–46.
Teggi R, Gatti O, Cangiano J, Fornasari F, Bussi M. Functional head impulse test with and without optokinetic stimulation in subjects with persistent postural perceptual dizziness (PPPD): preliminary report. Otol Neurotol. 2020;41:e70–5.
Indovina I, Riccelli R, Chiarella G, Petrolo C, Augimeri A, Giofrè L, Lacquaniti F, Staab J, Passamonti L. Role of the insula and vestibular system in patients with chronic subjective dizziness: an fMRI study using sound-evoked vestibular stimulation. Front Behav Neurosci. 2015;9:334.
Riccelli R, Passamonti L, Toschi N, Nigro S, Chiarella G, Petrolo C, Lacquaniti F, Staab J, Indovina I. Altered insular and occipital responses to simulated vertical self-motion in patients with persistent posturalperceptual dizziness. Front Neurol. 2017;8:529.
Passamonti L, Riccelli R, Lacquaniti F, Staab JP, Indovina I. Brain responses to virtual reality visual motion stimulation are affected by neurotic personality traits in patients with persistent postural-perceptual dizziness. J Vestib Res. 2019;28:369–78.
Roberts R, Ahmad H, Patel M, Dima D, Ibitoye R, Sharif M, Leech R, Arshad Q, Bronstein A. An fMRI study of visuo-vestibular interactions following vestibular neuritis. Neuroimage Clin. 2018;20:1010–7.
Van Ombergen A, Heine L, Jillings S, Roberts RE, Jeurissen B, van Rompaey V, Mucci V, Vanhecke S, Sijbers J, Vanhevel F, et al. Altered functional brain connectivity in patients with visually induced dizziness. Neuroimage Clin. 2017;14:538–45.
Lee J-O, Lee E-S, Kim J-S, Lee Y-B, Jeong Y, Choi BS, Kim J-H, Staab JP. Altered brain function in persistent postural perceptual dizziness: a study on resting state functional connectivity. Hum Brain Mapp. 2018;39:3340–53.
Li K, Si L, Cui B, Ling X, Shen B, Yang X. Altered spontaneous functional activity of the right precuneus and cuneus in patients with persistent postural-perceptual dizziness. Brain Imaging Behav. 2020;14:2176–86.
Li K, Si L, Cui B, Ling X, Shen B, Yang X. Altered intra- and inter-network functional connectivity in patients with persistent postural-perceptual dizziness. Neuroimage Clin. 2020;26:102216.
Na S, Im JJ, Jeong H, Lee E-S, Lee T-K, Chung Y-A, Song I-U. Cerebral perfusion abnormalities in patients with persistent postural-perceptual dizziness (PPPD): a SPECT study. J Neural Transm. 2019;126:123–9.
Nigro S, Indovina I, Riccelli R, Chiarella G, Petrolo C, Lacquaniti F, Staab JP, Passamonti L. Reduced cortical folding in multi-modal vestibular regions in persistent postural perceptual dizziness. Brain Imaging Behav. 2019;13:798–809.
Wurthmann S, Naegel S, Steinberg BS, Theysohn N, Diener H-C, Kleinschnitz C, Obermann M, Holle D. Cerebral gray matter changes in persistent postural perceptual dizziness. J Psychosom Res. 2017;103:95–101.
von Söhsten Lins EMD, Bittar RSM, Bazán PR, Júnior EA, Staab JP. Cerebral responses to stationary emotional stimuli measured by fMRI in women with persistent postural-perceptual dizziness. Int Arch Otorhinolaryngol. 2021;25:e355–64.
Breinbauer HA, Contreras MD, Lira JP, Guevara C, Castillo L, Ruëdlinger K, Muñoz D, Delano PH. Spatial navigation is distinctively impaired in persistent postural perceptual dizziness. Front Neurol. 2020;10:1361. https://doi.org/10.3389/fneur.2019.01361.
Rizk HG, Sharon JD, Lee JA, Thomas C, Nguyen SA, Meyer TA. Cross-sectional analysis of cognitive dysfunction in patients with vestibular disorders. Ear Hearing. 2020;41:1020–7.
Shepard NT, Telian SA. Programmatic vestibular rehabilitation. Otolaryngol Head Neck Surg. 1995;112(1):173–82.
Thompson KJ, Goetting JC, Staab JP, Shepard NT. Retrospective review and telephone follow-up to evaluate a physical therapy protocol for treating persistent postural-perceptual dizziness: a pilot study. J Vestib Res. 2015;25(2):97–103.
Nada EH, Ibraheem OA, Hassaan MR. Vestibular rehabilitation therapy outcomes in patients with persistent postural-perceptual dizziness. Ann Otol Rhinol Laryngol. 2019;128(4):323–9.
Min S, Kim JS, Park HY. Predictors of treatment response to pharmacotherapy in patients with persistent posturalperceptual dizziness. J Neurol. 2021;268(7):2523–32.
Yu Y-C, Xue H, Zhang Y-X, Zhou J. Cognitive behavior therapy as augmentation for sertraline in treating patients with persistent postural-perceptual dizziness. BioMed Res Int. 2018;2018:Article ID 8518631.
Kuwabara J, Kondo M, Kabaya K, Watanabe W, Shiraishi N, Sakai M, Toshishige Y, Ino K, Nakayama M, Iwasaki S, Akechi T. Acceptance and commitment therapy combined with vestibular rehabilitation for persistent postural-perceptual dizziness: a pilot study. Am J Otolaryngol. 2020;41(6):102609.
Axer H, Finn S, Wasserman A, Guntinas-Lichius O, Klingner CM, Witte OW. Multimodal treatment of persistent postural–perceptual dizziness. Brain Behav. 2020;10(12):e01864.
Acknowledgement
Dr Staab was supported by grant W81XWH1810760 from the U.S. Army Medical Research and Materiel Command via the Congressionally Directed Medical Research Program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Staab, J.P. (2023). Persistent Postural-Perceptual Dizziness. In: Crane, B.T., Lustig, L., de Souza, C. (eds) Disorders of the Vestibular System. Springer, Cham. https://doi.org/10.1007/978-3-031-40524-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-40524-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40523-5
Online ISBN: 978-3-031-40524-2
eBook Packages: MedicineMedicine (R0)